Abstract
Background: Digital ulcers (DUs) are among the most debilitating vascular complications in SSc and are commonly attributed to microvascular damage. However, recent evidence suggests a potential involvement of macrovascular abnormalities, including subclinical atherosclerosis and altered hemodynamic parameters. Objectives: This study aimed to investigate the association between a history of DUs and macrovascular involvement in SSc patients through carotid and vertebral Doppler ultrasonography, with a focus on hemodynamic indices such as Peak Systolic Velocity (PSV), End-Diastolic Velocity (EDV), Resistive Index (RI), and Intima–Media Thickness (IMT). Methods: A cross-sectional study was conducted on 107 SSc patients. Clinical, serological, cardiovascular, and metabolic data were collected, and carotid–vertebral ultrasound was performed. Patients were stratified based on DU history. Statistical analyses assessed associations between DU status and carotid–vertebral US findings. Results: Patients with DUs showed a significantly higher PSV in both right (86.9 ± 67.9 vs. 64.2 ± 20.5 cm/s, p = 0.010) and left ICA (78.9 ± 29.6 vs. 63.4 ± 18.2 cm/s, p = 0.002). Right ICA RI vas elevated in the DU group (p = 0.021). PSV in the external carotid arteries was also bilaterally increased in DU patients (p < 0.005). DU-positive patients had a higher prevalence of left carotid plaques (p = 0.012) and right-sided ICA RI > 0.75 (p = 0.01). Logistic regression identified DU history as an independent predictor of PSV at ICA (β = 31.89, p = 0.043) and carotid plaque presence at any side (OR 14.34, p = 0.012). Conclusions: A history of digital ulcers in SSc patients is associated with altered carotid hemodynamics and an increased subclinical atherosclerotic burden. These findings suggest that DUs may reflect not only microvascular damage, but also macrovascular dysfunction, supporting the need for integrated vascular assessment in SSc clinical practice.
1. Introduction
Historically, vasculopathy in systemic sclerosis (SSc) was considered a microcirculatory disorder, however, accumulating evidence suggests that medium- and large-caliber arteries may also be implicated, revealing a complex scenario that extends beyond microcirculation [1,2]. Indeed, microvascular damage represents the hallmark of the disease, with endothelial dysfunction playing a pivotal role, even in the early stages [3]. The main clinical manifestations of SSc-related vasculopathy include Raynaud’s phenomenon (RP) and puffy hands, which are observed even in patients with a very early diagnosis of SSc (VEDOSS), while fingertip pitting scars, digital ulcers (DUs), telangiectasias, and pulmonary arterial hypertension (PAH) tend to appear in established forms of the disease [4,5].
Insightfully, DUs are among the most debilitating complications of SSc and have been widely acknowledged as markers of the severity of SSc-related vasculopathy. They may provoke significant pain, impaired hand function, increased risk of infection, and reduced quality of life in affected patients [6]. DUs are defined as the loss of epidermal continuity extending into the dermis, with different degrees of exposure of the underlying tissues, potentially evolving towards gangrene and digital loss [7].
In SSc, DU development has predominantly been attributed to microvascular injury, however, in the general population, the primary causes of digital ischemia include arterial abnormalities, extrinsic vascular compression, thromboembolic events, and atherosclerosis, with the latter predominantly resulting from plaque accumulation in large-caliber arteries [8,9,10,11].
In this context, recent research has shown that endothelial dysfunction is also present in the brachial arteries and correlates with microvascular damage at the nailfold level, suggesting a continuum of vascular injury spanning both micro- and macrovascular beds [12]. As shown in the Italian observational multicenter GIRRCS study, a slight increase in clinical and subclinical atherosclerosis was displayed by SSc patients compared to available controls. In addition, the authors demonstrated that both traditional cardiovascular risk factors and SSc-specific features, such as ischemic digital ulcers, played a synergistic role in the development of cardiovascular complications [13].
To detect pre-atherosclerotic changes, most studies have employed B-mode vascular ultrasound at carotid and peripheral artery beds [14]. These non-invasive techniques have proven effective in identifying early vascular abnormalities, such as Intima–Media Thickness (IMT) and arterial stiffness, which have been consistently observed at higher rates in SSc. Notably, these findings occur even in the absence of traditional cardiovascular risk factors and despite a relatively low incidence of clinically overt cardiovascular events in SSc patients [15].
However, Doppler ultrasound at both the carotid and peripheral artery levels could provide various hemodynamically significant indices other than IMT, including Peak Systolic Velocity (PSV), End-Diastolic Velocity (EDV), and Resistive Index (RI). These hemodynamic indices have already been validated as predictors of macrovascular dysfunction in other populations, such as Type 2 Diabetes Mellitus patients [16]. Briefly, PSV reflects the maximum blood flow velocity during systole and it is particularly useful in identifying areas of arterial narrowing [17]. EDV, on the other hand, represents blood flow velocity during diastole, and it is particularly sensitive to downstream vascular resistance [18]. RI, quantifying the resistance to blood flow within a vessel, is used to evaluate end-organ perfusion [19].
Lastly, given the conflicting data regarding which SSc-specific features best explain clinical or subclinical atherosclerosis and macrovascular impairment, our study investigates the association between DUs, widely recognized as a clinical surrogate of microvascular injury, and Doppler ultrasound indices of the carotid and vertebral arteries. Utilizing non-invasive Doppler hemodynamic measurements, including cIMT, PSV, EDV, and RI, our objective is to elucidate the emerging interplay between microvascular and macrovascular compartments. By employing widely accessible and routinely performed ultrasound imaging techniques, we further aim to support the integration of macrovascular ultrasound assessment into clinical practice for SSc patients.
2. Materials and Methods
2.1. Study Population and Sample Definition
This cross-sectional observational study involved a cohort of one-hundred and seven SSc patients attending the Scleroderma Unit of ASST Ovest Milanese, Legnano Hospital (Milan, Italy) comprising participants aged ≥18 years able to provide informed consent. Patients were selected based on their fulfillment of the 2013 ACR/EULAR for a definitive diagnosis of SSc [20]. Patients with severe heart failure, a history of congenital heart disease, current malignancies, or anti-neoplastic treatment and individuals who had undergone cardiac surgery, percutaneous coronary, carotid and vertebral intervention, pacemaker implant, or carotid–vertebral endarterectomy were excluded from the study. Severe cognitive impairment and pregnancy status served as further exclusion criteria.
Participants were recruited from September 2024 to May 2025, and the study was conducted in respect of the ethical guidelines proposed in the Declaration of Helsinki, with the approval of the Ethic Committee of Milan Area 3 (protocol number S00125/2023, 29 May 2023).
2.2. Data Collection
Patients’ demographic and anthropometric characteristics were extracted from medical records. Data collection included information on age, sex, weight, height, Body Mass Index, Body Surface Area, and previous or smoking status. Data concerning age at enrollment and SSc diagnosis were also gathered from clinical records, as well as disease duration.
Based on data from previous evaluations, all patients’ disease-specific characteristics were assessed, including the presence of RP, puffy hands, telangiectasias, prior and current history of DUs, fingertip pitting scars, sclerodactyly, skin sclerosis, calcinosis, microstomia and microcheilia, and musculoskeletal and upper and/or lower gastrointestinal involvements. Interstitial Lung disease (ILD), PAH, and cardiomyopathy were detected through chest High-Resolution Computed Tomography (HRCT), Right Heart Catheterization (RHC), and cardiac Magnetic Resonance Images (cMRI), respectively. The Modified Rodnan Skin Score (mRSS) was employed to assess skin sclerosis extension [21].
Moreover, for the main purpose of the study, the presence of prior and current DUs, defined as the presence of painful, well-demarcated areas of skin loss and/or ulceration occurring on the tips of the fingers or toes and resulting from ischemia due to severe microvascular damage, was recorded and selected as a hallmark of advanced microvascular involvement in our population for comparison analysis.
Serological classification based on positivity for anticentromere antibodies (ACA), anti-topoisomerase I (anti-Scl70) antibodies, and anti-RNA polymerase III antibodies (ARA) was collected from patient medical history. Based on the most recent assessment, Nailfold Videocapillaroscopy (NVC) patterns were classified according to the Cutolo criteria and categorized into early, active, and late patterns [22].
Current medication status with a potential influence on macro- and microvascular functionality was gathered, including anti-hypertensive treatment (such as calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), beta-blockers, and diuretics), low-dose aspirin, intravenous Iloprost, endothelin receptor antagonists (ERAs), phosphodiesterase type 5 inhibitors (PDE5 is), and lipid-lowering treatment. Data on glucocorticoid usage and immunosuppressive treatments were also evaluated.
2.3. Cardiovascular and Atherosclerotic Risk Assessment
Data regarding comorbidities such as Type 2 Diabetes Mellitus, Systemic Arterial Hypertension, Arrhythmias, Hyperuricemia, and Dyslipidemia, with a known and established influence on the cardiovascular system, were taken into account, as well as a previous familial or personal history of cardio- and cerebrovascular events. Patients were clinically evaluated to obtain information regarding the presence of cardiopalmus, angina pectoris, and heart-related dyspnea.
All recruited participants underwent standardized measurements of hemodynamic parameters at rest, in a quiet environment, thirty minutes prior to the execution of the carotid–vertebral Doppler ultrasound. Specifically, systolic and diastolic blood pressure levels, as well as heart rate (HR), were recorded in duplicate, with each measurement taken five minutes apart using an automated oscillometric sphygmomanometer on the dominant arm after at least 10 minutes of supine rest [23]. Cardiovascular risk scores were calculated using the validated Framingham and ASCVD (Atherosclerotic Cardiovascular Disease) risk equations, incorporating clinical variables such as age, sex, blood pressure, lipid profile, diabetes status, and smoking habits, in accordance with ACC/AHA guidelines [24,25,26].
2.4. Biochemical and Metabolic Assessments
Venous blood samples were collected within the same month as the carotid and vertebral Doppler ultrasound examination. All laboratory analyses were performed in the institutional central laboratory, following standardized protocols. The metabolic and cardiovascular profile included measurements of total cholesterol, HDL cholesterol (HDL), LDL cholesterol (LDL), triglycerides, fasting glucose, high-sensitivity troponin T (hs-TnT), N-terminal pro–brain natriuretic peptide (NT-proBNP), uric acid, c-reactive protein (CRP), and hemoglobin (Hb). Units of measurement were as follows: cholesterol and triglycerides (mg/dL), glucose (mg/dL), troponin T (ng/L), NT-proBNP (pg/mL), uric acid (mg/dL), Hb (g/dL), and CRP (mg/L). Lipid parameters were assessed enzymatically, cardiac markers by electrochemiluminescence immunoassay, and Hb using an automated hematology analyzer.
Based on these parameters, the following metabolic indices were further calculated: (1) the triglyceride–glucose index (TyG index) was derived by taking the natural logarithm of the product of fasting triglyceride and fasting glucose levels divided by two [27]; (2) the LDL/HDL ratio was calculated by dividing the concentration of LDL cholesterol by that of HDL cholesterol [28]; (3) the triglyceride/HDL ratio (TG/HDL) was obtained by dividing serum triglycerides by HDL cholesterol [29]; (4) the Atherogenic Index of Plasma (AIP) was expressed as the base-10 logarithm of the ratio between serum triglycerides and HDL cholesterol [30]; and (5) the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated, where available, by multiplying fasting glucose (in mg/dL) by fasting insulin (in µU/mL) and dividing the result by 405 [31].
2.5. Ultrasound Examination
Ultrasound examination of the supra-aortic vessels was performed using a GE Vivid T8 ultrasound system equipped with a high-frequency linear transducer (8 MHz). All patients were examined in the supine position, with the neck slightly extended and rotated contralaterally to the side under evaluation to optimize image acquisition. For the common carotid artery (CCA), internal carotid artery (ICA), external carotid artery (ECA), and vertebral artery (VA), both transverse and longitudinal scans were performed using B-mode imaging, color Doppler, and pulsed-wave Doppler techniques.
Intima–Media Thickness (IMT) was measured in the longitudinal plane of the CCA, on the far wall, approximately 1 cm proximal to the carotid bifurcation [32]. The IMT value was calculated as the mean of three separate consecutive measurements. Atherosclerotic plaques were defined as focal structures that protrude into the arterial lumen by at least 0.5 mm or 50% of the surrounding IMT value, or that exhibit a thickness greater than 1.5 mm, measured from the intima–lumen interface to the media–adventitia interface [33]. For each vessel, Peak Systolic Velocity (PSV) and End-Diastolic Velocity (EDV) were recorded using pulsed-wave Doppler, maintaining an angle of insonation between 45° and 60°. In addition, Resistance Indices (RIs) were calculated for the CCA, ICA, and ECA to assess vascular resistance and aid in hemodynamic interpretation. The RI was calculated using the following formula: “RI = (PSV − EDV)/PSV”, and the validated cut-off of 0.75 was considered for the analysis [34].
In the presence of atherosclerotic plaques, the degree of stenosis was first assessed morphologically according to the criteria of the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [35]. A complementary hemodynamic evaluation was also performed based on the classification proposed by Grant et al. and applied in cases of stenosis >50% or PSV >125 cm/s [36].
As most participants were undergoing monthly Iloprost infusion, US examination was performed two weeks after the last infusion to avoid any influence on SBP, DBP, and HR in examined velocities. Moreover, to avoid intra- and interobserver bias, the images were acquired and further evaluated two-fold by two experienced sonography examiners (F.L., seven years of experience, and L.C., five years of experience) who were blinded to patient data and characteristics.
2.6. Statistical Analysis
Continuous variables are expressed as mean ± standard deviation (SD) when normally distributed, or as median with interquartile range (IQR) in the case of non-normal distributions. Categorical variables are reported as absolute frequencies and percentages. Comparisons between continuous variables were performed using either the Student’s t-test or the Mann–Whitney U test, depending on the underlying data distribution. For categorical variables, the Chi-squared test or Fisher’s exact test was applied, as appropriate based on the sample size and expected frequencies. Linear regression analysis determined predictors for mean PSV at ICA and ECA. Additionally, a binary logistic regression model was developed to investigate potential risk factors associated with the presence of atherosclerotic plaques at any site. Covariates included in both analyses were selected a priori based on their well-established roles in plaque formation, as supported by evidence from the literature. The variables for the binary logistic regression comprised age, sex, BMI, LDL/HDL ratio, Framingham risk score, ASCVD risk score, and SBP, and as per the main purpose of the study, history of DUs was also incorporated. For the linear regression model, disease duration, AIP and SSc subtype according to the LeRoy classification were further included.
Statistical significance was defined as a p-value of ≤0.05 or when the 95% confidence interval excluded zero. The data analyses were conducted using IBM SPSS Statistics software, version 27 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Patient Clinical Features
A total of 107 patients were enrolled, with 76 (71.0%) having no history of DUs and 31 (29.0%) reporting past DUs. Female prevalence was significantly higher in the non-DUs group (96.1% vs. 71.0%, p < 0.001). Mean age at enrollment was similar between groups (62.3 ± 12.0 vs. 58.8 ± 12.7 years, p = 0.189), but patients with DUs were diagnosed earlier (43.4 ± 15.2 vs. 49.9 ± 13.2 years, p = 0.041). Disease duration, BMI, and BSA showed no significant differences (Table 1).

Table 1.
Clinical characteristics.
Regarding autoantibodies, anti-Scl-70 positivity was more frequent in the DUs group (35.5% vs. 7.9%, p = 0.001), whereas ACA and ARA antibodies were comparable. Skin involvement, measured by the mRSS, was greater in the DUs group (8.5 ± 8.4 vs. 2.1 ± 2.3, p < 0.001). According to the LeRoy classification, dcSSc was more common in the DUs group (38.7% vs. 1.3%, p = 0.001), while the limited and sine scleroderma subsets predominated in non-DU patients. NVC patterns also differed: early/active patterns were more frequent in non-DU patients (76.3% vs. 45.2%, p = 0.002), and late patterns prevailed among DU patients (54.8% vs. 23.7%, p = 0.002). Moreover, several clinical features correlated with a history of DUs, including puffy hands (p = 0.005), telangiectasias (p = 0.001), pitting scars (p < 0.001), sclerodactyly (p = 0.001), calcinosis (p = 0.003), friction rubs (p = 0.001), and microstomia (p < 0.001).
Upper gastrointestinal involvement was more prevalent in DU patients (80.6% vs. 56.6%, p = 0.019), while lower gastrointestinal symptoms, arthritis, renal crisis, cardiomyopathy, and PAH did not differ significantly. Lastly, ILD was notably more frequent in the DUs group (48.4% vs. 11.8%, p < 0.001).
Regarding therapies, antihypertensive medication usage and lipids and uric acid lowering therapies were comparable. The only differences emerged with the use of ERAs and sildenafil, as expected for the prevention of DUs (p < 0.001 for both) (Supplementary Table S1).
3.2. Cardiovascular Risk Assessment and Metabolic Indices
Patients with DUs more commonly reported angina pectoris (19.4% vs. 5.3%, p = 0.023) and hyperuricemia (16.1% vs. 1.3%, p = 0.003). However, no differences emerged for dyspnea, cardiopalmus, arrhythmias, hypertension, dyslipidemia, and T2 DM, as shown in Table 2.

Table 2.
Atherosclerotic risk factors and metabolic indices.
Notably, metabolic parameters, including total cholesterol, HDL, LDL, triglycerides, fasting glucose, insulin, and calculated indices such as the TyG index, HOMA-IR, LDL/HDL ratio, TG/HDL ratio, and Atherogenic Index of Plasma (AIP), showed no significant differences between groups. Similarly, inflammatory and cardiac biomarkers (CRP, hs-TnT, NT-proBNP, and uric acid) were comparable. Blood pressure and heart rate, measured twice five minutes apart, did not differ significantly. Moreover, Framingham risk scores were higher in DU-positive patients (p = 0.048), indicating an increased cardiovascular risk, whereas ASCVD risk scores were similar, as shown in Figure 1.
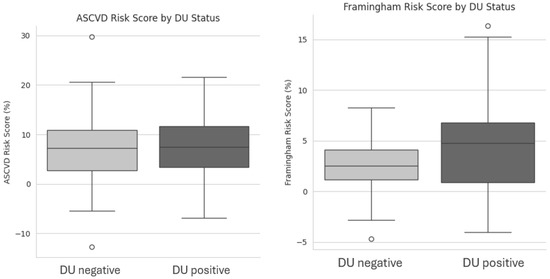
Figure 1.
Box plot showing differences between DU negative and DU positive patients on ASCVD Risk Score and Framingham risk scores. Acronyms. DU = Digital Ulcers; ASCVD = Atherosclerotic Cardiovascular Disease.
3.3. Carotid Ultrasound Findings
Carotid ultrasound revealed a higher prevalence of atherosclerotic plaques in the left carotid artery in DU patients (51.6% vs. 26.3%, p = 0.012). Similarly, right carotid plaques were more frequent in those with DUs (45.2% vs. 31.6%), but without statistical significance. DU patients exhibited a greater prevalence of plaque bilateral localization (38.7% vs. 13.2%, p = 0.003), while the two groups did not differ when any side (left, right, or both) was considered, however, a trend toward significance was detected (58.1% vs. 38.2%, p = 0.059), as shown in Figure 2.
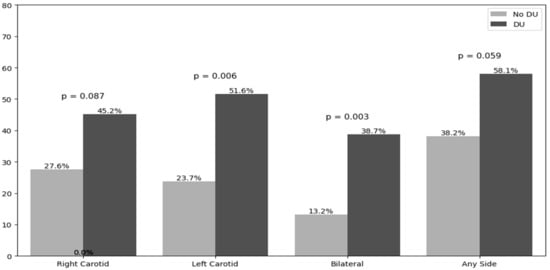
Figure 2.
Atherosclerotic plaque distribution according to the side. Acronyms. DUs = Digital Ulcers.
On the left side, plaque prevalence in the carotid bulb was 11.8% in the non-DUs group and 25.8% in the DUs group, without reaching statistical significance. Plaques at the bulb–ICA transition were detected in 3.9% of non-DU and 9.7% of DU patients, while isolated ICA plaques were found in 7.9% and 16.1% of patients. In contrast, the right side revealed that carotid bulb plaques were significantly more frequent in patients with a history of DUs compared to those without (29.0% vs. 11.8%, p = 0.02). Plaques at the bulb–ICA site were seen in 9.7% of DU patients and 6.6% of non-DU patients (p = 0.69), and ICA plaques were seen in 29.0% vs. 9.2%, respectively (p = 0.07), showing a trend toward significance (Figure 3).
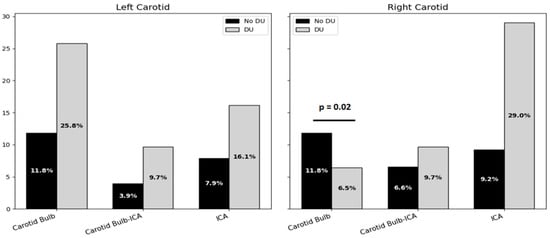
Figure 3.
Bar chart on atherosclerotic plaque localization on both sides of carotid arteries. Acronyms. DUs = Digital Ulcers; ICA = Internal Carotid Artery.
Moreover, the number of patients reporting a stenosis percentage at the plaque site comprising between 0 and 49% amounted to 18 out of 76 patients in the non-DUs group and 10 out of 31 in the DUs group (p = 0.4673), however, patients with a grade of stenosis more than 50% were 2/76 in the non-DUs group and 4/31 in the DUs group (p = 0.057), showing a trend toward significance in the latter group.
3.4. Doppler Hemodynamic Parameters
From a vascular standpoint, no significant differences were observed in EDV or cIMT measurements, as shown in Table 3. However, patients with DUs exhibited higher carotid blood flow velocities, still falling within the normal physiological range (PSV < 125 cm/s), with a significant bilateral increase in PSV of the ICA (right: 86.9 ± 67.9 vs. 64.2 ± 20.5 cm/s, p = 0.01; left: 78.9 ± 29.6 vs. 63.4 ± 18.2 cm/s, p = 0.001), as well as PSV in the ECA (right: 75.0 ± 24.2 vs. 87.7 ± 25.3 cm/s, p = 0.018; left: 71.7 ± 20.1 vs. 86.1 ± 24.1 cm/s, p = 0.002).

Table 3.
Doppler ultrasonographic hemodynamic parameters at carotid and vertebral levels.
Moreover, significant increases in RI were noted in the right ICA in the DUs group compared to DU-negative controls (p = 0.021 and p = 0.013, respectively). Finally, the ICA/CCA PSV ratio on the right side was significantly elevated in DU patients (1.48 ± 1.21 vs. 1.16 ± 0.33; p = 0.043).
Furthermore, on the right side, DU patients more frequently had an elevated pulsatility index (PI > 1.2) and Resistive Index (RI > 0.75) in the ICA (35.5% vs. 13.5%, p = 0.01), along with more carotid stenosis (12.9% vs. 2.7%, p = 0.04). On the left side, an elevated PI and RI in the ICA were also more common in DU patients (35.5% vs. 8.1%, p < 0.001). No differences were found for cIMT > 0.9 mm, or elevated RI in the CCA or ECA, between the groups.
Furthermore, on the right side, DU patients more frequently had an elevated Resistive Index (RI > 0.75) in the ICA (35.5% vs. 13.5%, p = 0.01), along with more carotid stenosis (12.9% vs. 2.7%, p = 0.04). On the left side, an elevated RI in the ICA was also more common in DU patients (35.5% vs. 8.1%, p < 0.001). No differences were found for CCA IMT > 0.9 mm or increased RI in the CCA or ECA between the groups, as shown in Figure 4.
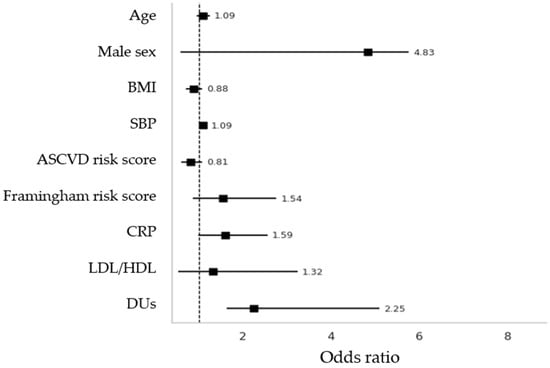
Figure 4.
Binary logistic regression with adjusted OR with “plaques at any site” was considered as dependent variable. BMI = Body Mass Index; SBP = Systolic Blood Pressure; CRP = C-Reactive Protein; LDL = Low-density lipoprotein; HDL = High-density lipoprotein; ASCVD = Atherosclerotic Cardiovascular Disease.
3.5. Regression Analyses
Firstly, two multivariable linear regressions were performed to identify predictors of mean PSV at the ICA and ECA (Table 4).

Table 4.
Linear regression model predicting mean PSV at ICA and ECA as dependent variables (cm/sec).
For the ICA, a history of DUs was an independent predictor of a higher PSV (β = 31.89, p = 0.043). Traditional cardiovascular risk factors were not significant. In the ECA model, age predicted a lower PSV (β = −1.59, p = 0.022), while ASCVD risk score had a borderline positive association (β = 2.53, p = 0.055). DUs were not a significant determinant.
Secondly, in the binary logistic regression model employing plaques at any site as a dependent variable, it was revealed that both SBP and DUs were significantly associated with plaque presence (adjusted OR 1.09, p = 0.019; adjusted OR 2.25, p = 0.015, respectively) (Figure 4). Other included variables were age, sex, BMI, Framingham and ASCVD risk scores, and LDL/HDL ratio, which failed to prove associations.
4. Discussion
Our study provides evidence for the relationship between microvascular damage and macrovascular impairment in SSc from a peculiar clinical standpoint. Previous evidence has underscored that SSc patients exhibit increased cardiovascular mortality compared to healthy controls, and cardiac alterations can also be found in milder forms of the disease despite a lower prevalence of traditional cardiovascular risk factors across these populations [37,38]. In fact, cardiovascular mortality in SSc is estimated to be attributable to atherosclerotic events in up to 29% of cases, according to EUSTAR data, signifying a shift from SSc-specific causes (e.g., renal crisis and pulmonary hypertension) towards more generalized vascular complications [39,40,41].
Various studies have tried to define the SSc-related features which best contribute to macrovascular impairment and cardiovascular event prediction. For instance, a study by Caimmi et al., using ultrasound to analyze different medium-large vessels beds, such as carotid, upper, and lower limb arteries, revealed an association with Forced Vital Capacity, Diffusing Capacity of the lungs for Carbon Monoxide, limited cutaneous SSc, and calcinosis in defining macrovascular impairment [42]. In this context, particular interest has gained regarding the potential interconnection between microvascular changes, defined per the late NVC pattern or reduced capillary density, and altered endothelial function, detected as flow-mediated vasodilatation at the brachial arteries and arterial stiffness [12].
From a clinical perspective, the role of DUs in predicting macrovascular involvement in patients with SSc remains a subject of debate, with contrasting evidence reported in the literature. For instance, a large cohort study conducted on Japanese SSc patients failed to demonstrate a significant association between DUs and the development of atherosclerotic plaques. In contrast, data emerging from the GIRRCS study identified DUs as independent predictors of overt clinical atherosclerosis, although no significant link was found with subclinical atherosclerotic changes [13,43]. In view of this apparent dichotomy, we observed a higher prevalence of atherosclerotic plaques, particularly in the left carotid artery and bilaterally, among patients with a documented history of DUs.
In contrast to the Japanese study, despite its large sample size, our approach incorporated both hemodynamic assessments and cIMT evaluations, providing a more comprehensive analysis. The Japanese study primarily focused on the binary presence or absence of plaques, without integrating these additional parameters [43]. Furthermore, while the GIRRCS study emphasized the role of DUs in predicting clinically overt atherosclerosis, our findings suggest that DUs may also serve as a valuable predictor of subclinical carotid plaque formation, thereby reinforcing their significance as a marker of subtle macrovascular disease in SSc [13].
Moreover, although increased cIMT has long been recognized as a marker of cardiovascular morbidity and mortality, our findings suggest that alone, it may not suffice to stratify vascular risk in SSc patients [44]. Previous studies by Bartoli et al., Soltesz et al., and more recently Sedky Abdou et al. reported significantly increased cIMT in SSc patients compared to healthy controls, consistent with early arterial rearrangement toward increased stiffness. However, these changes do not always correlate with plaque presence or Doppler hemodynamic indices [45,46,47].
To clarify, in our analysis, only a few plaques determined significant hemodynamic alterations in the vascular beds under study, pointing to the presence of subclinical atheromatous process at this level. Moreover, this was elucidated by the comparable values of bilateral cIMT between the two groups, failing to reach the standardized cut-off of 0.9 mm indicative of atherosclerotic processes in the general population. This observation suggests that cIMT alone is not capable of defining plaque formation. This aligns with the previous findings of Frerix et al., who demonstrated discordance between plaque burden and cIMT in both SSc and systemic lupus erythematosus (SLE), suggesting that plaque formation may occur independently of intima–media thickening [48]. Moreover, Schiopu’s work noted increased expressions of serum proteins, including IL-2, IL-6, CRP, keratinocyte growth factor, intercellular adhesion molecule 1, endoglin, plasminogen activator inhibitor 1, and insulin-like growth factor binding protein 3, associated with carotid plaques in an SSc population, while myeloid progenitor inhibitory factor 1, serum amyloid A, thrombomodulin, N-terminal pro-brain natriuretic peptide (BNP), and Clara cell secretory protein 16 kD correlated with cIMT. Notably, these molecules are implicated in both fibrosis and vasculopathy processes, highlighting the presence of other intrinsic SSc-related mechanisms at play [49].
Supporting this hypothesis, the Doppler ultrasound findings in our cohort demonstrated an increased PSV in both the ICA and ECA among patients with DUs. Although these PSV values did not exceed clinically significant thresholds, their elevation, particularly when accompanied by a higher RI in the ICA, may reflect early alterations in arterial wall properties. Specifically, such changes suggest a reduction in arterial compliance in response to increased distal vascular resistance, even in the absence of critical stenosis.
Notably, the more prominent PSV alterations in the ICA and ECA, rather than in the CCA, are of particular interest. In this regard, vascular stiffness and endothelial dysfunction could preferentially affect arteries that are anatomically closer to the microvascular beds. The ICA and ECA serve as proximal conduits to microcirculation, and alterations in their hemodynamics may represent an indirect sign of impaired endothelial function. Thus, the closer the arterial segment is to the microvascular environment, the greater its susceptibility to resistance-related changes.
Definitively, a history of DUs might exert a proactive effect on determining these hemodynamic alterations occurring at the most distal branches of the carotid instead of CCA, which may reflect functional vessel stiffening and precede clinically overt atherosclerosis or ischemic events. These alterations have also been proven from a mechanistic point of view by Rollando D. et al., who demonstrated the presence of early-stage endothelial dysfunction at the brachial arteries in SSc, with parameters of altered FMD correlated to NVC changes [12]. In fact, endothelial cell injury induced by anti-endothelial antibodies, ischemia/reperfusion damage, and immune-mediated cytotoxicity represent the main causes of vascular injury, together with an impaired vascular repair mechanism, which determines defective vasculogenesis [50].
Collectively, these observations and ours reinforce the hypothesis that macrovascular impairment in SSc stems from a dual pathogenic origin: one is established by classical atherosclerosis, and the other is SSc-specific fibrotic vasculopathy. Remarkably, the observed increase in PSV, not paralleled by changes in EDV, points to a mechanism beyond simple luminal narrowing due to atherosclerosis, as increases in EDV are exclusively reported in proximity to atherosclerotic plaques [51].
Moreover, our research revealed that in DU patients, despite the presence of macrovascular alterations, no differences in classical cardiometabolic risk factors were found. Parameters such as the atherogenic index of plasma, TyG, HOMA-IR, and lipid ratios (TG/HDL and LDL/HDL) were similar between the groups, as were the rates of hypertension, diabetes, smoking, and dyslipidemia. DU patients had a higher cardiovascular risk as estimated by the Framingham score, but not by the ASCVD risk estimator. These discrepancies point to the inadequacy of traditional cardiovascular risk models in capturing the unique vascular pathology of SSc. Traditional models are primarily designed based on general population data, emphasizing risk factors such as lipid abnormalities, metabolic syndrome, and lifestyle-related contributors [52]. However, in SSc, the pathogenesis of vascular disease appears to follow a distinct trajectory, where immune-mediated endothelial dysfunction, vascular remodeling, and progressive fibrosis play central roles, often independently of typical metabolic derangements.
Another key consideration is the method of vascular assessment we employed. The use of carotid ultrasound and Doppler imaging provides a more sensitive evaluation of subclinical vascular pathology than broad estimators like Framingham or ASCVD risk scores do. In fact, Sanz Perez I et al. found that carotid ultrasound and coronary artery calcium (CAC) scoring were more effective in detecting subclinical atherosclerosis in SSc than conventional risk charts [53].
Our study presents several strengths. Firstly, the comprehensive evaluation of both microvascular (DUs and NVC) and macrovascular (carotid ultrasound, Doppler hemodynamics, and cIMT) parameters within the same cohort allowed for an integrated assessment of vascular pathology from a real-life clinical perspective. Second, the rigorous ultrasound methodology applied, with blinded dual-operator assessments, improved the reliability of imaging data and minimized operator bias.
Despite these strengths, several limitations should be acknowledged. For instance, the lack of standardized cut-off values for Doppler indices in SSc populations adds complexity to interpreting results and comparing findings across different studies. Future efforts should aim to elucidate common accepted thresholds in this cohort.
Although our primary objective was to explore whether clinical signs of microvascular dysfunction—specifically DUs—might serve as predictors of macrovascular alterations in SSc, our findings prompt the need for a more comprehensive assessment of endothelial function. In this regard, the inclusion of functional vascular tests such as FMD and arterial stiffness measurements would offer a more direct evaluation of endothelial health and vascular compliance.
Furthermore, it could be valuable to extend such vascular assessment to more distal arterial branches, such as the ophthalmic artery. Moreover, to elucidate the potential interconnection of macro- and microvasculopathy at the cerebral level, as recent evidence from optical coherence tomography angiography studies has shown a reduced retinal vascular density in patients with SSc and other autoimmune diseases, the employment of these advanced imaging modalities might provide further insight into this continuum [54,55,56].
The cross-sectional design of the study inherently restricts causal inferences regarding the temporal relationship between microvascular damage, macrovascular impairment, and cardiovascular events. Additionally, the single-time-point measurements may have missed dynamic changes in metabolic status or vascular health over time. Longitudinal follow-up would be necessary to clarify whether the observed vascular changes predict future cardiovascular morbidity and mortality in SSc. Finally, monocentric recruitment could limit the generalizability of these findings.
5. Conclusions
In conclusion, the data supports the concept that macrovascular disease in SSc arises from both atherosclerotic and fibrotic mechanisms. Traditional cardiovascular risk scores and metabolic parameters fail to account for this vascular burden, emphasizing the need for SSc-specific vascular assessment strategies. Incorporating DU status and non-invasive vascular imaging into routine clinical practice could allow for the earlier identification of patients at elevated risk, opening a window for timely preventive interventions and potentially improving cardiovascular outcomes in this high-risk population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/clinpract15080152/s1, Table S1: Medication Usage.
Author Contributions
E.C.: conceptualization, software, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing. F.L.: methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft. L.C. (Luca Clerici): methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft. E.Z.: resources, data curation, writing—original draft. G.C.M.: resources, data curation, writing—original draft. F.C.: resources, data curation, writing—original draft. D.B.: resources, data curation, writing—original draft. L.C. (Laura Castelnovo): resources, data curation, writing—original draft. A.T.: resources, data curation, writing—original draft. M.I.: methodology, writing—review and editing. M.S.C.: methodology, writing—review and editing. P.M.L.F.: conceptualization, validation, resources, writing—review and editing, supervision. A.M.: conceptualization, validation, resources, supervision, writing—review and editing, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Milan Area 3, protocol number S00125/2023, 29 May 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request. The data is not publicly available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 95%CI | 95% Confidence Interval |
| ACA | Anti-Centromere Antibodies |
| ACC/AHA | American College of Cardiology/American Heart Association |
| ACEis | Angiotensin-Converting Enzyme Inhibitors |
| ACR/EULAR | American College of Rheumatology/European League Against Rheumatism |
| AIP | Atherogenic Index of Plasma |
| ARA | Anti-RNA Polimerase III Antibodies |
| ARBs | Angiotensin II Receptor Blockers |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| ASST | Azienda Socio-Sanitaria Territoriale |
| β-coeff. | Beta Coefficient |
| β-stand | Standardized Beta Coefficient |
| BMI | Body Mass Index |
| BSA | Body Surface Area |
| CCA | Common Carotid Artery |
| CCBs | Calcium Channel Blockers |
| cIMT | Carotid Intima–Media Thickness |
| cMRI | Cardiac Magnetic Resonance Imaging |
| CRP | C-Reactive Protein |
| DBP | Diastolic Blood Pressure |
| DUs | Digital Ulcers |
| ECA | External Carotid Artery |
| EDV | End-Diastolic Velocity |
| ERAs | Endothelin Receptor Antagonists |
| GIRRCS | Gruppo Italiano per la Ricerca e la Ricerca Clinica sulla Sclerodermia |
| HDL | High-Density Lipoprotein |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| HR | Heart Rate |
| HRCT | High-Resolution Computed Tomography |
| hs-TnT | High-Sensitivity Troponin T |
| ICA | Internal Carotid Artery |
| ILD | Interstitial Lung Disease |
| IMT | Intima–Media Thickness |
| IQR | Interquartile Range |
| LDL | Low-Density Lipoprotein |
| mRSS | Modified Rodnan Skin Score |
| NASCET | North American Symptomatic Carotid Endarterectomy Trial |
| NT-proBNP | N-terminal pro Brain Natriuretic Peptide |
| NVC | Nailfold Videocapillaroscopy |
| PAH | Pulmonary Arterial Hypertension |
| PDE5 i | Phosphodiesterase Type 5 Inhibitors |
| PSV | Peak Systolic Velocity |
| RI | Resistive Index |
| RP | Raynaud’s Phenomenon |
| SBP | Systolic Blood Pressure |
| Scl-70 | Anti-Topoisomerase I Antibodies |
| SSc | Systemic Sclerosis |
| TG | Triglycerides |
| Tyg | Triglyceride–Glucose Index |
| VEDOSS | Very Early Diagnosis of Systemic Sclerosis |
References
- Mok, M.Y.; Lau, C.S. The burden and measurement of cardiovascular disease in SSc. Nat. Rev. Rheumatol. 2010, 6, 430–434. [Google Scholar] [CrossRef]
- Keret, S.; Rimar, D.; Lansiaux, P.; Feldman, E.; Lescoat, A.; Milman, N.; Farge, D.; MATHEC working group. Differentially expressed genes in systemic sclerosis: Towards predictive medicine with new molecular tools for clinicians. Autoimmun. Rev. 2023, 22, 103314. [Google Scholar] [CrossRef] [PubMed]
- Bandini, G.; Bellando Randone, S.; Manetti, M.; Dagna, L.; Matucci Cerinic, M.; Moggi Pignone, A. Endotheliopathy in systemic sclerosis: From endothelium-dependent vasodilation to the dysfunction of the vascular reserve, is the paradise lost? Arthritis Res. Ther. 2025, 27, 107. [Google Scholar] [CrossRef] [PubMed]
- Lescoat, A. Very Early Diagnosis of Systemic Sclerosis: Deciphering the heterogeneity of systemic sclerosis in the very early stages of the disease. J. Scleroderma Relat. Disord. 2023, 8, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, M.T.; Hou, Y.; Wang, Q.; Hu, C.J.; Song, N.; Zhao, J.L.; Zeng, X.F.; Zhang, F.C. Clinical characteristics of systemic sclerosis patients with digital ulcers in China. Clin. Exp. Rheumatol. 2013, 31 (Suppl. S76), 46–49. [Google Scholar]
- Mouthon, L.; Mestre-Stanislas, C.; Bérezné, A.; Rannou, F.; Guilpain, P.; Revel, M.; Pagnoux, C.; Guillevin, L.; Fermanian, J.; Poiraudeau, S. Impact of digital ulcers on disability and health-related quality of life in systemic sclerosis. Ann. Rheum. Dis. 2010, 69, 214–217. [Google Scholar] [CrossRef]
- Mihai, C.; Distler, O.; Gheorghiu, A.M.; Constantin, P.I.; Dobrota, R.; Jordan, S.; Smith, V.; Hachulla, E.; Henes, J.; Siegert, E.; et al. EUSTAR collaborators, Incidence and risk factors for gangrene in patients with systemic sclerosis from the EUSTAR cohort. Rheumatology 2020, 59, 2016–2023. [Google Scholar] [CrossRef]
- Patnaik, E.; Lyons, M.; Tran, K.; Pattanaik, D. Endothelial Dysfunction in Systemic Sclerosis. Int. J. Mol. Sci. 2023, 24, 14385. [Google Scholar] [CrossRef]
- McMahan, Z.H.; Wigley, F.M. Raynaud’s phenomenon and digital ischemia: A practical approach to risk stratification, diagnosis and management. Int. J. Clin. Rheumtol. 2010, 5, 355–370. [Google Scholar] [CrossRef]
- Edjlali-Goujon, M.; Alison, D. Acute Digital Ischaemia-What The Radiologist Needs to Know. Eur. Cardiol. 2011, 7, 10–13. [Google Scholar]
- Pahwa, R.; Jialal, I. Atherosclerosis; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Rollando, D.; Bezante, G.P.; Sulli, A.; Balbi, M.; Panico, N.; Pizzorni, C.; Negrini, S.; Brunelli, C.; Barsotti, A.; Cutolo, M.; et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J. Rheumatol. 2010, 37, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Liakouli, V.; Verde, I.; Ruscitti, P.; Di Vico, C.; Ruggiero, A.; Mauro, D.; Forte, G.; Navarini, L.; Di Donato, S.; Bearzi, P.; et al. Clinical and subclinical atherosclerosis in patients with systemic sclerosis: An observational, multicentre study of GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale). Clin. Exp. Rheumatol. 2024, 42, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Bedi, R.; Nagra, A.; Fukumoto, T.; Lynum, S.; Sengupta, P.; Aw, J.; Mefford, I.; Panwar, S.R.; Bansal, N.; Insaan, P.; et al. Detection of subclinical atherosclerosis in peripheral arterial beds with B-mode ultrasound: A proposal for guiding the decision for medical intervention and an artifact-corrected volumetric scoring index. Glob. Heart 2014, 9, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Leening, M.J.; Norby, F.L.; Roetker, N.S.; Hofman, A.; Franco, O.H.; Pan, W.; Polak, J.F.; Witteman, J.C.; Kronmal, R.A.; et al. Carotid Intima-Media Thickness and Arterial Stiffness and the Risk of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study, Multi-Ethnic Study of Atherosclerosis (MESA), and the Rotterdam Study. J. Am. Heart Assoc. 2016, 5, e002907. [Google Scholar] [CrossRef]
- Bill, O.; Mazya, M.V.; Michel, P.; Prazeres Moreira, T.; Lambrou, D.; Meyer, I.A.; Hirt, L. Intima-Media Thickness and Pulsatility Index of Common Carotid Arteries in Acute Ischaemic Stroke Patients with Diabetes Mellitus. J. Clin. Med. 2022, 12, 246. [Google Scholar] [CrossRef]
- Morales, M.M.; Anacleto, A.; Filho, C.M.; Ledesma, S.; Aldrovani, M.; Wolosker, N. Peak Systolic Velocity for Calcified Plaques Fails to Estimate Carotid Stenosis Degree. Ann. Vasc. Surg. 2019, 59, 1–4. [Google Scholar] [CrossRef]
- Alexandrov, A.V.; Tsivgoulis, G.; Rubiera, M.; Vadikolias, K.; Stamboulis, E.; Molina, C.A.; Alexandrov, A.W. TUCSON Investigators. End-diastolic velocity increase predicts recanalization and neurological improvement in patients with ischemic stroke with proximal arterial occlusions receiving reperfusion therapies. Stroke 2010, 41, 948–952. [Google Scholar] [CrossRef]
- Delong, C.; Sharma, S. Physiology, Peripheral Vascular Resistance; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef]
- Cutolo, M.; Matucci Cerinic, M. Nailfold capillaroscopy and classification criteria for systemic sclerosis. Clin. Exp. Rheumatol. 2007, 25, 663–665. [Google Scholar]
- Rehman, S.; Hashmi, M.F. Blood Pressure Measurement; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hemann, B.A.; Bimson, W.F.; Taylor, A.J. The Framingham Risk Score: An appraisal of its benefits and limitations. Am. Heart Hosp. J. 2007, 5, 91–96. [Google Scholar] [CrossRef]
- Duttagupta, S.; Thachathodiyl, R.; Rameshan, A.; Venkatachalam, A.; Georgy, S.; Ts, D.; Menon, J. Effectiveness of Framingham and ASCVD Risk Scores in Predicting Coronary Artery Disease-A Comparative Study with Syntax Score. J. Assoc. Physicians India. 2022, 69, 11–12. [Google Scholar]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, H.; Sun, W.; An, X.; Lian, F. Triglyceride glucose (TyG) index: A promising biomarker for diagnosis and treatment of different diseases. Eur. J. Intern. Med. 2025, 131, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.; Pintó, X.; Muñoz, A.; Zúñiga, M.; Rubiés-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernández-Mijares, A.; González-Santos, P.; et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar] [PubMed] [PubMed Central]
- Babic, N.; Valjevac, A.; Zaciragic, A.; Avdagic, N.; Zukic, S.; Hasic, S. The Triglyceride/HDL Ratio and Triglyceride Glucose Index as Predictors of Glycemic Control in Patients with Diabetes Mellitus Type 2. Med. Arch. 2019, 73, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Can, G.; Kaya, H.; Hergenç, G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J. Clin. Lipidol. 2010, 4, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Majid, H.; Masood, Q.; Khan, A.H. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR): A Better Marker for Evaluating Insulin Resistance Than Fasting Insulin in Women with Polycystic Ovarian Syndrome. J. Coll Physicians Surg. Pak 2017, 27, 123–126. [Google Scholar] [PubMed]
- Seekircher, L.; Tschiderer, L.; Lind, L.; Safarova, M.S.; Kavousi, M.; Ikram, M.A.; Lonn, E.; Yusuf, S.; Grobbee, D.E.; Kastelein, J.J.P.; et al. Intima-media thickness at the near or far wall of the common carotid artery in cardiovascular risk assessment. Eur. Heart J. Open 2023, 3, oead089. [Google Scholar] [CrossRef]
- Touboul, P.J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez Hernandez, R.; et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar]
- Valaikiene, J.; Schuierer, G.; Ziemus, B.; Dietrich, J.; Bogdahn, U.; Schlachetzki, F. Transcranial color-coded duplex sonography for detection of distal internal carotid artery stenosis. AJNR Am. J. Neuroradiol. 2008, 29, 347–353. [Google Scholar] [CrossRef]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators; Barnett, H.J.M.; Taylor, D.W.; Haynes, R.B.; Sackett, D.L.; Peerless, S.J.; Ferguson, G.G.; Fox, A.J.; Rankin, R.N.; Hachinski, V.C.; et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991, 325, 445–453. [Google Scholar] [PubMed]
- Grant, E.G.; Benson, C.B.; Moneta, G.L.; Alexandrov, A.V.; Baker, J.D.; Bluth, E.I.; Carroll, B.A.; Eliasziw, M.; Gocke, J.; Hertzberg, B.S.; et al. Carotid artery stenosis: Gray-scale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003, 229, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Jones, X.M.; Bottini, N.; Boin, F.; Marbán, E. Cardiac involvement in systemic sclerosis: A critical review of knowledge gaps and opportunities. J. Scleroderma Relat. Disord. 2025, 10, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Capparelli, E.; Zaccara, E.; Suardi, I.; Laria, A.; Castelnovo, L.; Mauric, E.; Bompane, D.; Tamburello, A.; Iacovantuono, M.; Chimenti, M.S.; et al. Uncovering Subclinical Cardiac Involvement in VEDOSS: An Echocardiographic Driven Study. Sclerosis 2025, 3, 7. [Google Scholar] [CrossRef]
- Sherif, A.A.; Gilvaz, V.J.; Abraham, S.; Saji, A.M.; Mathew, D.; Isath, A.; Rajendran, A.; Contreras, J.; Lanier, G.M.; Reginato, A.M. Systemic sclerosis is associated with increased in-patient mortality in patients hospitalized for heart failure. ESC Heart Fail. 2024, 11, 1900–1910. [Google Scholar] [CrossRef]
- Tyndall, A.J.; Bannert, B.; Vonk, M.; Airò, P.; Cozzi, F.; Carreira, P.E.; Bancel, D.F.; Allanore, Y.; Müller-Ladner, U.; Distler, O.; et al. Causes and risk factors for death in systemic sclerosis: A study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann. Rheum. Dis. 2010, 69, 1809–1815. [Google Scholar] [CrossRef]
- Györfi, A.H.; Filla, T.; Polzin, A.; Tascilar, K.; Buch, M.; Tröbs, M.; Matei, A.E.; Airo, P.; Balbir-Gurman, A.; Kuwert, F.; et al. Evaluation of Systemic Sclerosis Primary Heart Involvement and Chronic Heart Failure in the European Scleroderma Trials and Research Cohort. J. Am. Heart Assoc. 2025, 14, e036730. [Google Scholar] [CrossRef]
- Caimmi, C.; De Marchi, S.; Bosello, S.L.; Giuggioli, D.; Caramaschi, P.; Di Giorgio, A.; Spinella, A.; Astorino, G.; Canestrari, G.; Cocchiara, E.; et al. Ultrasonography involvement of carotid, upper and lower limb arteries in a large cohort of systemic sclerosis patients. Int. J. Rheum. Dis. 2020, 23, 681–692. [Google Scholar] [CrossRef]
- Motegi, S.; Toki, S.; Hattori, T.; Yamada, K.; Uchiyama, A.; Ishikawa, O. No association of atherosclerosis with digital ulcers in Japanese patients with systemic sclerosis: Evaluation of carotid intima-media thickness and plaque characteristics. J. Dermatol. 2014, 41, 604–608. [Google Scholar] [CrossRef]
- Sayan, M.; Raaj, K.B.; Hooijenga, P.; Cassidy, S.; Nova, A.; De Ciutiis, I.; Wang, T.; Kroeger, C.M.; Stamatakis, E.; Masedunskas, A.; et al. Carotid intima-media thickness, cardiovascular disease, and risk factors in 29,000 UK Biobank adults. Am. J. Prev. Cardiol. 2025, 22, 101011. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Blagojevic, J.; Bacci, M.; Fiori, G.; Tempestini, A.; Conforti, M.L.; Guiducci, S.; Miniati, I.; Di Chicco, M.; Del Rosso, A.; et al. Flow-mediated vasodilation and carotid intima-media thickness in systemic sclerosis. Ann. N. Y. Acad. Sci. 2007, 1108, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Sedky Abdou, M.M.; El Desouky, S.M.; Helmy El Kaffas, K.M.; Ahmed Hassan, A.M. Premature atherosclerosis in systemic sclerosis patients: Its relation to disease parameters and to traditional risk factors. Int. J. Rheum. Dis. 2017, 20, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Soltész, P.; Kerekes, G.; Dér, H.; Szücs, G.; Szántó, S.; Kiss, E.; Bodolay, E.; Zeher, M.; Timár, O.; Szodoray, P.; et al. Comparative assessment of vascular function in autoimmune rheumatic diseases: Considerations of prevention and treatment. Autoimmun. Rev. 2011, 10, 416–425. [Google Scholar] [CrossRef]
- Frerix, M.; Stegbauer, J.; Kreuter, A.; Weiner, S.M. Atherosclerotic plaques occur in absence of intima-media thickening in both systemic sclerosis and systemic lupus erythematosus: A duplex sonography study of carotid and femoral arteries and follow-up for cardiovascular events. Arthritis Res. Ther. 2014, 16, R54. [Google Scholar] [CrossRef]
- Schiopu, E.; Au, K.M.; McMahon, M.A.; Kaplan, M.J.; Divekar, A.; Singh, R.R.; Furst, D.E.; Clements, P.J.; Ragvendra, N.; Zhao, W.; et al. Prevalence of subclinical atherosclerosis is increased in systemic sclerosis and is associated with serum proteins: A cross-sectional, controlled study of carotid ultrasound. Rheumatology 2014, 53, 704–713. [Google Scholar] [CrossRef]
- Cannarile, F.; Valentini, V.; Mirabelli, G.; Alunno, A.; Terenzi, R.; Luccioli, F.; Gerli, R.; Bartoloni, E. Cardiovascular disease in systemic sclerosis. Ann. Transl. Med. 2015, 3, 8. [Google Scholar]
- Strosberg, D.S.; Haurani, M.J.; Satiani, B.; Go, M.R. Common carotid artery end-diastolic velocity and acceleration time can predict degree of internal carotid artery stenosis. J. Vasc. Surg. 2017, 66, 226–231. [Google Scholar] [CrossRef]
- Oreska, S.; Tomcik, M. Atherosclerosis and Cardiovascular Risk in Systemic Sclerosis. In Systemic Sclerosis; InTech: London, UK, 2017. [Google Scholar]
- Sanz Pérez, I.; Martínez Valle, F.; Guillén-Del-Castillo, A.; Roque Pérez, A.; Cuéllar Calàbria, H.; Pizzi, M.N.; Fernández Codina, A.; Callejas-Moraga, E.; Orozco Gálvez, O.; Fonollosa Pla, V.; et al. Subclinical cardiovascular disease and Systemic Sclerosis: A comparison between risk charts, quantification of coronary calcium and carotid ultrasonography. Autoimmun. Rev. 2018, 17, 900–905. [Google Scholar] [CrossRef]
- Cutolo, C.A.; Cere, A.; Toma, P.; Cannavacciuolo, T.; Toma, C.; Balito, S.; Gerli, V.; Smith, V.; Sulli, A.; Paolino, S.; et al. Peripheral and ocular microvascular alterations in systemic sclerosis: Observations from capillaroscopic assessments, perfusion peripheral analysis, and optical coherence tomography angiography. Rheumatol. Int. 2024, 44, 107–118. [Google Scholar] [CrossRef]
- Triggianese, P.; D’Antonio, A.; Nesi, C.; Kroegler, B.; Di Marino, M.; Conigliaro, P.; Modica, S.; Greco, E.; Nucci, C.; Bergamini, A.; et al. Subclinical microvascular changes in ANCA-vasculitides: The role of optical coherence tomography angiography and nailfold capillaroscopy in the detection of disease-related damage. Orphanet J. Rare Dis. 2023, 18, 184. [Google Scholar] [CrossRef]
- Ferrigno, S.; Conigliaro, P.; Rizza, S.; Longo, S.; Nesi, C.; Carlucci, F.; Bergamini, A.; Mancino, R.; Nucci, C.; Federici, M.; et al. Relationship between retinal microvascular impairment and subclinical atherosclerosis in SLE. Lupus Sci. Med. 2023, 10, e000977. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).