Abstract
Currently, the rising prevalence of resistant Candida species, particularly Candida albicans, as well as non-albicans isolates such as Candida glabrata and Candida krusei, represent challenges in their management. In this review, we aimed to explore the current management of fluconazole-resistant vulvovaginal candidiasis (FRVVC). Identified studies focused on alternative antifungal therapies, including boric acid, nystatin, and newer agents like oteseconazole and ibrexafungerp. The findings highlight the need for tailored treatment regimens, considering the variability in resistance patterns across regions. Unprofessional as well as professional overuse of antifungals for vulvovaginal symptoms that are not caused by Candida infections should be combatted and banned as much as possible. Instead of high-dose maintenance regimens using weekly doses of 150 to 200 mg of fluconazole for 6 months or longer, it is advisable to use an individualised degressive regimen (ReCiDiF regimen) in order to tailor the treatment of a particular patient to the lowest dosage possible to keep the diseases controlled. Additionally, this report underscores the impact of antibiotic use on the microbiota, which can raise the possibility of VVC and lead to fluconazole resistance, emphasizing the necessity for cautious antibiotic prescribing practices.
1. Introduction
Vulvovaginal candidiasis (VVC) is a commonly encountered disease that affects about three out of four women during their lifetime [1,2,3]. A subset of these patients has recurrent episodes of this condition termed recurrent vulvovaginal candidiasis (RVVC). The condition is diagnosed by the presentation of a minimum of three to four episodes of VVC in a single year, impacting approximately 5–8% of women in their reproductive years [1,2,3]. The primary pathogen responsible for VVC is Candida albicans, which accounts for more than 90% of cases, although non-albicans Candida (NAC) species are increasingly recognized as contributors to these infections [1,2,3,4,5]. Some contraceptives, pregnancy, use of poorly ventilated clothing, HIV infection, immunosuppressive agents, diabetes mellitus, antibiotic use, atopic disease, and genetic predisposition are common factors that promote RVVC [6,7]. According to some, even bacterial vaginosis (BV), oral sex, and the use of soap are predisposing factors, but the list is long and the evidence is scarce [8,9].
Among antifungal treatments, fluconazole is commonly utilized due to its effectiveness and ease of use. Nevertheless, the rising frequency of fluconazole-resistant C. albicans (FRCA) presents a growing obstacle to successful treatment [5,10,11,12]. Historically, fluconazole resistance in C. albicans was deemed uncommon, representing less than 5% of isolates with RVVC [13,14]; however, there has been a notable increase in recent years [15,16]. Several mechanisms have been implicated in fluconazole-resistant VVC (FRVVC), including altered ergosterol synthesis, altered sterol and azole import, the overexpression of membrane transporters, as well as genomic and chromosomic variations reducing susceptibility to therapy [17].
Research indicates a growing incidence of FRCA among women with RVVC, particularly those with significant prior exposure to fluconazole [1,2,3]. This increase is likely linked to the extensive usage of fluconazole for both acute and maintenance treatment of VVC, which exerts selective pressure favouring resistant strains [1,2,3]. The median minimum inhibitory concentration (MIC) of fluconazole for resistant isolates varies considerably, often requiring the consideration of alternative therapeutic approaches [1,2,3,4,5].
The inherent fluconazole resistance among NAC species has also become a growing issue, especially as these species are being increasingly identified in VVC cases [10,11,15,16,18,19]. C. albicans was traditionally recognized as the primary cause of VVC; NAC species were once considered responsible for a smaller fraction of cases [20,21,22]. In recent years, however, clinicians have encountered a notable prevalence of NAC isolates, such as C. glabrata (Nakaseomyces glabrata) and C. krusei (Pichia kudriavzevii) [23], in some particular areas, with some studies indicating their involvement in up to half of RVVC cases [7,24,25]. Similarly to FRCA, the increasing prevalence of fluconazole-resistant NAC species is closely linked to the widespread use of fluconazole [7,24,25]. This extensive use has contributed to fluconazole resistance, particularly for C. glabrata and C. krusei, which are inherently less susceptible to azoles or exhibit cross-resistance to other azole drugs [7,24,25].
This resistance complicates treatment strategies, as these species often require alternative antifungal therapies that are less commonly used and may have more side effects. Managing VVC that is resistant to fluconazole necessitates the use of alternative antifungal treatments, given the limited effectiveness of standard therapies [7,24,25]. Treatment options include (a) boric acid, which may accomplish high clinical and mycological cure rates and offers symptomatic relief as well as helps reduce recurrence rates; (b) nystatin, which may serve as a viable alternative, particularly for NAC infections; (c) a switch to vaginal capsules or creams containing antifungal agents, sometimes in combination, which can provide targeted treatment with more favourable safety profiles than oral formulations; (d) topical antifungals like itraconazole and ketoconazole, which require careful monitoring for hepatic toxicity and other adverse effects when used orally, thereby rendering them less suitable for prolonged use; (e) augmentation of local fungicidal effect with potentiators like ibuprofen or domiphen bromide, (f) newer agents, such as oteseconazole or ibrexafungerp, which may provide more effective management and (g) combination therapies; e.g., an azole combined with a non-azole therapy [1,2,3,4,25,26,27,28].
We hereby expand on previous findings and guidelines regarding the management of VVC, particularly fluconazole-resistant infections [7]. This study, therefore, seeks to investigate the recent literature to evaluate treatment options and clinical outcomes for patients with FRVVC. Finally, we end with discussing some essential preventive measures to dam the antifungal resistance epidemic.
2. Materials and Methods
We performed extensive searches in PubMed, Medline, and Cochrane databases from 1 January 2000 to 1 September 2024. The search strategy included the following parameters: Vulvovaginal Candidosis OR Vulvovaginal Candidiasis OR Fluconazole Resistant OR Recurrent Vulvovaginal Candidiasis OR Fluconazole Refractory AND Therapy AND Outcomes. A total of 933 studies were selected for further examination. After a closer examination of these 933 articles, 826 were excluded as they were out of scope (unrelated topic or duplicates). Only English-language studies were examined further. Moreover, 26 articles were reviewed for final-stage selection after 81 studies were excluded due to language criteria or work unrelated to VVC or non-clinical articles. Ultimately, 16 articles on the management of fluconazole-resistant clinical cases were specifically included in this work. Only articles since 2002 were included to account for the evolution of clinical practice when managing recurrent VVC. Three independent researchers participated in this selection process. An illustration of included studies is visualized in Figure 1.
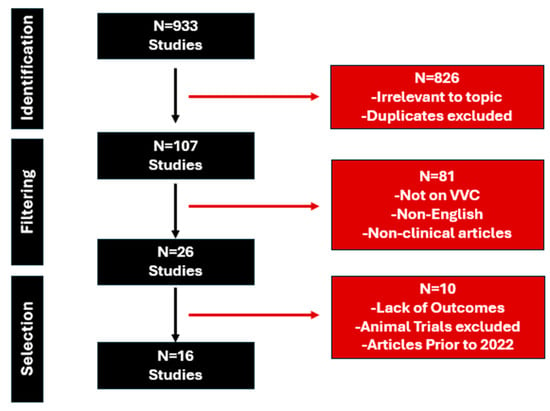
Figure 1.
Diagram of included studies.
3. Results
Fluconazole resistance was defined in all studies through in vitro analysis. The overall most widely used methodology was in vitro antifungal susceptibility testing of different variations (n = 10). A representative sample (n = 8) of recent epidemiological studies on the frequency of fluconazole-resistant VVC is presented in Table 1. As fluconazole resistance would vary significantly for older publications, only articles published in the last decade were included from different countries/regions to better capture the global epidemiological footprint of fluconazole resistance in VVC patients. Data from prevalence studies highlight the variability in fluconazole resistance among different Candida species and across various regions. C. albicans was the most prevalent species in all the regions studied, with its prevalence ranging from 32.4% in China to 88.2% in Iran (Table 1). Resistance rates for C. albicans vary significantly, with the lowest being approximately 2% in Ethiopia and the UK and no resistance detected in Iran [26,27]. However, in the USA, C. albicans showed a resistance rate of 23% at a neutral pH of 7.0, which increased to 52% at a lower pH of 4.5, indicating that conditions significantly influence resistance (Table 1).
C. glabrata was the second most common species in several regions, with a notable prevalence of 43% in Turkey, 13.6% in Greece, and 6.8% in the UK (Table 1). C. krusei consistently showed high resistance across the studies, with a 100% resistance rate reported in both Turkey and Ethiopia [26,29]. The data underscore the importance of regional surveillance, as resistance patterns differ, necessitating tailored antifungal strategies depending on the specific Candida species and local resistance profiles.

Table 1.
Representative studies on the prevalence of fluconazole-resistant VVC.
Table 1.
Representative studies on the prevalence of fluconazole-resistant VVC.
| Author | Year | Origin | Study Design | Candida Species | Diagnosis Method | Prevalence of Resistance |
|---|---|---|---|---|---|---|
| J. D. Sobel [30] | 2023 | USA | Longitudinal, Observational study over a 10-year period | C. albicans was the dominant species (76.3% of positive yeast isolates) | Antibiotic susceptibility tests in line with guidelines MICs for pH 7.0 and 4.5 | pH 7.0: 23% of isolates were resistant (MIC ≥ 8 mg/mL) pH 4.5: resistance rates were 52% of the isolates |
| S. Maraki [31] | 2019 | Greece | 6-year Observational study | C. albicans (75.6%) C. glabrata (13.6%) | Isolation on Sabouraud dextrose agar, identification using VITEK card. | Overall resistance rates: 6.6% to fluconazole |
| D.N. Anh [32] | 2021 | Vietnam | Cross-sectional study | C. albicans (51.37%) C. parapsilosis (25.88%) C. glabrata (11.37%) C.tropicalis (4.31%) C. krusei (3.92%) C. africana (1.57%) S. cerevisiae (0.78%) C. nivariensis (0.39%) C. lusitaniae (0.39%) | Direct microscopic examination (10% KOH) Species identification was performed using morphological tests, PCR, and sequencing | Resistance rate to fluconazole was 4.35% |
| W. Wang [19] | 2024 | China | Retrospective Observational study | C. albicans (32.40%) C. tropicalis (17.80%) C. glabrata (13.70%) C. parapsilosis (8.63%) | Antifungal susceptibility testing using ATB® FUNGUS 3. | C.albicans exhibited a fluconazole resistance rate of 5.2%. C. tropicalis showed significant resistance to fluconazole of 38.3% |
| A. Bitew and Y. Abebaw [26] | 2018 | Ethiopia | Cross-sectional study | C. albicans (58.6%) C. krusei (17.2%) C. dubliniensis (9.2%) Other included C. glabrata, C. tropicalis | Susceptibility testing using VITEK system | Highest resistance was observed against fluconazole (17.2% overall). C. krusei: 100% resistance to fluconazole. C. albicans: 2% resistance rate to both fluconazole |
| A. Rezaei-Matehkolae [27] | 2016 | Iran | Cross-sectional study | C. albicans (88.2%) C. glabrata (8.8%) C. kefyr (2.9%) | Classical mycological tests, PCR-RFLP method for molecular identification | Resistance was not detected among the isolates for fluconazole |
| F.G. Hösükoğlu [29] | 2022 | Turkey | Observational study | C. albicans (47%) C. glabrata (43%) C. kefyr (5%) C. krusei (2%) C. tropicalis (2%) C. guilliermondii (1%) | Antifungal susceptibility of these isolates using the reference broth microdilution method as per CLSI guidelines | C. albicans: 21.3% resistant C. krusei: 100% resistant (intrinsic resistance) |
| Ratner JC [33] | 2024 | UK | Retrospective Observational study | C. albicans: 87.4% N. glabrata: 6.8% P. kudriavzevii: 0.55% C. dubliniensis: 1.64% Meyerozyma guilliermondii: 0.27% Clavispora lusitaniae: 0.82% C. parapsilosis: 2.19% C.tropicalis: 0.27% | Fluconazole resistance assessed using the disc diffusion method and Sensititre YeastOne assay as per CLSI | Resistant species C. albicans:
Nakaseomyces glabrata [Candida glabrata]:
Pichia kudriavzevii [Candida krusei]:
|
The included studies on the management of fluconazole resistance in Candida infections reveal a comprehensive investigation into various Candida species, with C.albicans being included in all studies but 1, while the most identified NAC species like C.glabrata was present in 16 studies (Table 2). Resistance was predominantly determined through in vitro antifungal susceptibility testing (six studies), broth microdilution (three studies), E-test methods (one study), clinical symptoms (two studies), and the use of a Candifast kit (one study) [1,2,18,19,20,21,22,24,33,34,35,36,37,38,39,40]. Notably, all studies but one included a fluconazole arm in their study design. Following resistance diagnosis, treatments varied widely, including the use of boric acid, ketoconazole, itraconazole, otesoconazole, voriconazole, nystatin, amphotericin B, flucytosine, and ibrexafungerp.

Table 2.
Representative studies on the management of fluconazole-resistant VVC.
When ranking the effectiveness of various treatments for fluconazole-resistant Candida infections, O-oteseconazole stands out as the most effective, with a weighted overall success rate of 88% and a 71% clinical cure rate [19,38]. Boric acid follows with a weighted success rate of 77%, making it a strong treatment option for fluconazole-resistant cases [2,18,34,36,37,40,41]. Voriconazole comes next with a 73% clinical cure rate [7]. Ibrexafungerp also shows effectiveness, with a 70% clinical cure rate by day 25 [34]. Finally, nystatin offers a mycological cure rate of 56% in fluconazole-resistant cases [1]. The worst-performing studies in terms of treatment failures include a broad range of Candida species, particularly NAC species like C. glabrata and C. krusei, which are known for their resistance to common antifungal treatments [7,25]. Richter SS et al. had the highest failure rate at 51.4%, particularly struggling with C. glabrata [22]. Fan S et al. further illustrate the treatment difficulties of fluconazole-resistant isolates, where nearly half of the cases failed to respond to nystatin [1]. These studies underscore the necessity of utilizing multiple antifungal agents and tailored treatment regimens to effectively manage resistant cases.
4. Discussion
We aimed to explore the current literature pertaining to FRVVC and its management. Data from prevalence studies highlighted the variability in fluconazole resistance among different Candida species and across various regions. Following resistance diagnosis, treatments varied widely, including the use of boric acid, ketoconazole, itraconazole, otesoconazole, voriconazole, nystatin, amphotericin B, flucytosine, and ibrexafungerp. Otesoconazole and boric acid proved to be the more effective, followed by voriconazole, ibrexafungerp, and nystatin.
In cases of fluconazole-resistant VVC (FRVVC), alternative treatment plans, such as a degressive dosing regimen, are currently being explored [7,10,25]. This approach starts with a standard fluconazole dose and gradually reduces the frequency of administration over time [7,10,25]. The objective is to control Candida overgrowth and prevent recurrences by maintaining sufficient antifungal levels while minimizing the risk of drug resistance and side effects from long-term, high-dose therapy [7,10,25]. This strategy aims to manage and prevent recurrent infections while reducing the overall antifungal load. The approach to managing RVVC after a maintenance regimen depends on the frequency of recurrences. If recurrences are infrequent, each episode can be treated on a case-by-case basis. However, if frequent recurrences return, it is advised to reinitiate, extend, or modify the induction and maintenance regimens to better control the condition [7,25,34]. In clinical settings, experts have noted that treatment durations typically may last up to half a year, although approximately 60% of women relapse after discontinuing maintenance treatment [14,42,43]. In more challenging cases, maintenance treatment may extend beyond 6 months, sometimes continuing for years, which is not unusual in clinical practice for severe RVVC cases [37,38,41]. Within the ReCiDiF regimen, the dosing schedule is progressively reduced as symptoms come under control, with the regimen usually lasting for a year for patients who respond well. These patients can stop treatment after this period [10,14,42,43]. For those considered “suboptimal responders,” the regimen may require further adjustment and an extended duration [10,14,42,43]. In some cases, patients may need to continue taking one tablet per month or every two weeks, depending on how frequently they relapse and how well they respond during the treatment course [10,14,42,43]. As a primary measure, there is a proper diagnosis prior to treatment that determines whether this is professionally installed or driven by peer or pharmacist advice without examination, which is imperative in order to diminish the improper and frequent use of antifungals for reasons that may not be Candida infection. It is known that patients very often misdiagnose themselves based on their assumption of what causes their vulvovaginal discomfort [44].
Topical treatments, including vaginal creams with azole antifungals, are frequently used in managing VVC, especially FRVVC [7,10,25]. These creams deliver medication directly to the infection site, achieving higher local drug concentrations while minimizing systemic side effects [7,10,25]. This approach is especially beneficial in cases where oral antifungals, such as fluconazole, have failed due to resistance [7,10,25]. Clotrimazole, miconazole, terconazole, and boric acid are recommended for recurrent VVC caused by both C. albicans and NAC species [45]. Boric acid has shown high cure rates and low short-term recurrence rates in FRCA infections [2]. Topical therapy, except for boric acid due to teratogenic effects [46], is preferred for pregnant women, breastfeeding mothers, and those with potential drug interactions or previous adverse effects from oral azoles [47]. The prolonged contact with the affected area enhances the potential for eradicating resistant strains, particularly when combined with additional therapies like boric acid or probiotics [7,10,25]. This multi-faceted treatment aims to counter various risk factors for recurrence, including the intestinal Candida reservoir, mycotic biorhythm, biofilm formation, and the presence of non-albicans species or G. vaginalis [48]. The use of topical antifungals can also be potentiated by the addition of adjuvant substances, such as domiphen bromide or low doses of ibuprofen [28,49,50] (ref). In humans, a combination of different doses of domiphen bromide with miconazole in a vaginal cream was recently tested in a phase 1 trial by our group, resulting in promising amelioration of the latter’s effect on the candida vulvovaginitis, as compared with miconazole cream with the additional domiphen bromide.
Probiotics have gained attention as a complementary treatment aimed at restoring and maintaining a balanced vaginal microbiome [7,25]. In particular, those containing Lactobacillus species have been investigated as both a preventive and adjunctive treatment for VVC [7,10,25]. These beneficial bacteria help sustain an acidic vaginal environment and compete with Candida species, potentially lowering the infection risk [7,10,25]. Although evidence from randomized controlled trials is still evolving, the use of probiotics alongside antifungal therapy has shown the potential to decrease the recurrence of VVC by strengthening the vaginal ecosystem’s natural defences [7,25]. However, current evidence is not yet robust enough to be universally included in clinical guidelines, and additional research is necessary to better define the role of probiotics in preventing and treating FRVVC [7,10,25,51].
The use of systemic antibiotics has been associated with disruptions in the vaginal microbiome, increasing the risk of VVC, particularly in women who experience recurrent infections [7,10,25]. Moreover, we are now aware of the rising levels of azole antifungal resistance, as shown by in vitro testing of Candida in women with complicated RVVC [33]. However, we need more information on how this affects patient management, as treating these infections is challenging. The exact causes of this increase are still uncertain, but it appears to be linked to a policy shift that promoted the empirical treatment of VVC based on symptoms and signs or over-the-counter drugs rather than prescriptions following wet mount microscopy or yeast cultures or seeking medical care. Empirical treatment of VVC should be discouraged to prevent further growth of FRCA and fluconazole-resistant NAC species. The clinical management of fluconazole-resistant VVC encompasses not only the identification of suitable antifungal therapies but also the education of patients and their adherence to treatment protocols [31,32,52]. Regular follow-up is crucial for assessing treatment efficacy and addressing potential side effects. Furthermore, healthcare providers should stress the importance of avoiding unnecessary antifungal use to mitigate the risk of developing resistance [29,34,35,53].
In the era of rising antifungal resistance, with an increased need for safe regimens with minor adverse effects, minimal drug–drug interactions, and longer half-times that allow less frequent administration and outpatient care, new agents in currently used classes of antifungals and new compounds with novel mechanisms of action have now been developed. A number of those have already been approved by the European Medical Agency and the U.S. Food and Drug Administration for the treatment of VVC, including oteseconazole and ibrexafungerp. In phase III studies by Martens et al. and Sobel/Donders et al., oteseconazole showed very promising results [38,54], leading to a recurrence rate as low as 4 to 5% in cases of FRVVC. Also, in comparison with fluconazole, clinical and mycological cure rates were higher in the oteseconazole arms (71.3 and 82.5% with oteseconazole versus 56.0 and 59.1 in the fluconazole arm, respectively) [19]. In the same context, the need for rescue medication and clinical cure rates on day 25 favored ibrexafungerp in a recent randomized clinical trial by Nyirjesy et al. [34].
It would be interesting to explore how other new agents, now tested for invasive fungal infection, would perform in mucocutaneous disease, including VVC. Fosmanogepix is a novel Gwt1 inhibitor [55] that blocks inositol acylation during GPI-anchored protein biosynthesis and offers an oral availability of over 90% beyond its intravenous formulation. It is active against many pathogenic molds and yeast, including most Candida species, including strains resistant to echinocandins and triazoles [56]. However, Candida krusei and related species exhibit intrinsic resistance to fosmanogepix [57]. In phase II trials for candidemia, fosmanogepix was shown to be safe and well-tolerated, achieving an 80% treatment success rate and an 85% survival rate at day 30 [58,59]. Currently, a phase III clinical trial is evaluating the efficacy and safety of fosmanogepix for candidemia and invasive candidiasis. Rezafungin is a new long-acting echinocandin, enabling once-weekly intravenous administration with front-loaded dosing. It has shown in vitro efficacy against several Candida species while two recent trials, a phase II (STRIVE) and a phase III (ReSTORE) study, compared rezafungin to caspofungin for treating candidemia and invasive candidiasis [60,61,62,63]. Rezafungin proved non-inferior to caspofungin, meeting the EMA’s global cure endpoint on day 14 and the FDA’s all-cause mortality endpoint on day 30, leading to its approval for invasive candidiasis [60]. Of note, its efficacy remained consistent across various Candida species, including fluconazole-resistant and FKS mutant strains, regardless of MIC values [64]. Lastly, ATI-2307 (or T-2307) is an investigational antifungal agent classified as an aromatic diamidine. It works by inhibiting respiratory chain complexes III and IV, disrupting the mitochondrial membrane potential in yeast cells [65]. This drug shows broad-spectrum activity, targeting Candida species, including strains resistant to echinocandins and azoles [66,67]. Though still in the early stages of development or study, so far, available data on all these regimens have shown promising outcomes, so one can wonder whether their use can expand in FRVVC.
Limitations
Our study suffers a number of limitations. Although FRCA or fluconazole-resistant NAC isolates were noted in the studies explored, it remains unknown whether fungi were acting as colonizers or actual pathogens. In this context, C. glabrata has been identified as a non-pathogenic saprophyte, especially among people living with HIV or diabetes and those who are immunocompromised [68]. In the majority of studies, no data are provided regarding microscopic examination that could identify the presence of pseudomycelia; hence, the presence of an infection was recorded based on culture data only. Similarly, no data on repeated or confirmative testing or compliance with guidelines [42,69,70] on the diagnosis of RVVC was noted, leading to overdiagnosis or misinterpretation. Also, in the studies where MIC was measured, we have to keep in mind that fluconazole resistance through in vitro susceptibility testing does not always correlate with phenotypic resistance, unlike in antibiotic resistance testing of bacteria. The trailing growth phenomenon that Candida exhibits due to the diversity of resistance mechanisms and its ability of biofilm formation prevents directly translating in vitro susceptibility to in vivo efficacy [71,72]. On the other hand, negative cultures may not always reflect active disease but rather recent treatment, bad swab quality, the presence of growth inhibitors, or missed sample location. Although our review spans the last 25 years of practice, the non-inclusion of English-language papers and databases other than PubMed, Medline, and Cochrane may have led to important experiences being overlooked. Lastly, at the time of the publication of this review, it is possible that the rapidly evolving field of antifungal therapeutics has developed more regimens than discussed herein [73].
5. Conclusions
The management of VVC, particularly in women prone to frequent relapses, remains a significant challenge despite the efficacy of current antifungal treatments like fluconazole and itraconazole. While these therapies can alleviate acute infections, a substantial proportion of patients experience only temporary symptom relief, necessitating long-term maintenance strategies to prevent recurrent episodes. However, even with carefully monitored regimens, a significant number of women continue to suffer from multiple recurrences, highlighting the limitations of existing approaches.
The rising prevalence of FRVVC further complicates this issue, underscoring the urgent need for alternative therapeutic strategies and robust antifungal stewardship. Clinicians must adopt a comprehensive approach that balances effective treatment with efforts to reduce the emergence of antifungal resistance. This includes prioritizing topical therapies whenever feasible, optimizing treatment regimens to preserve the natural vaginal flora, and minimizing the unnecessary use of systemic antifungal medications. A healthy vaginal microbiome and restored immune function are crucial in preventing recurrences and maintaining long-term vaginal health.
Emerging treatments, such as newer antifungal agents like oteseconazole and ibrexafungerp, show promise in addressing these challenges and expanding the therapeutic options available to both patients and clinicians. Additionally, the development of a vaccine currently undergoing clinical trials is a highly encouraging step forward, offering the potential for transformative progress in preventing VVC altogether. However, the success of these advancements will depend on appropriate usage guided by well-informed clinical oversight and antifungal stewardship to ensure sustainable outcomes.
Ultimately, addressing the growing issue of recurrent and resistant VVC requires an integrated strategy that combines effective treatment, patient education, systematic monitoring, and continued research into innovative therapies. By adopting a forward-looking and balanced approach, we can improve outcomes for women while safeguarding the effectiveness of antifungal treatments for future generations.
Author Contributions
K.A. (Karolina Akinosoglou) and G.G.G.D. conceived the idea; A.L., K.A. (Konstantinos Asimos) and F.D. performed the literature search; K.A. (Karolina Akinosoglou) and A.L. wrote manuscript and drew the figures and tables; F.D. and G.G.G.D. critically corrected manuscript; K.A. (Karolina Akinosoglou) and A.L. revised manuscript; K.A. (Karolina Akinosoglou) oversaw the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fan, S.; Liu, X.; Wu, C.; Xu, L.; Li, J. Vaginal nystatin versus oral fluconazole for the treatment for recurrent vulvovaginal candidiasis. Mycopathologia 2015, 179, 95–101. [Google Scholar] [CrossRef] [PubMed]
- File, B.; Sobel, R.; Becker, M.; Nyirjesy, P. Fluconazole-Resistant Candida albicans Vaginal Infections at a Referral Center and Treated with Boric Acid. J. Low. Genit. Tract. Dis. 2023, 27, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Muraglia, R.; Dietz, J.P.; Sobel, J.D.; Wagner, J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: Results from an internet panel survey. J. Low. Genit. Tract. Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Aballea, S.; Guelfucci, F.; Wagner, J.; Khemiri, A.; Dietz, J.P.; Sobel, J.; Toumi, M. Subjective health status and health-related quality of life among women with Recurrent Vulvovaginal Candidosis (RVVC) in Europe and the USA. Health Qual. Life Outcomes 2013, 11, 169. [Google Scholar] [CrossRef]
- Mendling, W.; Brasch, J. Guideline vulvovaginal candidosis (2010) of the german society for gynecology and obstetrics, the working group for infections and infectimmunology in gynecology and obstetrics, the german society of dermatology, the board of german dermatologists and the german speaking mycological society. Mycoses 2012, 55, 1–13. [Google Scholar] [CrossRef]
- Boyd Tressler, A.; Markwei, M.; Fortin, C.; Yao, M.; Procop, G.W.; Soper, D.E.; Goje, O. Risks for Recurrent Vulvovaginal Candidiasis Caused by Non-Albicans Candida Versus Candida albicans. J. Womens Health 2021, 30, 1588–1596. [Google Scholar] [CrossRef]
- Donders, G.; Sziller, I.O.; Paavonen, J.; Hay, P.; de Seta, F.; Bohbot, J.M.; Kotarski, J.; Vives, J.A.; Szabo, B.; Cepuliene, R.; et al. Management of recurrent vulvovaginal candidosis: Narrative review of the literature and European expert panel opinion. Front. Cell Infect. Microbiol. 2022, 12, 934353. [Google Scholar] [CrossRef]
- Geiger, A.M.; Foxman, B. Risk factors for vulvovaginal candidiasis: A case-control study among university students. Epidemiology 1996, 7, 182–187. [Google Scholar] [CrossRef]
- Sobel, J.D.; Vempati, Y.S. Bacterial Vaginosis and Vulvovaginal Candidiasis Pathophysiologic Interrelationship. Microorganisms 2024, 12, 108. [Google Scholar] [CrossRef]
- Donders, G.; Bellen, G.; Byttebier, G.; Verguts, L.; Hinoul, P.; Walckiers, R.; Stalpaert, M.; Vereecken, A.; Van Eldere, J. Individualized decreasing-dose maintenance fluconazole regimen for recurrent vulvovaginal candidiasis (ReCiDiF trial). Am. J. Obstet. Gynecol. 2008, 199, 613.e1–613.e9. [Google Scholar] [CrossRef]
- Sobel, J.D.; Wiesenfeld, H.C.; Martens, M.; Danna, P.; Hooton, T.M.; Rompalo, A.; Sperling, M.; Livengood, C., 3rd; Horowitz, B.; Von Thron, J.; et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004, 351, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Guimaraes, T.; Camargo, L.F.; Richtmann, R.; Queiroz-Telles, F.; Salles, M.J.; Cunha, C.A.; Yasuda, M.A.; Moretti, M.L.; Nucci, M. Brazilian guidelines for the management of candidiasis—A joint meeting report of three medical societies: Sociedade Brasileira de Infectologia, Sociedade Paulista de Infectologia and Sociedade Brasileira de Medicina Tropical. Braz. J. Infect. Dis. 2013, 17, 283–312. [Google Scholar] [CrossRef]
- Collins, L.M.; Moore, R.; Sobel, J.D. Prognosis and Long-Term Outcome of Women with Idiopathic Recurrent Vulvovaginal Candidiasis Caused by Candida albicans. J. Low. Genit. Tract. Dis. 2020, 24, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Sobel, R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert. Opin. Pharmacother. 2018, 19, 971–977. [Google Scholar] [CrossRef]
- Rosa, M.I.; Silva, B.R.; Pires, P.S.; Silva, F.R.; Silva, N.C.; Silva, F.R.; Souza, S.L.; Madeira, K.; Panatto, A.P.; Medeiros, L.R. Weekly fluconazole therapy for recurrent vulvovaginal candidiasis: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 132–136. [Google Scholar] [CrossRef]
- Watson, C.; Calabretto, H. Comprehensive review of conventional and non-conventional methods of management of recurrent vulvovaginal candidiasis. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 262–272. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Marchaim, D.; Lemanek, L.; Bheemreddy, S.; Kaye, K.S.; Sobel, J.D. Fluconazole-resistant Candida albicans vulvovaginitis. Obstet. Gynecol. 2012, 120, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Ruan, H.; Xiong, Z.; Wang, W.; Qiu, J.; Song, W.; Zhang, C.; Xue, F.; Qin, T.; et al. Oteseconazole versus fluconazole for the treatment of severe vulvovaginal candidiasis: A multicenter, randomized, double-blinded, phase 3 trial. Antimicrob. Agents Chemother. 2024, 68, e0077823. [Google Scholar] [CrossRef]
- Fan, S.R.; Liu, X.P. In vitro fluconazole and nystatin susceptibility and clinical outcome in complicated vulvovaginal candidosis. Mycoses 2011, 54, 501–505. [Google Scholar] [CrossRef]
- Maftei, N.M.; Arbune, M.; Georgescu, C.V.; Elisei, A.M.; Iancu, A.V.; Tatu, A.L. Vulvovaginal Candidiasis in Pregnancy-Between Sensitivity and Resistance to Antimycotics. J. Xenobiot. 2023, 13, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.S.; Galask, R.P.; Messer, S.A.; Hollis, R.J.; Diekema, D.J.; Pfaller, M.A. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J. Clin. Microbiol. 2005, 43, 2155–2162. [Google Scholar] [CrossRef]
- Borman, A.M.; Johnson, E.M. Name changes for fungi of medical importance, 2018 to 2019. J. Clin. Microbiol. 2021, 59, e01811. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.C.; Dean, G.; Soni, S.; Sundaram, S.; Fearnley, N.; Wilson, J.D. Outcomes and experiences of using oral voriconazole with or without concomitant topical agents to treat refractory vulvovaginal yeast infections. Int. J. STD AIDS 2022, 33, 1134–1141. [Google Scholar] [CrossRef]
- Neal, C.M.; Martens, M.G. Clinical challenges in diagnosis and treatment of recurrent vulvovaginal candidiasis. SAGE Open Med. 2022, 10, 20503121221115201. [Google Scholar] [CrossRef]
- Bitew, A.; Abebaw, Y. Vulvovaginal candidiasis: Species distribution of Candida and their antifungal susceptibility pattern. BMC Womens Health 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Rezaei-Matehkolaei, A.; Shafiei, S.; Zarei-Mahmoudabadi, A. Isolation, molecular identification, and antifungal susceptibility profiles of vaginal isolates of Candida species. Iran. J. Microbiol. 2016, 8, 410–417. [Google Scholar]
- Tits, J.; Cools, F.; De Cremer, K.; De Brucker, K.; Berman, J.; Verbruggen, K.; Gevaert, B.; Cos, P.; Cammue, B.P.A.; Thevissen, K. Combination of Miconazole and Domiphen Bromide Is Fungicidal against Biofilms of Resistant Candida spp. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Hösükoğlu, F.; Ekşi, F.; Erinmez, M.; Uğur, M. An Epidemiologic Analysis of Vulvovaginal Candidiasis and Antifungal Susceptibilities. Infect. Microbes Dis. 2022, 4, 131–136. [Google Scholar] [CrossRef]
- Sobel, J.D.; Sebastian, S.; Boikov, D.A. A longitudinal study on fluconazole resistance in Candida albicans vaginal isolates. Mycoses 2023, 66, 563–565. [Google Scholar] [CrossRef]
- Maraki, S.; Mavromanolaki, V.E.; Stafylaki, D.; Nioti, E.; Hamilos, G.; Kasimati, A. Epidemiology and antifungal susceptibility patterns of Candida isolates from Greek women with vulvovaginal candidiasis. Mycoses 2019, 62, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Anh, D.N.; Hung, D.N.; Tien, T.V.; Dinh, V.N.; Son, V.T.; Luong, N.V.; Van, N.T.; Quynh, N.T.N.; Van Tuan, N.; Tuan, L.Q.; et al. Prevalence, species distribution and antifungal susceptibility of Candida albicans causing vaginal discharge among symptomatic non-pregnant women of reproductive age at a tertiary care hospital, Vietnam. BMC Infect. Dis. 2021, 21, 523. [Google Scholar] [CrossRef] [PubMed]
- Ratner, J.C.; Wilson, J.; Roberts, K.; Armitage, C.; Barton, R.C. Increasing rate of non-Candida albicans yeasts and fluconazole resistance in yeast isolates from women with recurrent vulvovaginal candidiasis in Leeds, United Kingdom. Sex. Transm. Infect. 2024. [Google Scholar] [CrossRef] [PubMed]
- Nyirjesy, P.; Schwebke, J.R.; Angulo, D.A.; Harriott, I.A.; Azie, N.E.; Sobel, J.D. Phase 2 Randomized Study of Oral Ibrexafungerp Versus Fluconazole in Vulvovaginal Candidiasis. Clin. Infect. Dis. 2022, 74, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sobel, J.D.; Bhargava, P.; Boikov, D.; Vazquez, J.A. Vaginitis due to Candida krusei: Epidemiology, clinical aspects, and therapy. Clin. Infect. Dis. 2002, 35, 1066–1070. [Google Scholar] [CrossRef]
- Kalkan, Ü.; Yassa, M.; Sandal, K.; Tekin, A.B.; Kilinç, C.; Gülümser, Ç.; Tug, N. The efficacy of the boric acid-based maintenance therapy in preventing recurrent vulvovaginal candidiasis. J. Exp. Clin. Med. 2021, 38, 461–465. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Sobel, J.D. Vulvovaginal Candidiasis Caused by Non-albicans Candida Species: New Insights. Curr. Infect. Dis. Rep. 2010, 12, 465–470. [Google Scholar] [CrossRef]
- Martens, M.G.; Maximos, B.; Degenhardt, T.; Person, K.; Curelop, S.; Ghannoum, M.; Flynt, A.; Brand, S.R. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am. J. Obstet. Gynecol. 2022, 227, 880.e1–880.e11. [Google Scholar] [CrossRef]
- Nyirjesy, P.; Alexander, A.B.; Weitz, M.V. Vaginal Candida parapsilosis: Pathogen or bystander? Infect. Dis. Obstet. Gynecol. 2005, 13, 37–41. [Google Scholar] [CrossRef]
- Ray, D.; Goswami, R.; Banerjee, U.; Dadhwal, V.; Goswami, D.; Mandal, P.; Sreenivas, V.; Kochupillai, N. Prevalence of Candida glabrata and its response to boric acid vaginal suppositories in comparison with oral fluconazole in patients with diabetes and vulvovaginal candidiasis. Diabetes Care 2007, 30, 312–317. [Google Scholar] [CrossRef]
- Iavazzo, C.; Gkegkes, I.D.; Zarkada, I.M.; Falagas, M.E. Boric acid for recurrent vulvovaginal candidiasis: The clinical evidence. J. Womens Health 2011, 20, 1245–1255. [Google Scholar] [CrossRef]
- Saxon Lead Author, G.; Edwards, A.; Rautemaa-Richardson, R.; Owen, C.; Nathan, B.; Palmer, B.; Wood, C.; Ahmed, H.; Ahmad Patient Representatives, S.; FitzGerald Ceg Editor, M. British Association for Sexual Health and HIV national guideline for the management of vulvovaginal candidiasis (2019). Int. J. STD AIDS 2020, 31, 1124–1144. [Google Scholar] [CrossRef] [PubMed]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Ledger, W.J. Determining the cause of vulvovaginal symptoms. Obstet. Gynecol. Surv. 2008, 63, 445–464. [Google Scholar] [CrossRef]
- Phillips, N.A.; Bachmann, G.; Haefner, H.; Martens, M.; Stockdale, C. Topical Treatment of Recurrent Vulvovaginal Candidiasis: An Expert Consensus. Womens Health Rep. 2022, 3, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Acs, N.; Banhidy, F.; Puho, E.; Czeizel, A.E. Teratogenic effects of vaginal boric acid treatment during pregnancy. Int. J. Gynaecol. Obstet. 2006, 93, 55–56. [Google Scholar] [CrossRef]
- Faro, S. Systemic vs. topical therapy for the treatment of Vulvovaginal Candidiasis. Infect. Dis. Obstet. Gynecol. 1994, 1, 202–208. [Google Scholar] [CrossRef]
- Genovese, C.; Cianci, A.; Corsello, S.; Ettore, G.; Mattana, P.; Tempera, G. Combined systemic (fluconazole) and topical (metronidazole + clotrimazole) therapy for a new approach to the treatment and prophylaxis of recurrent candidiasis. Minerva Ginecol. 2019, 71, 321–328. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Miranda, I.M.; Silva-Dias, A.; Silva, A.P.; Rodrigues, A.G.; Pina-Vaz, C. Ibuprofen potentiates the in vivo antifungal activity of fluconazole against Candida albicans murine infection. Antimicrob. Agents Chemother. 2015, 59, 4289–4292. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Sansonetty, F.; Rodrigues, A.G.; Martinez, D.E.O.J.; Fonseca, A.F.; Mardh, P.A. Antifungal activity of ibuprofen alone and in combination with fluconazole against Candida species. J. Med. Microbiol. 2000, 49, 831–840. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Schinas, G.; Polyzou, E.; Tsiakalos, A.; Donders, G.G.G. Probiotics in the Management of Vulvovaginal Candidosis. J. Clin. Med. 2024, 13, 5163. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Resistance to Fluconazole of Candida albicans in Vaginal Isolates: A 10-Year Study in a Clinical Referral Center. Antimicrob. Agents Chemother. 2023, 67, e0018123. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, C.; Li, Z.; Ji, B.; Man, S.; Yi, M.; Li, R.; Hao, M.; Wang, S. Epidemiology and antifungal susceptibility of fungal infections from 2018 to 2021 in Shandong, eastern China: A report from the SPARSS program. Indian J. Med. Microbiol. 2024, 47, 100518. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Donders, G.; Degenhardt, T.; Person, K.; Curelop, S.; Ghannoum, M.; Brand, S.R. Efficacy and Safety of Oteseconazole in Recurrent Vulvovaginal Candidiasis. NEJM Evid. 2022, 1, EVIDoa2100055. [Google Scholar] [CrossRef]
- Miyazaki, M.; Horii, T.; Hata, K.; Watanabe, N.A.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; Inoue, S.; Matsukura, M.; et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 2011, 55, 4652–4658. [Google Scholar] [CrossRef]
- Pfaller, M.; Huband, M.; Bien, P.A.; Carvalhaes, C.G.; Klauer, A.; Castanheira, M. In vitro activity of manogepix and comparators against infrequently encountered yeast and mold isolates from the SENTRY Surveillance Program (2017–2022). Antimicrob. Agents Chemother. 2024, 68, e0113223. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Huband, M.D.; Rhomberg, P.R.; Bien, P.A.; Castanheira, M. Activities of Manogepix and Comparators against 1,435 Recent Fungal Isolates Collected during an International Surveillance Program (2020). Antimicrob. Agents Chemother. 2022, 66, e0102822. [Google Scholar] [CrossRef]
- Pappas, P.G.; Vazquez, J.A.; Oren, I.; Rahav, G.; Aoun, M.; Bulpa, P.; Ben-Ami, R.; Ferrer, R.; McCarty, T.; Thompson, G.R.; et al. Clinical safety and efficacy of novel antifungal, fosmanogepix, for the treatment of candidaemia: Results from a Phase 2 trial. J. Antimicrob. Chemother. 2023, 78, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Pappas, P.G.; Boffard, K.; Paruk, F.; Bien, P.A.; Tawadrous, M.; Ople, E.; Wedel, P.; Oborska, I.; Hodges, M.R. Clinical Efficacy and Safety of a Novel Antifungal, Fosmanogepix, in Patients with Candidemia Caused by Candida auris: Results from a Phase 2 Trial. Antimicrob. Agents Chemother. 2023, 67, e0141922. [Google Scholar] [CrossRef]
- Thompson, G.R., 3rd; Soriano, A.; Honore, P.M.; Bassetti, M.; Cornely, O.A.; Kollef, M.; Kullberg, B.J.; Pullman, J.; Hites, M.; Fortun, J.; et al. Efficacy and safety of rezafungin and caspofungin in candidaemia and invasive candidiasis: Pooled data from two prospective randomised controlled trials. Lancet Infect. Dis. 2024, 24, 319–328. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Carvalhaes, C.; Messer, S.A.; Rhomberg, P.R.; Castanheira, M. Activity of a Long-Acting Echinocandin, Rezafungin, and Comparator Antifungal Agents Tested against Contemporary Invasive Fungal Isolates (SENTRY Program, 2016 to 2018). Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R.; Soriano, A.; Skoutelis, A.; Vazquez, J.A.; Honore, P.M.; Horcajada, J.P.; Spapen, H.; Bassetti, M.; Ostrosky-Zeichner, L.; Das, A.F.; et al. Rezafungin Versus Caspofungin in a Phase 2, Randomized, Double-blind Study for the Treatment of Candidemia and Invasive Candidiasis: The STRIVE Trial. Clin. Infect. Dis. 2021, 73, e3647–e3655. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R., 3rd; Soriano, A.; Cornely, O.A.; Kullberg, B.J.; Kollef, M.; Vazquez, J.; Honore, P.M.; Bassetti, M.; Pullman, J.; Chayakulkeeree, M.; et al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): A multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet 2023, 401, 49–59. [Google Scholar] [CrossRef]
- Locke, J.B.; Pillar, C.M.; Castanheira, M.; Carvalhaes, C.G.; Andes, D.; Aram, J.A.; Andrzejewski, C.; Bartizal, K.; Das, A.F.; Sandison, T.; et al. Outcomes by Candida spp. in the ReSTORE Phase 3 trial of rezafungin versus caspofungin for candidemia and/or invasive candidiasis. Antimicrob. Agents Chemother. 2024, 68, e0158423. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Miyazaki, T.; Fukuda, Y.; Mitsuyama, J.; Saijo, T.; Shimamura, S.; Yamamoto, K.; Imamura, Y.; Izumikawa, K.; Yanagihara, K.; et al. The Novel Arylamidine T-2307 Selectively Disrupts Yeast Mitochondrial Function by Inhibiting Respiratory Chain Complexes. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Fothergill, A.W.; Bocanegra, R.; Olivo, M.; McCarthy, D.I.; Fukuda, Y.; Mitsuyama, J.; Patterson, T.F. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against echinocandin-resistant Candida glabrata. J. Antimicrob. Chemother. 2016, 71, 692–695. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Fothergill, A.W.; Bocanegra, R.; Olivo, M.; McCarthy, D.I.; Kirkpatrick, W.R.; Fukuda, Y.; Mitsuyama, J.; Patterson, T.F. The novel arylamidine T-2307 maintains in vitro and in vivo activity against echinocandin-resistant Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 1341–1343. [Google Scholar] [CrossRef]
- Roetzer, A.; Gabaldón, T.; Schüller, C. From Saccharomyces cerevisiae to Candida glabrata in a few easy steps: Important adaptations for an opportunistic pathogen. FEMS Microbiol. Lett. 2011, 314, 1–9. [Google Scholar] [CrossRef]
- Farr, A.; Effendy, I.; Frey Tirri, B.; Hof, H.; Mayser, P.; Petricevic, L.; Ruhnke, M.; Schaller, M.; Schaefer, A.P.A.; Sustr, V.; et al. Guideline: Vulvovaginal candidosis (AWMF 015/072, level S2k). Mycoses 2021, 64, 583–602. [Google Scholar] [CrossRef]
- Sherrard, J.; Wilson, J.; Donders, G.; Mendling, W.; Jensen, J.S. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int. J. STD AIDS 2018, 29, 1258–1272. [Google Scholar] [CrossRef]
- Rex, J.H.; Nelson, P.W.; Paetznick, V.L.; Lozano-Chiu, M.; Espinel-Ingroff, A.; Anaissie, E.J. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 1998, 42, 129–134. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, D.M.; Coste, A.; Ischer, F.; Jacobsen, M.D.; Odds, F.C.; Sanglard, D. Genetic dissection of azole resistance mechanisms in Candida albicans and their validation in a mouse model of disseminated infection. Antimicrob. Agents Chemother. 2010, 54, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.G.; Grinceviciene, S.; Bellen, G.; Jaeger, M.; Ten Oever, J.; Netea, M.G. Is non-response to fluconazole maintenance therapy for recurrent Candida vaginitis related to sensitization to atopic reactions? Am. J. Reprod. Immunol. 2018, 79, e12811. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).