Abstract
As pests are an important factor in reducing crop yields, pest control is an important measure in preventing reductions in crop yields. With the aim of ending the use of chemical pesticides, biological control and genetically modified methods are now considered more reasonable pest control strategies. The bacterium Bacillus thuringiensis (Bt) can produce crystal proteins that have specific toxicity to lepidopteran insects, and so it has been applied as a microbial insecticide in the control of crop pests for several decades. With the development of plant genetic engineering, Bt genes encoding insecticidal crystal protein have been introduced into many crop species for pest control. This article indicates that, after years of experiments and research, Bt transgenic rice is close to becoming a commercial insect-resistant rice, and many studies have shown that transgenic rice has pronounced abilities in the control of pests such as yellow stem borers (Scirpophaga incertulas, YSB), striped stem borers (Chilo suppressalis, SSB), and rice leaf rollers (Cnaphalocrocis medinalis, RLR); moreover, it does not obviously differ from non-transgenic rice in terms of safety. This paper suggests that transgenic Bt rice has application potential and commercial value.
1. Introduction
Rice is one of the most important food crops in the world, being the staple food for about half of the world’s population [1]. Rice is also one of the food crops most seriously affected by pests, and the annual yield loss caused by insect pests is about 10% [2]. Therefore, effective pest control is particularly important. Chemical insecticides are mainly used to control pests during production. This control method is not only expensive, but also causes environmental pollution, pesticide residues, pest resistance, and other problems. Since the Bt insecticidal protein was found to kill pests selectively, it has been widely used as a biological agent in crop pest control. The creation of the first transgenic tobacco in 1983 heralded the arrival of the era of plant genetic engineering; since then, the transgenic research of plants has entered a stage of vigorous development [3]. The development of plant transgenic technology provides a new method for the control of agricultural pests. The genetically modified crops are mainly designed with a focus on improving traits to obtain ideal results. This has led to the development of crops with improved yield, quality and tolerance to biotic and abiotic stresses. With the introduction of favorable traits into crops, biotechnology has opened up a way for transgenic crops to integrate into sustainable food production systems [4]. Using plant transgenic technology, researchers have transferred the Bt gene into crops to control target pests. So far, it has been reported that transgenic rice with the Bt gene can effectively control pests such as yellow stem borers (Scirpophaga incertulas), striped stem borers (Chilo suppressalis), and rice leaf rollers (Cnaphalocrocis medinalis). In this paper, the breeding, resistance phenotype, and relative safety of different forms of Bt transgenic rice are established and interrogated.
2. Bt Gene
Bacillus thuringiensis (Bt) is a kind of Gram-positive bacteria widely found in soil. It produces a large number of parasporal crystals in the process of spore formation. These crystals are composed of proteins with highly specific insecticidal activity, and they are referred to as insecticidal crystal protein (ICP). A 1989 study proposed that, according to their structural characteristics and insecticidal specificity, Bt proteins can be divided into five categories: Cry Ⅰ (specific toxicity for Lepidoptera insects), Cry Ⅱ (specific toxicity for Lepidoptera and Diptera insects), CryIII (specific toxicity for Coleoptera insects), Cry Ⅳ (specific toxicity for Diptera insects), and Cyt (cytolytic crystal proteins) [5]. With the discovery of more and more Bt genes, it was found that there was no consistency between amino acid sequence homology and insecticidal activity. The discovery of new genes made the defects of the above classification methods increasingly obvious, so it is difficult to accurately classify new genes. In 1998, Crickmore et al. proposed using the homology of the amino acid sequence of Bt proteins as the only basis for the classification of Bt genes. A homology of amino acid sequences less than 45%, which is the first classification level, is expressed in Arabic numerals. A sequence homology higher than 45% but less than 78%, which is the second classification level, is expressed in uppercase English letters. A sequence homology higher than 78% but less than 95%, which is the third classification level, is expressed in lowercase English letters; finally, a sequence homology more than 95%, which is the fourth classification level, is represented by Arabic numerals [6]. An example is the cry1Aa1 gene. Since the first Bt gene was discovered and cloned in 1981 [7], new Bt genes have been continuously discovered. By September 2016, the cloned Bt genes fell into 77 categories, with a total of 825 gene sequences (including 787 cry genes and 38 cyt genes) (http://www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt/toxins2.html, accessed on 5 May 2023). These Bt proteins can act on target insects such as Lepidoptera, Diptera, and Coleoptera, as well as nematodes. Moreover, Cyt has cytolytic properties (Figure 1). The nomenclature and statistics of the Bt gene in crop application are shown in Table 1.
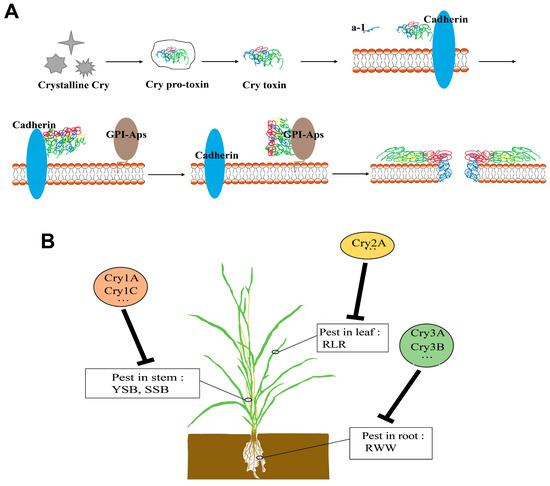
Figure 1.
The Insecticidal Mechanism of Bt Protein and Its Application in Rice. (A) Models of the mode of action of Cry toxins: (1) Toxin dissolved in the midgut. (2) Activated by midgut protease. (3) Binding to primary receptor cadherin. (4) Step 3 induces the cleavage of helix a-1 and triggers toxin oligomerization. (5) Toxin oligomers bind to a second receptor (glycosylphosphatidylinositol-anchored proteins, GPI-Aps), such as aminopeptidase or alkaline phosphatase, which is anchored by glycosylphosphatidylinositol in the membrane. (6) The toxin enters the cell membrane, forming a hole, killing insect cells; (B) Bt protein’s application in rice.

Table 1.
Nomenclature and application of Bt gene.
3. Research Progress of Transgenic Rice That Is Resistant to Lepidoptera Pests
Lepidoptera pests (such as YSB, SSB damage rice stems, RLR damage rice leaves) harm rice and affect its physiological development. At present, many different Bt genes have been applied to various varieties of rice, including indica rice, japonica rice and hybrid rice (Table 2).

Table 2.
Application of Bt gene in rice.
3.1. A Close-To-Commercial Insect-Resistant Rice Strain: Huahui No.1
Since the first case of insect-resistant rice with a modified Cry1Ab gene was reported [17], more and more studies have reported the successful breeding of insect-resistant upland rice with the Bt gene. Huahui No.1 is the first transgenic rice line with the Bt gene being tested in the field in China. The Cry1Ab/1Ac fusion gene vector driven by an ActinI promoter and the vector containing a hygromycin phosphotransferase gene (hpt) were transformed into the rice restorer line MH63 by particle bombardment. Through the self-separation of offspring, a good homozygous line without a selective marker gene was screened, namely TT51-1(Huahui No.1). According to the results of the Western Blot analysis, it was estimated that the ratio of Cry1Ab/1Ac protein to total soluble protein in the transgenic plants was about 0.02%. A field insect resistance test in Wuhan in 1999 investigated the damage caused by rice leaf rollers under natural infestation. The results showed that the TT51-1 plants had significantly fewer damaged leaves than the control variety MH63 (30.5), and the percentage of damaged tillers (0.7%) was also significantly lower than that of the control varieties (85.9%). Under the conditions of artificial inoculation and natural infestation, the dead heart rate (0–0.2%) and white ear rate (0–0.4%) caused by stem borers (C. suppressalis and S. incertulas) in Huahui No.1 and its hybrid combination Bt Shanyou 63 were significantly lower than that of the control varieties MH63 and SY63 (the dead heart rate and white ear rate were 1.1~41.8% and 10.3~94.2%, respectively). No pesticides were sprayed during the whole growth period; the results of the investigation of agronomic characteristics showed that there was no significant difference between Bt SY63 and the control SY63 for most variables, but the yield increased by 28.9%. The results further showed that the transgenic Huahui No.1 and its hybrid combination Bt SY63 had good resistance to rice leaf rollers, striped rice borers, and yellow rice borers across the whole growth period [9]. In 2014 and 2020, Huahui No.1 and Bt SY 63 obtained safety certificates for production and application in Hubei Province, which allowed their commercialization to progress steadily.
On 20 January 2018, the transgenic insect-resistant rice “Huahui No.1” passed the safety review procedures of the FDA and EPA in the United States, which means that “Huahui No.1” rice and its products can be exported to the United States and directly sold to ordinary consumers in the country’s market. The data from the safety evaluation of “Huahui No.1” granted by the FDA showed that there was no substantial difference between “Huahui No.1” rice and the original variety in terms of safety, nutritional composition, anti-nutritional factors, and other related parameters, either as human food or animal feed (https://www.fda.gov/media/110390/download; accessed on 5 May 2023).
3.2. Cultivation of Insect-Resistant Transgenic Rice with the New Bt Gene
Bt transgenic crops have been commercially cultivated for 20 years, but most of the Bt genes used in these crops are Cry1A (Cry1Ab, Cry1Ac, Cry1Ab/Cry1Ac) [18,19,20,21,22,23,24,25,26]. Some studies have shown that Cry1Ab and Cry1Ac have the same receptor in insects and exhibit cross resistance. Once the target insect develops resistance to one of these proteins, then all these proteins will lose their insect resistance [27,28]. Therefore, it is of great significance to find a new type of Bt insect resistance gene. The homology of the Cry1C, Cry2A, and Cry1A proteins was found to be very low; they have different receptors in insects and do not exhibit cross resistance [29,30,31].
Chen et al. modified the wild type cry2Aa gene according to the codon preference of rice, added a non-translational sequence (5′UTR) at the 5′ end to improve the gene expression, and added a 3′ tailed recognition sequence to synthesize a new cry2A* gene. Then, the cry2A* gene was driven by a maize Ubiquitin promoter to construct a transformation vector and introduced into indica rice restorer line MH 63 through Agrobacterium tumefaciens-mediated genetic transformation. Through Southern Blotting and a field investigation of transgenic offspring, four transgenic single-copy homozygous families with good resistance were screened (T2A-1, T2A-2, T2A-3, and T2A-4). The results of the indoor feeding of the first instar yellow rice borer showed that all the larvae of the four transgenic families died, while the mortality rate of the control variety MH63 was only 10%. Four transgenic families showed good insecticidal effects. In 2004, field insect resistance identification was carried out in the field. A combination of natural infestation and artificial inoculation (i.e., access to the first instar S. incertulas during the tillering stage) was adopted. The results of the first investigation showed that the number of damaged leaves per tiller of four transgenic families was 0.01~0.02, which was significantly lower than that of MH 63 (1.17). The dead heart rate of the transgenic families was 5.36–7.48%, which was significantly lower than that of MH63 (17.24%). The results of the second investigation showed that the number of damaged leaves per tiller of the four transgenic families was 0.01~0.08, which was significantly lower than that of MH 63 (1.13). The dead heart rate and white ear rate of transgenic families were 0.98–4.18% and 0.00–0.50%, respectively, significantly lower than the values of 17.37% and 5.57% of the control MH63. The results of the indoor and field experiments showed that the four transgenic families had good resistance to YSB, SSB, and RLR [10].
Tang et al. modified the wild-type cry1Ca5 gene according to the codon preference of rice, adding nos Terminator at the 3′ end, and then synthesizing a new cry1C* gene. Genetic transformation mediated by Agrobacterium tumefaciens was introduced into the indica rice restorer line MH63. Through a series of molecular detections and field investigations, five transgenic single-copy homozygous families with good insect resistance were selected. After an indoor inoculation test and the determination of the Cry1C* protein content, the family with the best performance, T1C-19, was selected for follow-up field experiments. The results of the field resistance test of T1C-19 and its hybrid combinations showed that T1C-19 and its hybrid combinations had good resistance to YSB, SSB, and RLR [11].
3.3. Cultivation of Bivalent Bt Insect-Resistant Rice
As is the case for chemical insecticides, the large-scale cultivation of Bt transgenic crops also leads to resistance in the target insects, resulting in reduced insect resistance and even the loss of the insecticidal effect of Bt transgenic crops. In the 20 years since the initial commercial cultivation of Bt crops, a variety of resistant insects have been found in the field. Plutella xylostella was the first insect found to be resistant to the Bt protein (Cry1A) in the field [27]. Then, Helicoverpa armigera and Heliothis virescens were found to be resistant to the Bt protein [32]. In addition, Helotropha leucostigma was the first stem-boring insect found to be resistant to the Bt protein [33]. It has been suggested that there is a risk of insects evolving populations resistant to transgenic rice with the Bt gene in the field. In view of the risk of insect resistance to Bt crops, a series of insect resistance management (IRM) strategies have been advanced, including the high-dose strategy, the shelter strategy, the high-dose/shelter strategy, and the gene aggregation strategy. In view of the particular context of the decreasing area of cultivated land in China, the gene aggregation strategy is the most effective one. The gene aggregation strategy refers to the aggregation of two or more different types of insect-resistant genes in the same crop; for instance, the aggregation of Bt genes and non-Bt insect-resistant genes, or the aggregation of Bt genes without cross-resistance. Because the probability of insect resistance to two or more insecticidal proteins is much lower than that of resistance to a single gene, the gene aggregation strategy can effectively delay the emergence of resistant insect populations, improving the useful life of transgenic insect-resistant crops.
In their study of insect-resistant rice in China, Yang et al. used Minghui 63 insect-resistant materials transformed with four univalent Bt genes (cry1Ab (named 1Ab), cry1Ac (named 1Ac), cry1C* (named 1C) and cry2A* (named 2A)) as parents to carry out reciprocal polymerization with five combinations (1Ab+1C, 1Ab+2A, 1Ac+1C, 1Ac+2A, 1C+2A) to aggregate different types of Bt genes together. Ten bivalent Bt gene insect-resistant combinations were obtained. Then, the homozygous lines of bivalent Bt were screened using the PCR method. The results of the indoor inoculation test showed that the resistance of almost all bivalent strains to the first instar C. suppressalis and S. incertulas was consistent with or significantly increased relative to the univalent Bt strain, while most bivalent Bt strains had higher resistance to the second instar C. suppressalis and S. incertulas than the univalent Bt strain. In the field experiments, both the bivalent Bt homozygous lines and univalent Bt homozygous lines showed good resistance to YSB, SSB, and RLR under both natural and artificial treatments; in particular, the bivalent Bt strain polymerized cry2A* made up for the lack of resistance of cry2A* univalent lines to RLR. Under normal pesticide spraying in the field, the yields of most bivalent Bt lines were not significantly different from that of the control MH63. A few bivalent Bt lines with some differences showed good recovery of yield characters in their hybrid combinations (with Zhenshan 97A) and have good application prospects [12].
To improve the insect resistance of water-saving and drought-resistant rice (WDR), Ye et al. used Huahui No. 1 (TT51) as the cry1Ab/Ac gene donor, crossed it with water-saving and drought-resistant rice recovery line variety Hanhui No. 3, screened the target gene by molecular marker-assisted selection technology, and finally obtained the Hanhui 3T of the new insect-resistant recovery line. Subsequently, the hybrid rice combination Huhan 5A/Hanhui 3T was also found to have high resistance to Cnaphalocrocis medinalis, which laid a material foundation for the cultivation of new rice varieties with water-saving, drought-resistant and insect-resistant properties [13].
3.4. The Tissue-Specific Expression of Bt in Insect-Resistant Rice
In recent years, there have been many breakthroughs and much progress in transgenic rice with the Bt gene, but there are still many deficiencies. One obvious problem is that, in most studies, the promoters used to drive Bt gene expression are constitutive promoters, such as the ActinI promoter [9], the Ubiquitin promoter [10,11], and so on. These constitutive expression promoters lead to the expression of the Bt gene in all rice plant tissues, which increases the metabolic burden of the rice itself. In addition, since rice is the part we eat directly, the presence of the Bt protein in the endosperm raises concerns about its edible safety, which will further affect the commercial production of the rice. Using tissue-specific promoters to drive the expression of the Bt gene and reduce the content of the Bt protein in endosperm is a very effective strategy.
Qiu et al. used a maize-derived PEPC promoter to drive cry1Ab/Ac fusion gene expression to transform the Japonica rice variety Nipponbare. Western Blot analysis of this transgenic rice showed that high expression levels of the Cry1Ab/Ac protein could be detected in the leaves and stems, but the Cry1Ab/Ac protein could not be detected in mature seeds [14]. In the vector constructed by Ye et al., cry1C* gene expression was driven by the rbcS promoter from Nipponbare. The vector was transformed into the Japonica variety Zhonghua 11. After a series of subsequent tests, six single-copy homozygous families (RJ2, RJ3, RJ4, RJ5, RJ6, and RJ7) were selected for subsequent field resistance determination. In the field experiment, under artificial inoculation, the rolled leaves caused by C. medinalis damage in six transgenic families were very weak; the percentage of damaged tillers per plant was 0.005–1.41% with less than 0.1 leaves per tiller. However, Zhonghua 11 seriously suffered, and the damaged tiller ratio and proportion of damaged leaves per tiller were 37.1% and 4.25%, respectively.
In addition, the rate of white ear caused by C. suppressalis and S. incertulas of the six transgenic families was 0.82% and 2.13%, respectively, which was significantly lower than the value of 36.25% for Zhonghua 11 in the control. The results of Cry1C protein determination showed that the content of Cry1C protein in the leaves of the 6 families was 0.87 μg g-1~3.13μg g-1 at the tillering stage and 0.71 μg g-1~0.86 μg g-1 at the filling stage. Meanwhile, the content of Cry1C protein in the endosperm was very low; the lowest content was less than 0.001 μg g-1μg g−1 (RJ6) and less than 1/600 Cry1C* protein content in the leaves during the filling stage. The content of Bt protein in the endosperm is well controlled and has good application prospects [15].
3.5. Expression of Hybrid Fusion Protein in Transgenic Rice
Xu et al. transformed cry1Ab and vip3A toxin fusion genes into rice. The transgenic plants expressing the fusion protein had high resistance to the two main rice pests Chilo suppressalis and Cnaphalocrocis medinalis, while their agronomic traits were not significantly different from those of non-transgenic rice [34].
Boddupally et al. used the DI and DII domains of Bt Cry1Ac and the carbohydrate-binding domain of garlic lectin (ASAL) to construct fusion genes and generate transgenic rice lines to evaluate the efficacy of Cry1Ac: ASAL fusion protein against YSB, RLR and brown planthopper (Nilaparvata lugens, BPH). The results showed that the transgenic rice lines had a significant control effect on lepidopteran pests YSB and RLR, and also had a good effect on the toxic hemipteran pest BPH [35].
4. Research Status of the Safety of Transgenic Rice
With the development of genetic engineering technology, the improvement of crop characteristics has been greatly accelerated; as a result, genetically modified corn, cotton, and soybeans have been commercially planted in many countries around the world. Since Yang et al. bred the first transgenic rice with the Bt gene [36] in 1989, researchers have developed a variety of transgenic rice plants with the Bt gene, including cry1Ab [23,37], cry1Ac [25,38], cry1Ab/Ac [9], cry1C [11,15], and cry2A [10]. In 2009, the Chinese Ministry of Agriculture issued a safety certificate in Hubei Province for the production and application of Huahui No.1, which expresses a Cry1Ab/Ac fusion protein, and its corresponding hybrid combination Bt Shanyou 63, which was approved again in 2014. However, due to the pressure of public opinion in China and some experts’ concerns about the safety of GM rice, it has not been approved for commercial cultivation. In the past decade, there have been many research reports on the safety of GM rice, which can provide a reference for government departments to approve the commercial cultivation of GM rice.
4.1. Edible Safety
As rice is a food crop that people use directly, consumers’ primary concern is its edible safety. An important principle in food safety assessments is the principle of substantive equivalence. A variety of biochemical methods have been used to compare nutrients between transgenic rice and its receptor parents. In the process of the evaluation, the subjects of the experiment were mainly mice. Compared with the control varieties, the rice transformed with Cry1Ab or CpTI+Cry1Ab showed no differences in terms of the main nutrients (such as crude protein, crude fat, free amino acids, and mineral elements) and physicochemical properties (such as the starch content, gelatinization temperature, and starch viscosity) [39,40].
In the 90-day feeding experiment, Cao et al. [41] used 1H-NMR to detect the metabolic molecules in the urine of mice fed with rice containing Cry2A and their corresponding control rice; they further analyzed their metabolic groups. Through multivariate analysis and analysis of variance, it was found that, although there were differences between them, these differences had no biological significance. The team also achieved similar results in rice containing the Cry1C protein [42]. In the 90-day experiment, Yuan et al. studied the feces of mice fed with Cry2A and its control MH63 rice using the real-time PCR method and detected their intestinal colonies (Lactobacillus, Bifidobacterium, Escherichia coli, Enterococcus, and Clostridium perfringens). There was no difference in the number of single bacteria and total bacteria. In addition, there were no differences in the microbial community’s composition, intestinal permeability, epidermal structure, fecal enzymes, bacterial activity, or intestinal immunity [43,44].
The 90-day indoor feeding test of mice showed that the Cry1Ab rice flour did not affect the development of the mice. There were no differences in the animals’ behavior, body weight, organ weight, or blood indexes between the transgenic rice flour and control rice flour. A small number of blood index differences were within the normal reference value [45,46]. Zhu et al. fed Xenopus laevis with food containing 30% Huahui No.1 and MH63 rice. The results of the 90-day experiment showed that there were no significant differences in body weight, body length, animal behavior, organ weight, liver and kidney function, or microstructure of tested tissues between Huahui No.1 containing the Cry1Ab/1Ac protein and the control MH63 [47]. In the 90-day experiment and a long-term experiment with two generations of mice, Wang et al. fed the animals with 60% MH63 and Huahui No. 1 rice. There were no significant differences in body weight, food consumption, reproductive data, or the organ/body weight ratio between the two groups. However, there were differences in some hematological and serum chemical parameters, and in the brain, heart, liver, spleen, stomach, small intestine, and thymus. There were no histological abnormalities in the ovaries, testes, or other organs, indicating that Huahui No.1 did not affect the reproductive systems of the mice [48,49]. In the 78-week experiment conducted by Zhang et al., Sprague–Dawley (SD) mice were fed with rice containing Cry1Ac, sck, and their controls; there were no significant differences in body weight, food consumption, mortality, tumor incidence, or pathological parameters [50].
4.2. Environmental Security
4.2.1. Effects on Non-Target Insects
Rice planthoppers and leafhoppers are both significant rice pests. As common feeding pests of rice, they are important non-target herbivores of transgenic rice with Bt resistance. Bai et al. found that the development of brown planthoppers was not affected by feeding on Bt rice material containing the Cry protein [51]. Years of studies in the same place have shown that the populations of rice planthoppers and leafhoppers are the same in Bt rice fields and non-transgenic rice fields [52,53]. Lu et al. found that there was no significant difference in biological parameters such as the egg period, adult fresh weight, life span, and spawning period between the black-tailed leafhopper fed with T1C-19 (Cry1C) or T2A-1 (Cry2A) rice and the control MH63. Moreover, there were no significant differences in the population density and dynamics of adults and nymphs between the transgenic (T1C-19 and T2A-1) rice field and the control rice field [54]. The effect of the transgenic rice containing cry1Ab on the white-backed planthopper was similar, and the laboratory results showed that there were no significant differences in the egg stage, nymph survival rate, or female fecundity. In addition, the results of the field sampling survey showed that there were no significant differences in nymph and adult density between the transgenic materials and control materials. However, the results of the vacuum extractor showed that the adult density of white-backed planthoppers in the transgenic rice field was slightly lower than that in the control field; this finding needs to be further verified by long-term and large-scale field investigations [55].
Han et al. investigated the effect of T2A-1 on Hylyphantes graminicola, which preys on brown planthoppers. There were no differences in survival rate, development time, body weight, or fecundity after H. graminicola preyed on brown planthoppers on T2A-1 and MH63 plants, and Cry2A protein was not accumulated in the body. The results of the field investigation from 2011 to 2013 showed that there was no significant difference in population density between the T2A-1 and MH63 fields [56]. The team studied the effect of Cry2A on Anagrus nilaparvatae parasitizing brown planthopper eggs for up to seven generations in the laboratory. There were no significant differences in survival rate, development time, female-to-male ratio, longevity, or fecundity between the brown planthopper eggs parasitizing T2A-1 and those on the control MH63. In addition, even if the A. nilaparvatae was directly fed with a high dose of the Cry2Aa protein, its survival rate and fecundity were not significantly affected [57].
Li et al. found that rice expressing the Cry2A protein had no effect on the growth and development of Chrysopa sinica larvae [58]. In addition, in laboratory experiments, adults of C. sinica fed on rice pollen containing Cry2A showed no differences in terms of their survival rate, pre-oviposition, fecundity, or dry weight compared with those fed on non-transgenic control materials [59]. Li et al. found that the larval stage of the Propylaea japonica fed with Cry1C or Cry2A rice pollen was longer than that of the control pollen, but the purified Cry1C or Cry2A was added to the rape pollen and then fed upon by the P. japonica larvae. There was no difference in the larval stage between the tortoise ladybug and the control, indicating that the tortoise ladybug was not sensitive to Cry1C or Cry2A [60].
Sun et al. investigated the effects of Bt rice (T1C-19) on the main stored pest, Rhyzopertha dominica, and its parasitoid Nasonia. An analysis of the electronic nose and electronic tongue showed that the brown rice of T1C-19 was like that of the control MH63, and the GC-MC results showed that the types of volatiles were similar. Moreover, the contents of most species were the same, and there were no differences in the densities of stone cells and epidermal hairs. R. dominica and Nasonia had no selectivity to the brown rice of transgenic rice and control rice. These results show that T1C-19 had no negative effect on the behaviors of transgenic rice and control rice [61].
Ren et al. studied the effects of three transgenic Bt rice lines KMD1, KMD2 and G8-7 on the biological parameters and population dynamics of the non-target insect Rhopalosiphum maidis. There was no significant difference in aphid survival rate between Bt rice and non-Bt rice. The developmental duration of R. maidis fed on KMD1 and KMD2 was not significantly different from that of the parent Xiushui 11. Two years of field investigation showed that Bt rice did not significantly affect the population dynamics of R. maidis compared with non-Bt rice [62].
4.2.2. Effects of Bt Rice on the Root Ecosystem
Another important aspect of the environmental impact of transgenic rice is the effect of Bt protein on soil microorganisms and its residues in the soil. It was found that the straw of the KMD transgenic rice was not toxic to a variety of culturable microorganisms in paddy fields [63]. The results of laboratory studies showed that mixed Bt (Cry1Ab) rice straw and non-transgenic rice straw had no decisive effect on soil microorganisms including bacteria, actinomycetes, and fungi [64]. Wang et al. investigated tadpoles and young frogs in a Huahui No. 1 rice field and a Minghui 63 rice field. No significant difference was found for tadpole density and young frog weight between the two rice fields, and no Cry1Ab/1Ac protein was detected in tissue samples of tadpoles and young frogs, indicating that Cry1Ab/1Ac had no significant effect on frogs in rice fields [65].
Wang et al. mixed rice straw containing Cry1Ab KMD with five different soils, and the semi-residue time (half of the initial content of the experiment) of the Cry1Ab protein in soil was 11.5d~34.3d. Cry1Ab degraded most rapidly in alkaline soil; in contrast, it took the longest to degrade in acidic soil. In addition, after five months, almost all Cry1Ab in the five kinds of soil degraded [66]. Li et al. found that, after the rice harvest, the Cry1Ab content in the rice stalks and roots rapidly degraded to less than 50% within a month. As the temperature decreases, the degradation rate slows down. After the temperature rises in the spring, the degradation continues. In addition, the Cry1Ac protein degraded rapidly in soil, but then entered a long stable period; meanwhile, Cry1Ac degraded slowly and continuously in sterilized water and was completely degraded at 115 days, reflecting the need for timely ploughing and irrigation after the rice harvest to promote the degradation process of Cry1Ac protein [67].
Wang et al. planted transgenic Bt rice lines cry1Ab/1Ac Minghui 63 (Huahui 1) and cry2A Minghui 63 in the same field for 9 years. Cry proteins in rhizosphere soil were determined at the tillering stage and 60 days after harvest. The Cry protein content of seedlings, flowering and mature stages was measured in the first year (2012) and the last year (2020) of the experiment. Cry protein could be detected in the tillering stage of Bt rice, but it had degraded 60 days after harvest, and the concentration of Cry protein in soil would not accumulate in multiple planting years [68].
5. Prospects
Since the commercial cultivation of genetically modified crops began in 1996, the planting area of genetically modified crops has increased year on year. More and more countries have planted modified crops, mainly soybeans, corn, cotton, rape, and so forth. The large-scale planting of genetically modified crops has not only reduced the use of chemical pesticides, but has also generated huge economic benefits. The growing global population has amplified food shortages around the world and ushered in the development of genetically modified (GM) crops to overcome these challenges [69]. At present, commercially grown crops are mainly eaten indirectly or used as raw processing materials, so they are relatively easily accepted by ordinary consumers. However, regarding genetically modified rice for direct consumption, people’s concerns about safety—and especially safety for consumption—have always been difficult to dispel. The current research results show that transgenic rice is not associated with food safety problems; in particular, the idea that it affects fertility has been thoroughly disproven.
In terms of environmental safety, no obvious adverse effects have been found so far. In general, GM rice that is approved by the state is as safe as traditional rice. As far as the specific situation of China is concerned, the pressure of public opinion and ordinary consumers’ understanding of GM rice have not reached suitable levels, so the acceptance of GM rice is still relatively low; as such, the government and researchers need to strengthen science popularization, so that consumers know more about GM. The general public also needs to actively try to understand it, rather than following the opinions of others. It will take a long time for transgenic rice to move from the research and development stage to commercial cultivation.
Author Contributions
Conceptualization, A.Y. and C.L.; figures and manuscript-original draft preparation, C.L., J.W. and F.L.; manuscript-review and editing, A.Y. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 31701399), the Science and Technology Major Program of Hubei Province (2022ABA001, 2021ABA011), and the Wuhan Science and Technology Major Project for Biological Breeding (2022021302024850).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- de Sousa, I.G.; Oliveira, J.; Mexia, A.; Barros, G.; Almeida, C.; Brazinha, C.; Vega, A.; Brites, C. Advances in Environmentally Friendly Techniques and Circular Economy Approaches for Insect Infestation Management in Stored Rice Grains. Foods 2023, 12, 511. [Google Scholar] [CrossRef]
- Chen, H.; Lin, Y.; Zhang, Q. Review and prospect of transgenic rice research. Chin. Sci. Bull. 2009, 54, 4049–4068. [Google Scholar]
- Barton, K.A.; Binns, A.N.; Matzke, A.J.; Chilton, M.D. Regeneration of intact tobacco plants containing full length copies of genetically engineered T-DNA, and transmission of T-DNA to R1 progeny. Cell 1983, 32, 1033–1043. [Google Scholar] [CrossRef]
- Abdul, A.M.; Brini, F.; Rouached, H.; Masmoudi, K. Genetically engineered crops for sustainably enhanced food production systems. Front. Plant Sci. 2022, 13, 1027828. [Google Scholar]
- Höfte, H.; Whiteley, H. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 1989, 53, 242–255. [Google Scholar] [CrossRef]
- Crickmore, N.; Zeigler, D.; Feitelson, J.; Schnepf, E.; Van, R.J.; Lereclus, D.; Baum, J.; Dean, D. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 807–813. [Google Scholar] [CrossRef]
- Schnepf, H.E.; Whiteley, H. Cloning and expression of the Bacillus thuringiensis crystal protein gene in Escherichia coli. Proc. Natl. Acad. Sci. USA 1981, 78, 2893–2897. [Google Scholar] [CrossRef]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.; Bonning, B. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2021, 186, 107438. [Google Scholar] [CrossRef]
- Tu, J.; Zhang, G.; Datta, K.; Xu, C.; He, Y.; Zhang, Q.; Khush, G.S.; Datta, S.K. Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis δ-endotoxin. Nat. Biotechnol. 2000, 18, 1101–1104. [Google Scholar] [CrossRef]
- Chen, H.; Tang, W.; Xu, C.; Li, X.; Lin, Y.; Zhang, Q. Transgenic indica rice plants harboring a synthetic cry2A* gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theor. Appl. Genet. 2005, 111, 1330–1337. [Google Scholar] [CrossRef]
- Tang, W.; Chen, H.; Xu, C.; Li, X.; Lin, Y.; Zhang, Q. Development of insect-resistant transgenic indica rice with a synthetic cry1C* gene. Mol. Breed. 2006, 18, 1–10. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, H.; Tang, W.; Hua, H.; Lin, Y. Development and characterisation of transgenic rice expressing two Bacillus thuringiensis genes. Pest Manag. Sci. 2011, 67, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Lou, J.; Gao, N.; Yang, X.; Deng, J. Production and Insect-Resistant Characterization of Hanhui 3T a Derivative Line of Huahui No.1. J. Nucl. Agric. Sci. 2022, 36, 1–6. [Google Scholar]
- Qiu, C.; Sangha, J.S.; Song, F.; Zhou, Z.; Yin, A.; Gu, K.; Tian, D.; Yang, J.; Yin, Z. Production of marker-free transgenic rice expressing tissue-specific Bt gene. Plant Cell Rep. 2010, 29, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Huang, H.; Yang, Z.; Chen, T.; Liu, L.; Li, X.; Chen, H.; Lin, Y. Development of insect-resistant transgenic rice with Cry1C*-free endosperm. Pest Manag. Sci. 2009, 65, 1015–1020. [Google Scholar] [CrossRef]
- Qi, Y.; Ye, S.; Lu, Y.; Jin, Q.; Zhang, X. Development of Marker-Free Transgenic Cry1Ab Rice with Lepidopteran Pest Resistance by Agrobacterium Mixture-Mediated Co-transformation. Rice Sci. 2009, 16, 181–186. [Google Scholar] [CrossRef]
- Fujimoto, H.; Itoh, K.; Yamamoto, M.; Kyozuka, J.; Shimamoto, K. Insect resistant rice generated by introduction of a modified δ-endotoxin gene of Bacillus thuringiensis. Nat. Biotechnol. 1993, 11, 1151–1155. [Google Scholar] [CrossRef]
- Wünn, J.; Klöti, A.; Burkhardt, P.K.; Biswas, G.C.G.; Launis, K.; Iglesias, V.A.; Potrykus, I. Transgenic indica rice breeding line IR58 expressing a synthetic crylA (b) gene from Bacillus thuringiensis provides effective insect pest control. Nat. Biotechnol. 1996, 14, 171–176. [Google Scholar] [CrossRef]
- Ghareyazie, B.; Alinia, F.; Menguito, C.A.; Rubia, L.G.; de Palma, J.M.; Liwanag, E.A.; Cohen, M.B.; Khush, G.S.; Bennett, J. Enhanced resistance to two stem borers in an aromatic rice containing a synthetic cryIA (b) gene. Mol. Breed. 1997, 3, 401–414. [Google Scholar] [CrossRef]
- Nayak, P.; Basu, D.; Das, S.; Basu, A.; Ghosh, D.; Ramakrishnan, N.A.; Ghosh, M.; Sen, S.K. Transgenic elite indica rice plants expressing CryIAc ∂-endotoxin of Bacillus thuringiensis are resistant against yellow stem borer (Scirpophaga incertulas). Proc. Natl. Acad. Sci. USA 1997, 94, 2111–2116. [Google Scholar] [CrossRef]
- Wu, C.; Fan, Y.; Zhang, C.; Oliva, N.; Datta, S. Transgenic fertile japonica rice plants expressing a modified cryIA (b) gene resistant to yellow stem borer. Plant Cell Rep. 1997, 17, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Sardana, R.; Kaplan, H.; Altosaar, I. Agrobacterium-transformed rice plants expressing synthetic cryIA (b) and cryIA (c) genes are highly toxic to striped stem borer and yellow stem borer. Proc. Natl. Acad. Sci. USA 1998, 95, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.Y.; Shu, Q.Y.; Yao, H.W.; Cui, H.R.; Cheng, X.Y.; Hu, C.; Xia, Y.W.; Gao, M.W.; Altosaar, I. Field evaluation of resistance of transgenic rice containing a synthetic cry1Ab gene from Bacillus thuringiensis Berliner to two stem borers. J. Econ. Entomol. 2001, 94, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Maqbool, S.B.; Riazuddin, S.; Sticklen, M.B. Expression of synthetic cry1Ab and cry1Ac genes in basmati rice (Oryza sativa L.) variety 370 via Agrobacterium-mediated transformation for the control of the European corn borer (Ostrinia nubilalis). Vitr. Cell. Dev. Biol. Plant 2002, 38, 213–220. [Google Scholar] [CrossRef]
- Khanna, H.K.; Raina, S.K. Elite Indica transgenic rice plants expressing modified Cry1Ac endotoxin of Bacillus thuringiensis show enhanced resistance to yellow stem borer (Scirpophaga incertulas). Transgenic. Res. 2002, 11, 411–423. [Google Scholar] [CrossRef]
- Ramesh, S.; Nagadhara, D.; Pasalu, I.; Kumari, A.P.; Sarma, N.; Reddy, V.; Rao, K. Development of stem borer resistant transgenic parental lines involved in the production of hybrid rice. J. Biotechnol. 2004, 111, 131–141. [Google Scholar] [CrossRef]
- Ballester, V.; Granero, F.; Tabashnik, B.E.; Malvar, T.; Ferré, J. Integrative model for binding of Bacillus thuringiensis toxins in susceptible and resistant larvae of the diamondback moth (Plutella xylostella). Appl. Environ. Microb. 1999, 65, 1413–1419. [Google Scholar] [CrossRef]
- Li, H.; González-Cabrera, J.; Oppert, B.; Ferré, J.; Higgins, R.A.; Buschman, L.L.; Radke, G.A.; Zhu, K.Y.; Huang, F. Binding analyses of Cry1Ab and Cry1Ac with membrane vesicles from Bacillus thuringiensis resistant and susceptible Ostrinia nubilalis. Biochem. Biophys. Res. Commun. 2004, 323, 52–57. [Google Scholar]
- Zhao, J.Z.; Li, Y.X.; Collins, H.L.; Cao, J.; Earle, E.D.; Shelton, A.M. Different cross-resistance patterns in the diamondback moth (Lepidoptera: Plutellidae) resistant to Bacillus thuringiensis toxin Cry1C. J. Econ. Entomol. 2001, 94, 1547–1552. [Google Scholar] [CrossRef]
- Luo, S.; Wu, K.; Tian, Y.; Liang, G.; Feng, X.; Zhang, J.; Guo, Y. Cross-resistance studies of Cry1Ac-resistant strains of Helicoverpa armigera (Lepidoptera: Noctuidae) to Cry2Ab. J. Econ. Entomol. 2007, 100, 909–915. [Google Scholar] [CrossRef]
- Caccia, S.; Hernández-Rodríguez, C.S.; Mahon, R.J.; Downes, S.; James, W.; Bautsoens, N.; Van, R.J.; Ferre, J. Binding site alteration is responsible for field-isolated resistance to Bacillus thuringiensis Cry2A insecticidal proteins in two Helicoverpa species. PLoS ONE 2010, 5, e9975. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carrière, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 2008, 26, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, J. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S. Afr. J. Plant Soil 2007, 24, 147–151. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, J.; Lin, H.; Gao, J.; Shen, Z. Characterization of transgenic rice expressing fusion protein Cry1Ab/Vip3A for insect resistance. Sci. Rep. 2018, 8, 15788. [Google Scholar] [CrossRef] [PubMed]
- Boddupally, D.; Tamirisa, S.; Gundra, S.; Vudem, D.; Khareedu, V. Expression of hybrid fusion protein (Cry1Ac::ASAL) in transgenic rice plants imparts resistance against multiple insect pests. Sci. Rep. 2018, 8, 8458. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Guo, S.; Chen, X.; Fan, Y. Transgenic rice plants produced by direct uptake of δ-endotoxin protein gene from Bacillus thuringiensis into rice protoplasts. Sci. Agric. Sin. 1989, 22, 605–611. [Google Scholar]
- Wu, G.; Cui, H.; Ye, G.; Xia, Y.; Sardana, R.; Cheng, X.; Li, Y.; Altosaar, I.; Shu, Q. Inheritance and expression of the cry1Ab gene in Bt (Bacillus thuringiensis) transgenic rice. Theor. Appl. Genet. 2002, 104, 727–734. [Google Scholar] [CrossRef]
- Han, L.; Wu, K.; Peng, Y.; Wang, F.; Guo, Y. Efficacy of transgenic rice expressing Cry1Ac and CpTI against the rice leaffolder, Cnaphalocrocis medinalis (Guenee). J. Invertebr. Pathol. 2007, 96, 71–79. [Google Scholar] [CrossRef]
- Wu, D.; Shu, Q.; Ye, Q.; Lei, Z.; Ma, C.; Xia, Y. Comparative studies on major nutritional components and physicochemical properties of the transgenic rice with a synthetic cry1Ab gene from Bacillus thuringiensis. J. Food Biochem. 2003, 27, 295–308. [Google Scholar] [CrossRef]
- Li, X.; Huang, K.; He, X.; Zhu, B.; Liang, Z.; Li, H.; Luo, Y. Comparison of nutritional quality between Chinese indica rice with sck and cry1Ac genes and its nontransgenic counterpart. J. Food Sci. 2007, 72, S420–S424. [Google Scholar] [CrossRef]
- Cao, S.; Xu, W.; Luo, Y.; He, X.; Yuan, Y.; Ran, W.; Liang, L.; Huang, K. Metabonomics study of transgenic Bacillus thuringiensis rice (T2A-1) meal in a 90-day dietary toxicity study in rats. Mol. Biosyst. 2011, 7, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; He, X.; Xu, W.; Luo, Y.; Yuan, Y.; Liu, P.; Cao, B.; Shi, H.; Huang, K. Safety assessment of transgenic Bacillus thuringiensis rice T1c-19 in Sprague–Dawley rats from metabonomics and bacterial profile perspectives. IUBMB Life 2012, 64, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, W.; Luo, Y.; Liu, H.; Lu, J.; Su, C.; Huang, K. Effects of genetically modified T2A-1 rice on faecal microflora of rats during 90 day supplementation. J. Sci. Food Agric. 2011, 91, 2066–2072. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, W.; He, X.; Liu, H.; Cao, S.; Qi, X.; Huang, K.; Luo, Y. Effects of genetically modified T2A-1 rice on the GI health of rats after 90-day supplement. Sci. Rep. 2013, 3, 1962. [Google Scholar]
- Wang, Z.H.; Wang, Y.; Cui, H.r.; Xia, Y.W.; Altosaar, I.; Shu, Q.Y. Toxicological evaluation of transgenic rice flour with a synthetic cry1Ab gene from Bacillus thuringiensis. J. Sci. Food Agric. 2002, 82, 738–744. [Google Scholar] [CrossRef]
- Schrøder, M.; Poulsen, M.; Wilcks, A.; Kroghsbo, S.; Miller, A.; Frenzel, T.; Danier, J.; Rychlik, M.; Emami, K.; Gatehouse, A. A 90-day safety study of genetically modified rice expressing Cry1Ab protein (Bacillus thuringiensis toxin) in Wistar rats. Food Chem. Toxicol. 2007, 45, 339–349. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Li, Y.; Wang, J.; Ding, J.; Chen, X.; Peng, Y. A 90 Day Safety Assessment of Genetically Modified Rice Expressing Cry1Ab/1Ac Protein using an Aquatic Animal Model. J. Agric. Food Chem. 2015, 63, 3627–3633. [Google Scholar] [CrossRef]
- Wang, E.H.; Yu, Z.; Hu, J.; Xu, H.B. Effects of 90-day feeding of transgenic Bt rice TT51 on the reproductive system in male rats. Food Chem. Toxicol. 2013, 62, 390–396. [Google Scholar] [CrossRef]
- Wang, E.H.; Yu, Z.; Hu, J.; Jia, X.D.; Xu, H.B. A two-generation reproduction study with transgenic Bt rice TT51 in Wistar rats. Food Chem. Toxicol. 2014, 65, 312–320. [Google Scholar]
- Zhang, M.; Zhuo, Q.; Tian, Y.; Piao, J.; Yang, X. Long-term toxicity study on transgenic rice with Cry1Ac and sck genes. Food Chem. Toxicol. 2014, 63, 76–83. [Google Scholar] [CrossRef]
- Bai, Y.; Jiang, M.; Cheng, J.; Wang, D. Effects of Cry1Ab toxin on Propylea japonica (Thunberg) (Coleoptera: Coccinellidae) through its prey, Nilaparvata lugens Stål (Homoptera: Delphacidae), feeding on transgenic Bt rice. Environ. Entomol. 2006, 35, 1130–1136. [Google Scholar] [CrossRef]
- Chen, M.; Ye, G.; Liu, Z.; Yao, H.; Chen, X.; Shen, Z.; Hu, C.; Datta, S. Field assessment of the effects of transgenic rice expressing a fused gene of cry1Ab and cry1Ac from Bacillus thuringiensis Berliner on nontarget planthopper and leafhopper populations. Environ. Entomol. 2006, 35, 127–134. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Z.; Ye, G.; Shen, Z.; Hu, C.; Peng, Y.; Altosaar, I.; Shelton, A.M. Impacts of transgenic cry1Ab rice on non-target planthoppers and their main predator Cyrtorhinus lividipennis (Hemiptera: Miridae)—A case study of the compatibility of Bt rice with biological control. Biol. Control 2007, 42, 242–250. [Google Scholar] [CrossRef]
- Lu, Z.; Tian, J.; Wang, W.; Xu, H.; Hu, C.; Guo, Y.; Peng, Y.; Ye, G. Impacts of Bt rice expressing Cry1C or Cry2A protein on the performance of nontarget leafhopper, Nephotettix cincticeps (Hemiptera: Cicadellidae), under laboratory and field conditions. Environ. Entomol. 2014, 43, 209–217. [Google Scholar] [CrossRef]
- Lu, Z.; Han, N.; Tian, J.; Peng, Y.; Cui, H.; Guo, Y.; Shen, Z.; Ye, G. Transgenic cry1Ab/vip3H+epsps rice with insect and herbicide resistance acted no adverse impacts on the population growth of a non-target herbivore, the white-backed planthopper, under laboratory and field conditions. J. Integr. Agric. 2014, 13, 2678–2689. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Wang, H.; Zhao, J.; He, Y.; Hua, H. Prey-mediated effects of transgenic cry2Aa rice on the spider Hylyphantes graminicola, a generalist predator of Nilapavarta lugens. Biol. Control 2015, 60, 251–261. [Google Scholar] [CrossRef]
- Han, Y.; Wang, H.; Chen, J.; Cai, W.; Hua, H. No impact of transgenic cry2Aa rice on Anagrus nilaparvatae, an egg parasitoid of Nilaparvata lugens, in laboratory tests. Biol. Control 2015, 82, 46–51. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Romeis, J.; Liu, Q.; Lin, K.; Chen, X.; Peng, Y. Bt rice expressing Cry2Aa does not cause direct detrimental effects on larvae of Chrysoperla sinica. Ecotoxicology 2013, 22, 1413–1421. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Romeis, J.; Chen, X.; Zhang, J.; Chen, H.; Peng, Y. Consumption of Bt rice pollen expressing Cry2Aa does not cause adverse effects on adult Chrysoperla sinica Tjeder (Neuroptera: Chrysopidae). Biol. Control 2012, 61, 246–251. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Chen, X.; Romeis, J.; Yin, X.; Peng, Y. Consumption of Bt rice pollen containing Cry1C or Cry2A does not pose a risk to Propylea japonica (Thunberg) (Coleoptera: Coccinellidae). Sci. Rep. 2015, 5, 7679. [Google Scholar] [CrossRef]
- Sun, X.; Yan, M.; Zhang, A.; Wang, M. Transgenic cry1C gene rough rice line T1C-19 does not change the host preferences of the non-target stored product pest, Rhyzopertha dominica (Fabricius) (Coleoptera: Bostrichidae), and its parasitoid wasp, Anisopteromalus calandrae (Howard) (Hymenoptera: Pteromalidae). Ecotox Environ. Saf. 2015, 120, 449–456. [Google Scholar]
- Ren, S.; Yang, F.; Gao, M.; Pu, D.; Shi, M.; Ye, G.; Shen, Z.; Chen, X. Effects of Transgenic Bt Rice on Nontarget Rhopalosiphum maidis (Homoptera: Aphididae). Environ. Entomol. 2016, 45, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ye, Q.; Min, H.; Duan, X.; Jin, W. Bt-transgenic rice straw affects the culturable microbiota and dehydrogenase and phosphatase activities in a flooded paddy soil. Soil Biol. Biochem. 2004, 36, 289–295. [Google Scholar]
- Wu, W.; Ye, Q.; Min, H.; Chen, H. Effect of cry1Ab toxin released from straw of Bt-transgenic rice on microflora and enzymatic activities in upland soil. Acta Pedol. Sin. 2003, 40, 606–612. [Google Scholar]
- Wang, J.; Chen, X.; Liang, Y.; Zhu, H.; Ding, J.; Peng, Y. Influence of transgenic rice expressing a fused Cry1Ab/1Ac protein on frogs in paddy fields. Ecotoxicology 2014, 23, 1619–1628. [Google Scholar] [CrossRef]
- Wang, H.; Ye, Q.; Wang, W.; Wu, L.; Wu, W. Cry1Ab protein from Bt transgenic rice does not residue in rhizosphere soil. Environ. Pollut. 2006, 143, 449–455. [Google Scholar] [CrossRef]
- Li, Y.; Wu, K.; Zhang, Y.; Yuan, G. Degradation of Cry1Ac protein within transgenic Bacillus thuringiensis rice tissues under field and laboratory conditions. Environ. Entomol. 2007, 36, 1275–1282. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Zhang, J.; Jia, R. [Accumulation of Cry proteins in soil released from Bt rice after planting for multiple years]. Ying Yong Sheng Tai Xue Bao 2022, 33, 119–125. [Google Scholar]
- Un Jan Contreras, S.; Gardner, C.M. Environmental fate and behaviour of antibiotic resistance genes and small interference RNAs released from genetically modified crops. J. Appl. Microbiol. 2022, 133, 2877–2892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).