Abstract
Soil microorganisms play an important role in regulating a variety of ecological functions. In recent years, the research on ecological restoration after mining has made people more aware of the importance of microbial diversity to ecosystem restoration. The present study investigated the effect of ecological restoration on microbial community structure and its relationship with soil physicochemical properties in the Dabaoshan mining area, China. High throughput sequencing technology was used to analyze and compare the microbial community composition of three types of soil (undamaged area, unrestoration area, and ecological restoration area). The contents of organic carbon, total nitrogen, and total phosphorus were 2.38–12.97 g/kg, 0.39–1.62 g/kg, and 0.99–1.51 g/kg, respectively. In different soil states, undamaged area and ecological restoration area were significantly higher than those in unrestoration area. The results showed that the structure of soil microbial community was significantly correlated with soil physicochemical properties, and formations in the repaired and unrepaired soils were different. Operational Taxonomic Unit (OTU) cluster analysis and diversity index analysis showed that soil microbial community changed at phylum and genus levels. The results showed that at the phylum level, all soil samples contained Firmicutes, Proteobacteria, and actinobacteria. Firmicutes and Proteobacteria of the ecological restoration area (ER1, ER2) were the highest in relative abundance compared with other samples, accounting for more than 45%. Proteobacteria and Acidobacteria were the dominant phylum in the undamaged area (UD), accounting for 32.7% and 22.3%, respectively. It can be seen that soil restoration produced a new dominant population, and Proteobacteria showed an absolute competitive advantage in the mining soil.
1. Introduction
Mineral resources not only bring great economic benefits to human beings, but also cause serious pollution to the environment of mining areas and damage to the ecosystem. During the mining process, the vegetation, soil, and landforms in the mining area are damaged [1]. Due to the unconscionable exploitation and utilization of mineral resources, soil pollution is becoming more and more serious [2]. Soil pollution in mining areas migrates under surface runoff and biogeochemistry, endangering the environmental quality of adjacent areas and endangering human health by contaminating food and drinking water [3,4,5]. In addition, the topsoil needs to be stripped during mining, resulting in serious damage to vegetation [6]. Mining reduces the diversity of the surrounding ecosystem. A large number of rare earth elements and radioactive substances produced in the mining process accumulate in the surrounding soil with surface runoff under the leaching of rain, causing harm to plants, animals, and microorganisms and reducing the diversity of the ecosystem [7,8,9]. In Minqin County, China, the over-mining for years has led to the collusive of underground brackish water layers and the increasing salinity of the water, which is neither drinkable nor suitable for irrigation, causing serious damage to the surface ecosystem and causing the death of 48,691 ha (=120,450 acres) of natural forests and 18,676 ha (=46,200 acres) of artificial forests [10]. The overexploitation of the Kamioka mine in Japan had led to serious Cadmium (Cd) pollution in the downstream. Local residents have suffered from the painful “Alitai disease” due to the long-term consumption of Cd contaminated food and water, which causes symptoms such as bone softening, spinal deformation, and osteoporosis [11]. Mining in Fuyang City, Zhejiang Province has led to the serious exceeding of the content of Plumbum (Pb), Zinc (Zn), Copper (Cu), and Chromium (Cr) in the soil, which has led to the lack of vegetation and serious soil erosion [12]. Therefore, it has become an increasingly prominent problem to repair mining pollution and alleviate the harm caused by mining pollution. Ecological restoration technology has been widely used to control mining pollution because of its effective ecological, economic, social, environmental, and safety benefits [13]. The literature confirms that ecological restoration is key to realize green and climate-smart mining [14,15,16]. As an appropriate means to offset the inevitable damage to natural areas, ecological restoration reduces the environmental risks caused by mining activities and, in fact, promotes the sustainable development of mineral resources [17,18]. The ecological restoration of mining areas mainly includes the treatment and restoration of polluted soil, the restoration of damaged vegetation, and the restoration of the original ecological landscape [1,19]. According to the literature, vegetation restoration in the mining area is the key to restore the ecological balance in the mining area [20]. Meanwhile, attention should be paid to the restoration of soil properties during the ecological restoration in the mining area [20]. Through the implementation of a series of ecological protections and restoration measures, such as planting flowers, turf slope consolidation, afforestation, and mine park greening, Zijin mine achieved a good ecological restoration effect, and the impact on the ecological environment of the mining area was been alleviated and restored [21]. In addition, the ecological restoration of the open pit coal mine in Inner Mongolia steppe area promoted the rapid improvement of plant community and soil physicochemical properties, and made the height, coverage, and aboveground biomass of plant community higher than or close to the original steppe [22]. The restoration of vegetation in the ecological restoration of the mining area improves the physicochemical properties of soil, improves the diversity of microbial community, and reduces the content of heavy metals in soil [20,23,24].
The ecological restoration process of mines is relatively long, and it is very necessary to monitor and evaluate the ecological characteristics of the restoration process. In the early stage, the evaluation and monitoring of the restoration effect of mining area mostly depend on the physicochemical properties of aboveground vegetation and soil [25,26]. Although soil microorganisms were affected by mining activities and had a regulatory role in ecosystem function, they were rarely monitored in prior research studies [27,28]. High-throughput sequencing technology has the advantages of fast speed, low cost, and high accuracy [29]. It has unique advantages in analyzing microbial community structure and provides a new tool for us to understand the characteristics of soil microbial community structure. In recent years, microbial diversity has become a standardized and cost-effective method for monitoring the ecosystem health of mine restoration [30,31]. Soil microorganisms are an important part of terrestrial ecosystem, among which bacteria are the most abundant and diversified [32]. The restoration of soil microbial community is a key process of soil restoration, which plays a positive role in realizing soil health and sustainable utilization [33]. Studies have shown that soil microorganisms play an important role in regulating various ecological functions [34,35]. By adding bacillus to the mining area, it provided nutrient elements for plant growth and promoted plant growth. At the same time, the growth of plants promoted the growth of microbes, and the two complement each other so that the ecosystem reached a self-sustaining state and maintained the durability of ecological restoration [36]. In addition, an ecological restoration project was implemented in a copper mine wasteland in northwest Spain, which enhanced the utilization rate of nutrients by soil microorganisms, improved the nutrient absorption of plants, and promoted plant growth [37]. The ecological restoration of ecosystem services and biodiversity is a key intervention to reverse the effects of human activities such as mining [38].
In this study, we conducted high-throughput sequencing of the bacterial 16S rRNA gene at four sampling sites in the Dabaoshan mining area for research on the characteristics of soil bacterial communities. In addition, soil physicochemical properties and heavy metal content were determined. This study explored the changes of soil physicochemical properties under different ecological restoration conditions and analyzed the diversity and structure characteristics of soil bacterial community under different ecological restoration conditions. Then, we monitored and evaluated the effect of mine ecological restoration. By studying the relationship between soil properties and soil microbial community, we can better understand and evaluate the ecological restoration effect of the mine. Collecting soil samples of four sample plots in the mining area for analysis of physicochemical properties and microbial diversity, it is beneficial to explore the effect of ecological restoration in the mining area and provide a reference for the ecological restoration of the Dabaoshan mining area.
2. Materials and Methods
2.1. Study Site and Sample Collection
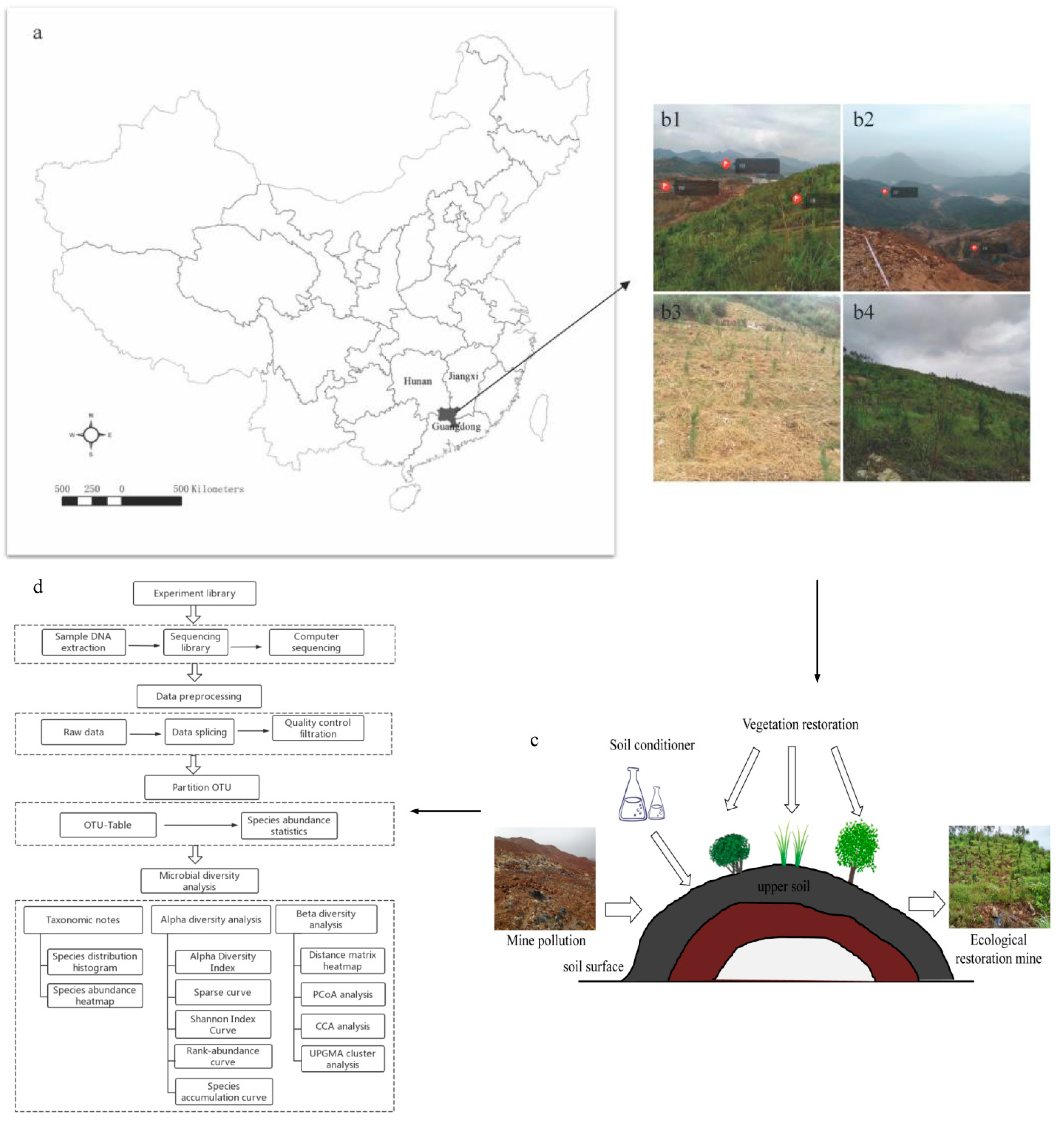
Dabaoshan (113°40′~113°43′ E, 24°30′~24°36′ N), located between Hunan, Jiangxi, and Guangdong, is a large open-pit polymetallic mine in Guangdong Province and an important raw material base for the iron and steel industry and non-ferrous metal industry in South China [39]; the serious heavy metal pollution in Dabaoshan Mining Area threatens the ecological security of the surrounding areas (Figure 1). Due to many years of large-scale open-pit mining and the lack of rational planning of civilian mining, serious ecological degradation and environmental pollution problems in the mining area have resulted, and biodiversity has been damaged. Sewage discharged from the Dabaoshan mining area led the river to turn red, the bottom sediment to turn black, and to the disappearance of fish and shrimp in the river. Meanwhile, many villagers had serious diseases such as liver disease and cancer due to the long-term consumption of food and water polluted by the waste water in the mining area [40,41,42]. Ecological restoration in mining areas is conducive to restoring the productivity of the soil in mining areas and maintaining the sustainable development of the ecosystem [43,44], which is an effective approach for mine treatment. Through vegetation restoration and added soil conditioner of the Dabaoshan mining area, the vegetation coverage of each restoration area showed a significant increase trend with the increase of restoration time [45]. In 2017, the mine-damaged area of Dabaoshan completed the re-greening of 42,000 square meters at 13 km of the eastern highway through the soil restoration project, which greatly reduced soil erosion from the source and prevented new pollution wastewater [46].
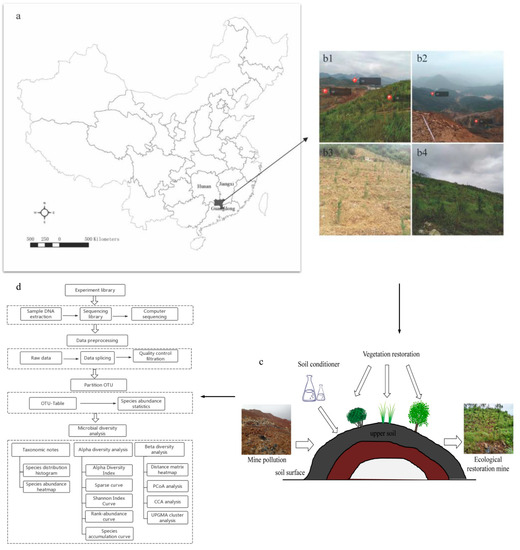
Figure 1.
Geographical location of the study region (a) and the landscape of sampling sites, of which (b) b1 was the study sample plot, b2 was the landscape view of UD and UR, and b3 (ER1) and b4 (ER2) were the landscape view of the ecological restoration area. (c) was the schematic diagram of the mine’s ecological restoration process. (d) was the 16S rRNA gene sequencing and data analysis process.
The above survey was completed in June 2018. The study sample plot was divided into an undamaged area (UD) (Figure 1(b1,b2)), an unrestoration area (UR) (Figure 1(b1,b2)), and two ecological restoration areas (ER1 and ER2) (Figure 1(b3,b4)). The details of the abovementioned ecological restoration process was described by Yang et al. [45], including soil improvement [47] and vegetation restoration [48]. Pinus elliottii, Boehmeria nivea, Rhus chinensis, Cinnamomum camphora, Sesbania cannabina, Schima superba, Erigeron acer, Solidago canadensis, and Miscanthus floridulus etc. were mainly planted in the ecological restoration project. About two years later, the vegetation covers the damaged landscape. The soil conditioner includes lime, nitrogen, and phosphate. ER1 had a low vegetation coverage rate and a low plant growth density (Figure 1(b3)), while ER2 had a good restoration effect and a high vegetation coverage rate (Figure 1(b4)). Soil sampling was carried out by soil ring knife method. Three soil cores (5 cm diameter, 0–10 cm) were randomly collected from each individual plot using a soil auger. The samples were kept cold in an ice chest during sampling and transport back to the laboratory. After returning to the laboratory, the undried soil samples were sieved (by 2 mm) to remove gravel and plant residues, homogenized thoroughly, and then compartmentalized into three portions for further analysis. The first portion was kept at −80 °C for subsequent DNA extraction. The second portion was retained after air-dried for analysis of chemical properties. The third portion was retained after air-drying for analysis of heavy metal content of soil. The three soil samples used in the experiment were not mixed.
2.2. Determination of Soil Physicochemical Properties and Heavy Metal Content
After drying and sieving the soil samples, the physicochemical properties were analyzed and the heavy metal content was determined. Soil moisture content is determined by drying the soil to a constant weight [49]. The organic carbon (SOC) was determined by potassium dichromate hydration heating method [50], the total nitrogen (TN) was determined by Kjeldahl method [50], and the total phosphorus (TP) was determined by molybdenum antimony colorimetry [51,52]; the content of heavy metals (Cu and Cd) was determined by the atomic adsorption spectrophotometer (AAS) after digestion with a mixture of HNO3-HCL-HCLO4 [52,53]. Three replicated tests were performed for all the above indicators.
2.3. 16S rRNA Gene Sequencing of Soil Bacteria
Genomic DNA was extracted according to the instructions of DNA extraction kit, and the integrity and purity of DNA were detected by agarose gel electrophoresis, while the concentration of DNA was detected by Nanodrop One. Using genomic DNA as template, primers with Barcode and Premix Taq (Takara) were used to amplify the V4 region of bacterial 16S rRNA gene. PCR reaction conditions: initialization at 94 °C for 5 min, denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s, extension at 72 °C for 30 s, 30 cycles; the final elongation was then performed at 72 °C for 10 min, with each sample repeated three times. The purified samples were sent to Guangdong Meige Gene Technology Co., Ltd. (Shenzhen, China) for sequencing using Illumina MiSeq high-throughput sequencing technology. The 16S rRNA high-throughput sequencing data were submitted to the NCBI Sequence Read Archive (SRA) with the BioProject ID PRJNA752830.
2.4. Statistical Analysis
Low-quality bases were removed according to the quality control information of the original sequence. The original sequences were denoised, sorted, and distinguished using Trimmomatic software, and then the primers were pruned. The UPARSE algorithm was used for Operational Taxonomic Unit (OTU) clustering at 97% consistency level, and the sequence with the highest occurrence frequency in each OTU was selected as the representative sequence of OTU. OTU annotation by using BLAST to compare the sequence database in QIIME, the taxonomic information of each OTU was obtained. The Venn diagram of OTU between samples was generated by Mothur software [51,54]. The optimized sequences were identified at the level of phylum, class, family and genus according to the reference sequences in the database. The software R was used to draw a Heatmap to compare and analyze the community composition, abundance, and diversity [29,55]. Community richness and diversity index (PD_whole_tree, Chao1, Observed_species, Shannon, and Simpson) and rarefaction curves were calculated in Mothur using the high-throughput sequencing data [33].
SPSS 20.0 (SPSS Inc., Chicago, IL, USA) software was employed to analysis the data. Canoco 5.0 software was employed to draw Canonical Correlation Analysis (CCA) diagram. One-way analysis of variance (ANOVA) was employed to examine the significant differences under the different sampling sites (soil physicochemical properties). The least significant difference (LSD) test was performed for the homogeneity of variance (p > 0.05).
3. Results and Analysis
3.1. Soil Physicochemical Properties and Heavy Metal Content
The effect of ecological restoration of the mining area on soil physicochemical properties is presented in Table 1. Soil physicochemical properties displayed significant differences across the four sites (p < 0.05) (except Total nitrogen). Soil organic carbon content, total phosphorus content, and total nitrogen content were related to soil fertility, soil phosphorus cycle, and soil nitrogen supply, respectively. The results showed that the contents of organic carbon, total nitrogen, and total phosphorus were 2.38–12.97 g/kg, 0.39–1.62 g/kg, and 0.99–1.51 g/kg, respectively. As can be seen from the table, the organic carbon content of each soil sample was UD > ER2 > ER1 > UR; the total nitrogen content was ER2 > UD > ER1 > UR; the total phosphorus content was ER2 > ER1 > UD > UR. The contents of total nitrogen and total phosphorus in the restored soil were higher than those in the undamaged area, which may be caused by the addition of nutrient species in the process of soil improvement. In addition, the soil moisture content of the undamaged area and ecological restoration area were higher than that of the unrestoration area. UR had the highest Cu and Cd contents in soil samples, which were 4.44 mg/kg and 0.51 mg/kg, respectively. Overall, UR had the lowest contents of organic carbon, total nitrogen, and total phosphorus, which may be due to heavy metal pollution affecting soil carbon, nitrogen, and phosphorus content.

Table 1.
Physicochemical properties and heavy metal content of soil samples. The data are presented with the mean ± SD of three replicates. Different letters indicate significant differences between the same indicators (p < 0.05).
3.2. Analysis of OTU Abundance and Species Community
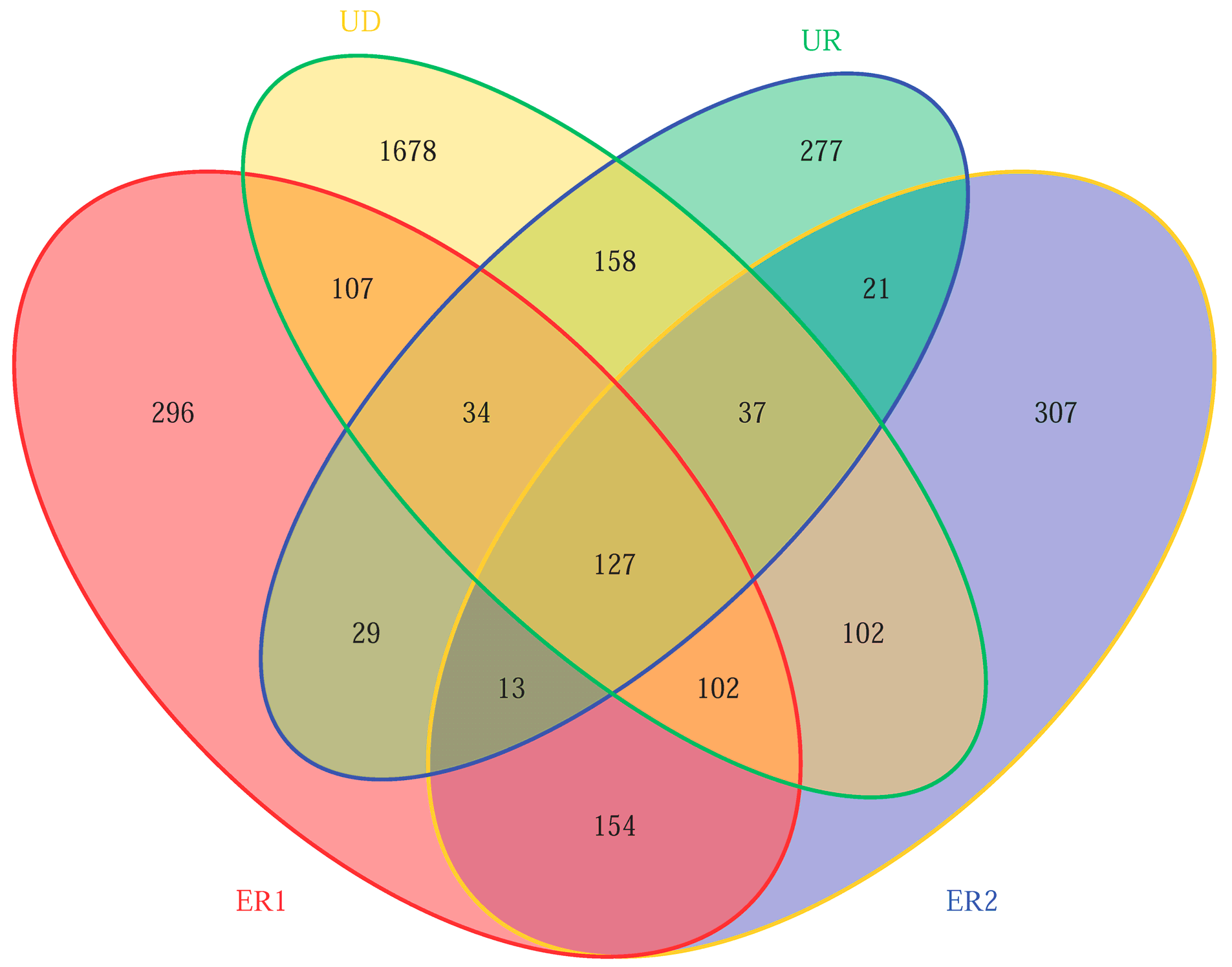
After filtering the low-quality sequences and chimeras, 305,165 high-quality sequences were retained from the integrated data set. A total of 5001 OTUs were detected according to 97% similarities. Results showed that the specific OTU numbers of ER1, ER2, UD, and UR were 296, 307, 1678, and 277, respectively, UD > ER2 > ER1 > UR, indicating that the soil microbial groups of virgin forest were the most abundant, while those of the unrestored soil after destruction were the least. The similarity of ER1 and ER2 microbial groups was high, indicating that there was no significant difference in microbial diversity in the restoration soil (Figure 2).
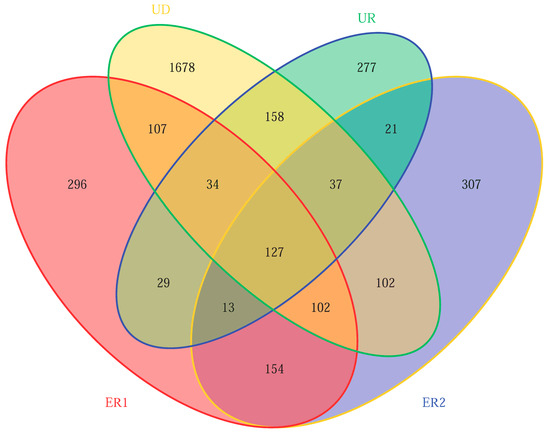
Figure 2.
Venn plot of microbial OTU quantity in soil samples.
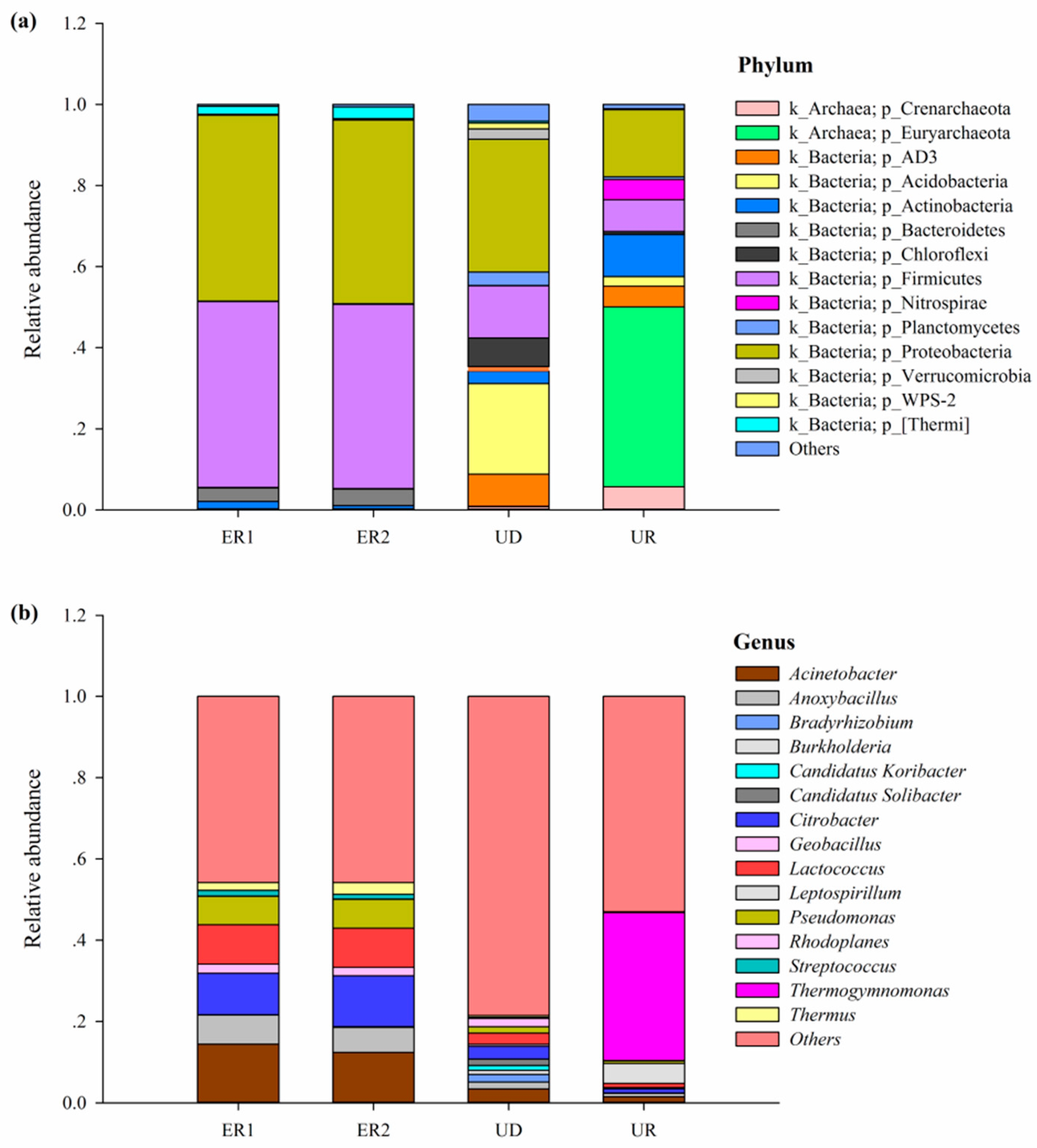
Sequence information was extracted from OTU according to the five levels of Phylum, Class, Order, Family, Genus, and species with relative abundance above 1% were selected to draw the relative abundance distribution map. Species with relative abundance less than 1% and classified as unclassified and unidentified were classified as others. The results showed that at the phylum level, all soil samples contained Firmicutes, Proteobacteria, and Actinobacteria. The relative abundances of Firmicutes and Proteobacteria of ER1 and ER2 were the highest, accounting for more than 45%, while Actinobacteria was the lowest. Among UR, Euryarchaeota had the highest abundance. The dominant phylum in UD were Proteobacteria and Acidobacteria, accounting for 32.7% and 22.3%, respectively. In general, the structural composition of microorganisms in UD and UR at the phylum level was more abundant than that of ER1 and ER2, while the species of ER1 and ER2 were consistent and the changes were not significant (Figure 3a).
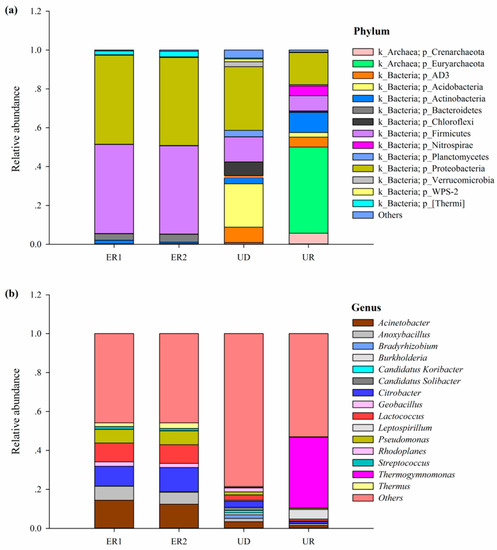
Figure 3.
Histogram of horizontal bacterial distribution in phylum (a) and genus (b).
According to the analysis of genus level species in each sample, all samples were distributed in 15 genera, which mainly included Acinetobacter, Anoxybacillus, Citrobacter, Lactococcus, and Pseudomonas. The highest abundance in UR was Thermogymnomonas, while Acinetobacter, Citrobacter, Lactococcus, Pseudomonas, and Anoxybacillus accounted for a large proportion in ER1 and ER2. In general, the composition of microorganisms in UD was the most abundant at genus level, and the species of ER1 and ER2 were consistent, while the species of UR were significantly different from those of ER1, ER2, and UD (Figure 3b).
3.3. Alpha Diversity Analysis
In the study of microbial diversity in soil, the abundance and diversity of microbial community can be reflected through the analysis of single sample diversity (Alpha diversity), including the Observed_species index, Chao1 index, Shannon index, Simpson index, and PD whole tree [56,57].
The results showed that the PD_Whole_tree, Chao1 and Observed_species of UD, ER1, ER2 were higher than UR, and Simpson index was lower than UR (Table 2), indicating that the diversity and richness of soil microorganisms in UD, ER1, ER2 were higher than UR, and soil pollution damaged the diversity and richness of microorganisms, which was consistent with most of the research results. Among them, the richness and diversity of UD were the highest, followed by ER1 and ER2, indicating that the richness and diversity of soil microorganisms after heavy metal contamination decreased compared with that of unpolluted soil microorganisms. In addition, the indexes of ER1 and ER2 did not change significantly, indicating that the diversity and richness of microbial communities in the two soils did not change significantly.

Table 2.
Index of the bacteria abundance and diversity in soil samples.
3.4. Beta Diversity Analysis
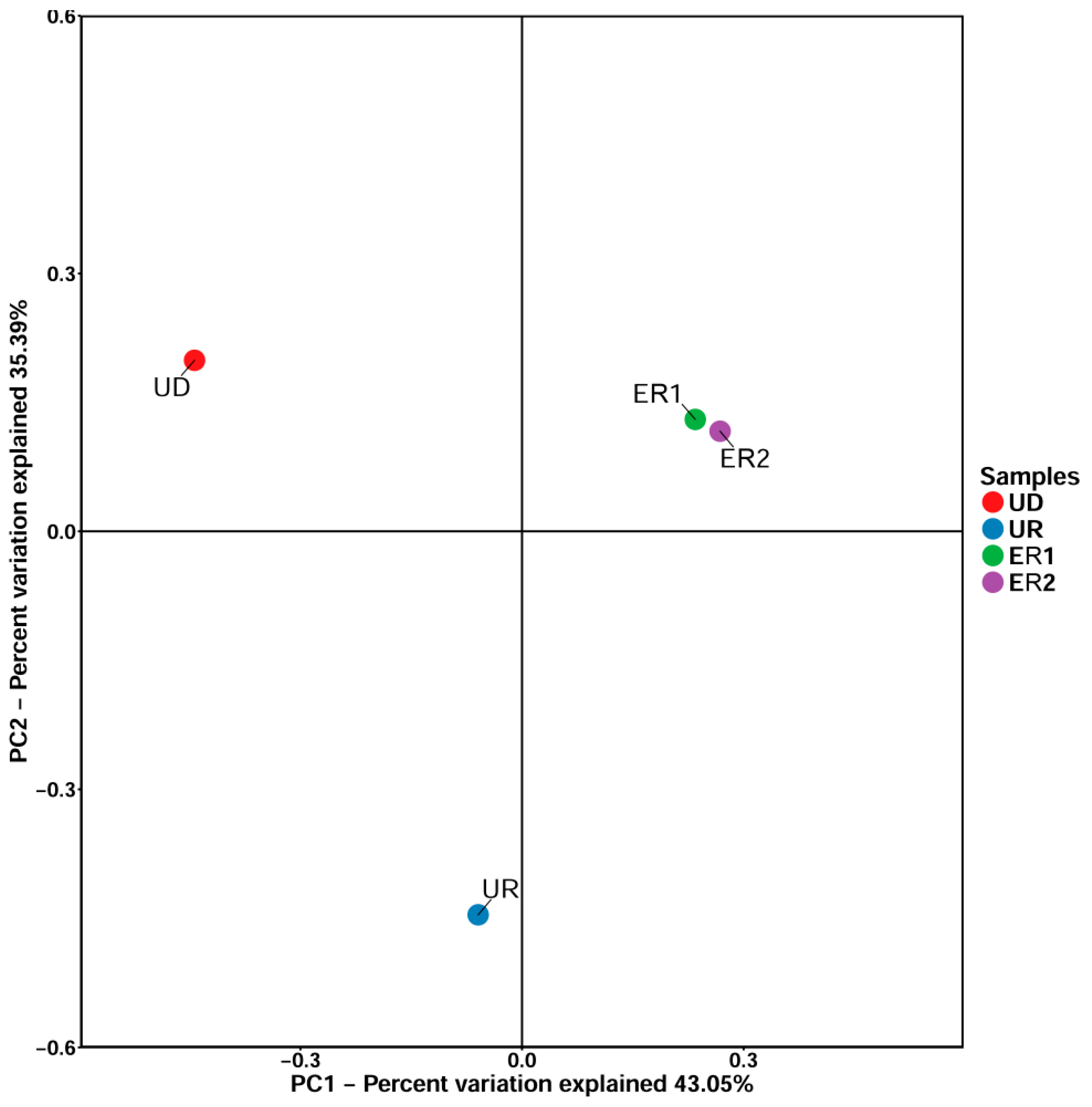
The principal coordinate analysis can be used to classify multiple samples to further display the diversity differences among samples. The closer the samples are on the coordinate map, the greater the similarity. The results of PCOA analysis showed that the principal component interpretation of PC1 and PC2 in soil samples were 43.05% and 35.39%, respectively (Figure 4). The coordinate distances of ER1 and ER2 were very similar, indicating that the similarity between the soil samples after restoration was obvious, and the diversity of microbial species among the groups was not significant. However, UD and UR coordinates were dispersed, indicating significant differences in microbial species composition and distribution among the groups, which might be due to heavy metal pollution that destroyed microbial diversity.
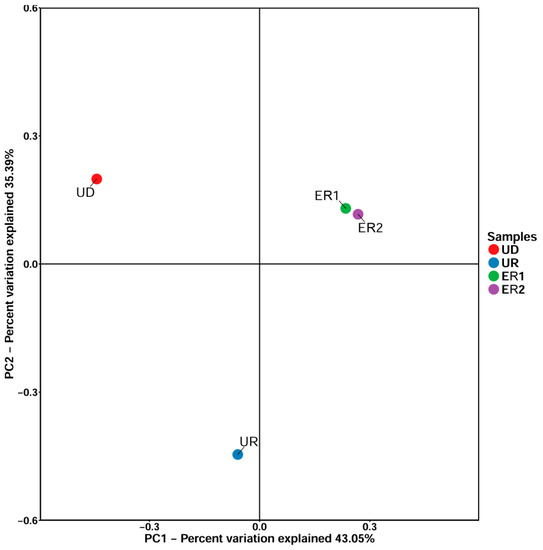
Figure 4.
PCOA analysis of soil samples.
3.5. Relationships between Bacterial Communities and Soil Physicochemical Properties
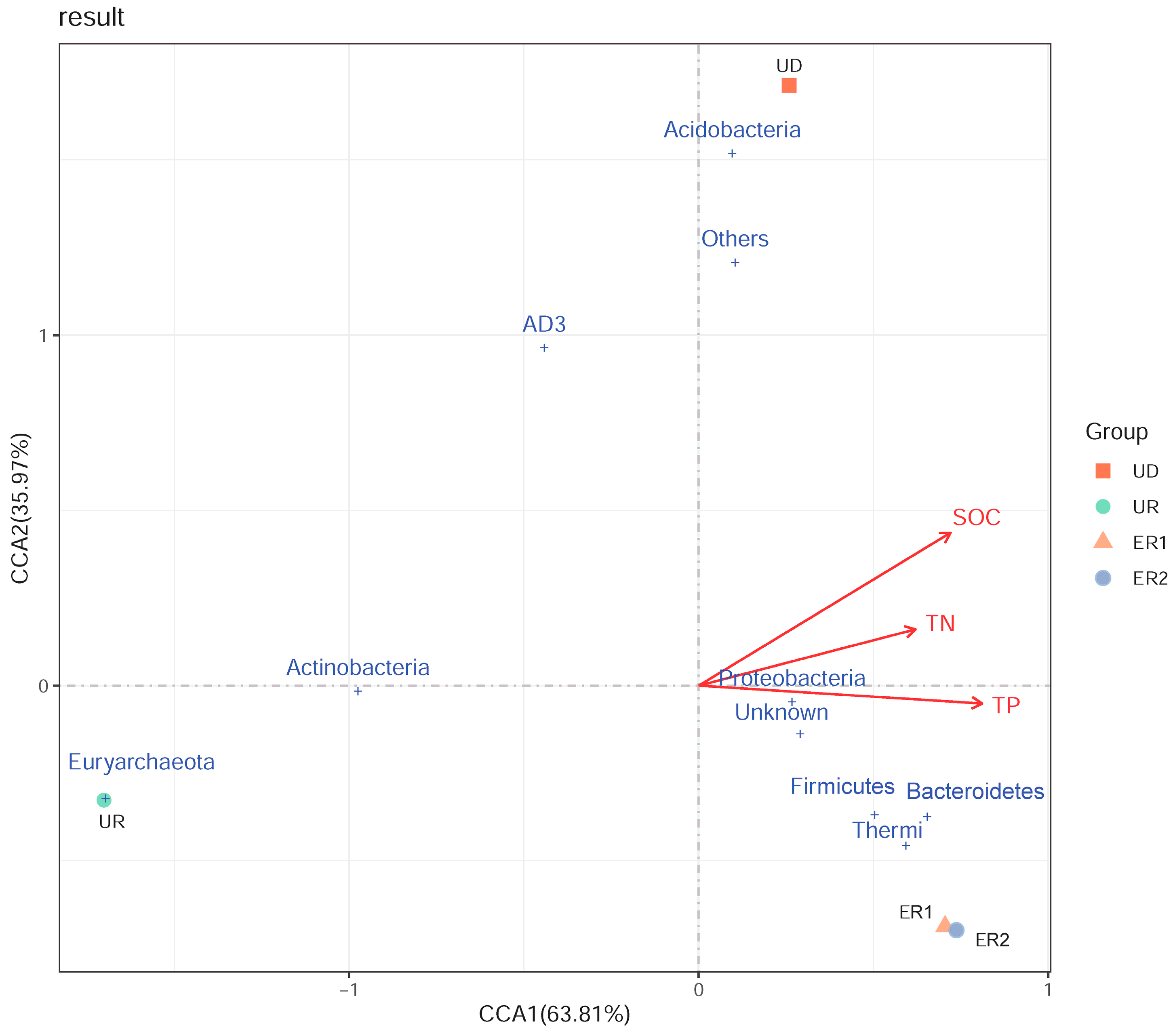
The correlation between bacterial communities’ structure and soil physicochemical properties was analyzed based on the dominant bacteria species at phylum level (Figure 5). The red arrows represent different environmental factors. The longer the ray of the environmental factor, the greater the influence of the factor. The angle between different physicochemical properties represents the correlation between the two factors, and the acute angle represents the positive correlation, while the obtuse angle represents the negative correlation. The results showed that the eigenvalues of the first and second axes are 63.81% and 35.97% respectively. The first two axes account for 99.78% of the total variation in the bacterial community. The CCA diagram clearly shows that SOC, TN, and TP were concentrated in the same direction. The environmental factor with the longest ray was SOC, indicating that SOC was the main factor leading to the difference of bacterial dominant groups, followed by TP. Proteobacteria, Firmicutes, and Bacteroidetes were highly correlated with SOC, TN, and TP. In addition, UD, ER1, and ER2 were positively correlated with environmental factors in the same direction, but negatively correlated with UR.
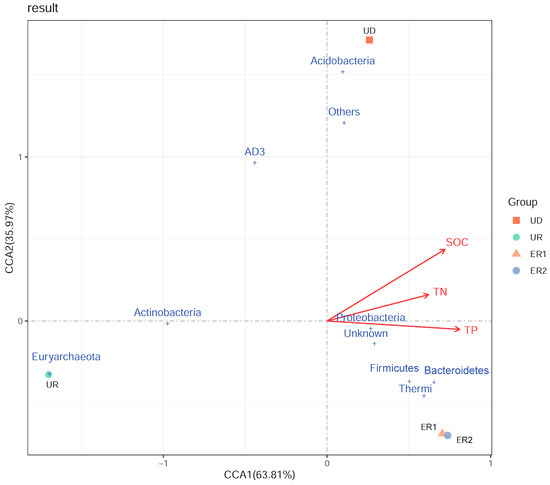
Figure 5.
CCA analysis of phylum horizontal bacterial species and physicochemical properties.
4. Discussion
Vegetation restoration is the key to ecological restoration in the mining area. Most natural ecosystem restoration is based on vegetation restoration [45]. Phytoremediation has been widely used in the ecological restoration of mining areas in recent years because of its advantages of economy, environmental friendliness, and lack of secondary pollution [58]. Due to the lack of nutrient elements and high toxicity of heavy metals in abandoned mining areas, soil amendments should be added to vegetation restoration [59]. Vegetation restoration fixed heavy metal ions through absorption, precipitation, chelation, complexation, and redox reaction to reduce their bioavailability and mobility [60]. The results showed that the heavy metal contents of UD, UR, ER1, and ER2 did not change significantly, indicating that there was no significant change in the content of heavy metals before and after vegetation restoration, which may be because heavy metals were fixed in the upper soil system and reduced the migration caused by wind erosion and water erosion. Soil amendments can form heavy metal organic complexes with heavy metal ions in the upper soil through absorption, precipitation, chelation, and redox reaction, thus reducing the availability of heavy metals [60,61]. ER1 and ER2 changed soil physicochemical properties and promoted the accumulation of nutrient elements and the decomposition of organic matter by adding soil amendments and vegetation restoration measures. In addition, the improvement of soil physicochemical properties directly affected the growth and activity of microorganisms [62]. In this study, the soil bacterial diversity of ER1 and ER2 increased, indicating that the ecological restoration of the mining area improved the physicochemical properties of the soil, and the content of nutrients in the soil increased, enabling more bacteria to adapt to the environment.
The diversity and composition of microbial communities are intricately related to soil physicochemical properties and different soil environments, and there are great differences in microbial diversity and species composition of different soils [63]. Organic carbon is the substrate and energy source of various physiological and biochemical processes of plants. Nitrogen and phosphorus are important components of organisms and also important limiting factors of soil productivity, affecting soil physicochemical properties [64]. Studies have shown that soil physicochemical properties such as organic carbon, total nitrogen, and total phosphorus are important conditions for the growth and development of soil microorganisms, which affect the community structure and function of soil microorganisms [65,66,67,68]. In this study, the contents of organic carbon, total nitrogen, and total phosphorus in different soil states were all higher than those in the unrestored soil after destruction, indicating that soil pollution affects the contents of carbon, nitrogen, and phosphorus, thus affecting soil microbial diversity and community structure, which is consistent with the results of previous studies [52,53]. Generally, soil moisture is an important factor regulating soil microbial community richness [33]. In this study, the soil moisture in the UR was the lowest, which may result in the soil microbial α-diversity index of unrestoration area being lower than that of ecological restoration area. Research showed that the soil moisture content in the abandoned mining areas was relatively low, which was unfavorable to plant growth [69]. In our research, the moisture content of the unrestoration area was lower than that of undamaged area and ecological restoration area, which was consistent with previous studies. Moreover, the moisture content in the ecological restoration area was higher than that in the undamaged area, indicating that ecological restoration is helpful to improve the moisture content of soil in mining area. In addition, in contaminated soil, microorganisms need more energy to survive under adverse conditions. Therefore, more energy was lost and less C, N, and P were composed of organic components [70]. Soil bacterial community structure was significantly correlated with soil physicochemical properties, resulting in two different growth condition in restored and unrestored soils [71].
Heavy metals have long-term effects on the community structure of microorganisms. Therefore, the response mechanism of microbial communities to heavy metals may be that heavy metal pollution to a certain extent changes the competitive relationship within and between the original populations, leading to the loss of the dominant role of the original dominant population [72,73], or the heavy metal resistance produced by some microbial products protects the microorganisms of some populations and changes the community diversity. In this study, the contents of Cu and Cd in UR were higher than those in UD, ER1, and ER2, the microbial diversity in UR decreased, and the dominant population also changed, indicating that heavy metals would indeed affect microbial diversity and inhibit microbial growth. Studies have shown that the decrease of microbial community diversity caused by heavy metal copper and chromium pollution can be compensated with the extension of time, though it will not be restored to the unpolluted state through this compensation effect, but generate new dominant populations [39].The results also showed that the dominant microorganisms in the repaired and unrepaired soils were different. At the same time, microbial diversity decreased with the increase of heavy metal content, which has been well proven in previous reports [74,75,76].
Soil is rich in microbial groups, which shows the complexity of soil microbial community structure [51]. The change of microbial community structure can be judged by Alpha diversity index and Beta diversity index [77]. In this study, the Alpha diversity index showed that the microbial diversity and richness of UD, ER1, and ER2 were higher than UR, indicating that the microbial diversity and richness of damaged unrestored soil decreased, which was consistent with other research results [53]. Beta diversity index showed that the coordinate distances of ER1 and ER2 were very similar, indicating that the similarity of soil samples after restoration was obvious among groups, and the diversity of microbial species among groups had little difference. Moreover, the values of ER1 and ER2 were higher than UR, indicating that mine restoration affected the microbial diversity of soil. In the process of soil investigation, it was found that UD was grew luxuriantly, was easy to grow, and developed roots and strong stress resistance, such as Pinus massoniana and Miscanthus floridulus. ER1 and ER2 were fast-growing, easy to survive, and strong stress-resistance plants, such as Cinnamomum camphora, Sesbania cannabina, Pinus elliottii, and Erigeron acer; following the principle of combination of vegetation and height, they increased the richness of species and formed rich plant communities. Previous studies have found that the development of plant communities can promote the development of microbial communities [78,79]. In this study, the microbial diversity of UD, ER1, and ER2 was higher than that of UR, indicating that plant community affected microbial diversity, and the development of plant communities were conducive to promoting the development of microbial communities. In addition, UD had the highest richness and diversity, followed by ER1 and ER2. The results showed that the factors affecting the composition and diversity of soil microbial community included natural factors and anthropogenic factors [51,80]. In the soil of the virgin forest formed under natural conditions, there are microbial communities specific to this environment [81]. The same is true of the soil microbial community structure after artificial restoration. In order to adapt to the impact of environmental damage, the resistance to adversity was enhanced special microbial community structure was formed [82,83,84]. At the phylum level, all soil samples contained Firmicutes, Proteobacteria, and Actinobacteria, which was consistent with the results of other soil microbial diversity studies [53,84] and indicates that these phylum are not exclusive to Dabaoshan mining area. Studies have shown that Proteobacteria was the dominant phylum in soil layers through microbiological analysis of antimony and arsenic contaminated soil [85]. At the same time, Proteobacteria was also the main dominant phylum of the leaf and rhizosphere bacterial communities of Aeusmus chinensis in the Tongwei mining area [86]. In this study, the structural composition of microorganisms in UD and UR at the phylum level was more abundant than that of ER1 and ER2, while the species categories of ER1 and ER2 were consistent and the change was not significant, indicating that the microorganisms in the damaged and unrestored soil still maintained a high diversity, which was consistent with previous studies [84]. Firmicutes and Proteobacteria of ER1 and ER2 were the highest in relative abundance compared with other samples, while Actinobacteria was the lowest in relative abundance. It can be seen that Firmicutes and Proteobacteria were the main active gates and representative gates of ecological restoration in the process of ecological restoration. Further study showed that there were corresponding rules at both the phylum level and the genus level.
CCA showed the relationship between relative abundance of dominant bacterial communities and soil physicochemical properties, indicating that the change of soil bacterial communities was responsive to the change of soil biochemical properties. The dominant bacterial communities (Proteobacteria, Firmicutes, Bacteroidetes) were significantly correlated with SOC, TN, and TP. The Actinobacteria in this study presented the opposite results to the Proteobacteria, with a higher abundance in the UD compared with the ecological restoration area. The Actinobacteria negatively correlated with all physicochemical properties, indicating that this phylum showed a negative response to Vegetation restoration. Acidobacteria were positively correlated with physicochemical properties and had a positive responsive to vegetation restoration. This could be because the Acidobacteria may be suitable for a weak acid environment and organic acids secreted by plant roots can reduce the pH value of soil, which is conducive to the growth of Acidobacteria [33]. These results indicated that there is a certain correlation between vegetation restoration and bacterial community structure change.
This study was only a case of Dabaoshan mining area, and the results contribute to a general understanding of the effects of the vegetation restoration; however, they cannot be considered general. In the future, a series of related studies should be conducted in the Dabaoshan mining area long term and under different periods of restoration to further explore the relationship between soil, vegetation, and microorganisms. At the same time, the characteristics of soil fungal community structure can be deeply discussed.
5. Conclusions
There is an intricate relationship between the diversity and composition of microbial community and soil physicochemical properties. The findings of this study suggest that vegetation restoration in the mining area of Dabaoshan can significantly improve OTUs, bacterial community richness, and diversity indices and soil physicochemical properties. In this study, the contents of organic carbon, total nitrogen, and total phosphorus in different soil states were positively correlated with microbial diversity, and the contents of carbon, nitrogen, and phosphorus in soil affected the microbial diversity and community structure. OTU cluster analysis and diversity index analysis showed that UD had the most abundant microbial groups, while ER1 and ER2 had little difference. CCA results showed that there was a strong correlation between bacterial community structure and physicochemical properties in ecological restoration areas (ER1 and ER2). SOC was the main factor leading to the difference of dominant bacterial communities, followed by TP. Different soil types had different microbial community characteristics, and the microbial community will have different responses to different soil environments. Restoration of contaminated soil can play a compensating role and generate new dominant populations. This study showed that Firmicutes and Proteobacteria were the main active phyla in the process of ecological restoration, and they were the representative phyla of ecological restoration. In general, this study offered some information on the diversity and community structure of soil microorganisms after ecological restoration in the Dabaoshan Mining area and demonstrated the advantages of high-throughput sequencing technology in the study of soil microbial communities. In the future, it is necessary to study the soil biochemical characteristics and microbial community characteristics in the vegetation restoration area of Dabaoshan mining area long term and under different periods of restoration, and to deeply explore the process and mechanism of vegetation restoration and its influence mechanism on soil microorganisms. At the same time, the characteristics of the soil fungal community structure can be deeply discussed.
Author Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by L.F. and Z.X.; L.F., W.Z. and W.F. were responsible for conceptualization; G.Y. and P.M. were responsible for methodology; Y.Z. was responsible for funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Key Projects of National Forestry and Grassland Bureau (201801), Natural Science Foundation of Hunan Province (2019JJ50027), Open Fund of Key Laboratory of Microbial Resources Collection and Preservation, Ministry of Agriculture and Rural Affairs (KLMRCP2021-07) and Experimental Demonstration Station (Base) Science and Technology Innovation and Achievement Transformation Project from Northwest Agriculture and Forestry University (TGZX2021-41).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All the related authors have known the informed consent.
Data Availability Statement
Data are publicly available with accession number BioProject ID PRJNA752830 and interested parties may contact the authors for more information.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Abbreviations
OTU: Operational Taxonomic Unit; ER, Ecological restoration area; UD, Undamaged area; UR, Unrestoration area; Cd, Cadmium; Pb, Plumbum; Zn, Zinc; Cu, Copper; Cr, Chromium; SOC, Organic carbon; TN, Total nitrogen; TP, Total phosphorus; CCA, Canonical Correlation Analysis; ANOVA, Analysis of variance; LSD, least significant difference; SD, standard deviation.
References
- Leng, W. Ecological damage status and common restoration technologies in Jinggong coal mine. Environ. Prot. Technol. 2019, 25, 47–54. [Google Scholar]
- Abdullah, M.; Fasola, M.; Muhammad, A.; Malik, S.A.; Bostan, N.; Bokhari, H.; Kamran, M.A.; Shafqat, M.N.; Alamdar, A.; Khan, M. Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures: A case study from severely contaminated areas. Chemosphere 2015, 119, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Jianqiao, Q.; Huarong, Z.; Xiuyu, Z. Microbial characteristics and heavy metal content in soil ecosystem of lead zinc mining area in northern Guangdong. J. Soil Water Conserv. 2012, 4, 221–225. [Google Scholar]
- Wenbo, Y.; Dan, L.; Danli, P. Progress in ecological treatment and environmental restoration technology of heavy metal mines. J. Zhejiang Agric. For. Univ. 2015, 32, 467–477. [Google Scholar]
- Cheng, H.; Huang, L.; Ma, P.; Shi, Y. Ecological Risk and Restoration Measures Relating to Heavy Metal Pollution in Industrial and Mining Wastelands. Int. J. Environ. Res. Public Health 2019, 16, 3985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, M.H. Ecological Restoration of Mine Degraded Soils, with Emphasis on Metal Contaminated Soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Liang, T.; Li, K.; Wang, L. State of rare earth elements in different environmental components in mining areas of China. Environ. Monit. Assess. 2014, 186, 1499–1513. [Google Scholar] [CrossRef]
- Min, C.; Dachao, Z.; Qingjiang, Z. Research progress on ecological restoration of abandoned land of ionic rare earth mines. Chin. J. Rare Earth 2017, 35, 461–468. [Google Scholar]
- Zhenya, W.; Xianping, L.; Jian, L. Progress in ecological restoration technology of abandoned land of rare earth mines in South China. Nonferrous Met. Sci. Eng. 2018, 9, 102–106. [Google Scholar]
- Liping, D. Impact of mining on ecological environment and ecological restoration of mining area—Taking Coal Mine as an example. Acad. Theory 2010, 18, 109–110. [Google Scholar]
- Yoshida, F.; Hata, A.; Tonegawa, H. Itai-Itai disease and the countermeasures against cadmium pollution by the Kamioka mine. Environ. Econ. Policy Stud. 1999, 2, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.; Yitai, C.; Shufeng, W. Accumulation and nutrient absorption of heavy metals Pb and Zn by 15 plants in abandoned tailings pond. Environ. Sci. 2012, 33, 2021–2027. [Google Scholar]
- Jiantao, L.; Hankui, Y. Ecological restoration technology of mine pollution. Hunan For. Sci. Technol. 2018, 45, 66–70. [Google Scholar]
- Chen, J.; Jiskani, I.M.; Jinliang, C.; Yan, H. Evaluation and future framework of green mine construction in China based on the DPSIR model. Sustain. Environ. Res. 2020, 30, 13. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, W.; Lu, X.; Jiskani, I.M.; Li, L. Evaluation Index System of Green Surface Mining in China. Miner. Metall. Process. 2020, 72, 45–46. [Google Scholar] [CrossRef]
- Jiskani, I.M.; Cai, Q.X.; Wei, Z.; Shah, S. Green and climate-smart mining: A framework to analyze open-pit mines for cleaner mineral production. Resour. Policy 2021, 71, 102007. [Google Scholar] [CrossRef]
- Jiskani, I.M.; Cai, Q.; Zhou, W.; Lu, X. Assessment of risks impeding sustainable mining in Pakistan using fuzzy synthetic evaluation. Resour. Policy 2020, 69, 101820. [Google Scholar] [CrossRef]
- Jiskani, I.M.; Shah, S.; Cai, Q.; Wei, Z.; Xiang, L. A multi-criteria based SWOT analysis of sustainable planning for mining and mineral industry in Pakistan. Arab. J. Geosci. 2020, 13, 1108. [Google Scholar] [CrossRef]
- Shoujun, Z. Research progress of ecological restoration in mining areas. Anhui Agric. Sci. 2013, 34, 276–277. [Google Scholar]
- Liu, Y.; Lei, S.; Gong, C. Comparison of plant and microbial communities between an artificial restoration and a natural restoration topsoil in coal mining subsidence area. Environ. Earth Sci. 2019, 78, 1–13. [Google Scholar] [CrossRef]
- Xin, Y.; Songlin, S.; Yangyang, G. Study on ecological impact and ecological restoration effect of Zijin mine. Environ. Ecol. 2019, 1, 84–90. [Google Scholar]
- Junfang, W. Study on Vegetation and Soil Characteristics of Ecological Restoration of Typical Open Pit Mines in Grassland Area of Inner Mongolia; Inner Mongolia Agricultural University: Hohhot, China, 2020. [Google Scholar]
- Cardoso, E.B.; Júnior, P.P.; de Cássia Soares da Silva, M.; Cerqueira, A.E.S.; Jordao, T.C.; Moreira, B.C.; Pereira, E.G.; Kasuya, M.C.M. Composition and diversity of prokaryotes at an iron ore post-mining site revealed the natural resilience 10 years after mining exploitation. Land Degrad. Dev. 2021, 32, 256–269. [Google Scholar] [CrossRef]
- Cui, J.-L.; Zhao, Y.-P.; Chan, T.-S.; Zhang, L.-L.; Tsang, D.C.W.; Li, X.-D. Spatial distribution and molecular speciation of copper in indigenous plants from contaminated mine sites: Implication for phytostabilization. J. Hazard. Mater. 2020, 381, 121208. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, M.R.; Neldner, V.J. Two-tiered methodology for the assessment and projection of mine vegetation rehabilitation against mine closure restoration goal. Ecol. Manag. Restor. 2015, 16, 215–223. [Google Scholar] [CrossRef]
- Ngugi, M.R.; Fechner, N.; Neldner, V.J.; Dennis, P.G. Successional dynamics of soil fungal diversity along a restoration chronosequence post-coal mining. Restor. Ecol. 2020, 28, 543–552. [Google Scholar] [CrossRef]
- Quideau, S.A.; Swallow, M.; Prescott, C.E.; Grayston, S.J.; Oh, S.-W. Comparing soil biogeochemical processes in novel and natural boreal forest ecosystems. Biogeosciences Discuss. 2013, 10, 7521–7548. [Google Scholar]
- Detheridge, A.P.; Comont, D.; Callaghan, T.M.; Bussell, J.; Brand, G.; Gwynn-Jones, D.; Scullion, J.; Griffith, G.W. Vegetation and edaphic factors influence rapid establishment of distinct fungal communities on former coal-spoil sites. Fungal Ecol. 2018, 33, 92–103. [Google Scholar] [CrossRef]
- Qiufang, W.; Lijiang, H.; Xinqiang, G.; Meiling, Z. High throughput sequencing analysis of microbial diversity in rhizosphere and non rhizosphere soil of beiai. J. Henan Agric. Univ. 2021, 2, 1–14. [Google Scholar]
- Wildman, H. Improving Mine Rehabilitation Success through Microbial Management. J. Environ. Solut. Oil Gas Min. 2014, 1, 32–46. [Google Scholar] [CrossRef]
- Yan, D.F.; Mills, J.G.; Gellie, N.; Bissett, A.; Lowe, A.J.; Breed, M.F. High-throughput eDNA monitoring of fungi to track functional recovery in ecological restoration. Biol. Conserv. 2018, 217, 113–120. [Google Scholar] [CrossRef]
- Gans, J.; Wolinsky, M.; Dunbar, J. Computational Improvements Reveal Great Bacterial Diversity and High Metal Toxicity in Soil. Science 2005, 309, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, X.; Hao, M.; Cui, Y.; Zhu, S.; Zhang, Y. Effects of Vegetation Restoration on Soil Bacterial Communities, Enzyme Activities, and Nutrients of Reconstructed Soil in a Mining Area on the Loess Plateau, China. Sustainability 2019, 11, 2295. [Google Scholar] [CrossRef] [Green Version]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA. 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Baquerizo, M.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Maestre, F.T. Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe. Ecol. Lett. 2017, 20, 1295–1305. [Google Scholar] [CrossRef] [Green Version]
- Jinbin, C.; Lin, L.; Jianhong, C. Application of microbial technology in mine ecological restoration. Energy Environ. 2020, 4, 102–103. [Google Scholar]
- Asensio, V.; Covelo, E.F.; Kandeler, E. Soil management of copper mine tailing soils—Sludge amendment and tree vegetation could improve biological soil quality. Sci. Total. Environ. 2013, 456–457, 82–90. [Google Scholar] [CrossRef]
- Van der Heyde, M.; Bunce, M.; Dixon, K.; Wardell-Johnson, G.; White, N.E.; Nevill, P. Changes in soil microbial communities in post mine ecological restoration: Implications for monitoring using high throughput DNA sequencing. Sci. Total. Environ. 2020, 749, 142262. [Google Scholar] [CrossRef]
- Shasha, X. Effect of Heavy Metal Pollution on Soil Microbial Community Structure in Mining Area; South China Agricultural University: Guangzhou, China, 2016. [Google Scholar]
- Xuexiu, C.; Xiaodong, S. Soil heavy metal pollution and food safety. Guide Environ. Sci. 2001, 20, 21–24. [Google Scholar]
- Chuxia, L.; Wenzhou, L.; Yonggui, W. Environmental impact of water discharge from Dabaoshan Mine. Agricultural ecosystem. J. Ecol. Environ. 2005, 14, 169–172. [Google Scholar]
- Yisheng, L.; Yi, G.; Kangwei, W. Etiological study of high incidence village of gastrointestinal malignancies in Guangdong. China Trop. Med. 2005, 5, 1139. [Google Scholar]
- Ingram, L.J.; Schuman, G.E.; Stahl, P.D.; Spackman, L.K. Microbial Respiration and Organic Carbon Indicate Nutrient Cycling Recovery in Reclaimed Soils. Soil Sci. Soc. Am. J. 2005, 69, 1737–1745. [Google Scholar] [CrossRef] [Green Version]
- Yuanyuan; Li; Hongyu; Wen; Longqian; Chen; Tingting; Yin, Succession of bacterial community structure and diversity in soil along a chronosequence of reclamation and re-vegetation on coal mine spoils in China. PLoS ONE 2014, 9, e115024.
- Shengxiang, Y. Ecological Restoration of Waste Dump of Dabaoshan Polymetallic Mine in Guangdong; Sun Yat-Sen University: Guangzhou, China, 2010. [Google Scholar]
- Huilu, C. Dabaoshan green is returning. Environment 2018, 476, 44–46. [Google Scholar]
- Yang, S.X.; Li, J.T.; Liao, B.; Zhang, J.T.; Shu, W.S. Effectiveness of amendments on re-acidification and heavy metal immobilization in an extremely acidic mine soil. J. Environ. Monit. JEM 2011, 13, 1849–2068. [Google Scholar] [CrossRef]
- Yang, S.X.; Liao, B.; Li, J.T.; Tao, G.; Shu, W.S. Acidification, heavy metal mobility and nutrient accumulation in the soil–plant system of a revegetated acid mine wasteland. Chemosphere 2010, 80, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Y.; Xu, Z.; Huang, H.; Yang, G. Morphological and Physiological Changes of Broussonetia papyrifera Seedlings in Cadmium Contaminated Soil. Plants 2020, 9, 1698. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Zhu, M.; Meadows, M.; Sun, L.; Wu, T.; Bu, X.; Xu, Y. Differential effects of various reclamation treatments on soil characteristics: An experimental study of newly reclaimed tidal mudflats on the east China coast. Sci. Total. Environ. 2021, 768, 144–996. [Google Scholar] [CrossRef] [PubMed]
- Shiquan, N.; Yang, L. Application of illuminamiseq high-throughput sequencing technology to analyze microbial diversity of saline alkali soil in Hexi Corridor. Microbiol. Bull. 2017, 9, 63–74. [Google Scholar]
- Zhang, F.-P.; Li, C.-F.; Tong, L.-G.; Yue, L.-X.; Li, P.; Ciren, Y.-J.; Cao, C.-G. Response of microbial characteristics to heavy metal pollution of mining soils in central Tibet, China. Appl. Soil Ecol. 2010, 45, 144–151. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, W.; Yang, Y.; Ma, J.; Li, S.; Wen, Z. Analysis of Soil and Microbial Characteristics and Microbial Response in Rare Earth Mining Areas in Jiangxi Province, China. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.; Lesniewski, R.A.; Oakley, B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 1471–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, W.X.C. Microbial Community Structure and Functional Diversity in Rhizosphere Soil of Three Plants in Fengfeng Mining Area; Hebei University: Baoding, China, 2019. [Google Scholar]
- Yanhui, D.; Yuchuan, W.; Qiufen, C. Microbial diversity in rhizosphere soil of continuous cropping quinoa based on high-throughput sequencing. Acta Agric. Boreali Sin. 2019, 34, 205–211. [Google Scholar]
- Farbo, M.G.; Urgeghe, P.P.; Fiori, S.; Marceddu, S.; Jaoua, S.; Migheli, Q. Adsorption of ochratoxin A from grape juice by yeast cells immobilised in calcium alginate beads. Int. J. Food Microbiol. 2016, 217, 29–34. [Google Scholar] [CrossRef]
- Li, M.S. Ecological restoration of mineland with particular reference to the metalliferous mine wasteland in China: A review of research and practice. Sci. Total Environ. 2006, 357, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Adriano, D.C.; Wenzel, W.W.; Vangronsveld, J.; Bolan, N.S. Role of assisted natural remediation in environmental cleanup. Geoderma 2004, 122, 121–142. [Google Scholar] [CrossRef]
- Bolan, N.S.; Duraisamy, V.P. Role of inorganic and organic soil amendments on immobilisation and phytoavailability of heavy metals: A review involving specific case studies. Aust. J. Soil Res. 2003, 41, 533–555. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Luan, T.; Jin, J.; Lan, C. Structure and function of microbial communities during the early stages of revegetation of barren soils in the vicinity of a Pb/Zn Smelter. Geoderma 2007, 136, 555–565. [Google Scholar] [CrossRef]
- Lei, Z.; Yusen, L.; Xiande, H. Spatial distribution of bacterioplankton community and its relationship with environmental factors in hongchaojiang reservoir. Acta Microbiol. Sin. 2020, 60, 2253–2264. [Google Scholar]
- Yajuan, M. Effects of Fertilization on Nutrient Absorption Characteristics and Ecological Stoichiometry of Carbon, Nitrogen and Phosphorus in Chinese Fir; Northwest University of Agriculture and Forestry Science and Technology: Yangling, China, 2015. [Google Scholar]
- Gao, T.; Wan, Z.; Liu, X.; Fu, J. Effects of heavy metals on bacterial community structure in the rhizosphere of Salsola collina and bulk soil in the Jinchuan mining area. Geomicrobiol. J. 2021, 38, 620–630. [Google Scholar] [CrossRef]
- Delong, S.; Fengming, C.; Li, L. Development status and Prospect of bio organic fertilizer in China. Chin. Soil Fertil. 2007, 6, 1–5. [Google Scholar]
- Shanshan, L. Mechanism Analysis of Farmland Soil Microbial Response to Climate Change Based on Metagenetics; Tsinghua University: Beijing, China, 2014. [Google Scholar]
- Shun, L.; Zhenhua, W.; Xiaomin, G. Characteristics of soil microbial community structure in different forest ages of Larix chenshanensis. J. Appl. Environ. Biol. 2016, 22, 510–517. [Google Scholar]
- Xuna, L. Study on Soil Characteristics and Vegetation Restoration of Abandoned Land in Mining Area; Inner Mongolia University: Hohhot, China, 2019. [Google Scholar]
- Mikanova, O. Effects of heavy metals on some soil biological parameters. J. Geochem. Explor. 2006, 88, 220–223. [Google Scholar] [CrossRef]
- Berbel-Rodríguez, N.; Soria, R.; Ortega, R.; Bastia, F.; Miralles, I. Quarry restoration treatments from recycled waste modify the physicochemical soil properties, composition and activity of bacterial communities and priming effect in semi-arid areas. Sci. Total Environ. 2021, 774, 145693. [Google Scholar] [CrossRef] [PubMed]
- Zhenggang, X.; Yunlin, Z.; Xiaomei, Z.; Yongli, X. Research Progress on effects of heavy metal pollution on soil microorganisms. J. Jiangxi Agric. 2014, 26, 53–55. [Google Scholar]
- Hui, K. Effects of Lead Zinc Tailings on Soil Microbial Community Structure and Diversity of Ligustrum Lucidum; Central South University of Forestry Science and Technology: Changsha, China, 2020. [Google Scholar]
- Renella, G.; Ortigoza, A.; Landi, L.; Nannipieri, P. Additive effects of copper and zinc on cadmium toxicity on phosphatase activities and ATP content of soil as estimated by the ecological dose (ED50). Soil Biol. Biochem. 2003, 35, 1203–1210. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.W.; Zhen-Cheng, S.U.; Zhang, C.G. Soil Microbial Characteristics Under Long-Term Heavy Metal Stress: A Case Study in Zhangshi Wastewater Irrigation Area, Shenyang. Pedosphere 2008, 18, 1–10. [Google Scholar] [CrossRef]
- Papa, S.; Bartoli, G.; Pellegrino, A.; Fioretto, A. Microbial activities and trace element contents in an urban soil. Environ. Monit. Assess. 2010, 165, 193–203. [Google Scholar] [CrossRef]
- Wei, Z.; Yu, D. Analysis of the succession of structure of the bacteria community in soil from long-term continuous cotton cropping in Xinjiang using high-throughput sequencing. Arch. Microbiol. 2018, 200, 653–662. [Google Scholar] [CrossRef]
- Pepper, I.L.; Zerzghi, H.G.; Bengson, S.A.; Iker, B.C.; Banerjee, M.J.; Brooks, J.P. Bacterial populations within copper mine tailings: Long-term effects of amendment with Class A biosolids. J. Appl. Microbiol. 2012, 113, 569–577. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Bond, P.L.; Lu, Y.; Vink, S. Bacterial diversity in response to direct revegetation in the Pb–Zn–Cu tailings under subtropical and semi-arid conditions. Ecol. Eng. 2014, 68, 233–240. [Google Scholar] [CrossRef]
- Hongjie, C.; Hongwei, N. Research progress on soil microbial diversity and its influencing factors. Land Nat. Resour. Res. 2015, 3, 85–88. [Google Scholar]
- Zahran, H.H. Diversity, adaptation and activity of the bacterial flora in saline environments. Biol. Fertil. Soils 1997, 25, 211–223. [Google Scholar] [CrossRef]
- Prasenjit, B.; Sumathi, S. Uptake of chromium by Aspergillus foetidus. J. Mater. Cycles Waste Manag. 2005, 7, 88–92. [Google Scholar] [CrossRef]
- Xiangwei, Z.; Yongming, L. Study on genetic diversity of microbial community in Heavy Metal Contaminated Farmland Soil. J. Environ. Sci. 2005, 2, 186–191. [Google Scholar]
- Jianhua, G.; Huaping, L.; Honghui, Z. Analysis of dominant population of microbial community in heavy metal contaminated soil in Dabaoshan. J. South China Agric. Univ. 2010, 3, 56–60. [Google Scholar]
- Bocong, H.; Jian, L.; Lingfei, L. Distribution and correlation of antimony and arsenic forms and bacterial community structure in vertical profile of paddy soil around antimony mine. J. Environ. Sci. 2019, 39, 1274–1283. [Google Scholar]
- Tong, J.; Yushan, Y.; Ruihong, W. Characteristics of bacterial community in the rhizosphere and leaf of Leymus chinensis. Environ. Sci. 2020, 41, 417–424. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).