Abstract
L-methioninase is an enzyme that has recently gained significant interest in the scientific community because of its potential as a targeted therapy for cancer. This study aims to isolate and identify extremophilic bacteria that could produce L-methioninase and to access the enzymatic potential of isolated bacteria under stress conditions, specifically in agro-industrial waste. In this study, a rare marine bacterium, Alcaligenes aquatilis BJ-1, exhibited the highest specific activity of 4.61 U/mg at an optimum pH of 8.3. The L-methioninase was purified 4.3-fold and 7.15-fold by acetone precipitation and Sephadex G-100 gel filtration chromatography, which revealed a molecular weight of 46 kDa. In addition, agriculture waste materials such as cottonseed oil cake had the highest L-methioninase production. Moreover, A. aquatilis BJ-1 can tolerate and produce enzymes in the presence of 10% NaCl, 6% KCl, and 4% MgSO4. Similarly, substrates such as L-asparagine, L-glutamine, L-alanine, and L-tyrosine were found suitable to increase enzyme production. The strain produced L-methioninase in the presence of various heavy metals. Maximum enzyme activity was found in Zn2+ at 0.1% (2.52 U/mL), Li2+ at 0.03% (2.90 U/mL), and Ni2+ at 0.01% (2.78 U/mL), as compared to the control (2.23 U/mL) without metal. Enzyme production was also observed at a high temperature (60 °C), with the produced enzymes possessing antioxidant properties. In addition, no hemolytic activity was observed. The results indicate that A. aquatilis BJ-1 is an appropriate bacterium for metal bioremediation procedures in unfavorable circumstances.
1. Introduction
Cancer is a multifaceted illness characterized by several mechanisms involving behavioral and metabolic changes in cells, allowing them to reproduce uncontrollably. Cancer development is also influenced by genetic, epigenetic, and other factors [1]. Conventional therapies such as surgery, chemotherapy, radiation, and ultrasound fail to completely eliminate cancer and frequently negatively affect healthy cells [2]. As a result, modern cancer treatments have focused on using medications, biological agents, and immune-related approaches. Using enzymes to prevent the accessibility of essential nutrients necessary for the rapid proliferation of tumor cells is a unique method for combating cancer. Anti-tumor enzymes are progressively gaining application in therapy. As previously stated, the medicinal use of enzymes is based on their ability to reduce the blood levels of nutrients required to develop tumors. Typically, such compounds are amino acids in nature. A few key characteristics required of these anticancer enzymes include the following: They need to be sufficiently stable in the bloodstream and other bodily fluids, have high activity at physiological pH, and possess a low Km value for the suitable substrates. They should not have to be inhibited by the product of the reaction even at elevated concentrations, and additionally should not require a cofactor along with an easily dissociable prosthetic group to function. Finally, they should have relatively few impurities in their preparations, such as endotoxins [3].
Recently, many anticancer enzymes have received more attention, including L-asparaginase (E.C.3.5.1.1), L-arginase (E.C.3.5.3.1), L-methioninase (E.C.4.4.1.11), and L-glutaminase (E.C.3.5.1.2) [4]. L-asparaginase is an anticancer enzyme that is used to treat acute myelocytic leukemia, Hodgkin’s disease, lymphosarcoma, and melanoma sarcomas [5]. Arginase cleaves the L-arginine amino acid into urea and ornithine, causing malignant cells to starve to death [6]. L-glutaminase converts L-glutamine into glutamic acid and ammonia, which are used in treating acute lymphoblastic leukemia [7]. Regrettably, these enzymes frequently cause destruction of the kidney and central nervous system in animal and human clinical trials [3]. Also, various problems and side effects are associated with using asparaginase, ranging from mild (headache, weakness, and anorexia) to severe (hepatic damage and anaphylactic shock). The use of additional enzymes to convert essential amino acids appears promising. As a result, discovering new biological sources of anticancer enzymes to overcome immunogenicity will be critical for developing cancer therapies.
L-methioninase (EC 4.4.1.11) is a homotetrameric, multifunctional enzyme that belongs to the γ family of pyridoxal-5-phosphate (PLP)-dependent enzymes, and the active methioninase tetramer consists of two sets of strongly associated catalytic dimers [8]. It catalyzes the conversion of L-methionine into α-ketobutyrate, methanethiol (MTL), and ammonia by γ-elimination mechanism [9]. L-methioninase can suppress a variety of cell lines, including lung, kidney, glioblastoma, and colon cancer cell lines [10]. L-methionine is a necessary amino acid for the survival and proliferation of human tumors and several cancer cell lines [11]. In contrast, healthy cells are independent of methionine because they possess active synthase. As a result, instead of methionine, they can thrive on a medium enriched with homocysteine, vitamin B12, and folic acid. The absence of the methionine synthase enzyme in many tumors, as opposed to normal cells, provides a basis for understanding the loss of the ability of tumor cells to rely on homocysteine [12,13]. L-methionine is, therefore, the key focus for tumor-specific therapies. In this context, the therapeutic use of L-methioninase to lower methionine in the plasma appears as a potential approach [10]. Lack of methionine in the blood causes the arrest of cancer cells in the S-G2 phase of the cell cycle, which results in the death of cells [14]. In addition to having antioxidant activity by reducing polyamines, L-methioninase has been suggested as a possible treatment for methionine auxotrophic malignancies and utilized as an antioxidant, antifungal, bactericide, and antiprotozoal agent [15,16,17,18]. In addition, L-methioninase has been used in the food industry. The L-methioninase product methanethiols combine with acyl-coenzyme A and produce various sulfur-containing chemical substances that play an essential part in the flavoring and aroma of cheese, for example, S-methyl thioester, dimethyl trisulfide (DMTS), and dimethyl disulfide (DMDS) [19,20].
L-methioninase has been reported in bacteria such as Brevibacterium linens [21], Aeromonas sp. [22], Ferroplasma acidarmanus [23], Serratia marcescens [24], Clostridium sporogenes [25], Lactococcus lactis [26], Pseudomonas putida [27], Streptomyces sp. [28], Micromonospora echinospora [29], Enterobacter Cloacae [30], Bacillus haynesii [9], Citrobacter freundii [31], Methylobacterium sp. [32], Idiomarina sp. [33], Treponema denticola [34], Hafnia alvei [35] and fungi include Aspergillus ustus [36], Aspergillus flavipes [37], Aspergillus fumigatus [38], Trichoderma harzianum [39], Fusarium nivale [40], F. oxysporum [40], F. solani [40], Penicillium digitatum [40], Saccharomyces cerevisiae [40], Geotrichum candidum [41], and Candida tropicalis [42]. A well-known characteristic of bacteria is that they produce a variety of secondary metabolites with antioxidant properties that particularly target tumor cells, resulting in the removal of tumors [43]. Though the production of methioninase from fungi has been shown to be easier and more effective, the allergic response caused by fungi is of major concern. Therefore, compared to fungal sources, exploring bacterial sources of L-methioninase appears safer [44].
Given the necessity for L-methioninase with high specificity, high thermostability, and stability over a wide range of pH levels in therapeutic and other industrial uses, one possibility could be to utilize bacterial sources. Therefore, the current study attempted to isolate potent L-methioninase-producing bacteria from Mangrove-associated sediment samples, screen for the highest L-methioninase production using agro-industrial waste material (groundnut and cotton seed oil cake), and analyze the effects of environmental stress in the presence of salts, temperature, and heavy metals. In addition, we studied the hemolytic and antioxidant activities of the enzyme for its use as an anticancer agent. As far as we know, this is the first report of Alcaligenes aquatilis species as an L-methioninase enzyme producer, which might be a promising alternative resource for cancer treatment.
2. Materials and Methods
The media, chemicals, and solvents used in this study were purchased from Hi-Media, Merck, and Sigma-Aldrich with purity of chemicals (99%), and solvents (AR grade).
2.1. Sample Collection, Isolation, and Identification of Microbes
Samples were collected from the Saurashtra coastal line of Gujarat (21.5136070 N 72.2570415 E), India. Mangrove-associated sediment samples were gathered in triplicate, and a sterile polyethylene Ziplock bag was used and transported to the laboratory under controlled conditions (in an icebox). The collected sample was used for the isolation of the microorganisms. 1 g of sediment was taken, resuspended in Zobell marine broth, and incubated for 48 h at 37 °C in shaking conditions to increase vegetative cell growth. After incubation, the media were serially diluted from 10−1 to 10−5. A 100 μL diluted sample was spread on Zobell marine agar plates and incubated in a bacteriological incubator at 37 °C. After incubation, isolated colonies were purified and stored at 4 °C for further use.
The bacterial isolate was subjected to molecular identification, carried out by 16S rRNA gene sequencing. The Sanger sequencing method was used for molecular identification, and sequencing was carried out at the Gujarat Biotechnology Research Centre (GBRC), Gandhinagar. The consensus sequence used the BLAST algorithm to compare to the NCBI database, and the sequence was deposited in the GenBank database. The phylogenetic tree was prepared using Mega X software.
2.2. Rapid Screening of Isolates
The method used to screen isolates for L-methioninase production was adapted from Mohkam et al. [44]. To perform the qualitative screening, isolated colonies were streaked onto a modified M9 medium (g/L) containing Na2HPO4 (6.0), L-methionine (10.0), MgSO4 (0.24), NaCl (0.5), KH2PO4 (3.0), D-glucose (2.0), CaCl2 (0.011), and bromophenol blue as an indicator. The pH was adjusted to 7.0, and 2.5% agar was added as a solidifying agent. The M9 medium plates were inoculated and incubated for 24–48 h at 37 °C. The next day, a color change from green to blue was observed evidencing the detection of L-methioninase production.
2.3. Production of L-Methioninase by Submerged Fermentation
A modified M9 medium was used to carry out the production of L-methioninase. The modification of the M9 medium using different raw materials, such as groundnut oil cake (GOC) and cottonseed oil cake (COC), was used to produce the enzyme. Subsequently, 2.5 g of each raw material was added to 90 mL of modified M9 medium, and the contents were mixed thoroughly and autoclaved at 121 °C and 15 psi for 15 min. Fermentation broth was inoculated and incubated at 37 °C in a rotatory shaker for enzyme production.
2.4. Extraction and Partial Purification of the Enzyme
After enzyme production, the fermentation broth was subjected to centrifugation at 4 °C at 10,000 rpm for 10 min (Eppendorf, Framingham, MA, USA). The supernatant was collected in sterile tubes and used as an enzyme sample to measure the enzyme activity.
The enzyme was partially purified by the solvent precipitation method with some modifications [45]. The crude extract of enzyme and chilled solvent (acetone) (1:4) were vigorously mixed in a magnetic stirrer at 4 °C. The mixture was centrifuged under the same conditions as above. The precipitate protein was dissolved in a 100 mM potassium phosphate buffer, pH 7.0, and stored at 4 °C.
2.5. Purification of L-Methioninase by Gel Filtration Chromatography and Molecular Weight Determination by SDS-PAGE
The partially purified enzyme was applied to a Sephadex G-100 column pre-equilibrated with 50 mM potassium phosphate buffer, pH 7.0. The fractions were eluted using 50 mM potassium phosphate buffer, pH 7.0, at a 1 mL/min flow rate, and a total of nine fractions were collected. Protein content and enzyme activity of all fractions were measured. Fractions (4, 5, and 6) with maximum enzyme activity were combined [37].
The purified enzyme molecular weight was measured using SDS-PAGE employing the protocol of Lammeali (1970) using standard protein ladders [46].
2.6. Enzyme Assay
Enzyme activity was measured by determining the liberated ammonia using the Nesslerization method. The supernatant containing the crude enzyme was utilized for the L-methioninase enzyme assay using direct Nesslerization; the method was modified from Spinnler et al. [47]. The experiment was carried out by the incubation of 1 mL of crude enzyme extract with 1 mL of 1% L-methionine (substrate) prepared in 100 mM potassium phosphate buffer, pH 7.0, containing 0.01 mM pyridoxal 5-phosphate (PLP). The reaction was performed at ambient temperature for 20 min. The enzyme-catalyzed process was terminated by the addition of 0.2 mL of 1.5 M trichloroacetic acid. The released ammonia was detected by the addition of 0.2 mL of Nessler’s reagent and measured at 425 nm in a UV–Visible spectrophotometer UV-1900i (Shimadzu, Kyoto, Japan). One unit (U) of L-methioninase was revealed as the amount of enzyme that liberates one micromole (μmol) of ammonia per minute in an optimal condition.
2.7. Confirmatory Test for L-Methioninase Enzyme
Several enzymes can transform amino acids or other nitrogenous substrates into ammonia. As a result, the formation of L-methioninase was further validated by determining the amount of methanthiols, and specific thiol groups were detected using 5,5-dithiobis-2-nitrobenzoic acid (DTNB). The produced methanethiols reacted with DTNB to yield thionitrobenzoic acid (TNB), quantified spectrophotometrically at 412 nm. The mixture contained 20 mM L-methionine in 100 mM potassium phosphate buffer, pH 7.0, 0.01 mM PLP, 0.25 mM DTNB, and the desired cell-free extract to prepare up to 1 mL as the final volume. The reaction was set at 37 °C for 20 min, and an increase in optical density by developing a yellow color was measured at 412 nm [48].
2.8. Estimation of Total Protein Content
The protein content of the enzyme sample was determined using the Lowry method [49]. A 200 g/mL bovine serum albumin concentration was used as a standard. The crude sample protein concentration was measured using the Folin–Ciocalteu reagent. The total protein concentration was expressed as mg/mL from the crude extract and purified sample. The unit enzyme activity in the crude extract was denoted as a unit per mL (U/mL), and the specific activity was denoted as a unit activity per milligram of protein (U/mg).
2.9. Effect of Biotic and Abiotic Factors
The effect of different parameters on bacterial growth and enzyme production was investigated by inoculating Alcaligenes aquatilis in a modified M9 medium and incubating it at 37 °C in a rotatory shaker. After incubation at 24, 48, 72, and 96 h, the pH of the fermentation medium of A. aquatilis was measured, and L-methioninase production was determined by Nessler’s method [47].
The effect of temperature was investigated by inoculating the M9 medium and incubating it at various temperatures such as 20, 30, 40, 50 °C, and 60 °C for 48 h. The cell-free supernatant was obtained after incubation and used as an enzyme sample to observe L-methioninase activity. The thermal stability of the L-methioninase enzyme was determined by pre-incubating the enzyme for 1 h at various temperatures (20 to 70 °C). After incubation, the enzyme was cooled, and an assay was performed to measure residual activity.
Similarly, the effects of heavy metals (i.e., MnSO4, ZnSO4, FeSO4, CuSO4, NiSO4, and BaCl2 at 0.1%; CoCl2 and LiSO4 at 0.03%; and HgSO4 and Lead acetate at 0.01%) were supplemented in different concentrations to evaluate growth and enzyme activity.
Other biotic factors, such as salt concentrations of NaCl (2–10%), KCl (2–6%), and MgSO4 (2–4%), were studied to check the salt tolerance capabilities of the isolate. Moreover, the effect of various amino acids as nitrogen sources were supplemented to assess the efficiency of L-methioninase initiation in the presence of L-asparagine, L-cysteine, L-glutamine, L-arginine, L-histidine, L-alanine, L-tyrosine, and L-serine, at a concentration of 5 g/L of each. M9 medium supplemented with L-methionine acted as a control. The medium was incubated in a rotatory shaker at 37 °C for 96 h, and the enzyme activity was measured as per the method described above [35,40,50].
2.10. Antioxidant Activity
The radical scavenging enzyme activity was evaluated using the 2,2-diphenyl-1-picryl-hydrazylhydrate (DPPH) assay with minor modifications in the method of Apostolou et al. [51]. Various enzyme concentrations were prepared using a phosphate buffer at pH 8.0. A mixture of 200 µL enzyme and 100 µL DPPH was reacted at ambient temperature for 30 min in the dark. A sample without an enzyme was used as a control. The absorbance at 517 nm was measured, and the following equation was used for the estimation of DPPH radical scavenging activity.
2.11. Hemolytic Activity of L-Methioninase
A hemolytic assay was used to examine the impact of L-methioninase on human red blood cells, and a qualitative hemolytic assay was performed [52]. Subsequently, 3% freshly defibrinated human blood was used to prepare agar plates. The 50 µL enzyme at various concentrations (U/mL) was placed into agar wells on blood agar, incubated at 37 °C for 24 h, and hemolysis activity was monitored. As a positive and negative control, 1% SDS and phosphate buffer were used, respectively. The crude enzyme was used to evaluate anticancer activity in the liver cancer cell line.
The quantitative hemolytic activity was carried out according to Bulmus et al. [53]. A 150 mM NaCl solution was used to wash blood multiple times, and the erythrocytes were pelleted and resuspended in a 100 mM sodium phosphate buffer, pH 7.0. The erythrocytes were incubated with various enzyme concentrations (U/mg) at 37 °C for 24 h. As a blank, phosphate buffer was used in place of the enzyme, and 1% SDS solution was used as a positive control. The solutions were spun down at 2500 rpm for 15 min after incubation. An absorbance at 541 nm was measured to evaluate the lysis of RBCs in the supernatant.
3. Results and Discussion
3.1. Isolation, Screening, and Identification of L-Methioninase-Producing Bacteria
A sample of sediment associated with mangroves was taken and inoculated with Zobell marine broth. A total of eight morphologically distinct isolates were obtained. All isolates were screened for L-methioninase production using a modified M9 medium containing L-methionine as the sole nitrogen source. The M9-modified medium’s color shift from green to blue indicated the production of ammonia due to an enzyme reaction (Figure 1). Among them, only one isolate was potentially found to produce the L-methioninase enzyme. Based on its morphological and physiological characteristics, it was observed to be a short, rod-shaped, Gram-negative bacterium that also produces pigments. Furthermore, molecular identification was carried out by targeting the 16S rRNA gene using 27 forward and 1492 reverse primers. Using the Sanger sequencing method, the identification was conducted at the Gujarat Biotechnology Research Centre (GBRC), Gandhinagar, Gujarat, India. The consensus sequence was compared to the NCBI database using the BLAST algorithm, and the isolate exhibited 99.71% similarity with Alcaligenes aquatilis strain NR_104977.1. The isolate that produced L-methioninase was identified as a strain of Alcaligenes aquatilis. This strain was named Alcaligenes aquatilis BJ-1, and the sequence was submitted to the NCBI database with accession no. OQ392439.1. A phylogenetic tree was constructed using the neighbor-joining method by MEGA11 software, and the tree showed relatedness with other strains. The tree revealed that Alcaligenes aquatilis BJ-1 and strain NR_104977.1 were closely related and formed a cluster, as depicted in Figure 2.
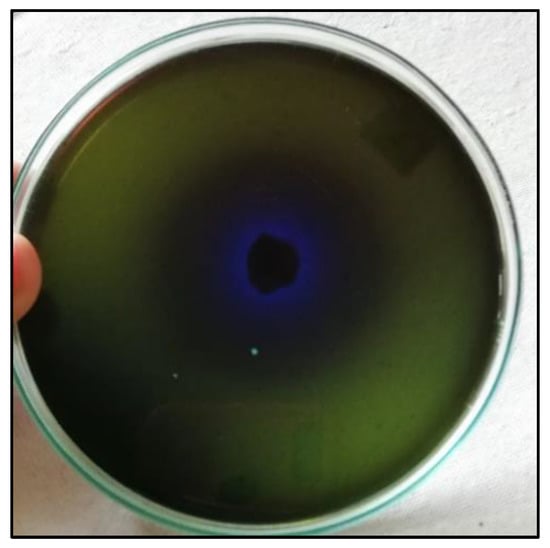
Figure 1.
Rapid screening of Alcaligenes aquatilis for production of L-methioninase enzyme using M9 medium after an incubation period of 48 h.
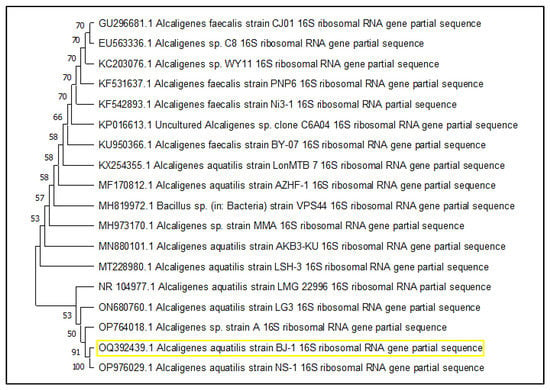
Figure 2.
The isolate was identified as Alcaligenes aquatilis BJ-1, the highlight OQ392439.1, by constructing a phylogenetic tree using the neighbor-joining method.
3.2. Production of L-Methioninase by Submerged Fermentation
L-methioninase production was carried out in a modified M9 medium under submerged fermentation conditions. L-methioninase production using submerged fermentation has several advantages over solid-state fermentation. Firstly, in submerged fermentation, the process is carried out in a liquid medium, which allows for better control of the fermentation conditions, such as pH, temperature, and oxygen supply. This precise control over the fermentation conditions helps optimize enzyme production and yield. Secondly, it has a higher productivity rate than solid-state fermentation because the liquid medium provides the microorganisms with better nutrient and oxygen availability. This results in increased microbial growth and enzyme production. Thirdly, submerged fermentation is easily scalable for commercial production as it can be readily adapted to larger bioreactors, making it suitable for industrial-scale production. Therefore, the advantages of submerged fermentation make it a more efficient and effective method for producing L-methioninase. Hence, L-methioninase production was carried out under controlled conditions. The experiment measured enzyme activity by determining the quantity of ammonia released using the Nessler method. The enzyme activity, growth, and pH of the fermentation media were monitored up to the 7th day. The growth of A. aquatilis was monitored at 600 nm. The enzyme activity varied between 0.22 and 1.14 U/mL, with the highest activity occurring on the fourth day at pH 8.3 and bacterial cell growth at 600 nm was 0.42 (Figure 3). However, as the incubation time increased, the activity of the enzyme decreased due to the excessive secretion of ammonia in the fermentation media, causing a rapid decline in enzyme activity. In a previous study, Abu-Tahon and Isaac [36] found that an alkaline environment (pH 8.5) resulted in maximum L-methioninase production using Aspergillus ustus. Bopaiah et al. [9] reported that the Bacillus haynesii JUB2 strain achieved maximum enzyme activity at pH 8.0.
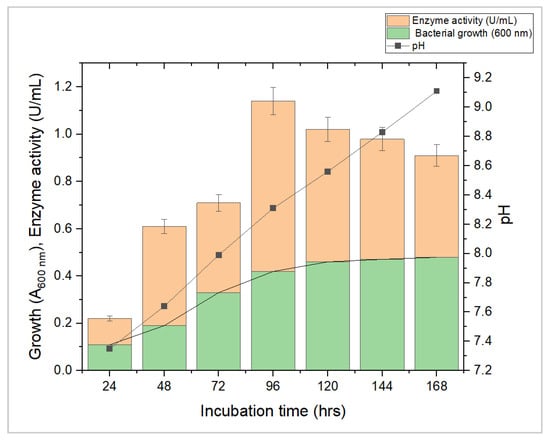
Figure 3.
Growth of Alcaligenes aquatilis, L-methioninase activity, and pH of production medium at different incubation times. All reactions were performed in triplicates, and results were displayed as the mean of the data obtained.
3.3. Production of L-Methioninase by Using Agro-Industrial Waste Material
In recent years, there has been growing attention toward utilizing agricultural waste for biotechnological applications such as agriculture, bioenergy, and pharmaceuticals. These wastes have varying nutritional compositions, such as high amounts of proteins, sugars, and minerals, making them suitable for microbial growth. The present study utilized cottonseed oil cake (COC) and groundnut oil cake (GOC) as substrates for L-methioninase enzyme production. The use of agricultural waste materials as a substrate for enzyme production has become increasingly popular due to several benefits, including the presence of essential nutrients such as minerals and nitrogen components, as well as being an economically viable, eco-friendly, sustainable, easily accessible, and cost-effective approach for value-added bioproduct development [54]. The current study focused on producing the L-methioninase enzyme from Alcaligenes aquatilis using COC and GOC as raw materials for carbon and energy sources. The current study showed that the use of agro-waste materials such as COC and GOC as substrates in the M9 medium led to an increase in L-methioninase enzyme production and specific activity. The M9 medium containing COC resulted in the highest enzyme activity and specific activity, followed by the M9 medium containing GOC. These results suggest that agro-waste materials can serve as a cost-effective and sustainable substrate for enzyme production. Previous studies have also reported using agro-waste materials for enzyme production, such as lipase, amylase, and asparaginase, and shown promising results [55,56,57].
Agricultural waste has been explored as an alternative and reliable carbon and energy source for developing valuable enzymes. The present study investigated COC and GOC as substrates for L-methioninase enzyme production. Results showed that M9 medium containing COC and GOC resulted in higher enzyme and specific activity than M9 medium without agro-waste, as shown in Figure 4. The protein content of the raw materials was also analyzed, with GOC found to have a higher protein content than COC due to its fatty acid content. The methionine content in GOC was also higher than in COC, ranging from 2–9% to 1–2%, which may affect enzyme production [50]. The study concluded that the amount of substrate in the media should be carefully controlled, as both excess and insufficient substrate levels may decrease enzyme yield. The optimum substrate level is required to produce the highest amount of the desired enzyme. The researcher suggests that the optimal level of the substrate should be used to achieve the best results. In this study, we focus on inducing an enzyme using different sources of amino acids. Essentially, our investigation presents an exploration of avenues to increase the production of the enzyme by providing the microorganism with the right amount and type of nutrients.
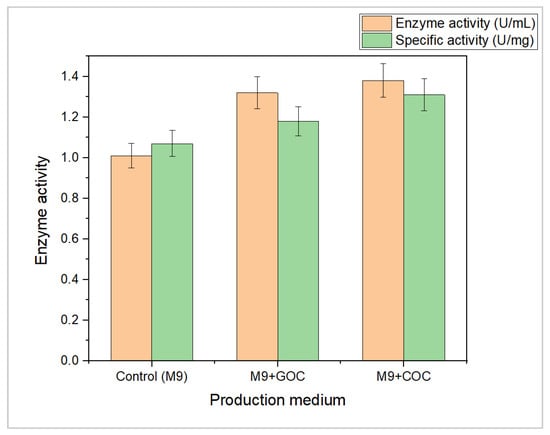
Figure 4.
Comparison of enzyme activity (U/mL) and specific activity (U/mg) of L-methioninase enzyme in agro-industrial waste materials [M9 medium (control); M9 + GOC (Groundnut oil cake); M9 + COC (cotton seed oil cake)]. Enzyme reactions were performed in triplicates, and results were given as the mean ± SD.
Several studies have reported that agro-waste materials are utilized to produce valuable bioproducts. Specifically, researchers in another study focused on producing two enzymes, L-asparaginase and lipase, using the microorganism Serratia marcescens. They found that using cottonseed oil cake and groundnut oil cake as carbon sources resulted in higher levels of L-asparaginase activity than other waste material types [8]. Cottonseed oil cake was found to exhibit the highest L-asparaginase activity of all the materials tested. On the other hand, groundnut oil cake was found to have the highest lipase activity due to its high crude protein content [58]. These findings suggest that using agro-waste materials can be a sustainable and cost-effective way to produce valuable enzymes.
3.4. Enzyme Extraction and Partial Purification
In the process described in the method, centrifugation separated the crude enzyme from the fermentation broth. Once this was completed, chilled acetone was added to the crude enzyme to isolate and concentrate it. The partially purified protein precipitates were then dissolved in a phosphate buffer solution. Furthermore, the amount of enzyme present in both the crude extract was measured and the sample purified using acetone. The measurements were taken to determine the enzyme concentration in each sample. The activity of the enzyme was measured in two different forms: the crude form and a partially purified form. The crude form had an activity of 1.01 U/mL, while the partially purified form had an activity of 0.6 U/mL. The specific activity of the enzyme is a measure of how active the enzyme is in relation to the amount of protein present. The specific activity of the crude enzyme was 1.07 U/mg, while the specific activity of the partially purified enzyme was 4.61 U/mg. This means that the partially purified enzyme was much more active than the crude enzyme relative to the amount of protein present. The partially purified enzyme was obtained by a process of purification that resulted in a 4.3-fold rise in the specific activity of the enzyme.
In other words, the purification process increased the concentration and activity of the enzyme, resulting in a 4.3-fold increase in its specific activity. This information is summarized in Table 1. Kavya and Nadumane [32] reported the partial purification of L-methioninase enzyme from two different strains, Methylobacterium sp., and Streptomyces sp., using acetone solvent. They achieved up to 3.46- and 1.74-fold purification of the enzyme, respectively. In comparison, the present study showed even better fold purification of the enzyme. The results suggest that the partially purified enzyme has the potential for further applications, and this indicates that using acetone as a solvent for the partial purification of L-methioninase could be a promising approach for its production and purification.

Table 1.
Purification profile of the L-methioninase enzyme from Alcaligenes aquatilis.
3.5. Purification of L-Methioninase by Gel Filtration Chromatography and Molecular Weight Determination by SDS-PAGE
First, the enzyme was purified through solvent precipitation using acetone. Then, the acetone-purified protein was further purified through gel filtration chromatography using Sephadex G-100. The enzyme was purified 7.15-fold with 7.66 U/mg specific activity by gel filtration chromatography (Table 1). According to earlier research, a 3.15-fold purification of L-methioninase was found in Streptomyces sp. through Sephadex-G column chromatography [57]. L-methioninase was purified 12.1-fold from Aspergillus flavipes by precipitation through ammonium sulfate, ion exchange, and gel filtration chromatography techniques [37]. Selim et al. [28] purified L-methioninase 2.55-fold from Streptomyces sp. DMMMH4 with a molecular mass of 47 kDa.
The purification method resulted in a pure enzyme showing a single band on gel with an approximate molecular weight of 46 kDa, as shown in Figure 5. The enzyme in this study had a molecular weight of 46 kDa, similar to that obtained from Trichoderma harzianum by Salim et al. [39]. The molecular mass of the enzyme is in accordance with that reported by L-methioninases purified from Aspergillus flavipes, which have a molecular mass of 47 kDa, and the enzyme purified from Citrobacter freundii, which has a molecular weight ranging from 43.0 to 45.0 kDa per subunit [37,59].
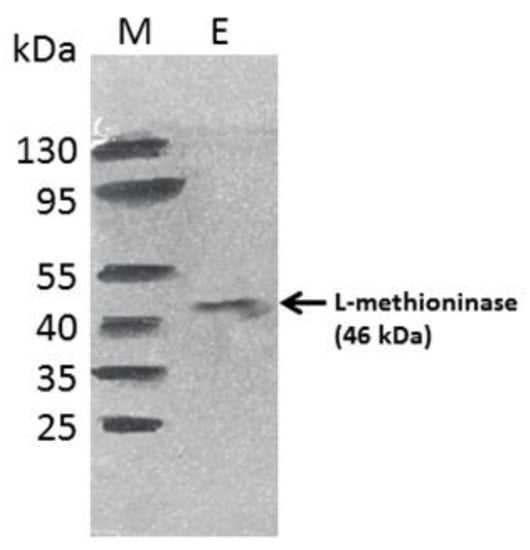
Figure 5.
SDS-PAGE analysis of the purified L-methioninase by A. aquatilis. M: Marker; E: Purified L-methioninase.
3.6. Effect of Biotic and Abiotic Factors on the Production of L-Methioninase
The growth and enzymatic mechanism of Alcaligenes aquatilis, a microorganism known to produce L-methioninase, can be influenced by factors such as pH, temperature, incubation time, and certain nutrients. The availability of carbon, nitrogen, and mineral salts can significantly impact microbial growth and enzymatic activity [60]. Providing sufficient supplementation of these nutrients can lead to increased production of L-methioninase by microbes. Therefore, this study aimed to investigate how varying these parameters could affect the growth and production of the enzyme. Hence, understanding the impact of biotic and abiotic factors on the growth and production of L-methioninase can be crucial in optimizing the conditions for its production and potential applications in fields such as cancer therapy and metabolic disorders.
A study investigated the effect of different mineral salts, namely NaCl, MgSO4, and KCl, on the activity of the L-methioninase enzyme, ranging from 2% to 10%, 1 to 6%, and 2% to 4%, respectively. The study subsequently found that NaCl had a significant effect on L-methioninase activity, with enzyme activity ranging from 0.36 to 1.07 units per milliliter (U/mL) at different pH values between 6.70 to 8.49. The highest enzyme activity was observed at pH 8.49 in the presence of a 2% NaCl concentration after 96 h of incubation. However, the researchers also found that when the concentration of NaCl increased exponentially, the enzyme activity decreased. This is likely due to the influence of the high salt concentration on the adaptation of the microorganism producing the enzyme, as well as the pH of the medium, which may not be suitable for the secretion of the L-methioninase enzyme. Therefore, this study highlights the importance of optimizing the concentration of mineral salts in the growth medium to achieve maximum activity of the L-methioninase enzyme.
When the effect of KCl was evaluated, the enzyme activity ranged from 0.17 to 0.90 U/mL at pH values from 6.47 to 7.88. The highest enzyme activity was reported at pH 7.88 after 96 h of incubation. Similarly, when the effect of MgSO4 was evaluated, the enzyme activity ranged from 0.24 to 1.01 U/mL, with pH values ranging from 6.93 to 8.11. The highest enzyme activity was reported at pH 8.11. Based on the results obtained from these experiments, the researchers concluded that pH plays an important role in L-methioninase enzyme production, and that enzyme activity is highest at pH values greater than 8.0. In contrast, enzyme activity decreased when the pH decreased below this value. They also found that the presence of salts such as NaCl, KCl, and MgSO4 had varying effects on enzyme activity depending on the pH of the growth medium.
Previous research has shown that L-asparaginase, which was used in cancer therapy, is most effective at a pH of 8 and loses its effectiveness when the pH drops below 8. This is because the enzyme has specific characteristics that make it effective at high pH levels [61,62]. Unlike L-asparaginase, L-methioninase remains active across all salt concentrations, indicating that the enzyme-producing organism, likely Alcaligenes aquatilis, is halotolerant (Figure 6A). This unique property of the enzyme may be attributed to the isolated strains from marine origin.
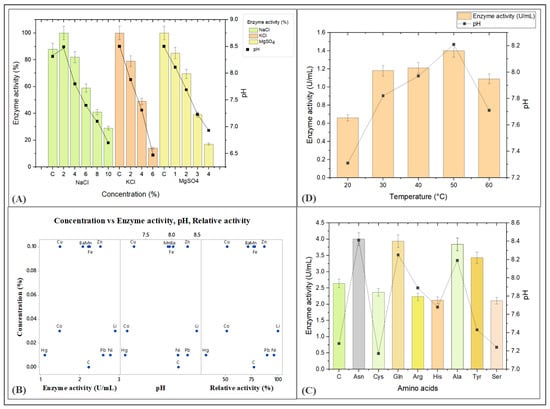
Figure 6.
The effects of biotic and abiotic factors, such as (A) effect of salts; (B) effect of different heavy metal concentrations; (C) effect of amino acids, and (D) effect of temperature, on the production of L-methioninase enzyme at various pH levels. Reactions were performed in triplicates, and results were shown as the mean of the data obtained.
The study examined the effects of different metal ions such as Mn2+, Zn2+, Fe2+, Cu2+, Ba2+, Co2+, Li2+, Pb2+, Ni2+, and Hg2+ on L-methioninase activity. The results showed that exposure to heavy metals positively affected both growth and enzyme activity. However, Li2+, Ni2+, Pb2+, and Zn2+ were found to be the most effective in terms of enzyme production, with enzyme levels of 2.9, 2.78, 2.6, and 2.52 U/mL, respectively. These metals also exhibited the highest relative enzyme activity, ranging from 86.89% to 100% (Figure 6B). The study suggests that certain metal ions can enhance the production and activity of the L-methioninase enzyme, which could have potential applications in various industries. The study found that the control group without added metals had a comparable activity level of 2.23 U/mL. In contrast, the steady-state activity for the metals Mn2+, Fe2+, and Ba2+ was reported to be 2.25, 2.22, and 2.07 U/mL, respectively.
The study found that adding certain metal ions did not have a negative effect on growth and enzyme activity but instead helped stabilize and maintain enzyme activity in the medium. The pH of the medium was also found to be stable around pH 8.0, which is optimal for enzyme stability. Some metals, like Ba2+, even help in enhancing enzyme activity at desired concentrations [63]. However, other metal ions like Cu2+, Co2+, and Hg2+ negatively impacted the growth, production, and relative activity of enzyme (Figure 6B). Two studies, one by Dange and Peshwe [64] and another by Dias et al. [65], found that the same substance (Cu2+ ions) was hindering the function of L-asparaginase in different species of Aspergillus fungi. This inhibition may occur because the substance causes protein particles to stick together (precipitate).
A previous study by Lincoln et al. [66] found that a substance called Hg2+ was hindering the activity of the enzyme L-asparaginase. Similarly, Abu-Tahon et al. [36] found that the enzymatic activity of alkaline L-methioninase from the fungus Aspergillus ustus was boosted slightly by the addition of Ni2+, while it was inhibited in the presence of copper (Cu2+), Mercury (Hg2+), and other metal ions. In another study by Selim et al. [42], it was observed that in Candida tropicalis, the activity of the enzyme was severely hindered by cadmium (Cd2+) and copper (Cu2+), while it was strengthened by nickel (Ni2+) and magnesium ions (Mg2+). Based on the results of these studies, it can be inferred that heavy metal ions such as Zn2+, Li2+, and Ni2+ are responsible for activating the enzymatic mechanism and aiding in the secretion of the enzyme. On the other hand, certain heavy metal ions such as Hg2+, Co2+, and Cu2+ are found to inhibit enzymatic activity. Overall, the presence of heavy metal ions has a significant impact on the functioning of enzymes and their activity, and their effects can vary depending on the specific enzyme and the type of metal ion involved.
To determine the impact of different amino acids on the production of L-methioninase, the production medium was supplemented with various nitrogen sources, namely L-asparagine, L-cysteine, L-glutamine, L-arginine, L-histidine, L-alanine, L-tyrosine, and L-serine, at a concentration of 0.5%. The aim was to assess how these amino acids affected enzyme induction. The results indicated that among the seven amino acids tested, L-asparagine was the most effective alternative to L-methionine (Figure 6C). To assess the impact of different amino acids on the activity of the enzyme, various amino acids were added to the production medium, and the mixture was incubated for 24–48 h. L-asparagine showed the highest enzyme activity among the different amino acids tested, showing 4 U/mL activity (Figure 6C). The production of the enzyme was also found to be increased when glutamine (3.94 U/mL), alanine (3.84 U/mL), and tyrosine (3.43 U/mL) were added to the medium, compared to the control, which only had methionine as the amino acid (with an activity of 2.64 U/mL). EL-Sayed [37] also found that L-methioninase from A. flavipes showed relative enzymatic activity for the same amino acids as our results: asparagine and glutamine. L-asparagine was found to promote the synthesis of methioninase, even in the absence of L-methionine. Similar results were reported for other microorganisms, such as Hafnia alvei, Pseudomonas putida, and Geotricum candidum, as reported by [41,67]. However, other studies showed that L-methionine was necessary for producing L-methioninase in the fungi A. flavipes and Yarrowia lipolytica, as reported [40,41].
To determine the effect of temperature on the production of L-methioninase, the fermentation medium was incubated at diverse temperatures ranging from 20 to 60 °C. The results showed that maximum enzyme production was observed at 50 °C (Figure 6D), which is not reported in earlier studies on other microorganisms such as Bacillus haynesii, Hafnia alvei, Brevibacterium linens, Pseudomonas putida, and Alcaligenes sp. These microorganisms were found to produce their maximum amounts of L-methioninase at 37 °C, as reported by [35,44]. Therefore, maintaining the fermentation temperature at 50 °C can be beneficial for maximizing L-methioninase production. Hence the Alcaligenes aquatilis, which are called thermotolerant microorganisms. They have various adaptations that enable them to withstand extreme heat, including specialized enzymes and heat shock proteins that protect the cells from damage. This microorganism has been studied due to its potential applications in biotechnology, such as producing heat-resistant enzymes and anticancer activity in high-temperature environments. The current investigation results indicate that Alcaligenes aquatilis can survive and grow well even at a high temperature of 60 °C and produce a significant amount of the L-methioninase enzyme.
The findings suggest that the L-methioninase enzyme produced by Alcaligenes aquatilis is thermostable because enzyme activity 1.31 U/mL was retained at 50 °C after an incubation of 60 min. The activity at zero time was taken as 100% (1.31 U/mL). Enzyme activity was retained as 81% (1.06 U/mL) at 60 °C after an incubation period of 30 min, as shown in Figure 7. Based on the above result, thermostability of the enzyme was found up to 50 °C, above which the enzyme activity declined. In previous studies on L-methioninase, the thermal stability of the enzyme was reported at 55 °C for 15 min in Candida tropicalis [42], at 70 °C for 30 min in Streptomyces sp. [32], and 50 °C for 20 min in A. fumigatus [64]. In this context, the A. aquatilis-producing enzyme was more thermally stable at 50 °C even with an increased incubation time of up to 60 min. Hence, the enzyme is relatively more vigorous and exhibits higher thermal capabilities.
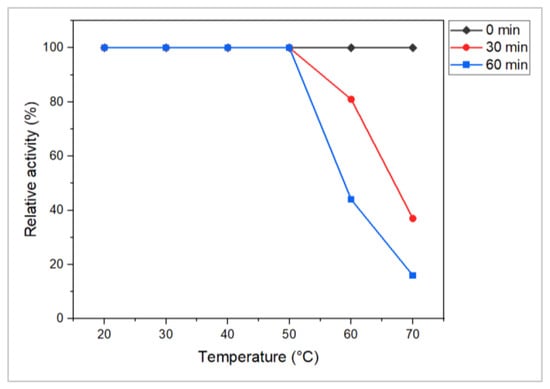
Figure 7.
Stability of L-methioninase enzyme from A. aquatilis at different temperatures. Residual enzyme activity is given as the percentage of initial enzyme activity at different times. Residual activity was measured by performing an enzyme assay after preincubating enzyme solution at different temperatures for different time intervals. Reactions were performed in triplicates, and results were shown as the mean of the data obtained.
3.7. Antioxidant Activity
The role of antioxidants in cancer treatment has been extensively studied, as they are believed to have the potential to cause tumor regression. In this study, the researchers evaluated the ability of L-methioninase to scavenge free radicals using the DPPH assay. In a 0.2 mL aliquot of enzyme with activity of 1.07 U/mL (M9 medium as a control), 1.18 U/mL (groundnut oil cake- GOC), and 1.31 U/mL (cottonseed oil cake-COC), the findings revealed percentage scavenging activity of 54.53%, 61.3%, and 59.2%, respectively (Figure 8). This indicates that the L-methioninase samples have strong free radical scavenging ability, suggesting their potential for use in anticancer applications. In other words, the results of the study indicate that L-methioninase has antioxidant properties that could be useful in fighting cancer. A study by El-Sayed [37] found that L-methioninase from A. flavipes showed free radical scavenging capacity, further supporting the potentiality of the enzyme as an anticancer agent.
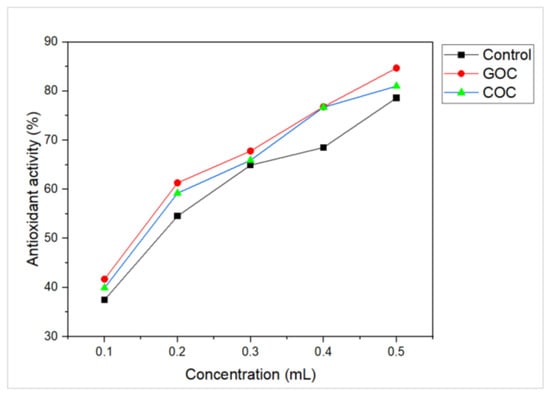
Figure 8.
Comparison of antioxidant activity (DPPH assay) of L-methioninase enzyme from Alcaligenes aquatilis produced using different raw materials (M9 medium- Control, GOC- Groundnut oil cake; COC- Cottonseed oil cake).
3.8. Hemolytic Activity
The L-methioninase was tested at different concentrations to determine whether it has the potential to damage human blood cells. The results of the hemolytic assays showed that the enzyme did not have any visible effect on the erythrocytes, suggesting that L-methioninase is safe against cancer. The crude extract and acetone-purified L-methioninase showed no hemolytic activity, indicating the enzyme has excellent safety properties. The experiment involved using L-methioninase in different concentrations to observe its impact on blood cells. Results showed that L-methioninase did not cause any hemolysis (destruction of red blood cells) when tested qualitatively, as no visible hemolytic zone was observed around the test well or the negative control well. However, a hemolytic zone around the well contained 1% SDS solution, which served as a positive control.
Moreover, when the experiment was repeated using various concentrations of crude and acetone-purified enzymes (1.07 and 4.61 U/mg), quantitative assays showed no significant effect on human erythrocytes. Hence, the results indicate that L-methioninase is safe to use in relation to human blood cells. During the experiment, it was observed that L-methioninase did not cause significant hemolysis after 48–72 h, whereas the positive control with 1% SDS showed clear hemolysis (Figure 9). Previous studies have also reported no hemolysis by purified L-methioninase [37]. These results indicate that L-methioninase is safe to use and does not damage human erythrocytes, making it a viable option for therapeutic purposes, which has been demonstrated in several studies where L-methioninase did not cause any visible hemolysis at various concentrations of the enzyme tested [37]. The study also showed the anticancer activity with crude enzyme (IC50 0.27 U/mL) in the liver cancer cell line (data not shown).
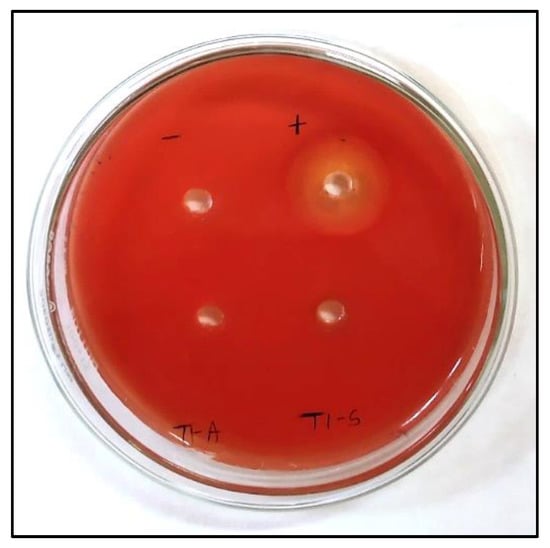
Figure 9.
A qualitative hemolysis test of L-methioninase enzyme on a blood agar plate demonstrates the absence of hemolysis in the negative controls (phosphate buffer), crude extract (T1-S), and acetone-purified enzyme (T1-A). In contrast, the positive controls exhibit a hemolytic zone (1% SDS).
Similarly, a study reported that L-methioninase from Bacillus spp. did not cause any hemolysis in human erythrocytes. In contrast, some studies have reported the hemolytic activity of L-methioninase, but it was found to be very low and not clinically significant. Therefore, the previous report suggests that L-methioninase has low or negligible hemolytic activity, making it a potentially safe option for therapeutic applications [37].
4. Conclusions
L-methioninase can be regarded as a potential target for anticancer treatment. Alcaligenes aquatilis species, isolated from sediment samples associated with mangroves, were used for the first time to produce the L-methioninase enzyme. In the current study, A. aquatilis was assessed for its ability to produce enzymes using agricultural waste materials and purified using solvent precipitation and gel filtration chromatography. Cottonseed oil cake was found to exhibit the highest enzyme activity as a raw material. The study concluded that agricultural waste material could present a cost-effective substrate for L-methioninase production.
Further, the strain was subjected to experiments to investigate the effects of temperature, salts, heavy metals, and other inducers on growth and enzyme production. L-methioninase production was shown to increase after using different amino acids, including L-asparagine, L-glutamine, L-alanine, and L-tyrosine. Enzyme activity was increased 4.3-fold using acetone precipitation and 7.15-fold using Sephadex G-100 gel filtration chromatography. The molecular mass of the purified enzyme was approximately 46 kDa. The findings showed that A. aquatilis could produce L-methioninase under stress conditions, making it appropriate for metal bioremediation procedures in unfavorable circumstances. L-methioninase from A. aquatilis was also found to have potential antioxidant properties, no hemolytic activities, and exhibited enzymatic stability at pH levels higher than the pH of blood plasma, making it suitable for purification and anticancer applications in the future. More research, however, is required to determine its safety and efficacy and optimize its use as a therapeutic agent.
Author Contributions
Methodology, data curation, writing—original draft preparation, B.J.; formal analysis, M.G.; validation, writing—review and editing, D.J.H.S., D.D. and Y.-Y.C.; Conceptualization and data curation, S.V. and P.D.; supervision, D.D. and Y.-Y.C.; project administration, P.D., D.J.H.S., D.D. and Y.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
We thank the Department of Education, Government of Gujarat, for providing the B.J. financial assistance (SHODH fellowship). We thank the Department of Life Sciences, Bhakta Kavi Narsinh Mehta University, and the Department of Biochemical Science and Technology, National Chiayi University, for providing the resources to carry out this work successfully.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Mittelstein, D.R.; Ye, J.; Schibber, E.F.; Roychoudhury, A.; Martinez, L.T.; Fekrazad, M.H.; Ortiz, M.; Lee, P.P.; Shapiro, M.G.; Gharib, M. Selective ablation of cancer cells with low intensity pulsed ultrasound. Appl. Phys. Lett. 2020, 116, 013701. [Google Scholar] [CrossRef]
- Torchilin, V.P. Enzymes in Medicine: Advantages and Disadvantages. In Immobilized Enzymes in Medicine; Progress in Clinical Biochemistry and Medicine, Vol 11; Springer: Berlin/Heidelberg, Germany, 1991; pp. 3–12. [Google Scholar] [CrossRef]
- Fernandes, H.S.; Silva Teixeira, C.S.; Fernandes, P.A.; Ramos, M.J.; Cerqueira, N.M.F.S.A. Amino acid deprivation using enzymes as a targeted therapy for cancer and viral infections. Expert Opin. Ther. Pat. 2017, 27, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Kiriyama, Y.; Kubota, M.; Takimoto, T.; Kitoh, T.; Tanizawa, A.; Akiyama, Y.; Mikawa, H. Biochemical characterization of U937 cells resistant to L-asparaginase: The role of asparagine synthetase. Leukemia 1989, 3, 294–297. [Google Scholar]
- Prajapati, B.; Supriya, N.R. Review on anticancer enzymes and their targeted amino acids. World J. Pharm. Res. 2017, 6, 268–284. [Google Scholar]
- El-Asmar, F.A.; Greenberg, D.M.; Amand, G.S. Studies on the mechanism of inhibition of tumor growth by the enzyme glutaminase. Cancer Res. 1966, 26, 116–122. [Google Scholar]
- Ghosh, S.; Murthy, S.; Govindasamy, S.; Chandrasekaran, M. Optimization of L-asparaginase production by Serratia marcescens (NCIM 2919) under solid state fermentation using coconut oil cake. Sustain. Chem. Process. 2013, 1, 9. [Google Scholar] [CrossRef]
- Bopaiah, B.B.K.; Kumar, D.A.N.; Balan, K.; Dehingia, L.; Reddy, M.K.R.V.; Suresh, A.B.; Nadumane, V.K. Purification, characterization, and antiproliferative activity of L-methioninase from a new isolate of Bacillus haynesii JUB2. J. Appl. Pharm. Sci. 2020, 10, 054–061. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, S.; Kanwar, S.S. L-methioninase: A therapeutic enzyme to treat malignancies. BioMed Res. Int. 2014, 2014, 506287. [Google Scholar] [CrossRef]
- Anderson, M.E. Glutathione: An overview of biosynthesis and modulation. Chem. Biol. Interact. 1998, 111, 1–14. [Google Scholar] [CrossRef]
- Kahraman, H.; Aytan, E.; Kurt, A.G. Production of methionine γ-lyase in recombinant Citrobacter freundii bearing the hemoglobin gene. BMB Rep. 2011, 44, 590–594. [Google Scholar] [CrossRef]
- Cellarier, E.; Durando, X.; Vasson, M.P.; Farges, M.C.; Demiden, A.; Maurizis, J.C.; Madelmont, J.C.; Chollet, P. Methionine dependency and cancer treatment. Cancer Treat. Rev. 2003, 29, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Altered methionine metabolism, DNA methylation and oncogene expression in carcinogenesis: A review and synthesis. Biochim. Biophys. Acta 1984, 738, 49–87. [Google Scholar] [CrossRef] [PubMed]
- Carr, K.A.; Arduino, M.J. Isolation and characterization of bacterial L-Methioninase as an effective anti-tumor agent. Bios 1981, 52, 13–22. Available online: http://www.jstor.org/stable/4607648 (accessed on 20 June 2023).
- Moinard, C.; Cynober, L.; de Bandt, J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef]
- Gilmour, S.K. Polyamines and nonmelanoma skin cancer. Toxicol. Appl. Pharmacol. 2007, 224, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Breillout, F.; Antoine, E.; Poupon, M.F. Methionine dependency of malignant tumors: A possible approach for therapy. J. Natl. Cancer Inst. 1990, 82, 1628–1632. [Google Scholar] [CrossRef]
- Yvon, M.; Thirouin, S.; Rijnen, L.; Fromentier, D.; Gripon, J.C. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl. Environ. Microbiol. 1997, 63, 414–419. [Google Scholar] [CrossRef]
- Amarita, F.; Yvon, M.; Nardi, M.; Chambellon, E.; Delettre, J.; Bonnarme, P. Identification and functional analysis of the gene encoding methionine-γ-lyase in Brevibacterium linens. Appl. Environ. Microbiol. 2004, 70, 7348–7354. [Google Scholar] [CrossRef]
- Dias, B.; Weimer, B. Purification and Characterization of l-Methionine γ-Lyase from Brevibacterium linens BL2. Appl. Environ. Microbiol. 1998, 64, 3327–3331. [Google Scholar] [CrossRef]
- Nakayma, T.; Esaki, N.; Lee, W.J.; Tanaka, I.; Tanaka, H.; Soda, K. Purification and properties of l-methionine γ-lyase from Aeromonas sp. Agric. Biol. Chem. 1894, 48, 2367–2369. [Google Scholar] [CrossRef]
- Khan, M.A.; Lopez-Munoz, M.M.; Kaspar, C.W.; Hung, K.F. Activities of methionine-γ-lyase in the acidophilic archaeon “Ferroplasma acidarmanus” strain fer1. Res. Rep. Biol. 2013, 4, 11–22. [Google Scholar] [CrossRef][Green Version]
- Sundar, W.A.; Nellaiah, H. A rapid method for screening of methioninase producing Serratia marcescens species from soil. Int. J. Pharm. Pharm. Sci. 2013, 5, 426–427. Available online: https://innovareacademics.in/journal/ijpps/Vol5Issue2/6615.pdf (accessed on 20 June 2023).
- Revtovich, S.; Anufrieva, N.; Morozova, E.; Kulikova, V.; Nikulin, A.; Demidkina, T. Structure of methionine γ-lyase from Clostridium sporogenes. Acta Crystallogr. F Struc. Biol. Commun. 2016, 72, 65–71. [Google Scholar] [CrossRef]
- Martínez-Cuesta, M.C.; Peláez, C.; Eagles, J.; Gasson, M.J.; Requena, T.; Hanniffy, S.B. YtjE from Lactococcus lactis IL1403 is a C-S lyase with α, γ-elimination activity toward methionine. Appl. Environ. Microbiol. 2016, 72, 4878–4884. [Google Scholar] [CrossRef]
- Fukumoto, M.; Kudou, D.; Murano, S.; Shiba, T.; Sato, D.; Tamura, T.; Harada, S.; Inagaki, K. The role of amino acid residues in the active site of L-methionine γ-lyase from Pseudomonas putida. Biosci. Biotechnol. Biochem. 2012, 76, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.H.; Elshikh, H.H.; Saad, M.M.; Mostafa, E.E.; Mahmoud, M.A. Purification and characterization of a novel thermo stable L-methioninase from Streptomyces sp. DMMMH4 and its evaluation for anticancer activity. J. Appl. Pharm. Sci. 2016, 6, 53–60. [Google Scholar] [CrossRef]
- Song, H.; Xu, R.; Guo, Z. Identification and characterization of a methionine γ-lyase in the calicheamicin biosynthetic cluster of Micromonospora echinospora. Chembiochem 2015, 16, 100–109. [Google Scholar] [CrossRef]
- Prihanto, A.A.; Nursyam, H.; Yufidasari, H.S.; Lutfiana, L. Mangrove ecosystem as a source of L-methioninase-producing bacteria. Malays. Appl. Biol. 2018, 47, 141–145. Available online: https://www.mabjournal.com/images/47_3_June_2018/47_03_18.pdf (accessed on 20 June 2023).
- Nikulin, A.; Revtovich, S.; Morozova, E.; Nevskaya, N.; Nikonov, S.; Garber, M.; Demidkina, T. High-resolution structure of methionine γ-lyase from Citrobacter freundii. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 211–218. [Google Scholar] [CrossRef]
- Kavya, D.; Nadumane, V.K. Identification of highest L-Methioninase enzyme producers among soil microbial isolates, with potential antioxidant and anticancer properties. J. Appl. Biol. Biotechnol. 2020, 8, 21–27. [Google Scholar] [CrossRef]
- Huang, K.Y.; Hu, H.Y.; Tang, Y.L.; Xia, F.G.; Luo, X.Q.; Liu, J.Z. High-level expression, purification and large-scale production of L-methionine γ-lyase from Idiomarina as a novel anti-leukemic drug. Mar. Drugs 2015, 13, 5492–5507. [Google Scholar] [CrossRef] [PubMed]
- Fukamachi, H.; Nakano, Y.; Okano, S.; Shibata, Y.; Abiko, Y.; Yamashita, Y. High production of methyl mercaptan by L-methionine-α-deamino-γ-mercaptomethane lyase from Treponema denticola. Biochem. Biophys. Res. Commun. 2005, 331, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, W.A. Bacterium Hafnia alvei secretes L-methioninase enzyme: Optimization of the enzyme secretion conditions. Saudi J. Biol. Sci. 2020, 27, 1222–1227. [Google Scholar] [CrossRef]
- Abu-Tahon, M.A.; Isaac, G.S. Purification and characterization of new alkaline L-methioninase from Aspergillus ustus AUMC 1051 grown under solid-state fermentation conditions. Egypt. J. Bot. 2016, 56, 785–798. [Google Scholar] [CrossRef]
- El-Sayed, A.S. Purification and characterization of a new L-methioninase from solid cultures of Aspergillus flavipes. J. Microbiol. 2011, 49, 130–140. [Google Scholar] [CrossRef]
- Hendy, M.H.; Hashem, A.H.; Sulieman, W.B.; Sultan, M.H.; Abdelraof, M. Purification, Characterization and anticancer activity of L-methionine γ-lyase from thermo-tolerant Aspergillus fumigatus. Microb. Cell Fact. 2023, 22, 1–11. [Google Scholar] [CrossRef]
- Salim, N.; Santhiagu, A.; Joji, K. Purification, characterization and anticancer evaluation of l-methioninase from Trichoderma harzianum. 3 Biotech 2020, 10, 501. [Google Scholar] [CrossRef]
- Khalaf, S.A.; El-Sayed, A.S. L-Methioninase production by filamentous Saccharomyces: I-screening and optimization under submerged conditions. Curr. Microbiol. 2009, 58, 219–226. [Google Scholar] [CrossRef]
- Bonnarme, P.; Arfi, K.; Dury, C.; Helinck, S.; Yvon, M.; Spinnler, H.E. Sulfur compound production by Geotrichum candidum from L-methionine: Importance of the transamination step. FEMS Microbiol. Lett. 2001, 205, 247–252. [Google Scholar] [CrossRef]
- Selim, M.H.; Karm Eldin, E.Z.; Saad, M.M.; Mostafa, E.S.E.; Shetia, Y.H.; Anise, A.A.H. Purification, characterization of L-methioninase from Candida tropicalis, and its application as an anticancer. Biotechnol. Res. Int. 2015, 2015, 173140. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Mohkam, M.; Taleban, Y.; Golkar, N.; Berenjian, A.; Dehshahri, A.; Mobasher, M.A.; Ghasemi, Y. Isolation and identification of novel L-methioninase producing bacteria and optimization of its production by experimental design method. Biocatal. Agric. Biotechnol. 2020, 26, 101566. [Google Scholar] [CrossRef]
- Nejadi, N.; Masti, S.M.; Tavirani, M.R.; Golmohammadi, T. Comparison of three routine protein precipitation methods: Acetone, TCA/acetone wash and TCA/acetone. Arch. Adv. Biosci. 2014, 5. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Spinnler, H.E.; Berger, C.; Lapadatescu, C.; Bonnarme, P. Production of sulfur compounds by several yeasts of technological interest for cheese ripening. Int. Dairy J. 2001, 11, 245–252. [Google Scholar] [CrossRef]
- Arfi, K.; Tâche, R.; Spinnler, H.E.; Bonnarme, P. Dual influence of the carbon source and L-methionine on the synthesis of sulphur compounds in the cheese-ripening yeast Geotrichum candidum. Appl. Microbiol. Biotechnol. 2003, 61, 359–365. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. Available online: https://www.jbc.org/article/S0021-9258(19)52451-6/pdf (accessed on 20 June 2023). [CrossRef]
- El-Sayed, A.S. L-methioninase production by Aspergillus flavipes under solid-state fermentation. J. Basic Microbiol. 2009, 49, 331–341. [Google Scholar] [CrossRef]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Hayes, A.W.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced D.N.A. damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef]
- Huang, S.; Pan, S.; Chen, G.; Huang, S.; Zhang, Z.; Li, Y.; Liang, Z. Biochemical characteristics of a fibrinolytic enzyme purified from a marine bacterium, Bacillus subtilis HQS-3. Int. J. Biol. Macromol. 2013, 62, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bulmus, V.; Woodward, M.; Lin, L.; Murthy, N.; Stayton, P.; Hoffman, A. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J. Control. Release 2003, 93, 105–120. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of agro-waste into value-added bioproducts and bioactive compounds: Micro/nano formulations and application in the agri-food-pharma sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Ramachandran, S.; Patel, A.K.; Nampoothiri, K.M.; Francis, F.; Nagy, V.; Szakacs, G.; Pandey, A. Coconut oil cake—A potential raw material for the production of α-amylase. Bioresour. Technol. 2004, 93, 169–174. [Google Scholar] [CrossRef]
- Rekha, K.S.S.; Lakshmi, M.V.C.; Devi, V.S.; Siddartha Kumar, M. Production and optimization of lipase from Candida rugosa using groundnut oilcake under solid state fermentation. Int. J. Res. Eng. Technol. 2012, 1, 571–577. [Google Scholar] [CrossRef]
- Abdelraof, M.; Selim, M.H.; Elsoud, M.M.A.; Ali, M.M. Statistically optimized production of extracellular l-methionine γ-lyase by Streptomyces Sp. DMMMH60 and evaluation of purified enzyme in sub-culturing cell lines. Biocatal. Agric. Biotechnol. 2019, 18, 101074. [Google Scholar] [CrossRef]
- Joseph, B.; Upadhyaya, S.; Ramteke, P. Production of cold-active bacterial lipases through semisolid state fermentation using oil cakes. Enzym. Res. 2011, 2011, 796407. [Google Scholar] [CrossRef]
- Manukhov, I.V.; Mamaeva, D.V.; Rastorguev, S.M.; Faleev, N.G.; Morozova, E.A.; Demidkina, T.V.; Zavilgelsky, G.B. A gene encoding l-methionine γ-lyase is present in Enterobacteriaceae family genomes: Identification and characterization of Citrobacter freundii l-methionine γ-lyase. J. Bacteriol. 2005, 187, 3889–3893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reddy, M.S.; Kanwal, H.K. Influence of carbon, nitrogen sources, inducers, and substrates on lignocellulolytic enzyme activities of Morchella spongiola. J. Agric. Food Res. 2022, 7, 100271. [Google Scholar] [CrossRef]
- Yadav, N.; Sarkar, S. Production of L-asparaginase by Fusarium oxysporum using submerged fermentation. Int. J. Pharm. Sci. Invent. 2014, 3, 32–40. Available online: http://www.ijpsi.org/Papers/Vol3(6)/G0361032040.pdf (accessed on 20 June 2023).
- Fatima, N.; Khan, M.M.; Khan, I.A. L-asparaginase produced from soil isolates of Pseudomonas aeruginosa shows potent anticancer activity on HeLa cells. Saudi J. Biol. Sci. 2019, 26, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, M.M.A.A.; Awad, M.F.; El-Shenawy, F.S.; El-Bondkly, A.M.A. Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J. Biol. Sci. 2021, 28, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Dange, V.; Peshwe, S. Purification and biochemical characterization of L-asparaginase from Aspergillus niger and evaluation of its antineoplastic activity. Int. J. Sci. Res. 2015, 4, 564–569. Available online: https://www.ijsr.net/getabstract.php?paperid=OCT141573 (accessed on 20 June 2023).
- Dias, F.F.G.; Santos Aguilar, J.G.D.; Sato, H.H. L-Asparaginase from Aspergillus spp.: Production based on kinetics, thermal stability and biochemical characterization. 3 Biotech 2019, 9, 289. [Google Scholar] [CrossRef]
- Lincoln, L.; Niyonzima, F.N.; More, S.S. Purification and properties of a fungal L-asparaginase from Trichoderma viride pers: SF GREY. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 310–316. [Google Scholar]
- Tan, Y.; Xu, M.; Tan, X.; Tan, X.; Wang, X.; Saikawa, Y.; Nagahama, T.; Sun, X.; Lenz, M.; Hoffman, R.M. Overexpression and large-scale production of recombinant L-methionine-α-deamino-γ-mercaptomethane-lyase for novel anticancer therapy. Protein Expr. Purif. 1997, 9, 233–245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).