Abstract
Background. The Tumor-Node-Metastasis system does not include additional prognostic factors present in the Lymph Node Metastasis (LNM) such as extra-capsular extension (ECE), which is associated with decreased survival. There are not studies addressing this topic in rectal cancer patients with preoperative chemoradiotherapy (nCRT) and total mesorectal excision (TME). Aim. We aimed to examine the survival influence of ECE in patients with stage III rectal cancer who received nCRT followed by surgery. Methods. A retrospective study of 126 patients prospectively collected with rectal cancer in clinical stage III rated with nCRT and TME from 2010 to 2015 was performed. Results. In total, 71.6% of cases had 1 to 3 lymph node metastases, most tumors were grade 2 (52.4%), 25.4% had good pathologic response, 77.8% had a good quality TME, and the median tumor budding count was 4/0.785 mm2. Forty-four (34.9%) patients had ECE+, which was associated with a higher nodal stage (pN2), perineural invasion and a higher lymph node retrieval. The factors associated with the survival were a higher pathologic T stage, higher pathological N stage, high-grade tumors, and perineural invasion. The ECE did not decrease the 5–year survival with a similar median survival (86.5 months for the ECE+ group vs. 84.1 for the ECE–). Conclusion. Our results demonstrate that ECE has no impact on overall survival in rectal cancer patients who received nCRT and this was independent of nodal stage or number of lymph nodes examined.
1. What Does This Paper Add to the Literature?
Extra-capsular extension of nodal metastasis in rectal cancer treated with chemoradiotherapy failed to predict mortality. Even our results are negative, and it is important to show that this feature has not got prognostic implications in this scenario.
2. Introduction
Rectal carcinoma is a cancer with a high incidence and mortality in western countries. As almost any tumor, the clinical stage at diagnosis is the best indicator of prognosis and most patients present with lymph node metastasis (LNM), but little attention has been paid to its histologic features.
Despite the fact that the Tumor-Node-Metastasis (TNM) system includes factors such as tumor deposits in the nodal category, it does not include additional prognostic factors present in the LNM, such as extra-capsular extension (ECE), defined as the dissemination of neoplastic cells through the nodal capsule into the adipose tissue (Figure 1). ECE is an important prognostic factor in cancers of the unknown primary [1], breast [2], head-and-neck [3], and vulva [4]. A systematic review about ENE as a prognostic factor in gastrointestinal neoplasms demonstrated their association with decreased survival in colorectal cancer (four studies, n = 502) [5]. Another systematic review of 13 studies (n = 1336) combining the colon and rectum finds a Hazard Ratio (HR) for death of 1.6 (95% confidence interval (CI): 1.32–2.17) for ECE+, but has moderate heterogeneity (I2 = 46%), which could be related with the combination of colon cancer with rectal cancer and some included studies addressed tumor deposits instead of ECE [6].
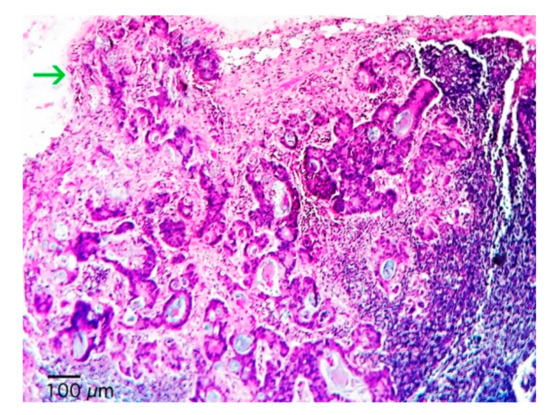
Figure 1.
Photomicrography of a lymph node with partial substitution of a rectal adenocarcinoma. The metastatic glands predominate in the subcapsular region, invades the nodal capsule and extents into perinodal soft tissues (arrow) (hematoxylin and eosin, 100×).
Several works that studied ECE combined rectal with colon cancer [7,8,9]; the results should be interpreted with caution and additional research needs to be performed focusing attention on specific cancers (colon and rectum) because these tumors behave differently. In rectal cancer, two works addressed specifically ECE in rectal cancer treated with surgery and postoperative chemo-radiotherapy or surgery alone [10,11] showing the adverse prognostic impact of ECE. However, these are studies carried out decades ago when total mesorectal excision (TME) was not the standard of care, and, nowadays neoadjuvant chemoradiotherapy (nCRT) has importantly improved overall survival in these patients.
Our aim was to prove the survival influence of ECE in patients with stage III rectal cancer treated with nCRT followed by surgery with TME. To our knowledge, this is the first study addressing this topic in this clinical scenario.
3. Methods
Study design and sample size calculation. We designed a retrospective study. For a two-tail significance level of 0.05, a power of 80% and a minimum detectable difference of 40% in the survival between the group of patients with rectal cancer with nodal metastasis with and without ECE, the number of patients needed for the study are 114 cases [12]. Anticipating a loss of 10% of cases for the analysis, the final sample is of 126 cases. This work was approved by the ethics committee of the National Cancer Institute (Reference number: Rev218/18).
Population. The 126 cases were retrieved consecutively from the pathological files in our Institution from 2005 to 2015. The inclusion criteria were adult patients (>18 years old) affected by rectal adenocarcinoma following nCRT of at least 3 chemotherapy cycles with a complete course of 50.4 Gy. Furthermore, we selected cases with LNM and with complete histopathologic material for the analysis. We excluded cases with complete pathologic response.
Variables. Pathologic and clinical variables were retrieved from the clinical files and pathologic reports. All specimens were processed systematically according to a systematic protocol and were examined by two pathologists blinded to clinical data. All cases with ECE were reviewed in a confrontation microscope. ECE was defined as any tumor cell or group infiltrating the nodal capsule of a metastatic node and contiguously invading the perinodal soft tissues (Figure 2a,b). In a case of a discontinuous tumor nodule adjacent to an LNM, several step sections were performed to demonstrate (or not) the contiguity between LNM and ECE. If the LNM was not contiguous with the perinodal tumor cells (Figure 2b) it was classified as a tumor deposit according to the AJCC 8 definition [13].
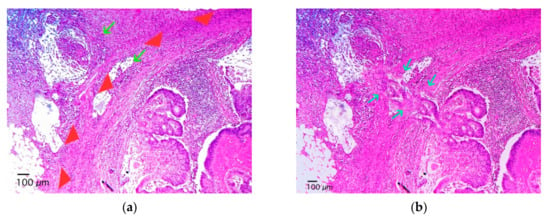
Figure 2.
Photomicrography of a lymph node with adenocarcinoma metastasis. In cases where neoplastic nests (arrows) are outside the nodal capsule (arrowheads) with no apparent connection to intranodal metastatic nests (a) we performed additional deep sections to test if there is a connection between the extracapsular nests and the intranodal nests (b), the arrows show the connection between intranodal tumor cells and extranodal tumor cells), if so, the case was classified as lymph node metastasis with extra-capsular extension; on the contrary, it was considered a tumor deposits, if also some nodal, vascular, or neural structure is not identified.
Statistical analysis. We used the Kolmogorov–Smirnov test to determine normality and we used the median and interquartile range (IQR), count, and percentage. For the comparison of variables, we applied the Mann–Whitney U test for numerical variables and the Chi-square test or Fischer’s exact test for the categorical ones. The variables were analyzed between patients with ECE (ECE+) and patients without ECE (ECE–). We performed a survival analysis with the Kaplan and Meier method and compared survival estimates with the log-rank test. All analyses were computed with SPSS version 22.0 (IBM, Armonk, NY, USA) with a two-tailed statistical significance set at p-value < 0.05.
4. Results
In Table 1 are the details of the clinicopathologic characteristics of the 126 patients. From the total, 71.6% had 1 to 3 LNM, most tumors were grade 2 (52.4%), 25.4% had good pathologic response, 77.8% had a good quality TME, and the median tumor budding count was 4/0.785 mm2.

Table 1.
Clinicopathologic characteristics of 126 patients with residual rectal cancer post neoadjuvant chemoradiotherapy with lymph node metastasis.
All patents had >10 lymph node resected. Forty-four (34.9%) patients had ECE+ and this was associated with a higher pathological nodal stage (pN2) in 45.5% vs. 14.6% for ECE−, perineural invasion (50% in ECE+ vs. 17.1 in ECE−) and a higher lymph node resected (median of 16 vs. 13 lymph nodes for ECE+ and ECE−, respectively) (Table 2).

Table 2.
Clinicopathologic characteristics of 126 patients with residual rectal cancer post neoadjuvant chemoradiotherapy and lymph node metastasis grouped according extracapsular extension.
At a median follow-up of 39 (IQR 25–90) months, 24 (19%) patients died. The factors associated with the survival were a higher pathologic T stage, higher pathological N stage, high-grade tumors, and perineural invasion. The ECE did not decrease the 5–year survival with a similar median survival (86.5 months for ECE+ group vs. 84.1 for the ECE−). All variables in the survival analysis are in Table 3. Furthermore, when stratifying the EEC by the lymph node stage, no significant differences were found (p = 0.570), nor were they found when stratifying by the number of positive lymph nodes (1 vs.> 1, p = 0.961).

Table 3.
Clinicopathologic variables and survival in 63 patients with residual rectal cancer post neoadjuvant chemoradiotherapy and lymph node metastasis.
5. Discussion
We show that patients with rectal cancer treated with nCRT and surgery who had LNM have similar survival between ECE+ and ECE–. This contrasts with previous reports on colorectal cancer (studies including both colon and rectal cancer without nCRT), where ECE is associated with an increased risk of mortality (HR = 1.69, 95%CI: 1.32–2.17, p < 0.0001, I2 = 46%) [6]. However, we think that it is somewhat logical because populations and our sample are not comparable because of the use of nCRT, and we insist that is important to address this topic because most patients with rectal cancer present in locoregional advanced disease then will receive nCRT. Following nCRT and surgery, it is important to identify which patients need additional therapy and pathologic parameters could be the answer.
There is still controversy about the amount of tissue that needs to be resected, i.e., the extent of resection with respect to the regional lymphatics, lymph nodes, and their features.
The fact that we have not found differences with the presence or not of ECE is of practical utility for pathologists because although we do our best to identify all the prognostic factors recommended in the evaluation of such cancer, we can obviate the ECE in the evaluation and report. An important implication regards the surgical pathology approach and the gross sampling. Indeed, based on the shown importance of ECE in colon cancer, and knowing that ECE can be very focal, a mandatory consequence is that all the lymph nodes with their surrounding adipose tissue must be completely included. A common gross approach tends to start with the manual isolation of lymph nodes, or with the sampling of only a portion of lymph nodes with metastatic aspect. However, based on our results, in rectal cancer a complete inclusion of all the lymph nodes, even if very large, and of the perinodal fatty tissue is unnecessary.
ECE, indicated recently as a prognostic factor for cancers in several organs [14,15,16], has also been taken into account in the last staging systems of squamous cell carcinoma of the vulva [17]. It is likely that the better prediction of prognosis of vulvar cancer by these new staging systems could be partly due even to the consideration of the importance of ECE. Our results in rectal cancer show different outcomes.
Why was ECE not associated with prognosis in our study while the others were? In addition to the reason initially stated (we are in a different clinical context) the answer perhaps lies in the fact that the definition of ECE has also been different in the various studies, as shown in the meta-analyses [5,6], where they have included as ECE cases that correspond to tumor deposits. Indeed, in rectal cancer evaluation of tumor deposits, tumor budding, and several other parameters are difficult to evaluate in the setting of nCRT because discontinuity between neoplastic cells could be explained by tumor response. To avoid this confusion, we use a strict definition of ECE demonstrating contiguity between the LNM and the nest of neoplastic cells in ECE.
An additional strength of our study is that surgery quality is well-described, with near 80% of patients with adequate quality of TME, and all patients had >10 lymph nodes. The TME is the standard of care and maybe the most significant contribution to advancing rectal cancer care which has shown universal reproducible reductions in local recurrence and improvement in disease-free and overall survival [18]. Despite its importance, TME is not frequently considered as a variable of interest in prognostic studies about rectal cancer.
The limitations of our study are inherent to a retrospective series (biases in the administration of the treatment, variability in surgery techniques, technology trough the time, etc.).
6. Conclusions
Our results show that ECE has no impact on overall survival in rectal cancer patients who received nCRT and this finding was independent of the nodal stage or the number of lymph nodes examined. Additional studies with a larger number of patients and other populations and therapeutic regimens are needed to prove these findings.
Author Contributions
All authors meet the criteria for authorship as per the guidelines of the International Committee of Medical Journal Editors (ICMJE). Conception and design: All authors. Financial support: L.S.L.-S., C.Z.-N., C.I.S.-A., R.A.S.-H. Provision of study materials or patients: All authors. Collection and assembly of data: All authors. Data analysis (statistical analysis) and interpretation: L.S.L.-S. Manuscript writing: All authors. Final revision and approval of manuscript: All authors. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Coster, J.R.; Foote, R.L.; Olsen, K.D.; Jack, S.M.; Schaid, D.J.; DeSanto, L.W. Cervical nodal metastasis of squamous cell carcinoma of unknown origin: Indications for withholding radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 743–749. [Google Scholar] [CrossRef]
- Leonard, C.; Tompkin, J.; Zhen, B.; Zehn, B.; Waitz, D.; Norton, L. Are axillary recurrence and overall survival affected by axillary extranodal tumor extension in breast cancer? Implications for radiotherapy. J. Clin. Oncol. 1995, 13, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Tiwari, R.; Nauta, J.J.; van der Waal, I.; Snow, G.B. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993, 71, 452–456. [Google Scholar] [CrossRef]
- Van der Velden, J.; van Lindert, A.C.M.; Lammes, F.B.; ten Kate, F.J.; Sie-Go, D.M.; Oosting, H.; Heintz, A.P.M. Extracapsular growth of lymph node metastasis in squamous cell carcinoma of the vulva: The impact on recurrence and survival. Cancer 1995, 75, 2885–2890. [Google Scholar] [CrossRef]
- Wind, J.; Lagarde, S.M.; Ten Kate, F.J.; Ubbink, D.T.; Bemelman, W.A.; van Lanschot, J.J.B. A systematic review on the significance of extracapsular lymph node involvement in gastrointestinal malignancies. Eur. J. Surg. Oncol. 2007, 33, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Nottegar, A.; Pea, A.; Solmi, M.; Stubbs, B.; Capelli, P.; Scarpa, A. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: A systematic review with meta-analysis. Ann. Oncol. 2016, 27, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Tabe, Y.; Yajima, R.; Yamaguchi, S.; Tsutsumi, S.; Asao, T.; Kuwano, H. Process of distant lymph node metastasis in colorectal carcinoma: Implication of extracapsular invasion of lymph node metastasis. BMC Cancer 2011, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Tabe, Y.; Yajima, R.; Yamaguchi, S.; Tsutsumi, S.; Asao, T.; Kuwano, H. Extracapsular invasion as a risk factor for disease recurrence in colorectal cancer. World J. Gastroenterol. 2011, 17, 2003–2006. [Google Scholar] [CrossRef] [PubMed]
- Komuta, K.; Okudaira, S.; Haraguchi, M.; Furui, J.; Kanematsu, T. Identification of extracapsular invasion of the metastatic lymph nodes as a useful prognostic sign in patients with resectable colorectal cancer. Dis. Colon Rectum 2001, 44, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Heide, J.; Krull, A.; Berger, J. Extracapsular spread of nodal metastasis as a prognostic factor in rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 773–778. [Google Scholar] [CrossRef]
- Brabender, J.; Bollschweiler, E.; Hölscher, A.H.; Strobel, K.; Gutschow, C.; Prenzel, K.; Vallböhmer, D. The prognostic impact of extracapsular lymph node involvement in rectal cancer patients: Implications for staging and adjuvant treatment strategies. Oncol. Lett. 2012, 3, 825–830. [Google Scholar] [PubMed]
- Schoenfeld, D.; Richter, J. Nomograms for calculating the number of patients needed for a clinical trial with survival as an endpoint. Biometrics 1982, 38, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Brookland, M.K.; Washington, M.K.; Gershenwald, J.E. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Alakus, H.; Hölscher, A.H.; Grass, G.; Hartmann, E.; Schulte, C.; Drebber, U.; Baldus, S.E.; Bollschweiler, E.; Metzger, R.; Mönig, S.P. Extracapsular lymph node spread: A new prognostic factor in gastric cancer. Cancer 2010, 116, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, S.M.; ten Kate, F.J.; de Boer, D.J.; Busch, O.R.; Obertop, H.; van Lanschot, J.J. Extracapsular lymph node involvement in node-positive patients with adenocarcinoma of the distal esophagus or gastroesophageal junction. Am. J. Surg. Pathol. 2006, 30, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Griebling, T.L.; Ozkutlu, D.; See, W.A.; Cohen, M.B. Prognostic implications of extracapsular extension of lymph node metastases in prostate cancer. Mod. Pathol. 1997, 10, 804–809. [Google Scholar] [PubMed]
- Luchini, C.; Nottegar, A.; Solmi, M.; Sergi, G.; Manzato, E.; Capelli, P.; Scarpa, A.; Veronese, N. Prognostic implications of extranodal extension in node-positive squamous cell carcinoma of the vulva: A systematic review and meta-analysis. Surg. Oncol. 2016, 25, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Knol, J.; Keller, D.S. Total Mesorectal Excision Technique-Past, Present, and Future. Clin. Colon Rectal Surg. 2020, 33, 134–143. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).