Abstract
Growth in cattle population is associated with increased manure generation whose current management in low-income countries is associated with health and environmental problems as well as low utilization rates. This trend can be reversed by promoting better manure management technologies. This study assessed vermicomposting as one of the technologies to manage organic wastes, using the case study in Uganda. A vermicomposting system using cattle manure and earthworms (Eudrilus euginea) was monitored for one year with the harvesting of products (compost, earthworm biomass) after every three months. Vermicompost samples from the beginning of the experiment and after every harvest were analyzed for the following parameters: pH, ash content, volatile and total solids, nutrients N, P, K, and C. Emissions of CO2, CH4, NH3, and N2O were also measured. Material flow analysis was used to determine the flows and retention of nutrients within the system. Results showed that total solids, ash, N, P, and K content significantly increased, while contents of volatile solids and C, as well as the pH, significantly decreased over time. Of the materials that entered the vermicomposting system, 46% went to vermicompost, 2% into earthworms, and 52% was lost to the atmosphere. Substance flow analysis showed that 30% of C went to vermicompost, 69% was emitted to the atmosphere, and 2% ended up in earthworms while 75% of N was transferred to vermicompost, 7% went to earthworms, and 18% escaped into the atmosphere. The cumulative emissions were 102 g CO2 kg−1 waste, 7.6 g CH4 kg−1 waste, and 3.943 × 10−5 g N2O kg−1 waste on a dry basis, while NH3 was not detected throughout the measurement time. Compared to other manure management methods, vermicomposting demonstrated good potential in conserving nutrients as well as reducing greenhouse gas emissions.
1. Introduction
Livestock is amongst the fastest growing agricultural subsectors in low and medium income (LMI) countries [1]. This growth can be attributed to high demand for animal protein as a result of population increase and improvements in the economic wellbeing of the citizens of these countries [2]. Cattle farming, in particular, is of great importance across the globe. Taking the example of Uganda, cattle population is growing at a rate of 3% per annum [3,4] leading to increased production of cattle manure. Cattle manure has many uses; it improves soil properties, provides crop nutrients, and acts as a source of energy and construction material [2]. However, studies from Uganda show that many households, particularly in urban and peri-urban areas, do not use this manure as fertilizer but solely discard it [5]. This poses a threat to both human health and the environment. According to Teenstra et al. [2], improper manure management contributes up to 10% of the total livestock emissions. This, therefore, calls for appropriate manure management strategies that reduce negative impacts on the environment [6,7].
Vermicomposting, which is the biodegradation of organic wastes using earthworms and microorganisms [8,9,10], has been reported as an effective and relatively inexpensive cattle manure management technology [11,12]. The technology is also considered a bioecologically sustainable process in which renewable biological resources with an added value are produced [13]. In this aspect, vermicompost, a plant-nutrient-rich product [14], and earthworm biomass that can be used as animal, poultry or aquaculture feed, are produced. Vermicomposting takes place in aerobic environments. In the process, carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), ammonia (NH3), and other volatile substances are emitted by microbes [15]. Such emissions are detrimental to the environment by contributing to global warming, eutrophication, and acidification of ecosystems [16]. Quantifying these emissions aids in developing emissions mitigation measures [17] as well as improving the accuracy of the parameters required for compost emission models [18]. These models, together with life cycle assessment, can be useful tools in analyzing and reporting the environmental performance of processes [19]. Gaseous emissions from vermicomposting of various substrates, such as sewage sludge [20], agricultural wastes [21], vegetable waste, cattle manure, sawdust [22], duck manure [23], fruit and vegetable home leftovers [24], woodchips [25], sheep manure [26], and household wastes [27], have been looked at before. In Uganda, gaseous emissions (CH4, N2O, and NH3) from vermicomposting systems using cattle manure as a substrate were analyzed by Komakech et al. [28]. However, in their study, emissions were measured only once throughout the course of a single vermicomposting cycle. Therefore, no information on the variation in emissions at different stages of vermicomposting was obtained. This study was, therefore, done to address this gap.
Effective waste management cannot be achieved without a thorough knowledge of the material flows into, within, and out of a waste management system and its processes [29]. A method to achieve this objective is material flow analysis (MFA), which has been applied to analyze processes such as composting facilities [30,31], water and wastewater treatment [32,33], and combined anaerobic and pre-composting of household wastes [34]. However, there is scanty information on MFAs of vermicomposting systems. Therefore, a vermicomposting experiment was performed, analyzed, and validated through MFA. Specifically, the variation of physio-chemical parameters and gaseous emissions in the vermicomposting units over time was monitored. The novelty of the study is represented by the approach used in capturing the variation of key parameters during the vermicomposting period. Results can guide farmers and agronomists on how to properly monitor their vermicomposting systems and the expected nutrient compositions at different vermicomposting stages. On the other hand, waste managers, environmentalists, and policymakers can be informed with the results of emissions and nutrient distributions for better environmental management.
2. Materials and Methods
2.1. Experiments
2.1.1. Experimental Setup
The vermicomposting units were set up at Makerere University Agricultural Research Institute Kabanyolo (MUARIK) (0°28′06′′ N, 32°36′24′′ E) which is about 15 km from the city center of Kampala, Uganda. The cattle manure used in the vermicomposting units was obtained from the dairy farm at MUARIK. Vermicomposting units were constructed following the specifications by Lalander et al. [35] as follows: Three units (for replication purposes) made from wooden pallet frames from Albizia coriaria tree (local name Mugavu), each unit with a base pallet, two support pallets and a top pallet (Figure 1) of uniform dimensions (1 m × 1 m × 0.3 m). The base pallet consisted of netting at the bottom to prevent rodents from entering, and it was filled with bedding material to provide favorable conditions for the earthworms. The top pallet was also covered by a netting together with a sisal sack to limit the amount of light entering the units.
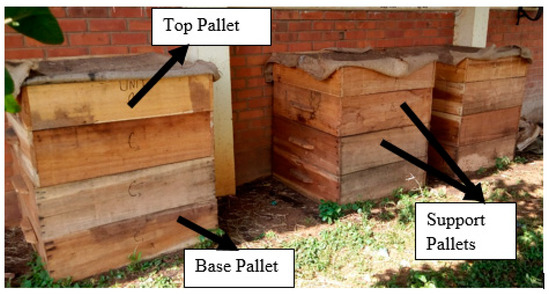
Figure 1.
Vermicompost experimental setup.
One kg of earthworms (Eudrilus euginea) was introduced into each vermicomposting unit to provide an initial stocking density of 1 kg/m2. Then, into each unit, 10 kg of cattle manure (stored under shade for one week to reduce both its ammonia content and temperatures) was added. The feeding frequency of cattle manure into the units was dependent on the consumption rate of the earthworms. The moisture content within the vermicomposting units was maintained at 60% to 70% by sprinkling water into the units. The experiment ran continuously for a year (December 2017–December 2018), harvesting the products after every three months (12 weeks). Hence, the experiment covered four runs (R1, R2, R3, and R4), each run lasting three months (12 weeks). The period of three months is suggested by Mainoo et al. [36] and Yadav et al. [37] to be enough for the stabilization of vermicompost. The initial stock of earthworms that was used for starting the process was obtained from a vendor. The amount of cattle manure and water added was recorded.
2.1.2. Sample Handling and Chemical Analysis
All solid and liquid inputs (manure, worms, water) and outputs (vermicompost, worms) were measured directly by weighing using a GLOBE digital weighing scale model 821–018 (Adam Equipment Inc., Oxford, UK). For each run, the physio-chemical properties of cattle manure were measured at the start of the experiment and after every month (four weeks) until the end of the experiment. Five samples were randomly taken and mixed to form a composite sample. The composite samples were then packed in dry plastic zip lock bags and delivered to the laboratory for analysis. The physio-chemical properties analyzed were pH, total solids (TS), ash content, volatile solids (VS), total organic carbon (TOC), total Kjeldahl nitrogen (TKN), total phosphorous (TP) and total potassium (TK). These were determined at the soil laboratory of the School of Agricultural Sciences at the College of Agricultural and Environmental Sciences, Makerere University.
pH was determined by dissolving and stirring 10 g of a sample in distilled water and using a digital pH meter model HI 96107 (Hanna Instruments, Padova, Italy) to indicate the pH of the solution after standing for one hour. TS was determined as the remaining weight after oven heating 10 g of a sample at 105 °C for 16 h [38]. Ash content was determined as the remaining weight after heating 5 g of the oven-dried sample in a muffle furnace at 550 °C for 6 h [39]. VS was taken as the lost weight after determining the ash content. TKN was determined following procedures as specified by Okalebo et al. [40]. TOC was determined according to the method by Walkley and Black [41]. The method involved adding 5.0 and 7.5 mL of aqueous potassium dichromate (K2Cr2O7) and sulfuric acid (H2SO4), respectively, to 0.5 g of the prepared sample in a block digester tube. This was subsequently placed in a pre-heated block at 145 °C to 155 °C for 0.5 h. After cooling, the digestate was titrated with ferrous ammonium sulfate solution until a color change from green to brown was attained. The amount of K2Cr2O7 consumed during the chemical reaction indicated the total organic carbon content of the feedstock. TK was determined using a flame photometer after digesting an oven-dried sample for 2 h at 360 °C, while TP was determined by an Atomic Absorption Spectrophotometer using procedures from Okalebo et al. [40]. C/N ratio was obtained by dividing the total organic carbon composition of the samples by their nitrogen content.
2.1.3. Emission Measurement
Emissions of carbon-dioxide (CO2), methane (CH4), ammonia (NH3), and nitrous oxide (N2O) were measured using the static chamber method [23]. A cylindrical metallic drum with a height of 60 cm and an open bottom of diameter 57.5 cm was obtained and used as a chamber. On top of this drum, a circular hole of about 5 mm diameter was drilled and used as the port for gas sampling. During gas sampling, the drum was inserted about 2 cm deep into the surface of the vermicompost in the units. The port was then tightly sealed using duct tape to avoid any gas leakage. After 30 min, the gas sample concentration (C) was taken. A period of 30 minutes is reported to give more precise results compared to the results of longer durations [27]. The concentration of the gas (C) was used to calculate its emission rate, according to Chan et al. [27] using Equations (1)–(3).
where Er = emission rate (mg h−1 m−2), C = concentration of the gas (ppm), MW = molecular weight of the gas, V = volume of the air above the waste surface inside the drum (m3), 0.08206 = ideal gas constant, T = temperature (K), Af = area of the waste surface inside the drum (m2), D = duration of sampling (h), Efd = emission factor per day (g Mg−1 d−1), Av = total area of the vermicomposting unit (m2), Mv = total mass of the waste in the vermicomposting unit (Mg), Ef = emission factor (g Mg−1), P = vermicomposting period (d).
Gas samples were taken between 1200 and 1400 h since this time range comes with moderate temperatures and hence, is suggested as representative of the entire day [42]. Gas concentrations for CO2, NH3, and CH4 were analyzed on-site from each experimental unit using a Geotech gas analyzer model GA5000 (Italy). For N2O, gas samples were taken using 60 mL syringes and brought to the laboratory at the Department of Chemistry, College of Natural and Applied Sciences, Makerere University, Kampala, Uganda. Samples were then analyzed using a Shimadzu GC-2030 gas chromatograph (Japan) following procedures as specified by Ermolaev et al. [15]. Gas measurement was done at the end of every fourth week (week 4, 8, and 12) for the four runs (R1, R2, R3, and R4). Gaseous emissions were then summed up to give the cumulative emissions during the process of vermicomposting [23]. From the cumulative emissions, global warming potential (GWP) of the vermicomposting system was calculated using environmental factors of 1, 28, and 265 for CO2, CH4, and N2O, respectively [43].
2.2. Statistical Analysis
The variation of physio-chemical properties of the vermicompost within runs and over time (week of measurement) was analyzed using two-way ANOVA. In addition, the differences in runs and the effect of the week of measurement on emission rates of different gases were also assessed using a two-way ANOVA with GenStat (14th edition) software. Statistical tests were considered significant at p < 0.05. If variation in effects was significant, Tukey test was used to determine the differences in means amongst the runs.
2.3. MFA Modelling
Results from chemical analysis and weight measurement were used to perform a MFA of the studied vermicomposting system. A MFA is the spatial and temporal systematic assessment of flows and stocks of materials within a system or process [29]. In this study, a MFA was used to indicate mass and nutrient distribution/retention within the vermicomposting system. This was executed using STAN software. Within STAN, substance flows were determined by multiplying measured substance concentrations by the corresponding material flows. Ash content, TP, and TK were taken as representative of non-volatile substances [34]. Hence, their loss into the atmosphere was assumed to be zero [34,44]. TS, VS, TOC, and TKN were considered volatile, and their loss to the atmosphere was determined by STAN software. Standard errors of all measured parameters were used as uncertainties in the software. Carbon and nutrient concentrations in water were assumed to be zero in all cases while those of earthworms were based on literature values [35,45,46]. A temporal boundary of three months was chosen, thereby corresponding to the duration of one harvesting period during the experiments. The spatial boundary (Figure 2) was limited to one vermicomposting unit where consumption of manure by earthworms took place. All mass/substance flows within the study were based on a wet weight basis and were expressed in kg/a.
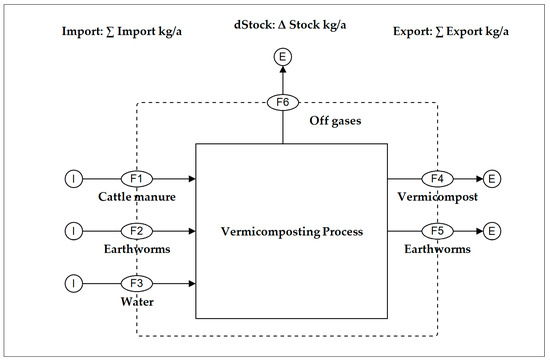
Figure 2.
STAN illustration of the analyzed vermicomposting system.
3. Results and Discussions
3.1. Material Inflows and Outflows from the Vermicomposting System
The material flows in (cattle manure and water) and out (vermicompost and earthworm biomass) of the vermicomposting process are shown in Table 1 for the different runs. For each run, one kg of earthworms was initially added at the beginning of the vermicomposting process. Earthworm mass had increased by a factor of 5.7 to 7.4 for the different runs at the end of the experiment. The amount of cattle manure added to each run every week was increased up to week 9 due to the growth in the worm population. However, the added amount was deliberately reduced in the 10th week of the experiment to allow ample time for the complete consumption of all manure and for the harvest of a homogenous product at the end of week 12. The earthworm biomass inoculum for R1 which had previously been reared in a banana plantation, required about one week to acclimatize to the new environment. This thus explains why no manure and water were added in week two for R1, unlike in the other runs. For all runs, the water added each week varied depending on the moisture content within the vermicomposting units and weather conditions of the day.

Table 1.
Material inflow and outflow of the vermicomposting system for the four runs.
The harvested vermicompost and earthworm biomass were both significantly different (p < 0.05) across the four runs (Table 1). For all four runs, the amount of harvested vermicompost was about 52% less than the added cattle manure. This is in agreement with Lalander et al. [35] who reported 46% material reduction during vermicomposting of cattle manure and food waste. The weight reduction may be attributed to degradation of cattle manure by earthworms, as reported by Ndegwa and Thompson [47].
3.2. Physio-Chemical Characteristics
The mean variation of physio-chemical parameters over time for the four runs is presented in Table 2. Differences in physio-chemical properties were significant (p < 0.05) between weeks of measurement but not significant (p > 0.05) between runs. There was also no significant (p > 0.05) interaction between runs and weeks of measurement. The initial characteristics of cattle manure added to the vermicomposting units are presented at week 0 while those of the harvested vermicompost are presented at week 12. From Table 2, it can be observed that pH, TOC, VS, and C/N ratio significantly decreased (p < 0.05) while TS, ash content, TKN, TP, and TK significantly increased (p < 0.05) over time.

Table 2.
Variation of physio-chemical parameters with vermicomposting time.
The overall decrease in pH was about 20% which is more than the 15% and 14% reduction reported by Yadav et al. [37] and Bhat et al. [48], respectively, during vermicomposting of cow dung. A fall in pH during vermicomposting may be attributed to mineralization of phosphorus and nitrogen compounds and production of fulvic and humic acids [47].
The overall increase in TS content was about 41%. This is in agreement with Lalander et al. [35] who reported a 50% increase in TS content during vermicomposting of cattle manure and food waste. An increasing TS content (reduction of moisture) is indicative of the progress in composting [49], which may be attributed to a high consumption rate of cattle manure by earthworms, thereby making it friable [50].
There was a 20% total decrease in VS content from week 0 to week 12. This decrease is lower than the 58% reported by Lalander et al. [35] during vermicomposting of cow manure and the 33% reported by Varma et al. [51] during vermicomposting of water hyacinth and cow manure. A reduction in VS is attributed to the bioconversion of waste material by earthworms, and it indicates the degree of maturity of the product [47]. This thus shows that the harvested vermicompost for this study was matured.
The overall decrease in TOC was about 41%. This was higher than the 36% and 21% reduction reported by Yadav et al. [37] and Xie et al. [52], respectively, during vermicomposting of cow dung. The decrease in the amount of carbon may be attributed to its role as an energy source for both earthworms and microorganisms [53].
TKN in vermicompost was about 48% more than that of initial cattle manure (Table 2). This was comparable to the 49% increase reported by Bhat et al. [48] but lower than the 237% reported by Yadav et al. [37] during vermicomposting of cattle dung. Increase in total nitrogen content of vermicompost may be attributed to earthworm activity, such as the addition of mucus, growth-stimulating hormones, nitrogenous excretory substances, and microbe mediated transformations [48]. The further increase in nitrogen content in the last weeks of the experiment (Table 2) could be attributed to decomposing earthworms as proposed by Atiyeh et al. [54]. Furthermore, an increase in nitrogen content over time may also be attributed to a decreasing pH since nitrogen is easily lost as volatile ammonia at high pH values [55].
This study established an overall C/N ratio reduction of about 60%. This is lower than the 81% but greater than the 35% and 25% reduction reported by Yadav et al. [37], Taeporamaysamai and Ratanatamskul [56], and Xie et al. [52], respectively during vermicomposting of cow dung. A fall in C/N is associated with a loss of carbon as well as an increase in TKN. C/N ratio is an indicator for the maturity of compost/vermicompost [57] and usually values <12 signify compost maturity and stabilization [58]. Hence, it is indicated from the C/N ratio of 10 obtained in week 12 that the vermicompost at the end of the experiments reached full maturity (Table 2).
TP registered an overall increase of about 120%. This is more than the 83% and 99% increase reported by Bhat et al. [48] and Yadav and Garg [12], respectively, during vermicomposting of cow dung. The increase in TP may be attributed to the presence of earthworm gut phosphatase, and phosphorous solubilizing microorganisms in the worm casts that enhance the release of phosphorus in various forms [59]. Ghosh et al. [60] reported the presence of phytase enzymes in vermicompost that also enhances mineralization of phosphorus as time progresses. In addition, mineralization and mobilization of organic matter by the combined effect of microorganisms and fecal phosphate activity of earthworms probably increases TP content in the final vermicompost [12]. These factors could thus have caused an overall increase in TP.
The overall increase in TK was about 47% which is greater than the 31% and 34% increase reported by Yadav and Garg [12] and Lalander et al. [35], respectively. The increase in TK could be attributed to the solubilizing of insoluble potassium as a result of acid production by microorganisms [61]. TK content within vermicompost is also directly related to phosphatase enzymes present in the digestive systems of earthworms and bacteria [62]. In addition, the gut of an earthworm has a big population of microflora which could enhance the release of potassium in vermicompost [63]. These factors could thus have contributed to an overall increase in TK over time.
3.3. Gaseous Emissions
The change in the mean composition of gaseous emissions over the course of the experiment is shown in Table 3. A two-way ANOVA indicated that there was no significant difference (p > 0.05) in the composition between the different runs but a significant difference (p < 0.05) in the different weeks of measurement. CO2 increased by 1.1% from week 4 to week 8 and then decreased by 0.8% from week 8 to week 12. CH4 was highest in week 4, decreased slightly by 0.06% in week 8, and further decreased by 0.06% in week 12. The concentration of N2O was greatest in week 4, decreased in week 8 by 2.25 ppm, and was not detected in week 12. The same trend was observed for the emission factors (Figure 3). The cumulative emission factors were 102 g CO2 kg−1 DM, 7.6 g CH4 kg−1 DM, and 40 mg N2O kg−1 DM.

Table 3.
Composition of gaseous emissions during vermicomposting time.
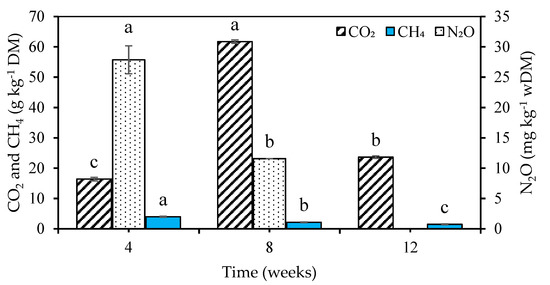
Figure 3.
Gaseous emission factors. Same bars with different letters are significantly different (p < 0.05).
Ammonia (NH3) emissions were not detected during the measurement period for the four runs. This could be attributed to relatively low temperatures and pH throughout the experiment. According to Velasco-Velasco et al. [26], ammonia volatilization is promoted by relatively high temperatures and pH, and this usually occurs in the first week of vermicomposting. High temperatures (>45 °C) and pH (>9) promote NH3 volatilization due to an inhibition of nitrification [22]. However, vermicomposting is a mesophilic process (<30 °C) implying that NH3 volatilization is less likely to occur [64]. The absence of NH3 emissions in this study could also be associated with the high earthworm density in the vermicomposting units. In their experiment measuring gaseous emissions during vermifiltration of pig slurry, Robin et al. [65] reported an absence of NH3 emissions in their units, and this was attributed to the presence of a big earthworm population.
Emissions of methane (CH4) decreased over time. The high CH4 content in week 4 may be attributed to low oxygen concentrations resulting from the relatively high moisture content of the cattle manure substrate in the vermicomposting units compared to the other weeks with measurements (Table 3). Low oxygen content and high moisture content cause anaerobic conditions in vermicomposting units, hence stimulate the development of methanogenic bacteria that produce CH4 [66,67]. A reduction in the CH4 concentration in week 8 and further in week 12 may be attributed to an increase in the earthworm population. The burrowing activity of earthworms increases aeration, which inhibits those anaerobic conditions in which CH4 producing methanogens would thrive [23]. Earthworms also fragment large substrate particles and hinder the development of anaerobic conditions that promote methane production [20]. The cumulative CH4 emissions measured in this study (7.6 g CH4 kg−1 DM) were greater than the 0.011 g CH4 kg−1 DM reported by Komakech et al. [28] during vermicomposting of cow manure and food waste. This could be the result of higher uncertainty since measurements were performed only on a single day by Komakech et al. [28].
Emissions of nitrous oxide (N2O) decreased over time. This may be attributed to an increasing density of earthworms within the vermicomposting units. The burrowing action of these earthworms reduces anaerobic denitrification processes within the earthworm guts, hence reducing N2O emissions [27]. The decrease in emissions of N2O by earthworms may also be as a result of the earthworms gut content being lower in available nitrogen than the fresh organic matter that was added [65]. In addition, Wang et al. [23] reported that the addition of earthworms in treatment units of duck manure reportedly decreased cumulative N2O emissions by 7% when compared to units with no earthworms within a comparative period of 45 days. The cumulative N2O emissions of this study were higher than the 7.5 mg kg−1 DM [22] but lower than 180 mg kg−1 DM [23] measured during vermicomposting of vegetable waste and duck manure, respectively. This could have been caused by the lower nitrogen content of cattle manure (1.4%) used in this study compared to that of duck manure (2.3%) as reported in Wang et al. [23].
The low concentrations of CO2 at the start of the experiment may be attributed to the then low rate of biological degradation of organic matter [68]. After the initial decrease in CO2 concentration, it increased. This was probably caused by earthworms facilitating the enzymatic and microbial degradation of organic matter resulting in the transformation of wastes into organic humic forms with a significant release of CO2 [67]. The decrease in CO2 emissions in week 12 of this study may be attributed to stabilization of the compost as reported by Nada et al. [25]. The cumulative CO2 emissions obtained in this study (102 g CO2 kg−1 DM) were less than the 264 g CO2 kg−1 DM reported by Wang et al. [23] during vermicomposting of duck manure. This might have been caused by the addition of red straw in the duck manure vermicomposting system which caused longer durations of higher temperatures, in turn, promoting duck manure decomposition [23] and high CO2 emissions.
From the cumulative emission factors (Figure 3) and using the environmental factors of 1, 28, and 265 for CO2, CH4, and N2O, respectively [43], the study established a total GWP of 324 kg CO2 eq t−1 waste. From active composting of feedlot cattle manure, Hao et al. [69] reported a GWP of 401.4 kg CO2 eq t−1 waste. During composting of straw bedded and wood-chip bedded cattle manure, Hao et al. [70] reported GWP of 368.4 kg CO2 eq t−1 waste and 349.2 kg CO2 eq t−1 waste, respectively. On the other hand, GWP from dairy cattle manure composting, stockpiling, and anaerobic digestion (slurry) was 605.08 kg CO2 eq t−1 waste, 741.4 kg CO2 eq t−1 waste, and 605 kg CO2 eq t−1 waste, respectively, after three months of evaluation [71]. The GWP from beef cattle manure management using the same management methods for the same evaluation period were 388.2 kg CO2 eq t−1 waste, 433.81 kg CO2 eq t−1 waste, and 372.18 kg CO2 eq t−1 waste, respectively [71]. It can thus be concluded that vermicomposting is a better cattle manure management technology in terms of mitigating greenhouse gas emissions.
3.4. Material and Substance Flows
The MFA was based on the average mass flows (Table 1) and average nutrient composition of cattle manure (week 0) and vermicompost (week 12) for the four runs as indicated in Table 2. The nutrient composition of earthworms was derived from literature, as shown in Table 4.

Table 4.
Nutrient composition of African nightcrawlers (Eudrilus eugenia).
Resulting material flows are shown in Figure 4 for mass flows and Figure 5 for TOC and TKN flows. The MFAs for VS, TK, TP, and ash content are shown in the Supplementary Materials.
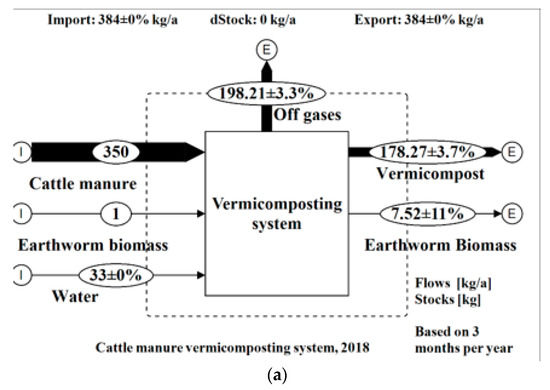
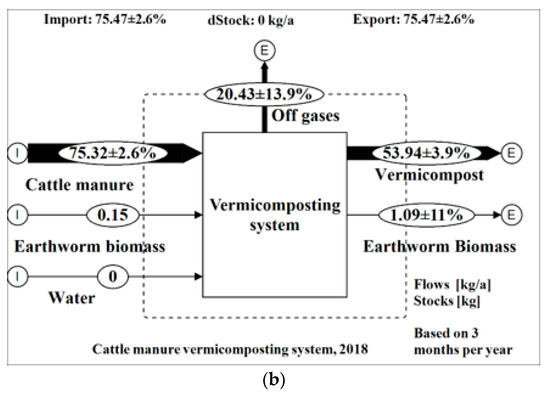
Figure 4.
Mass flows of the vermicomposting system on (a) wet basis; (b) dry basis.
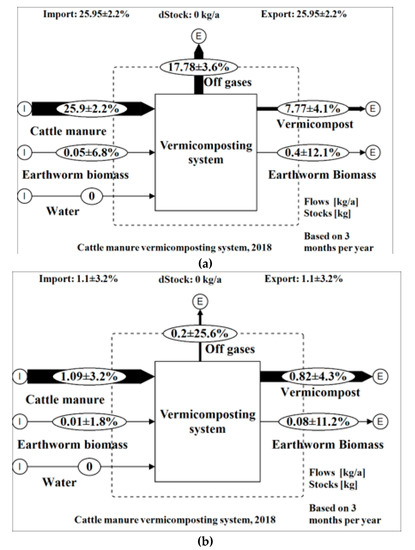
Figure 5.
(a) Total organic carbon (TOC) and (b) total Kjeldahl nitrogen (TKN) flow analyses of a vermicomposting system (wet weight basis).
The MFA showed results with low uncertainties (< ± 15%) for all the flows except for N emissions into the atmosphere with an uncertainty of 25.6%. A total wet weight (ww) of 384 kg of materials (cattle manure, water, and earthworm biomass) entered into the vermicomposting unit of which 46% was transformed into vermicompost, 2% into earthworm biomass, and 52% was lost to the atmosphere in the form of various gases. Through data reconciliation and error propagation performed by the software STAN based on all the inputs of materials and substances as well as their outputs, the predicted mean output of vermicompost was about 178 kg as opposed to a mean of 168 kg of the four runs (6% increase). This may be attributed to precision errors by the weighing scales used in the field. On a dry weight basis, 75.33 kg of total solids entered into the vermicomposting system of which 72% was transformed into vermicompost, 28% lost to the atmosphere, and 0.2% was transformed to earthworm biomass. About 89% of weight was lost to the atmosphere in the form of water vapor. The flows of nutrients within the vermicomposting systems are shown in Table 5. Inputs are shown as total kilograms/year and transferred in percentage to the outputs.

Table 5.
Distribution of nutrients in the vermicomposting system.
The calculated N loss of 18.18% to atmosphere is less than the 22.2% reported by Nigussie et al. [22] during vermicomposting of cattle manure and vegetable waste. However, the calculated C loss of 68.49% is greater than the 45.5% reported by Nigussie et al. [22]. Of the C losses, 30% was in the form of CO2, and 2.2% was lost as CH4. Of the N losses, only 0.28% was lost as N2O. However, it is assumed that most of the lost nitrogen to the atmosphere is in the form of N2 with no impact on the climate [30,72]. The C and N losses that are not accounted for may be attributed to the difficult collection of homogeneous and representative samples for gaseous emissions over a long period [73]. There is also the possibility that gaseous emissions were emitted before the start of the monitoring campaign and in-between the sampling. Furthermore, Whittaker et al. [74] recently reported that the static chamber technique may not completely account for spatial and temporal gaseous variations which may lead to an underestimation of emission rates. Possibly C and N were also lost via volatile compounds [72].
The nitrogen loss uncertainty (±25.6%) is less than that reported by Jensen et al. [34] of 126% uncertainty for N emission to the atmosphere during combined dry anaerobic digestion and post-composting of household wastes. This high uncertainty was associated with few and uncertain measurements of N emissions. Hence, they suggested that frequent emission measurements onsite would reduce these uncertainties. When compared to other manure management methods (Table 6), vermicomposting proved to enable high retention of nutrients. Higher losses of carbon to the atmosphere in this study (68.52%) are most likely due to easily degradable initial C content in cattle manure.

Table 6.
Nutrient losses associated with other manure management methods.
4. Conclusions
Vermicomposting showed a higher potential in retaining nutrients within cattle manure than other manure management methods, such as composting, stockpiling, and anaerobic digestion. In addition, a product (vermicompost) rich in macronutrients N, P, and K was produced. In terms of environmental performance, gaseous emissions associated with vermicomposting were low compared to other methods of manure management, such as composting and stockpiling. This, in turn, leads to low GWP, hence protecting the environment from adverse effects of climate change. It can thus be concluded that vermicomposting can be adopted as an environmentally sustainable manure management technology. Vermicomposting is also an economically sustainable technology as the major costs involved are associated with training personnel to run the system effectively as well as labor costs. However, more research is required to establish the exact economics in terms of costs and benefits of vermicomposting systems.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/11/19/5173/s1, Figure S1: Substance flow analysis of (a) TP and (b) TK of the vermicomposting system. Figure S2: Substance flow analysis of (a) VS and (b) ash content of the vermicomposting system.
Author Contributions
Conceptualization, J.L., J.K., A.J.K., and J.J.; methodology, J.J., J.L., A.A., A.J.K., and J.K.; software, J.J.; validation, J.L., A.A., A.J.K., and J.K.; formal analysis, J.J. and J.W.; investigation, J.J.; data curation, J.J.; writing—original draft preparation, J.J.; writing—review and editing, A.J.K., J.L., J.K., J.W., and A.A.; visualization, J.J.; supervision, A.J.K., J.K., and J.L.; project administration, J.L., J.K., and A.J.K.; funding acquisition, J.L., J.K., and A.J.K.
Funding
This research is part of the project “Capacity building on the water-energy-food security Nexus” (CAPNEX), project #158 of the Austrian Partnership Programme in Higher Education and Research for Development (APPEAR), funded by the Austrian Development Cooperation. The authors further acknowledge the TU Wien University Library for financial support through its Open Access Funding Programme.
Acknowledgments
We thank Charles Aziz, John Tuwijukye, Emmanuel Nabyama, and Bakka for the technical support rendered during experimentation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Teenstra, E.; Vellinga, T.V.; Aktasaeng, N.; Amatayaku, W.; Ndambi, A.; Pelster, D.; Germer, L.; Jenet, A.; Opio, C.; Andeweg, K. Global Asessment of Manure Management Policies and Practices; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2014. [Google Scholar]
- UBOS. Uganda Bureau of Statistics 2017 Statistical Abstract. Available online: https://www.ubos.org/wp-content/uploads/publications/03_20182017_Statistical_Abstract.pdf (accessed on 23 March 2019).
- Kiggundu, N.; Ddungu, S.P.; Wanyama, J.; Cherotich, S.; Mpairwe, D.; Zziwa, E.; Mutebi, F.; Falcucci, A. Greenhouse gas emissions from Uganda’s cattle corridor farming systems. Agric. Syst. 2019, 176, 102649. [Google Scholar] [CrossRef]
- Lederer, J.; Karungi, J.; Ogwang, F. The potential of wastes to improve nutrient levels in agricultural soils: A material flow analysis case study from Busia District, Uganda. Agric. Ecosyst. Environ. 2015, 207, 26–39. [Google Scholar] [CrossRef]
- Komakech, A.J.; Sundberg, C.; Jönsson, H.; Vinnerås, B. Life cycle assessment of biodegradable waste treatment systems for sub-Saharan African cities. Resour. Conserv. Recycl. 2015, 99, 100–110. [Google Scholar] [CrossRef]
- Vu, T.; Vu, D.; Jensen, L.S.; Sommer, S.G.; Bruun, S. Life cycle assessment of biogas production in small-scale household digesters in Vietnam. Asian Austral. J. Anim. 2015, 28, 716. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Chia, S.R.; Yen, H.W.; Nomanbhay, S.; Ho, Y.C.; Show, P.L.J.S. Transformation of Biomass Waste into Sustainable Organic Fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef]
- Dominguez, J.; Edwards, C. Vermicomposting organic wastes: A review. In Soil Zoology for Sustainable Development in the 21st Century; Shakir Hanna, S., Mikhail, W.Z., Eds.; Geocities: Cairo, Egypt, 2004; pp. 369–395. [Google Scholar]
- Nagavallemma, K.; Wani, S.; Lacroix, S.; Padmaja, V.; Vineela, C.; Rao, M.B.; Sahrawat, K. Vermicomposting: Recycling wastes into valuable organic fertilizer. J. Agric. Environ. Int. Dev. 2004, 99. [Google Scholar] [CrossRef]
- Mahaly, M.; Senthilkumar, A.K.; Arumugam, S.; Kaliyaperumal, C.; Karupannan, N. Vermicomposting of distillery sludge waste with tea leaf residues. Sustain. Environ. Res. 2018, 28, 223–227. [Google Scholar] [CrossRef]
- Yadav, A.; Garg, V. Biotransformation of bakery industry sludge into valuable product using vermicomposting. Bioresour. Technol. 2019, 274, 512–517. [Google Scholar] [CrossRef]
- Kopeć, M.; Gondek, K.; Mierzwa-Hersztek, M.; Antonkiewicz, J. Factors influencing chemical quality of composted poultry waste. Saudi J. Biol. Sci. 2018, 25, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Abbasi, S.J.S. Efficacy of the vermicomposts of different organic wastes as “clean” fertilizers: State–of–the–art. Sustainability 2018, 10, 1205. [Google Scholar] [CrossRef]
- Ermolaev, E.; Sundberg, C.; Pell, M.; Jönsson, H. Greenhouse gas emissions from home composting in practice. Bioresour. Technol. 2014, 151, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Fantozzi, F.; Bartocci, P.; D’Alessandro, B.; Testarmata, F.; Fantozzi, P. Carbon footprint of truffle sauce in central Italy by direct measurement of energy consumption of different olive harvesting techniques. J. Clean. Prod. 2015, 87, 188–196. [Google Scholar] [CrossRef]
- Andersen, J.K.; Boldrin, A.; Samuelsson, J.; Christensen, T.H.; Scheutz, C. Quantification of greenhouse gas emissions from windrow composting of garden waste. J. Environ. Qual. 2010, 39, 713–724. [Google Scholar] [CrossRef]
- Bartocci, P.; Fantozzi, P.; Fantozzi, F. Environmental impact of Sagrantino and Grechetto grapes cultivation for wine and vinegar production in central Italy. J. Clean. Prod. 2017, 140, 569–580. [Google Scholar] [CrossRef]
- Lv, B.; Zhang, D.; Cui, Y.; Yin, F. Effects of C/N ratio and earthworms on greenhouse gas emissions during vermicomposting of sewage sludge. Bioresour. Technol. 2018, 268, 408–414. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Zang, B.; Zhang, Z. The maturity and CH4, N2O, NH3 emissions from vermicomposting with agricultural waste. Compost. Sci. Util. 2017, 25, 262–271. [Google Scholar] [CrossRef]
- Nigussie, A.; Kuyper, T.W.; Bruun, S.; de Neergaard, A. Vermicomposting as a technology for reducing nitrogen losses and greenhouse gas emissions from small-scale composting. J. Clean. Prod. 2016, 139, 429–439. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.; Xu, X.; Jiang, X.; Zheng, B.; Liu, X.; Pan, X.; Kardol, P. Emissions of ammonia and greenhouse gases during combined pre-composting and vermicomposting of duck manure. Waste Manag. 2014, 34, 1546–1552. [Google Scholar] [CrossRef]
- Lleó, T.; Albacete, E.; Barrena, R.; Font, X.; Artola, A.; Sánchez, A. Home and vermicomposting as sustainable options for biowaste management. J. Clean. Prod. 2013, 47, 70–76. [Google Scholar] [CrossRef]
- Nada, W.M.; van Rensburg, L.; Claassens, S.; Blumenstein, O. Vermicomposting as a Sustainable Procedure for the Reduction of Carbon Dioxide Emissions. Dyn. Soil Dyn. Plant 2012, 6, 43–50. [Google Scholar]
- Velasco-Velasco, J.; Parkinson, R.; Kuri, V. Ammonia emissions during vermicomposting of sheep manure. Bioresour. Technol. 2011, 102, 10959–10964. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Sinha, R.K.; Wang, W. Emission of greenhouse gases from home aerobic composting, anaerobic digestion and vermicomposting of household wastes in Brisbane (Australia). Waste Manag. Res. 2011, 29, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Komakech, A.; Zurbrügg, C.; Miito, G.; Wanyama, J.; Vinnerås, B. Environmental impact from vermicomposting of organic waste in Kampala, Uganda. J. Environ. Manag. 2016, 181, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.H.; Rechberger, H. Practical Handbook of Material Flow Analysis: For Environmental, Resource, and Waste Engineers; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ng, C.G.; Yusoff, S. Life cycle inventory of institutional medium-scaled co-composting of food waste and yard waste in tropical country. Sains. Malays. 2015, 44, 517–527. [Google Scholar]
- Padeyanda, Y.; Jang, Y.-C.; Ko, Y.; Yi, S. Evaluation of environmental impacts of food waste management by material flow analysis (MFA) and life cycle assessment (LCA). J. Mater. Cycles Waste 2016, 18, 493–508. [Google Scholar] [CrossRef]
- Chèvre, N.; Guignard, C.; Rossi, L.; Pfeifer, H.R.; Bader, H.P.; Scheidegger, R. Substance flow analysis as a tool for urban water management. Water Sci. Technol. 2011, 63, 1341–1348. [Google Scholar] [CrossRef]
- Yoshida, H.; Christensen, T.H.; Guildal, T.; Scheutz, C. A comprehensive substance flow analysis of a municipal wastewater and sludge treatment plant. Chemosphere 2015, 138, 874–882. [Google Scholar] [CrossRef]
- Jensen, M.B.; Møller, J.; Scheutz, C. Assessment of a combined dry anaerobic digestion and post-composting treatment facility for source-separated organic household waste, using material and substance flow analysis and life cycle inventory. Waste Manag. 2017, 66, 23–35. [Google Scholar] [CrossRef]
- Lalander, C.H.; Komakech, A.J.; Vinnerås, B. Vermicomposting as manure management strategy for urban small-holder animal farms–Kampala case study. Waste Manag. 2015, 39, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Mainoo, N.O.; Barrington, S.; Whalen, J.K.; Sampedro, L. Pilot-scale vermicomposting of pineapple wastes with earthworms native to Accra, Ghana. Bioresour. Technol. 2009, 100, 5872–5875. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Gupta, R.; Garg, V.K. Organic manure production from cow dung and biogas plant slurry by vermicomposting under field conditions. Int. J. Rec. Org. Waste Agric. 2013, 2, 21. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; NREL Technical Report No. NREL/TP–510–42621; National Renewable Energy Laboratory: Golden, CO, USA, 2008; pp. 1–6. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of ash in biomass; Technical Report No. NREL/TP–510–42622; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Okalebo, J.; Gathua, K.; Woomer, P. Laboratory Methods of Plant and Soil Analysis: A Working Manual; TSBF-UNESCO: Nairobi, Kenya, 2002. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Collier, S.M.; Ruark, M.D.; Oates, L.G.; Jokela, W.E.; Dell, C.J. Measurement of greenhouse gas flux from agricultural soils using static chambers. J. Vis. Exp. 2014, 90, e52110. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2014: Synthesis Report. Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/AR5_SYR_FINAL_SPM.pdf (accessed on 16 September 2019).
- Andersen, J.K.; Boldrin, A.; Christensen, T.H.; Scheutz, C. Mass balances and life cycle inventory of home composting of organic waste. Waste Manag. 2011, 31, 1934–1942. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.B.; Allen, M.E.; Ullrey, D.E. Feeding captive insectivorous animals: Nutritional aspects of insects as food. Nutrition Advisory Group Handbook, Fact Sheet 003. Available online: https://nagonline.net/wp-content/uploads/2014/01/NAG-FS003-97-Insects-JONI-FEB-24-2002-MODIFIED.pdf (accessed on 16 September 2019).
- Jiménez, E.I.; García, V.P. Relationships between organic carbon and total organic matter in municipal solid wastes and city refuse composts. Bioresour. Technol. 1992, 41, 265–272. [Google Scholar] [CrossRef]
- Ndegwa, P.; Thompson, S. Integrating composting and vermicomposting in the treatment and bioconversion of biosolids. Bioresour. Technol. 2001, 76, 107–112. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Vig, A.P. Potential utilization of bagasse as feed material for earthworm Eisenia fetida and production of vermicompost. Springerplus 2015, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Tatàno, F.; Pagliaro, G.; Di Giovanni, P.; Floriani, E.; Mangani, F. Biowaste home composting: Experimental process monitoring and quality control. Waste Manag. 2015, 38, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Sonowal, P.; Khwairakpam, M.; Kalamdhad, A.S. Vermicomposting of solid pulp and paper mill sludge (SPPMS) using Eudrilus Eugeniae Earthworm. Int. J. Environ. Sci. 2014, 5, 502–514. [Google Scholar]
- Varma, V.S.; Kalamdhad, A.S.; Khwairkpam, M. Feasibility of Eudrilus eugeniae and Perionyx excavatus in vermicomposting of water hyacinth. Ecol. Eng. 2016, 94, 127–135. [Google Scholar] [CrossRef]
- Xie, D.; Wu, W.; Hao, X.; Jiang, D.; Li, X.; Bai, L. Vermicomposting of sludge from animal wastewater treatment plant mixed with cow dung or swine manure using Eisenia fetida. Environ. Sci. Pollut. Res. Int. 2016, 23, 7767–7775. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.; Rao, R. Bioconversion of garden waste, kitchen waste and cow dung into value-added products using earthworm Eisenia fetida. Saudi J. Biol. Sci. 2013, 20, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, R.M.; Domínguez, J.; Subler, S.; Edwards, C.A. Changes in biochemical properties of cow manure during processing by earthworms (Eisenia andrei, Bouché) and the effects on seedling growth. Pedobiologia 2000, 44, 709–724. [Google Scholar] [CrossRef]
- Elvira, C.; Sampedro, L.; Benitez, E.; Nogales, R. Vermicomposting of sludges from paper mill and dairy industries with Eisenia andrei: a pilot-scale study. Bioresour. Technol. 1998, 63, 205–211. [Google Scholar] [CrossRef]
- Taeporamaysamai, O.; Ratanatamskul, C. Co-composting of various organic substrates from municipal solid waste using an on-site prototype vermicomposting reactor. Int. Biodeterior. Biodegrad. 2016, 113, 357–366. [Google Scholar] [CrossRef]
- Soobhany, N.; Mohee, R.; Garg, V.K. Recovery of nutrient from Municipal Solid Waste by composting and vermicomposting using earthworm Eudrilus eugeniae. J. Environ. Chem. Eng. 2015, 3, 2931–2942. [Google Scholar] [CrossRef]
- Tumuhairwe, J.B.; Tenywa, J.S.; Otabbong, E.; Ledin, S. Comparison of four low-technology composting methods for market crop wastes. Waste Manag. 2009, 29, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Deka, H.; Deka, S.; Baruah, C.; Das, J.; Hoque, S.; Sarma, N. Vermicomposting of distillation waste of citronella plant (Cymbopogon winterianus Jowitt.) employing Eudrilus eugeniae. Bioresour. Technol. 2011, 102, 6944–6950. [Google Scholar] [CrossRef]
- Ghosh, S.; Goswami, A.J.; Ghosh, G.K.; Pramanik, P. Quantifying the relative role of phytase and phosphatase enzymes in phosphorus mineralization during vermicomposting of fibrous tea factory waste. Ecol. Eng. 2018, 116, 97–103. [Google Scholar] [CrossRef]
- Garg, P.; Gupta, A.; Satya, S. Vermicomposting of different types of waste using Eisenia foetida: A comparative study. Bioresour. Technol. 2006, 97, 391–395. [Google Scholar] [CrossRef]
- Alavi, N.; Daneshpajou, M.; Shirmardi, M.; Goudarzi, G.; Neisi, A.; Babaei, A.A. Investigating the efficiency of co-composting and vermicomposting of vinasse with the mixture of cow manure wastes, bagasse, and natural zeolite. Waste Manag. 2017, 69, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.; Ghosh, G.; Ghosal, P.; Banik, P. Changes in organic–C, N, P and K and enzyme activities in vermicompost of biodegradable organic wastes under liming and microbial inoculants. Bioresour. Technol. 2007, 98, 2485–2494. [Google Scholar] [CrossRef]
- Nigussie, A. Closing the Nutrient Loops in (peri-) Urban Farming Systems through Composting. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2017. [Google Scholar]
- Robin, P.; Germain, P.; Lecomte, M.; Landrain, B.; Li, Y.; Cluzeau, D. Earthworm effects on gaseous emissions during vermifiltration of pig fresh slurry. Bioresour. Technol. 2011, 102, 3679–3686. [Google Scholar]
- Shen, Y.; Ren, L.; Li, G.; Chen, T.; Guo, R. Influence of aeration on CH4, N2O and NH3 emissions during aerobic composting of a chicken manure and high C/N waste mixture. Waste Manag. 2011, 31, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Swati, A.; Hait, S. Greenhouse Gas Emission During Composting and Vermicomposting of Organic Wastes–A Review. CLEAN–Soil Air Water 2018, 46, 1700042. [Google Scholar] [CrossRef]
- Sánchez, A.; Artola, A.; Font, X.; Gea, T.; Barrena, R.; Gabriel, D.; Sánchez-Monedero, M.Á.; Roig, A.; Cayuela, M.L.; Mondini, C. Greenhouse gas from organic waste composting: Emissions and measurement. In CO2 Sequestration, Biofuels and Depollution; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Cham, Switzerland, 2015; pp. 33–70. [Google Scholar]
- Hao, X.; Chang, C.; Larney, F.J.; Travis, G.R. Greenhouse gas emissions during cattle feedlot manure composting. J. Environ. Qual. 2001, 30, 376–386. [Google Scholar] [CrossRef]
- Hao, X.; Chang, C.; Larney, F.J. Carbon, nitrogen balances and greenhouse gas emission during cattle feedlot manure composting. J. Environ. Qual. 2004, 33, 37–44. [Google Scholar] [CrossRef]
- Pattey, E.; Trzcinski, M.; Desjardins, R. Quantifying the reduction of greenhouse gas emissions as a resultof composting dairy and beef cattle manure. Nutr. Cycl. Agroecosyst. 2005, 72, 173–187. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; de Neergaard, A.; Jensen, L.S. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 2014, 97, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, H.; Viguria, M.; López, D.M.; Merino, P. Ammonia and greenhouse gases losses from mechanically turned cattle manure windrows: A regional composting network. J. Environ. Manag. 2017, 203, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.; Yates, N.E.; Powers, S.J.; Donovan, N.; Misselbrook, T.; Shield, I. Testing the use of static chamber boxes to monitor greenhouse gas emissions from wood chip storage heaps. BioEnergy Res. 2017, 10, 353–362. [Google Scholar] [CrossRef]
- Shah, G.M.; Groot, J.; Oenema, O.; Lantinga, E. Covered storage reduces losses and improves crop utilisation of nitrogen from solid cattle manure. Nutr. Cycl. Agroecosyst. 2012, 94, 299–312. [Google Scholar] [CrossRef]
- Larney, F.J.; Buckley, K.E.; Hao, X.; McCaughey, W.P. Fresh, stockpiled, and composted beef cattle feedlot manure. J. Environ. Qual. 2006, 35, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.C., Jr.; Pecchia, J.A.; Rigot, J.; Keener, H.M. Mass and nutrient losses during the composting of dairy manure amended with sawdust or straw. Compost. Sci. Util. 2004, 12, 323–334. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).