Abstract
Background: The management of resected stage III colorectal cancer (CRC) has long been reliant on fluoropyrimidine-based adjuvant chemotherapy. However, the 10–15% of patients with mismatch repair-deficient (dMMR) tumors derive limited benefit from this approach. While immunotherapy has revolutionized the treatment of metastatic dMMR CRC, its role in the early-stage setting is rapidly evolving, creating a paradigm shift. Methods: A systematic literature review was conducted to identify pivotal clinical trials evaluating therapeutic strategies for non-metastatic dMMR CRC. Databases including PubMed/MEDLINE and conference proceedings from the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) were searched up to June 2025. The review focused on phase II and III trials reporting on disease-free survival (DFS), pathological complete response (pCR), and safety. Study selection followed PRISMA guidelines. Results: The systematic review identified 14 key studies that were included for narrative synthesis. The evidence base encompassed three areas: (1) Foundational adjuvant chemotherapy trials (e.g., MOSAIC, IDEA); (2) Pivotal metastatic trials (e.g., KEYNOTE-177) validating immunotherapy efficacy in dMMR CRC; and (3) Modern trials in non-metastatic disease. The phase III ATOMIC trial demonstrated that adding atezolizumab to mFOLFOX significantly improved 3-year DFS versus chemotherapy alone (86.4% vs. 76.6%; Hazard Ratio [HR] 0.50, 95% Confidence Interval [CI] 0.34–0.72; p < 0.001). Concurrently, phase II neoadjuvant immunotherapy trials (e.g., NICHE-2) reported remarkable pCR rates of 68% and a 3-year DFS of 100%, with a more favorable safety profile compared to chemoimmunotherapy. Conclusions: The landscape for non-metastatic dMMR CRC is shifting from a chemotherapy-based model to an immunotherapy paradigm. The ATOMIC trial establishes adjuvant chemoimmunotherapy as a new standard, while robust neoadjuvant data suggest a potential future where short-course, chemotherapy-free immunotherapy could become a preferred strategy. Ongoing trials directly comparing these approaches are awaited.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related mortality worldwide. In 2022, it was estimated that there were 1,926,118 new diagnoses of CRC and 903,859 related deaths globally [1]. Since 1990, the management of resected stage III CRC has been transformed by adjuvant chemotherapy, beginning when Dr. Moertel demonstrated that 5-fluorouracil (5FU) led to a 33% reduction in the risk of death [2]. A subsequent major advance came with the MOSAIC trial, published in 2004, which confirmed that 6 months of FOLFOX chemotherapy resulted in a significant improvement in disease-free survival (DFS) and overall survival (OS) compared to LV5FU2 [3]. For low-risk stage III CRC, the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) collaboration demonstrated in 2018 the non-inferiority of 3 months of chemotherapy compared to 6 months, with a lower frequency and severity of adverse events, particularly neuropathy [4]. However, despite adjuvant chemotherapy, 30% of stage III CRC patients will experience recurrence. Consequently, there is an unmet need for more effective strategies to improve patient outcomes.
Approximately 10 to 15% of patients diagnosed with CRC have mismatch repair deficiency (dMMR), representing 300,000 new cases each year. dMMR cancers are biologically distinct; given their DNA repair defect, they accumulate mutations that enhance the anti-tumor immune response [5]. These cancers do not derive any benefit from 5FU monotherapy, which should not be used in these patients. In the metastatic setting, immunotherapy has demonstrated meaningful improvements in progression-free survival (PFS), OS, and quality of life for patients with advanced dMMR CRC [6,7].
Recently, the second interim analysis of the ATOMIC trial, presented by Dr. Sinicrope on 1 June 2025, at the ASCO annual meeting (Plenary session, LBA 1001), showed that mFOLFOX plus atezolizumab significantly improved DFS compared to mFOLFOX alone in patients with R0-resected stage III dMMR CRC [8]. Nevertheless, the optimal therapeutic strategy for this population is not yet fully defined. Critical uncertainties persist, including the identification of patients who require treatment, the potential of adjuvant immunotherapy to improve cure rates, the role of adjuvant chemotherapy in the context of effective immunotherapy, and the comparative efficacy of neoadjuvant versus adjuvant immunotherapy.
Therefore, this systematic review aims to critically appraise and synthesize the current evidence regarding the efficacy and safety of immunotherapy in the management of non-metastatic dMMR CRC. We will systematically evaluate and compare the two emerging paradigms, adjuvant chemoimmunotherapy (as exemplified by the ATOMIC trial) and neoadjuvant immunotherapy (as investigated in the NICHE-2 and other trials), against the historical data of adjuvant chemotherapy alone (MOSAIC and IDEA). The objective is to provide a clear overview of this treatment landscape, discuss the implications for current clinical practice, and highlight key unanswered questions to guide future research.
2. Materials and Methods
A systematic literature review was conducted to identify pivotal clinical trials evaluating therapeutic strategies for mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) non-metastatic CRC. Databases including PubMed/MEDLINE were searched, along with proceedings from the annual meetings of the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO), up to June 2025. The search strategy combined keywords related to the following concepts: “colorectal cancer”, “stage II/III”, “non-metastatic”, “dMMR”, “MSI-H”, “immunotherapy”, “immune checkpoint inhibitors”, “neoadjuvant”, “adjuvant”, “atezolizumab”, “pembrolizumab”, “nivolumab”, and “ipilimumab”.
2.1. Eligibility Criteria
Studies were eligible if they (1) involved adult patients with resectable, non-metastatic dMMR/MSI-H CRC; (2) evaluated immunotherapy (in the neoadjuvant, adjuvant, or perioperative setting) or were historical adjuvant chemotherapy trials that defined the previous standard of care; (3) were Phase II or III clinical trials; and (4) reported relevant efficacy outcomes (DFS, pathological complete response [pCR], OS). Preclinical studies, Phase I trials, case reports, and publications in languages other than English were excluded.
2.2. Study Selection and Data Extraction
The study selection process followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Figure 1). The completed PRISMA 2020 checklist is provided as Supplementary File S1. After duplicate removal, titles and abstracts were screened for relevance, followed by a full-text review of potentially eligible articles. The final selection was based on the predefined eligibility criteria. Data from the included studies were extracted into a standardized table, capturing key information such as study design, patient population, intervention, comparator, and primary efficacy and safety outcomes.
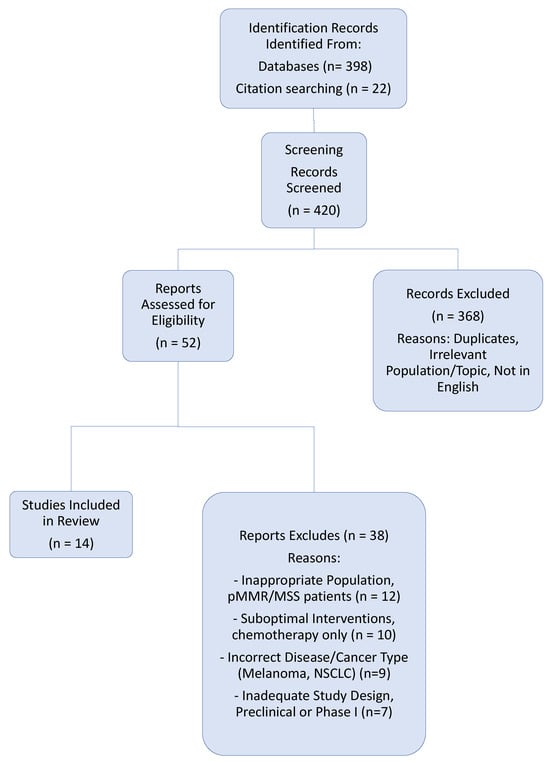
Figure 1.
PRISMA Flow Diagram: Identification and Selection of Studies.
2.3. Protocol Registration
This systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD420251174716. The protocol was developed prior to the data synthesis but after the initial literature search had commenced.
2.4. Data Synthesis
A quantitative meta-analysis was not performed due to the significant clinical heterogeneity of interventions (chemotherapy alone, chemoimmunotherapy, neoadjuvant immunotherapy alone) and settings (adjuvant vs. neoadjuvant) among the included studies. Therefore, a narrative synthesis was conducted. The results are presented to chronologically and thematically illustrate the evolution of the treatment paradigm, from the establishment of adjuvant chemotherapy to the current era of immunotherapy. While the present study adopted a narrative approach, the similarity of the endpoints in the studies included suggests that a quantitative meta-analysis could be conducted in the future to present robust pooled estimations.
3. Results
3.1. Study Selection
The initial database and manual search identified 420 records. After the removal of duplicates and the screening of titles and abstracts, 52 full-text articles were assessed for eligibility. Of these, 38 were excluded with reasons, the most common being incorrect disease focus (other cancer types), inappropriate patient population (pMMR/MSS tumors), or suboptimal interventions (chemotherapy-only trials not considered foundational). The final review included 14 pivotal studies for qualitative synthesis (Table 1 and Table 2). The selection process is detailed in the PRISMA flow diagram (Figure 1).

Table 1.
Evolution of Adjuvant Therapy in Stage III Colon Cancer: Key Clinical Trials.

Table 2.
Pivotal Clinical Trials in the Evolution of Treatment for Non-Metastatic dMMR CRC.
3.2. Overview of Included Studies and Synthesized Findings
The 14 included studies were categorized into three groups that chart the evolution of treatment for non-metastatic dMMR/MSI-H CRC (Table 1): (1) Foundational Adjuvant Chemotherapy Trials: This group includes landmark studies such as Moertel et al. (establishing 5-FU), MOSAIC (establishing FOLFOX), and the IDEA collaboration (refining treatment duration). These trials set the historical standard against which new strategies are measured. The MOSAIC trial demonstrated a significant DFS benefit (78.2% vs. 72.9%) specifically in the stage III population, while the benefit in stage II was not statistically significant. Crucially, pooled analyses like Sargent et al. demonstrated the lack of benefit from 5-FU in dMMR/MSI-H tumors, highlighting a critical unmet need. (2) Immunotherapy in Metastatic dMMR CRC: Pivotal trials like KEYNOTE-177 (pembrolizumab) and CheckMate 8HW (nivolumab + ipilimumab) provided the foundational proof-of-concept for the high efficacy of immune checkpoint blockade in dMMR CRC, supporting their investigation in earlier disease stages. (3) Modern Trials in Non-Metastatic Disease: This group forms the core of the current paradigm shift. It is subdivided into two approaches: Adjuvant Chemoimmunotherapy: The Phase III ATOMIC trial demonstrated that adding one year of atezolizumab to adjuvant mFOLFOX6 resulted in a statistically significant and clinically meaningful improvement in 3-year DFS compared to chemotherapy alone (86.4% vs. 76.6%; HR 0.50; 95% CI 0.34–0.72). Neoadjuvant Immunotherapy: Multiple Phase II trials reported groundbreaking efficacy. The NICHE-2 trial, using nivolumab + ipilimumab, showed a pCR rate of 68% and a 3-year DFS of 100%. Similarly, the Cercek et al. trial in locally advanced dMMR rectal cancer demonstrated a remarkable pCR rate of 74%, with most patients achieving a clinical complete response and avoiding surgery and radiotherapy. Other studies like NICHE-3, IMHOTEP, and NEOPRISM-CRC have further confirmed high pCR rates (53–68%) with neoadjuvant immunotherapy. Collectively, these regimens demonstrate a consistently more favorable safety profile with notably lower rates of high-grade adverse events compared to chemotherapy-containing regimens (Table 1, Table 2 and Table 3).

Table 3.
Efficacy and Safety of Neoadjuvant Immunotherapy in Non-Metastatic dMMR Colorectal Cancer.
4. Discussion
4.1. The Landmark ATOMIC Trial: Establishing a New Standard with Adjuvant Chemoimmunotherapy
Alliance A021502 (ATOMIC) was a randomized, multicenter, open-label phase III study that enrolled patients (n = 712) with histologically confirmed, completely resected (R0) stage III dMMR CRC. Both sporadic cases and those associated with Lynch syndrome were included. Patients who had received prior systemic therapy or radiotherapy were excluded. Prespecified stratification factors were T stage, N stage, and tumor location. Patients were randomized 1:1 to receive either mFOLFOX6 plus atezolizumab (an anti–PD-L1 antibody) for 6 months, followed by atezolizumab monotherapy for an additional 6 months (investigational arm), or mFOLFOX6 for 6 months (comparator arm). The primary endpoint was DFS, and the key secondary endpoints were OS and safety [8].
Demographics and patient characteristics were well-balanced between the two arms. Notably, more than 45% of patients in the ATOMIC trial had low-risk disease; according to the IDEA collaboration findings, these patients would now typically receive only 3 months of CAPEOX chemotherapy. The study met its primary endpoint, demonstrating a clinically and statistically significant 10% absolute improvement in 3-year DFS for mFOLFOX plus atezolizumab compared to mFOLFOX alone (86.4% vs. 76.6%; HR 0.50, 95% CI 0.34–0.72; p < 0.001). The benefit of atezolizumab was observed across most subgroups. Notably, this therapeutic benefit appeared relevant regardless of whether the mismatch repair deficiency was of hereditary (Lynch syndrome) or somatic origin. However, 13% of patients experienced a recurrence despite receiving 6 months of chemotherapy plus one year of immunotherapy. Overall survival data are not yet mature. Regarding safety, atezolizumab and chemotherapy are generally manageable. However, grade 3/4 adverse events were more frequent in the atezolizumab arm, including peripheral neuropathy, hypothyroidism, diarrhea, and non-febrile neutropenia. Additionally, six (1.7%) treatment-related deaths were reported in the atezolizumab arm compared to three (0.6%) in the control arm [8].
4.2. The Paradigm Shift: Neoadjuvant Immunotherapy as a Potentially Transformative Strategy
The ATOMIC trial establishes adjuvant chemoimmunotherapy as a new, more effective standard. However, the treatment landscape for non-metastatic dMMR CRC is now evolving with two distinct pathways, as neoadjuvant immunotherapy emerges as a potentially transformative strategy. This approach finds strong support from successful applications in other cancer types, particularly non-small cell lung cancer (NSCLC) where the phase III CheckMate 816 trial demonstrated significantly improved outcomes with neoadjuvant nivolumab plus chemotherapy, leading to regulatory approval and establishing a new paradigm in thoracic oncology. Similarly, the NADINA trial in melanoma showed two cycles of neoadjuvant nivolumab–ipilimumab outperformed one year of adjuvant nivolumab, creating a new treatment standard in that disease [17,18]. In colorectal cancer itself, the NICHE-2 trial reported a 68% pathological complete response rate and 95% major pathological response with neoadjuvant nivolumab–ipilimumab, achieving 100% 3-year DFS despite 65% of patients having T4 tumors, while the Cercek trial in locally advanced dMMR rectal cancer demonstrated a 74% complete response rate with dostarlimab monotherapy, enabling organ preservation and showing 92% 2-year DFS [9,15].
The scientific basis strongly supports beginning immunotherapy before surgery, as giving checkpoint inhibitors while the primary tumor remains enables the immune system to identify a wide spectrum of tumor targets, generating more vigorous and varied activation of tumor-specific T-cells that can eliminate micrometastatic disease throughout the body [19]. Research from NSCLC and initial data from dMMR CRC trials verify that the neoadjuvant approach creates a stronger and broader T-cell response than usually possible after tumor removal [20,21]. This scientific advantage leads to important clinical benefits including the possibility of avoiding chemotherapy while providing better safety, as brief immunotherapy courses can achieve substantial results while avoiding neuropathy, hematological toxicity, and other treatment-related side effects that greatly affect quality of life (Table 3). This strategy also shows exceptional effectiveness against high-risk disease, controlling locally advanced tumors as effectively as adjuvant chemoimmunotherapy methods while additionally permitting treatment response evaluation where tissue examination at surgery provides an immediate indicator for adjusting therapy, allowing treatment de-escalation for complete responders or additional therapy for those with poor response or R1/2 disease, creating a more customized treatment strategy compared to fixed-duration regimens [8,9].
4.3. Immunotherapy Alone in dMMR Stage III CRC
Chemotherapy has limited efficacy in dMMR stage III CRC [16]. In contrast, neoadjuvant immunotherapy has demonstrated high efficacy and a favorable safety profile in phase II trials (Table 3). For instance, the NICHE-2 study reported a pCR rate of 68% and a 3-year DFS of 100%, while the Cercek Cohort-1 study in locally advanced rectal cancer reported a pCR rate of 74% [9,15]. A recent study including a large number of patients treated with neoadjuvant pembrolizumab (IMHOTEP) (n = 87) reported a lower pCR rate compared with NICHE-2 (53%), suggesting lower efficacy of pembrolizumab in the neoadjuvant setting [9,14].
Notably, despite NICHE-2 including 65% of patients with T4-stage disease, the 3-year DFS was 100%, underscoring the profound efficacy of this neoadjuvant immunotherapy protocol in high-risk, locally advanced dMMR tumors [9]. The safety profile of these regimens is encouraging, with NICHE-2 and IMHOTEP reporting only 4% and 9% high-grade adverse events, respectively [9,14].
4.4. Limitations of the Current Evidence
A key limitation of the ATOMIC trial was the absence of an immunotherapy-only arm, leaving unanswered the fundamental question of whether chemotherapy is a necessary component of treatment for all patients [8]. This critical question is being addressed by the ongoing AZURE-2 trial, a phase III study randomizing patients to a chemotherapy-free regimen of perioperative Dostarlimab or to the current standard of care from MOSAIC/IDEA, adjuvant FOLFOX/CAPEOX, which will definitively determine if a chemotherapy-free approach is non-inferior. Beyond this specific design limitation, other considerations warrant attention. The influence of factors such as the prevalence of Lynch syndrome and underlying tumor heterogeneity on the outcomes within the ATOMIC cohort requires further exploration. Furthermore, while the 100% 3-year DFS in the NICHE-2 trial is remarkable, it applies to the per-protocol population that underwent surgery, and longer-term follow-up across all these studies is essential to confirm the durability of responses and OS benefits [9]. Acknowledging these limitations and awaiting the results of definitive trials like AZURE-2 are crucial next steps in refining this rapidly evolving treatment paradigm.
4.5. Implications for Clinical Practice and Future Research
MMR deficiency status is a highly predictive biomarker for immunotherapy response and should be routinely analyzed in all non-metastatic colorectal cancer patients prior to surgery. The ATOMIC trial establishes that adding adjuvant atezolizumab to mFOLFOX cures more patients with stage III R0-resected dMMR/MSI-H CRC, demonstrating a 10% absolute improvement in 3-year DFS and represents a potential new standard of care.
Based on current evidence, we propose the following treatment algorithm (Figure 2): (1) For patients with clinically low-risk tumors (cT3/N0), we recommend upfront surgery. If pathological staging confirms stage III disease, treatment with 3 months of adjuvant chemotherapy (per IDEA collaboration data) plus one year of atezolizumab is advised. (2) For patients with clinically high-risk tumors (cT4/N+) at elevated risk of R1 resection, we recommend short-course neoadjuvant immunotherapy followed by surgery. If R1 resection is confirmed, post-operatively, adjuvant chemotherapy plus atezolizumab, should be administered, with a chemotherapy duration of 6 months (Figure 2).
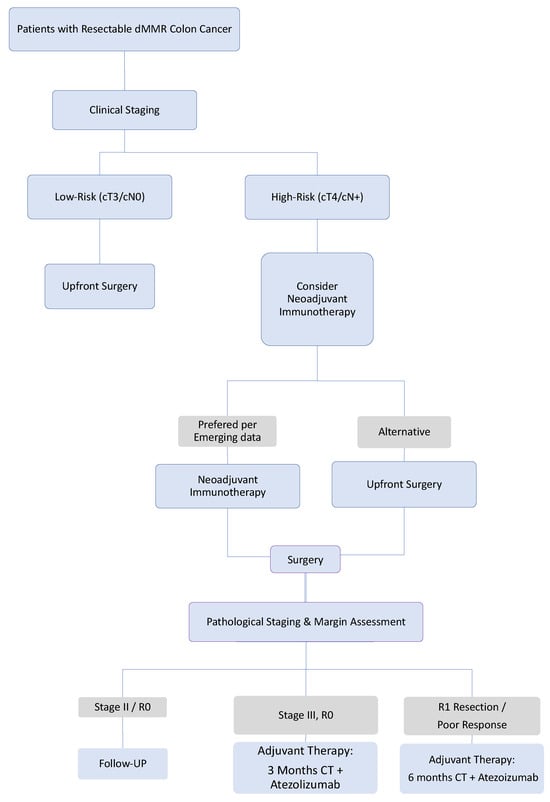
Figure 2.
Proposed Treatment Recommendations for Stage III Resectable dMMR Colon Cancer. This algorithm synthesizes current evidence (ATOMIC) with promising emerging strategies (neoadjuvant immunotherapy). The pathways involving neoadjuvant immunotherapy are considered investigative and await validation from definitive randomized trials. The choice of strategy should involve shared decision-making with the patient, considering the distinct efficacy and toxicity profiles of each approach. The critical need for biomarker development to guide these choices is emphasized. Abbreviation: CT: Chemotherapy, dMMR: Mismatch repair deficiency.
There is an urgent need to develop reliable predictive biomarkers to prevent overtreatment and to better select patients who will benefit from neoadjuvant or adjuvant strategies.
5. Conclusions
The treatment paradigm for resected stage III dMMR CRC is undergoing a fundamental transformation, moving beyond the long-standing era of fluoropyrimidine-based chemotherapy. The recent results from the ATOMIC trial establish a new benchmark for the adjuvant setting, demonstrating that adding atezolizumab to mFOLFOX significantly improves DFS and offers a new, more effective standard of care. Concurrently, the remarkable efficacy and favorable safety profile of short-course neoadjuvant immunotherapy, as exemplified by the NICHE-2 trial, present it as a compelling and potentially chemotherapy-free alternative that can induce high rates of pathological complete response and possibly prevent recurrence altogether.
This evolution creates a critical dilemma for clinicians: choosing between the proven, enhanced efficacy of adjuvant chemoimmunotherapy and the highly effective, less toxic, and organ-preserving potential of a neoadjuvant immunotherapy-first approach. The answer to this question is not yet clear and underscores an urgent need for predictive biomarkers to guide personalized therapy selection. Future results from ongoing trials, such as AZURE-2, which directly compare perioperative immunotherapy to standard chemotherapy, are essential to definitively determine the optimal sequence, combination, and duration of treatment.
Ultimately, the management of non-metastatic dMMR CRC has entered a new era of personalization. The determination of MMR status is now a critical gateway, not just for prognosis but also for selecting between two powerful, and concurrently emerging, paradigm-shifting strategies: adjuvant chemoimmunotherapy and neoadjuvant immunotherapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gastroent16040043/s1, Table S1: PRISMA 2020 Checklist for “The New Horizon for Non-Metastatic dMMR Colorectal Cancer: A Systematic Review of the Adjuvant Chemoimmunotherapy and Neoadjuvant Immunotherapy Revolution”.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data availability is not applicable to this article as no new data were created or analyzed in this study. All data presented in this systematic review were extracted from previously published clinical trials, which are cited in the references.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Abbreviations
The following abbreviations are used in this manuscript:
| Abbreviation | Explanation |
| 5-FU | 5-fluorouracil |
| ASCO | American Society of Clinical Oncology |
| ATOMIC | Adjuvant Trial of MImmunotherapy in Colon cancer |
| CAPEOX | Capecitabine and Oxaliplatin |
| CI | Confidence Interval |
| CRC | Colorectal Cancer |
| CR | Complete Response |
| CT | Chemotherapy |
| dMMR | Mismatch Repair-deficient |
| DFS | Disease-Free Survival |
| ESMO | European Society for Medical Oncology |
| FOLFOX | 5-Fluorouracil, Leucovorin, and Oxaliplatin |
| HR | Hazard Ratio |
| IDEA | International Duration Evaluation of Adjuvant Chemotherapy |
| LV5FU2 | Leucovorin and 5-Fluorouracil |
| MMR | Mismatch Repair |
| MPR | Major Pathological Response |
| MSI-H | Microsatellite Instability-High |
| MSS | Microsatellite Stable |
| NSCLC | Non-Small-Cell Lung Cancer |
| OS | Overall Survival |
| pCR | Pathological Complete Response |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-Free Survival |
| pMMR | Proficient Mismatch Repair |
| PN | Peripheral Neuropathy |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| R0 | Complete Resection |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Goodman, P.J.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 1990, 322, 352–358. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Sobrero, A.F.; Shields, A.F.; Yoshino, T.; Paul, J.; Taieb, J.; Souglakos, J.; Shi, Q.; Kerr, R.; Labianca, R.; et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N. Engl. J. Med. 2018, 378, 1177–1188. [Google Scholar] [CrossRef]
- Johannet, P.; Rousseau, B.; Aghajanian, C.; Foote, M.B.; Diaz, L.A. Therapeutic targeting of mismatch repair-deficient cancers. Nat. Rev. Clin. Oncol. 2025, 22, 734–759. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; Alcaide-Garcia, J.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy in microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer: 5-year follow-up from the randomized phase III KEYNOTE-177 study. Ann. Oncol. 2025, 36, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Elez, E.; Van Cutsem, E.; Jensen, L.H.; Bennouna, J.; Mendez, G.; Schenker, M.; de la Fouchardiere, C.; Limon, M.L.; Yoshino, T.; et al. Nivolumab plus Ipilimumab in Microsatellite-Instability-High Metastatic Colorectal Cancer. N. Engl. J. Med. 2024, 391, 2014–2026. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Ou, F.S.; Arnold, D.; Peters, W.; Behrens, R.J.; Lieu, C.H.; Matin, K.; Cohen, D.J.; Potter, S.L.; Frankel, W.L.; et al. Randomized trial of standard chemotherapy alone or combined with atezolizumab as adjuvant therapy for patients with stage III deficient DNA mismatch repair (dMMR) colon cancer (Alliance A021502; ATOMIC). J. Clin. Oncol. 2025, 43, LBA1. [Google Scholar] [CrossRef]
- Chalabi, M.; Verschoor, Y.L.; Tan, P.B.; Balduzzi, S.; Van Lent, A.U.; Grootscholten, C.; Dokter, S.; Büller, N.V.; Grotenhuis, B.A.; Kuhlmann, K.; et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair–Deficient Colon Cancer. N. Engl. J. Med. 2024, 390, 1949–1958. [Google Scholar] [CrossRef]
- Wang, F.; Chen, G.; Qiu, M.; Ma, J.; Mo, X.; Liu, H.; Li, Y.; Ding, P.; Wan, X.; Hu, Y.; et al. Neoadjuvant Treatment of IBI310 Plus Sintilimab in Locally Advanced MSI-H/dMMR Colon Cancer: A Randomized Phase 1b Study. Cancer Cell 2025, 43, 1958–1967. [Google Scholar] [CrossRef]
- de Gooyer, P.G.; Verschoor, Y.L.; van den Dungen, L.D.; Balduzzi, S.; Marsman, H.A.; Geukes Foppen, M.H.; Grootscholten, C.; Dokter, S.; den Hartog, A.G.; Verbeek, W.H.; et al. Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: A phase 2 trial. Nat. Med. 2024, 30, 3284–3290. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Kang, L.; Zhang, J.; Wu, Z.; Wang, H.; Huang, M.; Lan, P.; Wu, X.; Wang, C.; Cao, W.; et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): A single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 38–48. [Google Scholar] [CrossRef]
- Shiu, K.K.; Jiang, Y.; Saunders, M.; Seligmann, J.F.; Iveson, T.; Wilson, R.H.; Graham, J.S.; Khan, K.H.; Militello, A.M.; Irvine, S.; et al. NEOPRISM-CRC: Neoadjuvant pembrolizumab stratified to tumour mutation burden for high-risk stage 2 or stage 3 deficient-MMR/MSI-high colorectal cancer. J. Clin. Oncol. 2024, 42, 3500. [Google Scholar] [CrossRef]
- de la Fouchardiere, C.; Zaanan, A.; Cohen, R.; Le Sourd, S.M.; Tougeron, D.; Soularue, E.; Dubreuil, O.; Williet, N.; Samalin-Scalzi, E.; Piessen, G.; et al. IMHOTEP phase II trial of neoadjuvant pembrolizumab in dMMR/MSI localized cancers: Results of the digestive non-colorectal cancer cohorts. Ann. Oncol. 2024, 35, S899–S900. [Google Scholar] [CrossRef]
- Cercek, A.; Foote, M.B.; Rousseau, B.; Smith, J.J.; Shia, J.; Sinopoli, J.; Weiss, J.; Lumish, M.; Temple, L.; Patel, M.; et al. Nonoperative Management of Mismatch Repair–Deficient Tumors. N. Engl. J. Med. 2025, 392, 2297–2308. [Google Scholar] [CrossRef]
- Sargent, D.J.; Marsoni, S.; Monges, G.; Thibodeau, S.N.; Labianca, R.; Hamilton, S.R.; French, A.J.; Kabat, B.; Foster, N.R.; Torri, V.; et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J. Clin. Oncol. 2010, 28, 3219–3226. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.D.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Wang, C.; Lu, S.; Felip, E.; Swanson, S.J.; Brahmer, J.R.; et al. Overall Survival with Neoadjuvant Nivolumab plus Chemotherapy in Lung Cancer. N. Engl. J. Med. 2025, 392, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; Van De Wiel, B.A.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.R.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved Efficacy of Neoadjuvant Compared to Adjuvant Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Cell 2021, 39, 593–595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).