Abstract
Myocardial Bridging (MB) is typically a benign congenital coronary anomaly. MB can infrequently result in complications such as myocardial ischemia, arrhythmias, and sudden cardiac death. Recent studies suggest an underlying genetic component for MB involving DES, FBN1, SCN2B, or NOTCH1. The role of percutaneous coronary intervention (PCI) in managing MB, compared to optimal medical therapy (OMT), remains uncertain. Our study used the National Inpatient Sample (NIS) Database to identify patients aged 18 or older with myocardial bridging who were managed with PCI versus medical therapy. We compared the outcomes between both groups including in-hospital mortality, the trend of management of MB and other in-hospital outcomes or complications. Our results showed no statistically significant difference between both subgroups when comparing in-hospital mortality and secondary outcomes of cardiac arrest and the development of an acute kidney injury (AKI). Patients with myocardial bridging treated with PCI had a higher risk of developing cardiogenic shock, requiring LVAD, and requiring the use of intra-aortic balloon pump (IABP) compared to the medical therapy subgroup. Our study suggests the decision to perform PCI in myocardial bridging patients should be individualized such as in patients with refractory symptoms despite medical therapy or those with known high-risk features.
1. Introduction
Myocardial bridging (MB) is a congenital coronary anomaly in which a segment of the normal epicardial coronary artery courses within the myocardium (i.e., intra-myocardial course). The condition generally has a benign course [1]. Indeed, bridged segments of coronary arteries are narrowed during systole, while most of the coronary blood flow occurs during diastole. Myocardial bridging is a congenital anomaly and, in some cases, may be hereditary. However, the genetic basis of MB is currently underrecognized. Recent studies suggested some genetic associations (e.g., DES, FBN1, SCN2B, or NOTCH1) for myocardial bridging [2,3]. Generally, percutaneous coronary intervention (PCI) is not advised due to the high risk of in-stent restenosis, stent perforation, or stent fracture, while medical management is recommended in symptomatic myocardial bridging [4,5,6,7,8]. In this study of a nationwide cohort, we evaluated the baseline characteristics and trends of patients with myocardial bridging. We also analyzed clinical outcomes and in-hospital mortality of myocardial bridging patients treated with medical therapy versus PCI.
2. Methods
2.1. Data Source
Our study is sourced from the National Inpatient Sample (NIS) database, one of the largest publicly available inpatient databases in the United States. When weighted, it provides estimated projections of 35 million hospitalizations nationwide.
2.2. Study Population
Using the NIS database from 2016 to 2020, we identified patients aged 18 and above with myocardial bridging using the International Classification of Disease, Tenth Edition, Clinical Modification (ICD-10-CM) code I25.41. Following that, we categorized these patients into PCI and medical therapy arms, respectively. Patients who did not undergo PCI were considered to be in the medical therapy group. We excluded patients with missing information on age, gender, and mortality. Patients who underwent coronary artery bypass graft (CABG) during index hospitalization were excluded from this study.
2.3. Study Outcomes and Definitions
Our primary outcome is in-hospital mortality. Secondary outcomes include the trend of management of MB and other in-hospital outcomes or complications, which include cardiac arrest, cardiogenic shock, acute kidney injury (AKI), and use of mechanical circulatory support such as left ventricular assist device (LVAD), extracorporeal membrane oxygenation (ECMO) and intra-aortic balloon pump (IABP), cost of hospitalizations, and length of stay.
2.4. Statistical Analysis
We employed the discharge weight provided to produce a national estimate of these hospitalizations. Categorical variables are presented as frequencies and/or percentages and compared using a chi-square test. Continuous variables were summarized with means and their respective standard deviations, and comparisons of these variables were made using linear regression. We adhered rigorously to the data agreement with the Healthcare Cost and Utilization Project (HCUP) and avoided reporting variables with less than or equal to 10 counts in either arm. Complete case analyses were performed since the amount of missing data is minimal (<5%). We implemented multivariate logistic regression to analyze these outcomes, using variables that were significant in univariate analysis, and incorporated these into the construction of the model. Variables with a two-sided p-value of less than 0.05 were considered statistically significant in our study. All analyses were conducted using STATA v17.0 (StataCorp, College Station, TX, USA).
3. Results
We analyzed 15,510 patients diagnosed with myocardial bridging. Of these patients, 11,875 (76.6%) were managed with medical therapy, while 3635 (23.4%) were managed with PCI. The number of myocardial bridging patients who were treated with medical therapy has increased over time until 2019 (Figure 1).
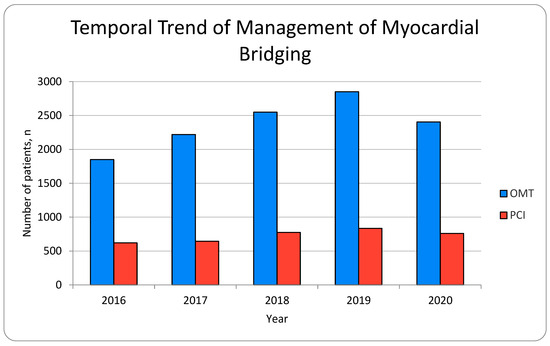
Figure 1.
Temporal trend of management of myocardial bridging.
In the medical therapy subgroup of patients, the mean age was 64.5 years, and 68.9% were male. In the PCI subgroup of patients, the mean age was 65.9 years, and 73.3% were male. The highest ethnicity of patients in both subgroups was White, followed by Black and Hispanic. Table 1 demonstrates the baseline characteristics of patients with myocardial bridges treated with medical therapy or PCI. A comparison of the baseline comorbidities of both subgroups demonstrates that patients managed with medical therapy had statistically significant higher percentages of comorbidities, including cardiac arrhythmias, valvular heart disease, pulmonary circulatory diseases, peripheral vascular disease, hypertension (complicated and uncomplicated), chronic lung disease, uncomplicated diabetes mellitus, CKD, rheumatologic disorders, coagulopathies, fluid and electrolyte disorders, history of smoking, and acute myocardial infarction (Table 2). Table 3, Table 4 and Table 5 demonstrated acute myocardial infarction and mortality predictors.

Table 1.
Baseline characteristics of myocardial bridge between medical therapy and PCI.

Table 2.
Outcomes of myocardial bridge between medical therapy and PCI.

Table 3.
Acute myocardial infarction predictors.

Table 4.
Predictors of mortality in myocardial bridge patients.

Table 5.
Predictors of mortality in myocardial bridge patients.
Comparing the PCI subgroup to the medical therapy subgroup, there was no statistically significant difference between both subgroups when comparing in-hospital mortality (adjusted OR of 1.39 (0.81–2.40), p = 0.232), cardiac arrest (adjusted OR of 1.68 (0.94–3.03), p = 0.083), and the development of AKI (adjusted OR of 0.93 (0.70–1.23), p = 0.595). Patients with myocardial bridging treated with PCI had a higher risk of developing cardiogenic shock (adjusted OR of 2.04 (1.40–2.99), p < 0.001), requiring LVAD (adjusted OR of 15.22 (5.54–41.79), p < 0.001), and use of IABP (adjusted OR of 2.24 (1.34–3.74), p = 0.002) compared to the medical therapy subgroup. The cost of hospitalization was higher in the PCI subgroup (30,884 +/− 25,100 vs. 27,787 +/− 32,418 p = 0.009), while the length of stay was shorter (6.13 +/− 6.67 days vs. 4.53 +/− 5.25 days, p < 0.001) compared to the medical therapy subgroup.
4. Discussion
In our study, we found no statistically significant differences in in-hospital mortality, cardiac arrest, or development of AKI between patients with myocardial bridging who were treated with medical therapy vs. PCI. Interestingly, myocardial bridging patients who were treated with PCI were associated with cardiogenic shock, the use of LVAD, and IABP. Notably, all poor early outcomes were less favorable in the PCI group. Even with adjustment, the OR for mortality with PCI was 30% higher, though the p value was not significant. However, we were unable to identify why patients were managed with PCI.
Recent advances in genetic research have significantly enhanced our understanding of the molecular basis of myocardial bridging. A 2023 systematic review studied several genes that may be associated with myocardial bridge development, including DES (E434K), FBN1 (I1175M), SCN2B (E31D), and NOTCH1 (R2313Q), along with others, such as COMMD10, MACROD2, SLMAP, MYH7, and DPP6 [2]. Among the mutations discovered, MYH7, SCNH2B, COMMD10, NOTCH1, FBN1, and MACROD2 have been proposed as candidate genes for future research, as these genes have vital roles in coronary artery development and migration, and the authors hypothesized that the disruptions in these genes may play a role in the pathogenesis in myocardial bridging. For instance, the NOTCH gene plays an important role in cardiac development through the proliferation and differentiation of cardiomyocytes, and its mutation has been implicated in congenital heart diseases such as hypoplastic left heart syndrome. In a young female who died from sudden cardiac death, a post-mortem autopsy discovered myocardial bridging, and a mutation in the NOTCH1 gene was found. Furthermore, in adults, the NOTCH signaling pathway is activated in response to myocardial insult, suggesting that the gene may have a role in cardiac repair through angiogenesis. Though, at this point, there was no direct link between the NOTCH gene and the development of a myocardial bridge, it is plausible that the NOTCH1 gene may contribute to this anomaly, and further research is required to support this hypothesis.
Evidence suggests familial clustering of cardiac diseases in approximately 20% of patients with myocardial bridges and other coronary anomalies [9]. Among patients with anomalous coronary arteries and myocardial bridges, a significant family history involving both 1st-degree and 2nd-degree relatives has been documented. However, direct inheritance of myocardial bridging itself appears rare, suggesting complex inheritance patterns rather than simple Mendelian inheritance, likely involving multiple genetic and environmental factors [9].
Myocardial bridging is known to cause angina-type symptoms and can result in significant myocardial ischemia. Several studies have analyzed the underlying hemodynamic mechanisms through which myocardial bridging can result in angina and ischemia [10,11]. One study followed 42 symptomatic patients with myocardial bridging through quantitative coronary angiography and intracoronary Doppler flow velocity measurements [12]. This study found an overall delay in the diastolic lumen diameter gain of the bridged segments and noted an increase in diastolic intracoronary Doppler flow velocities [10]. These findings, in combination with a reduced coronary flow reserve and the presence of associated angina symptoms in myocardial bridging, suggest there is a significant obstruction to coronary flow in the presence of myocardial bridging [10]. A separate study of 12 symptomatic patients with myocardial bridging similarly found via quantitative coronary angiography a noticeable reduction in the systolic vessel diameter, persistent diastolic diameter reduction, increased diastolic blood flow velocities, retrograde flow, and a reduced flow reserve within the bridged segments [11]. Interestingly, these underlying hemodynamic differences improved after intracoronary stent placement [11].
While no studies have directly compared medical therapy vs. PCI outcomes in treating myocardial bridging, aggressive risk factor modification through pharmacotherapy, such as beta-blockers or non-dihydropyridine calcium-channel blockers, have been the mainstay of therapy. PCI may be considered in myocardial bridging patients who are not a candidate for surgical myotomy or coronary artery bypass graft surgery (CABG) and to ameliorate symptoms for those who failed medical therapy [12,13]. CABG with an isolated left internal mammary artery to the LAD artery may be considered [11,12]. Evidence on the choice of surgical treatment for the treatment of symptomatic myocardial bridging is limited. CABG has been suggested over myotomy in patients with extensive or deep myocardial bridges, defined as >25 mm and >5 mm, respectively, or when there is failure of the coronary segment to decompress during diastole [14,15].
One study by Ernst and colleagues analyzed 15 patients with symptomatic myocardial bridging after drug-eluting stent implantation in the absence of coronary atherosclerotic disease, and they found that a significant improvement in symptoms was achieved in all patients, but there was a high rate of in-stent restenosis and target lesion revascularization (18.7%) after 1 year [16]. In a small study (70 myocardial bridging patients), for those who received drug-eluting stents for a LAD artery lesion, rates of target lesion revascularization at 1 year were 24% [17]. Another study by Hao and colleagues analyzed 330 patients presenting with acute coronary syndrome after drug-eluting stent placement and found a higher incidence of major adverse cardiac events and in-stent restenosis in those with myocardial bridging compared to patients without myocardial bridging [18]. A separate study previously cited compared hemodynamic measurements before and after intracoronary stent placement to bridged segments [11]. Patients with coronary stent placement had immediate normalization of intracoronary blood flow velocity profiles and intracoronary pressures and did not have systolic or diastolic lumen diameter reduction 7 weeks after stent placement [11]. Haager, Schwarz, and colleagues similarly analyzed the long-term follow-up of symptoms in patients with stent implantation for symptomatic myocardial bridging and found persistent benefits after stent placement at 6 months and 2 years after [19]. However, 5 of 11 patients (36%) followed in this study were found to have in-stent stenosis, with 4 of these patients undergoing repeat target vessel revascularization [19].
The effectiveness of medical therapy with beta-blockers has also been independently analyzed. One study followed 15 symptomatic patients with myocardial bridging without evidence of angiographic coronary artery disease who were treated with intravenous beta-blocker medication during atrial pacing [20]. While similar to other studies that noted reduced systolic and diastolic coronary lumen diameter in myocardial bridging, short-term intravenous beta-blocker therapy decreased the noted coronary artery diameter reduction during both systole and diastole, produced a return to baseline diastolic peak flow velocities, and abolished noted ST segment changes and symptoms [20]. This study strongly supports a similar underlying mechanism by which (1) myocardial bridging changes the hemodynamics of bridged segments and (2) beta-blockers enact therapeutic and hemodynamic effects on myocardial bridges compared to coronary artery stent placement.
While different modalities of treatment for the presence of myocardial bridging are available, there are no practice guidelines providing recommendations on the management of myocardial bridging [4]. Treatment for myocardial bridging is primarily based on individual profiles. Treatment for myocardial bridging with PCI has been reserved for patients with refractory symptoms despite medical therapy, although studies looking at the efficacy of stent placement report high rates of in-stent restenosis, stent fractures, and stent perforation [4,5,6,7,8]. Myocardial bridging patients who undergo PCI should expect revascularization rates to be high even with drug-eluting stents. In theory, a drug-coated balloon (DCB) could be a potential PCI strategy for atherosclerosis located in the myocardial bridging segment [21]. Despite these limitations, there have been prior efforts to prognosticate and guide therapeutic options for patients with myocardial bridging [22]. One retrospective study proposed a myocardial bridging classification for symptomatic patients based on angiographic findings of ischemia and altered intracoronary hemodynamics [22]. This specific study compared patients without coronary artery disease or myocardial bridging to patients with myocardial bridging and analyzed differences in clinical symptoms, objective signs of ischemia via stress test, and intracoronary Doppler flow measurements and coronary flow reserves [22]. Outcome analysis from this study separated based on one of three classifications classification suggests that patients with myocardial bridging and objective signs of ischemia (Category I) or myocardial bridging and altered intracoronary hemodynamics (Category II) may have an improved response to medical therapy with either beta-blockers or calcium channel antagonists, while patients with myocardial bridging but no objective signs of ischemia (Category III) may not [22]. A similar approach would be useful to distinguish patients who may benefit from PCI therapy from those who may not. Further prospective studies and clinical trials with PCI using DCB in treating myocardial bridging are urgently needed.
Our study has certain limitations. First, the outcomes analyzed in our study look at complications at one point in time and do not characterize long-term follow-up. Second, other complications, such as events of in-stent restenosis, stent perforation, and stent thrombosis, were not characterized in our database. Third, the lack of anatomical or imaging data in the database prevents differentiation of myocardial bridging subtypes or confirmation that PCI was performed on the myocardial bridge itself rather than on co-existing atherosclerotic lesions. Although we carefully selected cases coded for myocardial bridging and PCI, the absence of procedural and imaging details limits definitive conclusions about procedural intent and outcomes. Therefore, these results should be interpreted in the context of the potential selection bias. However, the data still allow for population-level analysis and may reflect current practice patterns and associated outcomes. Despite these limitations, while our results show no differences in mortality or cardiac arrest, the higher percentages of cardiogenic shock and higher use of LVAD and IABP in the PCI subgroup suggest that patients may benefit from a more nuanced approach to the treatment of myocardial bridging and more studies are needed to help guide future clinical guidelines. However, this retrospective study does not imply causation. It is possible that myocardial bridging patients who were very sick and presented cardiogenic shock more often underwent PCI and possibly required IABP rather than medical therapy. Third, we could not identify vessels of the myocardial bridge that could potentially change the outcomes based on PCI or medical therapy. Fourth, details of medical therapy are not defined in the database, and while we assume it includes beta-blockers, calcium channel blockers, and possibly other agents, this assumption introduces potential variability. Furthermore, regional variations in practice patterns, influenced by institutional expertise, resource availability, and geographic disparities, may have contributed to differences in PCI utilization and outcomes. Acknowledging these factors provides important context for interpreting findings and highlights the need for prospective studies to guide standardized management strategies.
In conclusion, our study found no significant difference in in-hospital mortality between myocardial bridging patients treated with PCI and those managed with medical therapy. PCI in myocardial bridging patients should be based on individual profiles. In patients with myocardial bridging with refractory angina despite medical therapy or high-risk features (e.g., proximal LAD, cardiogenic shock, ventricular tachycardia, and resuscitated cardiac arrest), PCI may be considered. Our study is among the first to provide large-scale, nationally representative data comparing the outcomes of PCI versus medical therapy in myocardial bridging. Future research should prioritize long-term outcomes and comparative effectiveness trials to identify the optimal management strategy for myocardial bridging. Prospective studies are needed to evaluate the role of PCI, particularly with advancements in drug-eluting stents and drug-coated balloons, and to compare their success rates against medical therapy. Incorporating detailed patient data, such as symptom severity and refractoriness to medical treatment, could further inform PCI decision making and improve personalized management in this population.
Author Contributions
Methodology, S.P.A.; Software, Z.W.; Validation, Z.W.; Formal analysis, S.P.A., Y.K.Q. and Z.W.; Investigation, Y.K.Q.; Writing—original draft, C.K. and F.A.P.; Writing—review & editing, C.K., F.A.P., Y.K.Q., Z.W., N.G., S.K.S., C.J.L., H.V.S. and E.R.S.; Visualization, C.K., S.K.S., C.J.L., H.V.S. and E.R.S.; Supervision, C.K., N.G., S.K.S., C.J.L., H.V.S. and E.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Patient consent was waived due to data being used being part of a publicly available database.
Data Availability Statement
Data available upon requested.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Evbayekha, E.O.; Nwogwugwu, E.; Olawoye, A.; Bolaji, K.; Adeosun, A.A.; Ajibowo, A.O.; Nsofor, G.C.; Chukwuma, V.N.; Shittu, H.O.; Onuegbu, C.A.; et al. A Comprehensive Review of Myocardial Bridging: Exploring Diagnostic and Treatment Modalities. Cureus 2023, 15, e43132. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.; Murdock, P.; Ramanathan, A.; Sathyamoorthy, M. A Contemporary Review of the Genomic Associations of Coronary Artery Myocardial Bridging. Genes 2023, 14, 2175. [Google Scholar] [CrossRef]
- Yang, T.L.; Ting, J.; Lin, M.R.; Chang, W.C.; Shih, C.M. Identification of Genetic Variants Associated with Severe Myocardial Bridging through Whole-Exome Sequencing. J. Pers. Med. 2023, 13, 1509. [Google Scholar] [CrossRef] [PubMed]
- Sternheim, D.; Power, D.A.; Samtani, R.; Kini, A.; Fuster, V.; Sharma, S. Myocardial Bridging: Diagnosis, Functional Assessment, and Management: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 2196–2212. [Google Scholar] [CrossRef]
- Tandar, A.; Whisenant, B.K.; Michaels, A.D. Stent fracture following stenting of a myocardial bridge: Report of two cases. Catheter. Cardiovasc. Interv. 2008, 71, 191–196. [Google Scholar] [CrossRef]
- Srinivasan, M.; Prasad, A. Metal fatigue in myocardial bridges: Stent fracture limits the efficacy of drug-eluting stents. J. Invasive Cardiol. 2011, 23, E150–E152. [Google Scholar]
- Broderick, T.M.; Kereiakes, D.J.; Whang, D.D.; Toltzis, R.J.; Abbottsmith, C.W. Myocardial Bridging May Predispose to Coronary Perforation During Rotational Atherectomy. J. Invasive Cardiol. 1996, 8, 161–163. [Google Scholar] [PubMed]
- Zhang, M.; Kang, W.C.; Moon, C.I.; Han, S.H.; Ahn, T.H.; Shin, E.K. Coronary artery perforation following implantation of a drug-eluting stent rescued by deployment of a covered stent in symptomatic myocardial bridging. Korean Circ. J. 2010, 40, 148–151. [Google Scholar] [CrossRef]
- Agrawal, H.; Mery, C.M.; Sexson, T.S.K.; Fraser, C.D.; McKenzie, E.D.; Qureshi, A.M.; Molossi, S. Familial clustering of cardiac conditions in patients with anomalous aortic origin of a coronary artery and myocardial bridges. Cardiol. Young 2018, 28, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.R.; Klues, H.G.; vom Dahl, J.; Klein, I.; Krebs, W.; Hanrath, P. Functional characteristics of myocardial bridging. A combined angiographic and intracoronary Doppler flow study. Eur. Heart J. 1997, 18, 434–442. [Google Scholar] [CrossRef]
- Klues, H.G.; Schwarz, E.R.; vom Dahl, J.; Reffelmann, T.; Reul, H.; Potthast, K.; Schmitz, C.; Minartz, J.; Krebs, W.; Hanrath, P. Disturbed intracoronary hemodynamics in myocardial bridging: Early normalization by intracoronary stent placement. Circulation 1997, 96, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Corban, M.T.; Hung, O.Y.; Eshtehardi, P.; Rasoul-Arzrumly, E.; McDaniel, M.; Mekonnen, G.; Timmins, L.H.; Lutz, J.; Guyton, R.A.; Samady, H. Myocardial bridging: Contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J. Am. Coll. Cardiol. 2014, 63, 2346–2355. [Google Scholar] [CrossRef]
- Lee, M.S.; Chen, C.H. Myocardial Bridging: An Up-to-Date Review. J. Invasive Cardiol. 2015, 27, 521–528. [Google Scholar] [PubMed]
- Attaran, S.; Moscarelli, M.; Athanasiou, T.; Anderson, J. Is coronary artery bypass grafting an acceptable alternative to myotomy for the treatment of myocardial bridging? Interact. Cardiovasc. Thorac. Surg. 2013, 16, 347–349. [Google Scholar] [CrossRef]
- Pratt, J.W.; Michler, R.E.; Pala, J.; Brown, D.A. Minimally invasive coronary artery bypass grafting for myocardial muscle bridging. Heart. Surg. Forum 1999, 2, 250–253. [Google Scholar]
- Ernst, A.; Bulum, J.; Šeparović, H.J.; Lovrić, B.M.; Strozzi, M. Five-year angiographic and clinical follow-up of patients with drug-eluting stent implantation for symptomatic myocardial bridging in absence of coronary atherosclerotic disease. J. Invasive Cardiol. 2013, 25, 586–592. [Google Scholar] [PubMed]
- Tsujita, K.; Maehara, A.; Mintz, G.S.; Doi, H.; Kubo, T.; Castellanos, C.; Liu, J.; Yang, J.; Oviedo, C.; Franklin-Bond, T.; et al. Impact of myocardial bridge on clinical outcome after coronary stent placement. Am. J. Cardiol. 2009, 103, 1344–1348. [Google Scholar] [CrossRef]
- Hao, Z.; Xinwei, J.; Ahmed, Z.; Huanjun, P.; Zhanqi, W.; Yanfei, W.; Chunhong, C.; Chan, Z.; Liqiang, F. The Outcome of Percutaneous Coronary Intervention for Significant Atherosclerotic Lesions in Segment Proximal to Myocardial Bridge at Left Anterior Descending Coronary Artery. Int. Heart. J. 2018, 59, 467–473. [Google Scholar] [CrossRef]
- Schwarz, E.R.; Klues, H.G.; vom Dahl, J.; Klein, I.; Krebs, W.; Hanrath, P. Functional, angiographic and intracoronary Doppler flow characteristics in symptomatic patients with myocardial bridging: Effect of short-term intravenous beta-blocker medication. J. Am. Coll. Cardiol. 1996, 27, 1637–1645. [Google Scholar] [CrossRef]
- Haager, P.K.; Schwarz, E.R.; vom Dahl, J.; Klues, H.G.; Reffelmann, T.; Hanrath, P. Long term angiographic and clinical follow up in patients with stent implantation for symptomatic myocardial bridging. Heart 2000, 84, 403–408. [Google Scholar] [CrossRef]
- Shen, L.; Xu, K.; Zhang, W.; Zheng, X.; He, B. Drug-Coated Balloon Treatment for ACS Induced by Myocardial Bridging: An Intravascular Ultrasound-Guided PCI. CJC Open 2021, 3, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.R.; Gupta, R.; Haager, P.K.; vom Dahl, J.; Klues, H.G.; Minartz, J.; Uretsky, B.F. Myocardial bridging in absence of coronary artery disease: Proposal of a new classification based on clinical-angiographic data and long-term follow-up. Cardiology 2009, 112, 13–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).