Abstract
The extensive use of pharmaceuticals and personal care products (PPCPs) causes high concentrations of pharmaceutical metabolites to exist in aquatic environments. Though the removal of parent PPCPs has raised concerns, the degradation of pharmaceutical metabolites was rarely investigated. In this study, the degradation of 4-acetylaminoantipyrine (4-AAA), a typical dipyrone metabolite frequently detected worldwide in surface water and wastewater, was initially studied using persulfate (PDS)-based advanced oxidation processes (AOPs). Compared with commonly used activation methods of alkali, ultrasonic, ultraviolet, and Fe2+, 4-AAA achieved its best degradation (98.9%) within 30 min in a thermally activated PDS system due to the promotion of both radical production and the reaction rate with the rise in temperature. The optimum degradation of 4-AAA could be achieved with the temperature of 80 °C regardless of initial pH values, indicating a wide suitable pH range. Moreover, over 80% of the degradation of 4-AAA could be achieved with the presence of Cl− (0–16 mM), HCO3− (0–8 mM), and humic acid (0–30 mg/L), further indicating the application potential of the system. Both sulfate radicals (SO4•−) and hydroxyl radicals (•OH) contributed to 4-AAA degradation and the contribution of •OH increased with the pH rising from 3 to 11 due to the transformation from SO4•− when reacting with OH−. Three hydroxylated and ring-opening intermediates were detected during the 4-AAA degradation. The ECOSAR prediction indicated that the acute toxicity of most intermediates decreased than 4-AAA while the chronic toxicity increased, which suggested the transformation of intermediates should be further focused on in SO4•− and •OH based AOPs. This study would provide technical reference for the control of 4-AAA in wastewater treatment processes, raise concerns on the influence of PPCPs metabolites, and throw light on reducing the harm of PPCPs and their metabolites in aquatic environments.
1. Introduction
In recent years, pharmaceuticals and personal care products (PPCPs) have raised increasing concerns due to their frequent detection in aquatic environments and potential threats to human health [1]. Though a large number of studies have been conducted with the aim to remove parent PPCPs during water treatment processes, various hydrolyzed or metabolized pharmaceuticals are also extracted from the human body via feces and urine and eventually discharged into the wastewater treatment system. Compared with parent PPCPs, metabolites are usually water-stabilized. Previous studies indicated that some metabolites (e.g., the primary metabolites of carbamazepine) have larger toxicities and higher concentrations than the parent PPCPs due to the transformation of parent PPCPs in biotic and abiotic wastewater treatment processes [2]. Thus, metabolites of PPCPs might also show a risk to both the environment and human health, whose removal and transformation during water treatment processes are necessarily focused on. However, compared with parent PPCPs, researches on the efficient degradation of PPCP metabolites are still limited.
Among these metabolites, 4-acetylaminoantipyrine (4-AAA) is the typical PPCP metabolite whose parent PPCP is dipyrone, a commonly used analgesic and antipyretic drug. After oral intake, dipyrone is hydrolyzed to 4-methylaminoantipyrine (4-MAA) in the human body, then 4-MAA is further metabolized to 4-aminoantipyrine (4-AA), which is finally acetylated to 4-AAA in the liver [3]. Thus, though dipyrone is highly consumed in clinical use, 4-AAA is more frequently detected in worldwide aquatic environments under high concentration ranges of µg/L level [4,5,6,7,8]. Conventional biological wastewater treatment processes show limited effects on the removal of 4-AAA due to its strong antimicrobial activity [9,10]. Normally, around 50% of 4-AAA could be removed during wastewater treatment processes [5,7]. Recent research reported that only less than 11% of 4-AAA was degraded in a municipal wastewater treatment plant with detected concentrations higher than 390 ng/L in both influents and effluents [11]. Under these conditions of high concentrations and frequent detections, it is urgent to develop effective treatment methods besides conventional biological treatments for 4-AAA removal to avoid its potential harm to the environment and human bodies.
However, studies have rarely focused on the degradation of 4-AAA in water, except for their gradual removal through photolysis and with the addition of Cl2 and O3 once reported [3,12,13]. Considering the limited degradation effects of 4-AAA in conventional wastewater treatment processes, advanced oxidation processes (AOPs) with the production of strong oxidizing free radicals would be the promising way. In the past two decades, SO4•−-based AOPs have been developed and shown good effects on the removal of various refractory organic contaminants due to the selective reactivity and relative longer half-life of SO4•− [14,15]. With the usage of persulfate (PDS) as the oxidant, various methods including heat, UV radiation, alkaline treatment, ultrasonic, electricity, and transition metal species could be adapted for PDS activation to produce SO4•− [16,17,18,19,20,21]. Among these methods, thermally activated PDS shows its unique advantages. Researchers have found that high temperatures (usually >50 °C) could promote the homolysis of the O−O bonds in PDS to produce SO4•− and increase the reaction rate for pollutants oxidation at the same time [18,22,23,24]. Especially, many industrial processes would produce large amounts of waste heat with temperature ranges from 60 to 120 °C, which has been an attractive source of heat for PDS activation in recent studies [25]. Based on above analysis, thermally activated PDS has potential application in 4-AAA removal. With the potential harm of 4-AAA and research on its removal being scarce, the degradation of 4-AAA using thermally activated PDS deserves further investigation.
In this article, the degradation of 4-AAA was initially studied in thermally activated PDS systems. To provide a systematic evaluation on the degradation efficiency, response surface methodology (RSM) were adapted to analyze the interactive and synergistic effects of various activation conditions on 4-AAA removal [26]. The aims of this research are (1) to investigate the degradation efficiency of 4-AAA and obtain the optimal reaction conditions in thermally activated PDS system, (2) to explore the major reactive species and possible degradation pathways of 4-AAA, and (3) to investigate the effects of water constitutes on 4-AAA degradation for the evaluation of practical treatment potential. This study would provide technical support and a theoretical basis for the effective control of 4-AAA in aquatic environments.
2. Material and Methods
2.1. Chemicals
4-acetylaminoantipyrine (4-AAA) and ferrous sulfate (FeSO4) were purchased from J&K scientific Ltd. (Shanghai, China). Potassium persulfate (K2S2O8, PDS) was provided by Beijing Innochem Science & Technology Co., Ltd. (Beijing, China). Sodium hydroxide, sodium bicarbonate, sodium chloride and sulfuric acid were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Methanol (MeOH) and tert-butyl alcohol (TBA) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China), and humic acid (HA) was supplied by Shanghai yuanye Bio-Technology Co., Ltd. (Shanghai, China). Chemicals were at least of analytical grade and used as received.
2.2. Experimental Procedures
Batch-scale experiments were conducted in 250 mL Erlenmeyer flasks with a total volume of 100 mL reaction solution. 4-AAA was first added to the reaction system and quickly mixed to keep the concentration of 40.77 µM. For scavenging experiments and water- matrix influence experiments, a desired concentration of alcohol (MeOH or TBA), anions (provided by NaCl or NaHCO3), or HA was also added with 4-AAA. The mixed solution was heated to 80 °C with a water bath and then the stock solution of the PDS was added to initiate the reaction (detailed schematic for experimental reactors in Figure S1). Initial values of pH were adjusted to 7.0 by H2SO4 or NaOH just before the reaction started. The samples were withdrawn at predetermined time intervals and quenched by excess MeOH before analysis.
2.3. Analytical Methods
The concentration of 4-AAA was measured using high-performance liquid chromatography (HPLC, Thermo Fisher 3000) with a reversed-phase C18 column (4.6 mm × 150 mm × 5 µm) at 270 nm. The mobile phase was composed of water (1‰ formic acid) and methanol (1‰ formic acid) mixed at a ratio of 40:60 (v:v) with a flow rate of 0.4 mL/min.
Oxidation intermediates of 4-AAA were analyzed using ultra-performance liquid chromatography-ion trap-mass spectrometry (UPLC-Orbitrap-MS, Thermo Scientific) equipped with a Waters Acquity UPLC BEH C8 column (2.1 × 100 mm, 1.7 μm particle size) under a positive ion mode. A gradient mobile phase program was used for separation. Detailed separation conditions for UPLC and operating parameters for MS were provided in Text S1.
The changes of total organic carbon (TOC) during 4-AAA degradation were detected using the TOC analyzer (TOC-Control L/V, Shimadzu).
2.4. Response Surface Methodology Analysis
Traditional laboratory experiments mainly focused on the effect of certain single reaction parameters on degradation efficiency. To investigate the interacting effects of reaction parameters (PDS concentrations, values of initial pH, and temperature) on the removal rate of 4-AAA in thermally activated PDS system, response surface methodology (RSM) was used due to its advantages of reducing experimental errors [21,27,28]. To build the quadratic regression model using multiple independent variables and determine the optimal levels as well as interactions of such variables, a three factor, five-level experiments with 25 runs were designed and the five coded levels of each variable were designated as −2, −1, 0, +1, and +2 (Table 1). Design Expert 8.0.6 software was used to design, calculate, and analyze the experimental data in RSM.

Table 1.
Level and encoding of experimental factors using RSM.
3. Results and Discussion
3.1. Degradation of 4-AAA Using Thermally Activated PDS with Changes of Single Reaction Conditions
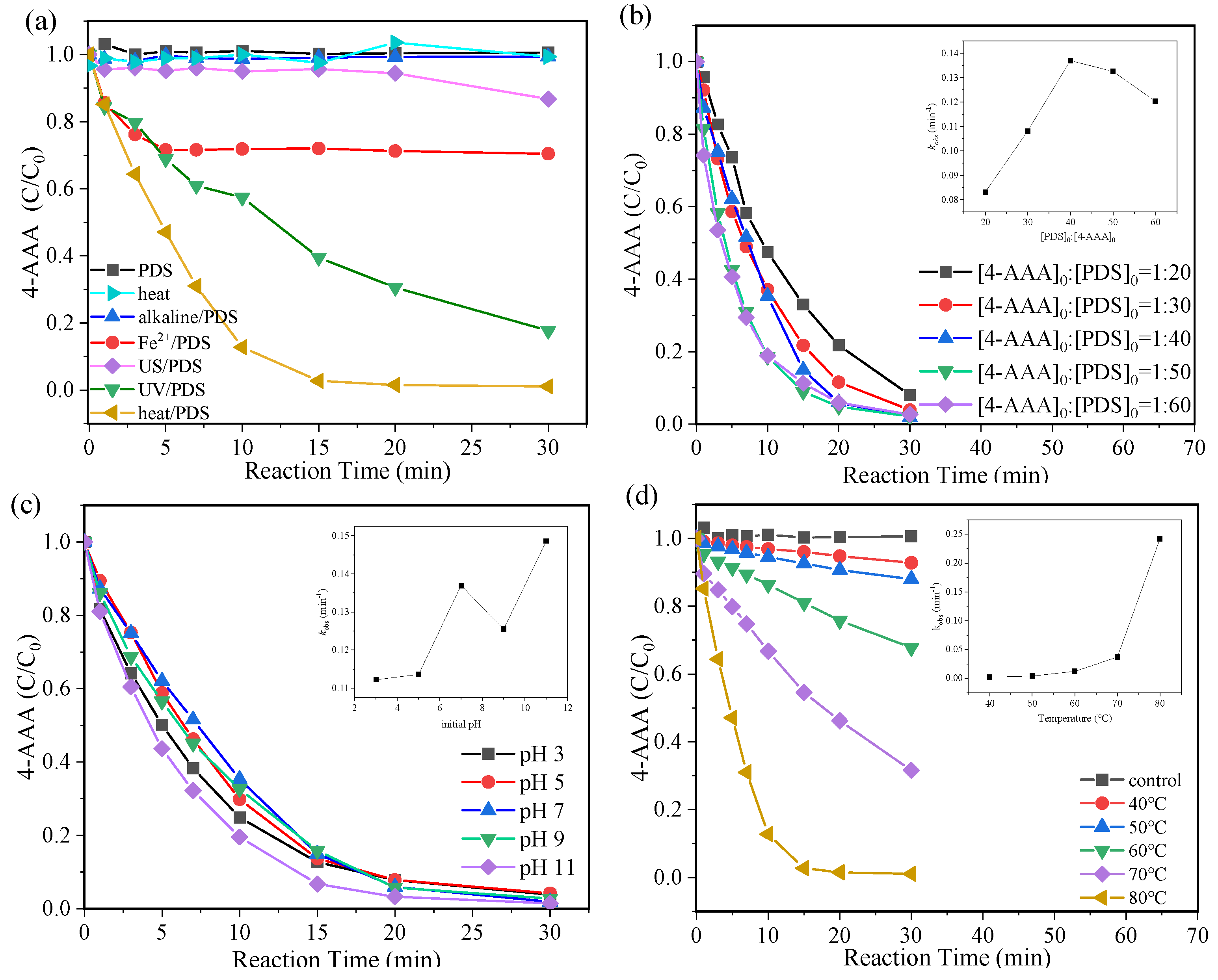
To achieve the efficient degradation of 4-AAA, five activation methods (i.e., heat, alkaline, Fe2+, ultrasonic, and UV) were tested under the commonly reported conditions [20,29,30,31]. As shown in Figure 1a, 4-AAA was hardly degraded in alkaline/PDS (0.63%) and ultrasonic (US)/PDS (13.26%) systems, while it achieved the fastest degradation in thermally activated PDS systems with the removal process almost completed in 15 min. For the whole reaction time of 30 min, the ultimate 4-AAA degradation efficiency using thermally activated PDS was 98.9%, which was significantly higher than that using UV/PDS (82.27%) and Fe2+/PDS (29.59%). Considering the slight degradation of 4-AAA with single PDS and heat, it could be deduced that the combination of heat and PDS resulted in PDS activation for efficient 4-AAA degradation, which showed advantages differing from the commonly used PDS activation methods.
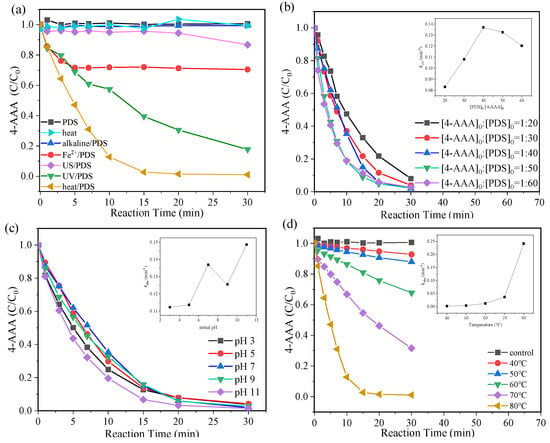
Figure 1.
Degradation of 4-AAA with various activation methods (a) and different PDS concentrations (b), initial pH (c) and temperature (d) in thermally activated PDS systems. Experimental conditions: [4-AAA]0 = 40.77 µM, [PDS]0 = 1.63 mM, pH0 = 7. For various activation methods in Figure 1a: pH0 = 11 for alkaline/PDS, T = 80 °C for heat and heat/PDS, [FeSO4]0 = 1.63 mM for Fe2+/PDS, ultrasonic frequency was 40 kHz for US/PDS, and λ = 253.7 nm for UV/PDS.
The effects of reaction conditions in thermally activated PDS systems were further investigated to obtain the optimal reaction parameters for 4-AAA removal. After the reaction of 30 min, the degradation of 4-AAA was partly improved from 91.96% to 98.48% with the molar ratio of [4-AAA]0:[PDS]0 increasing from 1:20 to 1:40 (Figure 1b), while further increasing the molar ratio to 1:60 hardly improved 4-AAA degradation (Figure 1b). The pseudo first-order kinetic fitting results also indicated that the value of pseudo first-order rate constants (kobs) was almost doubled when [4-AAA]0:[PDS]0 increased from 1:20 to 1:40 and then it dropped with further increases in PDS concentrations due to the competitive consumption of reactive species by excess PDS (Figure 1b). Differently, more than 95% of the 4-AAA could be degraded in 30 min regardless of the initial value of pH from 3 to 11 with the value of kobs fluctuating in the range of 0.11–0.15 min−1, indicating a broad range of suitable initial pH in thermally activated PDS system (Figure 1c). Particularly, changes in temperature showed significant effects on 4-AAA degradation (Figure 1d). The removal of 4-AAA improved from 7.2% to 68.4% in 30 min when the temperature rose from 40 °C to 70 °C. Moreover, when the temperature further increased to 80 °C, 4-AAA could be almost completely degraded in 15 min with the value of kobs 104 times higher than that of 40 °C. Through the PDS activation process, the rise in temperatures would both promote SO4•− production (Equation (1) [32]) and improve its reaction rate with target pollutants, eventually resulting in a large improvement of 4-AAA degradation efficiency. Since the degradation of 4-AAA followed the pseudo first-order kinetics in thermally activated PDS systems (Figure S2), the apparent activation energy (Ea) of 4-AAA could be calculated to be 104.88 kJ/mol using the Arrhenius equation based on a series of temperatures and related kobs (Figure S3). Compared with previous research, the Ea of 4-AAA was lower than that of other typical PPCPs such as bisoprolol (119.8 kJ/mol), sulfamethazine (126 kJ/mol), and iohexol (122.2 kJ/mol) [33,34,35], further indicating the advantages of thermally activated PDS systems on 4-AAA degradation.
3.2. Optimization of 4-AAA Degradation Conditions in Thermally Activated PDS System
Despite the efficiencies of thermally activated PDS systems, the above experimental results could only show the influence of each single factor on 4-AAA degradation, while the interactions among these experimental parameters and their influence on 4-AAA degradation were unable to be provided. To obtain the optimal reaction conditions with the consideration of multiple experimental parameters, RSM was used to establish the polynomial regression model with multiple independent variables of temperature, initial pH, and PDS concentrations during the 4-AAA degradation process.
3.2.1. Predicting the Optimal 4-AAA Degradation Conditions with Response Surface Methodology
Based on the key factors of temperature, initial pH, and PDS concentrations, three factor, five-level experiments with 25 runs were designed and the degradation efficiencies of 4-AAA were all detected (Table 2).

Table 2.
Experimental scheme and response of 4-AAA removal.
A polynomial regression equation was built through RSM on the basis of the aforementioned experimental matrix (Equation (2)) to describe the simultaneous function of temperature, initial pH, and PDS concentrations on 4-AAA removal.
where Y represented the removal of 4-AAA in 20 min, A, B, and C represented temperature, initial pH and PDS concentrations, respectively.
Y = 28.89 + 42.02A + 1.8B + 9.76C + 0.55AB + 4.17AC + 0.65BC + 26.83A2 + 0.94B2 − 4.28C2
The linear terms, quadratic terms, and interaction terms of A, B, and C were all considered relevant to 4-AAA removal in thermally activated PDS system. The analysis of variance (ANVOA) and regression coefficients were obtained to further evaluate the model (Table 3). The “F-value” and “p values” were used to evaluate the significance level of the model. The “F-value” of the model was 81.61 (>1) and “p value” was less than 0.0001, indicating that the model was statistically significant and there was only 0.01% chance that the “F-value” could have occurred as the result of noise. Among all the terms in the model, the “p values” of A, C, and A2 were less than 0.0001, indicating that A, C, and A2 showed a more significant impact on the response value. Especially, A and C had significant linear effects and A2 showed significant quadratic effects on the degradation efficiency of 4-AAA. Thus, temperature (A) was identified as the most important factor affecting 4-AAA degradation in thermally activated PDS systems, which was in accordance with previous research on the removal of polyvinyl alcohol and acid orange 7 using thermally activated PDS [36,37].

Table 3.
Analysis of variance (ANVOA) for the optimized RSM model.
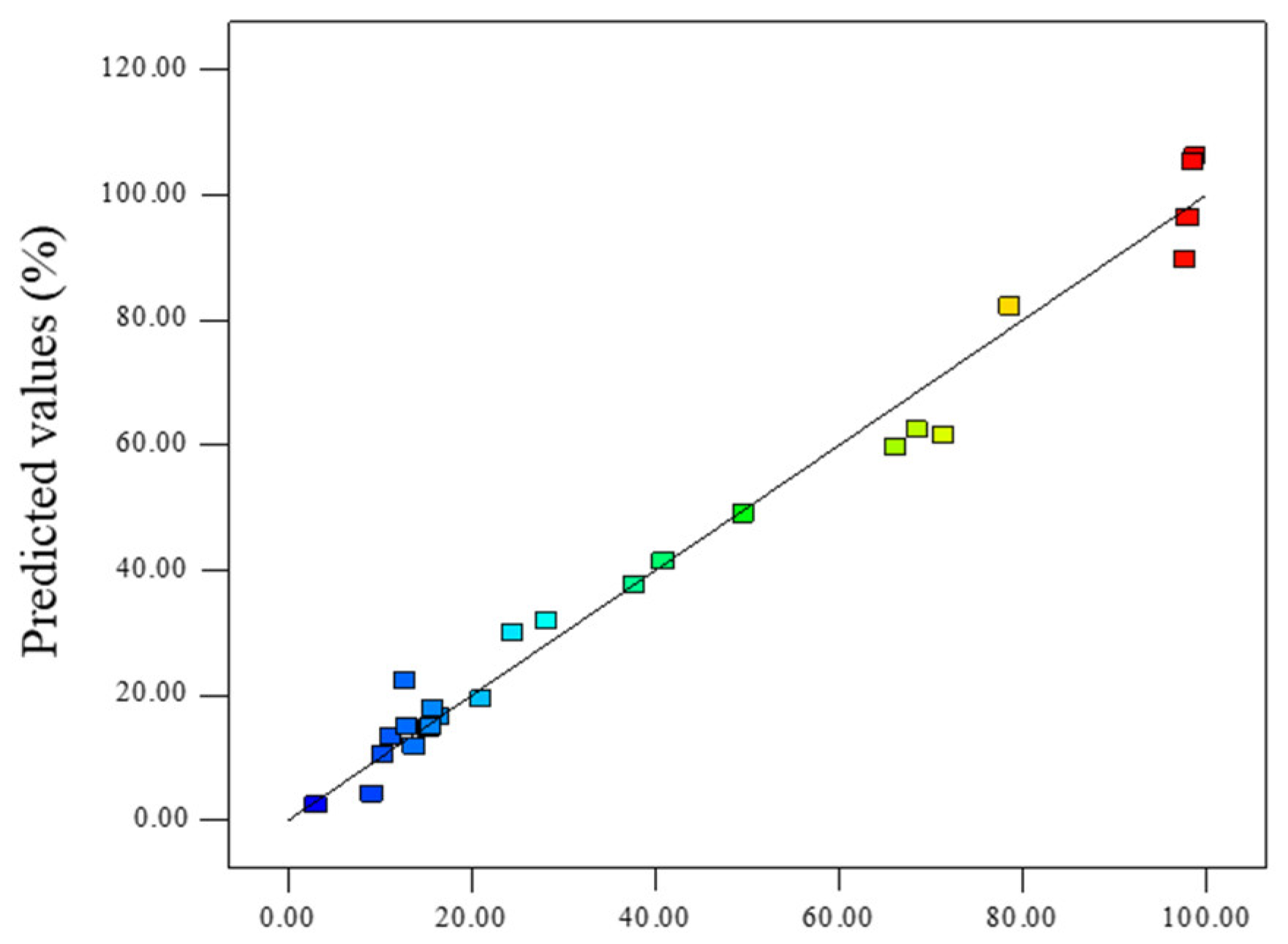
The predicted values of 4-AAA removal rate using the model were further calculated. As shown in Figure 2, the predicted results could well overlap the experimental data points (R2 = 0.9800) and the value of R2pred was 0.9352, indicating that 93.52% of changes on the response surface could be predicted using this model. These results further proved the validity of the RSM model to fit with the experimental results. According to the RSM model, three optimal experimental parameters were obtained and the degradation efficiencies of 4-AAA were predicted (Table 4). To examine the validity of the optimal conditions, experiments were performed under the same conditions and the degradation of 4-AAA were measured (Figure S4). For the three optimal conditions with the pH values of 3.73, 7.34, and 11.00, respectively, the removal rate of 4-AAA by experiments were 98.38%, 98.48%, and 94.34%, whose relative deviations from the model predicted results were 2.3%, 1.9%, and 11.1%, respectively. This result was also in accordance with the single factor experiments showed in Figure 1c that the efficient removal of 4-AAA could be achieved with a wide range of initial pHs. Therefore, the model could reflect the influence of various factors and obtain the optimum reaction conditions on 4-AAA degradation in thermally activated PDS systems. The optimum reaction conditions also indicated that with the temperature of 79 °C and PDS concentration of about 49 times higher than that of 4-AAA, the 4-AAA could achieve effective degradation under the initial pHs of acid, medium, and alkaline, further proving the broad applicability of thermally activated PDS on the degradation of 4-AAA.
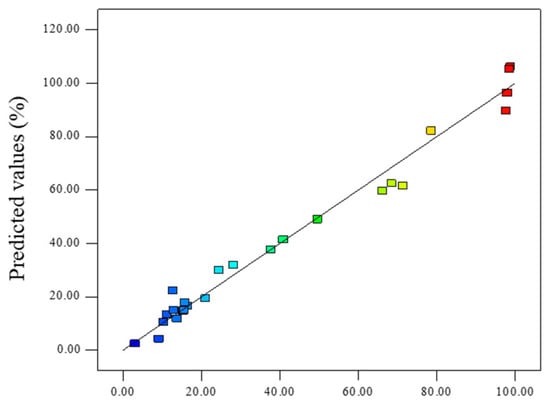
Figure 2.
Comparison of the actual 4-AAA degradation efficiency and the predicted 4-AAA degradation efficiency.

Table 4.
The optimal 4-AAA degradation conditions in thermal/PS system using RSM.
3.2.2. Analyzing the Interaction of Reaction Conditions for 4-AAA Degradation Using Response Surface Methodology
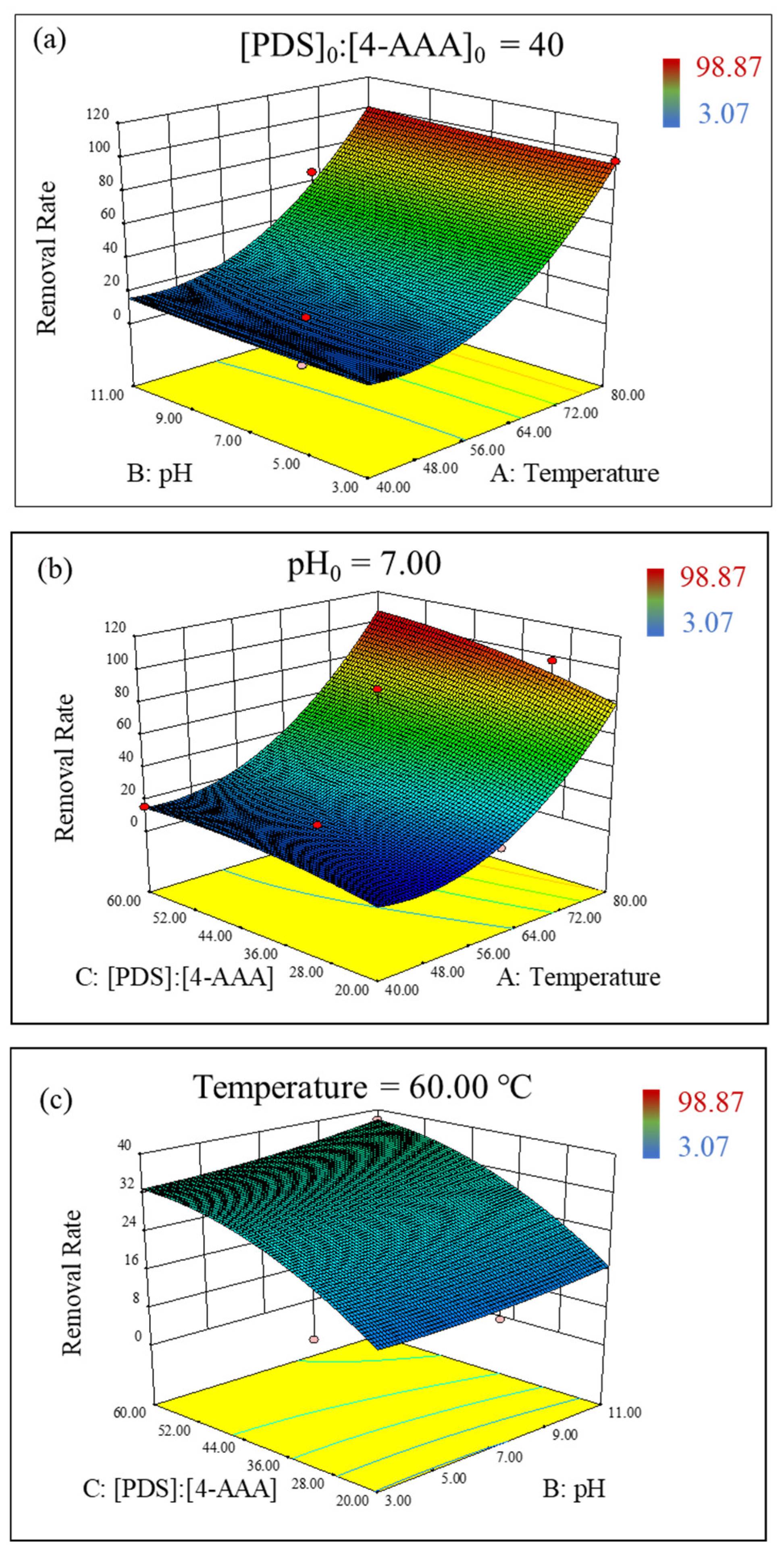
Based on the established model of Equation (2), the interacting influence of temperature, pH, and PDS concentrations on 4-AAA degradation in thermally activated PDS systems could be elucidated. The 3D surface plots of the RSM study indicated that the degradation rate of 4-AAA would increase with the increasing temperature and initial pH, but the influence of the temperature on the 4-AAA removal was more significant (Figure 3a). Similarly, the increase in temperature and PDS concentrations improved the degradation of 4-AAA (Figure 3b). Though the effect of temperature was significant, the influence of PDS concentrations on 4-AAA degradation was only obvious at high temperature. Considering that increasing temperature could promote PDS activation, the rise of PDS concentrations under high temperatures would increase free radical (e.g., SO4•−) production as well as the collision between radicals and 4-AAA molecules, eventually achieving a higher 4-AAA removal rate. Yazici Guvenc also found that a SO4•− formation at high temperatures could be strengthened by increased PDS concentrations [38]. As for pH and PDS concentrations, the interaction between pH and PDS concentrations on 4-AAA removal gradually became significant under high PDS concentrations (Figure 3c). Since more free radicals were generated with high PDS dosages, the transformation between radicals under different initial pHs (e.g., the transformation of SO4•− to •OH with the presence of OH− [39]) would be more significant to affect 4-AAA degradation. In a word, temperature played a dominant role for 4-AAA removal in thermally activated PDS systems and the impact of the initial pH was minimum, which were in accordance with the aforementioned results of single factor experiments.
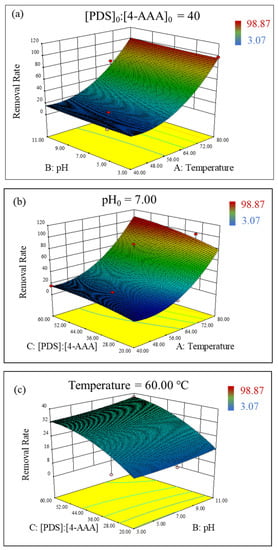
Figure 3.
Interaction between temperature and pH (a), PDS concentrations and temperature (b), pH and PDS concentrations (c) on the removal rate (%) of 4-AAA with 3D response surface in thermally activated PDS systems.
3.3. Exploration of Reaction Mechanism of 4-AAA in Thermally Activated PDS Systems
3.3.1. Identifying the Major Reactive Species for 4-AAA Degradation
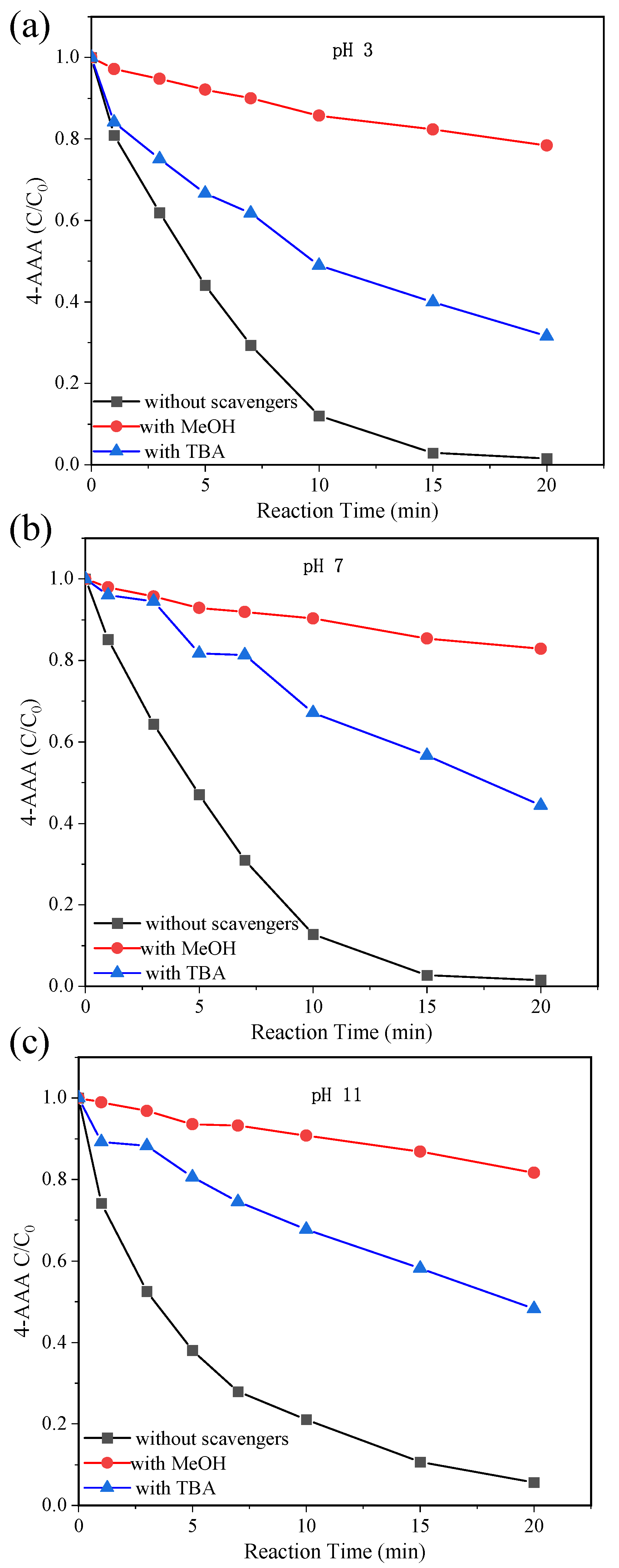
Above analysis showed the advantage of thermally activated PDS systems on 4-AAA removal and the dominant role of temperature. To elucidate the inner reaction mechanism for deeply understating the degradation of 4-AAA, major reactive species for 4-AAA degradation in thermally activated PDS system were identified with scavenging experiments. MeOH and TBA were chosen as scavengers to distinguish the contribution of SO4•− and •OH in 4-AAA degradation. As could be seen in Figure 4a–c, only around 20% of the 4-AAA was degraded with excess MeOH added in thermally activated PDS systems, while the degradation of 4-AAA was only partly inhibited with the addition of excess TBA regardless of initial values of pH. Since MeOH could scavenge both SO4•− and •OH ( = 2.5 × 107 M−1 s−1, k•OH MeOH = 9.7 × 108 M−1 s−1) and TBA could only effectively scavenge •OH ( = 8 × 105 M−1 s−1, k•OH TBA = 6 × 108 M−1 s−1) [40,41], the changes of degradation efficiency with MeOH and TBA indicated that both SO4•− and •OH contributed to 4-AAA degradation in thermally activated PDS systems. As aforementioned, SO4•− was produced through the activation of PDS and •OH could be generated from the reaction between SO4•− and OH− (Equation (3) [39]). With the initial values of pH increasing from 3 to 7 to 11, the degradation of 4-AAA decreased 27.74%, 42.9%, and 45.05% with the addition of excess TBA, respectively, indicating the increasing contribution of •OH to 4-AAA degradation in thermally activated PDS systems, which further proved the transformation of SO4•− to •OH with the reaction of OH−. The increased contribution of •OH with the rise of pH was also found for bisoprolol degradation in thermally activated PDS systems [33]. Thus, though both SO4•− and •OH contributed to 4-AAA degradation, the contribution of •OH could increase with higher initial values of pH in thermally activated PDS systems.
SO4•− + OH− → •OH + SO42−
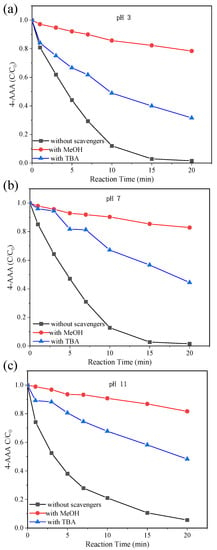
Figure 4.
Scavenging experiments for 4-AAA degradation in thermally activated PDS systems with initial pH of 3 (a), 7 (b), and 11 (c). Experimental conditions: [4-AAA]0 = 40.77 µM, [PDS]0 = 1.63 mM, [MeOH]0 = [TBA]0 = 2.4 M, T = 80 °C.
3.3.2. Identifying the Degradation Intermediates of 4-AAA Using Thermally Activated PDS
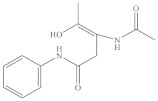
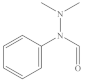
With the reaction of both SO4•− and •OH, the degradation intermediates of 4-AAA were investigated to explore the possible reaction pathway. A total of three reaction intermediates were detected in thermally activated PDS systems (Table 5 and Figure S5), indicating that the hydroxylation of the aromatic ring (P-262) and the pyrazolinone ring opening reaction (P-235 and P-165) were two possible parallel reaction pathways for 4-AAA degradation in thermally activated PDS systems and the N–N bonds in the hydrazine group were the major active site reacting with SO4•− and •OH. Though the degradation intermediates of 4-AAA by AOPs were rarely reported in detail in previous studies, similar hydroxylated and ring-opening intermediates were once found during the degradation of other phenazone drugs and their metabolites such as 4-MAA by AOPs [3], further proving the possible degradation pathway of 4-AAA. Accordingly, the removal of total organic carbon (TOC) was around 36.1% when 98.48% of the 4-AAA was degraded (Figure S6). Thus, 4-AAA was mostly converted to the abovementioned intermediates during the reaction in thermally activated PDS systems, rather than mineralizing into CO2 and H2O.

Table 5.
Identified intermediates for 4-AAA degradation in thermally activated PDS systems.
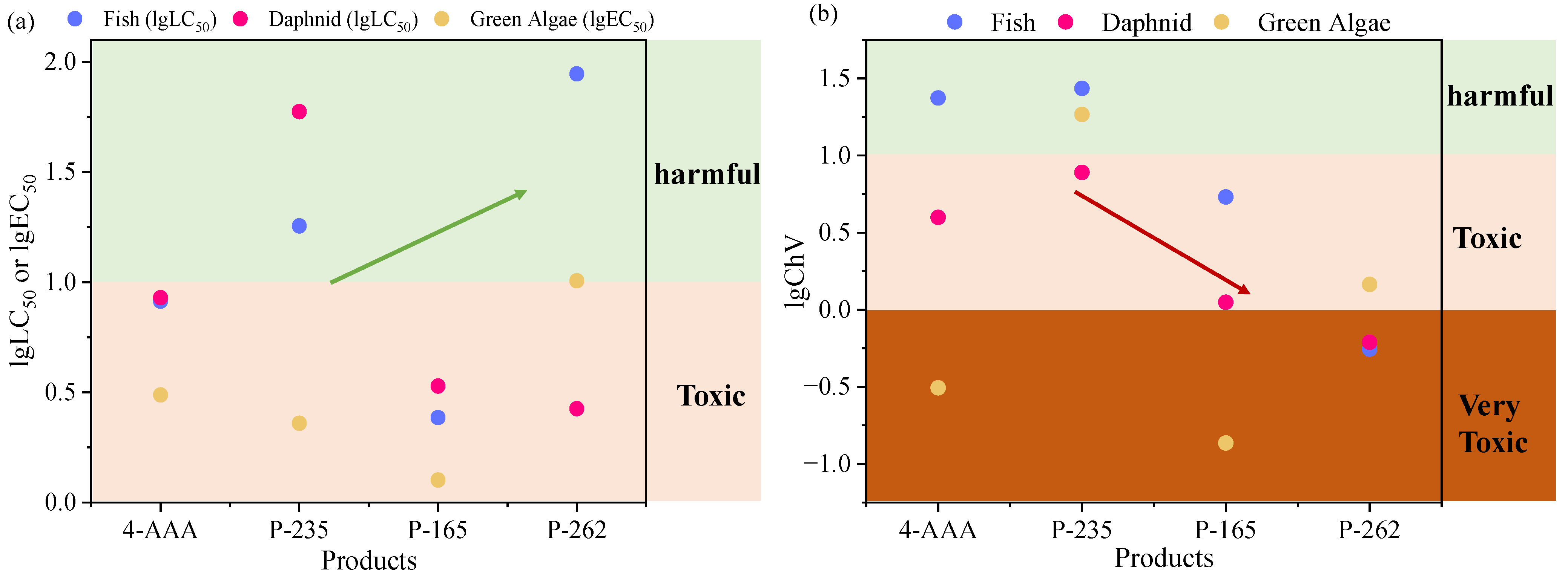
Since the presence of 4-AAA were related to the drug metabolism process in the human body, the existence of 4-AAA and its degradation intermediates in aquatic environments would show a potential risk of being accumulated through food chains and eventually threaten human health. To investigate the toxicity changes of 4-AAA during degradation in thermally activated PDS systems, ECOSAR V 2.0 was used to provide the predicted acute and chronic toxicities of 4-AAA and aforementioned intermediates. As showed in Figure 5, the LC50 values of 4-AAA for fish and daphnia and the EC50 value for green algae were 8.190, 8.506, and 3.070 mg/L and the ChV values of 4-AAA for fish, daphnia, and green algae were 23.590, 3.960, and 0.311 mg/L, respectively, indicating that the acute toxicity of 4-AAA was in the category of toxic substance (1–10 mg/L) and the chronic toxicity of 4-AAA for green algae was assigned to be a very toxic level (0–1 mg/L) [42]. With the degradation in thermally activated PDS systems, the LC50 and EC50 values of P-235 and P-262 mostly increased and were assigned to harmful levels, indicating the decrease in acute toxicity compared with 4-AAA. While the ChV of intermediates decreased even to very toxic levels except for P-235, which suggested the increase in chronic toxicity of most intermediates compared with 4-AAA. Among the three degradation intermediates, P-165 showed the highest level of both acute and chronic toxicities, whose values were higher than the parent compound of 4-AAA. Thus, the production and transformation of P-165 should be focused on in further treatment processes.
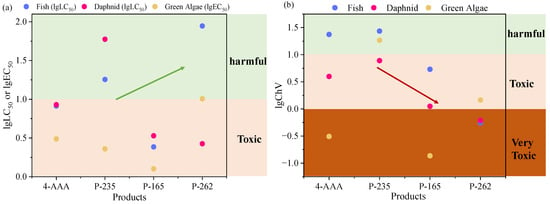
Figure 5.
Predicted acute (a) and chronic (b) toxicity of 4-AAA and its degradation intermediates based on ECOSAR V 2.0 estimation.
3.4. Effects of Water Constitutions on Degradation of 4-AAA Using Thermally Activated PDS
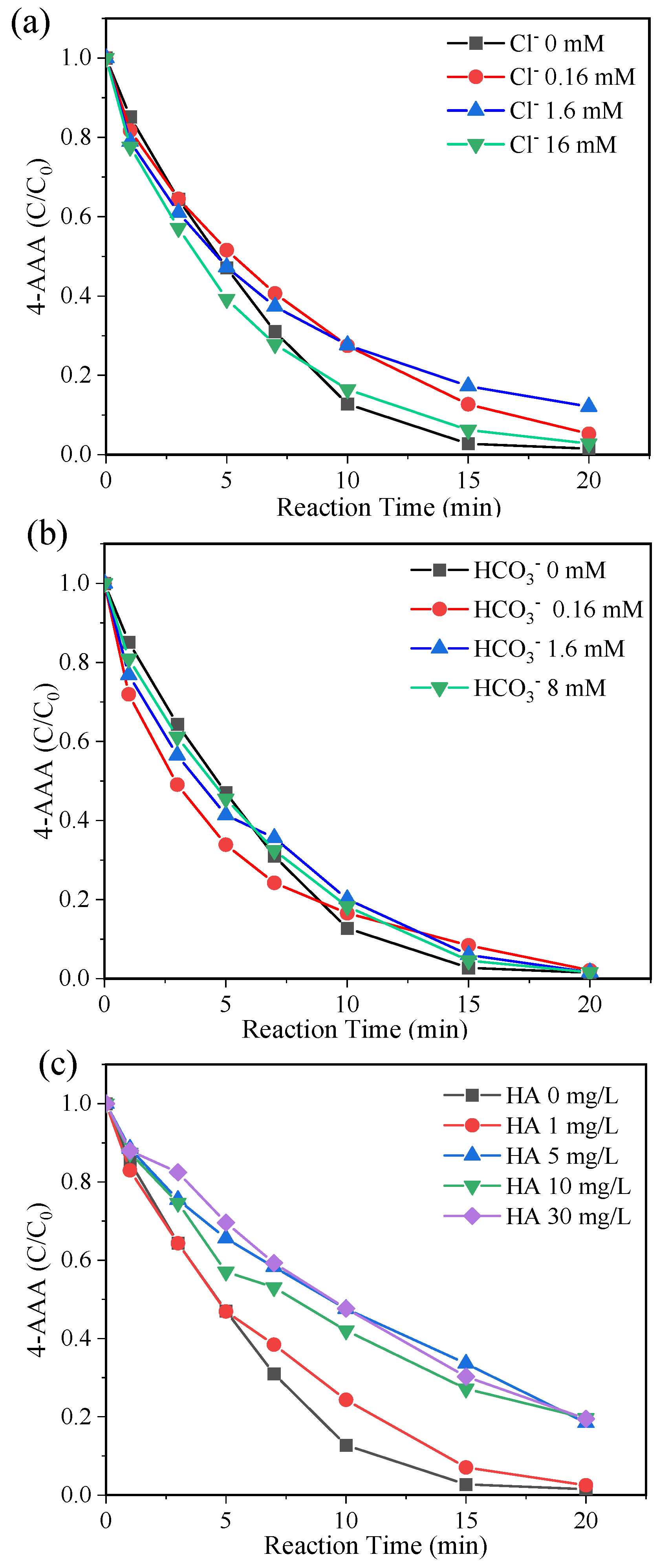
With the high degradation efficiency of 4-AAA in thermally activated PDS systems, the effects of water constitutions on 4-AAA degradation were investigated to evaluate the systematic applicability. The degradation of 4-AAA changed slightly with the presence of commonly existing anions Cl− and HCO3− (Figure 6a,b), while its removal rate decreased from 98.48% to around 81% with the addition of humic acid ranging from 5 to 30 mg/L (Figure 6c). The addition of Cl− would produce Cl• and Cl2•− through the reactions with SO4•− (Equation (4) and (5)) [43,44]. Though these reactions competed with 4-AAA for SO4•− consumption, the produced Cl• and Cl2•− showed a high reactive selectivity to electron-rich functional groups [45], which might also effectively react with 4-AAA due to the contained electron-donating group of acetyl. Compared with Cl•, the production of CO3•− through reactions of •OH and SO4•− with HCO3− were significantly slower (Equation (6) and (7)) [46], resulting in the limited scavenging ability of •OH and SO4•− compared with 4-AAA. Thus, the degradation of 4-AAA was less influenced by Cl− and HCO3− in thermally activated PDS systems. Different from Cl− and HCO3−, the diverse electron-rich functional groups of humic acid (e.g., phenolic compounds) resulted in its reducibility and strong radical scavenging capacity with the reaction rate constant around 108 MC−1 s−1 toward both •OH and SO4•− [47]. Thus, the degradation of 4-AAA was partly inhibited with the presence of humic acid in thermally activated PDS systems. Despite this inhibition effect, more than 80% of the 4-AAA could be degraded after 20 min of reaction, indicating that thermally activated PDS systems could show good effects on 4-AAA removal with the presence of commonly existing water constitutions, which were scarcely investigated in previous research on 4-AAA oxidation processes. Besides, thermal activated PDS systems exhibited higher or identical degradation efficiencies on 4-AAA than the previously studied solar UV, Cl2, and O3 systems under the optimum reaction conditions (Table 6), indicating its promising application potential in drinking water and wastewater treatment processes.
SO4•− + Cl− → Cl• + SO42− k = 2.7 × 108 M−1 s−1
Cl• + Cl− → Cl2•− k = 8 × 109 M−1 s−1
HCO3− + •OH → CO3•− + H2O k = 8.5 × 106 M−1 s−1
HCO3− + SO4•− → CO3•− + HSO4− k = 2.8 × 106 M−1 s−1
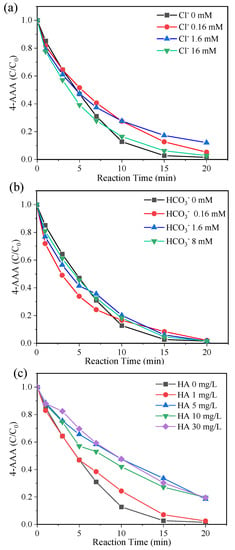
Figure 6.
Degradation of 4-AAA with different concentrations of Cl− (a), HCO3− (b), and humic acid (c) in thermally activated PDS systems. Experimental conditions: [4-AAA]0 = 40.77 µM, [PDS]0 = 1.63 mM, pH0 = 7, T = 80 °C.

Table 6.
Summary of previous research on the removal of 4-AAA.
4. Conclusions
In this article, efficient 4-AAA degradation was achieved using a thermally activated PDS system. Compared with other common activation methods of PDS such as UV, US, alkaline, and iron species, 4-AAA obtained its highest removal rate (98.9%) in thermally activated PDS systems with a reaction time of 30 min. Temperature was the dominant factor affecting 4-AAA degradation due to the promotional production of SO4•− and •OH through PDS activation. The degradation of 4-AAA was slightly influenced with the presence of Cl− and HCO3− and over 80% of 4-AAA could be removed with the existence of humic acid, indicating that the degradation process was less affected by water constitutions.
As the commonly detected antibiotic metabolism in municipal wastewater, only a limited removal of 4-AAA could be achieved in conventional biological treatment processes. Moreover, the efficient degradation method of 4-AAA was rarely reported in previous studies. This study demonstrated that thermally activated persulfate systems were useful for 4-AAA degradation, which would provide technical support on the removal of 4-AAA in drinking water and wastewater treatment processes. Besides, the acute and chronic toxicities of 4-AAA intermediates were initially focused on. The hydroxylated and pyrazolinone ring-opening intermediates of 4-AAA might show an identical or higher predicted chronic toxicity. Thus, the following transformation of these intermediates from 4-AAA and other PPCP metabolites after AOPs should be further focused on in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su142114300/s1, Figure S1: Schematic of experimental reactors; Figure S2: Pseudo first order reaction kinetic fitting results for 4-AAA degradation with different PDS concentrations (a), initial pH (b) and temperature (c) in thermally activated PDS systems; Figure S3: Calculation of Ea of 4-AAA in thermally activated PDS systems; Figure S4: Validation experiments of the optimal conditions by RSM model; Figure S5: Chromatography and mass spectrometry of reaction products and structural formula; Figure S6: TOC changes in degradation of 4-AAA by thermal/PS system; Text S1: Separation conditions and operating parameters for UPLC-Orbitrap-MS.
Author Contributions
Conceptualization, Q.W.; Data curation, X.W.; Formal analysis, S.L. and Z.L.; Investigation, S.L.; Methodology, Q.W.; Resources, C.C.; Supervision, Y.Z.; Validation, X.W.; Writing—original draft, Z.L.; Writing—review & editing, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Science Foundation of China University of Petroleum, grant number 01JB20220078 and 2462022XKBH002.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.J.; Sirtori, C.; Mezcua, M.; Fernandez-Alba, A.R.; Aguera, A. Photodegradation study of three dipyrone metabolites in various water systems: Identification and toxicity of their photodegradation products. Water Res. 2008, 42, 2698–2706. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.J.; Gómez-Ramos, M.M.; Malato, O.; Mezcua, M.; Férnandez-Alba, A.R. Rapid automated screening, identification and quantification of organic micro-contaminants and their main transformation products in wastewater and river waters using liquid chromatography–quadrupole-time-of-flight mass spectrometry with an accurate-mass database. J. Chromatogr. A 2010, 1217, 7038–7054. [Google Scholar] [CrossRef] [PubMed]
- Heeb, F.; Singer, H.; Pernet-Coudrier, B.; Qi, W.; Liu, H.; Longree, P.; Muller, B.; Berg, M. Organic micropollutants in rivers downstream of the megacity Beijing: Sources and mass fluxes in a large-scale wastewater irrigation system. Environ. Sci. Technol. 2012, 46, 8680–8688. [Google Scholar] [CrossRef]
- Wiegel, S.; Aulinger, A.; Brockmeyer, R.; Harms, H.; Loffler, J.; Reincke, H.; Schmidt, R.; Stachel, B.; von Tumpling, W.; Wanke, A. Pharmaceuticals in the river Elbe and its tributaries. Chemosphere 2004, 57, 107–126. [Google Scholar] [CrossRef]
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef]
- Wielens Becker, R.; Ibanez, M.; Cuervo Lumbaque, E.; Wilde, M.L.; Flores da Rosa, T.; Hernandez, F.; Sirtori, C. Investigation of pharmaceuticals and their metabolites in Brazilian hospital wastewater by LC-QTOF MS screening combined with a preliminary exposure and in silico risk assessment. Sci. Total Environ. 2020, 699, 134218. [Google Scholar] [CrossRef]
- Bai, X.; Liang, W.; Sun, J.; Zhao, C.; Wang, P.; Zhang, Y. Enhanced production of microalgae-originated photosensitizer by integrating photosynthetic electrons extraction and antibiotic induction towards photocatalytic degradation of antibiotic: A novel complementary treatment process for antibiotic removal from effluent of conventional biological wastewater treatment. J. Environ. Manag. 2022, 308, 114527. [Google Scholar] [CrossRef]
- Kamaruddin, M.A.; Ibrahim, M.H.; Thung, L.M.; Emmanuel, M.I.; Niza, N.M.; Shadi, A.M.H.; Norashiddin, F.A. Sustainable synthesis of pectinolytic enzymes from citrus and Musa acuminata peels for biochemical oxygen demand and grease removal by batch protocol. Appl. Water Sci. 2019, 9, 68. [Google Scholar] [CrossRef]
- Asghar, M.A.; Zhu, Q.; Sun, S.; Peng, Y.; Shuai, Q. Suspect screening and target quantification of human pharmaceutical residues in the surface water of Wuhan, China, using UHPLC-Q-Orbitrap HRMS. Sci. Total Environ. 2018, 635, 828–837. [Google Scholar] [CrossRef]
- Favier, M.; Dewil, R.; Van Eyck, K.; Van Schepdael, A.; Cabooter, D. High-resolution MS and MS(n) investigation of ozone oxidation products from phenazone-type pharmaceuticals and metabolites. Chemosphere 2015, 136, 32–41. [Google Scholar] [CrossRef]
- Sieira, B.J.; Quintana, J.B.; Cela, R.; Rodil, R. Reaction of phenazone-type drugs and metabolites with chlorine and monochloramine. Sci. Total Environ. 2021, 757, 143770. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, K.-M.; Kim, H.-E.; Lee, H.-J.; Lee, C.; Lee, C. Disintegration of Waste Activated Sludge by Thermally-Activated Persulfates for Enhanced Dewaterability. Environ. Sci. Technol. 2016, 50, 7106–7115. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Transition metal/UV-based advanced oxidation technologies for water decontamination. Appl. Catal. B-Environ. 2004, 54, 155–163. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Peroxymonosulfate: A Novel Advanced Oxidation Process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef]
- Wei, Z.; Villamena, F.A.; Weavers, L.K. Kinetics and Mechanism of Ultrasonic Activation of Persulfate: An in Situ EPR Spin Trapping Study. Environ. Sci. Technol. 2017, 51, 3410–3417. [Google Scholar] [CrossRef]
- Shakeri, E.; Mousazadeh, M.; Ahmadpari, H.; Kabdasli, I.; Jamali, H.A.; Graça, N.S.; Emamjomeh, M.M. Electrocoagulation-flotation treatment followed by sedimentation of carpet cleaning wastewater: Optimization of key operating parameters via RSM-CCD. Desalination Water Treat. 2021, 227, 163–176. [Google Scholar] [CrossRef]
- Li, J.; Zou, J.; Zhang, S.; Cai, H.; Huang, Y.; Lin, J.; Li, Q.; Yuan, B.; Ma, J. Sodium tetraborate simultaneously enhances the degradation of acetaminophen and reduces the formation potential of chlorinated by-products with heat-activated peroxymonosulfate oxidation. Water Res. 2022, 224, 119095. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zou, J.; Lin, J.; Li, Q.; Li, J.; Huang, Y.; Yang, H.; Yuan, B.; Ma, J. Elimination of acetaminophen in sodium carbonate-enhanced thermal/peroxymonosulfate process: Performances, influencing factors and mechanism. Chem. Eng. J. 2022, 449, 137765. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Lin, J.; Li, J.; Huang, Y.; Zhang, S.; Yuan, B.; Ma, J. Sodium hydroxide-enhanced acetaminophen elimination in heat/peroxymonosulfate system: Production of singlet oxygen and hydroxyl radical. Chem. Eng. J. 2022, 429, 132438. [Google Scholar] [CrossRef]
- Guo, Y.; Liang, H.; Bai, L.; Huang, K.; Xie, B.; Xu, D.; Wang, J.; Li, G.; Tang, X. Application of heat-activated peroxydisulfate pre-oxidation for degrading contaminants and mitigating ultrafiltration membrane fouling in the natural surface water treatment. Water Res. 2020, 179, 115905. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Ji, Q.Q.; Lai, B. Degradation of p-nitrophenol (PNP) in aqueous solution by Fe-0-PM-PS system through response surface methodology (RSM). Appl. Catal. B-Environ. 2017, 200, 633–646. [Google Scholar] [CrossRef]
- Naghdali, Z.; Sahebi, S.; Mousazadeh, M.; Jamali, H.A. Optimization of the Forward Osmosis Process Using Aquaporin Membranes in Chromium Removal. Chem. Eng. Technol. 2019, 43, 298–306. [Google Scholar] [CrossRef]
- Umamaheswari, J.; Bharathkumar, T.; Shanthakumar, S.; Gothandam, K.M. A feasibility study on optimization of combined advanced oxidation processes for municipal solid waste leachate treatment. Process Saf. Environ. Prot. 2020, 143, 212–221. [Google Scholar] [CrossRef]
- Furman, O.S.; Teel, A.L.; Watts, R.J. Mechanism of Base Activation of Persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- Luo, C.; Gao, J.; Wu, D.; Jiang, J.; Liu, Y.; Zhou, W.; Ma, J. Oxidation of 2,4-bromophenol by UV/PDS and formation of bromate and brominated products: A comparison to UV/H2O2. Chem. Eng. J. 2019, 358, 1342–1350. [Google Scholar] [CrossRef]
- Tsitonaki, A.; Petri, B.; Crimi, M.; Mosbaek, H.; Siegrist, R.L.; Bjerg, P.L. In Situ Chemical Oxidation of Contaminated Soil and Groundwater Using Persulfate: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 55–91. [Google Scholar] [CrossRef]
- Ghauch, A.; Tuqan, A.M. Oxidation of bisoprolol in heated persulfate/H2O systems: Kinetics and products. Chem. Eng. J. 2012, 183, 162–171. [Google Scholar] [CrossRef]
- Fan, Y.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Hou, Y.-Z.; Lin, Y.-L.; Deng, Y.-G.; Hua, S.-J.; Du, Y.-F.; Chen, C.-W.; Wu, C.-H. Investigation of iohexol degradation kinetics by using heat-activated persulfate. Chem. Eng. J. 2020, 379, 122403. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Kim, H.-W.; Park, J.-M.; Park, H.-S.; Yoon, C. Oxidation of polyvinyl alcohol by persulfate activated with heat, Fe2+, and zero-valent iron. J. Hazard. Mater. 2009, 168, 346–351. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Yazici Guvenc, S. Optimization of COD removal from leachate nanofiltration concentrate using H2O2/Fe+2/heat—Activated persulfate oxidation processes. Process Saf. Environ. Prot. 2019, 126, 7–17. [Google Scholar] [CrossRef]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Rate Constants and Mechanism of Reaction of SO4.- with Aromatic Compounds. J. Am. Chem. Soc. 1977, 126, 7–17. [Google Scholar]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (-OH/O- in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate Constants for Reactions of Inorganic Radicals in Aqueous-Solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, C.; Fang, G.; Chu, L.; Gu, C.; Gao, J. Persistent Free Radicals from Low-Molecular-Weight Organic Compounds Enhance Cross-Coupling Reactions and Toxicity of Anthracene on Amorphous Silica Surfaces under Light. Environ. Sci. Technol. 2021, 55, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Fessenden, R.W. Flash photolysis of transient radicals. 1. X2- with X = Cl, Br, I, and SCN. J. Phys. Chem. 1985, 89, 2330–2335. [Google Scholar] [CrossRef]
- McElroy, W.J. A laser photolysis study of the reaction of sulfate(1-) with chloride and the subsequent decay of chlorine(1-) in aqueous solution. J. Phys. Chem. 1990, 94, 2435–2441. [Google Scholar] [CrossRef]
- Fang, J.; Fu, Y.; Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Jiang, J.; Ma, J.; Liu, G.; Cao, Y.; Liu, W.; Li, J.; Pang, S.; Kong, X.; et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate. Water Res. 2017, 118, 196–207. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Guan, J.; Westerhoff, P.; Yang, X. Kinetics and Transformations of Diverse Dissolved Organic Matter Fractions with Sulfate Radicals. Environ. Sci. Technol. 2022, 56, 4457–4466. [Google Scholar] [CrossRef]
- Vasiliadou, I.A.; Sanchez-Vazquez, R.; Molina, R.; Martinez, F.; Melero, J.A.; Bautista, L.F.; Iglesias, J.; Morales, G. Biological removal of pharmaceutical compounds using white-rot fungi with concomitant FAME production of the residual biomass. J. Environ. Manag. 2016, 180, 228–237. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).