Abstract
Bempedoic acid is a new drug that improves the control of cholesterol levels, either as monotherapy or in combination with existing lipid-lowering therapies, and shows clinical efficacy in cardiovascular disease patients. Thus, patients with comorbidities and under multiple therapies may be eligible for bempedoic acid, thus facing the potential problem of drug–drug interactions (DDIs). Bempedoic acid is a prodrug administered orally at a fixed daily dose of 180 mg. The dicarboxylic acid is enzymatically activated by conjugation with coenzyme A (CoA) to form the pharmacologically active thioester (bempedoic acid–CoA). This process is catalyzed by very-long-chain acyl-CoA synthetase 1 (ACSVL1), expressed almost exclusively at the hepatic level. Bempedoic acid–CoA is a potent and selective inhibitor of ATP citrate lyase (ACL), a key enzyme in the biosynthetic pathway of cholesterol and fatty acids. The drug reduces low-density lipoprotein–cholesterol (LDL-C) (20–25%), non-high-density lipoprotein–cholesterol (HDL-C) (19%), apolipoprotein B (apoB) (15%), and total cholesterol (16%) in patients with hypercholesterolemia or mixed dyslipidemia. The drug has a favorable pharmacokinetics profile. Bempedoic acid and its metabolites are not substrates or inhibitors/inducers of cytochrome P450 (CYP450) involved in drug metabolism. On the other hand, bempedoic acid–glucuronide is a substrate for organic anion transporter 3 (OAT3). Bempedoic acid and its glucuronide are weak inhibitors of the OAT2, OAT3, and organic anion-transporting polypeptide 1B1 (OATP1B1) and 1B3 (OATP1B3). Thus, bempedoic acid could inhibit (perpetrator) the hepatic uptake of OATP1B1/3 substrate drugs and the renal elimination of OAT2 and OAT3 substrates and could suffer (victim) the effect of OAT3 transporter inhibitors, reducing its renal elimination. Based on these pharmacological characteristics, here, we describe the potential DDIs of bempedoic acid with concomitant medications and the possible clinical implications.
1. Introduction
Lipid-lowering drugs represent the first line of intervention for primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD). A Cholesterol Treatment Trialist (CTT) meta-analysis from randomized clinical trials firmly established that lowering low-density lipoprotein–cholesterol (LDL-C) with standard statin regimens reduced the 5-year relative incidence of major coronary events, coronary revascularizations, and ischemic strokes by about 20% for each mmol/L reduction in LDL-C, and that additional reductions, obtained with more intensive statin regimens, further reduced the incidence of these major vascular events [1]. In addition, the clinical benefit of LDL-C reduction with statin therapy was confirmed in individuals who had a 5-year risk lower than 10%, even in those who had no previous history of vascular disease, diabetes, or chronic kidney disease [2].
The combination of statins with either ezetimibe, which reduces the intestinal uptake of dietary and biliary cholesterol by inhibiting the Niemann–Pick C1-like protein 1 (NPC1L1) or monoclonal antibodies anti-proprotein convertase subtilisin/kexin type 9 (PCSK9), further reduced the LDL-C levels and major cardiovascular events [3,4,5]. Thus, the clinical data demonstrated that, independently from the pharmacological approach, the more aggressive lowering of LDL-C is associated with increased benefits in reducing atherosclerotic disease burden, supporting the notion of ‘the lower, the better’ [6,7]. Indeed, in patients with ASCVD, the long-term achievement of lower LDL-C levels with monoclonal anti-PCSK9 evolocumab, down to <20 mg/dL (<0.5 mmol/L), has been associated with a lower risk of cardiovascular outcomes with no significant safety concerns [8]. The most recently approved hypocholesterolemic drug is represented by the small molecule bempedoic acid [9]. Bempedoic acid has shown effective hypocholesterolemic activity, either as monotherapy or in combination with existing lipid-lowering therapy, in a broad spectrum of patients at high cardiovascular risk [10,11,12]. This drug has been demonstrated to significantly reduce the major cardiovascular events in statin-intolerant patients, either in primary or secondary prevention [13,14]. Here, we described the pharmacological properties of bempedoic acid and the potential drug–drug interactions that might occur in patients with multiple comorbidities.
2. Mechanism of Action of Bempedoic Acid
Bempedoic acid is a prodrug that requires enzymatic activation by the very-long-chain acyl–CoA synthetase 1 (ACSVL1, encoded by the SLC27A2 gene) enzyme, almost exclusively expressed in the liver (Figure 1). Thus, dicarboxylic acid is conjugated with coenzyme A (CoA) to form the pharmacologically active thioester (bempedoic acid–CoA) in the liver where it acts as a potent and selective inhibitor of ATP citrate lyase (ACLY), a key enzyme in the biosynthetic pathway of cholesterol and fatty acids [9]. Thus, bempedoic acid and statins act on two different enzymatic steps in the same metabolic pathway. For this reason, the lipid-powering effect of bempedoic acid appears to be more pronounced in statin-intolerant patients [10]. Concentration–response studies have shown that bempedoic acid–CoA inhibits cholesterol synthesis in cultured hepatocytes, with a IC50 value equal to 10 µM [9]. In response to cholesterol biosynthesis, hepatocyte activates the transcription factor sterol regulatory element-binding protein 2 (SREBP2), which drives the expression of LDL receptors and PCSK9. Thus, the upregulation of LDL receptors increases the LDL catabolism with a concomitant reduction in plasma LDL cholesterol levels.
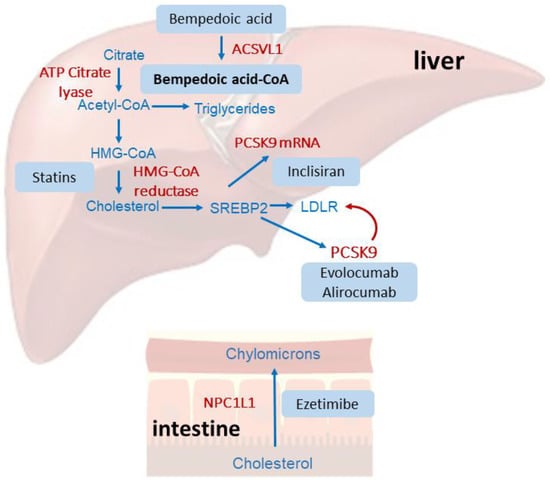
Figure 1.
Schematic representation of the mechanism of action of bempedoic acid compared to statins, ezetimibe, and anti-PCSK9 therapies. Statins, by inhibiting the hydroxy-methyl-glutaryl–CoA (HMG-CoA) reductase, reduce cholesterol biosynthesis in the liver, determining the activation of the transcription factor SREBP2, which drives the expression of the LDL receptor. Statins also induce the expression of PCSK9, which can degrade the LDL receptor and thus partially reduce the hypocholesterolemic effect of statins. Monoclonal antibodies (evolocumab and alirocumab) bind and inhibit PCSK9, while inclisiran reduces its synthesis, interfering with its mRNA. Bempedoic acid acts by inhibiting the ACLY, and thus reduces cholesterol biosynthesis, which activates a similar cellular response to that observed with statins. Finally, ezetimibe interacts with the intestinal cholesterol transporter NPC1L1, reducing its absorption from the diet.
3. Pharmacokinetic Properties
3.1. Absorption
The administration of multiple doses of bempedoic acid (180 mg/day) resulted in Cmax and AUC values of between 24.8 ± 6.9 μg/mL and 348 ± 120 μg·h/mL, respectively (Table 1) [15]. After 7 days, at a steady state, no time-dependent changes were observed in the pharmacokinetic profile of the drug [16]. Bempedoic acid is a weak acid with high solubility at intestinal pHs, resulting in rapid oral absorption from the gastrointestinal tract. The pH-dependent solubility characteristics and high membrane permeability are clinically manifested in rapid oral absorption, with a median time-to-peak concentration of 3.5 h [16,17,18,19]. The bioavailability of bempedoic acid is 95%, and it is not influenced by the presence of food (Table 1) [20].

Table 1.
Pharmacokinetic characteristics of bempedoic acid.
3.2. Distribution
Bempedoic acid has a volume of distribution of 18 L and a plasma protein binding of 99% (Table 1) [15,16].
3.3. Metabolism
Bempedoic acid is reversibly converted to the active metabolite ETC15228 (AUC of the metabolite is 18% compared to bempedoic acid). ETC15228, a keto metabolite of bempedoic acid, is converted into the active molecule thioester CoA conjugate by the same mechanism of activation of bempedoic acid [21]. Metabolism of ETC15228 by ACSVL1 leads to the formation of ETC15228–CoA, a selective inhibitor, in the liver of the ACLY, with a potency similar to bempedoic acid–CoA. Although ETC15228 thioester and bempedoic acid–CoA are equipotent, ETC15228 plasma exposure is approximately 72% lower than bempedoic acid levels with a mean steady state Cmax of 2.8 ± 0.9 ng/mL compared to a Cmax of 20.6 ± 6.1 μg/mL [20]. The corresponding time–concentration profile of ETC15228 indicates a Tmax of 11.0 h with an elimination half-life (t1/2) of 31.1 h, significantly slower than the t1/2 of 19–21 h of bempedoic acid (Table 1) [15].
Bempedoic acid and its metabolites are not substrates or inhibitors/inducers of cytochrome P450 (CYP450) involved in drug metabolism but are metabolized through conjugation with glucuronic acid by UDP-Glucuronosyltransferase-2B7 (UGT2B7) (Figure 2) [15,20]. Based on AUCinf exposure, the metabolites bempedoic acid–glucuronide, ETC15228, and ETC15228–glucuronide represent approximately 28.9%, 12.1%, and 10.4%, respectively, of the total plasma radioactivity detectable after administration of 180 mg of [14C]–bempedoic acid [20].
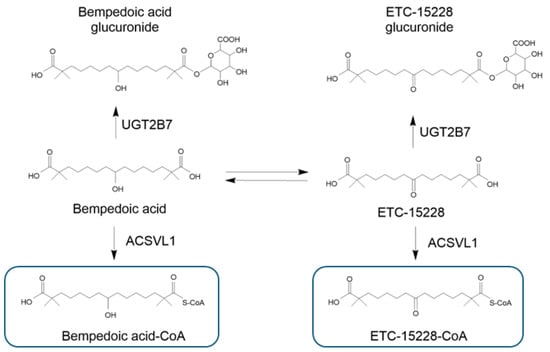
Figure 2.
Main metabolites of bempedoic acid. Bempedoic acid and its keto metabolite ECT-15228 are both substrates of the UGT2B7 enzyme, which forms the two glucuronide derivatives. The enzyme ACSVL1 also converted the bempedoic acid and ECT-15228 into their active metabolites. UGT-2B7: UDP-Glucuronosyltransferase-2B7; ACSVL1: very-long-chain acyl–CoA synthetase 1. Bempedoic acid–CoA and ETC-15228–CoA are the active metabolites formed selectively in the liver.
3.4. Elimination
At steady state, after once-daily administration, the bempedoic acid total clearance is equal to 11.2 mL/min. Renal clearance of the parental drug represents less than 2% of the total clearance. However, between 70% and 30% of the total dose is found in the urine and feces, respectively, although bempedoic acid represents less than 5% of the administered dose (feces and urine). The half-life of bempedoic acid at a steady state is 19–21 h [20].
After administration of [14C]–bempedoic acid, the majority of the radioactivity is found in urine; thus, renal clearance is the most representative route of elimination for the drug. The most abundant analyte in urine is bempedoic acid–glucuronide (Figure 2), which represents approximately 37.3% of the total radioactivity excreted in 120 h [20]. The metabolite ETC15228–glucuronide and bempedoic acid are also excreted in urine, and represent from 10% to 14% and from 0.9% to 3.3% of the total radioactivity, respectively [20].
The clearance of bempedoic acid was studied in patients with mild, moderate, and severe renal impairment [18]. Although exposure (AUC) to bempedoic acid was increased approximately two-fold in subjects with renal impairment, compared to subjects with normal renal function, no safety problems with the drug were observed. This is due to the fact that, despite an increase in AUC in subjects with renal insufficiency, Cmax values did not change compared to subjects with normal renal function. A possible explanation relies in the fact that, in conditions of renal insufficiency, bempedoic acid is mostly eliminated via the bile in the form of glucuronide, with subsequent enterohepatic recirculation and lengthening of the plasma half-life after a reduction in clearance. No dosage adjustment of bempedoic acid is suggested in patients with mild or moderate renal insufficiency [15].
4. General Considerations on DDI with Bempedoic Acid
DDIs can be related to the pharmacokinetics or pharmacodynamics of a drug. Taking into consideration the pharmacodynamics of bempedoic acid, it is conceivable to hypothesize a pharmacological interaction with drugs or phytotherapies that act on the same ACLY enzyme or that inhibit its activation from prodrug to active drug by ACSVL1.
Considering the pharmacokinetic profile, bempedoic acid is neither a substrate nor an inhibitor of P-glycoprotein [16]; therefore, no interactions with inducers or inhibitors of this transporter are expected. Furthermore, bempedoic acid is a weak acid with high solubility at intestinal pH; for this reason, interactions with proton pump inhibitors (PPIs) or other antacids are not expected.
Bempedoic acid, as well as its active metabolite and glucuronide form, are not substrates of commonly characterized drug transporters, with the exception of bempedoic acid–glucuronide, which is an OAT3 substrate [22]. However, bempedoic acid and its glucuronide are weak inhibitors of OATP1B1/3, while only bempedoic acid is an inhibitor of the OAT2 and OAT3 transporters [15]. These characteristics indicate that bempedoic acid could inhibit (perpetrator) the liver uptake of OATP1B1/3 substrate drugs and the renal elimination of OAT2/3 substrate drugs. Furthermore, bempedoic acid itself could suffer (victim) the effect of OAT3 inhibitors, reducing its renal clearance. Probenecid, an inhibitor of UGT and OAT3 transporters, has been studied to evaluate the potential effect of OAT3 inhibitors on the pharmacokinetics of bempedoic acid. Administration of 180 mg of bempedoic acid with steady-state probenecid resulted in a 1.7-fold increase in bempedoic acid exposure and a 1.9-fold increase in bempedoic acid active metabolite exposure [17]. These elevations are not considered to be clinically meaningful and do not impact dosing recommendations [15,17]. Indeed, human pharmacokinetics of bempedoic acid are linear over the dose range of 120–240 mg, and doses up to 240 mg/day (1.3 times the approved recommended dose) have been administered in clinical trials, with no evidence of dose-limiting toxicity [15,20]. In addition, a single dose of bempedoic acid was also well-tolerated in subjects with severe renal impairment that experienced a 2.2-fold increase in drug exposure [18]. Thus, relevant interactions between bempedoic acid and other OAT3 inhibitors are not expected to be clinically relevant.
Finally, by inhibiting the OAT2/3 renal transporter, bempedoic acid determines a minor and reversible increase in uric acid and creatinine plasma levels, effects that can be added to those related to other drugs. The minor increase incidence of cholelithiasis in patients treated with bempedoic acid, observed exclusively in the phase 3 clinical trial CLEAR outcomes [13], could deteriorate by the co-treatment with other drugs with similar side effects [13], such as the peroxisome proliferator-activated receptor α (PPARα) agonist fenofibrate [23,24].
5. Pharmacodynamic DDIs with Bempedoic Acid
As previously described, bempedoic acid acts by inhibiting the ACLY and, thus, cholesterol biosynthesis. Possible DDIs may derive from drugs or chemical entities that act on the same enzyme and thus interfere with bempedoic acid. In addition, molecules that inhibit or are a substrate of ACSVL1 may interfere with bempedoic acid activation and, thus, its activity. For instance, ACLY is inhibited by hydroxycitric acid present in Garcinia cambogia, a natural product utilized to reduce bodyweight and plasma cholesterol levels [25]. Thus, the addition of this nutraceutical product may potentially interfere with the activity of bempedoic acid and should be avoided. Additionally, omega 3 fatty acids are substrates of ACSVL1 [9], the hepatic enzyme required for the activation of bempedoic acid. It is, therefore, reasonable to predict a possible interaction between bempedoic acid and omega 3, including the most recently approved highly purified eicosapentaenoic acid ethyl ester, icosapent ethyl [26].
6. Pharmacokinetics DDIs with Bempedoic Acid
To report a most comprehensive revision of the DDIs with bempedoic acid, we screened for therapies that have either an inhibitor/inducer activity on drug transporter OAT3 or that are a substrate for OATP1B1/B3 and OAT2/3.
6.1. Potential DDIs with Lipid-Lowering Drugs
To reach the appropriate LDL-C level, the current guidelines indicate the use of combination therapies with different lipid-lowering drugs, including statins, ezetimibe, PCSK9 inhibitors, fenofibrate, and bempedoic acid [27]. In addition, lifestyle changes should be pursued to control plasma lipid levels. From these recommendations, it is important to rule out or take into consideration the potential DDIs with functional foods or hypocholesterolemic drugs with bempedoic acid.
Considering the inhibitory activity of bempedoic acid on liver transporters OATP1B1/B3, drugs that are a substrate for these two transporters may undergo significantly higher exposure, with a possible increase in their side effects. Among many classes of drugs, it is important to remember that all statins are substrates of OATP1B1 and OATP1B3 and are susceptible of single nucleotide polymorphisms (SNPs) determining a partial loss-of-function phenotype [28], an effect mainly observed with simvastatin [29]. The pharmacokinetic interactions between bempedoic acid 180 mg and simvastatin 40 mg, atorvastatin 80 mg, pravastatin 80 mg, and rosuvastatin 40 mg were evaluated in clinical trials [15]. Administration of a single 40 mg dose of simvastatin with 180 mg of steady-state bempedoic acid resulted in a two-fold increase in simvastatin acid exposure. Elevations from 1.4-fold to 1.5-fold in AUC of atorvastatin, pravastatin, and rosuvastatin (administered as single doses) and/or their major metabolites were observed when co-administered with bempedoic acid (Table 2). From this evidence, the simvastatin dose should be limited to 20 mg daily (or 40 mg daily for patients with severe hypercholesterolemia and who are at a high risk of cardiovascular complications) when combined with bempedoic acid. In contrast, the interactions with atorvastatin, rosuvastatin, and pravastatin were not considered to be clinically relevant, and thus their combination with bempedoic acid is possible. In addition, both lovastatin and its analog monacolin K present in the red yeast rice preparations are predicted to not interact with bempedoic acid.

Table 2.
Potential DDIs of bempedoic acid with lipid-lowering agents.
6.2. Potential DDIs with Immune-Modulating and Antineoplastic Agents
It is important to point out that bempedoic acid and its glucuronide derivative are inhibitors but not substrates of OATP1B1/1B3; therefore, a variation in their systemic exposures is not expected with inhibitors of these transporters (Table 3). Thus, bempedoic acid, differently from statins, can be administered with cyclosporine, which is an inhibitor of CYP3A4, P-gp, and OATP1B1/1B3 [36,37,38]. Similarly, everolimus and tacrolimus are inhibitors of OATP1B1 and should not alter the pharmacokinetics of bempedoic acid [39,40].
The immunomodulators leflunomide and teriflunomide are both inhibitors of OAT3 [41,42], being the first that also affect OATP1B1/B3 transporters [41]. For this double action, teriflunomide increased systemic exposure of rosuvastatin by 2.5-fold, and it is indicated to reduce the dose of statin by 50% when co-administered with this immunomodulator [41]. The inhibition of renal OAT3 could also increase the systemic exposure of bempedoic acid–glucuronide, while the plasma levels of uric acid could potentially be controlled by leflunomide, whose treatment is associated with its higher fractional excretion [43].
Mycophenolate mofetil, another immunomodulator, is a substrate for OAT1, OAT3, MRP2, and BCRP [44], and its co-administration could determine a significant drug interaction with bempedoic acid by increasing its systemic exposure. This combination could also be associated with a higher incidence of hyperuricemia and gout (Table 3) [44,45].
Darolutamide, an androgen receptor inhibitor, is an inhibitor of OATP1B1/B3; indeed, the co-administration of rosuvastatin should be avoided. Similar interactions can be expected with other statins, and bempedoic acid could be a valid therapeutic alternative in these patients [46]. Enzalutamide is metabolized by CYP2C8 and CYP3A4 and can induce different enzymes and drug transporters, such as CYP3A4, CYP2B6, CYP2C9, CYP2C19, UGT1A1, MRP2, OATP1B1, and P-gp (Table 3) [47]. For this reason, it is expected to interact with statins metabolized by CYP3A4 (atorvastatin and simvastatin), and the induction of OATP1B1 could potentiate their hepatic uptake, while bempedoic acid should not be affected by these changes.
Tucatinib, the HER2 inhibitor, can induce several enzymes (CYP2B6, CYP3A4, CYP2C9, CYP2C19, or UGT1A1) and inhibits the drug transporters MRP2, OCT1, and OAT3 (Table 3) [48]. Thus, tucatinib and bempedoic acid are both inhibitors, but not substrates of OAT3, and their pharmacokinetics should not be altered when administered simultaneously.

Table 3.
Potential DDIs of bempedoic acid with immunosuppressant and antineoplastic agents.
Table 3.
Potential DDIs of bempedoic acid with immunosuppressant and antineoplastic agents.
| Drug | Effect on CYP450 and Drug Transporters | Effect on Bempedoic Acid | Effect on Interacting Drug | Expert Opinion |
|---|---|---|---|---|
| Immunosuppressant drugs | ||||
| Cyclosporine | Inhibitor of CYP3A4 and P-gp, OATP1B1/1B3 [36,37,38]. | No significant effect predicted. | No significant effect predicted. | Bempedoic acid inhibits OATP1B1/1B3, but it is not a substrate of these transporters. Thus, no interaction is predicted with OATP1B1/1B3 inhibitors or inducers. |
| Tacrolimus | Metabolized by CYP3A4 and substrate of P-gp [36,37,38]. | No significant effect predicted. | No significant effect predicted. | No drug interaction. |
| Everolimus, sirolimus | Everolimus is a substrate of CYP3A4 and weak inhibitor of P-gp and CYP2D6 [49]. Sirolimus is metabolized by CYP3A4 and is a substrate of P-gp [50]. Both are inhibitors of OATP1B1 [39,40]. | No significant effect predicted. | No significant effect predicted. | Bempedoic acid inhibits OATP1B1/1B3, but it is not a substrate of these transporters. Thus, no interaction is predicted with OATP1B1/1B3 inhibitors or inducers. |
| Leflunomide | Metabolized by CYP1A2, CYP2C19, and CYP3A4. Inhibitor of OAT3 [42]. | Leflunomide could increase the exposure of bempedoic acid. | No significant effect predicted. | It is recommended to have caution when administered with an OAT3 substrate like bempedoic acid–glucuronide. |
| Teriflunomide | Inhibitor of CYP2C8. Inducer of CYP1A2. Inhibitor of OAT3, OATP1B1/B3 [41]. | Teriflunomide could increase the exposure of bempedoic acid. | No significant effect predicted. | The inhibition of OAT3 could increase the AUC of bempedoic acid–glucuronide and potentially the plasma levels of uremic acid and creatinine. |
| Mycophenolate mofetil | Metabolized by UGT1A9. Substrate of OAT1, OAT3, MRP2, and BCRP [44]. | No significant effect predicted. | Bempedoic acid could increase the exposure of mycophenolate mofetil. | Bempedoic acid could reduce the renal excretion of mycophenolate mofetil by competing with OAT1 and OAT3. Both drugs increase uric acid plasma levels. Higher incidence of gout is predicted. |
| Anticancer drugs | ||||
| Tamoxifen | Metabolized by CYP3A4. CYP2D6 metabolism leads to the formation of the active molecule endoxifen [51]. Inhibitor of P-gp and is a substrate of OATP1A2 [52]. | No significant effect predicted. | No significant effect predicted. | No drug interaction. |
| Taxanes (paclitaxel, docetaxel) | Paclitaxel is a substrate of CYP2C8 and CYP3A4. Docetaxel is a substrate of CYP3A4. Both are substrates of OATP1B1/1B3. | No significant effect predicted. | Bempedoic acid could increase the exposure of docetaxel and paclitaxel. | Bempedoic acid inhibits OATP1B1/1B3 and may increase the exposure of docetaxel and paclitaxel. |
| Darolutamide | Metabolized by CYP3A4 and UGT1A9. Substrate of P-gp and BCRP. Inhibitor of OATP1B1/B3 [46]. | No significant effect predicted. | No significant effect predicted. | Co-administration of rosuvastatin with darolutamide should be avoided unless there is no alternative therapy. Consider drugs which are not substrates for OATP1B1 and OATP1B3. Bempedoic acid inhibits OATP1B1/1B3, but it is not a substrate of these transporters. Thus, no interaction is predicted with OATP1B1/1B3 inhibitors or inducers. |
| Imatinib, crizotinib and tucatinib | Inhibitor of CYP3A4 and P-gp. Imatinib inhibits CYP2D6, UGT2B17, and, partially, UGT1A1 [53]. Tucatinib could induce CYP2B6, CYP3A4, CYP2C9, CYP2C19, or UGT1A1, and inhibits MRP2, OCT1, and OAT3 [48]. | Tucatinib could increase the exposure of bempedoic acid. | No significant effect predicted. | The inhibition of OAT3 could increase the AUC of bempedoic acid–glucuronide and potentially the plasma levels of uremic acid and creatinine. |
| Enzalutamide | Metabolized by CYP2C8 and CYP3A4. Inducer of CYP3A4, CYP2B6, CYP2C9, CYP2C19, UGT1A1, MRP2, OATP1B1, and P-gp [47]. Possible inhibition of MRP2, OCT1, and OAT3. | Enzalutamide could increase the exposure of bempedoic acid. | No significant effect predicted. | Induction of OATP1B1 is not expected to potentiate the hepatic uptake and activity of bempedoic acid. Enzalutamide is an OAT3 inhibitor. This could increase the AUC of bempedoic acid–glucuronide and potentially the plasma levels of uremic acid and creatinine. |
6.3. Potential DDIs with Antiviral, Antibiotic, and Antifungal Agents
The combination lopinavir/ritonavir strongly inhibits CYP3A4 and the hepatic transporters OATP1B1/B3 (Table 4) [54,55]. These effects may determine a clinically relevant interaction with statins, but not with bempedoic acid, which could be considered a valid alternative for HIV patients with hypercholesterolemia. Asunaprevir, glecaprevir, grazoprevir, and voxilaprevir are anti-HCV drugs and substrates for OATP1B1 and OATP1B3. Thus, bempedoic acid is expected to increase their exposure and side effects [15]. The antiretroviral drug dolutegravir is another strong inhibitor of the OAT3 transporter, and its co-administration could prolong the exposure of bempedoic acid (Table 4) [56].
A single dose of rifampicin has been shown to inhibit the drug transporters OATP1B1/B3, while multiple doses determine a strong induction of CYP3A4, CYP2C19, P-gp, and UGT [57]. All of these effects are not expected to have an impact on the pharmacokinetics of bempedoic acid. Similar conclusions can be drawn for erythromycin and clarithromycin, strong inhibitors of CYP3A4, P-gp, and OATP1B1/B3 [58,59,60].

Table 4.
Potential DDIs of bempedoic acid with antiviral, antibiotic, and antifungal agents.
Table 4.
Potential DDIs of bempedoic acid with antiviral, antibiotic, and antifungal agents.
| Drug | Effect on CYP450 and Drug Transporters | Effect on Bempedoic Acid | Effect on Interacting Drug | Expert Opinion |
|---|---|---|---|---|
| Antivirals, antibiotics, antifungals | ||||
| Dolutegravir/lamivudine/abacavir | No inhibition of CYP450. Dolutegravir inhibits OAT1 and OAT3. | Dolutegravir may increase plasma concentrations of drugs excreted through OAT3, such as bempedoic acid. | No significant effect predicted. | The inhibition of OAT3 could increase the AUC of bempedoic acid–glucuronide and potentially the plasma levels of uremic acid and creatinine. |
| Atazanavir and ritonavir | Potent inhibitors of CYP3A4. Inhibitors of glucuronidation [61,62]. | Possible reduction in glucuronide metabolite of bempedoic acid. | No significant effect predicted. | Inhibition of glucuronidation could prolong the half-life of bempedoic acid. |
| Lopinavir and ritonavir | Potent inhibitors of CYP3A4. Inhibitors of OATP1B1 and OATP1B3 [54,55]. | No significant effect predicted. | No significant effect predicted. | Bempedoic acid inhibits OATP1B1/1B3, but it is not a substrate of these transporters. Thus, no interaction is predicted with OATP1B1/1B3 inhibitors or inducers. |
| Asunaprevir, glecaprevir, grazoprevir and voxilaprevir | Substrate of OATP1B1 and OATP1B3 | No significant effect predicted. | Possible increase in their exposure. | Bempedoic acid, by inhibiting OATP1B1/1B3, may increase the exposure of anti-HCV drugs. |
| Antibiotic | ||||
| Rifampicin | Single dose inhibits OATP1B1 and OATP1B3. Strong inducer of CYP3A4, CYP2C19, P-gp and UGT [57]. | No significant effect predicted. | No significant effect predicted. | Bempedoic acid inhibits OATP1B1/1B3, but it is not a substrate of these transporters. Thus, no interaction is predicted with OATP1B1/1B3 inhibitors or inducers. The induction of UGT could increase the metabolism of bempedoic acid. |
| Erythromycin and clarithromycin | Inhibitors of CYP3A4 and P-gp. Inhibitors of OATP1B1 and OATP1B3 [58,59,60,63]. | No significant effect predicted. | No significant effect predicted. | Bempedoic acid inhibits OATP1B1/1B3, but it is not a substrate of these transporters. Thus, no interaction is predicted with OATP1B1/1B3 inhibitors or inducers. |
| Antifungal | ||||
| Ketoconazole, itraconazole and voriconazole | Potent inhibitors of CYP3A4, P-gp, and BCRP. Ketoconazole is a potent inhibitor of OATP1B1, OATP1B3, OAT3, OCT1, and OCT2, and a weak inhibitor of OAT [64]. | No significant effect predicted. | No significant effect predicted. | An increase in systemic exposure of bempedoic acid–glucuronide may be expected by OAT3 inhibition of ketoconazole. Itraconazole and voriconazole do not appear to inhibit this transporter [65,66]. |
6.4. Potential DDIs with Antidiabetic Agents
The sodium glucose co-transporter 2 (SGLT2) inhibitor empagliflozin is a substrate of the OAT3, OATP1B1, and OATP1B3 transporters; thus, its elimination can be inhibited by bempedoic acid, an OAT3 inhibitor (Table 5). Nevertheless, this interaction is not predicted to be relevant, since probenecid, a potent OAT3 inhibitor, and gemfibrozil, another potent inhibitor of OAT3 and OATP1B1/1B3, did not show a significant interaction with empagliflozin [67]. Others SGLT2 inhibitors (ertugliflozin, dapagliflozin, and canagliflozin) are not substrates of OAT3, thus excluding any potential interaction with bempedoic acid (Table 5). Considering other antidiabetic drugs, among the dipeptidyl peptidase 4 (DDP4) inhibitors, sitagliptin is a substrate of both P-gp and OAT3 [68]. Bempedoic acid, by inhibiting OAT3, could increase the systemic exposure of sitagliptin. No interaction is expected with other DDP4 inhibitors (Table 5).
Phase 1 clinical trials have not found significant pharmacokinetic interaction between metformin and bempedoic acid [15,19]. This could have been predicted by taking into consideration the fact that metformin is metabolized by UGT1A1, UGT1A3, and by CYP3A4 and it is substrate for MRP2 and BCRP [69]. In addition, no changes were observed on the glycemic control of metformin when administered with bempedoic acid. In this regard, it is important to point out that metformin is an indirect activator of the AMP-dependent kinase (AMPK) by changing the ratio of ATP/AMP [70], while bempedoic acid–CoA has been suggested to directly activate this metabolic pathway [9].

Table 5.
Possible DDIs of bempedoic acid with antidiabetic agents.
Table 5.
Possible DDIs of bempedoic acid with antidiabetic agents.
| Drug | Effect on CYP450 and Drug Transporters | Effect on Bempedoic Acid | Effect on Interacting Drug | Expert Opinion |
|---|---|---|---|---|
| Antidiabetic drugs | ||||
| Metformin | Metabolized by UGT1A1 and partially by UGT1A3 and CYP3A4. Substrate of MATE-1, MATE-2K, and OCT2 [69]. | No significant effect predicted. | No significant effect predicted. | No pharmacokinetic and pharmacodynamic interaction observed between metformin and bempedoic acid [15,19]. |
| Glyburide | Substrate of CYP2C9. Substrate and inhibitor of OATP1B1/1B3. | No significant effect predicted. | Possible increase in glyburide exposure. | Bempedoic acid inhibits OATP1B1/1B3, but it is not a substrate of these transporters. Thus, no significant interaction is predicted. |
| Repaglinide | Substrate of CYP2C8 and OATP1B1/1B3. | No significant effect predicted. | Possible increase in repaglinide exposure. | Bempedoic acid inhibits OATP1B1/1B3, but it is not a substrate of these transporters. Thus, no significant interaction is predicted. |
| SGLT2 (ertugliflozin, dapagliflozin, empagliflozin, canagliflozin) | Metabolized by UGT. Empagliflozin is a substrate of OAT3 and OATP1B1/1B3 [67,71]. The dapagliflozin metabolite is a substrate of OAT3 [72]. | No significant effect predicted. | No significant effect predicted. | Although empagliflozin is a substrate for OAT3, OATP1B1, and OATP1B3, no significant interaction is predicted with bempedoic acid. Indeed, the strong OAT3 inhibitor probenecid showed a minimal effect on the exposure of empagliflozin [67]. |
| DPP4 inhibitors (sitagliptin, vildagliptin, saxagliptin, linagliptin, alogliptin) | Saxagliptin is metabolized by CYP3A4/5. Linagliptin is a weak inhibitor of CYP3A4 and substrate of P-gp [73]. Sitagliptin is a substrate of P-gp and OAT3 [68]. | No significant effect predicted. | Possible increase in sitagliptin exposure. | Bempedoic acid, by inhibiting the OAT3, could reduce the clearance of sitagliptin. No interactions are predicted with other DDP4 inhibitors. |
| GLP1 agonists (liraglutide, exenatide, semaglutide) | Proteolytic metabolism. | No significant effect predicted. | No significant effect predicted. | No drug interaction. |
6.5. Potential DDIs of Bempedoic Acid with Cardiovascular Drugs
Warfarin has several drawbacks, such as a delayed onset of anticoagulant action, a narrow therapeutic index, and an unpredictable and variable response related to single nucleotide polymorphisms in CYP2C9 and VKORC1. Due to the narrow therapeutic index, warfarin is extremely susceptible to DDIs with inhibitors and inducers of CYP2C9. Although bempedoic acid should not alter the exposure of warfarin, their combination deserves a close monitoring of INR (Table 6). Differently, no DDIs are predicted in bempedoic acid and direct oral anticoagulants (DOAC), whose pharmacokinetics are mainly determined by the expression and function of P-gp [74].

Table 6.
Possible DDIs of bempedoic acid and cardiovascular drugs.
Safe co-administration can also be predicted with antiarrhythmic agents, while the systemic exposure of bosentan, a drug approved for the treatment of pulmonary arterial hypertension, can be increased by bempedoic acid, since it is a substrate of OATP1B1 and OATP1B3 (Table 6) [15].
6.6. Potential DDIs of Bempedoic Acid with Antidepressant, Antipsychotic, and Antiepileptic Drugs
Different classes of antidepressant and antipsychotic drugs are extensively metabolized by CYP450 enzymes and may inhibit CYP2D6; however, both selective serotonin reuptake inhibitors (SSRI) and tricyclic antidepressants (TCA) are not influenced by the inhibition of OATP1B1/B3 and OAT3 transporters and, therefore, can be administered with bempedoic acid. Two antipsychotic drugs, clozapine and olanzapine, are associated with dyslipidemias, and, thus, the use of bempedoic acid can be envisioned to control plasma lipid levels (Table 6). Antiepileptic drugs, including carbamazepine, phenobarbital, and phenytoin, are considered to be strong inducers of CYP450 and P-gp and can interact with statins [76], while no changes in the pharmacokinetics of bempedoic acid are expected (Table 7). Finally, St. John’s Wort is a strong inducer of different cytochromes and P-gp, while its active constituent, hyperforin, has been found to inhibit OATP1B1/B3. Thus, this phytotherapy is contraindicated with statins, but can be considered in the presence of bempedoic acid.

Table 7.
Possible DDIs of bempedoic acid with antidepressant, antipsychotic, and antiepileptic agents.
6.7. Bempedoic Acid and Uric Acid: Potential DDI
Considering bempedoic acid’s side effects, its administration in combination with loop and thiazide diuretics may determine a further increase in uric acid levels and, potentially, cases of gout [79]. Other drugs that can increase the uric acid levels and incidences of gout are reported in Table 8.

Table 8.
Drugs that can increase the uric acid levels and incidences of gout in the presence of bempedoic acid.
7. Conclusions
Bempedoic acid represents a novel and effective hypocholesterolemic agent to be prescribed either as monotherapy or in combination with existing lipid-lowering therapies in a broad spectrum of patients at high cardiovascular risk. The drug has shown an excellent safety profile with selective activation in hepatocytes, avoiding side effects on skeletal muscles and glycemic homeostasis. Its use is associated with increased plasma levels of creatinine and uric acid due to the mild and reversible inhibition of the renal transporters OAT2 and OAT3. The drug has a favorable pharmacokinetics profile whose metabolism and disposition do not rely on CYP450 and the P-gp transporter, although it is a weak inhibitor of hepatic transporters OATP1B1 and OATP1B3. Thus, its use could be safer than statins, considering their muscle-related side effects and their CYP450-mediated metabolism. Although direct clinical evidence of DDIs with bempedoic acid is limited to statins [15], metformin [19], and probenecid [17], the results of a 2-year follow-up of a real-world evaluation of the effectiveness and safety of bempedoic acid reported a good safety profile for the drug. Overall, 0.3% (5/973) of patients had serious ADRs considered to be related to bempedoic acid. Among these adverse drug reactions, myalgia was the most common, occurring in 3.5% (34/973) of patients [92]. Thus, the safety profile of bempedoic acid in this real-world population is consistent with that observed in the CLEAR clinical trial program [11,13,93], suggesting a minor incidence of clinically relevant drug interactions in complicated and polytreated patients.
Bempedoic acid–glucuronide is also a substrate of OAT3, and thus any drugs with inhibitory actions on this transporter might reduce its clearance and potentially increase the plasma levels of uremic acid and creatinine. Given its favorable profile, bempedoic acid can be easily associated with other therapies and potentially be utilized in substitutions with statins in cases of intolerance.
Author Contributions
Writing—original draft preparation, N.F.; writing—review and editing, A.C. and E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by Daiichi-Sankyo Italia S.p.A.
Conflicts of Interest
The authors declare that this study received funding from Daiichi-Sankyo Italia S.p.A. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
References
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef]
- Kastelein, J.J.; Akdim, F.; Stroes, E.S.; Zwinderman, A.H.; Bots, M.L.; Stalenhoef, A.F.; Visseren, F.L.; Sijbrands, E.J.; Trip, M.D.; Stein, E.A.; et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 2008, 358, 1431–1443. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Ference, B.A.; Braunwald, E.; Catapano, A.L. The LDL cumulative exposure hypothesis: Evidence and practical applications. Nat. Rev. Cardiol. 2024, 21, 701–716. [Google Scholar] [CrossRef]
- Gaba, P.; O’Donoghue, M.L.; Park, J.G.; Wiviott, S.D.; Atar, D.; Kuder, J.F.; Im, K.; Murphy, S.A.; De Ferrari, G.M.; Gaciong, Z.A.; et al. Association Between Achieved Low-Density Lipoprotein Cholesterol Levels and Long-Term Cardiovascular and Safety Outcomes: An Analysis of FOURIER-OLE. Circulation 2023, 147, 1192–1203. [Google Scholar] [CrossRef]
- Pinkosky, S.L.; Newton, R.S.; Day, E.A.; Ford, R.J.; Lhotak, S.; Austin, R.C.; Birch, C.M.; Smith, B.K.; Filippov, S.; Groot, P.H.E.; et al. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nat. Commun. 2016, 7, 13457. [Google Scholar] [CrossRef]
- Banach, M.; Duell, P.B.; Gotto, A.M., Jr.; Laufs, U.; Leiter, L.A.; Mancini, G.B.J.; Ray, K.K.; Flaim, J.; Ye, Z.; Catapano, A.L. Association of Bempedoic Acid Administration With Atherogenic Lipid Levels in Phase 3 Randomized Clinical Trials of Patients With Hypercholesterolemia. JAMA Cardiol. 2020, 5, 1124–1135. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Laufs, U.; Ray, K.K.; Leiter, L.A.; Bays, H.E.; Goldberg, A.C.; Stroes, E.S.; MacDougall, D.; Zhao, X.; Catapano, A.L. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur. J. Prev. Cardiol. 2020, 27, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Nicholls, S.J.; Li, N.; Louie, M.J.; Brennan, D.; Lincoff, A.M.; Nissen, S.E.; CLEAR OUTCOMES Committees and Investigators. Efficacy and safety of bempedoic acid among patients with and without diabetes: Prespecified analysis of the CLEAR Outcomes randomised trial. Lancet Diabetes Endocrinol. 2024, 12, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N. Engl. J. Med. 2023, 388, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Menon, V.; Nicholls, S.J.; Brennan, D.; Laffin, L.; Ridker, P.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Cho, L.; et al. Bempedoic Acid for Primary Prevention of Cardiovascular Events in Statin-Intolerant Patients. JAMA 2023, 330, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Nilemdo: Summary of Product Characteristics. 2024. Available online: https://www.ema.europa.eu/en/documents/product-information/nilemdo-epar-product-information_en.pdf (accessed on 15 September 2024).
- Nilemdo. 2024. Available online: https://www.ema.europa.eu/en/documents/assessment-report/nilemdo-epar-public-assessment-report_en.pdf (accessed on 15 September 2024).
- Hanselman, J.C.; MacDougall, D.; Emery, M.G.; Sasiela, W.; Amore, B.M. A phase 1 drugdrug interaction study assessing the effects of steady state probenicid on single-dose bempedoic acid pharmacokinetics in healthy subjects. Clin. Pharmacol. Ther. 2020, 107, S43. [Google Scholar]
- Amore, B.M.; Sasiela, W.J.; Ries, D.K.; Tresh, P.; Emery, M.G. Pharmacokinetics of bempedoic acid in patients with renal impairment. Clin. Transl. Sci. 2022, 15, 789–798. [Google Scholar] [CrossRef]
- Emery, M.G.; Hanselman, J.H.; MacDougall, D.; Amore, B.; Sasiela, W.; McGonigal, J. Effect of bempedoic acid on the pharmacokinetics and bempedoic acid (BA) effect on metformin (MET) pharmacokinetics (PK) and pharmacodynamics (PD) in patients with type 2 diabetes (T2D): In vitro–in vivo correlation. J. Clin. Pharmacol. Ther. 2020, 107, S43. [Google Scholar]
- Amore, B.M.; Cramer, C.; MacDougall, D.; Emery, M.G. The Disposition and Metabolism of Bempedoic Acid, a Potent Inhibitor of ATP Citrate Lyase, in Healthy Human Subjects. Drug Metab. Dispos. 2023, 51, 599–609. [Google Scholar] [CrossRef]
- Oniciu, D.C.; Dasseux, J.L.; Yang, J.; Mueller, R.; Pop, E.; Denysenko, A.; Duan, C.; Huang, T.B.; Zhang, L.; Krause, B.R.; et al. Influence of various central moieties on the hypolipidemic properties of long hydrocarbon chain diols and diacids. J. Med. Chem. 2006, 49, 334–348. [Google Scholar] [CrossRef]
- Nexletol. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211616s000lbl.pdf (accessed on 15 September 2024).
- Ferri, N.; Corsini, A. Mechanism of bempedoic acid induced cholelithiasis: A role for statins to limit this adverse effect? Pharmacol. Res. 2023, 196, 106900. [Google Scholar] [CrossRef]
- Caroli-Bosc, F.X.; Le Gall, P.; Pugliese, P.; Delabre, B.; Caroli-Bosc, C.; Demarquay, J.F.; Delmont, J.P.; Rampal, P.; Montet, J.C. Role of fibrates and HMG-CoA reductase inhibitors in gallstone formation: Epidemiological study in an unselected population. Dig. Dis. Sci. 2001, 46, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Group, S.C.; Link, E.; Parish, S.; Armitage, J.; Bowman, L.; Heath, S.; Matsuda, F.; Gut, I.; Lathrop, M.; Collins, R. SLCO1B1 variants and statin-induced myopathy—A genomewide study. N. Engl. J. Med. 2008, 359, 789–799. [Google Scholar] [CrossRef]
- Niemi, M.; Pasanen, M.K.; Neuvonen, P.J. Organic anion transporting polypeptide 1B1: A genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol. Rev. 2011, 63, 157–181. [Google Scholar] [CrossRef]
- Corsini, A.; Bellosta, S. Drug-drug interaction with statins. Expert. Rev. Clin. Pharmacol. 2008, 1, 105–113. [Google Scholar] [CrossRef]
- Prueksaritanont, T.; Subramanian, R.; Fang, X.; Ma, B.; Qiu, Y.; Lin, J.H.; Pearson, P.G.; Baillie, T.A. Glucuronidation of statins in animals and humans: A novel mechanism of statin lactonization. Drug Metab. Dispos. 2002, 30, 505–512. [Google Scholar] [CrossRef]
- Prueksaritanont, T.; Tang, C.; Qiu, Y.; Mu, L.; Subramanian, R.; Lin, J.H. Effects of fibrates on metabolism of statins in human hepatocytes. Drug Metab. Dispos. 2002, 30, 1280–1287. [Google Scholar] [CrossRef]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions. Expert Opin. Drug Saf. 2012, 11, 933–946. [Google Scholar] [CrossRef]
- Ferri, N.; Bellosta, S.; Baldessin, L.; Boccia, D.; Racagni, G.; Corsini, A. Pharmacokinetics interactions of monoclonal antibodies. Pharmacol. Res. 2016, 111, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Leqvio: Summary of Product Characteristics. 2024. Available online: https://www.ema.europa.eu/en/documents/product-information/leqvio-epar-product-information_en.pdf (accessed on 15 September 2024).
- Hebert, M.F. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv. Drug Deliv. Rev. 1997, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Yigitaslan, S.; Erol, K.; Cengelli, C. The Effect of P-Glycoprotein Inhibition and Activation on the Absorption and Serum Levels of Cyclosporine and Tacrolimus in Rats. Adv. Clin. Exp. Med. 2016, 25, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Cakaloglu, Y.; Tredger, J.M.; Devlin, J.; Williams, R. Importance of cytochrome P-450IIIA activity in determining dosage and blood levels of FK 506 and cyclosporine in liver transplant recipients. Hepatology 1994, 20, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Farasyn, T.; Crowe, A.; Hatley, O.; Neuhoff, S.; Alam, K.; Kanyo, J.; Lam, T.T.; Ding, K.; Yue, W. Preincubation With Everolimus and Sirolimus Reduces Organic Anion-Transporting Polypeptide (OATP)1B1- and 1B3-Mediated Transport Independently of mTOR Kinase Inhibition: Implication in Assessing OATP1B1- and OATP1B3-Mediated Drug-Drug Interactions. J. Pharm. Sci. 2019, 108, 3443–3456. [Google Scholar] [CrossRef]
- Picard, N.; Levoir, L.; Lamoureux, F.; Yee, S.W.; Giacomini, K.M.; Marquet, P. Interaction of sirolimus and everolimus with hepatic and intestinal organic anion-transporting polypeptide transporters. Xenobiotica 2011, 41, 752–757. [Google Scholar] [CrossRef]
- Teriflunomide: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/teriflunomide-mylan-epar-product-information_en.pdf (accessed on 15 September 2024).
- Arava: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/arava-epar-product-information_en.pdf (accessed on 15 September 2024).
- Choe, J.Y.; Kim, S.K. Association between serum uric acid and inflammation in rheumatoid arthritis: Perspective on lowering serum uric acid of leflunomide. Clin. Chim. Acta 2015, 438, 29–34. [Google Scholar] [CrossRef]
- Mycophenolate Mofetil: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/cellcept-epar-product-information_en.pdf (accessed on 15 September 2024).
- Ding, X.; Zhu, X.; Zhang, Y.; Zhang, L.; Cheng, M.; Yu, Y.; Xu, L.; Li, G. Influence of serum uric acid levels in response to the conversion from mycophenolate mofetil to mizoribine in kidney transplant recipients. Transplant. Proc. 2013, 45, 190–193. [Google Scholar] [CrossRef]
- Nubeqa: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/nubeqa-epar-product-information_en.pdf (accessed on 15 September 2024).
- Xtandi: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/xtandi-epar-product-information_en.pdf (accessed on 15 September 2024).
- Tukysa: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/tukysa-epar-product-information_en.pdf (accessed on 15 September 2024).
- Afinitor: Summary of Product Characteristics. 2024. Available online: https://www.ema.europa.eu/en/documents/product-information/afinitor-epar-product-information_en.pdf (accessed on 15 September 2024).
- Rapamune: Summary of Product Characteristics. 2024. Available online: https://www.ema.europa.eu/en/documents/product-information/rapamune-epar-product-information_en.pdf (accessed on 15 September 2024).
- Tamoxifene, R. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_001561_033688_RCP.pdf&retry=0&sys=m0b1l3 (accessed on 15 September 2024).
- Keller, D.N.; Medwid, S.J.; Ross, C.D.; Wigle, T.J.; Kim, R.B. Impact of organic anion transporting polypeptide, P-glycoprotein, and breast cancer resistance protein transporters on observed tamoxifen and endoxifen concentration and adverse effects. Pharmacogenet Genom. 2023, 33, 10–18. [Google Scholar] [CrossRef]
- Glivec: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/glivec-epar-product-information_en.pdf (accessed on 15 September 2024).
- Kaletra: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/kaletra-epar-product-information_en.pdf (accessed on 15 September 2024).
- Annaert, P.; Ye, Z.W.; Stieger, B.; Augustijns, P. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica 2010, 40, 163–176. [Google Scholar] [CrossRef]
- Tivicay: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/tivicay-epar-product-information_en.pdf (accessed on 15 September 2024).
- Howard, P.; Twycross, R.; Grove, G.; Charlesworth, S.; Mihalyo, M.; Wilcock, A. Rifampin (INN Rifampicin). J. Pain. Symptom Manag. 2015, 50, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Ketek: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/ketek-epar-product-information_en.pdf (accessed on 15 September 2024).
- Higgins, J.W.; Ke, A.B.; Zamek-Gliszczynski, M.J. Clinical CYP3A inhibitor alternatives to ketoconazole, clarithromycin and itraconazole, are not transported into the liver by hepatic organic anion transporting polypeptides and organic cation transporter 1. Drug Metab. Dispos. 2014, 42, 1780–1784. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Maeda, K.; Shitara, Y.; Sugiyama, Y. Drug-drug interaction between pitavastatin and various drugs via OATP1B1. Drug Metab. Dispos. 2006, 34, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Atazanavir: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/atazanavir-krka-epar-product-information_en.pdf (accessed on 15 September 2024).
- Norvir: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/norvir-epar-product-information_en.pdf (accessed on 15 September 2024).
- Claritromicina, R. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_004852_044779_RCP.pdf&retry=0&sys=m0b1l3 (accessed on 15 September 2024).
- Ketoconazole: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/ketoconazole-hra-epar-product-information_en.pdf (accessed on 15 September 2024).
- Vfend: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/vfend-epar-product-information_en.pdf (accessed on 15 September 2024).
- Fungitraxx: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/fungitraxx-epar-product-information_en.pdf (accessed on 15 September 2024).
- Macha, S.; Koenen, R.; Sennewald, R.; Schone, K.; Hummel, N.; Riedmaier, S.; Woerle, H.J.; Salsali, A.; Broedl, U.C. Effect of gemfibrozil, rifampicin, or probenecid on the pharmacokinetics of the SGLT2 inhibitor empagliflozin in healthy volunteers. Clin. Ther. 2014, 36, 280–290.e281. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.Y.; Bleasby, K.; Yabut, J.; Cai, X.; Chan, G.H.; Hafey, M.J.; Xu, S.; Bergman, A.J.; Braun, M.P.; Dean, D.C.; et al. Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J. Pharmacol. Exp. Ther. 2007, 321, 673–683. [Google Scholar] [CrossRef]
- Shin, E.; Shin, N.; Oh, J.H.; Lee, Y.J. High-Dose Metformin May Increase the Concentration of Atorvastatin in the Liver by Inhibition of Multidrug Resistance-Associated Protein 2. J. Pharm. Sci. 2017, 106, 961–967. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Jardiance: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/jardiance-epar-product-information_en.pdf (accessed on 15 September 2024).
- Obermeier, M.; Yao, M.; Khanna, A.; Koplowitz, B.; Zhu, M.; Li, W.; Komoroski, B.; Kasichayanula, S.; Discenza, L.; Washburn, W.; et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab. Dispos. 2010, 38, 405–414. [Google Scholar] [CrossRef]
- Trajenta: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/trajenta-epar-product-information_en.pdf (accessed on 15 September 2024).
- Ferri, N.; Colombo, E.; Tenconi, M.; Baldessin, L.; Corsini, A. Drug-Drug Interactions of Direct Oral Anticoagulants (DOACs): From Pharmacological to Clinical Practice. Pharmaceutics 2022, 14, 1120. [Google Scholar] [CrossRef]
- Ferri, N.; Corsini, A. Nuovi anticoagulanti orali: Considerazioni di farmacologia clinica. G. Ital. Di Di Cardiol. 2015, 16, 3S–16S. [Google Scholar]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions: An update. Expert Opin. Drug Saf. 2018, 17, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.J.; Barecki-Roach, M.; Johnson, W.W. Quantitative characterization of direct P-glycoprotein inhibition by St John’s wort constituents hypericin and hyperforin. J. Pharm. Pharmacol. 2004, 56, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.F.; Acharya, M.R.; Desai, N.; Figg, W.D.; Sparreboom, A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol. Ther. 2005, 4, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.; Yip, K.; Pillinger, M.H.; Toprover, M. Lowering and Raising Serum Urate Levels: Off-Label Effects of Commonly Used Medications. Mayo Clin. Proc. 2022, 97, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Caspi, D.; Lubart, E.; Graff, E.; Habot, B.; Yaron, M.; Segal, R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum. 2000, 43, 103–108. [Google Scholar] [CrossRef]
- Ohtsu, N.; Anzai, N.; Fukutomi, T.; Kimura, T.; Sakurai, H.; Endou, H. Human renal urate transpoter URAT1 mediates the transport of salicylate. Nihon Jinzo Gakkai Shi 2010, 52, 499–504. [Google Scholar]
- El-Sheikh, A.A.; van den Heuvel, J.J.; Koenderink, J.B.; Russel, F.G. Effect of hypouricaemic and hyperuricaemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br. J. Pharmacol. 2008, 155, 1066–1075. [Google Scholar] [CrossRef]
- Ben Salem, C.; Slim, R.; Fathallah, N.; Hmouda, H. Drug-induced hyperuricaemia and gout. Rheumatology 2017, 56, 679–688. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Z.; Yang, Y.; Xu, Y.; Li, G.; Liu, T. Ticagrelor-related gout: An underestimated side effect. Int. J. Cardiol. 2015, 192, 11–13. [Google Scholar] [CrossRef]
- Jutabha, P.; Anzai, N.; Wempe, M.F.; Wakui, S.; Endou, H.; Sakurai, H. Apical voltage-driven urate efflux transporter NPT4 in renal proximal tubule. Nucleosides Nucleotides Nucleic Acids 2011, 30, 1302–1311. [Google Scholar] [CrossRef]
- Hagos, Y.; Stein, D.; Ugele, B.; Burckhardt, G.; Bahn, A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J. Am. Soc. Nephrol. 2007, 18, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.; Searle, M.; O’Donnell, J.; Chapman, P. Gout in solid organ transplantation: A challenging clinical problem. Drugs 2005, 65, 2593–2611. [Google Scholar] [CrossRef]
- Bahn, A.; Hagos, Y.; Reuter, S.; Balen, D.; Brzica, H.; Krick, W.; Burckhardt, B.C.; Sabolic, I.; Burckhardt, G. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J. Biol. Chem. 2008, 283, 16332–16341. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Fogh-Andersen, N.; Leyssac, P.P.; Strandgaard, S. Glomerular and tubular function in renal transplant patients treated with and without ciclosporin A. Nephron 1998, 80, 450–457. [Google Scholar] [CrossRef]
- Kanbay, M.; Akcay, A.; Huddam, B.; Usluogullari, C.A.; Arat, Z.; Ozdemir, F.N.; Haberal, M. Influence of cyclosporine and tacrolimus on serum uric acid levels in stable kidney transplant recipients. Transplant. Proc. 2005, 37, 3119–3120. [Google Scholar] [CrossRef] [PubMed]
- Hosoyamada, M.; Takiue, Y.; Shibasaki, T.; Saito, H. The effect of testosterone upon the urate reabsorptive transport system in mouse kidney. Nucleosides Nucleotides Nucleic Acids 2010, 29, 574–579. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Wouter Jukema, J.; Koskinas, K.; Ray, K.; Averna, M.; Stulnig, T.; Vanassche, T.; Climente, M.; Lamparter, M.; Soronen, J.; et al. Real-world effectiveness and safety of bempedoic acid in Europe: Final 2-year results from the MILOS German cohort. In Proceedings of the DGK Hertztage, Hamburg, Germany, 26–28 September 2024. [Google Scholar]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M.; Trial, C.H. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. N. Engl. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).