Effect of Acid and Thermo-Mechanical Attacks on Compressive Strength of Geopolymer Mortar with Different Eco-Friendly Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design and Sample Preparation
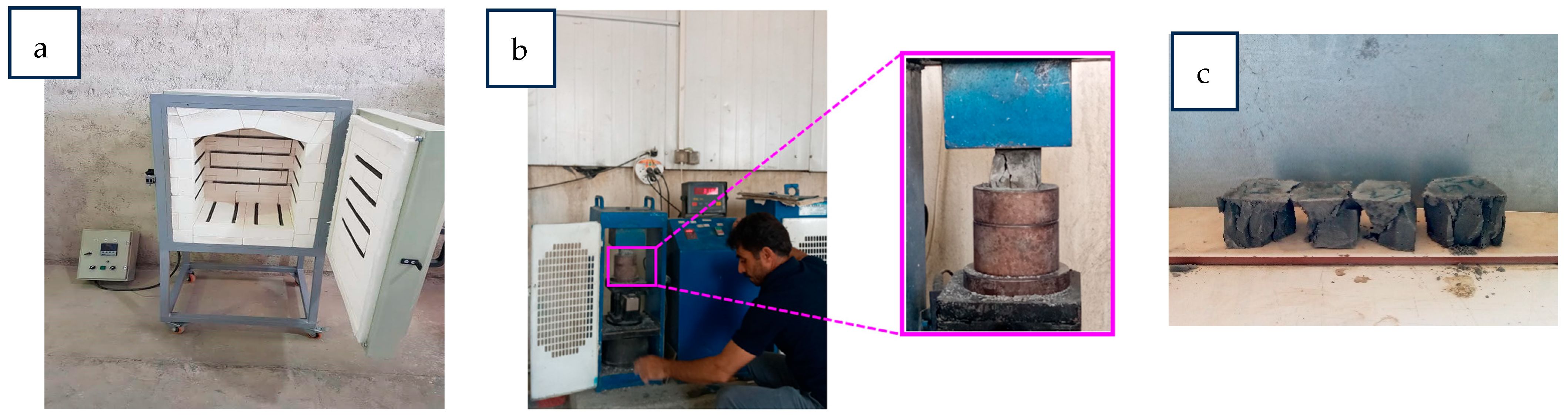
2.3. Details of the Testing Procedure
2.3.1. Compressive Strength
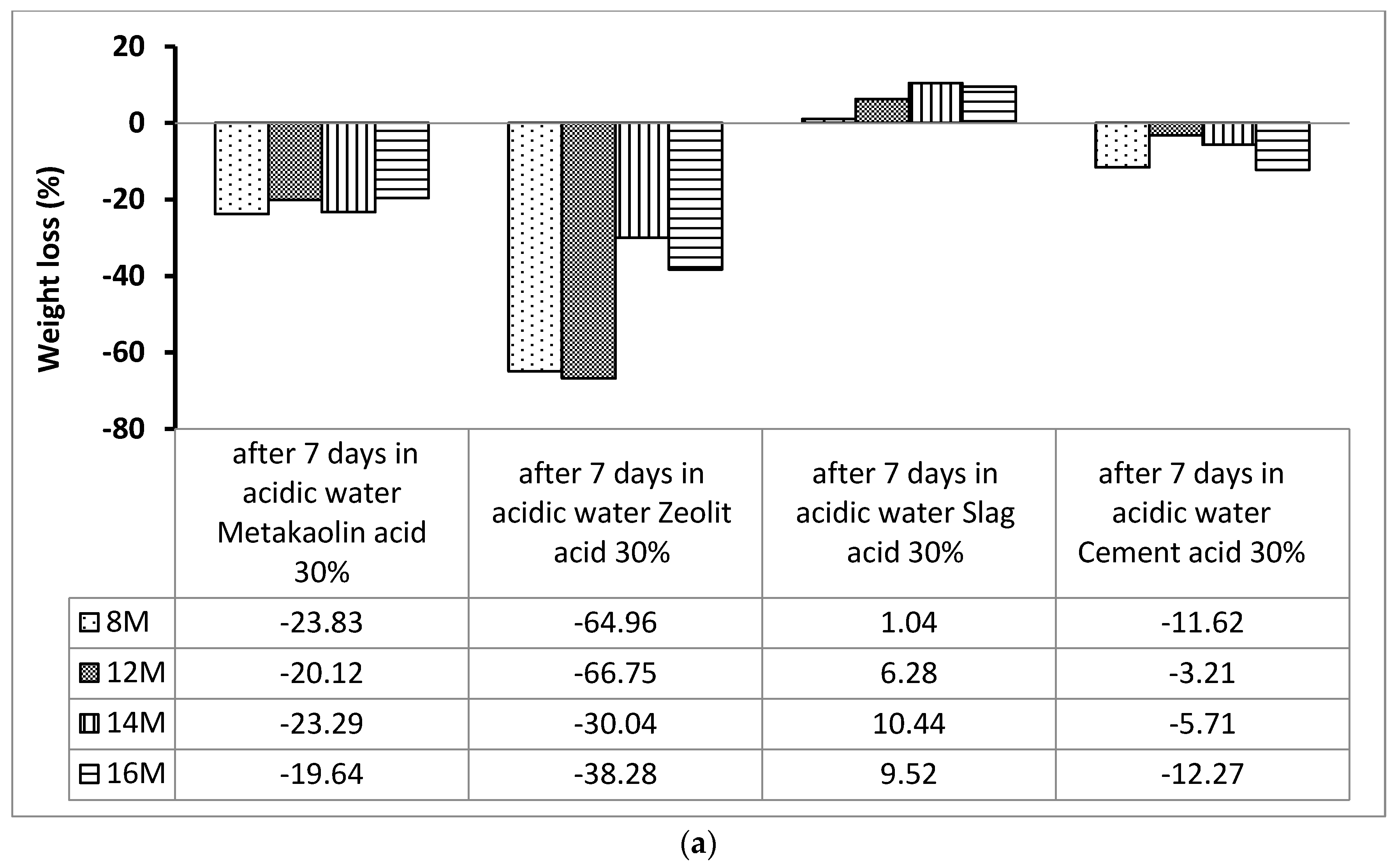
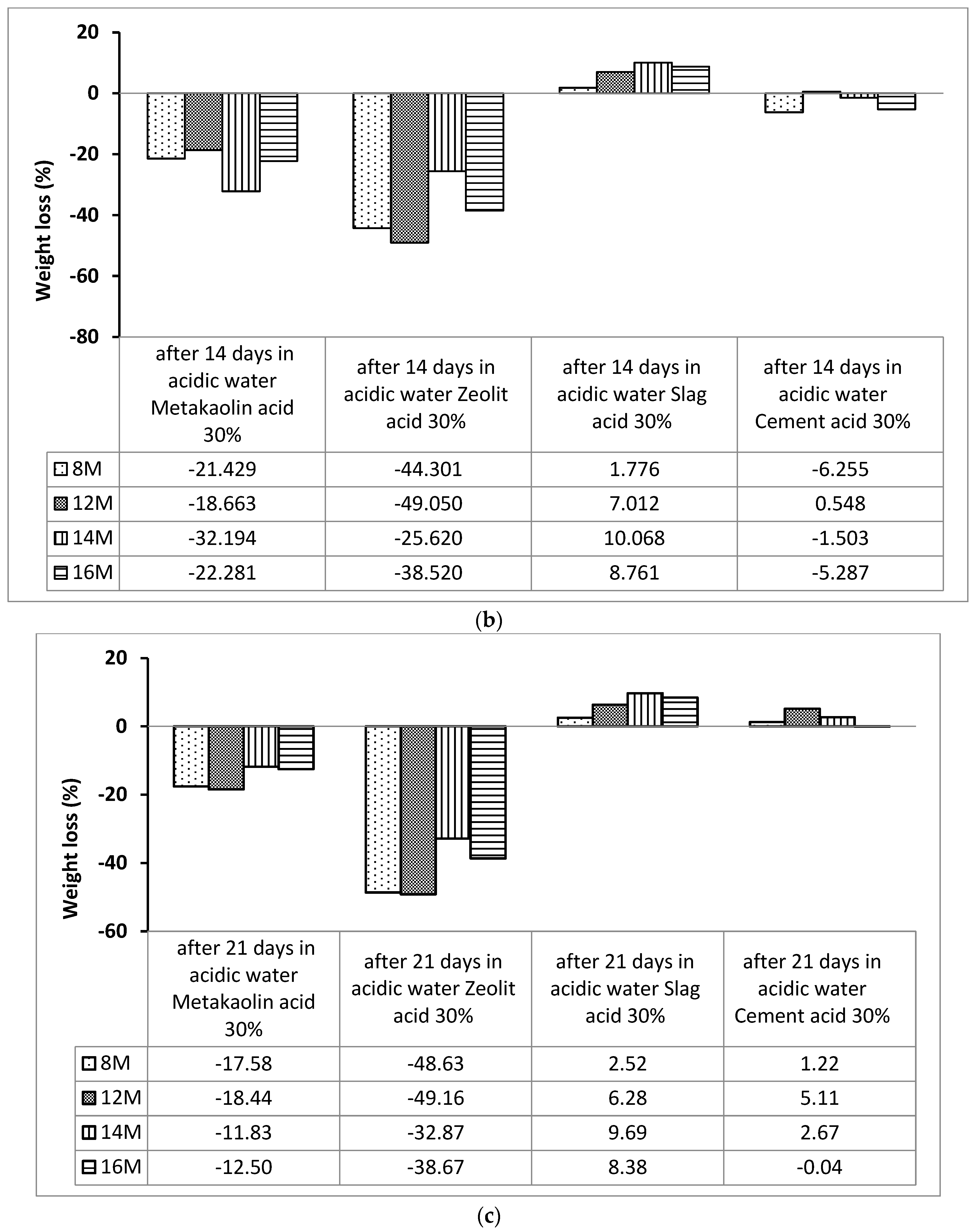
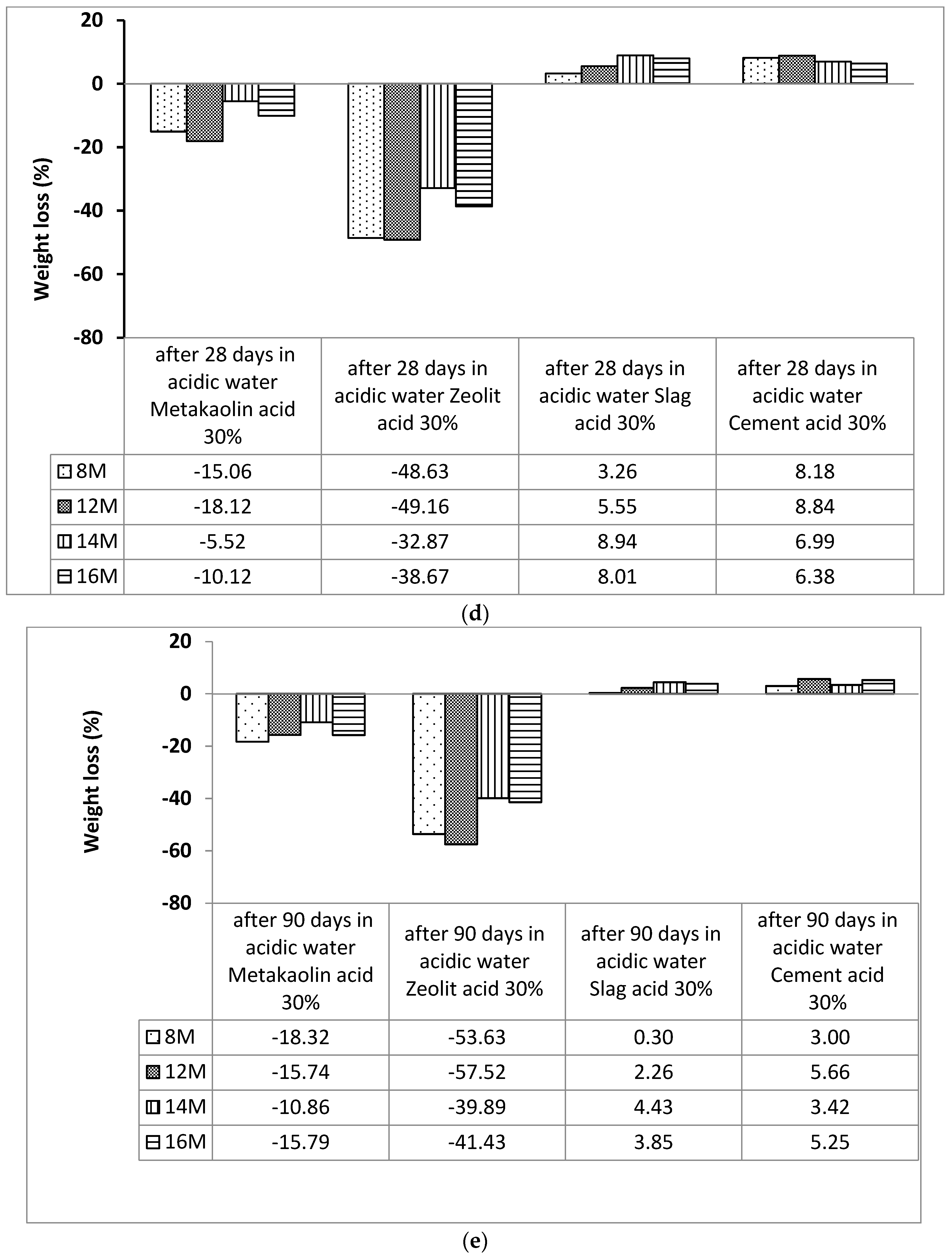
2.3.2. Acid Attack
2.3.3. Thermal Deformation
2.3.4. SEM Analysis
3. Results and Discussion
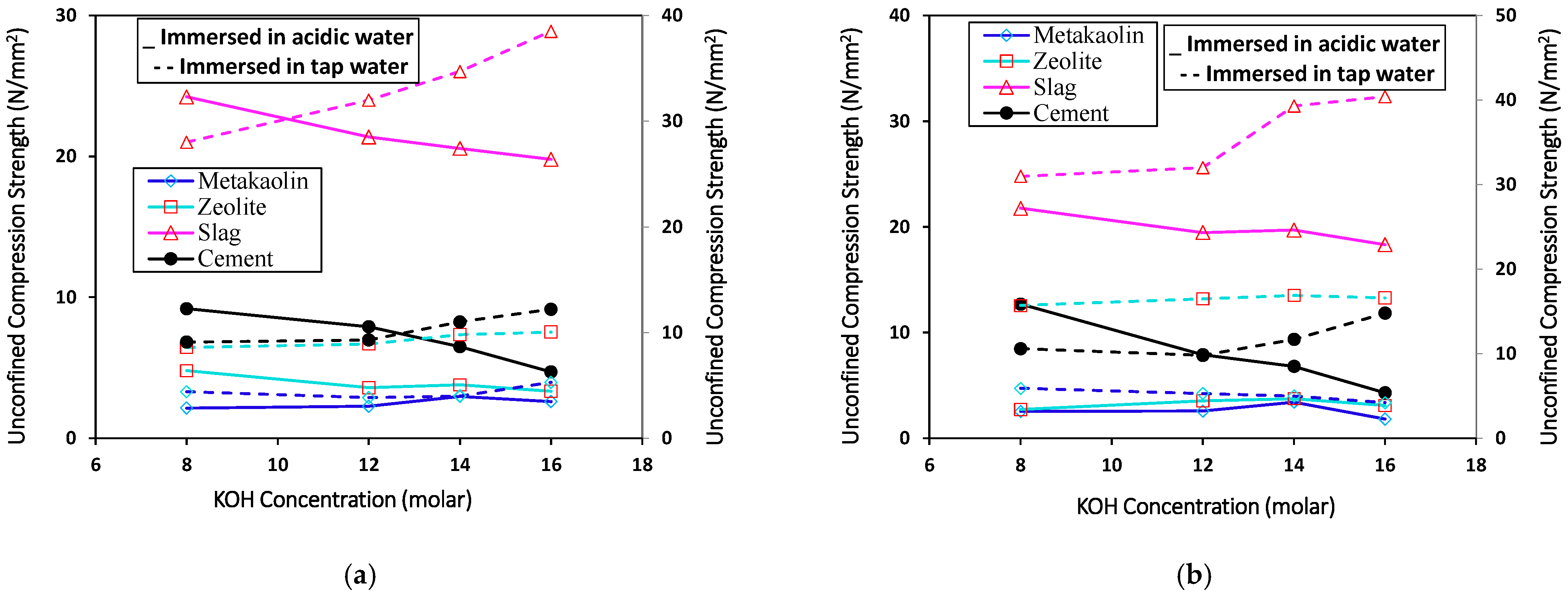
3.1. Acid Attack
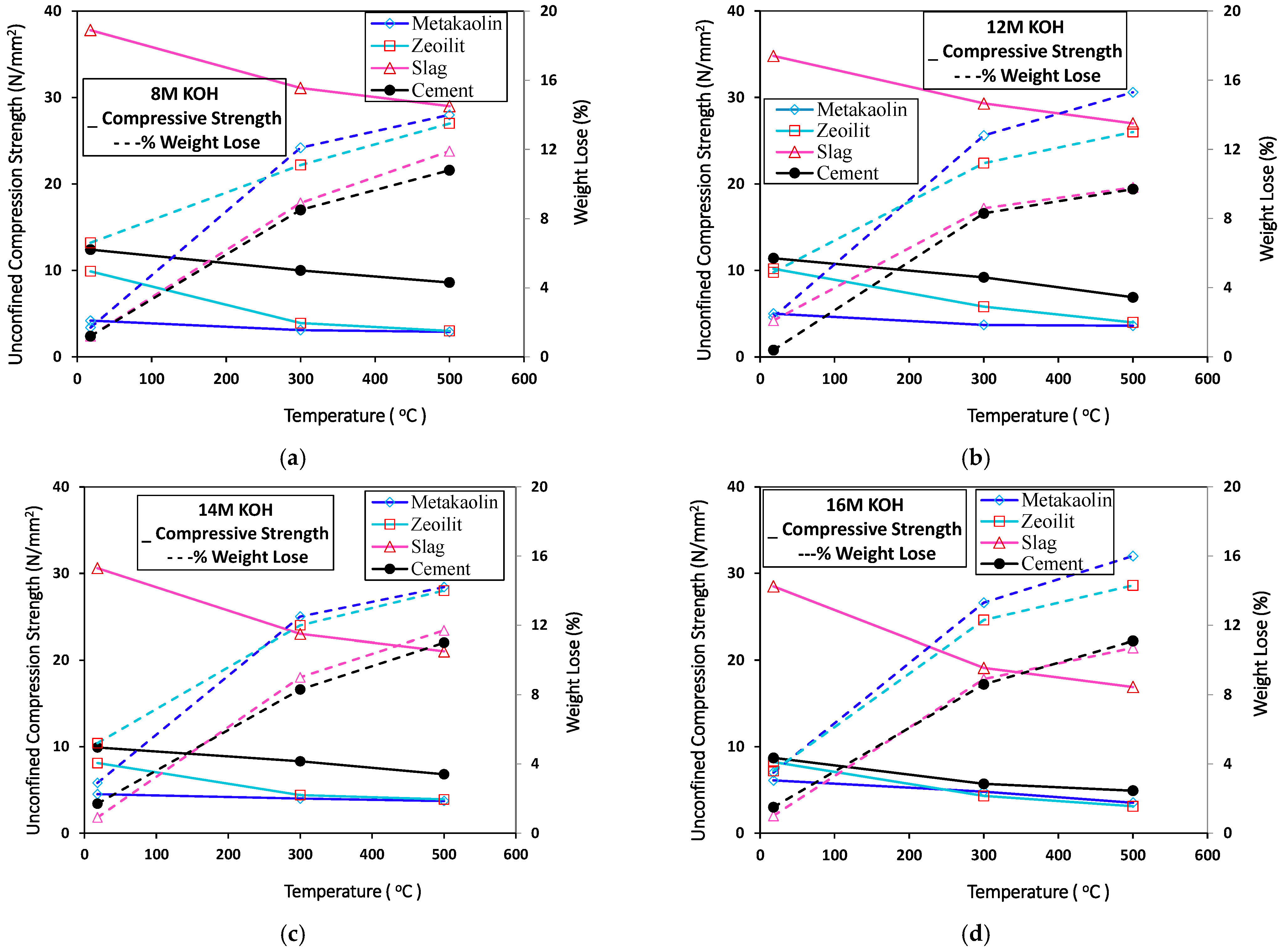
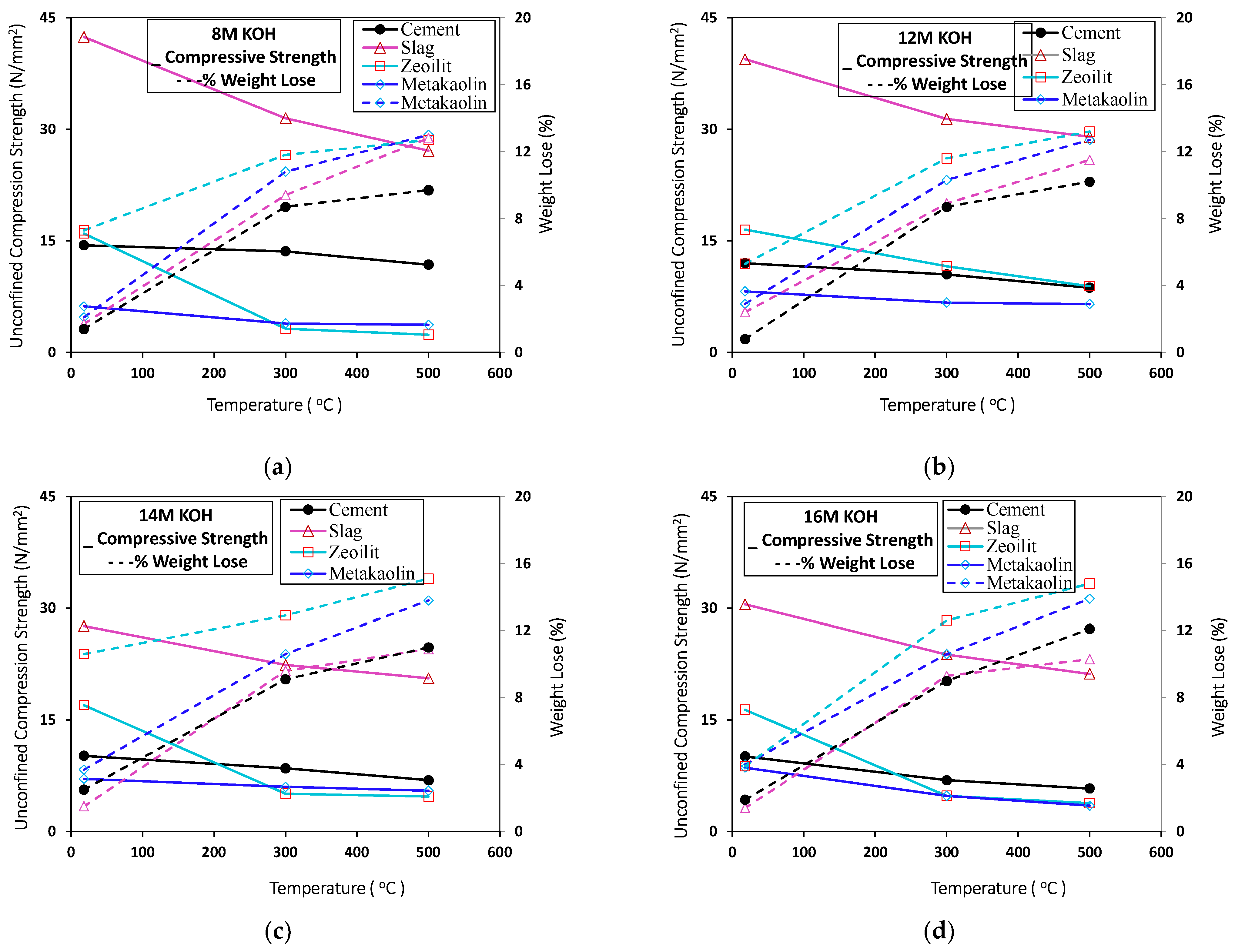
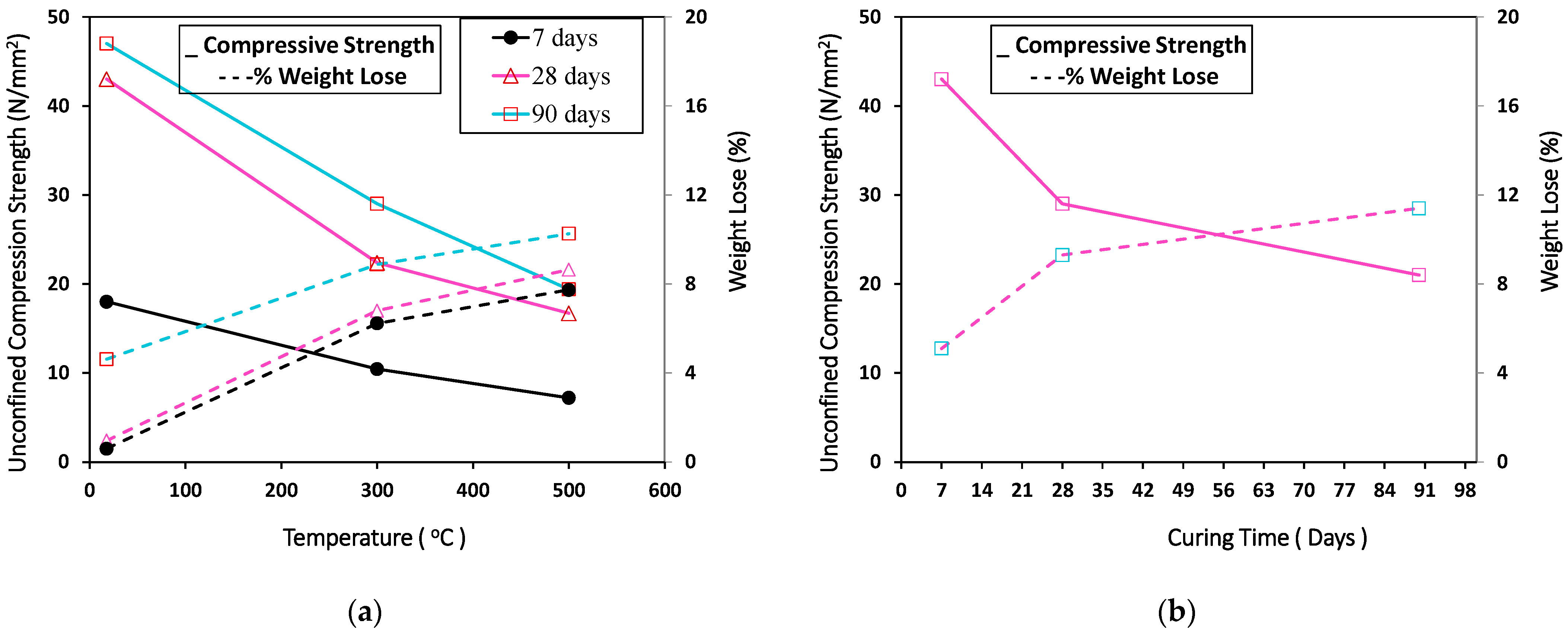
3.2. Effect of High Temperature
3.3. Comparing the Standard Mortar with Geopolymer Mortars
3.4. SEM
4. Practical Implications and Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosoaga, A.; Masek, O.; Oakey, J.E. CO2 Capture Technologies for Cement Industry. Energy Procedia 2009, 1, 133–140. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A. Mineralogical and microstructural characterisation of alkali-activated fly ash/slag pastes. Cem. Concr. Compos. 2003, 25, 287–292. [Google Scholar] [CrossRef]
- Naik, T.R. Sustainability of concrete construction. Pract. Period. Struct. Des. Constr. 2008, 13, 98–103. [Google Scholar] [CrossRef]
- Shaikh, F.U.A. Mechanical and durability properties of fly ash geopolymer concrete containing recycled coarse aggregates. Int. J. Sustain. Built Environ. 2016, 5, 277–287. [Google Scholar] [CrossRef]
- Aly, A.M.; El-Feky, M.S.; Kohail, M.; Nasr, E.-S.A.R. Performance of geopolymer concrete containing recycled rubber. Constr. Build. Mater. 2019, 207, 136–144. [Google Scholar] [CrossRef]
- Mirzababaei, M.; Karimiazar, J.; Sharifi Teshnizi, E.; Arjmandzadeh, R.; Bahmani, S.H. Effect of nano-additives on the strength and durability characteristics of marl. Minerals 2021, 11, 1119. [Google Scholar] [CrossRef]
- Belferrag, A.; Kriker, A.; Abboudi, S.; Tié Bi, S. Effect of granulometric correction of dune sand and pneumatic waste metal fibers on shrinkage of concrete in arid climates. J. Clean. Prod. 2016, 112, 3048–3056. [Google Scholar] [CrossRef]
- Belferrag, A.; Kriker, A.; Youcef, F.; Abboudi, S.; Tié Bi, S. Thermal Conductivity of Dune Sand Concrete Reinforced with Pneumatic Waste Metal Fibers. Int. J. Thermophys. 2022, 43, 140. [Google Scholar] [CrossRef]
- Qi, A.; Liu, X.; Wang, Z.; Chen, Z. Mechanical properties of the concrete containing ferronickel slag and blast furnace slag powder. Constr. Build. Mater. 2020, 231, 117120. [Google Scholar] [CrossRef]
- Amin, M.N.; Ahmad, W.; Khan, K.; Sayed, M.M. Mapping Research Knowledge on Rice Husk Ash Application in Concrete: A Scientometric Review. Materials 2022, 15, 3431. [Google Scholar] [CrossRef]
- Gerges, N.N.; Issa, C.A.; Sleiman, E.; Aintrazi, S.; Saadeddine, J.; Abboud, R.; Antoun, M. Eco-Friendly Optimum Structural Concrete Mix Design. Sustainability 2022, 14, 8660. [Google Scholar] [CrossRef]
- Sandanayake, M.; Bouras, Y.; Haigh, R.; Vrcelj, Z. Current sustainable trends of using waste materials in concrete—A decade review. Sustainability 2020, 12, 9622. [Google Scholar] [CrossRef]
- Karimizad, N.; Teshnizi, E.S.; Mahdad, M.; Karimiazar, J. Investigating the design features of CSG dams. JOJ Sci. 2020, 2, 60–66. [Google Scholar] [CrossRef]
- Sharifi Teshnizi, E.; Hoseini, Z.; Babakhani, P.; Mohamadi Anaie, H. Geodetic and Geotechnical Monitoring in Shallow and Urban Tunnels; Azarin Mehr: Tehran, Iran, 2021; ISBN 978-622-685544-0. [Google Scholar]
- Abharian, S.; Sarfarazi, V.; Rasekh, H.; Behzadinasab, M. Effects of concrete/gypsum bedding layers and their inclination angles on the tensile failure mechanism: Experimental and numerical studies. Case Stud. Constr. Mater. 2022, 17, e01272. [Google Scholar] [CrossRef]
- Ahmad, J.; Kontoleon, K.J.; Majdi, A.; Naqash, M.T.; Deifalla, A.F.; Ben Kahla, N.; Isleem, H.F.; Qaidi, S.M.A. A Comprehensive Review on the Ground Granulated Blast Furnace Slag (GGBS) in Concrete Production. Sustainability 2022, 14, 8783. [Google Scholar] [CrossRef]
- Cui, Y.; Gao, K.; Zhang, P. Experimental and statistical study on mechanical characteristics of geopolymer concrete. Materials 2020, 13, 1651. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M.M. Thermo-mechanical characteristics of geopolymer mortar. Constr. Build. Mater. 2019, 213, 100–108. [Google Scholar] [CrossRef]
- Amin, M.; Elsakhawy, Y.; Abu el-hassan, K.; Abdelsalam, B.A. Behavior evaluation of sustainable high strength geopolymer concrete based on fly ash, metakaolin, and slag. Case Stud. Constr. Mater. 2022, 16, e00976. [Google Scholar] [CrossRef]
- Rashad, A.M.; Ezzat, M.; ElNagar, A.M.; El-Nashar, M.H. Valorization of limestone powder as an additive for fly ash geopolymer cement under the effect of the simulated tidal zone and seawater attack. Constr. Build. Mater. 2023, 369, 130616. [Google Scholar] [CrossRef]
- Gultekin, A.; Ramyar, K. Investigation of high-temperature resistance of natural pozzolan-based geopolymers produced with oven and microwave curing. Constr. Build. Mater. 2023, 365, 130059. [Google Scholar] [CrossRef]
- Tan, J.; Dan, H.; Ma, Z. Metakaolin based geopolymer mortar as concrete repairs: Bond strength and degradation when subjected to aggressive environments. Ceram. Int. 2022, 48, 23559–23570. [Google Scholar] [CrossRef]
- Lăzărescu, A.V.; Ionescu, B.A.; Hegyi, A.; Florean, C. Alkali-activated fly ash based geopolymer paving blocks: Green materials for future conservation of resources. Int. J. Conserv. Sci. 2022, 13, 175–186. [Google Scholar]
- Li, Z.; Lu, T.; Liang, X.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Mohammadifar, L.; Miraki, H.; Rahmani, A.; Jahandari, S.; Mehdizadeh, B.; Rasekh, H.; Samadi, P.; Samali, B. Properties of Lime-Cement Concrete Containing Various Amounts of Waste Tire Powder under Different Ground Moisture Conditions. Polymers 2022, 14, 482. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Yazdi, A.; Teshnizi, E.S. Improvement of collapsing problematic soils on the sabzevar-mashhad railway route (northeast of Iran) using traditional additives. Nexo Rev. Cient. 2023, 36, 383–403. [Google Scholar] [CrossRef]
- Hardjito, D.; Rangan, B.V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Research Report GC 1; Faculty of Engineering Curtin University of Technology: Perth, Australia, 2005; p. 103. [Google Scholar]
- Hadi, M.N.S.; Al-Azzawi, M.; Yu, T. Effects of fly ash characteristics and alkaline activator components on compressive strength of fly ash-based geopolymer mortar. Constr. Build. Mater. 2018, 175, 41–54. [Google Scholar] [CrossRef]
- van Jaarsveld, J.G.S.; Van Deventer, J.S.J. Effect of the alkali metal activator on the properties of fly ash-based geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Schwartzman, A. The potential use of geopolymeric materials to immobilise toxic metals: Part II. Material and leaching characteristics. Miner. Eng. 1999, 12, 75–91. [Google Scholar] [CrossRef]
- Alouani, M.E.L.; Alehyen, S.; Achouri, M.E.L.; Hajjaji, A.; Ennawaoui, C.; Taibi, M. Influence of the nature and rate of alkaline activator on the physicochemical properties of fly ash-based geopolymers. Adv. Civ. Eng. 2020, 2020, 8880906. [Google Scholar] [CrossRef]
- Panias, D.; Giannopoulou, I.P.; Perraki, T. Effect of synthesis parameters on the mechanical properties of fly ash-based geopolymers. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 301, 246–254. [Google Scholar] [CrossRef]
- Sharma, P.K.; Singh, J.P.; Kumar, A. Effect of particle size on physical and mechanical properties of fly ash based geopolymers. Trans. Indian Inst. Met. 2019, 72, 1323–1337. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Factors affecting the performance of metakaolin geopolymers exposed to elevated temperatures. J. Mater. Sci. 2008, 43, 824–831. [Google Scholar] [CrossRef]
- Li, M.; Luo, R.; Qin, L.; Liu, H.; Duan, P.; Jing, W.; Zhang, Z.; Liu, X. High temperature properties of graphene oxide modified metakaolin based geopolymer paste. Cem. Concr. Compos. 2022, 125, 104318. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Topark-Ngarm, P.; Chindaprasirt, P.; Sata, V. Setting time, strength, and bond of high-calcium fly ash geopolymer concrete. J. Mater. Civ. Eng. 2015, 27, 4014198. [Google Scholar] [CrossRef]
- Kuri, J.C.; Hosan, A.; Shaikh, F.U.A.; Biswas, W.K. The Effect of Recycled Waste Glass as a Coarse Aggregate on the Properties of Portland Cement Concrete and Geopolymer Concrete. Buildings 2023, 13, 586. [Google Scholar] [CrossRef]
- Dai, W.; Wang, Y. Silica Fume Enhances the Mechanical Strength of Alkali-Activated Slag/Fly Ash Pastes Subjected to Elevated Temperatures. Fire 2023, 6, 252. [Google Scholar] [CrossRef]
- Wang, B.; Feng, H.; Huang, H.; Guo, A.; Zheng, Y.; Wang, Y. Bonding Properties between Fly Ash/Slag-Based Engineering Geopolymer Composites and Concrete. Materials 2023, 16, 4232. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, X.; Wang, F.; Wang, J. Mechanical properties and durability of geopolymer recycled aggregate concrete: A review. Polymers 2023, 15, 615. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Xia, J. Compressive Strength and Chloride Resistance of Slag/Metakaolin-Based Ultra-High-Performance Geopolymer Concrete. Materials 2022, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Al Bakri, A.M.M.; Kamarudin, H.; Bnhussain, M.; Rafiza, A.R.; Zarina, Y. Effect of Na2SiO3/NaOH ratios and NaOH molearities on compressive strength of flyash- based geopolymer. ACI Mater. J. 2012, 109, 503. [Google Scholar]
- Arunachelam, N.; Maheswaran, J.; Chellapandian, M.; Ozbakkaloglu, T. Effective Utilization of Copper Slag for the Production of Geopolymer Concrete with Different NaOH Molarity under Ambient Curing Conditions. Sustainability 2022, 14, 16300. [Google Scholar] [CrossRef]
- Sherwani, A.F.H.; Younis, K.H.; Arndt, R.W.; Pilakoutas, K. Performance of Self-Compacted Geopolymer Concrete Containing Fly Ash and Slag as Binders. Sustainability 2022, 14, 15063. [Google Scholar] [CrossRef]
- Sharifi Teshnizi, E.; Reisi Dehkordi, M.S.; Moaiedi Kia, A.; Jamgohari, Y. The Strategic Management of Ballast (Design, Maintenance); Supreme National Defense University: Tehran, Iran, 2021; ISBN 978-622-6350-21-1. [Google Scholar]
- Alanazi, H.; Hu, J.; Kim, Y.-R. Effect of slag, silica fume, and metakaolin on properties and performance of alkali-activated fly ash cured at ambient temperature. Constr. Build. Mater. 2019, 197, 747–756. [Google Scholar] [CrossRef]
- Zhu, Y.; Longhi, M.A.; Wang, A.; Hou, D.; Wang, H.; Zhang, Z. Alkali leaching features of 3-year-old alkali activated fly ash-slag-silica fume: For a better understanding of stability. Compos. Part B Eng. 2022, 230, 109469. [Google Scholar] [CrossRef]
- Supit, S.W.M.; Pandei, R.W. Effects of metakaolin on compressive strength and permeability properties of pervious cement concrete. J. Teknol. 2019, 81. [Google Scholar] [CrossRef]
- Saboo, N.; Shivhare, S.; Kori, K.K.; Chandrappa, A.K. Effect of fly ash and metakaolin on pervious concrete properties. Constr. Build. Mater. 2019, 223, 322–328. [Google Scholar] [CrossRef]
- Mohd Nasir, N.A.; Abu Bakar, N.; Safiee, N.A.; Abdul Aziz, F.N.A. Permeation-durability properties of metakaolin blended concrete containing rubber. Eur. J. Environ. Civ. Eng. 2022, 26, 5113–5128. [Google Scholar] [CrossRef]
- Liang, X.; Ji, Y. Mechanical properties and permeability of red mud-blast furnace slag-based geopolymer concrete. SN Appl. Sci. 2021, 3, 23. [Google Scholar] [CrossRef]
- Moradikhou, A.B.; Safehian, M. Comparison of Mechanical Strengths and Resistance to Acidic Conditions, Permeability and Resistance to Elevated Temperatures of Geopolymer Concrete and Conventional Concrete. Adv. Res. Civ. Eng. 2021, 3, 27–37. [Google Scholar]
- Lim, Y.Y.; Pham, T.M.; Jangra, P.; Arora, S. Influence of Portland cement on performance of fine rice husk ash geopolymer concrete: Strength and permeability properties. Constr. Build. Mater. 2021, 300, 124321. [Google Scholar]
- Sharifi Teshnizi, E.; Arjmandzadeh, R.; Reisi Dehkordi, M.S.; Jollaie, A. Introduction to Building Materials and Concrete Technology; Sokhanvaran Publisher: Tehran, Iran, 2019; ISBN 978-622-215-207-9. [Google Scholar]
- Sharifi Teshnizi, E.; O’Kelly, B.C.; Karimiazar, J.; Moosazadeh, S.; Arjmandzadeh, R.; Pani, A. Effects of cement kiln dust on physicochemical and geomechanical properties of loess soil, Semnan Province, Iran. Arab. J. Geosci. 2022, 15, 1482. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Wongpa, J.; Kiattikomol, K.; Jaturapitakkul, C.; Chindaprasirt, P. Compressive strength, modulus of elasticity, and water permeability of inorganic polymer concrete. Mater. Des. 2010, 31, 4748–4754. [Google Scholar] [CrossRef]
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I.; Wardhono, A. Long term durability properties of class F fly ash geopolymer concrete. Mater. Struct. 2015, 48, 721–731. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 1143–1148. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, G. The shrinkage of alkali activated fly ash. Cem. Concr. Res. 2015, 68, 75–82. [Google Scholar] [CrossRef]
- Rangan, B.V. Mix design and production of flyash based geopolymer concrete. Indian Concr. J. 2008, 82, 7–15. [Google Scholar]
- Afshar, A.; Jahandari, S.; Rasekh, H.; Rahmani, A.; Saberian, M. Effects of Different Coatings, Primers, and Additives on Corrosion of Steel Rebars. Polymers 2023, 15, 1422. [Google Scholar] [CrossRef] [PubMed]
- Neupane, K.; Chalmers, D.; Kidd, P. High-strength geopolymer concrete-properties, advantages and challenges. Adv. Mater. 2018, 7, 15–25. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Ekolu, S.O. Method for comprehensive mix design of fly ash geopolymer mortars. Constr. Build. Mater. 2019, 202, 704–717. [Google Scholar] [CrossRef]
- Abdellatief, M.; Alanazi, H.; Radwan, M.K.H.; Tahwia, A.M. Multiscale characterization at early ages of ultra-high performance geopolymer concrete. Polymers 2022, 14, 5504. [Google Scholar] [CrossRef]
- John, S.K.; Nadir, Y.; Girija, K. Effect of source materials, additives on the mechanical properties and durability of fly ash and fly ash-slag geopolymer mortar: A review. Constr. Build. Mater. 2021, 280, 122443. [Google Scholar] [CrossRef]
- Kotop, M.A.; El-Feky, M.S.; Alharbi, Y.R.; Abadel, A.A.; Binyahya, A.S. Engineering properties of geopolymer concrete incorporating hybrid nano-materials. Ain Shams Eng. J. 2021, 12, 3641–3647. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.M.Y.; Almakayeel, N.M.; Alghamdi, S. Review on the relationship between nano modifications of geopolymer concrete and their structural characteristics. Polymers 2022, 14, 1421. [Google Scholar] [CrossRef]
- Abd, D.M.; Al-Khalid, H. Fatigue Characterization of WMA and Modeling Using Artificial Neural Networks. J. Mater. Civ. Eng. 2022, 34, 4021467. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Ahmed Shaikh, F.U. Tensile strain hardening behavior of PVA fiber-reinforced engineered geopolymer composite. J. Mater. Civ. Eng. 2015, 27, 4015001. [Google Scholar] [CrossRef]
- Sata, V.; Sathonsaowaphak, A.; Chindaprasirt, P. Resistance of lignite bottom ash geopolymer mortar to sulfate and sulfuric acid attack. Cem. Concr. Compos. 2012, 34, 700–708. [Google Scholar] [CrossRef]
- ASTM C33/C33M; Standard Specification for Concrete Aggregates. ASTM: West Conshohocken, PA, USA, 2017.
- Alanazi, H.; Yang, M.; Zhang, D.; Gao, Z. Bond strength of PCC pavement repairs using metakaolin-based geopolymer mortar. Cem. Concr. Compos. 2016, 65, 75–82. [Google Scholar] [CrossRef]
- C109/C109M; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM: West Conshohocken, PA, USA, 2016.
- ASTM C267-97; Standard Test Methods for Chemical Resistance of Mortars, Grouts and Monolithic Surfacings and Polymer Concretes. ASTM: West Conshohocken, PA, USA, 1997.
- ASTM C1012-95a; Standard Test Method for Length Change of Hydroulic-Cement Mortars Exposed to a Sulfate Solution. ASTM: West Conshohocken, PA, USA, 2004; pp. 450–456.
- ASTM C157; Standard Test Method for Length Change of Hardened Hydraulic-Cement Mortar and Concrete. ASTM: West Conshohocken, PA, USA, 2010.
- Oelkers, E.H. General kinetic description of multioxide silicate mineral and glass dissolution. Geochim. Cosmochim. Acta 2001, 65, 3703–3719. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Yi, M.; Cai, Q.; Zhang, Z. Mechanical and durability properties of metakaolin blended with slag geopolymer mortars used for pavement repair. Constr. Build. Mater. 2021, 281, 122566. [Google Scholar] [CrossRef]
- Hartman, R.L.; Fogler, H.S. Reaction kinetics and mechanisms of zeolite dissolution in hydrochloric acid. Ind. Eng. Chem. Res. 2005, 44, 7738–7745. [Google Scholar] [CrossRef]
- Hartman, R.L.; Fogler, H.S. Understanding the dissolution of zeolites. Langmuir 2007, 23, 5477–5484. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, H.L.; Yin, H.; Waller, A.; Khosravi, A.; Lind, M.L. Impact of acids on the structure and composition of Linde Type A zeolites for use in reverse osmosis membranes for recovery of urine-containing wastewaters. Microporous Mesoporous Mater. 2015, 201, 50–60. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Ariffin, M.A.M.; Bhutta, M.A.R.; Hussin, M.W.; Mohd Tahir, M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Deb, P.S.; Sarker, P.K.; Barbhuiya, S. Sorptivity and acid resistance of ambient-cured geopolymer mortars containing nano-silica. Cem. Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.J.G.; Jäger, C.; Brouwers, H.J.H.; Kühne, H.C. Sulfuric acid resistance of one-part alkali-activated mortars. Cem. Concr. Res. 2018, 109, 54–63. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Zhang, H.; Li, M.; Wu, Y.; Guo, L.; Wang, W.; Duan, P.; Zhang, W.; Zhang, Z. Thermal stability and microstructure of metakaolin-based geopolymer blended with rice husk ash. Appl. Clay Sci. 2020, 196, 105769. [Google Scholar] [CrossRef]
- Saridemir, M.; Severcan, M.H.; Ciflikli, M.; Celikten, S.; Ozcan, F.; Atis, C.D. The influence of elevated temperature on strength and microstructure of high strength concrete containing ground pumice and metakaolin. Constr. Build. Mater. 2016, 124, 244–257. [Google Scholar] [CrossRef]
- Hussin, M.W.; Bhutta, M.A.R.; Azreen, M.; Ramadhansyah, P.J.; Mirza, J. Performance of blended ash geopolymer concrete at elevated temperatures. Mater. Struct. Constr. 2015, 48, 709–720. [Google Scholar] [CrossRef]
- Akca, A.H.; Zihnioǧlu, N.Ö. High performance concrete under elevated temperatures. Constr. Build. Mater. 2013, 44, 317–328. [Google Scholar] [CrossRef]
- Alexander, A.E.; Shashikala, A.P. Studies on the microstructure and durability characteristics of ambient cured FA-GGBS based geopolymer mortar. Constr. Build. Mater. 2022, 347, 128538. [Google Scholar] [CrossRef]
- Payakaniti, P.; Chuewangkam, N.; Yensano, R.; Pinitsoontorn, S.; Chindaprasirt, P. Changes in compressive strength, microstructure and magnetic properties of a high-calcium fly ash geopolymer subjected to high temperatures. Constr. Build. Mater. 2020, 265, 120650. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition-A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Klima, K.M.; Schollbach, K.; Brouwers, H.J.H.; Yu, Q. Enhancing the thermal performance of Class F fly ash-based geopolymer by sodalite. Constr. Build. Mater. 2022, 314, 125574. [Google Scholar] [CrossRef]


| Component (%) | Al2O3 | SiO2 | CaO | Fe2O3 | Na2O | MgO | SO3 | K2O | L.O.I * |
|---|---|---|---|---|---|---|---|---|---|
| Metakaolin | 40.43 | 56.2 | 0.17 | 0.59 | 0.04 | 0.32 | - | 0.73 | 1.52 |
| Zeolite | 8.9 | 58.85 | 10.8 | 6.0 | 0.23 | - | 1.3 | 5.32 | 8.6 |
| Slag | 11.32 | 36.2 | 36.9 | 0.63 | 0.42 | 11.31 | 2.73 | 0.49 | - |
| Cement | 5.53 | 23.13 | 58.95 | 3.51 | 0.33 | 1.18 | 2.19 | 0.85 | 4.33 |
| Fineness Modulus | Density (g/cm3) | Specific Gravity | Cumulative Mass Percentage (%) | |||||
|---|---|---|---|---|---|---|---|---|
| >2.38 mm | 2.38–1.18 | 1.18–0.6 | 0.6–0.3 | 0.3–0.15 | <0.15 mm | |||
| 2.25 | 1.69 | 2.65 | 0 | 19.5 | 54.5 | 68.1 | 80.1 | 100 |
| Mix Design | Geopolymer (gr) | KOH (Powder) (g) | Na2SiO3 (Powder) (g) | Water (g) | Sand (g) | |
|---|---|---|---|---|---|---|
| MK-1 | Metakaolin | 800 | 202 | 105 | 360 | 2200 |
| MK-2 | 800 | 303 | 105 | 360 | 2200 | |
| MK-3 | 800 | 354 | 105 | 360 | 2200 | |
| MK-4 | 800 | 404 | 105 | 360 | 2200 | |
| MZ-5 | Zeolite | 800 | 202 | 105 | 360 | 2200 |
| MZ-6 | 800 | 303 | 105 | 360 | 2200 | |
| MZ-7 | 800 | 354 | 105 | 360 | 2200 | |
| MZ-8 | 800 | 404 | 105 | 360 | 2200 | |
| MS-9 | Slag | 800 | 202 | 105 | 360 | 2200 |
| MS-10 | 800 | 303 | 105 | 360 | 2200 | |
| MS-11 | 800 | 354 | 105 | 360 | 2200 | |
| MS-12 | 800 | 404 | 105 | 360 | 2200 | |
| Standard mortar | Cement | 800 | - | - | 360 | 2200 |
| MC-13 | 800 | 202 | 105 | 360 | 2200 | |
| MC-14 | 800 | 303 | 105 | 360 | 2200 | |
| MC-15 | 800 | 354 | 105 | 360 | 2200 | |
| MC-16 | 800 | 404 | 105 | 360 | 2200 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teshnizi, E.S.; Karimiazar, J.; Baldovino, J.A. Effect of Acid and Thermo-Mechanical Attacks on Compressive Strength of Geopolymer Mortar with Different Eco-Friendly Materials. Sustainability 2023, 15, 14407. https://doi.org/10.3390/su151914407
Teshnizi ES, Karimiazar J, Baldovino JA. Effect of Acid and Thermo-Mechanical Attacks on Compressive Strength of Geopolymer Mortar with Different Eco-Friendly Materials. Sustainability. 2023; 15(19):14407. https://doi.org/10.3390/su151914407
Chicago/Turabian StyleTeshnizi, Ebrahim Sharifi, Jafar Karimiazar, and Jair Arrieta Baldovino. 2023. "Effect of Acid and Thermo-Mechanical Attacks on Compressive Strength of Geopolymer Mortar with Different Eco-Friendly Materials" Sustainability 15, no. 19: 14407. https://doi.org/10.3390/su151914407
APA StyleTeshnizi, E. S., Karimiazar, J., & Baldovino, J. A. (2023). Effect of Acid and Thermo-Mechanical Attacks on Compressive Strength of Geopolymer Mortar with Different Eco-Friendly Materials. Sustainability, 15(19), 14407. https://doi.org/10.3390/su151914407







