Abstract
The market for plant-based meat alternatives is growing to meet consumer demands for a more sustainable, ethical, and healthy diet, as well as to address global food security issues linked to an increasing global population and climate change. Increased consumption of plant-based meat products raises questions about potential food safety risks, including concerns about allergenicity, toxicity, foodborne pathogens, and adequate nutritional composition. From a public health perspective, there has been limited research on the nutritional and health aspects of plant-based meat products, and studies of potential food safety risks of these novel protein sources are not well documented. Much of the research on the nutrition and safety of these foods has been commissioned or funded by companies developing these products, or by other organizations promoting them. This article reviews the existing literature and analyses the potential food safety and health risks associated with plant-based meat products, including nutritional, chemical, microbiological, and allergen concerns. This review has revealed several research gaps that merit further exploration to inform the conversation around the future development and commercialization of plant-based meat substitutes. Further research, technological advancements, food standards, and risk assessment and a multidisciplinary approach are essential to address safety concerns and facilitate the responsible use of new-generation plant-based meat alternatives, particularly for emerging foods with limited knowledge of their risks and benefits.
1. Introduction
Growing consumer demand for more plant-based diets is propelling advancements in the development of plant-based meat alternatives (PBMAs), which are typically formulated from plant proteins and other ingredients [1].
The food industry is developing a variety of PBMA products that are usually designed to mimic the appearance, texture, mouthfeel, and taste of real meat products. Consumers are increasingly adopting these products because of their concerns about the health [2], environmental [3], and animal welfare [4] impacts of traditional meat. The current patterns of meat consumption have been linked to undesirable environmental consequences (like greenhouse gas emissions, pollution, and biodiversity loss), as well as deleterious human health effects (like cardiovascular disease, cancer, and obesity) [5]. Transitioning to a more plant-based diet could eliminate or reduce these problems [6].
PBMAs are being marketed as a means of promoting the transition away from animal-based products, and to establish a contemporary food system that benefits humans, animals, and the environment. Embracing a plant-centric diet in the wealthiest nations, despite representing just 16 percent of the world’s population, has the potential to reduce greenhouse gas emissions by approximately 61 percent, while also enhancing carbon sequestration [7]. The meat substitute segment is the highest contributor to the rise of the plant-based market. In 2020, the global consumption of alternative proteins reached approximately 13 million metric tons [8]. The PBMA product category is poised to exhibit distinctive physical, functional, nutritional, and sensory characteristics. To achieve this in a cost-effective manner on a mass scale, the food industry must adeptly discern the right ingredient combinations and manufacturing processes to match the market trends. The projected growth for the global market for plant-based foods (primarily PBMAs and beverages) indicates an estimated value of USD 162 billion by 2030, a substantial increase from the USD 4.6 billion recorded in 2018 [9] and the USD 29.4 billion registered in 2020 [10]. These significant growth figures show a strong long-term outlook for investments in alternative proteins, despite their still small sector [11] and the fact that consumer demand is currently outpacing the industry’s supply chain capabilities [12].
The increasing introduction of PBMAs into the food supply chain brings potential new food safety and nutritional risks that could lead to new health problems. This is why a profound understanding of the molecular and physicochemical attributes of plant-derived ingredients is imperative. Consequently, it is important that the emerging plant-based food industry carefully craft its products and considers food safety and nutrition issues and promotes healthiness. These issues depend on the nature of the ingredients and processes used to create PBMAs, necessitating control over their nutrient composition, digestibility, and bioaccessibility [1] and must, therefore, be considered on a case-by-case basis [6]. Researchers have highlighted the importance of providing consumers with information about the nutritional quality of PMBAs when compared to real meat, so they can make more informed decisions [13,14].
This review aims to critically evaluate the potential safety and nutritional risks associated with the production and consumption of PBMA products. It highlights potential safety and nutritional risks associated with the main production stages of these products: (1) protein isolation and functionalization; (2) product formulation; (3) processing; and (4) storage.
2. Methodology
A systematic literature review based on scientific articles published between January 2018 and May 2023 was carried out. Two databases, Scopus and Web of Science, were searched for articles related to food safety risks and nutritional aspects of PBMAs. The review involved a careful analytical process selecting the concept grid and list of keywords to be explored, formulating the research inclusion and exclusion criteria for articles’ selection and elimination, and searching databases (Table 1). The study selection process was managed using the Covidence platform [15]. All the records obtained from the database searches using Scopus and Web of Science were uploaded to Covidence. All the duplicated records were automatically removed. According to the set eligibility criteria, the title and abstracts were screened first and then, if selected, the full texts were screened. The review aimed to (a) identify all previously published work on the safety and nutritional risks linked with plant-based meat alternatives; (b) determine the consensus and controversies related to these issues; (c) aggregate empirical findings to support evidence-based issues.

Table 1.
Inclusion and exclusion criteria used during the literature search.
Articles were evaluated based on their final content, which resulted in further exclusion. A total of 326 articles were available from the search of the databases, and after the removal of duplicates and screening of the titles and abstracts, 48 articles were selected for inclusion. These articles all dealt with potential safety and nutrition risks associated with plant-based meat alternatives.
The search screened categorically and descriptively the available literature to collect information and finalize the sample for the study (n = 48). Table 2 below outlines the selected articles. The selected articles focused on the nutritional risks (n = 27) and the safety risks (n = 6) of PBMAs, with some of them sharing information and analysis on both risks (n = 15). The published studies were from around the world, including Australia, USA, France, Italy, Spain, Brazil, and Sweden.

Table 2.
Summary characteristics of the included articles related to PBMAs’ nutrition risks aspects discussed.
As outlined in Table 3 below, there were only six (n = 6) studies identified focused on the PBMAs safety risks associated with a variety of aspects, such as microbial proliferation, pathogenic bacteria, mycotoxins, and allergies. These aspects are discussed later in the paper in more detail.

Table 3.
Summary characteristics of the included articles related to PBMAs’ safety risks aspects discussed.
Some of the articles picked out (n = 15) discussed issues related to both nutritional and safety issues associated with PBMAs. These are outlined in Table 4 below. Among the discussed risks are nutrients deficiency, allergens, sodium level, additives, digestive issues, and so on. These topics will be covered in greater detail later.

Table 4.
Summary characteristics of the included articles related to both PBMAs’ safety and nutrition risks aspects discussed.
Figure 1 uses a PRISMA flow chart to document the selection criteria used in this study. The chart maps out the number of recorded studies and visually illustrates the flow of the studies selections though each phase of the review process in sequential order. From a total number of n = 327 articles initially identified, the final list of articles and topics related to safety and nutritional risks were reduced to n = 48 articles. These are discussed in association with the main PBMAs production stages, such as (1) protein isolation and functionalization; (2) product formulation; (3) processing; and (4) storage.
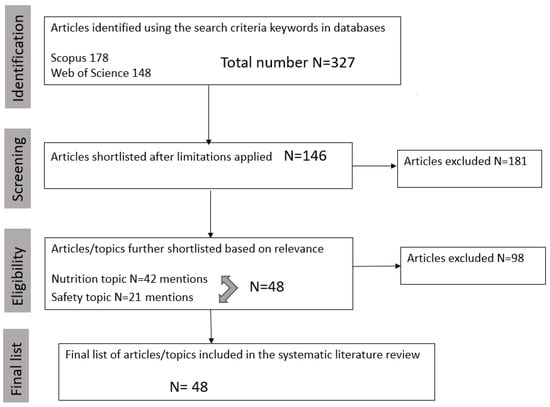
Figure 1.
Prisma flow graph of the procedures used to select papers on the safety and nutrition of plant-based meat alternatives included in this review.
3. Results and Discussion
The new PBMAs are meant to be serving as substitutes for traditional meat products. Their nutritional profile, health implications, and safety risks are contingent on the specific ingredients they contain and the type of processing operations used to manufacture them [36]. The fact that they possess distinct compositions and structures compared to real meat products means that PBMAs are also probable to yield divergent effects on nutrition, health, and safety impacts.
3.1. How Are Plant-Based Meats Made
While there has been a significant surge in awareness of plant-based meat alternatives in recent years, it is important to note that these products have been in production and consumption for well over a millennium [62]. In antiquity, Asian civilizations pioneered various meat analogues, crafting relatively straightforward derivatives from soybeans (like tofu and tempeh) or wheat (like seitan) [53,63]. Importantly, these early versions were not specifically intended and designed as direct replacements for meat [58]. Several decades ago, texturized vegetable protein (TVP) emerged as a meat substitute, created through the extrusion of defatted soy meal, soy protein concentrates, or wheat gluten [64].
In recent times, a fresh wave of new-generation plant-based meat alternatives have emerged, primarily targeted at omnivores who seek the appealing sensory attributes of meat. Currently, PBMA products are mainly available in the form of burger patties, mince, sausages, and nuggets, although there is a growing interest in developing more intricate and complex products, like chicken breast, beefsteak, or pork chop analogs [65]. PBMAs are typically engineered to be environmentally sustainable, while faithfully replicating the appealing appearance, feel, and taste of authentic meat products [34]. While some of them are formulated to closely mimic the nutritional composition of real meat, others may not prioritize this aspect. Moreover, most PBMAs fall into the category of ultra-processed foods, which means they may be digested and absorbed at a different rate compared to real meat. This can potentially lead to alterations in their nutritional and health effects [66]. As the popularity of plant-based diets increases, concerns and questions emerge regarding the potential safety and nutritional risks associated with transitioning from an omnivorous diet to one that is more plant based. As mentioned earlier, safety and nutritional issues can stem from the ingredients and processing operations methods employed in the production of PBMAs products [67]. Numerous potential challenges exist in the realms of food safety and nutrition when it comes to plant-based food. These challenges encompass various aspects, including the presence of different types of chemical and microbial contaminants present in the ingredients used, concerns about food adulteration issues, elevated levels of food additives, the use of genetically modified ingredients components, mislabeling, the introduction of new sources of allergens, potential vitamin or mineral deficiencies, and changes in macronutrient composition (such as protein, carbohydrate, or fat content). It is, therefore, of outmost importance to consider these issues when developing the next generation of plant-based foods. This ensures that they not only align with environmental sustainability goals, but are safe and nutritious, and beneficial for consumers and the environment.
The production of PBMAs consists of four main stages [68]:
- Protein isolation and functionalization: Plant proteins are extracted and purified to produce flours, concentrates, or isolates. In some cases, they undergo additional processing methods, like hydrolysis, conjugation, or denaturation, to enhance their functionality.
- Product formulation: The plant proteins are blended with a variety of other components, including carbohydrates, lipids, salts, flavors, and colors, to craft plant-based meat analogues that simulate the look, feel, texture, taste, flavor and functionality of actual meat products. Moreover, nutrients, like vitamins, minerals, dietary fibers, or nutraceuticals, may be incorporated to align with or surpass the nutritional composition of real meat.
- Processing: The mixture of ingredients goes through a sequence of processing operations that promote the development of meat-like structures and properties. These operations often involve mixing, extrusion, shearing, molding, and cutting.
- Storage: The product, packaging materials, and environmental conditions must be carefully designed to ensure the safety and quality of PBMAs throughout storage, transportation, and distribution. Achieving this involves careful management and control of microbiological, chemical, and physical deterioration processes.
The ultimate goal of this process is to produce a final product that faithfully replicates the desirable quality and sensory characteristics of real meat, while also ensuring its safety and nutritional value. Currently, extrusion technologies are the most widely used method of creating PBMAs that mimic the properties of actual meat. However, ongoing developments include other alternative technologies, such as shear cells, 3D printing, recombinant proteins, and mycelium [53,69]. In the following sections, we consider some of the potential health and safety risks associated with the main production stages of PBMAs. Certain safety considerations regarding novel protein sources are inherent to the product itself, but numerous potential risks can also arise from the methods of production and the conditions under which they are processed [70].
3.2. Protein Isolation and Functionalization
A process for isolating protein can be perceived as a series of interconnected stages, where the protein’s purity increases progressively through each step. These include: (a) sourcing suitable protein and obtaining the material; (b) extracting from the source; (c) segregating from non-protein elements, like nucleic acids and lipids; (d) concentrating the primary protein and segmenting it into fractions with distinct proteins; and (e) culminating in refined methods, like chromatography, that yield the ultimate end product [71].
Proteins play a pivotal role in shaping the technological, physicochemical, and sensory characteristics of plant-based food products. For instance, they have a profound impact on attributes, such as structure, texture, appearance, chewiness, water retention, and nutritional qualities, of PBMAs [72,73]. The choice of an appropriate plant protein is crucial, as it can impart a range of desirable functional attributes to the final product, including thickening, gelling, emulsifying, structuring, foaming, and fluids retention [1].
Plant proteins are typically derived from sources like soybeans, peas, or corn to produce flours, concentrates, or isolates. Extraction and purification can be accomplished through a diverse array of techniques. There methods encompass conventional approaches utilizing aggressive chemicals, like acids, bases, and/or organic solvents; eco-friendly green extraction methods involving single or mixed enzymes; and cutting-edge physical extraction techniques, such as ultrasound-, pulse electric field-, microwave-, and high pressure-assisted extraction [74,75]. Notably, it is important to highlight that a significant number of the techniques employed for proteins extraction were not originally intended to optimize and enhance their functionality. Instead, these methods were optimized for the extraction of oil or starch from plant materials. Consequently, the functionality of proteins may be compromised, as they can become denatured or aggregated during the isolation process.
3.3. Product Formulations
Product formulation entails understanding how materials interact to produce enhanced properties, optimize processing efficiency, and deliver active ingredients effectively. In the context of food, formulation pertains to the art of crafting, planning, or evolving food items, with the aim of incorporating specific functionalities. These functionalities can span from conferring extra nutritional advantages to enriching food products. PBMAs are distinctly defined as products meticulously crafted to emulate the color, flavor, taste, aroma, consistency, texture, and visual characteristics of animal-derived products to match or at least closely align with the sensory encounter of consuming meat products. PBMAs are formulated to simulate the physicochemical and organoleptic characteristics of traditional meat products.
During this stage of production, plant proteins are combined with an array of other functional ingredients to achieve the desired appearance, feel, texture, and taste of the end product. These additional components include flavorings, colorings, emulsifiers, texture modifiers, gelling agents, and binding agents [50]. Due to the diverse formulation of plant-based meat substitutes involving elements like proteins, water, fats, carbohydrates, flavor constituents, binding agents, and colorants, their operational characteristics assume a crucial role in dictating their attributes [76]. Some consumers have voiced concerns regarding the extensive use of additives in PMBAs, classifying them as ultra-processed foods [66,77]. A number of the components present in these intricately formulated food products have raised nutritional concerns, including saturated fats, highly refined flours, and high salt content [41,50]. Fortification practices vary between products, and so each product should be considered on a case-by-case basis.
Concerns have also been raised by some researchers regarding the inclusion of leghemoglobin in certain PBMAs. Leghemoglobin is an iron-containing hemeprotein that can be derived from soybean root nodules and is used to impart the desirable red color and meaty flavors typically associated with the hemoglobin in real meat [2]. Given the challenges in obtaining sufficient quantities from soybeans, this protein is often produced through genetically engineered yeast cultures. Some researchers have drawn connections between higher heme iron intake, elevated body iron stores, and an increased risk of developing type 2 diabetes [78]. Nevertheless, there is limited scientific evidence indicating that the levels of these proteins used in plant-based foods pose health risks.
Concerns have also been voiced regarding the potential health risks linked to the consumption of PBMAs that incorporate a mixture of numerous additives, encompassing flavorings, colorings, binding agents, preservatives, and sweeteners. Nevertheless, there is scant evidence to suggest that this poses a significant health concern. Further research on the nutritional quality and safety of PBMAs is clearly required [67].
Certain PBMAs exhibit higher levels of saturated fat compared to conventional meat products, as well as other plant-based protein sources, like beans and lentils [18,24,54]. Indeed, some researchers argue that PBMAs exceed the recommended dietary intake for saturated fat [54] and contain approximately the same number of calories and saturated fat as livestock meat [21]. As a result, there are concerns about the potential health implications of elevating saturated fats intake in the diet. Nevertheless, it is worth noting that plant-based meat products can be reformulated to lower their saturated fats contain [1]. Moreover, there is currently ongoing debate among nutrition scientists regarding the adverse nutritional effects of saturated fats [32,41,79].
Some PBMAs contain notable elevated salt levels, which could raise health concern. Increased dietary salt levels may potentially elevate the risk of conditions, such as high blood pressure, cardiovascular disease, osteoporosis, kidney disease, and stomach cancer [14,17,80]. A high sodium content is viewed as nutritionally undesirable and, over time, may potentially increase the long-term risk of cardiovascular problems for individuals with prolonged overconsumption [81]. It may, therefore, be crucial for manufacturers to consider lowering the salt levels in their PBMA products.
PBMAs frequently feature an extensive list of ingredients [26] encompassing isolated macronutrients (proteins, fats, and carbohydrates), micronutrients (vitamins and minerals), flavoring agents, colors, emulsifiers, salt, and plant-based extracts [50,82]. The potential health effects and impact of many of these ingredients and their combinations in PBMAs remain largely unknown and uncertain.
The nutritional profile of PBMAs depends on the ingredients used by the manufacturer and can vary considerably between products. Plant proteins are often considered to have a lower nutritional quality than animal ones because of their lack of some essential amino acids and lower digestibility [5]. In addition, plant-based foods often contain lower levels of key micronutrients (e.g., iron and vitamin B12) than the animal-based foods they are designed to replace [83]. PBMAs are lower in calcium, potassium, magnesium, zinc, and vitamin B12 [31,54,56]. Moreover, many plant-based meat alternatives are higher in sodium and saturated fat compared to meat [24,41,54]. Consequently, there may be some nutritional concerns from switching from an omnivore to a plant-based diet. But, these concerns can often be addressed with appropriate nutritional fortification of plant-based foods, which is being carried out by some of the major producers of these products.
3.4. Food Safety and Nutritional Concerns
Several food safety issues can be linked with this stage of PBMA creation, including the presence of allergens, bacteria, mycotoxins, anti-nutrients, thermally induced carcinogens, and natural toxins, which are discussed in detail in the following sections.
3.4.1. Allergens
The presence of allergens in PBMAs is a major food safety concern [47,59]. This is particularly important due to the worldwide rise of food-related allergies over the past few decades [84,85,86]. Development of food allergies is believed to be based on individual reactions to food, rather than being inherited. Both food allergies and adverse reactions to food with life-threatening consequences can arise at any age and may disappear or stay throughout a person’s life. Allergenic assessment of new foods is, therefore, critical to ensure they do not pose any risks [16]. Even a small amount of certain food ingredients can cause symptoms that range from minor (such as itching, swelling, and stomachache) to severe (such as anaphylaxis) [87,88].
Common components of PBMAs can cause allergic reactions in some people [44]. For instance, many plant proteins are known allergens, including legumes and cereals, such as soy, pea, wheat, rye, barley, and lupin proteins [47,49,55,59,60,89]. The severity of the food-induced allergic reactions is impacted by the dose of the product used [47]. Some consumers are concerned that certain kinds of plant proteins obtained from genetically modified (GM) sources may introduce new allergy risks [90]. Consequently, these new proteins should undergo rigorous assessment for potential allergenicity before the foods are made available for widespread consumption [91]. The likelihood of prompting a protein-related allergic reaction is related to its resistance to digestion by the proteases in the gastrointestinal tract [91]. For transparency and avoiding consumers concerns, a clear labeling of such genetically modified food ingredients used in a product formulation is necessary [92]. Nonetheless, the health and environmental risks linked to the consumption of genetically modified foods still remain a contentious subject of debate, necessitating further research [93].
Potentially, there is also the possibility that heightened consumption of products containing substantial amounts of soy, pea, wheat, and other plant proteins could provoke and trigger allergic reactions in individuals who have not previously experienced issues with these foods [47,59,60]. High-protein pea ingredients, such as concentrated pea protein, are being formulated into PBMAs and could increase the risk of allergies [94]. Peas, being a legume, also can cause allergenicity like peanuts and lentils [95,96]. The ongoing current trend of incorporating pea protein concentrates and pea protein isolates into various foods to add bulk and increase protein content levels could potentially lead to consumption-induced allergic reactions [97,98]. Reports from individuals with peanut allergies have indicated and reported post-consumption allergic symptoms, implying that the similarity between pea and peanut proteins might trigger cross-reactions [99]. With its increased exposure, peas can develop into a more frequently encountered allergenic food [47,59,94].
Wheat proteins used in PBMAs are also a common allergen capable of inducing life-threatening severe anaphylaxis reactions [100,101,102], as well as less serious reactions, but with still undesirable symptoms [84]. These proteins often serve as binders in various PBMA products. Consumers with wheat allergies and celiac disease may also have an adverse allergic reaction to gluten, a protein found in grains, such as wheat, barley, and rye. Consequently, it is important to select proteins that have a low allergenicity when formulating plant-based foods and to ensure careful processing and labeling. The Codex Alimentarius Commission includes a priority allergen list within its General Standards for the Labeling of Prepackaged foods. This list is developed and formulated based on predetermined criteria, which take into account global allergen prevalence and other established factors [103,104].
3.4.2. Bacteria
Throughout history, bacteria have been responsible for a disproportionate share of human diseases and fatalities. Understanding the various types of bacteria linked to food is of paramount importance for ensuring food safety. Pathogenic bacteria originating from the raw ingredients can be present in PBMA products [44]. Bacteria are usually inactivated during food processing operations (such as extrusion and cooking); however, studies have shown that some endospore-forming bacteria, (e.g., Clostridium spp. or Bacillus spp.) and other bacteria (e.g., Lactobacillus sakei and Enterococcus faecium) can live through the heating regime [105] or can be present in the final product due to post-extrusion process re-contamination [44,48,51].
Bacteria not only can encompass infectious pathogens, but also toxin-producing strains, which similarly pose significant safety concerns within the field of food microbiology.
3.4.3. Toxins
Substances, whether they are of natural or artificial origin, can pose risks when the level of exposure reaches a certain threshold.
Mycotoxins
Mycotoxins are hazardous toxic compounds produced by a variety of fungal (mold) species [106]. Among food commodities, the predominant mycotoxins include aflatoxins and ochratoxins, produced by Aspergillus species, as well as ochratoxins and patulin, synthesized by Penicillium. Additionally, Fusarium species generate fumonisins, deoxynivalenol, and zearalenone. Globally, mycotoxins, such as fumonisins, patulin, aflatoxins, and ochratoxins, among others, are accountable for a multitude of acute and chronic human illnesses [107].
Many edible plants are susceptible to contamination with mycotoxins, which are harmful to humans when ingested in sufficient quantities [45,108]. Indeed, many important agricultural crops have been reported to be contaminated with mycotoxins [103,109]. They are present in some of the raw ingredients used to formulate PBMAs, such as legumes (soy), cereals (oat, rice), and nuts (almond, walnut) [110]. Mycotoxins, being “mutagenic, teratogenic, and carcinogenic”, are potent toxins with harmful health effects in people [107]. The severity of the toxicity is based on the exposure time, mycotoxin amount, and consumers’ sensitivity [46,110].
Similar to bacteria, mycotoxins are heat resistant within the range of conventional food-processing temperatures and cannot be completely destroyed [110,111]. Ochratoxin A has been detected in plant-based foods and ingredients [112], which highlights the potential for mycotoxin contamination of PBMAs [45,46]. Another mycotoxin, Fumonisin FB1, is predominantly found in soybeans, corn, rice, beer, sorghum, cowpea seeds, and beans [110,113]. A proper regulatory framework for mycotoxins in PBMAs is a necessary step for minimizing mycotoxins contaminations and adverse health effects, such as development of life-threatening illnesses (e.g., liver cancer) [45].
Natural Toxins
Several ingredients used in the creation and formulation of plant-based foods may harbor natural toxins. These substances are typically metabolic byproducts produced by plants as a defense mechanism against various threats, such as bacteria, fungi, insects, and predators. Common examples of these natural toxins in plants encompass lectins found in green, red, and white kidney beans; cyanogenic glycosides present in bitter apricot seeds, bamboo shoots, cassava, and flaxseeds; glycoalkaloids within potatoes; 4’-methoxypyridoxine derived from ginkgo seeds; colchicine in fresh lily flowers; and muscarine found in some wild mushrooms [114]. As a result, it becomes imperative that all plant-derived ingredients employed in product formulation undergo meticulous selection and processing procedures to prevent, eliminate, or deactivate and neutralize these toxins.
Some scholars have raised awareness regarding the utilization of carrageenan. Carrageenan is sourced from seaweed and used as an ingredient component in food products. Carrageenan, a polysaccharide, is occasionally employed in PBMAs to serve as a thickener, gelling agent, stabilizer, or binder [115]. Although food-grade carrageenan is considered safe for consumption, there have been suggestions that carrageenan might contribute to gastrointestinal inflammation, disruptions in intestinal microflora, and irritable bowel syndrome, as well as the development of colon cancer and various other health issues [116,117]. Additionally, it is possible for carrageenan to accumulate elevated concentrations of heavy metals when obtained from contaminated or polluted seawater, potentially presenting a health hazard [117,118]. Nevertheless, the existing scientific consensus about the potential health risks associated with carrageenan remains limited [50,119,120]. Despite this, the consumer desire for cleaner labels on their food products is promoting the PBMAs industry to reduce the number of ingredients of concern in their products [121]. In addition, updated natural toxins regulations and risk assessments are needed for all PBMAs present and future products to minimize food safety risks [45].
Synthetic Toxins
Some of the ingredients used to formulate PBMAs may contain synthetic toxins, such as pesticide residues [44]. Employing organic solvents, like hexane, during the process of protein extraction can lead to both environmental and health issues, especially if relatively substantial residual amounts persist in the end product [122]. Concerns about the chemical hexane use in processing soy protein isolates are raised due to its neurotoxin nature [52]. Nonetheless, there is currently a lack of precise data available regarding the quantities of hexane used in the production of soy and pea protein isolates for plant-based alternatives, as well as the residual amounts that may persist in the final product [123]. This underscores the importance of conducting further research in this particular area.
3.4.4. Thermally Induced Carcinogens
Thermal processing used to reduce microbial contamination or cook foods may induce the formation of carcinogens in cooked PBMAs, just as they do in real meat products, [14]. Currently, there is little information about the formation and effects of thermally induced substances and hazardous chemicals in PBMAs [52]. Clearly, more studies are needed to verify the likely safety risk of the chemicals produced by high-temperature processing in plant-based meats [14,44].
3.4.5. Antinutrients
Some plant-based ingredients, such as legumes, contain antinutrients that can adversely affect human health, such as protease inhibitors, phytic acid, lectins, oxalates, goitrogens, saponins, phytoestrogens, phytates, and tannins [124]. These antinutrients can reduce the bioavailability of key nutrients by restricting protein digestion or mineral absorption [68,124,125]. Antinutrients naturally occur in some plants and may not be fully removed or deactivated during extraction and extrusion processes [125,126]. For instance, studies have shown that some may be present in maize, soybean, and cassava starch ingredients [127]. Lectins are of concern in the production of soybean and rice milk substitutes [67], but may also be a concern for the production of PBMAs. Some researchers have identified factors in plant-based proteins that may decrease nutrient bioavailability that include structures that are resistant to proteolysis, certain protein conformations, and the presence of antinutrients [53]. Therefore, testing for anti-nutrients is an important aspect when developing plant-based proteins [128].
3.5. Nutritional Profile
There is a potential impact on human health and nutrition from switching from animal-based to plant-based foods related to differences in their compositions, structures, and processing. Many of the studies included in this review have reported that PBMAs are highly processed foods that contain a longer list of ingredients than the equivalent animal-based products [32,36,38,58], which could lead to different nutritional outcomes.
As mentioned earlier, the composition of PBMAs can vary considerably between products, which means their health effects may be different. There may also be differences in the digestibility and absorption of PBMA and meat products [16,34]. A variety of processing operations are used to create PBMAs, which determine the composition and digestibility of the final product, including dehulling, soaking, blanching, pH adjustment, enzyme treatments, shearing, thermal processing, and size reduction [129]. Because they are highly processed materials, food producers can control the nutritional profile and digestibility of PBMAs, thereby modulating their health effects [34]. For instance, their macronutrient and micronutrient levels can be controlled, and they can be fortified with other health promoting ingredients.
The nutritional profile of PBMAs could be improved by reducing their salt content [17,18,39,49,54,130], as high sodium intake is associated with an increased risk of cardiovascular diseases [131,132]. In general, however, the amount of salt present is likely to depend on the particular type of plant-based food being considered and their producers’ product formulation.
PBMAs may contain soy isoflavones compounds, such as phytoestrogens, which have been linked to some health concerns [133]. Generally, estrogens are considered to have beneficial effects in preventing cardiovascular disease, osteoporosis, breast cancer, and menopausal symptoms. However, it is unclear whether ingested phytoestrogens behave like endogenous estrogens in the human body [133]. Some researchers reported that when phytoestrogens are excessively consumed, they may provoke adverse health effects on reproductive health [67].
Plant proteins possess distinct amino acid profiles compared to meat proteins, potentially influencing their nutritional implications. Some of them may be deficient in one or more essential amino acids vital for human health, which the human body cannot synthesize on its own, such as methionine, lysine, and tryptophan) [72]. In principle, this deficiency could raise health concerns, but the majority of vegan or vegetarian diets incorporate a wide variety of a diverse range of protein sources, generally providing adequate levels of these essential amino acids, rendering this matter largely non-consequential.
PBMA products contain different carbohydrate ingredients from starches, flours, and binding agents. Although starchy foods are an essential part of a nutritious diet, providing energy and fiber, they can be detrimental for human health, e.g., for people with medical conditions such as diabetes [134]. Also, when cooked at high temperatures (e.g., frying, roasting, and baking), starchy, plant-based foods produce some potentially harmful chemicals, such as acrylamide [135], which could be the case with cooked PBMAs. Limiting consumer exposure to acrylamide could be achieved by avoiding high-temperature cooking and practicing storing foods in a cool, dry place.
Meatless products can be designed and formulated using liquid smoke flavorings, which have been documented to be associated with potential carcinogenicity when consumed regularly at sufficient quantities [130]. Furthermore, plant-based meat alternatives have also been observed to be deficient in certain specific amino acids and their derivatives, including creatine, taurine, and anserine. These compounds are believed to play an important, meaningful role for human health, as they can have an impact on both brain and muscle functions [19].
Food manufacturers used various additives to improve the look, feel, and taste of foods. Among these are binding ingredients or gums, which serve as emulsifiers, stabilizers, binders, and thickeners, such as methylcellulose, acacia gum, xanthan gum, carrageenan, and others. The health and safety of these products have been challenged, and consumers are demanding cleaner labels [121]. Despite no real health risks or concerns having been detected [50], food processors are already trying to limit or eliminate the use of these ingredients in many processed food products [121].
Enhancing the nutritional profiles of PBMAs can be achieved through fortification with specific nutritional components as desired. Certain essential minerals, like iron, zinc, magnesium, and calcium, may exhibit reduced bioavailability in some of the ingredients present in these alternatives [136,137]. Consequently, it is important to develop strategies to increase the bioavailability [41], as well as to have in vitro and in vivo methods to measure the bioavailability of minerals and other nutrients in PBMAs.
Many kinds of natural colorants (e.g., leghemoglobin, red beet, red cabbage, etc.) and flavorings (e.g., herbs and spices) are used in PBMAs to reproduce the desirable color and flavor of real meat [14]. These ingredients are often less stable than synthetic alternatives and may chemically degrade during food processing, leading to unacceptable changes in quality attributes [28,82]. At present, there has been little research on the potential safety aspects of the chemical degradation of natural colorants or flavorings in plant-based foods.
3.6. Processing
Processing influences changes of the nutritional, physical, and chemical properties of foods [50]. Many technological and food engineering approaches exist to create the plant proteins texture, but balancing the processing methods to achieve all the desired mechanical properties and, at the same time, to retain the final product nutritional value still remains difficult [68]. Ishaq and colleagues highlighted that contemporary methods of structuring have substantially enhanced the operational capabilities of plant-based meat substitutes. Nonetheless, significant efforts are still required to enhance their operational efficiency, sensory qualities, safety, and the choice of appropriate components [28].
Typically, plant proteins, commonly in a defatted state, are combined with water, carbohydrates, salts, flavorings, and edible fats. This mixture is then subjected to a twin-screw extruder operating at high temperatures and varying moisture levels. This process encourages the proteins to form a meat-like fibrous structure, resulting in the creation of a meat substitute suitable for various food applications [55]. PBMAs processing concerns are discussed in more detail.
According to the description of ultra-processed, PBMA products fit entirely into the portrayal, as they are “formulations made mostly or entirely from substances derived from foods and additives, with little if any intact (whole) food” [77]. Ultra-processed foods consumption is associated with many adverse health consequences, such as obesity, cardiovascular disease, cancer, type 2 diabetes, and all-cause mortality [23]. On top of the highest degree of processing, PBMAs are inclined to contain a greater diversity of other ingredients needed to mimic the characteristics and sensory attributes of conventional animal-based products. This all provides grounds for a variety of sources, from where prospective hazards can develop and arise. The argument of the ultra-processed nature of the PBMAs and its connection to a potential elevation risk of health-related harm, affecting the consumer motivation for their regular consumption, is an imminent part of the discussion around these alternative products’ pivotal place in the transition toward more sustainable dietary change [36,138,139].
Different degrees of processing have a serious impact on health and nutrition, as many nutrients, vitamins, and minerals can be destroyed or removed during the process. Greater understanding of the possible impact is necessary, as even adding some specific ingredients designed to enhance nutritional quality (e.g., fortification) can lead to reduced product desirability among consumers [13]. Processing can increase or decrease the bioavailability, digestibility, nutritional, and functional characteristics of particular foods and ingredients [62,128]. To this should be added the long ingredients list and the tolerance for discrepancies from the food label laws worldwide regarding the actual nutritional value and the values described in the PBMAs food labels [39].
Like meat products, it is also important to subject plant-based meat analogues to sufficient and adequate thermal processing prior to consumption to ensure and guarantee their microbiological safety [43]. This thermal treatment can be conducted in a factory, restaurant, or even at home. The potential for microbial proliferation and presence of yeast and Enterobacteriaceae after hot meals preparation with PBMAs also needs special attention, as some researchers observed it to be slightly higher than that of meat-containing food [43]. For consumers, adhering to the food preparation instructions provided by the manufacturer on food labels is important because legumes, grains, and vegetables have the potential to be contaminated with pathogenic bacteria [48]. Observing sound food safety practices ensures that these foods pose no harm to consumers when prepared and consumed in accordance with their intended use.
Most PBMAs are processed using extrusion [140], such as dry extrusion, wet or high moisture extrusion, and thermal extrusion, or power heated [141,142]. Conventional dry extrusion is an established processing technique well suited for producing minced meat substitutes. Nonetheless, emerging high-moisture extrusion technology enables the creation of appealing whole pieces of alternative meats. Extrusion represents a high-temperature and high-pressure technique used to achieve the desired form and texture of products, while also simultaneously decreasing the microbial load [143]. The precise time and temperature factors involved are considered and regarded as critical control points. Additional research investigation into non-protein constituents, advancements, and innovations in production technologies for alternative protein products, improvements, and enhancements in overall appearance and flavor, rigorous control over biological and chemical safety, and the careful selection of protein sources are all essential for addressing food safety and quality issues and for the ongoing continuous expansion and diversification of protein offerings in the marketplace.
There are several chemical hazards that could potentially arise from the processing of PBMAs. Known carcinogens, such as the heterocyclic aromatic amines, nitrosamines, and polycyclic aromatic hydrocarbons, could be produced during thermal processing of PBMAs, just as they are produced in real meat products [14]. Similarly, other heat-induced contaminants could be produced in PMBAs during thermal processing, such as glycidyl fatty acid esters, 2-monochloropropanediol (2-MCPD), and 3-monochloropropanediol (3-MCPD) [144,145]. However, the production and effects of these kinds of toxic compounds in PBMA requires further investigation.
The utilization of lipid sources containing trans-fatty acids, which are formed during partial hydrogenation of vegetable oils, may also adversely affect the healthiness of PMBAs [146]. However, many countries around the world have legislation in place to ban industrially produced artificial trans-fatty acids from their food products [147]. Food processing may lead to the loss of certain beneficial nutrients and phytochemicals in plant-based foods, which could reduce their potential health benefits. In general, van Vliet et al. [19] advises against classifying plant-based alternatives as nutritionally equivalent to their corresponding animal-based counterparts [37]. Their metabolomics study indicates that the animal-based product (beef) and the PBMAs are more likely to be complementary, rather than identical or interchangeable, in terms of providing beneficial nutrients.
Another issue of concern is the digestion and bioavailability of PBMA products [41,148]. Some researchers believe that the industrial processing of plant-derived ingredients to form meat alternatives may not necessarily be unfavorable, as it has the potential to promote and encourage favorable alterations and positive changes in protein digestibility, nutrient bioavailability, and the human gut microbiome [25,41]. Others believe that the novel protein sources can potentially trigger adverse allergen and other reactions and, therefore, incontestably require thorough risk assessment [148], as well as an overall multidisciplinary approach [149].
3.7. Storage
The influence of microbial contamination on the safety of PBMAs is another issue of concern. Like meat products, PBMA products should be stored under appropriate conditions (e.g., temperature, humidity, packaging) to reduce the growth of undesirable microbial contaminants [105]. PBMAs are generally not strongly associated with concerns related to pathogenic diseases. However, they can potentially cause foodborne illnesses. For instance, they may become contaminated with pathogens through contact with sources like animal manure, water, or other foods [53]. PBMAs typically have a neutral pH, high moisture content, and a favorable nutrient profile, which makes them highly susceptible to microbial growth and spoilage [51,105].
At present, there is currently a lack of research on the microbial contamination and safety of plant-based foods, and more research is clearly needed [92,150]. Researchers have employed meta-genetics to examine shifts in the quantities and types of microorganisms in plant-based products during storage [151]. Some studies have noted the increased prevalence of particular microbes towards the end of the product’s shelf-life period, including Latilactobacillus sakei, Enterococcus faecium [152], and Enterobacteriaceae and yeasts [153]. These microbes may be present as a result of post-processing contamination of heat-treated PBMAs. Improved knowledge and understanding of the types of microorganisms present is crucial for ensuring the safety of PBMAs.
4. Studies Comparison
In the analysis of 48 studies concerning the safety and nutritional risks of plant-based meat alternatives, three distinct categories of research were identified, each contributing to evidence-based insights on these aspects. The majority of studies within the reviewed literature (27 in total) primarily concentrated on nutritional risks. Additionally, there were 15 articles that provided comprehensive information and analysis covering both nutritional and safety concerns. However, it is worth noting that a relatively small number of studies (six in total) specifically delved into the safety risks associated with plant-based meat alternatives.
In the assessment of these issues, a noteworthy number of consensuses and only a few points of controversy emerged, particularly within the studies that centered on nutritional risks (27 in total). The areas of agreement were predominantly related to several key concerns regarding plant-based meat alternatives (PBMAs). These included the higher levels of sodium, carbohydrates, and saturated fat content found in PBMAs, as well as the deficiency of essential nutrients, vitamins, and minerals in these products. This consensus was supported by numerous studies [17,18,19,21,22,24,26,28,34,36,41].
Another commonly shared concern revolved around the quality of protein in PBMAs and the necessity for optimizing the nutritional profiles and functional properties of these alternatives [29,30,31]. Additionally, there was a commonly acknowledged consensus regarding the importance of product information disclosure by manufacturers, particularly in relation to potential health aspects associated with PBMAs [21]. This included transparency in claims around the GMO-free claims [23].
Among the studies identified, there was a lack of consensus regarding the classification of plant-based meat alternatives (PBMAs) as ultra-processed products. Some publications argued against categorizing all PBMAs as detrimental to the human gut microbiome [25], suggesting that their consumption as part of a flexitarian diet could potentially promote positive changes in the microbiome. Conversely, other scholars contended that the higher degree of processing in PBMAs raises concerns about their long-term health implications [27,32,36,37,38,41], including organically certified PBMAs [42]. Additionally, the extensive list of ingredients in PBMAs was cited as a factor contributing to their classification as ultra-processed products [33,37].
The composition of ingredients in PBMAs and the role of excessive fibers also emerged as contentious topics within the discourse on plant-based meat alternatives. According to some researchers, an overabundance of fibers could negatively impact their taste and, consequently, their marketability and commercialization [39].
Among the studies that primarily focused on safety risks (six in total), the research was primarily centered on several critical aspects, including microbial proliferation, the formation of pathogenic bacteria, mycotoxins, chemical contaminants, and natural toxins [43,44,45,46,48]. It appeared that all of these studies were aligned in emphasizing the necessity for a comprehensive regulatory framework to address safety risks associated with plant-based meat alternatives (PBMAs), particularly in relation to mycotoxins [45,46], due to concerns about potentially severe health risks.
Another noteworthy and shared area of concern within these safety-focused studies revolved around the potential creation of PBMAs with allergenic potential [47]. This raised critical questions regarding the allergenicity of certain ingredients used in PBMA production and its implications for consumer safety.
In the studies that explored both nutritional and safety risks associated with PBMAs (n = 15), the authors echoed the concerns previously mentioned. Additionally, these mixed studies introduced a new perspective, emphasizing the need for further research to understand the implications of variations in protein digestion, absorption, amino acid profiles, and allergenicity on human health and well-being. This highlights the evolving nature of our understanding of the potential impacts of PBMAs on various aspects of human health, underlining the importance of continued investigation in this field.
5. Conclusions
Currently, there is insufficient information about the potential safety and nutritional health impacts of consuming plant-based meat alternatives with different nutritional profiles to animal-based ones, and further research is clearly needed. In addition, there are new safety concerns associated with incorporating next-generation plant-based foods into the human diet, which arise from the potential presence of allergens, chemical contaminants, antinutritional factors, and pathogenic microorganisms. The long-term health effects of regular PBMA consumption are also not yet comprehensively assessed, and further investigation is clearly required.
More research is needed to identify potential safety and health concerns associated with the new generation of PBMAs, as well as to develop technological innovations to mitigate any potentially adverse effects. Moreover, there is a need to establish appropriate food standards and guidelines, and to create adequate risk assessment and management methods. This is particularly relevant for emerging foods, where there is a limited understanding and knowledge of their risks and advantages. This lack of knowledge hampers harmonizing regulatory frameworks to address safety concerns and to guide the safe application of these products. Progress in this area will be made only if an integrated multidisciplinary approach is considered to help overcome the various challenges and enable the responsible advancement of next-generation plant-based meat alternatives.
In the future, it will be important to specifically design PBMA products to improve human health and well-being, as well as for being delicious and sustainable, which will require the development of new product formulations and processing methods. Further research on nutrient bioavailability, safety, costs, and consumer acceptance will shape the future of plant-based foods in future human diets.
Author Contributions
Conceptualization, D.B. and D.J.M.; methodology, D.B.; formal analysis, D.B.; investigation, D.B. and D.J.M.; writing—original draft preparation, D.B.; writing—review and editing, D.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data included within the text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McClements, D.J.; Grossmann, L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef]
- Hu, F.B.; Otis, B.O.; McCarthy, G. Can plant-based meat alternatives be part of a healthy and sustainable diet? JAMA 2019, 322, 1547–1548. [Google Scholar] [CrossRef]
- Boukid, F. Plant-based meat analogues: From niche to mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Hartmann, C.; Siegrist, M. Our daily meat: Justification, moral evaluation and willingness to substitute. Food Qual. Prefer. 2020, 80, 103799. [Google Scholar] [CrossRef]
- McClements, D.J. Meat Less: The Next Food Revolution; Springer Scientific: New York, NY, USA, 2023. [Google Scholar]
- Marinova, D.; Bogueva, D. Food in a Planetary Emergency; Springer Nature: Singapore, 2022; ISBN 9789811677069. [Google Scholar]
- Sun, Z.; Scherer, L.; Tukker, A.; Spawn-Lee, S.A.; Bruckner, M.; Gibbs, H.K.; Behrens, P. Dietary change in high-income nations alone can lead to substantial double climate dividend. Nat. Food 2022, 3, 29–37. [Google Scholar] [CrossRef]
- Morach, B.; Witte, B.; Walker, D.; von Koeller, E.; Grosse-Holz, F.; Rogg, J.; Brigl, M.; Dehnert, N.; Obloj, P.; Koktenturk, S.; et al. Food for Thought: The Protein Transformation. 2021. Available online: https://www.bcg.com/en-au/publications/2021/the-benefits-of-plant-based-meats (accessed on 19 September 2023).
- Gordon, W.; Gantori, S.; Gordon, J.; Leemann, R.; Boer, R. The Food Revolution: The Future of Food and the Challenges We Face. 2019. Available online: https://www.ubs.com/global/en/wealth-management/chief-investment-office/investment-opportunities/sustainable-investing/2019/food-revolution.html (accessed on 19 September 2023).
- Elkin, E. Plant-Based Food Sales to Increase Fivefold by 2030, BI Says. In Bloomberg. Available online: https://www.bloomberg.com/news/articles/2021-08-11/plant-based-food-sales-to-increasefivefold-by-2030-bi-says (accessed on 15 November 2022).
- O’Donnell, M.; Murray, S. A Deeper Dive into Alternative Protein Investments in 2022: The Case for Optimism. Good Food Institute. 16 February 2023. Available online: https://gfi.org/blog/alternative-protein-investments-update-and-outlook/ (accessed on 19 September 2023).
- Morrison, O. The Alternative Protein Space Is Coming to Terms with the Reality of What Is Needed to Build Self-Sustaining Businesses’: The Challenge of Scaling Up Plant-Based Production and Manufacturing. 28 April 2023. Available online: https://www.foodnavigator.com/Article/2023/04/28/The-challenge-of-scaling-up-plant-based-production-and-manufacturing (accessed on 10 June 2023).
- Jahn, S.; Furchheim, P.; Strässner, A.M. Plant-based meat alternatives: Motivational adoption barriers and solutions. Sustainability 2021, 13, 13271. [Google Scholar] [CrossRef]
- He, J.; Evans, N.M.; Liu, H.; Shao, S. A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2639–2656. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software. Available online: http://www.covidence.org (accessed on 10 June 2023).
- Ogawa, Y.; Donlao, N.; Thuengtung, S.; Tian, J.; Cai, Y.; Reginio, F.C.; Ketnawa, S.; Yamamoto, N.; Tamura, M. Impact of food structure and cell matrix on digestibility of plant-based food. Curr. Opin. Food Sci. 2018, 19, 36–41. [Google Scholar] [CrossRef]
- Curtain, F.; Grafenauer, S. Plant-based meat substitutes in the flexitarian age: An audit of products on supermarket shelves. Nutrients 2019, 11, 2603. [Google Scholar] [CrossRef]
- McClements, D.J. Future foods: Is it possible to design a healthier and more sustainable food supply? Br. Nutr. Found. Nutr. Bull. 2020, 45, 341–354. [Google Scholar] [CrossRef]
- Van Vliet, S.; Bain, J.R.; Muehlbauer, M.J.; Provenza, F.D.; Kronberg, S.L.; Pieper, C.F.; Huffman, K.M. A metabolomics comparison of plant-basedmeat and grass-fed meat indicates large nutritional differences despite comparable Nutrition Facts panels. Sci. Rep. 2021, 11, 13828. [Google Scholar] [CrossRef]
- Lee, H.J.; Yong, H.I.; Kim, M.; Choi, Y.S.; Jo, C. Status of meat alternatives and their potential role in the future meat market—A review. Asian-Australas. J. Anim. Sci. 2020, 33, 1533–1543. [Google Scholar] [CrossRef]
- Takefuji, Y. Sustainable protein alternatives. Trends Food Sci. Technol. 2021, 107, 429–431. [Google Scholar] [CrossRef]
- Harnack, L.; Mork, S.; Valluri, S.; Weber, C.; Schmitz, K.; Stevenson, J.; Pettit, J. Nutrient Composition of a Selection of Plant-Based Ground Beef Alternative Products Available in the United States. J. Acad. Nutr. Diet. 2021, 121, 2401–2408.e12. [Google Scholar] [CrossRef]
- Lacy-Nichols, J.; Hattersley, L.; Scrinis, G. Nutritional marketing of plant-based meat-analogue products: An exploratory study of front-of-pack and website claims in the USA. Public Health Nutr. 2021, 24, 4430–4441. [Google Scholar] [CrossRef]
- Alessandrini, R.; Brown, M.K.; Pombo-Rodrigues, S.; Bhageerutty, S.; He, F.J.; MacGregor, G.A. Nutritional Quality of Plant-Based Meat Products Available in the UK: A Cross-Sectional Survey. Nutrients 2021, 13, 4225. [Google Scholar] [CrossRef]
- Toribio-Mateas, M.A.; Bester, A.; Klimenko, N. Impact of Plant-Based Meat Alternatives on the Gut Microbiota of Consumers: A Real-World Study. Foods 2021, 10, 2040. [Google Scholar] [CrossRef]
- Cole, E.; Goeler-Slough, N.; Cox, A.; Nolden, A. Examination of the nutritional composition of alternative beef burgers available in the United States. Int. J. Food Sci. Nutr. 2022, 73, 425–432. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Srv, A.; Henry, C.J. Unknown impacts of plant-based meat alternatives on long-term health. Nat. Food 2022, 3, 90–91. [Google Scholar] [CrossRef]
- Ishaq, A.; Irfan, S.; Sameen, A.; Khalid, N. Plant-based meat analogs: A review with reference to formulation and gastrointestinal fate. Curr. Res. Food Sci. 2022, 5, 973–983. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Moshtaghian, H.; Bianchi, M.; Hallström, E. Nutritional assessment of plant-based meat analogues on the Swedish market. Int. J. Food Sci. Nutr. 2022, 73, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, L.; Li, H.; Ren, Y.; Chao, J.; Zhang, T.; Liu, X. Ingredients and Process Affect the Structural Quality of Recombinant Plant-Based Meat Alternatives and Their Components. Foods 2022, 11, 2202. [Google Scholar] [CrossRef]
- Harnack, L.J.; Reese, M.M.; Johnson, A.J. Are Plant-Based Meat Alternative Products Healthier Than the Animal Meats They Mimic? Nutr. Today 2022, 57, 195–199. [Google Scholar] [CrossRef]
- Penna Franca, P.A.; Duque-Estrada, P.; Fraga da Fonseca e Sá, B.; van der Goot, A.; Pierucci, A.P.T.R. Meat substitutes—Past, present, and future of products available in Brazil: Changes in the nutritional profile. Future Foods 2022, 5, 100133. [Google Scholar] [CrossRef]
- Cutroneo, S.; Angelino, D.; Tedeschi, T.; Pellegrini, N.; Martini, D.; SINU Young Working Group. Nutritional Quality of Meat Analogues: Results from the Food Labelling of Italian Products (FLIP) Project. Front. Nutr. 2022, 9, 852831. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; McClements, D.J. Applications of the INFOGEST In Vitro Digestion Model to Foods: A Review. Annu. Rev. Food Sci. Technol. 2023, 14, 135–156. [Google Scholar] [CrossRef]
- Lawrence, A.S.; Huang, H.; Johnson, B.J.; Wycherley, T.P. Impact of a Switch to Plant-Based Foods That Visually and Functionally Mimic Animal-Source Meat and Dairy Milk for the Australian Population—A Dietary Modelling Study. Nutrients 2023, 15, 1825. [Google Scholar] [CrossRef]
- Flint, M.; Bowles, S.; Lynn, A.; Paxman, J. Novel plant-based meat alternatives: Future opportunities and health considerations. Proc. Nutr. Soc. 2023, 82, 370–385. [Google Scholar] [CrossRef]
- Rizzolo-Brime, L.; Orta-Ramirez, A.; Puyol Martin, Y.; Jakszyn, P. Nutritional Assessment of Plant-Based Meat Alternatives: A Comparison of Nutritional Information of Plant-Based Meat Alternatives in Spanish Supermarkets. Nutrients 2023, 15, 1325. [Google Scholar] [CrossRef]
- Melville, H.; Shahid, M.; Gaines, A.; McKenzie, B.L.; Alessandrini, R.; Trieu, K.; Wu, J.H.Y.; Rosewarne, E.; Coyle, D.H. The nutritional profile of plant-based meat analogues available for sale in Australia. Nutr. Diet. 2023, 80, 211–222. [Google Scholar] [CrossRef]
- Romão, B.; Botelho, R.B.A.; Torres, M.L.; Maynard, D.D.C.; de Holanda, M.E.M.; Borges, V.R.P.; Raposo, A.; Zandonadi, R.P. Nutritional Profile of Commercialized Plant-Based Meat: An Integrative Review with a Systematic Approach. Foods 2023, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Salomé, M.; Mariotti, F.; Dussiot, A.; Kesse-Guyot, E.; Huneau, J.F.; Fouillet, H. Plant-based meat substitutes are useful for healthier dietary patterns when adequately formulated—An optimization study in French adults (INCA3). Eur. J. Nutr. 2023, 62, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- McClements, I.F.; McClements, D.J. Designing healthier plant-based foods: Fortification, digestion, and bioavailability. Food Res. Int. 2023, 169, 112853. [Google Scholar] [CrossRef]
- Rizzo, G.; Testa, R.; Dudinskaya, E.C.; Mandolesi, C.; Solfanelli, F.; Zanoli, R.; Schifani, G.; Migliore, G. Understanding the consumption of plant-based meat alternatives and the role of health-related aspects. A study of the Italian market. Int. J. Gastron. Food Sci. 2023, 32, 100690. [Google Scholar] [CrossRef]
- Toth, A.J.; Dunay, A.; Tattay, M.; Illes, C.B.; Bittsanszky, A.; Suth, M. Microbial spoilage of plant-based meat nalogues. Appl. Sci. 2021, 11, 8309. [Google Scholar] [CrossRef]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, O.; Dellafiora, L.; Dall’Asta, C. Assessing the Mycotoxin-related Health Impact of Shifting from Meat-based Diets to Soy-based Meat Analogues in a Model Scenario Based on Italian Consumption Data. Expo. Health 2022, 15, 661–675. [Google Scholar] [CrossRef]
- Mihalache, O.A.; Dellafiora, L.; Dall’Asta, C. A systematic review of natural toxins occurrence in plant commodities used for plant-based meat alternatives production. Food Res. Int. 2022, 158, 111490. [Google Scholar] [CrossRef]
- Kopko, C.; Garthoff, J.A.; Zhou, K.; Meunier, L.; O’Sullivan, A.J.; Fattori, V. Are alternative proteins increasing food allergies? Trends, drivers and future perspectives. Trends Food Sci. Technol. 2022, 129, 126–133. [Google Scholar] [CrossRef]
- Liu, Z.; Shaposhnikov, M.; Zhuang, S.; Tu, T.; Wang, H.; Wang, L. Growth and survival of common spoilage and pathogenic bacteria in ground beef and plant-based meat analogues. Food Res. Int. 2023, 164, 112408. [Google Scholar] [CrossRef]
- Fresán, U.; Mejia, M.A.; Craig, W.J.; Jaceldo-Siegl, K.; Sabaté, J. Meat Analogs from Different Protein Sources: A Comparison of Their Sustainability and Nutritional Content. Sustainability 2019, 11, 3231. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Luchansky, J.B.; Shoyer, B.A.; Jung, Y.; Shane, L.E.; Osoria, M.; Porto-Fett, A.C. Viability of Shiga toxin–producing Escherichia coli, Salmonella, and Listeria monocytogenes Within plant versus beef burgers during cold storage and following pan frying. J. Food Prot. 2020, 83, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Santo, R.E.; Kim, B.F.; Goldman, S.E.; Dutkiewicz, J.; Biehl, E.M.B.; Bloem, M.W.; Neff, R.A.; Nachman, K.E. Considering plant-based meat substitutes and cell-based meats: A public health and food systems perspective. Front. Sustain. Food Syst. 2020, 4, 134. [Google Scholar] [CrossRef]
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-based and cell-based approaches to meat production. Nat. Commun. 2020, 11, 6276. [Google Scholar] [CrossRef]
- Tso, R.; Forde, C.G. Unintended consequences: Nutritional impact and potential pitfalls of switching from animal- to plant-based Foods. Nutrients 2021, 13, 2527. [Google Scholar] [CrossRef]
- Sun, C.; Ge, J.; He, J.; Gan, R.; Fang, Y. Processing, Quality, Safety, and Acceptance of Meat Analogue Products. Engineering 2021, 7, 674–678. [Google Scholar] [CrossRef]
- Tyndall, S.M.; Maloney, G.R.; Cole, M.B.; Hazell, N.G.; Augustin, M.A. Critical food and nutrition science challenges for plant-based meat alternative products. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Pezzica, J.; Bolli, C.; Di Nicola, A.; Falai, A.; Giannese, D.; Cupisti, A. Processed plant-based foods for CKD patients: Good choice, but be aware. Int. J. Environ. Res. Public Health 2022, 19, 6653. [Google Scholar] [CrossRef]
- Nezlek, J.B.; Forestell, C.A. Meat Substitutes: Current Status, Potential Benefits, and Remaining Challenges. Curr. Opin. Food Sci. 2022, 47, 100890. [Google Scholar] [CrossRef]
- Shaghaghian, S.; McClements, D.J.; Khalesi, M.; Garcia-Vaquero, M.; Mirzapour-Kouhdasht, A. Digestibility and bioavailability of plant-based proteins intended for use in meat analogues: A review. Trends Food Sci. Technol. 2022, 129, 646–656. [Google Scholar] [CrossRef]
- Ahmad, M.; Qureshi, S.; Akbar, M.H.; Siddiqui, S.A.; Gani, A.; Mushtaq, M.; Hassan, I.; Dhull, S.B. Plant-based meat alternatives: Compositional analysis, current development and challenges. Appl. Food Res. 2022, 2, 100154. [Google Scholar] [CrossRef]
- Andreani, G.; Sogari, G.; Marti, A.; Froldi, F.; Dagevos, H.; Martini, D. Plant-Based Meat Alternatives: Technological, Nutritional, Environmental, Market, and Social Challenges and Opportunities. Nutrients 2023, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, W.; Aoyagi, A. History of Meat Alternatives (960 CE to 2014). 2014. Available online: https://www.soyinfocenter.com/books/179; https://www.soyinfocenter.com/pdf/179/MAL.pdf (accessed on 10 June 2023).
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Meat analogue as future food: A review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.E.; Franzen, K.L. Texturized proteins: Fabrication, flavoring, and nutrition. Crit. Rev. Food Sci. Nutr. 1978, 10, 147–207. [Google Scholar] [CrossRef]
- King, T.; Lawrence, S. Meat the Alternative-Australia’s $3 Billion Opportunity. 2019. Available online: https://www.foodfrontier.org/wp-content/uploads/2019/09/Meat_the_Alternative_FoodFrontier.pdf (accessed on 10 June 2023).
- McClements, D.J. Ultraprocessed plant-based foods: Designing the next generation of healthy and sustainable alternatives to animal-based foods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3531–3559. [Google Scholar] [CrossRef]
- Alfieri, F.; Rivero-Pino, F.; Zakidou, P.; Fernandez-Dumont, A.; Roldán-Torres, R. 4.04—Processes for Obtaining Plant-Based Dairy and Meat Substitutes. In Sustainable Food Science—A Comprehensive Approach; Ferranti, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 75–99. [Google Scholar] [CrossRef]
- Joshi, V.; Kumar, S. Meat Analogues: Plant based alternatives to meat products—A review. Int. J. Food Ferment. Technol. 2015, 5, 107–119. [Google Scholar] [CrossRef]
- Manski, J.M.; van Riemsdijk, L.E.; van der Goot, A.J.; Boom, R.M. Importance of intrinsic properties of dense caseinate dispersions for structure formation. Biomacromolecules 2007, 8, 3540–3547. [Google Scholar] [CrossRef]
- Van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of novel protein sources (Insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed protection. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Grant, G.A. Isolation/Purification of Proteins. In Encyclopedia of Cell Biology; Bradshaw, R.A., Stahl, P.D., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 66–74. [Google Scholar] [CrossRef]
- Loveday, S.M. Plant protein ingredients with food functionality potential. Nutr. Bull. 2020, 45, 321–327. [Google Scholar] [CrossRef]
- Zahari, I.; Östbring, K.; Purhagen, J.K.; Rayner, M. Plant-Based Meat Analogues from Alternative Protein: A Systematic Literature Review. Foods 2022, 11, 2870. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant Proteins for future foods: A roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Keppler, J.K.; van der Goot, A.J. Functionality of Ingredients and Additives in Plant-Based Meat Analogues. Foods 2021, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Rong, Y.; Rong, S.; Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: A systematic review and meta-analysis. BMC Med. 2012, 10, 119. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant-protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- WHO. Salt Reduction; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 17 November 2022).
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Plant-Based Meat Analogues. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–126. ISBN 9780128148747. [Google Scholar]
- Neufingerl, N.; Eilander, A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: A systematic review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- FAO/WHO. Risk Assessment of Food Allergens. Part 2: Review and Establish Threshold Levels in Foods for the Priority Allergens. Meeting Report. Food Safety and Quality Series No. 15. Rome. 2022. Available online: https://apps.who.int/iris/rest/bitstreams/1488221/retrieve (accessed on 10 June 2023).
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef]
- Berin, M.C.; Sampson, H.A. Food allergy: An enigmatic epidemic. Trends Immunol. 2013, 34, 390–397. [Google Scholar] [CrossRef]
- Kok, M.; Compagner, A.; Panneman, I.; Sprikkelman, A.; Vlieg-Boerstra, B. A Food, a Bite, a Sip: How Much Allergen Is in That? Nutrients 2021, 13, 587. [Google Scholar] [CrossRef]
- Jones, R. Why Food Allergies Are on the Rise? BBC. 26 October 2020. Available online: https://www.bbc.com/future/article/20201023-food-allergies-why-nut-dairy-and-food-allergy-are-rising (accessed on 10 June 2023).
- Lemken, D.; Spiller, A.; Schulze-Ehlers, B. More room for legume—Consumer acceptance of meat substitution with classic, processed and meat-resembling legume products. Appetite 2019, 143, 104412. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine (NASEM). Genetically Engineered Crops: Experiences and Prospects; National Academies Press: Washington, DC, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK424534/ (accessed on 10 June 2023).
- Dunn, S.E.; Vicini, J.L.; Glenn, K.C.; Fleischer, D.M.; Greenhowt, M. The allergenicity of genetically modified foods from genetically engineered crops. A narrative and systematic review. Ann. Asthma Allergy Immunol. 2017, 119, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Ho, H.K.; Leung, T.F. Genetically modified foods and allergy. Hong Kong Med. J. 2017, 23, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Boccia, F.; Punzo, G. A choice experiment on consumer perceptions of three generations of genetically modified foods. Appetite 2021, 161, 105158. [Google Scholar] [CrossRef]
- Taylor, S.L.; Marsh, J.T.; Koppelman, S.J.; Kabourek, J.L.; Johnson, P.E.; Baumert, J.L. A perspective on pea allergy and pea allergens. Trends Food Sci. Technol. 2021, 116, 186–198. [Google Scholar] [CrossRef]
- Morrison, O. Pea Protein Trend Sparks Allergy Warning. In Food Navigator. 16 March 2020. Available online: https://www.foodnavigator.com/Article/2020/03/16/Pea-protein-trend-sparks-allergy-warning (accessed on 21 October 2022).
- Wensing, M.; Knulst, A.C.; Piersma, S.; O’Kane, F.; Knol, E.F.; Koppelman, S.J. Patients with anaphylaxis to pea can have peanut allergy caused by cross-reactive IgE to vicilin (Ara h 1). J. Allergy Clin. Immunol. 2003, 111, 420–424. [Google Scholar] [CrossRef]
- Abrams, E.M.; Gerstner, T.V. Allergy to cooked, but not raw, peas: A case series and review. Allergy Asthma Clin. Immunol. 2015, 11, 10. [Google Scholar] [CrossRef]
- Fearn, H. Pea Protein Is Causing a Mighty Problem for People with Allergies. In HuffPost. 2021. Available online: https://www.huffingtonpost.co.uk/entry/peaprotein-allergy_uk_618ad212e4b055e47d80f1da (accessed on 16 November 2022).
- Allergen Bureau. Are Plant-Based Meat Alternatives Heralding New Allergen Risks? 24 June 2019. Allergen Bureau. Available online: https://allergenbureau.net/are-plant-based-meat-alternatives-heralding-new-allergen-risks/ (accessed on 10 June 2023).
- Christensen, M.J.; Eller, E.; Mortz, C.G.; Brockow, K.; Bindslev-Jensen, C. Exercise lowers threshold and increases severity, but wheat-dependent, exercise-induced anaphylaxis can be elicited at rest. J. Allergy Clin. Immunol. Pract. 2018, 6, 514–520. [Google Scholar] [CrossRef]
- Christensen, M.J.; Eller, E.; Mortz, C.G.; Brockow, K.; Bindslev-Jensen, C. Wheat-dependent cofactor-augmented anaphylaxis: A prospective study of exercise, aspirin, and alcohol efficacy as cofactors. J. Allergy Clin. Immunol. Pract. 2019, 7, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Khullar, K.; Saltzman, R.; Fiedler, J.; Garrett, J.P.; Naimi, D.R.; Spergel, J.M. Oral food challenge to wheat: A near-fatal anaphylaxis and review of 93 food challenges in children. World Allergy Organ. J. 2013, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. General Standard for the Labelling of Prepackaged Foods; FAO: Rome, Italy, 2018; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B1-1985%252FCXS_001e.pdf (accessed on 10 June 2023).
- FAO/WHO. Ad Hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens. Part 1: Review and Validation of Codex Priority Allergen List through Risk Assessment. Summary and Conclusions; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/3/cb4653en/cb4653en.pdf (accessed on 10 June 2023).
- Wild, F.; Czerny, M.; Janssen, A.M.; Kole, A.P.W.; Zunabovic, M.; Domig, K.J. The Evolution of a Plant-Based Alternative to Meat: From Niche Markets to Widely Accepted Meat Alternatives. Agro Food Ind. Hi Tech. 2014, 25, 45–49. [Google Scholar]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-Bijak, J.; Siadkowski, A.; Bijak, M. Molecular Aspects of Mycotoxins—A Serious Problem for Human Health. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Samota, M.K.; Kimar, A.; Silva, A.S.; Dubey, N.K. Fungal mycotoxins in food commodities: Present status and future concerns. Front. Sustain. Food Syst. 2023, 7, 1162595. [Google Scholar] [CrossRef]
- Bennett, J.W.; Kilch, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of foodcrops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Cinar, A.; Onbaşı, E. Mycotoxins: The Hidden Danger in Foods. In Mycotoxins and Food Safety; Sabuncuoğlu, S., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Piemontese, L.; Gambacorta, L.; Zivoli, R.; Longobardi, F. Food coloring agents and plant food supplements derived from Vitis vinifera: A new source of human exposure to ochratoxin A. J. Agric. Food Chem. 2015, 63, 3609–3614. [Google Scholar] [CrossRef]
- Scott, P.M. Recent research on fumonisins: A review. Food Addit. Contam. 2012, 29, 242–248. [Google Scholar] [CrossRef]
- Tang, A.S.P. Food Safety Focus. Centre for Food Safety: The Government of the Hong Kong Special Administrative Region. August 2007. Available online: https://www.cfs.gov.hk/english/multimedia/multimedia_pub/multimedia_pub_fsf_13_02.html# (accessed on 10 June 2023).
- Bixler, H.J. The carrageenan controversy. J. Appl. Phycol. 2017, 29, 2201–2207. [Google Scholar] [CrossRef]
- Cornucopia Institute (n.d.) Food Grade Carrageenan: Reviewing Potential Harmful Effects on Human Health. Available online: https://www.cornucopia.org/CornucopiaAnalysisofCarrageenanHealthImpacts042612.pdf (accessed on 10 June 2023).
- Almela, C.; Algora, S.; Benito, V.; Clemente, M.J.; Devesa, V.; Suner, M.A.; Velez, D.; Montoro, R. Heavy metal, total arsenic, and inorganic arsenic contents of algae food products. J. Agric. Food Chem. 2002, 50, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Besada, V.; Andrade, J.M.; Schultze, F.; González, J.J. Heavy metals in edible seaweeds commercialised for human consumption. J. Mar. Syst. 2009, 75, 305–313. [Google Scholar] [CrossRef]
- Watson, D.B. Public health and carrageenan regulation: A review and analysis. In Proceedings of the Nineteenth International Seaweed Symposium, Kobe, Japan, 26–31 March 2007; Springer: Dordrecht, The Netherlands, 2007; pp. 55–63. [Google Scholar]
- Liu, F.; Hou, P.; Zhang, H.; Tang, Q.; Xue, C.; Li, R.W. Food-grade carrageenans and their implications in health and disease. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3918–3936. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- U.S. Centers for Disease Control and Prevention. Public Health Statement for n-Hexane. Agency for Toxic Substances and Disease Registry: Toxic Substances Portal. 6 May 2014. Available online: https://wwwn.cdc.gov/TSP/PHS/PHS.aspx?phsid=391&toxid=68 (accessed on 10 June 2023).
- Cornucopia Institute. Guide to Hexane-Extracted Soy in Meat Alternatives. Hexane Project. 12 July 2018. Available online: https://www.cornucopia.org/hexane-guides/hexane_guide_meat_alternatives.html (accessed on 10 June 2023).
- Petroski, W.; Minich, D.M. Is there such a thing as “anti-nutrients”? A narrative review of perceived problematic plant compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Omosebi, M.O.; Osundahunsi, O.F.; Fagbemi, T.N. Effect of Extrusion on Protein Quality, Antinutritional Factors, and Digestibility of Complementary Diet from Quality Protein Maize and Soybean Protein Concentrate. J. Food Biochem. 2018, 42, e12508. [Google Scholar] [CrossRef]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for special dietary needs: Non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Medicine. Cancer Biologists Find DNA-Damaging Toxins in Common Plant-Based Foods. Johns Hopkins Medicine: News and Publications. 28 March 2013. Available online: https://www.hopkinsmedicine.org/news/media/releases/cancer_biologists_find_dna_damaging_toxins_in_common_plant_based_foods (accessed on 10 June 2023).
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Fahimi, S.; Singh, G.M.; Micha, R.; Khatibzadeh, S.; Engell, R.E.; Lim, S.; Danaei, G.; Ezzati, M.; Powles, J. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014, 37, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Pollan, M. Defense of Food: An Eater’s Manifesto; Penguin Putnam Inc.: New York, NY, USA, 2009. [Google Scholar]
- Seitz, A. Why Starchy Foods Matter, and Which Ones to Eat? 23 April 2023. Available online: https://www.medicalnewstoday.com/articles/starchy-foods (accessed on 10 June 2023).
- FSANZ. Acrylamide and Food. October 2016. Available online: https://www.foodstandards.gov.au/consumer/chemicals/acrylamide/Pages/default.aspx#:~:text=Acrylamide%20is%20a%20chemical%20that,cause%20cancer%20in%20laboratory%20animals (accessed on 10 June 2023).
- Antoine, T.; Icard-Vernière, C.; Scorrano, G.; Salhi, A.; Halimi, C.; Georgé, S.; Carrière, F.; Mouquet-Rivier, C.; Reboul, E. Evaluation of vitamin D bioaccessibility and mineral solubility from test meals containing meat and/or cereals and/or pulses using in vitro digestion. Food Chem. 2021, 347, 128621. [Google Scholar] [CrossRef]
- Gibson, R.S.; Heath, A.M.; Szymlek-Gay, E.A. Is iron and zinc nutrition a concern for vegetarian infants and young children in industrialized countries? Am. J. Clin. Nutr. 2014, 100, 459S–468S. [Google Scholar] [CrossRef]
- Alae-Carew, C.; Green, R.; Stewart, C.; Cook, B.; Dangour, D.; Scheelbeek, P.F. The role of plant-based alternative foods in sustainable and healthy food systems: Consumption trends in the UK. Sci. Total Environ. 2022, 807, 151041. [Google Scholar] [CrossRef]
- Leroy, F.; Cofnas, N. Should dietary guidelines recommend low red meat intake? Crit. Rev. Food Sci. Nutr. 2019, 60, 2763–2772. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Hamaker, B.R. Texturization of plant protein-based meat alternatives: Processing, base proteins, and other constructional ingredients. Future Foods 2023, 8, 100248. [Google Scholar] [CrossRef]
- Schmid, E.M.; Farahnaky, A.; Adhikari, B.; Torley, P.J. High moisture extrusion cooking of meat analogs: A review of mechanisms of protein texturization. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4573–4609. [Google Scholar] [CrossRef]
- Arora, S.; Kataria, P.; Nautiyal, M.; Tuteja, I.; Sharma, V.; Ahmad, F.; Haque, S.; Shahwan, M.; Capanoglu, E.; Vashishth, R.; et al. Comprehensive review on the role of plant protein as a possible meat analogue: Framing the future of meat. ACS Omega 2023, 8, 23305–23319. [Google Scholar] [CrossRef]
- Verma, T.; Subbiah, J. Conical twin-screw extrusion is an effective inactivation process for Salmonella in low-moisture foods at temperatures above 65 °C. LWT 2019, 114, 108369. [Google Scholar] [CrossRef]
- FAO; WHO. Evaluation of Certain Contaminants in Food. Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series No. 1002; WHO: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/bitstream/10665/254893/1/9789241210027-eng.pdf?ua=1#page=104%22%3E (accessed on 10 June 2023).
- Gao, B.; Li, Y.; Huang, G.; Yu, L. Fatty acid esters of 3-monochloropropanediol: A review. Annu. Rev. Food Sci. Technol. 2019, 10, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef] [PubMed]
- WHO. More than 3 Billion People Protected from Harmful Trans-Fat in Their Food; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/news/item/09-09-2020-morethan-3-billion-people-protected-from-harmful-transfat-in-their-food (accessed on 10 June 2023).
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.; Ramos, O.L.; Vicente, A.A. Emergent Food Proteins—Towards Sustainability, Health and Innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef]
- FAO. Thinking about the Future of Food Safety A Foresight Report; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/3/cb8667en/cb8667en.pdf (accessed on 10 June 2023).
- Rossi, F.; Felis, G.E.; Martinelli, A.; Calcavecchia, B.; Torriani, S. Microbiological characteristics of fresh tofu produced in small industrial scale and identification of specific spoiling microorganisms (SSO). LWT—Food Sci. Technol. 2016, 70, 280–285. [Google Scholar] [CrossRef]
- Duthoo, E.; De Reu, K.; Leroy, F.; Weckx, S.; Heyndrickx, M.; Rasschaert, G. To culture or not to culture: Careful assessment of metabarcoding data is necessary when evaluating the microbiota of a modified-atmosphere-packaged vegetarian meat alternative throughout its shelf-life period. BMC Microbiol. 2022, 22, 34. [Google Scholar] [CrossRef]
- Geeraerts, W.; De Vuyst, L.; Leroy, F. Ready-to-eat meat alternatives, a study of their associated bacterial communities. Food Biosci. 2020, 37, 100681. [Google Scholar] [CrossRef]
- Baylis, C.; Uyttendaele, M.; Joosten, H.; Davies, A. The Enterobacteriaceae and their significance to the food industry. In ILSI Europe; International Life Sciences Institute: Washington, DC, USA, 2011; pp. 9–12. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).