Abstract
Every year, millions of people worldwide suffer from bone tissue damage caused by bone trauma and surgical operations, as well as diseases such as osteoporosis, osteoarthritis, osteomyelitis, and periodontitis. Bone defect repair is one of the major challenges in the field of regenerative medicine. Although bone grafts are the gold standard for treating bone defects, factors such as donor sources and immune responses limit their application. Functionalized nanomaterials have become an effective means of treating bone diseases due to their good biocompatibility and osteoinductivity, anti-inflammatory, and antibacterial properties. Metal–organic frameworks (MOFs) are porous coordination polymers composed of metal ions and organic ligands, featuring unique physical properties, including a high surface area–volume ratio and porosity. In regenerative medicine, MOFs function as the functions of drug carriers, metal ion donors, nanozymes, and photosensitizers. When combined with other functional materials, they regulate cellular reactive oxygen species, macrophage phenotypic transformation, bone resorption, osteogenesis, and mineralization, providing a new paradigm for bone tissue engineering. This study reviews the classification of functionalized MOF composites in biomedicine and the application of their synthesis techniques in bone diseases. The unique in vivo and in vitro applications of MOFs in bone diseases, including osteoarthritis, osteoporosis, bone tumors, osteomyelitis, and periodontitis, are explored. Their properties include excellent drug loading and sustained release abilities, high antibacterial activity, and bone induction abilities. This review enables readers to better understand the cutting-edge progress of MOFs in bone regeneration applications, which is crucial for the design of and functional research on MOF-related nanomaterials.
1. Introduction
Bone is a highly dynamic balanced structure that involves the destruction of old bone and the regeneration of new bone [1]. It is composed of various functional cells, including osteoblasts, osteoclasts, endothelial cells, minerals, and extracellular matrix, endowing bones with weight-bearing, movement, and endocrine balance functions [2]. The problem of bone defect regeneration occurs not only in the context of bone trauma and surgical operations but also in bone diseases, including osteoporosis, osteoarthritis (OA), osteomyelitis, and periodontitis [3]. Importantly, these diseases are frequently associated with defects in the femur and alveolar bones, which present unique clinical challenges due to their structural and functional significance. Osteoporosis, for instance, is a systemic metabolic disorder characterized by reduced bone mass and bone microarchitecture deterioration, which significantly weaken bones, such as the femur and tibia, thereby increasing the risk of fractures and impairing load-bearing function [4]. OA is a degenerative joint disease characterized by cartilage degradation, subchondral bone remodeling, and synovitis, primarily affecting joint areas such as the knee and hip [5]. Osteomyelitis is a bacterial infection that commonly affects bones, such as the femur and tibia, leading to localized inflammation, bone necrosis, and chronic infection that complicates healing processes [6]. Bone tumors—including primary malignancies, such as osteosarcoma, and secondary metastatic lesions originating from cancers, such as breast and prostate cancer—predominantly form destructive osteolytic or osteoblastic lesions in bones, such as the femur, humerus, and tibia, compromising structural integrity and leading to severe functional deficits [7]. In addition, periodontitis is a chronic inflammatory condition triggered by bacterial biofilms that affects the alveolar bone surrounding teeth in the maxillofacial region, resulting in progressive tissue destruction and eventual tooth loss if untreated [8]. Due to the limited regenerative capacity of autologous bone, bone repair needs to be carried out through means such as autologous and allogeneic bone transplantation and tissue engineering. Autologous grafts have the disadvantages of difficulty acquisition and limited quantity [9]. Therefore, the development of functionalized nanomaterials and their modified allogeneic grafts has become an effective means for treating bone diseases. Functionalized bone materials need to have good biocompatibility, osteoinductivity, and antibacterial, anti-inflammatory, and immunomodulatory functions [10].
Metal–organic frameworks (MOFs) are porous coordination polymers with periodic network structures composed of metal ions and polydentate organic ligands [11,12]. These organic ligands also serve as linkers and coordinate with metal ions to form one-, two-, or three-dimensional networks. Generally, the ligands used in MOF organic linkers can be classified as nitrogen-centered ligands (pyridyl, imidazolyl, cyano, etc.), oxygen-centered ligands (aliphatic or aromatic carbolic acid, phosphonate, sulfonate, etc.), and other functional group-based linkers [13,14,15]. Therefore, the porosity and structure of a MOF can be tuned by decorating the ligand structures or using various metal ions. The distinctive structure properties of MOFs, including a high area–volume ratio and porosity, have attracted widespread attention. MOFs have not only been widely applied in gas storage, catalysis, sensors, and membranes [16,17,18,19,20] but also have revolutionary applications in the biomedical field, including as drug carriers, biological nanozymes, and photosensitizers, and in the treatment of cancer, bone disease, kidney disease, and so forth [21,22,23,24,25].
Recently, MOFs have been increasingly harnessed to address complex challenges in the treatment of bone disease. For instance, divalent MOFs, such as ZIF-8, HKUST-1, and Mg-MOF-74 have good stability and degradation kinetics, making them excellent drug carriers and controlled metal ion donors [26]. Catalytic MOFs, such as MIL-100 and Mn-TCPP, can simulate enzyme activity to drive Fenton-like reactions and generate reactive oxygen species (ROS) for therapeutic purposes, while photosensitive MOFs can integrate ligands, like tetrakis-(4-carboxyphenyl) porphyrin (TCPP), to achieve photodynamic therapy (PDT) for antibacterial and antitumor applications [27]. These biomedical functions, ranging from drug delivery to ROS modulation, position MOFs as versatile platforms for bone regeneration and disease management. By bridging the gaps in mechanical, biological, and therapeutic aspects, MOFs can provide innovative solutions for bone tissue engineering.
This review introduces the application classifications of MOFs in the biomedical field, including drug carriers, metal ion donors, nanozymes, and phototherapeutic agents. We also review the application of functionalized nanomaterials based on MOFs in bone regeneration for bone defects, OA, osteoporosis, bone tumors, osteomyelitis, and periodontitis. The biological regulatory effects of different types of MOFs in these diseases in the forms of nanoparticles, hydrogels, membranes, surface coating, and 3D printing, are discussed. This provides a therapeutic paradigm for the design of bone functional materials based on MOFs.
2. Classification of MOF Applications in Biomedicine
Due to the diversity of MOFs in terms of their chemical properties, size, preparation, and modification, with the diversification of synthesis and modification methods, MOF-based composites are increasingly being applied in the field of life sciences. Many excellent reviews summarizing and analyzing the application of MOFs in biology have been published, some of which are important in research advances. In this section, MOFs are classified into four types—drug carriers, metal ion donors, nanozymes, and phototherapeutic agents (Figure 1)—according to their primary roles in disease therapy and the impact of their functionalization on osteogenesis, antibacterial activity, and biocompatibility.
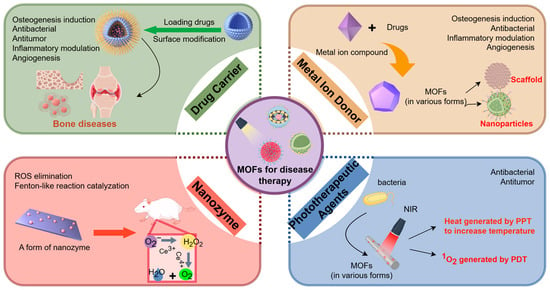
Figure 1.
The classification of MOFs applications in biomedicine includes drug carriers, metal ion donors, nanozymes, and phototherapeutic agents. The picture is made by Figdraw.
2.1. Drug Carriers
Since metal carboxylate-based MOFs were reported as pioneering examples of loading and releasing ibuprofen in drug delivery systems, MOFs have been recognized as excellent drug carriers due to their high porosity, large surface area, high load capacity, and customizable structure [12,28]. Using MOFs as drug carriers has attracted considerable interest in biological application. For instance, MOFs have been used to encapsulate and release therapeutic agents (e.g., BMP-6, dexamethasone, metformin, and quercetin) to treat bone regeneration (promoting osteogenic differentiation via PI3K/AKT pathways), skull defect repair, OA (reducing inflammation and cartilage degradation), and osteosarcoma (inducing immunogenic cell death and inhibiting tumor growth) [29,30,31,32,33]. The advantages of MOFs in drug delivery include improving therapeutic efficacy by stabilizing drugs with short half-lives and targeted delivery by stimulating reactivity or tissue-specific design [12]. Drugs can be loaded into MOFs via adsorption, encapsulation, non-covalent interactions, and covalent bonding [34]. These strategies can be achieved during MOF synthesis either through one-pot encapsulation (incorporating the drug directly into the framework) or post-synthetic modification (loading the drug into a preformed MOF) [35]. The drug release process mainly depends on the degradation of MOFs in response to environmental stimuli, such as pH [36], light [37,38], and temperature [39]. However, as exogenous nanoparticles (NPs), MOFs still face challenges, such as immunogenicity and macrophage phagocytosis, which reduce drug delivery efficiency and trigger inflammation. To address these issues, surface modifications using biocompatible molecules, such as polyethylene glycol (PEG) or stem cell membranes (SCMs), as well as optimizing particle size, shape, and surface charge, have proven to be effective strategies [40]. However, the partial metal ions and organic ligands used to construct MOFs can be toxic to health and the environment or show weak solubility. Therefore, the properties of MOFs, including their toxicity, water solubility, lipophilicity, structure homogeneity, and so on, should be considered to meet the medical requirements.
2.2. Metal Ion Donors
Metal ions play vital roles organisms, controlling many biological functions; signal transmission, osteogenic activity, enzyme catalysis, and the release of metal ions through biomaterials can activate related functions [41,42,43]. MOFs constructed through coordination between metal ions and organic ligands can control the release of metal ions during stimulus-responsive degradation [44]. When MOFs are prepared using biologically active metal ions, and the sustained release of metal ions (e.g., Zn2+, Mg2+, Cu2+) can provide therapeutic benefits for antibacterial, anti-inflammatory, and bone tissue engineering or exert systemic therapy in combination with other drugs [45,46,47,48]. For example, Zn2+ (from Cu-Mg-MOF, ZIF-8 composites, SCM/ZIF-8, etc.) promotes osteogenesis, angiogenesis, antibacterial activity, and anti-inflammatory effects [49,50], while Mg2+ (Cu-Mg-MOF, PLGA/Exo-Mg-GA MOF) enhances osteogenic/angiogenic gene expression and reduces inflammation [49,51]. Due to the risk of cytotoxicity arising from high ion concentrations, the release kinetics and dose need to be precisely regulated. The strength of metal–ligand bonds determines the structural stability of MOFs and has a key effect on ion release rates, which can be triggered by environmental factors, like pH, temperature, and ROS, triggering framework disintegration. The optimal balance between stability and controlled degradation ensures safe and effective therapeutic applications; for example, MOFs, including ZIF-8, ZIF-67, Mg-MOF-74, and HKUST-1, have been widely employed as ion donors [52]. However, as sustained release agents, most MOFs lack sufficient long-term biocompatibility and component synergy, which are challenges hindering their clinical translation.
2.3. Nanozymes
Nanozymes are artificial enzymes constructed based on nanomaterials to mimic the high catalytic efficiency properties of natural enzymes. In many catalytic fields, they are considered alternatives to natural enzymes due to their low cost, high stability, and excellent tolerance [53]. In recent decades, MOFs have also been widely used to prepare enzyme mimics. MOF nanozymes can be divided into two categories, according to their material composition. The first is the MOFs with catalytic activity, including two-dimensional (2D) MOF nanozymes and three-dimensional MOF nanozymes. For example, a 2D metal–organic skeleton-based nanozyme (Cu-TCPP(Fe)MOFs) was fabricated by coordinating between Cu(II) ions and peripheralcarboxyl groups of Fe(III) ion-centered metalloporphyrin to catalyze the decomposition of H2O2 [54]. The other class is composite nanozymes, which can be fabricated by combining MOFs and other functional materials to achieve the desired properties [55]. For example, Pt nanoparticles (Pt) have been loaded on the surface of MOFs based on Fe porphyrin and Zr4+ ions (PMOF(Fe)) to form Pt@PMOF(Fe), which has excellent catalase- and peroxidase-like activities [56]. The customizable structure provides MOFs with a variety of catalytic applications, and MOF-based nanozymes exhibit superior catalytic efficiency due to their high specific surface area and numerous binding sites. In addition, the robust synthetic framework exhibits greater stability against physicochemical degradation during transport to minimize loss of activity. These characteristics make MOF nanozymes a viable alternative to bioderived enzymes in catalytic applications [57]. Bio-MOF-1@AHTs, PEEK@ZIF-8(CEL), and UiO-66-modified titanium scaffolds enhance osteogenic gene expression (COL1, OPN, and BMP2), promote bone marrow mesenchymal stem cell (BMSCs) proliferation, and stimulate angiogenesis for bone integration [58,59,60]. For bone therapy, nanozymes based on MOFs focus on bone regeneration and OA treatment [61,62]. Although, nanocatalytic approaches based on MOFs offer a promising strategy for inflammatory disease treatment, among other things, translational studies are needed to evaluate their metabolic toxicity, long-term biocompatibility, immune responses, scalability, and so forth.
2.4. Phototherapeutic Agents
Phototherapy, including PDT and photothermal therapy (PTT), is a new and promising anticancer and antibacterial therapy with outstanding therapeutic effects and minor side effects on target tissues [63,64]. A specific light wavelength is used to activate the phototherapy agent when it is injected into the patient and selectively distributed in the target tissue to achieve the desired concentration, PDT produces ROS to interact with tissues, and PTT generates heat to increase the temperature; these are the most common mechanisms of phototherapy [65]. Therefore, the design and preparation of ideal photoactivatable agents are crucial for phototherapy, as photosensitizers (PSs) and photothermal agents (PTAs) are key components for PDT and PTT, respectively. MOFs with tunable structures have emerged as promising platforms for phototherapy [66]. The most common strategies for constructing MOF-based phototherapy agents are as follows: (1) PSs or PTAs are directly used as organic ligands to coordinate with metallic ions and clusters to fabricate MOF material. Porphyrins and their variations (such as TCPP, H2DBP, and TBBC) serve as photo-function groups and have been widely employed as organic ligands, especially for MOF-based PDT [63]. (2) Composite materials serve as phototherapeutic agents fabricated by PSs or PTAs and non-photo-function MOFs. This method allows for a greater variety of PSs and PTAs, in addition to porphyrins and their analogs. MOFs can be used as containers to load/encapsulate PSs or PTAs through non-covalent interactions; in addition, PSs and PTAs can be decorated om MOFs surfaces through covalent bonds [67,68]. For bone therapy, phototherapeutic agents based on MOFs exhibit antibacterial and osteogenic effects through mechanisms such as reactive oxygen species (ROS) generation, controlled ion release (e.g., Zn2+ and Cu2+), and the upregulation of genes (e.g., Runx2, ALP, Col I, and OCN) linked to bone formation [69,70,71]. As a new platform for phototherapeutic agents, MOFs not only inhibits the self-aggregation of photoresponsive molecules but also improves the efficiency of photoconversion. However, controlling selective light transmitted to organisms to trigger phototherapeutic agents remains challenging, as preventing light damage to normal tissues and guiding MOF aggregation can be difficult (Table 1).

Table 1.
The main types of MOFs and their function for disease therapy.
3. Synthesis Technology for MOF Composites in Bone Disease
At present, reconstructing large bone defects caused by infection, trauma, and tumor resection remains a significant clinical challenge [9]. Using biomaterials to simulate natural bone structures with similar mechanical and biological properties is a common strategy for bone defect repair [81]. However, considering the poor bone conduction and induction performance of the material, MOFs can be mixed with the substrates through various forms—such as surface coating, electrospinning, 3D printing, and other methods—to exert excellent bone defect repair performance in bone diseases, including OA, osteoporosis, bone tumors, periodontitis, and osteomyelitis (Figure 2).
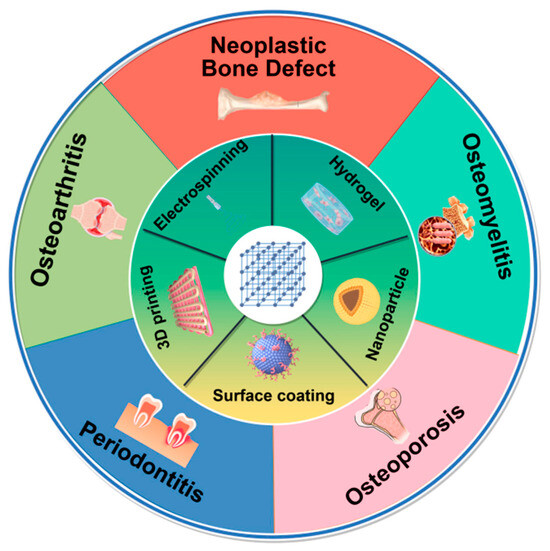
Figure 2.
The synthesis technology for MOF composites in bone defect repair of bone diseases, including surface coating, electrospinning, 3D printing, hydrogels, and nanoparticles. The picture is made by Figdraw.
3.1. Surface Coating Materials
ZIF-8 can be coated on the surface of biphase calcium phosphate ceramics (Osteon II) to enhance bone induction performance. ZIF8-Osteon improves the adhesion and growth of hADSC cells, while also increasing early and osteogenic markers, such as Col-1, OCN, and SPP1 to promote bone regeneration [82]. A novel bone integration-promoting implant was prepared by modifying bio-MOF-1 on the surface of alkali-treated titanium (Bio-MOF-1@AHTs). This material has good biocompatibility and osteogenic properties, can promote the ALP activity of BMSCs, and improve osteogenic and mineralization by upregulating osteogenic genes (COL1, OPN, and BMP2) [58]. Ti was coated with probiotic-derived membrane vesicles (OMVs), tinidazole, and Cu-TCPP nanosheets to obtain a composite material for antibacterial and osteogenic promotion (CuTOT-Ti). It can promote the osteogenic differentiation of MC3T3-E1 cells and the polarization of M1-to-M2 macrophages. In the bone defect model, ultrasound activation can inhibit S. aureus and promote bone formation, mineralization, and angiogenesis [83]. Using the photocatalytic properties of ZIF-8, iodine was loaded onto ZIF-8 and coated on a micro-arc-oxidized (MAO) titanium base surface for antibacterial and osteogenic applications. Under near-infrared (NIR) irradiation, MAO + ZI significantly increased reactive oxygen species (ROS) levels of the bacteria and reduced the number of bacterial colonies by 95.48%. It also promoted osteogenesis by upregulating osteogenic genes (Runx2, ALP, Col I, and OCN) in hBMSCs [84]. In addition, coating zinc-based implants with a Cu-Mg MOF promotes bone integration and angiogenesis. The implant can release Zn2+, Mg2+, and Cu2+; promote the osteogenesis of MC3T3-E1 cells; and increase the expression of osteogenic genes (COL-1, ALP, Runx-2, and OCN). It can also promote HUVECs proliferation and migration and the expression of angiogenesis-related genes (eNOS and VEGF). In addition, this implant can activate NO production to destroy the bacterial membrane, showing strong antibacterial activity against S. aureus and E. coli [49].
Polyetheretherketone (PEEK) has good biocompatibility and similar mechanical properties to bone tissue. However, due to bioinertness and surface hydrophobicity, it requires surface modifications for bone repair applications [85]. Deng et al. loaded simvastatin (SIM), ZIF-8, and polydopamine (PDA) to construct a functionalized PEEK implant with near-infrared-responsive antimicrobial and osteogenic properties. Under 808 nm irradiation, SIM@ZIF-8-PDA accelerated the release of Zn2+, which had an obvious antibacterial effect on Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus). In in vivo bone defect models, implants can effectively remove bacteria and promote bone regeneration [86]. Celecoxib (CEL) has been encapsulated in ZIF-8 and modified with DPA to PEEK to form a composite (PEEK@ZIF-8(CEL)). The implant promoted the proliferation of MC3T3-E1 cells and upregulated osteogenic genes to promote bone formation and mineralization [59].
3.2. Electrospinning Materials
MOFs can also be combined with other materials to form biofriendly bone-inducing materials through electrospinning. Zn-based MOF-derived nanocarbons (C-ZnO) can be mixed with poly(ε-caprolactone) (PCL) and form fiber scaffolds through electrospinning. This can promote the proliferation and osteogenic differentiation of MSCs, accompanied by the increased expression of laminin A/C and RUNX2 [87]. Toprak et al. encapsulated BMP-6 in ZIF-8 nanocrystals and incorporated them into PCL electrospun fibers, addressing BMP-6’s short half-life and localized efficacy. The PCL/BMP-6@ZIF-8 membrane enhances the proliferation, adhesion, and osteogenic differentiation of MC3T3-E1 cells. In vivo studies of rat skull defect models have shown that PCL/BMP-6@ZIF-8 significantly promotes bone regeneration [88].
Furthermore, mZIF-8/PLA has been prepared from ZIF-8 and polylactic acid (PLA) through electrospinning and simulated body fluid mineralization, showing good hydrophilicity and biocompatibility. The membrane can enhance the expression of osteogenic and vascularization genes in BMSCs, can promote M1-to-M2 polarization, and has good bone repair performance [51]. Exosomes secreted by human adipose-derived MSCs (hADSCs-Exos) can enhance the efficacy of bone regeneration biomaterials. The hADSCs-Exos has been encapsulated in poly(lactic-co-glycolic acid) (PLGA) and combined with a Mg2+-based organic framework and gallic acid to develop a scaffold (PLGA/Exo-Mg-GA MOF) through electrospinning. This scaffold has anti-inflammatory, osteogenic, and angiogenic properties. The release of Mg2+ can induce the expression of iNOS and COX-2 in RAW264.7 cells and inhibit proinflammatory mediators [48].
3.3. Hydrogel Materials
Hydrogel materials, such as gelatin methacrylate (GelMA) and methacrylate-modified hyaluronic acid (HAMA), can be coated with MOFs and other nanocomposites for bone defect repair. 2-ethylimidazole (elm)-doped ZIF-67 and GelMA have been photo-crosslinked in situ to form a hydrogel composite (GelMA@elm/ZIF-67) to achieve the controlled release of cobalt ions (Co2+). The release of Co2+ was sustained enough to correspond to the vascularization in the early stage of osteogenesis and increased the angiogenesis of the bone defect site by 17.03 ± 2.35%. It can also increase the activity of ALP and the expression of osteogenic gene in BMSCs to promote the formation of new bone [89]. ZIF-8 and dexamethasone (DEX) can be combined with wood aerogels to form DEX@ZIF-8, which can be used for skull defect repairs. The hydrogel enhances the adhesion and spread of rBMSCs and upregulates the expression of osteogenic genes (RUNX2, Col1A1) to promote osteogenic differentiation [90]. Moreover, ZIF-8-stabilized black phosphorus nanosheets (BPN) have been used to construct injectable nanocomposite hydrogels (GelMA/HAMA/BP@ZIF-8) by combining them with GelMA and HAMA. This hydrogel has good biocompatibility, can promote M1-to-M2 polarization of macrophages, and can reduce the expression of proinflammatory factors. Under NIR irradiation, GelMA/HAMA/BP@ZIF-8 has antibacterial and bone-forming capabilities, and it promotes late osteogenic differentiation and mineralization through the PI3K-Akt and calcium signaling pathways [30]. Metformin (Met) can be combined with ZIF-8 in GelMA to form a hydrogel for diabetic bone defects (GelMA/Met@ZIF-8) that simultaneously releases Met and Zn2+. At the same time, it promotes the transformation of M1 macrophages into M2 macrophages, reduces excess ROS production, maintains mitochondrial homeostasis, and thus, alleviates diabetic bone defects [31].
In tendon–bone regeneration, MOFs bind to cytokines and targeted peptides for the effective integration of tendon–bone interfaces. (DOPA)4-PEG5-N3 and DBCO-BMP-2 peptide have been used to construct a multifunctional hydrogel scaffold with GelMA (GM@MOF/DOPA-BMP-2) through a bioorthogonal click reaction. This scaffold can stimulate angiogenesis; can promote the osteogenic differentiation of BMSCs and the phenotype transformation of macrophages from M1 to M2; and has potential application value in tendon–bone reconstruction [91]. Ma et al. developed a double-targeted hydrogel scaffold for tendon–bone interface regeneration (LZIF-8/WMg-MOF@GEL) by combining tendon-targeting peptide-modified ZIF-8 and cartilage-targeting peptide-modified Mg-MOF into a GelMA matrix via UV polymerization crosslinking technology. The scaffold has sustained drug release, tissue targeting, and excellent tendon fibroblast biocompatibility, promoting tendon and bone tissue repair [92].
3.4. Three-Dimensional (3D) Printing Materials
Three-dimensional scaffolds with artificially controllable microstructures can bridge the complex morphology of bone defect edges, providing a potential strategy for bone tissue regeneration [93]. Extrusion-based 3D printing technology integrates ZIF-8 into a dicalcium phosphate dihydrate (DCPD) and PCL composite scaffold for bone regeneration. This scaffold has high-strength and a highly interconnected porous structure that sustainably releases Ca2+ and Zn2+, promoting the growth of BMSCs and new bone formation [94]. Modifying 3D-printed titanium scaffolds with UiO-66 can promote the optimal adhesion of BMSCs and HUVEC. Through the upregulation of osteogenic genes in BMSCs and enhancing the secretion of angiogenic factors in HUVEC, bone formation and angiogenesis can be promoted [60]. Furthermore, 3D-printed tricalcium β-phosphate (β-TCP) modified with Zn/Co-MOF can treat subchondral bone defects induced by osteoarthritis (OA). This scaffold has anti-inflammatory, antioxidant, and ROS scavenging capabilities that can promote the integration of subchondral bone defects [50].
3.5. Nanoparticle (NP) Materials
As ZIF-8 has a simple synthesis strategy, a strong loading capacity, and pH response degradation characteristics, it is widely used as an NP for drug and nucleic acid delivery in cells and in vivo [95]. Using the targeting properties of SCMs, ZIF-8 has been encapsulated in SCMs to enhance targeting and osteogenic regeneration. SCM/ZIF-8 can regulate the sustained release of Zn2+ and promote osteogenic differentiation and mineralization by upregulating the expression of OPN and OCN. In in vivo femur defect models, the material has good biocompatibility and promotes the formation of new bone tissue [74]. Furthermore, ZIF-8 loaded with dexamethasone (DEX) has been coated on SCMs to construct a bionic material for targeted bone repair (DEX@ZIF-8-SCM). These NPs can promote MSC growth, ALP activity, and OCN expression. Bone formation is accelerated through the phosphoinositol 3-kinase (PI3K)-AKT signaling pathway, thereby promoting bone tissue repair [96]. In addition, bimetallic MOF NPs (Pt@ZIF-8@La) have been constructed by encapsulating Pt nanozyme and lanthanum (La) with ZIF-8. These NPs have high active oxygen scavenging capacity, reduce cellular inflammation, and promote osteogenic differentiation and bone regeneration by upregulating the osteopontin (OPG)–nuclear factor-κB ligand (RANKL) ratio [61].
4. Applications of MOFs in Bone Disease Therapy
4.1. MOFs in Osteoarthritis (OA)
OA is a degenerative joint disease with pathological features, including cartilage destruction, subchondral bone remodeling, and synovitis. Approximately 250 million people worldwide currently suffer from OA, creating a significant social and economic burden [97,98]. MOF-based biomaterials based on tissue targeting and controlled slow release are becoming an important method for OA therapy [99] (Table 2).
The acidic substances produced by chondrocytes and synovial cells in the OA microenvironment make the pH lower than the normal physiological state. The acid reaction degradation properties of MOF materials, such as MIL-100(Fe) and ZIF-8 make them good carriers for sustained drug release [100,101]. Xiong et al. modified pH-responsive MIL-100(Fe) with hyaluronic acid (HA) and protocatechuic acid (PCA) (MOF@HA@PCA) to treat OA. These NPs have good biocompatibility, reduce IL-1β-induced chondrocyte inflammation and the expression of matrix-degrading enzymes (MMP1, MMP3, and MMP13), and promote Col2a1 and Acan expression. Cartilage degeneration can be alleviated by inhibiting the expression of MMP-13 in the cartilage tissue of OA rats [62]. Some studies have used ZIF-8 as a pH response vector. Quercetin, with anti-inflammatory and antiapoptotic properties, is delivered to chondrocytes via ZIF-8 (Qu@ZIF-8), which enhances chondrocyte autophagy by inhibiting the PI3K/Akt signaling pathway, promotes the expression of cartilage anabolic genes, and protects cartilage integrity [102]. MIL-101-NH2 combines with curcumin (CCM) and siHIF-2α to form pH-responsive NPs with a 59.7% controlled release effect under acidic conditions. These NPs have good biocompatibility and inhibit hypoxia-induced cartilage dysfunction in vivo [103]. In rheumatoid arthritis (RA), the CRIg-CD59 complement is encapsulated by ZIF-8 nanoparticles and forms a ZIF8@CRIg-CD59@HA@ZA complex with hydroxyapatite (HA) and zoledronic acid (ZA). In an acidic environment, the platform delivers CRIg-CD59 to the affected site of RA and can inhibit complement activation and repair VSIg4+ macrophage barrier function, while targeting osteoclasts to inhibit their differentiation [32]. This acid-sensitive property endows MOFs with excellent drug-sustained release performance, allowing them to be induced for release in acidic microenvironments.
Macrophages play an important role in OA. M1 macrophages are involved in tissue fibrosis, and M2 macrophages promote cartilage repair and alleviate arthritis [104]. Sun et al. developed a nanogel (KZIF@HA) for the sustained release of Kartogenin (KGN). Compared with KGN, KZIF@HA can increase cartilage tissue permeability by 40%. This nanogel promotes the polarization of M2 macrophages and the secretion of IL-10 and then inhibits JNK and ERK pathways in chondrocytes to promote chondrocyte anabolism and increase the secretion of type II collagen [105]. Folic acid (FA)-modified UiO-66 can target baicalin to M1 macrophages, effectively reducing ROS and promoting the polarization of M2 macrophages, thus alleviating synovial hyperplasia caused by OA [106]. These studies provide promising evidence for drug-loaded MOF nanoparticles in the treatment of OA by regulating the M1-M2 phenotypic transformation.
In addition, Liu et al. modified MOFs using biomimetic lubrication materials and developed a series of NPs with photothermal response and lubrication properties in OA treatment. The bionic lubrication nanosystem ZIF-8-PDA-HA was formed by combining HA and dopamine, which can load diclofenac sodium (DS) up to 99%. Under infrared irradiation, the lubrication system can reduce the friction coefficient indicated by wear, slow release DS, and enhance the expression of Col2α and Acan in chondrocytes [107]. Poly(ethylene glycol)-graft-poly(N-isopropylacrylamide) (PEG-g-PNIPAm) copolymer coated with MIL-101(Cr) forms a nanogel mixing system with a thermal response and lubrication properties. The expression of Sox-9, Col2α1, and aggrecan can be upregulated in TNF-α-induced chondrocytes, while the expression of MMP1, IL-6, and COX-2 can be downregulated, and ECM degradation can be inhibited [108]. Furthermore, by coating P(NIPAm-g-PEGMax) copolymer microgel on the surface of MIL-101(Cr), another heat-responsive drug-sustained release system was developed with good lubrication properties and OA therapeutic potential [109]. In addition to components with biological regulatory functions, combining materials with lubricating properties and MOFs can synergistically promote cartilage tissue regeneration in the in vivo environment.
Nanozyme is a kind of artificial enzyme with multi-enzyme activity that has the advantages of good biocompatibility, low cost, easy synthesis, etc. It has been widely applied in OA treatment [110]. Zhang et al. loaded Mn3O4 nanoparticles into UIO66 using the hydrothermal method and modified with mitochondrial-targeted triphenylphosphine (TPP) groups. Mn3O4/UIO-TPP clears mitochondrial ROS through the subcellular barrier, inhibits chondrocyte apoptosis and MMP13 expression, and effectively alleviates OA in rats [111]. The Cu-based Cu MOF nanozyme has better ROS clearance abilities than CuO and copper monoatomic nanozyme; it can promote the polarization of the macrophage M1 subtype into the M2 subtype, downregulate MMP13, and inhibit the degradation of cartilage matrix [112]. The ZIF-8 delivery platform (miR/IrO2@ZIF-8), loaded with IrO2 NPs and antisense oligonucleotide, can escape from lysosomes and penetrate human cartilage tissue to a depth of 1.5 mm, effectively reducing the expression of IL-6 and ADAMTS-5 in chondrocytes. The integrity of cartilage ECM can be protected by upregulating the expression of COLII and ACAN [110]. Antioxidant activity plays a key role in maintaining joint homeostasis. MOF materials can not only regulate ROS by loading nanozymes but also exert antioxidant capacity through specific metal atom sites and have great potential in alleviating the oxidative stress of OA.

Table 2.
Summary of the application of MOF-based composite materials for the treatment of osteoarthritis.
Table 2.
Summary of the application of MOF-based composite materials for the treatment of osteoarthritis.
| MOF Composites | Form of the Composites | Primary Role of MOFs | Loaded Drugs and Loading Efficiency | Metal Ions/Clusters | Cellular Biological Functions | Mechanism | In Vivo Effects | Refs |
|---|---|---|---|---|---|---|---|---|
| MIL-100(Fe)@HA@PCA | Nanoparticle | Drug carrier | Hyaluronic acid (~21.6%); protocatechuic acid (~19.4%) | Fe3+ | Promoting chondrocyte proliferation; relieving inflammation | / | Promoting cartilage regeneration: inhibit MMP-13 expression | [62] |
| Qu@ZIF-8 | Nanoparticle | Drug carrier | Quercetin (~23%) | Zn2+ | Promoting cartilage anabolism; anti-inflammatory; inhibiting IL-1β-induced apoptosis | Inhibiting PI3K/Akt signaling pathway | Maintaining cartilage structure integrity and glycosaminoglycan synthesis | [102] |
| MIL-101-NH2 | Nanoparticle | Drug carrier | Curcumin (~25.9%); siHIF-2α; hyaluronic acid | Fe3+ | Promoting cartilage anabolism; inhibiting inflammatory factors | / | Promoting cartilage anabolism; inhibiting inflammatory factors | [103] |
| ZIF8@CRIg-CD59@HA@ZA | Nanoparticle | Drug carrier | CRIg-CD59 (>90%); Zoledronic acid (~73.6%) | Zn2+ | Inhibiting bone resorption; repairing VSIg4+ macrophage barrier | / | Reducing synovial hyperplasia; protection of articular bone | [32] |
| KZIF@HA | Nanoparticle | Drug carrier | Kartogenin; hyaluronic acid | Zn2+ | Promoting cartilage anabolism; promoting M1-to-M2 macrophage polarization | Inhibiting JNK and ERK pathways in chondrocytes | Cartilage protection; inhibiting joint inflammation | [105] |
| Bai@FA-UIO-66-NH2 | Nanoparticle | Drug carrier | Baicalin | Zr4+ | Reducting ROS; promoting M1-to-M2 macrophage polarization | Modulation of immune homeostasis | Cartilage protection; reducing bone hyperplasia; recover subchondral bone structure | [106] |
| ZIF-8-PDA-HA | Nanoparticle | Drug carrier | Diclofenac sodium (~99%) | Zn2+ | Promoting cell proliferation; accelerated lubrication | / | / | [107] |
| MIL-101(Cr)@PEG-g-PNIPAm | Hydrogel | Drug carrier | Diclofenac sodium (~29.2%) | Cr3+ | Promoting cartilage anabolism; anti-inflammatory; accelerating lubrication | / | / | [108] |
| MIL-101(Cr)@P(NIPAm-gPEGMax) | Hydrogel | Drug carrier | Diclofenac sodium (~23.8%) | Cr3+ | Anti-inflammatory; accelerating lubrication | / | / | [109] |
| Mn3O4/UIO-TPP | Nanoparticle | Nanozyme | / | Mn3O4; Zr4+ | reducting mitochondrial ROS; inhibiting oxidative and inflammatory | / | Cartilage protection; inhibiting IL-6 and MMP-13 expression | [111] |
| Cu MOF | Nanoparticle | Nanozyme | / | Cu2+ | Reducting ROS; promoting M1-to-M2 macrophage polarization | / | Inhibiting ECM degradation; improving hypoxia; inhibiting synovitis | [112] |
| miR/IrO2@ZIF-8 | Nanoparticle | Nanozyme | AntagomiR-181a | Zn2+ | Reducting ROS; relieving inflammation; promoting cartilage anabolism; | / | Inhibiting ECM degradation; reducing bone hyperplasia; recover subchondral bone structure; inhibiting MMP-13 and ADAMTS-5 expression | [110] |
4.2. MOFs in Neoplastic Bone Defects
4.2.1. Breast Cancer-Induced Osteolysis
Bone metastasis in breast cancer is the main cause of death for this disease. Breast tumor cells promote osteoclast differentiation and osteolysis through IL-8 and other factors on the bone surface [113]. The release of nutrients from the bone matrix promotes the survival and invasion of breast tumor cells [114]. Inhibiting tumor cells and osteoclasts is an important strategy for treating breast cancer-induced osteolysis (Table 3).
UiO-66 NPs were combined with immunostimulatory cytosine–phosphate–guanosine(CpG) and ZA to form bone-targeting NPs (BT-isMOF). The system can strongly bind with calcium phosphate in bone tissue and inhibit osteoclast formation. At the same time, it can promote the polarization of M1 macrophages and the expression of pro-inflammatory factors and inhibit breast tumors. In vivo experiments show that BT-isMOF can effectively alleviate bone resorption caused by breast cancer bone metastasis [115]. Ge et al., utilizing the bone-targeting properties of ZA, simultaneously loaded the anticancer drug 5-fluorouracil (5-Fu) and the organic photothermal molecule indocyanine green (ICG) into ZIF-90 to develop a photothermal responsive bone-targeted drug delivery vector (5-Fu/ICG@ZIF-90-PEG-ZOL). Under NIR light, the NPs induced the apoptosis of MCF-7 cells by inhibiting DNA replication. NPs can reduce tumor volume and have no organ toxicity, showing an inhibitory effect on breast tumors in vivo [116]. Further, a bone-targeted ICG@Cu2-xSe-ZIF-8 composite was developed by combining Cu2-xSe chemodynamic therapy (CDT) with PTT to inhibit tumor growth and protect bone tissue. Under 808 nm laser irradiation, the complex can effectively inhibit the proliferation and invasion of MDA-MB-231 in vitro and inhibit osteoclastic differentiation. In a mouse model of breast cancer bone metastasis, NPs inhibit tumor growth and tumor cell-induced osteolysis [117]. Taking advantage of the PTT and CTT properties of MOF materials—combined with tissue-targeted peptides and drugs to precisely regulate the tumor site—it is possible to kill tumors while inhibiting osteolysis. This is a key advantage of MOF materials in the treatment of bone defects caused by tumors.
4.2.2. Osteosarcoma (OS)
OS is a malignant tumor that occurs in the bone, most commonly in children or adolescents, leading to 20,700 deaths in China every year [118]. Existing treatments for OS mainly include chemotherapy and surgery, usually requiring high doses of radiation for tumor ablation. Hypoxia is one of the reasons for the poor radiotherapy effect of osteosarcoma [119]. Antitumor NPs (HA@MOF/D-Arg) loaded with MIL-100(Fe), D-arginine (D-Arg), and HA can sensitize tumor cells to radiation. Under hypoxia conditions, NPs can enhance the production of free radicals in osteosarcoma (OS) cells, promote tumor ablation, and inhibit lung metastasis [120]. NPs formed by D-Arg with the chemotherapy drug telaprazine (TPZ) and Fe-based MOFs can catalyze the formation of NO from H2O2 to alleviate the hypoxia in OS cells and kill them by inducing DNA damage under X-ray irradiation [121]. Ge et al. developed a pH-responsive antitumor NPs (CUR-BMS1166@ZIF-8@PEG-FA, CBZP) by loading the immune checkpoint inhibitor BMS1166 and the autophagy activator curcumin (CUR) into ZIF-8. At pH = 6.5, CUR induced the expression of autophagy-associated protein ATG5 in OS cells and promoted HMGB1 release and calmodulin exposure, thus inducing autophagy dependent immunogenic cell death (ICD). In addition, the NPs inhibited the PD-1/PD-L1 axis by releasing BMS1166, forming a synergistic antitumor effect [122].
In addition to inhibiting OS formation, MOF composites can also simultaneously promote osteogenic differentiation and vascularization and inhibit osteolysis; they can jointly repair bone defects caused by OS. In terms of osteogenic differentiation, chitosan/NH2-MIL-125(Ti) scaffolds can be loaded with doxorubicin (DOX) to eliminate OS. Its superior biocompatibility and specific surface area can also enhance the adhesion, alkaline phosphatase (ALP) activity, and osteogenic differentiation of MC3T3-E1 cells [123]. UiO-66 NPs loaded with DOX (DOX@UiO-66-NH2) can release DOX in acidic environments, blocking nucleic acid synthesis and OS cell growth. They can also regulate the PI3K-Akt or MAPK signaling pathway to promote osteogenic differentiation [33]. A 3D-printed scaffold with Cu icons and MOFs can be used for OS treatment and bone repair. The scaffold not only eliminates tumor cells by generating reactive oxygen species (ROS) through the photothermal effect and Fenton reaction but also promotes osteogenic differentiation by upregulating the expression of COL1, OCN, BMP-2, and RUNX2 in hBMSCs [69]. As angiogenesis plays an important role in bone repair, a composite scaffold (CU-TCPP-TCP) was prepared by adding ultra-thin Cu-TCPP MOF nanosheets to a β-tricalcium phosphate (β-TCP) ceramic scaffold. Under NIR irradiation, the scaffold can eliminate residual OS cells and promote the osteogenesis and angiogenesis of hBMSCs and HUVECs, respectively, which jointly enhance new bone growth in vivo [124]. Qu et al. fabricated a photothermally responsive injectable multifunctional implant (Co-TCPP/CPC) by incorporating cobalt coordinated tetrakis (4-carboxyphenyl) porphyrin (Co-TCPP) into calcium phosphate cement (CPC). Under photothermal stimulation, OS cells can be ablated by inducing protein denaturation, and the expression of osteogenic and angiogenic factors can be up-regulated to promote bone defect repair [70]. In terms of inhibiting osteolysis in OS, Liu et al. developed a biomimetic multifunctional nanoplatform (PDA-MOF-E-M) that encapsulates Fe-MOF and erastin within the cell membrane of OS to achieve targeted therapy. This platform can induce ferroptosis in OS cells by promoting lipid peroxide accumulation. Beyond that, this platform inhibited NFATc1 expression and promoted TRAF3 expression, thereby inhibiting osteoclast differentiation and osteolysis under NIR irradiation [125]. Another nano-drug delivery system (V-RZCD) modified with vascular endothelial growth factor (VEGF) ligands can be targeted to treat OS and osteolysis. V-RZCD promotes the apoptosis of OS cells by downregulating Bcl-2 and increasing the expression of caspase-3. Furthermore, it inhibits osteoclast differentiation by regulating the RANKL/RANK/OPG signaling pathway, thereby protecting bone tissue structure [126]. Taken together, in the design of MOF composite materials for osteosarcoma treatment, in addition to considering the elimination of OS cells, multiple dimensions must be considered, such as promoting osteogenic differentiation, promoting vascularization, and inhibiting osteoclastic differentiation. These functions can endow MOFs with a synergistic effect in repairing of bone defects in OS.

Table 3.
Summary of the application of MOF-based composite materials for the treatment of neoplastic bone defects.
Table 3.
Summary of the application of MOF-based composite materials for the treatment of neoplastic bone defects.
| Neoplastic Bone Defects | MOF Composites | Form of the Composites | Primary Role of MOFs | Loaded Drugs | Metal Ions/Clusters | Cellular Biological Functions | Mechanism | In Vivo Effects | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Breast Cancer | BT-isUiO-66 | Nanoparticle | Drug carrier | Zoledronic acid (~3.14 wt%) | Zr4+ | Reducting ROS; inhibiting bone resorption; promoting M1 macrophage polarization | Activating TLR9 signaling transduction | Inhibiting tumor; protection of tibial tissue structure | [115] |
| 5-Fu/ICG@ZIF-90-PEG-ZOL | Nanoparticle | Drug carrier | 5-fluorouracil; zoledronic acid (~23.13%) | Zn2+ | Inducing apoptosis of MCF-7 cells under NIR | Inhibiting DNA replication | Bone-targeted; inhibiting tumor | [116] | |
| ICG@Cu2-xSe-ZIF-8 | Nanoparticle | Photosensitizer | / | Cu2-xSe | Promoting tumor cell apoptosis; inhibiting bone resorption | Inhibiting p65 and NFATc1 pathways | Inhibiting tumor; inhibiting cancer cells-induced osteolysis | [117] | |
| Osteosarcoma | HA@MOF/D-Arg | Nanoparticle | Drug carrier | D-arginine | Fe3+ | Promoting tumor cell apoptosis | Promoting DNA damage | Enhancing tumor ablation; preventing lung metastasis; alleviating tissue hypoxia | [120] |
| PDA-cloaked Fe-MOF | Nanoparticle | Drug carrier | D-arginine; tirapazamine | Fe3+ | Promoting tumor cell apoptosis | Producing ROS | Enhancing tumor ablation; preventing lung metastasis | [121] | |
| CUR-BMS1166@ZIF-8@PEG-FA | Nanoparticle | Drug carrier | Curcumin; BMS1166 | Zn2+ | Inducing immunogenic cell death | Activating autophagy; Inhibiting PD-1/PD-L1 signaling | Inhibiting tumor growth | [122] | |
| NH2-MIL-125(Ti) | Scaffold | Drug carrier | Doxorubicin | Ti4+ | Promoting tumor cell apoptosis; promoting osteogenesis | / | Inhibiting tumor growth; promoting bone repair | [123] | |
| DOX@UiO-66-NH2 | Nanoparticle | Drug carrier | Doxorubicin | Zr4+ | Promoting tumor cell apoptosis; promoting osteogenesis | Promoting osteoblast differentiation via activating PI3K-Akt and MAPK signaling pathways | Inhibiting tumor growth | [33] | |
| PCL@Cu-HHTP | Scaffold | Photosensitizer | / | Cu2+ | Promoting tumor cell apoptosis; promoting osteogenesis | / | Inhibiting tumor growth | [69] | |
| Cu-TCPP-TCP | Scaffold | Photosensitizer | / | Cu2+ | Promoting tumor cell apoptosis; promoting osteogenesis; promoting vascularization | / | Inhibiting tumor growth; promoting bone repair | [124] | |
| Co-TCPP/CPC | Scaffold | Photosensitizer | / | Co2+ | Promoting tumor cell apoptosis; promoting osteogenesis; promoting vascularization | / | Inhibiting tumor growth; promoting bone repair | [70] | |
| PDA-MOF-E-M | Nanoparticle | Photosensitizer | Erastin | Fe3+ | Promoting tumor cell apoptosis; inhibiting osteoclastic differentiation | / | Inhibiting tumor growth; inhibiting osteolysis | [125] | |
| V-RZCD | Nanoparticle | Drug carrier | Doxorubicin; zoledronic acid | Ca2+ | Promoting tumor cell apoptosis | / | Inhibiting tumor growth; inhibiting osteolysis | [126] |
4.3. MOFs in Osteoporosis
Osteoporosis is a chronic skeletal disorder marked by reduced bone density, bone microarchitecture deterioration, heightened bone fragility, and susceptibility to fractures [127]. Osteoporosis affects over 200 million individuals globally, leading to significant morbidity characterized by debilitating fractures and substantial physical limitations that impair quality of life [128]. In inflammatory, estrogenic, and age-related osteoporosis, imbalances in the expression of RANKL and OPG in the bone microenvironment lead to impaired osteoblast function and enhanced osteoclast formation, ultimately disrupting the balance of bone remodeling processes [129].
The release of active ingredients from biomaterials to support apatite formation is an important step in bone regeneration. Biocompatible MOFs composed of Sr2+ and Ca2+ can simulate the dissolution rate of these ions in body fluids and have good biocompatibility [130]. A complex of self-sacrificing MOFs with metal ions that promote osteoblast activity and bisphosphonate (BP) drugs that inhibit osteoclast activity can achieve controlled release for up to 20 days. For non-BP drugs, etidronate (ETID) and alendronate are released faster, while NER and PAM are released the slowest. In the case of compound BP drugs and metal ions, the sustained release effect of Ca-ETID is the best [131]. Hou et al. developed five Ca2+-based zwitterionic dicarboxylate MOFs to address osteoporosis. These bioMOFs can promote the expression of bone formation markers, such as ALP and Runx2 and promote osteogenic differentiation. In OVX mice, MOFs can increase serum OCN levels, promote collagen deposition, and improve bone microstructure, thus preventing bone loss [132]. Therefore, in the treatment of osteoporosis, the ionic composition of MOFs designed to release based on bone tissue composition plays an important role in bone regeneration.
In trauma and aging pathology, exposing MSCs to ROS results in cell senescence and weakened osteogenic differentiation, leading to osteoporosis [133]. Zhang et al. developed novel titanium–organic framework implants (AHT-Ce/Sr MOF) coated with Ce and Sr ions as bioactive molecules, which can be used for ROS regulation in MSCs. AHT-Ce/Sr MOF can reduce ROS levels by activating AMPK in MSCs and improve mitochondrial division, fusion, and autophagy to reverse aging. In vivo implantation experiments show that AHT-Ce/Sr MOF can effectively promote bone mineral and bone regeneration [134]. To take advantage of the acidic environmental characteristics of osteoclasts, an acid-responsive MOF system, ZIF8-NaHCO3@Cas9 (ZNC), was designed by incorporating sodium bicarbonate (NaHCO3) and RANKL-CRISPR/Cas9 plasmids for osteoporosis treatment. The ZNC NPs effectively neutralize the acidic microenvironment, improving transfection efficiency and inhibiting the formation of osteoclasts. In osteoblasts, ZNC NPs can reduce ROS levels and delay cell senescence. In addition, ZNC promotes osteogenic differentiation and mineralization by downregulating RANKL expression. In OVX mouse models, ZNC can promote bone mass and inhibit osteoclast and senescent cell formation [135]. Although MOFs of different ligands can be involved in stem cell osteogenic differentiation, senescence, and osteoclast formation in OP, more detailed molecular mechanisms have not yet been clarified, necessitating further exploration (Table 4).

Table 4.
Summary of the application of MOF-based composite materials for the treatment of osteoporosis.
4.4. MOFs in Periodontitis
Oral diseases such as periodontitis attract tremendous attention all over the world, Periodontitis alone accounts for over 5.4 million cases, making it the sixth most prevalent disease in the world [136]. Periodontitis is a chronic inflammatory disease characterized by the destruction of periodontal tissue, including gums, periodontal ligaments, and alveolar bone that often leads to tooth loss if untreated. This disease is primarily triggered by anaerobic bacterial biofilms, such as Porphyromonas gingivalis (P. gingivalis), which results in immune responses that exacerbate tissue damage [8]. The current treatment is mainly mechanical debridement and antibiotic therapy. While the difficulty in reaching certain periodontal areas and increased microbial resistance render existing approaches almost ineffective [137]; innovative biomaterials, such as MOFs, show potential in addressing these challenges (Table 5).

Table 5.
Summary of the application of MOF-based composite materials for the treatment of periodontitis.
Li et al. used ZIF-8 to encapsulate and deliver minocycline hydrochloride (Mino) to develop NPs with pH response properties. The NPs are effectively absorbed by human periodontal ligament cells (hPDLCs), and the release of Zn2+ and Mino reduces pro-inflammatory cytokines through the AKT/GSK3β/NRF2 pathway, which further reduces alveolar bone resorption and improves bone density in vivo [75]. ZIF-8 PDA formed a cell culture platform (PP/PDA/ZIF-8 membrane) on the surface of the polypropylene (PP) membrane with good wetness, roughness, and stiffness, promoting the proliferation of dental pulp stem cells (DPSCs). It also promotes osteogenic differentiation by regulating the expression of OPN and bone morphogenetic protein 2 (BMP2) [138]. Moreover, dexamethasone can be synthesized by combining ZIF-8 nanoparticles with polyphosphate methacrylate (PPEMA) and gelatin methacrylate (GelMA) to construct a nanohydrogel released in the acidic microenvironment of the periodontal pocket (DZIF@PGel). The continuous release of Zn2+ from ZIF-8 can destroy the bacterial biofilm and inhibit the growth of key periodontal pathogens. Hydrogels promote the proliferation of human gingival fibroblasts (HGFs) and BMSCs. They can also inhibit osteoclast activity in the inflammatory microenvironment and support bone regeneration in vivo [139]. These research results contribute to the development of more MOFs for regulating osteogenic and osteoclastic differentiation and bone remodeling, demonstrating the potential of MOFs in the effective treatment of periodontitis.
In addition, ZIF-8 loaded with miR-27a can be used as a coating material to release miR-27a and Zn2+ in a pH-responsive manner, reprograming the mitochondrial metabolism of macrophages to prevent peri-implant inflammation. L-MOF-agomir coating transforms RAW264.7 cells from the M1 phenotype into the M2 phenotype, a transition mediated by improved mitochondrial activity and a metabolic shift from glycolysis to oxidative phosphorylation, both of which are critical for reducing inflammation and promoting tissue repair. The supernatant secreted by macrophages also upregulates osteogenic markers, such as RUNX2 and ALP in BMSCs, indicating its potential for bone regeneration [140]. Quercetin can also be encapsulated in hydrogels with ZIF-8, and a five-in-one composite system has been developed (SFD/CS/ZIF-8@QCT). This system inhibits Porphyromonas gingivalis and demonstrates rapid and effective hemostatic capabilities, along with excellent blood compatibility. It reduces inflammatory factors by promoting M1-to-M2 macrophage polarization and immune reprogramming. By activating the PI3K/Akt/GSK-3β signaling pathway and inhibiting the production of ROS in PDLCs, it accelerates the periodontal repair process [141]. MOFs regulate macrophage inflammation, providing new ideas for the treatment of periodontitis. Through the regulation of the M1-M2 phenotypic transition, periodontal regeneration can be directly or indirectly affected.
In addition to ZIF-8, UiO-66 and CuTCPP can also be used as drug delivery carriers for periodontitis bone regeneration. UiO-66 is loaded with thymol and carvacrol to exert its antibacterial and anti-inflammatory properties. Car@UiO-66 and Thy@UiO-66 have good biocompatibility and enhance osteogenic activity and vascularization in hPDLCs. By inhibiting NF-κB signaling and reducing the expression of pro-inflammatory factors, macrophages can be polarized from the M1 phenotype into the M2 phenotype, showing a strong anti-inflammatory effect. In vivo experiments show that the composite hydrogel can promote the formation of new bone in alveolar bone defects and reduce inflammation [72]. Lam et al. designed hierarchical mesoporous zirconium-based MOF NPs (HMUiO-66-NH2) that slowly release zirconium ions (Zr4+) in acidic environments. These NPs break down hydrogen peroxide without producing harmful hydroxyl radicals, thereby reducing ROS levels. In addition, activating Wnt and TGF-β signaling pathways can enhance the osteogenic differentiation of BMSCs and protect bone structure defects caused by periodontitis [142]. In one study, CuTCPP modified by atomic-layer Fe2O3 was incorporated into the polyethylene glycol (PEG) matrix to form photoresponsive ointment (CuTCPP-Fe2O3), which can be used to treat periodontitis. CuTCPP-Fe2O3 exhibits strong antibacterial activity through ROS and ion release. In vivo studies have shown that this ointment reduces alveolar bone loss and tissue inflammation, promotes collagen deposition and angiogenesis, and alleviates periodontitis [73]. The MOF formed by Mg and gallic acid (GA) forms dynamic hydrogels with 4-formylphenylboronic acid, dextran, and carboxymethyl chitosan (CSBDX@MOF), which can deliver drugs in an acidic environment. The release of Mg2+ promotes osteogenic differentiation, while GA reduces oxidative stress and modulates the immune response by polarizing macrophages from M1 into M2. This hydrogel also demonstrates strong antibacterial action against P. gingivalis and Actinobacillus actinomycetemcomitans. In a rat model of periodontitis, CSBDX@MOF reduced alveolar bone loss and enhances bone regeneration and collagen deposition, which are key factors in tissue repair [143].
4.5. MOFs in Osteomyelitis
Osteomyelitis is a bacterial bone disease caused by trauma and joint replacement. If not treated in time, it will lead to sepsis, inflammation, and bone destruction [144]. Existing treatments consist mainly of long-term antibiotics and multiple debridement surgeries. However, due to antibiotic resistance and the pain caused by surgery, safer and more effective treatment options need to be considered [145]. Photodynamic therapy, photothermal therapy, and microwave therapy can effectively replace antibiotic-based therapies, have superior antibacterial and bone repair properties, and have become effective strategies for osteomyelitis treatment [71,146] (Table 6).
Among non-antibiotic antimicrobial therapies, sonodynamic therapy (SDT) has emerged as a reliable way to eliminate bacteria and repair tissue [147]. A zirconium-based porphyrin MOF (HNTM) is doped with Au and single atoms to provide it with SDT properties, and it is coated with a red blood cell (RBC) membrane to improve biocompatibility. The HNTM-Pt@Au system can effectively treat osteomyelitis induced by methicillin-resistant Staphylococcus aureus (MRSA); promote M1-to-M2 macrophage polarization by inhibiting iNOS expression and promoting TGF-β expression; and promote bone regeneration [148]. The piezoelectric material MoS2 forms a piezoelectric-enhanced SDT with HNTM and the RBC membrane, and its ROS and mechanical properties can effectively eliminate MRSA by promoting DNA damage, oxidative stress, tryptophan metabolism, and so on. In osteomyelitis rats, RBC-HNTM-MoS2 can relieve inflammation and promote the polarization of macrophage M1 into M2 [149]. Adding a sonosensitizer can effectively improve the antibacterial properties of photosensitive materials. HN-Ti3C2, consisting of HNTM and Ti3C2, can kill 99.75% of MRSA under low-intensity ultrasound (US) and promotes stem cell proliferation and osteogenic differentiation through the Wnt, calcium, and TGF-β signaling pathways. HN-Ti3C2 eliminates bacterial infection and promotes bone regeneration in a rat model of osteomyelitis [146]. Cerium-based MOF (CeTCPP) supported by Au NPs can also be used for SDT in osteomyelitis. Under US, Au NPs transform Ce3+ nodes into Ce4+ nodes and enhance ROS generation. Therefore, within 20 min of US, the antibacterial effect of CeTCPP-Au on Staphylococcus aureus and Escherichia coli can reach more than 99%, with good biocompatibility and deep antibacterial and anti-inflammatory effects in vivo [150]. Microwave (MV)-reactive materials are also an effective antibiotic-free treatment strategy. CNT-2D MOF formed by oxidized carbon nanotubes (CNT) and ultrasmall Cu-based 2D MOF can absorb microwaves and convert them into heat through heterojunction interface polarization. They can also destroy bacterial membranes through Cu ion release. This system showed antibacterial activity against seven pathogenic bacteria and good biocompatibility in vivo [151]. These methods of converting physical substances into biochemical signals through MOF complexes based on SDT and microwaves address the drawback that bone marrow infections require high-dose systemic antibiotic treatments, while also avoiding the limitations of antibiotic toxic side effects and bacterial resistance.

Table 6.
Summary of the application of MOF-based composite materials for the treatment of osteomyelitis.
Table 6.
Summary of the application of MOF-based composite materials for the treatment of osteomyelitis.
| MOF Composites | Form of the Composites | Primary Role of MOFs | Metal Ions/Clusters | Cellular Biological Functions | Mechanism | In Vivo Effects | Refs |
|---|---|---|---|---|---|---|---|
| HNTM-Pt@Au | Nanoparticle | Sound sensitizer | Pt, Au | Antibacterial; Promoting osteogenesis and mineralization; promoting M1-to-M2 macrophage polarization | Inhibiting iNOS and promoting TGF-β in macrophages | Inhibiting MRSA-infected osteomyelitis; preventing bone destruction | [148] |
| HNTM-MoS2 | Nanosheet | Sound sensitizer | MoS2 | Antibacterial; promoting M1-to-M2 macrophage polarization | / | Eliminating bone infection; inhibiting inflammation; inhibiting bone loss | [149] |
| HN-Ti3C2 | Nanosheet | Sound sensitizer | Ti3C2 | Antibacterial; Promoting osteogenesis and mineralization | Activating calcium, MAPK, and Wnt signaling pathways | Eliminating bone infection; inhibiting bone loss | [146] |
| CeTCPP-Au | Nanoparticle | Sound sensitizer | Au | Antibacterial | / | Eliminating bone infection; relieving inflammation | [150] |
| CNT-CuHHTP | Nanotube | Microwave dynamic therapy | Cu2+ | Antibacterial | / | Eliminating bone infection; relieving inflammation | [151] |
5. Conclusions and Prospects
With the development of tissue engineering technology, the use of functional MOF composites as emerging biomaterials for the treatment of bone diseases has become a potential trend in biomedical applications. MOFs can function as drug carriers, metal ion donors, nanozymes, and photosensitizers. When combined with other functional materials, they can regulate cellular reactive oxygen species, macrophage phenotypic transformation, bone resorption, osteogenesis, and mineralization, providing a new paradigm for bone tissue engineering. This study reviewed the progress in biomedical research progress on MOFs in diseases such as bone defects, osteoarthritis, osteoporosis, bone tumors, osteomyelitis, and periodontitis over the past five years.
The greatest advantage of materials based on MOF composites in the treatment of bone diseases is that they can be synthesized into different morphologies as needed, and they have shown improved drug solubility, loading, and release; targeting ability; ROS regulation ability; and pH and photothermal response, among other benefits. These factors help to significantly overcome the limitations of existing drugs and also increase the possibility of precise treatment. While the multifunctional properties of MOFs underscore their promising potential in bone-related biomedical applications, a comprehensive and critical evaluation across different MOF families reveals intricate variations in their performance, biocompatibility, and long-term suitability. ZIFs, for instance, have shown great chemical stability and good drug-loading capabilities, but their arbitrary breakdown routes can let imidazole derivatives out, hence generating questions about cytotoxic effects that might endanger cell survival [152]. While UiOs are known for their high thermal stability and chemical stability, emerging evidence suggests potential risks related to their long-term accumulation in tissues [153], which necessitates a deeper inquiry into their biodegradation kinetics and clearance mechanisms. Similarly, the MIL and HKUST frameworks have been shown to facilitate the effective delivery of therapeutic contents, but their degradation products may trigger tissue toxicity [154]. Therefore, it is necessary to conduct strict and systematic research under well-controlled experimental conditions to clarify the relationships between the physicochemical properties, biological interactions, and therapeutic effects of MOFs. This is crucial for determining the translational potential of MOFs in bone tissue engineering and regenerative medicine.
To date, there have been relatively few clinical experiments using MOFs as bone materials. Before MOF-based treatment methods can be translated into clinical bone regeneration applications, several practical challenges must still be addressed. The first issue is the safety of MOFs in vivo. At present, animal experiments are conducted through in situ implantation and blood injection. The organ distribution, metabolism, and release kinetics of MOFs and other functional materials in the body have not received much attention. These factors all determine the potential of MOFs in clinical applications. Therefore, it is necessary to establish a gold standard for biosafety to ensure the safety and efficacy of MOFs. The bottleneck of transformation lies in the unclear complex interaction relationship between the degradation kinetics of MOFs and the physiological microenvironment. In vitro models have shown that the controlled decomposition of biological systems, enzyme activity, and mechanical stress can produce unpredictable dissolution patterns, thereby altering the drug released dose and safety of drugs. The lack of correlation between pharmacokinetics and pharmacodynamics exacerbates this uncertainty. In addition, existing research lacks standardized protocols to track the biological distribution, metabolic pathways, and renal/liver clearance mechanisms of metal ions. Future research must focus on the frameworks of biocompatible metals (such as iron and calcium) and their combination with linkers that can be safely and predictably degraded in physiological environments. Combining complex surface modifications—such as bionic coatings that imitate natural tissue interfaces—can significantly prevent immune responses, thereby enhancing therapeutic effects and biocompatibility.
The second issue is the biological mechanism of MOF composites. Most studies have verified the roles of such composites both in vivo and in vitro, but they have not yet clearly explained the molecular switches and signaling pathways through which they exert their effects. These can be further explored through methods such as transcriptomics and proteomics.
The third issue is the fine regulation and standardization of MOF synthesis. The scalability of manufacturing comes at the cost of repeatability, where changes in particle size and morphology can alter performance. In clinical applications, stable and effective MOF complexes are required. Different laboratories may produce MOF NPs with different particle sizes and morphologies when using the same parameters, and all of these factors may affect the standardized production of MOFs. Therefore, it is necessary to develop more accurate MOF preparation methods and establish standards for the processing methods of MOF composite materials for clinical use.
In summary, the application of MOFs in bone diseases brings opportunities and challenges. By addressing the application limitations discussed above, MOFs are expected to become a key type of nanomaterial in the biomedical field, especially in advancing the treatment of bone-related diseases. In addition to their scientific prospects, the continuous development and clinical translation of MOF-based therapies may generate significant public health benefits through more effective, targeted, and personalized treatment strategies. These materials have the potential to shorten treatment time, reduce medical costs, and improve the quality of life of patients, especially among the aging population with an increasing prevalence of bone diseases. Furthermore, MOF research has promoted interdisciplinary collaboration between materials science, biology, and clinical medicine, enriching the scientific community and accelerating the development of next-generation bone defect treatment methodologies. With the progress in research, MOFs can also serve as a fundamental platform for the wider application of regenerative medicine and precision medicine, contribute long-term value to society, and shape new directions for biomedical innovation.
Author Contributions
Writing—original draft preparation, C.Y., Z.Y., R.E.H.-D., R.N.; writing—review and editing, X.L., J.S., Y.X., Z.Q.; supervision, X.L., J.S.; funding acquisition, X.L., J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82470926, 21901210), the Guangdong Basic and Applied Basic Research Foundation (2023A1515030047), the Natural Science Basic Research Plan in Shaanxi Province of China (2024JC-YBMS-605), and the Provincial College Student Innovation and Entrepreneurship Training Program (S202410699580).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wang, L.J.; You, X.L.; Zhang, L.L.; Zhang, C.Q.; Zou, W.G. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Rajawat, J. Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics. Int. J. Mol. Sci. 2021, 22, 3553. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Yin, S.H.; Lin, M.Z.; Chen, X.L.; Pan, Y.; Peng, Y.Q.; Sun, J.B.; Kumar, A.; Liu, J.Q. Current status and prospects of metal-organic frameworks for bone therapy and bone repair. J. Mater. Chem. B 2022, 10, 5105–5128. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Burley, G.; Lin, S.; Shi, Y.C. Osteoporosis pathogenesis and treatment: Existing and emerging avenues. Cell. Mol. Biol. Lett. 2022, 27, 72. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.M.; Ding, C.; et al. Osteoarthritis. Nat. Rev. Dis. Primers 2025, 11, 10. [Google Scholar] [CrossRef]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.D.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef]
- Coleman, R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006, 12, 6243S–6249S. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontology 2000 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A.R. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Wang, W.H.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Binaeian, E.; Nabipour, H.; Ahmadi, S.; Rohani, S. The green synthesis and applications of biological metal-organic frameworks for targeted drug delivery and tumor treatments. J. Mater. Chem. B 2023, 11, 11426–11459. [Google Scholar] [CrossRef] [PubMed]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal-Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, V.F.; Malek, N.I.; Kailasa, S.K. Review on Metal-Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega 2022, 7, 44507–44531. [Google Scholar] [CrossRef] [PubMed]
- Afshariazar, F.; Morsali, A. Mixed-valence metal-organic frameworks: Concepts, opportunities, and prospects. Chem. Soc. Rev. 2025, 54, 1318–1383. [Google Scholar] [CrossRef]
- Lin, Z.J.; Lü, J.; Hong, M.C.; Cao, R. Metal-organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar] [CrossRef]
- Qian, Q.H.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Bo, C.; Guo, S. Luminescent Ln-MOFs for Chemical Sensing Application on Biomolecules. ACS Sens. 2024, 9, 4402–4424. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic functional metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef]
- Jafarzadeh, M. Recent Progress in the Development of MOF-Based Photocatalysts for the Photoreduction of Cr(VI). ACS Appl. Mater. Interfaces 2022, 14, 24993–25024. [Google Scholar] [CrossRef]
- Roohollahi, H.; Zeinalzadeh, H.; Kazemian, H. Recent Advances in Adsorption and Separation of Methane and Carbon Dioxide Greenhouse Gases Using Metal-Organic Framework-Based Composites. Ind. Eng. Chem. Res. 2022, 61, 10555–10586. [Google Scholar] [CrossRef]
- Saboorizadeh, B.; Zare-Dorabei, R.; Safavi, M.; Safarifard, V. Applications of Metal-Organic Frameworks (MOFs) in Drug Delivery, Biosensing, and Therapy: A Comprehensive Review. Langmuir 2024, 40, 22477–22503. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, C.; Guo, J.; Zhang, X.; Wu, L.; Zhu, L.; Du, B. Research Progress of Metal-Organic Frameworks as Drug Delivery Systems. ACS Appl. Mater. Interfaces 2024, 16, 43156–43170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, X.; Su, Z. Design of metal-organic framework composites in anti-cancer therapies. Nanoscale 2021, 13, 12102–12118. [Google Scholar] [CrossRef]
- Deng, L.E.; Guo, M.L.; Deng, Y.J.; Pan, Y.; Wang, X.X.; Maduraiveeran, G.; Liu, J.Q.; Lu, C.Y. MOF-Based Platform for Kidney Diseases: Advances, Challenges, and Prospects. Pharmaceutics 2024, 16, 793. [Google Scholar] [CrossRef]
- Fatah, S.A.; Omer, K.M. Aptamer-Modified MOFs (Aptamer@MOF) for Efficient Detection of Bacterial Pathogens: A Review. ACS Appl. Mater. Interfaces 2025, 17, 11578–11594. [Google Scholar] [CrossRef]
- Anderson, S.L.; Stylianou, K.C. Biologically derived metal organic frameworks. Coord. Chem. Rev. 2017, 349, 102–128. [Google Scholar] [CrossRef]
- Lan, G.X.; Ni, K.Y.; Lin, W.B. Nanoscale metal-organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef]
- Huxford, R.C.; Della Rocca, J.; Lin, W. Metal–organic frameworks as potential drug carriers. Curr. Opin. Chem. Biol. 2010, 14, 262–268. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Cui, J.; Wang, S.; Han, Y.; Shao, H.; Wang, C.; Hu, Y.; Li, X.; Zhou, Q.; et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci. Adv. 2023, 9, eabo7868. [Google Scholar] [CrossRef]
- Li, Q.; Wang, R.J.; Xue, J.F.; Wang, R.Y.; Zhang, S.; Kang, H.; Wang, Y.; Zhu, H.D.; Lv, C.Z. ZIF-8-Modified Black Phosphorus Nanosheets Incorporated into Injectable Dual-Component Hydrogels for Enhanced Photothermal Antibacterial and Osteogenic Activities. ACS Appl. Mater. Interfaces 2024, 16, 32058–32077. [Google Scholar] [CrossRef]
- Lao, A.; Wu, J.; Li, D.; Shen, A.; Li, Y.; Zhuang, Y.; Lin, K.; Wu, J.; Liu, J. Functionalized Metal-Organic Framework-Modified Hydrogel That Breaks the Vicious Cycle of Inflammation and ROS for Repairing of Diabetic Bone Defects. Small 2023, 19, e2206919. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yu, H.; You, T.; Kong, X.; Wei, X.; Zheng, Z.; Zheng, L.; Feng, Z.; Huang, B.; Zhang, X.; et al. A Dual-Targeted Metal-Organic Framework Based Nanoplatform for the Treatment of Rheumatoid Arthritis by Restoring the Macrophage Niche. ACS Nano 2023, 17, 13917–13937. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Zeng, Y.X.; Pan, Z.X.; Feng, Z.Z.; Bao, Y.; Ye, Z.Y.; Li, Y.S.; Tang, J.Z.; Liu, X.J.; He, Y. Amino-Functionalized Zirconium-Based Metal-Organic Frameworks as Bifunctional Nanomaterials to Treat Bone Tumors and Promote Osteogenesis. ACS Appl. Mater. Interfaces 2023, 15, 53217–53227. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef]
- Wen, T.; Quan, G.; Niu, B.; Zhou, Y.; Zhao, Y.; Lu, C.; Pan, X.; Wu, C. Versatile Nanoscale Metal-Organic Frameworks (nMOFs): An Emerging 3D Nanoplatform for Drug Delivery and Therapeutic Applications. Small 2021, 17, 2005064. [Google Scholar] [CrossRef]
- Wan, Z.; Chung, C.H.Y.; Lau, C.M.L.; Chung, J.T.; Chau, Y.; Fan, Z.; Zhang, S.; Yao, S. Metal-Organic Frameworks-Based Microrockets for Controlled and Sustained Drug Release. Nano Lett. 2025, 25, 5989–5996. [Google Scholar] [CrossRef]
- Tohidi, S.; Aghaie-Khafri, M. Chitosan-Coated MIL-100(Fe) as an Anticancer Drug Carrier: Theoretical and Experimental Investigation. ACS Med. Chem. Lett. 2023, 14, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.D.; Chen, S.P.; Zhao, H.; Yang, Y.; Chen, X.Q.; Sun, J.; Fan, H.S.; Zhang, X.D. PPy@MIL-100 Nanoparticles as a pH- and Near-IR-Irradiation-Responsive Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 34209–34217. [Google Scholar] [CrossRef]
- Wan, M.; Song, S.; Feng, W.; Shen, H.; Luo, Y.; Wu, W.; Shen, J. Metal–Organic Framework (UiO-66)-Based Temperature-Responsive Pesticide Delivery System for Controlled Release and Enhanced Insecticidal Performance against Spodoptera frugiperda. ACS Appl. Bio Mater. 2022, 5, 4020–4027. [Google Scholar] [CrossRef]
- Long, Y.L.; Cheng, X.; Tang, Q.M.; Chen, L.L. The antigenicity of silk-based biomaterials: Sources, influential factors and applications. J. Mater. Chem. B 2021, 9, 8365–8377. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, K.; Wen, Z.; Liu, W.; Zhang, L.; Su, J. Double-edged effects and mechanisms of Zn(2+) microenvironments on osteogenic activity of BMSCs: Osteogenic differentiation or apoptosis. RSC Adv. 2020, 10, 14915–14927. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Perdikaki, A.; Galeou, A.; Pilatos, G.; Karatasios, I.; Kanellopoulos, N.K.; Prombona, A.; Karanikolos, G.N. Ag and Cu Monometallic and Ag/Cu Bimetallic Nanoparticle-Graphene Composites with Enhanced Antibacterial Performance. ACS Appl. Mater. Interfaces 2016, 8, 27498–27510. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.L.; Chen, X.R.; Sun, Y.; Yang, P.X.; Gu, X.S.; Dai, X. Application of metal-organic frameworks-based functional composite scaffolds in tissue engineering. Regen. Biomater. 2024, 11, rbae009. [Google Scholar] [CrossRef]
- Yao, S.; Chi, J.J.; Wang, Y.T.; Zhao, Y.J.; Luo, Y.; Wang, Y.A. Zn-MOF Encapsulated Antibacterial and Degradable Microneedles Array for Promoting Wound Healing. Adv. Healthc. Mater. 2021, 10, 2100056. [Google Scholar] [CrossRef]
- Lian, C.; Liu, J.; Wei, W.; Wu, X.; Goto, T.; Li, H.; Tu, R.; Dai, H. Mg-gallate metal-organic framework-based sprayable hydrogel for continuously regulating oxidative stress microenvironment and promoting neurovascular network reconstruction in diabetic wounds. Bioact. Mater. 2024, 38, 181–194. [Google Scholar] [CrossRef]
- Xue, Y.; Zhu, Z.; Zhang, X.; Chen, J.; Yang, X.; Gao, X.; Zhang, S.; Luo, F.; Wang, J.; Zhao, W.; et al. Accelerated Bone Regeneration by MOF Modified Multifunctional Membranes through Enhancement of Osteogenic and Angiogenic Performance. Adv. Healthc. Mater. 2021, 10, e2001369. [Google Scholar] [CrossRef]
- Kang, Y.; Xu, C.; Meng, L.; Dong, X.; Qi, M.; Jiang, D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 2022, 18, 26–41. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Tang, H.; Niu, X.; Yang, H.; Bai, Y.; Gu, X.; Zheng, Y. Fabrication of a Nanoscale Magnesium/Copper Metal–Organic Framework on Zn-Based Guided Bone Generation Membranes for Enhancing Osteogenesis, Angiogenesis, and Bacteriostasis Properties. ACS Appl. Mater. Interfaces 2024, 16, 5648–5665. [Google Scholar] [CrossRef]
- Shu, C.; Qin, C.; Chen, L.; Wang, Y.; Shi, Z.; Yu, J.; Huang, J.; Zhao, C.; Huan, Z.; Wu, C.; et al. Metal-Organic Framework Functionalized Bioceramic Scaffolds with Antioxidative Activity for Enhanced Osteochondral Regeneration. Adv. Sci. 2023, 10, 2206875. [Google Scholar] [CrossRef]
- Wang, B.; Xie, X.; Jiang, W.; Zhan, Y.; Zhang, Y.; Guo, Y.; Wang, Z.; Guo, N.; Guo, K.; Sun, J. Osteoinductive micro-nano guided bone regeneration membrane for in situ bone defect repair. Stem Cell Res. Ther. 2024, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, M.; Bennici, S.; Brendle, J.; Dutournie, P.; Limousy, L.; Pluchon, S. Systems for stimuli-controlled release: Materials and applications. J. Control. Release 2019, 294, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Zulpya, M.; Zhang, X.Y.; Xu, S.A.; Sun, J.; Dong, B.A. Recent Advances of Metal-Organic Frameworks-based Nanozymes for Bio-applications. Chem. Res. Chin. Univ. 2022, 38, 1324–1343. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, B.; Lang, L.M.; Liu, G.X.; Xia, X.H. Bioinspired Fabrication of Two-Dimensional Metal-Organic Framework-Based Nanozyme for Sensitive Colorimetric Detection of Glutathione. ACS Appl. Nano Mater. 2022, 5, 18761–18769. [Google Scholar] [CrossRef]
- Weng, Y.; Chen, R.; Hui, Y.; Chen, D.; Zhao, C.-X. Boosting Enzyme Activity in Enzyme Metal-Organic Framework Composites. Chem Bio Eng. 2024, 1, 99–112. [Google Scholar] [CrossRef]
- Ling, P.; Cheng, S.; Chen, N.; Qian, C.; Gao, F. Nanozyme-Modified Metal–Organic Frameworks with Multienzymes Activity as Biomimetic Catalysts and Electrocatalytic Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 17185–17192. [Google Scholar] [CrossRef]
- Niu, X.H.; Li, X.; Lyu, Z.Y.; Pan, J.M.; Ding, S.C.; Ruan, X.F.; Zhu, W.L.; Du, D.; Lin, Y.H. Metal-organic framework based nanozymes: Promising materials for biochemical analysis. Chem. Commun. 2020, 56, 11338–11353. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, S.; Xie, W.; Xue, Y.; Qiao, M.; Yang, X.; Zhang, X.; Wan, Q.; Wang, J.; Chen, J.; et al. Surface modification of the Ti surface with nanoscale bio-MOF-1 for improving biocompatibility and osteointegration in vitro and in vivo. J. Mater. Chem. B 2022, 10, 8535–8548. [Google Scholar] [CrossRef]
- Ge, Y.; Hu, L.; Liu, J.; Ma, F.; Zhang, J.; Wang, Y.; Tang, B.; Cao, S. Peek@ZIF-8(CEL) as a Novel Bone Implant for Large Defect Repair and Enhanced Bone Healing via a Long-Term Stable Bioactive Releaser. ACS Appl. Mater. Interfaces 2024, 16, 44127–44138. [Google Scholar] [CrossRef]
- Liu, L.; Wu, J.; Lv, S.; Xu, D.; Li, S.; Hou, W.; Wang, C.; Yu, D. Synergistic effect of hierarchical topographic structure on 3D-printed Titanium scaffold for enhanced coupling of osteogenesis and angiogenesis. Mater. Today Bio 2023, 23, 100866. [Google Scholar] [CrossRef]
- Pan, H.; Miao, X.; Deng, J.; Pan, C.; Cheng, X.; Wang, X. Bimetallic Metal-Organic Framework for Mitigating Aseptic Osteolysis. ACS Appl. Mater. Interfaces 2023, 15, 4935–4946. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Qin, Z.; Chen, H.; Lan, Q.; Wang, Z.; Lan, N.; Yang, Y.; Zheng, L.; Zhao, J.; Kai, D. pH-responsive and hyaluronic acid-functionalized metal-organic frameworks for therapy of osteoarthritis. J. Nanobiotechnol. 2020, 18, 139. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; Wu, S. The recent progress on metal-organic frameworks for phototherapy. Chem. Soc. Rev. 2021, 50, 5086–5125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, Y.; Yan, T.-H.; Li, J.; Li, Y.; Drake, H.F.; Zhong, H.; Jin, Y.; Zhao, R.; Zhou, H.-C. Metal-Organic Framework-Based Nanoheater with Photo-Triggered Cascade Effects for On-Demand Suppression of Cellular Thermoresistance and Synergistic Cancer Therapy. Adv. Healthc. Mater. 2022, 11, e2200004. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem.-Int. Ed. 2021, 60, 5010–5035. [Google Scholar] [CrossRef]
- Gao, D.; Gao, Y.; Shen, J.; Wang, Q. Modified nanoscale metal organic framework-based nanoplatforms in photodynamic therapy and further applications. Photodiagnosis Photodyn. Ther. 2020, 32, 102026. [Google Scholar] [CrossRef]
- Chien, W.C.; Cheng, P.H.; Cheng, X.J.; Chuang, C.C.; Huang, Y.T.; Anilkumar, T.S.; Liu, C.H.; Lu, Y.J.; Wu, K.C.W. MCP-1-Functionalized, Core-Shell Gold Nanorod@Iron-Based Metal-Organic Framework (MCP-1/GNR@MIL-100(Fe)) for Photothermal Therapy. ACS Appl. Mater. Interfaces 2021, 13, 52092–52105. [Google Scholar] [CrossRef]
- Huang, B.; Li, G.; Cao, L.; Wu, S.; Zhang, Y.; Li, Z.; Zhou, F.; Xu, K.; Wang, G.; Su, J. Nanoengineered 3D-printing scaffolds prepared by metal-coordination self-assembly for hyperthermia-catalytic osteosarcoma therapy and bone regeneration. J. Colloid Interface Sci. 2024, 672, 724–735. [Google Scholar] [CrossRef]
- Qu, Y.; Zhuang, H.; Zhang, M.; Wang, Y.; Zhai, D.; Ma, B.; Wang, X.; Qin, C.; Huan, Z.; Wu, C. Bone cements for therapy and regeneration for minimally invasive treatment of neoplastic bone defects. J. Mater. Chem. B 2021, 9, 4355–4364. [Google Scholar] [CrossRef]
- Jin, L.G.; Liu, H.P.; Wang, C.F.; Mao, C.Y.; Wu, S.L.; Zhang, Y.; Li, Z.Y.; Zhu, S.L.; Jiang, H.; Cui, Z.D.; et al. Interface/Dipole Polarized Antibiotics-Loaded Fe3O4/PB Nanoparticles for Non-Invasive Therapy of Osteomyelitis Under Medical Microwave Irradiation. Adv. Mater. 2024, 36, e2410917. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Huang, Y.; Hu, W.; Li, R.; Wang, J.; Han, M.; Li, Z. Evaluation of the Antibacterial, Anti-Inflammatory, And Bone-Promoting Capacity of UiO-66 Loaded with Thymol or Carvacrol. ACS Appl. Mater. Interfaces 2024, 16, 36017–36029. [Google Scholar] [CrossRef]
- Li, J.; Song, S.; Meng, J.; Tan, L.; Liu, X.; Zheng, Y.; Li, Z.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; et al. 2D MOF Periodontitis Photodynamic Ion Therapy. J. Am. Chem. Soc. 2021, 143, 15427–15439. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Liang, N.; Dong, M.; Feng, Z.; Meng, L.; Sun, C.; Wang, A.; Yu, X.; Wang, W.; Xie, J.; et al. Stem Cell Membrane-Encapsulated Zeolitic Imidazolate Framework-8: A Targeted Nano-Platform for Osteogenic Differentiation. Small 2022, 18, e2202485. [Google Scholar] [CrossRef]
- Li, Y.X.; Xu, C.C.; Mao, J.; Mao, L.X.; Li, W.Q.; Liu, Z.Y.; Shin, A.; Wu, J.Q.; Hou, L.L.; Li, D.J.; et al. ZIF-8-based Nanoparticles for Inflammation Treatment and Oxidative Stress Reduction in Periodontitis. ACS Appl. Mater. Interfaces 2024, 16, 36077–36094. [Google Scholar] [CrossRef]
- Travlou, N.A.; Algarra, M.; Alcoholado, C.; Cifuentes-Rueda, M.; Labella, A.M.; Lazaro-Martinez, J.M.; Rodriguez-Castellon, E.; Bandosz, T.J. Carbon Quantum Dot Surface-Chemistry-Dependent Ag Release Governs the High Antibacterial Activity of Ag-Metal-Organic Framework Composites. ACS Appl. Bio Mater. 2018, 1, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fang, J.; Wang, Y.; Sun, J.; Sun, Y.; Sun, X.; Qi, M.; Li, W.; Li, C.; Zhou, Y.; et al. Antibacterial Zeolite Imidazole Frameworks with Manganese Doping for Immunomodulation to Accelerate Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, e2101515. [Google Scholar] [CrossRef]
- Zhang, W.S.; Wang, B.J.; Xiang, G.L.; Jiang, T.Z.; Zhao, X. Photodynamic Alginate Zn-MOF Thermosensitive Hydrogel for Accelerated Healing of Infected Wounds. ACS Appl. Mater. Interfaces 2023, 15, 22830–22842. [Google Scholar] [CrossRef]
- Huang, S.; Ye, Y.; Jiang, C.; Wang, R.; Hu, W.; Raza, S.; Jie, O.; Pan, Y.; Liu, J. Current and promising applications of MOFs loaded with PTAs on photothermal therapy. React. Funct. Polym. 2023, 193, 105743. [Google Scholar] [CrossRef]
- Tong, P.H.; Yang, J.J.; Zhou, Y.F.; Tang, Y.F.; Tang, M.T.; Zang, Y.; Pan, Y.F.; Dong, L.W.; Tan, Y.X.; Nam, K.T.; et al. Metal-organic frameworks (MOFs) for phototherapy and synergistic phototherapy of cancer. Coord. Chem. Rev. 2025, 526, 216381. [Google Scholar] [CrossRef]
- Zhou, B.X.; Jiang, X.L.; Zhou, X.X.; Tan, W.Y.; Luo, H.; Lei, S.R.; Yang, Y. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: Therapeutic strategies and recent advances. Biomater. Res. 2023, 27, 86. [Google Scholar] [CrossRef] [PubMed]
- Fardjahromi, M.A.; Ejeian, F.; Razmjou, A.; Vesey, G.; Mukhopadhyay, S.C.; Derakhshan, A.; Warkiani, M.E. Enhancing osteoregenerative potential of biphasic calcium phosphates by using bioinspired ZIF8 coating. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 123, 111972. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yue, Y.; Wang, W.; Han, M.; Wan, X.; Li, Q.; Chen, X.; Cao, J.; Zhang, Y.; Li, J.; et al. Ultrasound-Activated Probiotics Vesicles Coating for Titanium Implant Infections Through Bacterial Cuproptosis-Like Death and Immunoregulation. Adv. Mater. 2024, 36, e2405953. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Zhang, Z.; Wang, Y.; Ye, Y.; Yinwang, E.; Liu, A.; Zhou, X.; Xu, J.; Zhou, C.; Sun, H.; et al. Iodine Immobilized Metal-Organic Framework for NIR-Triggered Antibacterial Therapy on Orthopedic Implants. Small 2021, 17, 2102315. [Google Scholar] [CrossRef]
- He, M.M.; Huang, Y.; Xu, H.; Feng, G.J.; Liu, L.M.; Li, Y.B.; Sun, D.; Zhang, L. Modification of polyetheretherketone implants: From enhancing bone integration to enabling multi-modal therapeutics. Acta Biomater. 2021, 129, 18–32. [Google Scholar] [CrossRef]
- Deng, Y.; Shi, J.; Chan, Y.K.; Bai, D.; Shu, R.; Shi, X.; Li, Y.; Li, L.; Yang, X.; Yang, W. Heterostructured Metal-Organic Frameworks/Polydopamine Coating Endows Polyetheretherketone Implants with Multimodal Osteogenicity and Photoswitchable Disinfection. Adv. Healthc. Mater. 2022, 11, e2200641. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, X.; Yang, H.; Li, L.; He, C.; Cheng, C.; Haag, R. ZnO/Nanocarbons-Modified Fibrous Scaffolds for Stem Cell-Based Osteogenic Differentiation. Small 2020, 16, e2003010. [Google Scholar] [CrossRef]
- Toprak, O.; Topuz, B.; Abou Monsef, Y.; Oto, C.; Orhan, K.; Karakecili, A. BMP-6 carrying metal organic framework-embedded in bioresorbable electrospun fibers for enhanced bone regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 120, 111738. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Zhu, Y.; Han, Y.; Shen, J.; Bao, B.; Gao, T.; Lin, J.; Huang, T.; Xu, J.; et al. Tunable and Controlled Release of Cobalt Ions from Metal-Organic Framework Hydrogel Nanocomposites Enhances Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 59051–59066. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, R.-D.; Su, S.-L.; Hao, Y.-L.; Zhou, F. Green synthesis of metal-organic framework loaded dexamethasone on wood aerogels for enhanced cranial bone regeneration. J. Mater. Chem. B 2023, 11, 9496–9508. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Hu, H.; Xu, Z.; Liu, J.; Chen, J.; Chen, B.; Shi, L.; Luo, H.; Chen, G.; et al. Engineered Metallic Ion-Based Hydrogel for Tendon-Bone Reconstruction. ACS Appl. Mater. Interfaces 2024, 16, 6837–6848. [Google Scholar] [CrossRef]
- Ma, J.; Yu, H.; Zhang, X.; Xu, Z.; Hu, H.; Liu, J.; Ren, P.; Kong, X.; Chen, J.; Yang, K.; et al. Dual-Targeted Metal Ion Network Hydrogel Scaffold for Promoting the Integrated Repair of Tendon-Bone Interfaces. ACS Appl. Mater. Interfaces 2024, 16, 5582–5597. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.J.; Johnson, B.N.; Jia, X.F. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Zhong, L.N.; Chen, J.Y.; Ma, Z.Y.; Feng, H.; Chen, S.; Cai, H.; Xue, Y.Y.; Pei, X.B.; Wang, J.; Wan, Q.B. 3D printing of metal-organic framework incorporated porous scaffolds to promote osteogenic differentiation and bone regeneration. Nanoscale 2020, 12, 24437–24449. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Hu, M.G.; Dong, M.R.; Yang, Z.H.; Zhan, X.; Chang, X.Y.; Lu, J.; Chen, X. Folic Acid Decorated Zeolitic Imidazolate Framework (ZIF-8) Loaded with Baicalin as a Nano-Drug Delivery System for Breast Cancer Therapy. Int. J. Nanomed. 2021, 16, 8337–8352. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Ren, N.; Feng, Z.; Sun, Z.; Dong, M.; Wang, W.; Liu, F.; Sun, C.; Zhou, W.; Xing, Z.; et al. Biomimetic Metal-Organic Frameworks as Targeted Vehicles to Enhance Osteogenesis. Adv. Healthc. Mater. 2022, 11, 2102821. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Hu, Y.; Zhang, H.; Wang, S.C.; Xu, K.; Su, J.C. Single-cell RNA sequencing in osteoarthritis. Cell Prolif. 2023, 56, e13517. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Chang, M.N.; Shi, D.; Yun, C.X.; Li, J.; Guo, H.T.; Lin, X. Therapeutic targets in aging-related osteoarthritis: A focus on the extracellular matrix homeostasis. Life Sci. 2025, 368, 123487. [Google Scholar] [CrossRef]
- Li, J.D.; Zhang, H.; Han, Y.F.; Hu, Y.; Geng, Z.; Su, J.C. Targeted and responsive biomaterials in osteoarthritis. Theranostics 2023, 13, 931–954. [Google Scholar] [CrossRef]
- Chen, H.M.; Qin, Z.N.; Zhao, J.M.; He, Y.; Ren, E.; Zhu, Y.; Liu, G.; Mao, C.B.; Zheng, L. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials 2019, 225, 119520. [Google Scholar] [CrossRef]
- Wang, D.D.; Zhou, J.J.; Chen, R.H.; Shi, R.H.; Xia, G.L.; Zhou, S.; Liu, Z.B.; Zhang, N.Q.; Wang, H.B.; Guo, Z.; et al. Magnetically guided delivery of DHA and Fe ions for enhanced cancer therapy based on pH-responsive degradation of DHA-loaded Fe3O4@C@MIL-100(Fe) nanoparticles. Biomaterials 2016, 107, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Tian, Z.; Sun, L.; Kong, W.; Yan, R.; Yu, Z.; Tian, Q.-W.; Liu, C. Quercetin-Loaded Zeolitic Imidazolate Framework-8 (ZIF-8) Nanoparticles Attenuate Osteoarthritis by Activating Autophagy via the Pi3k/Akt Signaling. ACS Appl. Mater. Interfaces 2024, 16, 40444–40454. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Hou, Y.-K.; Chen, M.-W.; Yu, X.-Z.; Chen, S.-Y.; Yue, Y.-R.; Guo, X.-T.; Chen, J.-X.; Zhou, Q. A pH-responsive metal-organic framework for the co-delivery of HIF-2α siRNA and curcumin for enhanced therapy of osteoarthritis. J. Nanobiotechnol. 2023, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, W.Y. Macrophage membrane-camouflaged biomimetic nanovesicles for targeted treatment of arthritis. Ageing Res. Rev. 2024, 95, 102241. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ding, S.-L.; Zhao, X.; Sun, D.; Yang, Y.; Chen, M.; Zhu, C.; Jiang, B.; Gu, Q.; Liu, H.; et al. Self-Reinforced MOF-Based Nanogel Alleviates Osteoarthritis by Long-Acting Drug Release. Adv. Mater. 2024, 36, e2401094. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Y.; Ruan, Z.; Zhang, S.; Feng, X.; Lu, C.; Zhao, J.; Yin, F.; Cao, C.; Zheng, L. Baicalin nanodelivery system based on functionalized metal-organic framework for targeted therapy of osteoarthritis by modulating macrophage polarization. J. Nanobiotechnol. 2024, 22, 221. [Google Scholar] [CrossRef]
- Gong, P.; Wang, M.; Wang, J.; Li, J.; Wang, B.; Bai, X.; Liu, J.; Liu, Z.; Wang, D.; Liu, W. A biomimetic lubricating nanosystem for synergistic therapy of osteoarthritis. J. Colloid Interface Sci. 2024, 672, 589–599. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Lin, X.; He, Z.; Zhang, H.; Ji, L.; Gong, P.; Zhou, F.; Liu, W. Dual-functional MOFs-based hybrid microgel advances aqueous lubrication and anti-inflammation. J. Colloid Interface Sci. 2023, 644, 200–210. [Google Scholar] [CrossRef]
- Tian, L.; Han, S.; Wu, W.; Li, Z.; He, Z.; Liu, C.; Xue, H.; Zhou, F.; Liu, W.; Liu, J. Dose-effect relationship of copolymer on enhancing aqueous lubrication of a hybrid osteoarthritis drug delivery nanocarrier. J. Colloid Interface Sci. 2025, 679, 788–797. [Google Scholar] [CrossRef]
- Wu, S.; Nan, F.; Zhang, K.; Hao, W.; Shi, D.; Li, Y.; Deng, W.; Jarhen, N.; Li, K.; Xiao, Y.; et al. A novel metal-organic framework encapsulated iridium oxide nanozyme enhanced antisense oligonucleotide combo for osteoarthritis synergistic therapy. Aggregate 2024, 5, e635. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, J.; Yao, Y.; Huang, L.; Zheng, L.; Zhao, J. Mitochondrial-targeting Mn3O4/UIO-TPP nanozyme scavenge ROS to restore mitochondrial function for osteoarthritis therapy. Regen. Biomater. 2023, 10, rbad078. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Sun, W.; Lin, J.; Fan, C.; Wang, C.; Zhang, Z.; Wang, Y.; Tang, Y.; Lin, Y.; Zhou, D. Using Cu-Based Metal-Organic Framework as a Comprehensive and Powerful Antioxidant Nanozyme for Efficient Osteoarthritis Treatment. Adv. Sci. 2024, 11, e2307798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, B.; Tan, Y.; Su, J.; Wang, Y.; Xu, J.; Tao, L.; Zhou, H.; Liu, L.; Li, X. Sinomenine inhibits osteolysis in breast cancer by reducing IL-8/CXCR1 and c-Fos/NFATc1 signaling. Pharmacol. Res. 2019, 142, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Liang, Y.J.; Lian, C.; Peng, F.L.; Xiao, Y.S.; He, Y.F.; Ma, C.X.; Wang, Y.; Zhang, P.Y.; Deng, Y.H.; et al. CST6 protein and peptides inhibit breast cancer bone metastasis by suppressing CTSB activity and osteoclastogenesis. Theranostics 2021, 11, 9821–9832. [Google Scholar] [CrossRef]
- Pang, Y.C.; Fu, Y.; Li, C.; Wu, Z.X.; Cao, W.C.; Hu, X.; Sun, X.C.; He, W.X.; Cao, X.K.; Ling, D.S.; et al. Metal-Organic Framework Nanoparticles for Ameliorating Breast Cancer-Associated Osteolysis. Nano Lett. 2020, 20, 829–840. [Google Scholar] [CrossRef]
- Ting, G.; Zhang, W.; Fei, G.; Zhu, L.; Ping, S.; Li, W.; Lin, G.; Wan, D.; Tao, Y.; Kai, Y. A bone-targeting drug delivery vehicle of a metal-organic framework conjugate with zoledronate combined with photothermal therapy for tumor inhibition in cancer bone metastasis. Biomater. Sci. 2022, 10, 1831–1843. [Google Scholar] [CrossRef]
- Zou, B.; Xiong, Z.; He, L.; Chen, T. Reversing breast cancer bone metastasis by metal organic framework-capped nanotherapeutics via suppressing osteoclastogenesis. Biomaterials 2022, 285, 121549. [Google Scholar] [CrossRef]
- Chen, W.Q.; Zheng, R.S.; Baade, P.D.; Zhang, S.W.; Zeng, H.M.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer Statistics in China, 2015. CA-A Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Zeng, W.; Wan, R.; Zheng, Y.H.; Singh, S.R.; Wei, Y.Y. Hypoxia, stem cells and bone tumor. Cancer Lett. 2011, 313, 129–136. [Google Scholar] [CrossRef]
- Du, C.; Zhou, M.; Jia, F.; Ruan, L.; Lu, H.; Zhang, J.; Zhu, B.; Liu, X.; Chen, J.; Chai, Z.; et al. D-arginine-loaded metal-organic frameworks nanoparticles sensitize osteosarcoma to radiotherapy. Biomaterials 2021, 269, 120642. [Google Scholar] [CrossRef]
- Wang, Y.; Williams, G.R.; Zheng, Y.; Guo, H.; Chen, S.; Ren, R.; Wang, T.; Xia, J.; Zhu, L.-M. Polydopamine-cloaked Fe-based metal organic frameworks enable synergistic multidimensional treatment of osteosarcoma. J. Colloid Interface Sci. 2023, 651, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.-X.; Zhang, T.-W.; Zhou, L.; Ding, W.; Liang, H.-F.; Hu, Z.-C.; Chen, Q.; Dong, J.; Xue, F.-F.; Yin, X.-F.; et al. Enhancement of anti-PD-1/PD-L1 immunotherapy for osteosarcoma using an intelligent autophagy-controlling metal organic framework. Biomaterials 2022, 282, 121407. [Google Scholar] [CrossRef]
- Zeng, Y.; Yuan, J.; Ran, Z.; Zhan, X.; Li, X.; Ye, H.; Dong, J.; Cao, G.; Pan, Z.; Bao, Y.; et al. Chitosan/NH2-MIL-125 (Ti) scaffold loaded with doxorubicin for postoperative bone tumor clearance and osteogenesis: An in vitro study. Int. J. Biol. Macromol. 2024, 263, 130368. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Ma, B.; Li, B.; Huan, Z.; Ma, N.; Zhu, H.; Chang, J.; Xiao, Y.; Wu, C. 3D printing of metal-organic framework nanosheets-structured scaffolds with tumor therapy and bone construction. Biofabrication 2020, 12, 025005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-j.; Chen, Q.-l.; Zhang, S.-f.; Song, K.; Han, Z.-t.; Liu, W.-b.; Zhang, X.-s.; Dong, S.h.; Hu, W.-h.; Han, Z.c.; et al. A multifunctional biomimetic nanoplatform for image-guideded photothermal-ferroptotic synergistic osteosarcoma therapy. Bioact. Mater. 2024, 36, 157–167. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Feng, W.; Feng, H.; Ding, Z.; Zhao, Q.; Li, X.; Tang, N.; Zhang, P.; Li, J.; et al. A Targeted Erythrocyte Membrane-Encapsulated Drug-Delivery System with Anti-osteosarcoma and Anti-osteolytic Effects. ACS Appl. Mater. Interfaces 2021, 13, 27920–27933. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, itc17–itc32. [Google Scholar] [CrossRef]
- Gao, Y.G.; Chen, N.; Fu, Z.D.; Zhang, Q. Progress of Wnt Signaling Pathway in Osteoporosis. Biomolecules 2023, 13, 483. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef]
- Matlinska, M.A.; Ha, M.; Hughton, B.; Oliynyk, A.O.; Iyer, A.K.; Bernard, G.M.; Lambkin, G.; Lawrence, M.C.; Katz, M.J.; Mar, A.; et al. Alkaline Earth Metal-Organic Frameworks with Tailorable Ion Release: A Path for Supporting Biomineralization. ACS Appl. Mater. Interfaces 2019, 11, 32739–32745. [Google Scholar] [CrossRef]
- Vassaki, M.; Papathanasiou, K.E.; Hadjicharalambous, C.; Chandrinou, D.; Turhanen, P.; Choquesillo-Lazarte, D.; Demadis, K.D. Self-sacrificial MOFs for ultra-long controlled release of bisphosphonate anti-osteoporotic drugs. Chem. Commun. 2020, 56, 5166–5169. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Luo, C.Z.; Xie, D.H.; Hu, J.J.; Chen, J.X.; Huang, N.H.; Wang, H.; Zhang, S.Q.; Zhang, Q. Convenient synthesis of zwitterionic calcium(II)-carboxylate metal organic frameworks with efficient activities for the treatment of osteoporosis. Int. J. Pharm. 2021, 608, 121083. [Google Scholar] [CrossRef]
- Riegger, J.; Schoppa, A.; Ruths, L.; Haffner-Luntzer, M.; Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell. Mol. Biol. Lett. 2023, 28, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, D.; Li, M.; He, Y.; He, T.; Chen, M.; Hu, Y.; Luo, Z.; Cai, K. Nanocatalytic Biofunctional MOF Coating on Titanium Implants Promotes Osteoporotic Bone Regeneration through Cooperative Pro-osteoblastogenesis MSC Reprogramming. ACS Nano 2022, 16, 15397–15412. [Google Scholar] [CrossRef]
- Lin, W.; Hu, S.; Li, K.; Shi, Y.; Pan, C.; Xu, Z.; Li, D.; Wang, H.; Li, B.; Chen, H. Breaking Osteoclast-Acid Vicious Cycle to Rescue Osteoporosis via an Acid Responsive Organic Framework-Based Neutralizing and Gene Editing Platform. Small 2024, 20, e2307595. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Susin, C. Periodontitis epidemiology: Is periodontitis under-recognized, over-diagnosed, or both? Periodontology 2000 2017, 75, 45–51. [Google Scholar] [CrossRef]
- Van Holm, W.; Carvalho, R.; Delanghe, L.; Eilers, T.; Zayed, N.; Mermans, F.; Bernaerts, K.; Boon, N.; Claes, I.; Lebeer, S.; et al. Antimicrobial potential of known and novel probiotics on in vitro periodontitis biofilms. npj Biofilms Microbiomes 2023, 9, 3. [Google Scholar] [CrossRef]
- Ejeian, F.; Razmjou, A.; Nasr-Esfahani, M.H.; Mohammad, M.; Karamali, F.; Warkiani, M.E.; Asadnia, M.; Chen, V. ZIF-8 Modified Polypropylene Membrane: A Biomimetic Cell Culture Platform with a View to the Improvement of Guided Bone Regeneration. Int. J. Nanomed. 2020, 15, 10029–10043. [Google Scholar] [CrossRef]
- Li, N.; Xie, L.; Wu, Y.; Wu, Y.; Liu, Y.; Gao, Y.; Yang, J.; Zhang, X.; Jiang, L. Dexamethasone-loaded zeolitic imidazolate frameworks nanocomposite hydrogel with antibacterial and anti-inflammatory effects for periodontitis treatment. Mater. Today Bio 2022, 16, 100360. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, Y.; Xue, H.; Yu, R.; Jin, X.; Wu, X.; Huang, H. Reprogramming mitochondrial metabolism of macrophages by miRNA-released microporous coatings to prevent peri-implantitis. J. Nanobiotechnol. 2023, 21, 485. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, Y.; Ji, C.; Zhu, H.; Lao, A.; Zhao, R.; Hu, Y.; Zhou, Y.; Zhou, J.; Lin, K.; et al. A five-in-one novel MOF-modified injectable hydrogel with thermo-sensitive and adhesive properties for promoting alveolar bone repair in periodontitis: Antibacterial, hemostasis, immune reprogramming, pro-osteo-/angiogenesis and recruitment. Bioact. Mater. 2024, 41, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.; Yao, Y.; Tang, C.; Wang, Y.; Yuan, Q.; Peng, L. Bifunctional mesoporous HMUiO-66-NH2 nanoparticles for bone remodeling and ROS scavenging in periodontitis therapy. Biomaterials 2025, 314, 122872. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yang, Y.; Ho, C.; Li, Z.; Chiu, W.; Li, A.; Dai, Y.; Li, W.; Zhang, X. Dynamic hydrogel-metal-organic framework system promotes bone regeneration in periodontitis through controlled drug delivery. J. Nanobiotechnol. 2024, 22, 287. [Google Scholar] [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef]
- Wang, H.; Mu, N.; He, Y.; Zhang, X.; Lei, J.; Yang, C.; Ma, L.; Gao, Y. Ultrasound-controlled MXene-based Schottky heterojunction improves anti-infection and osteogenesis properties. Theranostics 2023, 13, 1669–1683. [Google Scholar] [CrossRef]
- Song, Y.C.; Li, H.M.; Yuan, Y.; Zhang, D.; Wang, Z.; Qi, B.W.; Jiang, P.; Yu, A.X. Synergistic photothermal-sonodynamic therapy for antibacterial and immune reprogramming in chronic osteomyelitis. J. Control. Release 2025, 381, 113612. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, L.; Li, Z.; Liu, X.; Zheng, Y.; Feng, X.; Liang, Y.; Cui, Z.; Zhu, S.; Wu, S. Single-Atom Catalysis for Efficient Sonodynamic Therapy of Methicillin-Resistant Staphylococcus aureus-Infected Osteomyelitis. ACS Nano 2021, 15, 10628–10639. [Google Scholar] [CrossRef]
- Feng, X.; Ma, L.; Lei, J.; Ouyang, Q.; Zeng, Y.; Luo, Y.; Zhang, X.; Song, Y.; Li, G.; Tan, L.; et al. Piezo-Augmented Sonosensitizer with Strong Ultrasound-Propelling Ability for Efficient Treatment of Osteomyelitis. ACS Nano 2022, 16, 2546–2557. [Google Scholar] [CrossRef]
- Zheng, Q.Y.; Liu, X.M.; Gao, S.; Cui, Z.D.; Wu, S.L.; Liang, Y.Q.; Li, Z.Y.; Zheng, Y.F.; Zhu, S.L.; Jiang, H.; et al. Engineering Dynamic Defect of CeIII/CeIV-Based Metal-Organic Framework through Ultrasound-Triggered Au Electron Trapper for Sonodynamic Therapy of Osteomyelitis. Small 2023, 19, e2207687. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Wu, S.L.; Zheng, Y.F.; Wang, C.F.; Li, Z.Y.; Zhang, Y.; Zhu, S.L.; Jiang, H.; Cui, Z.D.; Liu, X.M. Enhancing Microwave Dynamic Effects via Surface States of Ultrasmall 2D MOF Triggered by Interface Confinement for Antibiotics-Free Therapy. Adv. Sci. 2023, 10, e2300084. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, F.; Ebrahem, N.E.; Abdelhamid, H.N. A comparative study of the toxic effect of ZIF-8 and ZIF-L on the colonization and decomposition of shaded outdoor mice carrions by arthropods. Sci. Rep. 2022, 12, 14240. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.Q.; Zheng, X.H.; Li, Z.S.; Xie, Z.G. Nanoscale Fluorescent Metal-Organic Framework@Microporous Organic Polymer Composites for Enhanced Intracellular Uptake and Bioimaging. Chem.-A Eur. J. 2017, 23, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, K.Y.A.; Chen, K.F.; Jiang, X.Y.; Lin, C.H. In vitro renal toxicity evaluation of copper-based metaleorganic framework HKUST-1 on human embryonic kidney cells. Environ. Pollut. 2021, 273, 116528. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).