Abstract
Viral diseases represent a major public health concerns and ever-present risks for developing into future pandemics. Antiviral antibody therapeutics, either alone or in combination with other therapies, emerged as valuable preventative and treatment options, including during global emergencies. Here we will discuss polyclonal and monoclonal antiviral antibody therapies, focusing on the unique biochemical and physiological properties that make them well-suited as therapeutic agents. We will describe the methods of antibody characterization and potency assessment throughout development, highlighting similarities and differences between polyclonal and monoclonal products as appropriate. In addition, we will consider the benefits and challenges of antiviral antibodies when used in combination with other antibodies or other types of antiviral therapeutics. Lastly, we will discuss novel approaches to the characterization and development of antiviral antibodies and identify areas that would benefit from additional research.
1. Introduction
Infectious diseases are a major global health burden with eight major diseases—HIV/AIDS, malaria, measles, hepatitis, dengue fever, rabies, tuberculosis and yellow fever—exacting a heavy toll in terms of human lives lost [1]. The coronavirus disease 2019 (COVID-19) pandemic further exacerbated the cost to human life and long-term health outcomes. Emerging and re-emerging viral diseases, such as Ebola, Zika, Lassa fever, measles, highly pathogenic avian influenza, etc., continue to pose a risk not only for local/regional outbreaks, but also for becoming the next pandemic. The availability of safe and effective prophylaxis and treatment options for these and other infectious diseases is a top public health priority. Antibody therapeutics have long been used in viral disease settings; for example, post-exposure prophylaxis for rabies or hepatitis B with respective hyper- or specific-immune globulin (IG, also known as immunoglobulin), or the use of monoclonal antibody (mAb) therapies for the prevention of respiratory syncytial virus (RSV) infection. Recent approvals of mAb therapies for human immunodeficiency virus type-1 (HIV-1) and Ebola virus (EBOV), as well as the rapid development and emergency use authorization of several mAbs against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for prophylaxis and treatment of COVID-19, further highlight the potential of these molecules, either alone or in combination with other therapies, to make a significant impact on public health. In this review, we will discuss the biochemical and physiological characteristics that render antibody molecules desirable therapeutics, pre-clinical assays that can be used to assess potency, discuss the benefits and challenges of antibody combination therapies, and highlight areas in need of additional research.
2. Antibodies as Therapeutics
With very few exceptions, antibody therapeutics approved to date are isotype G immunoglobulins (IgG). IgGs are protein macromolecules secreted in the blood of most vertebrates [2] through differentiated plasma B cells that have a high affinity and specificity for their respective antigen. The IgG molecules can then be purified from human or animal plasma to produce polyclonal immune globulin products. These types of products, such as diphtheria antitoxin [3], represent some of the first products to be licensed in the United States. In over a century of development, polyclonal products underwent tremendous advances in the manufacturing process and characterization of safety and efficacy attributes. In the last few decades, antibody therapeutic development shifted toward the development of IgG monoclonal antibodies that are engineered for in vitro expression in mammalian cell lines. Candidate antibodies are identified via traditional hybridoma technology, as well as increasingly through using mice engineered to express human VH and VL genes [4], phage or yeast display technologies [5], isolating virus antigen specific B cells from convalescent patients [6,7,8,9], or a combination of these technologies [10].
The structural and functional features of IgG antibodies render them well suited for use as therapeutics. Structurally, the molecule can be thought of as modular, with two identical heavy chains (HC) and two identical light chains (LC). The IgG HC comprises four domains: one variable (V) domain and three constant (CH1, CH2, and CH3) domains, with a hinge region between the CH1 and CH2 domains (Figure 1a). The LC comprises two domains: a variable (V) domain and a constant (CL) domain. The fragment antigen binding (Fab) region in each chain contains both V and constant (CH1 or CL) domains, with the former housing the complementarity determining regions (CDR) responsible for epitope recognition and antibody specificity. When properly folded, the CDRs of the HC and LC come together to form the antigen-binding site. The fragment crystallizable (Fc) region, comprising the HC CH2 and CH3 domains, is responsible for downstream processes (Fc effector functions) that result in immune activation and the ultimate destruction of the antigen. There are four different IgG subclasses (IgG1, IgG2, IgG3 and IgG4), with respective polymorphic variants [11]. Each subclass has different affinities for Fc receptors, which impacts their ability to engage different effector cells and mediate effector functions [12]. Most mAbs, including those directed against viral diseases, belong to the IgG1 subclass, since IgG1 antibodies have long half-lives and can efficiently mediate a wide variety of effector functions. In addition, IgG antibodies have a single N-glycan in the constant region. These biochemical properties (i.e., sequence and glycan structures) play an important role in physicochemical (i.e., stability, shelf-life), pharmacokinetic, and pharmacodynamic properties of the antibody therapeutic, and, thus, should be well characterized during development.
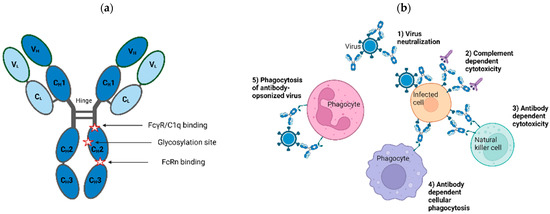
Figure 1.
Structural and functional features of isotype G immunoglobulins (IgG). (a) Structural features of IgG antibodies. IgG macromolecule is a tetramer of two identical heavy (H) and light (L) chains depicted in dark and light blue, respectively, each containing variable (VH, VL) and constant (CH, CL) regions, as shown. Glycosylation site and locations responsible for receptor and complement binding are marked. These regions can be engineered to modulate downstream properties of IgG products. (b) Antiviral functions of IgG antibodies. Antiviral pharmacologic properties of antibody therapies are as follows: (1) neutralization of viral entry to its cell target; (2) complement- and (3) antibody-mediated cytotoxicity of infected cells; (4) phagocytosis of infected cells; and (5) clearing of opsonized virus through phagocytosis. Figure created with BioRender.com, accessed on 17 May 2023.
The use of IgG products as prophylactic and therapeutic modalities for viral diseases is predicated on their ability to bind to one or more antigens on the surface of viral particles and/or infected cells via the antigen-binding sites. They can neutralize the ability of viruses to enter cells through blocking attachment or fusion, inactivating/disrupting virus particles, or triggering the killing of infected cells through Fc-mediated effector functions (Figure 1b; see [13,14,15,16] for a comprehensive review of the mechanism of virus neutralization). For the latter function, the antigen-antibody complex is recognized by effector molecules, such as the C1q component of complement or Fc gamma receptors (FcγRs) present on the surface of effector cells, giving rise to immune signaling cascades that culminate with the clearance of viruses and/or infected cells. In some cases, Fc effector functions are shown to enhance the antiviral activity of specific antibodies [16,17,18,19,20].
On the other hand, antibody-dependent enhancement (ADE) of viral infection or disease can also occur [21], as has been well documented in humans for dengue virus [22]. ADE can arise after natural infection, vaccination, or passive transfer of antibody therapies. It is widely thought that ADE occurs when antibodies of insufficient avidity or concentration are unable to neutralize the virus, but can facilitate the uptake of the virus-antibody complex through FcγR-bearing cells, such as monocytes, dendritic cells, or macrophages [23], resulting in increased viral production, enhanced immune activation (e.g., cytokine production), and more severe disease cases [24]. In addition to flaviviruses [25,26], ADE is observed for mAbs against influenza virus, HIV-1, and EBOV in cell culture, but not typically when tested in animal models or clinical trials, with a few exceptions [23,25]. When selecting antibodies best suited for use as an antiviral product, it is critical to optimize binding both to the antigen and FcγRs. For mAbs, the risk of ADE can be reduced through selection of a particular IgG subclass [27], modification of Fc glycans, or engineering substitutions into the Fc region that disrupt FcγR binding; however, these substitutions may also disrupt Fc effector functions that could contribute to clinical efficacy [28,29]. Although ADE in cell culture and animal studies was observed with antiviral specific polyclonal IGs [30], clinical ADE was not reported to our knowledge for any FDA-approved specific IG products.
During pharmaceutical development, mAb domains often undergo extensive biochemical engineering to optimize the properties of the antibody. For example, to humanize mAbs derived from mice or other species, the CDRs can be grafted onto the framework regions of V domains from other mAbs or germline V genes while retaining their antigen-binding properties in the context of a known protein fold [31]. In general, all mAb V regions are engineered to improve manufacturability and stability and optimize binding [32]. The Fc region can also be modified to alter pharmacokinetic properties and effector functions. On the other hand, although not subjected to Fc engineering, depending on the antigen or donor population, specific antiviral polyclonal IGs can be “enriched” for a particular isotype [33], subclass, or glycosylation signature, leading to different Fc effector functions compared to other polyclonal IG products. For example, IgG1 and IgG4 are the most prevalent subclasses following measles infection or vaccination, with significant differences in titers in infected versus vaccinated individuals [34]. In addition, anti-SARS-CoV-2 antibodies from convalescent donors have distinct glycosylation patterns depending on disease severity [35]. We will discuss some of the methods currently used to design, produce, and characterize antibody products, highlighting the differences between polyclonal and monoclonal antibody therapies.
2.1. Production and Characterization of Antibody Therapies
2.1.1. Specific Polyclonal Antibody Therapies
Specific polyclonal IG (SpIG) is used as the overarching term for all polyclonal preparations that are enriched for certain antiviral, antibacterial, or antitoxin antibodies. SpIGs are purified from plasma of humans who were vaccinated or recovered from a specific infection or animals that were vaccinated with a specific pathogen preparation or toxin. The first products were developed in 1898 and comprised little more than serum from horses vaccinated with virus preparations, bacterial toxins, or snake venom. In 1903, diphtheria antitoxin made from vaccinated horses became the first licensed product in the United States.
SpIGs from animal sources are produced via repeatedly immunizing donor animals. Advantages of large animal donors (horses, sheep, or cattle) include the ability to immunize more frequently (which increases the yield and avidity of specific antibodies), use experimental vaccinations, and safely collect larger volumes of plasma. A major disadvantage includes potential allergic reactions in patients due to animal proteins, including the antibodies. Animal-derived antibodies are often treated with pepsin or trypsin to remove the Fc portion and reduce immunogenicity. These fragments lack effector functions that could be important for antibody activity, depending upon the virus. An interesting strategy has been developed using transchromosomic cattle that produce full-length human IgG antibodies. The cattle are knocked out for bovine antibody heavy and lambda light chains, but contain an artificial chromosome encoding the respective human IgG chains. Chimeric antibodies consisting of human IgG heavy chains and bovine kappa light chains are removed during manufacturing [36]; thus, the resulting IG product manufactured from these bovines contain only human IgGs, lowering the risk of immunogenicity. These transchromosomic bovines were successfully hyperimmunized [37].
Research during World War II stimulated a major breakthrough in purification of IGs and other proteins from human plasma. IG purification methods are usually based on sequential alcohol precipitations, each with specific conditions of pH, ionic strength, temperature, protein concentration, and alcohol concentration [38,39]. For some products, purely chromatographic methods or caprylate precipitation methods have partially or completely supplanted alcohol precipitation. These changes are often driven by the need to increase yield of IgG, thus increasing product availability [40]. Nevertheless, alcohol-based fractionation remains the backbone of early steps in production of most IG products and is often combined with subsequent caprylate or polyethylene glycol precipitations. Modern IG products are further purified using column chromatography to remove unwanted plasma proteins or viral inactivating compounds used for upstream manufacturing steps. A minimum of two orthogonal, robust, dedicated viral clearance steps are performed, which often include solvent-detergent treatment and nanofiltration, as well as other virucidal (caprylate, heat treatment, low pH) and partitioning (chromatography, precipitations, depth filtration) steps. All viral clearance steps must be validated and found to be robust using scaled-down models of the manufacturing process and actual manufacturing intermediates spiked with virus as starting material. It should be emphasized that modern IG purification is highly complex with multiple steps, each of which must be controlled to result in a safe and intact product. Every manufacturing method is unique with respect to purification details and methodology (such as mixing speeds, equipment used, precipitation times, buffer types and concentrations, centrifugation vs. precipitation), as well as the equipment. Thus, each product is also unique with respect to levels and types of plasma protein impurities and IG stability.
Antibody enrichment for human antibodies is achieved through either immunizing donors or screening and selecting high-titer plasma from routine donations (as for Cytogam [41]) or convalescent donors (as for early versions of SARS-CoV-2 IG investigational products [42,43]). “Hyperimmune” polyclonal antibodies are derived from donors who were immunized intentionally for the purpose of obtaining high-titer plasma (e.g., rabies, vaccinia, or hepatitis B in humans). Nevertheless, convalescent plasma is often inaccurately referred to as “hyperimmune,” even though donors were not immunized. Under FDA-approved plasma center collection protocols, and after investigational safety studies are completed, hyperimmune plasma can be collected from consenting immunized donors. Human-derived, antiviral SpIG products licensed in the United States are shown in Table 1.

Table 1.
FDA-approved human polyclonal antibodies for prevention or treatment of viral diseases.
For purposes of final product testing, a validated bioassay demonstrating neutralization in cell culture or in animals is ideally performed for SpIG products. In special cases, adequate cell culture or animal models are not available at the time of licensure. In this situation, a binding assay is usually selected and validated for product release, contingent on discussions with FDA. Likewise, national or international IgG standards may be lacking. In these instances, an internal IgG standard is developed by the manufacturer.
Treatment Timing and Dosing for SpIG
Treatment timing relative to infection depends on demonstrable efficacy of the product for pre- or post-exposure prophylaxis. Pre- and post-exposure prophylaxis can be effective (if adequately dosed) largely because viral burdens are relatively low during early infection. Even if an infection was initiated, post-exposure prophylaxis attenuates disease severity of measles, HAV, and varicella zoster [47,53]. When vaccines are given concomitantly with specific IG, such as for rabies, passive immunization provides a defensive “bridge” that acts immediately to neutralize the virus until vaccine responses arise. It is important that the dose of rabies IG (RIG) is not so high that it suppresses the vaccine response. In such contexts, both a minimum and maximum potency should be defined to assure optimal function of both RIG and the vaccine. Pharmacokinetic studies performed in healthy immunocompetent human subjects are used to define the dose of SpIG that is needed to avoid suppression of vaccine responses, while still being able to provide protection until vaccine responses are sufficiently developed.
Treatment of symptomatic viral disease with SpIG is much more challenging and often ineffective. In these cases, the viral burden may exceed the capacity of the IG, viruses may be relatively inaccessible within infected cells or immune-privileged sites, and cellular immune responses may also be suppressed by the virus [59]. Notable lack of efficacy via specific IG for treatment of symptomatic infections, such as rabies, influenza, HAV, HBV, measles, and varicella, were observed. The time windows for effective post-exposure prophylaxis of each infection were established based on such failures. Treatment with CMVIG and HBVIG(IV) can prevent severe disease in transplanted patients but are not curative. Vaccinia Immune Globulin is used to treat severe complications (eczema vaccinatum and progressive vaccinia) resulting from live vaccinia virus vaccine (ACAM2000), which is used to prevent smallpox. The recently licensed replication-deficient vaccinia virus (Jynneos) also generates an immune response, and is thought to be incapable of causing eczema vaccinatum or progressive vaccinia. Both vaccines are indicated for prevention of smallpox. Jynneos is also licensed for prevention of monkeypox [60].
2.1.2. Monoclonal Antibodies
To date, the FDA has approved four mAb therapies to prevent or treat viral diseases (Table 2): palivizumab for prevention of RSV in pre-term infants and infants with other specific conditions, ibalizumab for treatment of HIV-1 in patients failing their current anti-retroviral regimen, and two products for treatment of Ebola virus disease resulting from Zaire ebolavirus. One of these products, known as Inmazeb, consists of three mAbs that target non-overlapping epitopes on EBOV glycoprotein, and represents the first co-formulated mAb cocktail approved by the FDA [61].

Table 2.
FDA-approved monoclonal antibodies for prevention or treatment of viral diseases.
Multiple mAbs are currently either in advanced stages of clinical development or were approved in other countries. Nirsevimab, which is a half-life extended mAb that targets the RSV fusion (F) protein [62], was recently approved by the European Medicines Agency for the prevention of RSV lower respiratory tract disease in neonates and infants during their first RSV season. In addition, three mAb products targeting the rabies virus glycoprotein were approved in other countries: two in India (Rabishield, a single mAb, and TwinRab, a cocktail of two mAbs [63]) and one in China (ormutivimab [64]).
Several mAbs and mAb combinations that target the SARS-CoV-2 spike protein were rapidly developed after the onset of the COVID-19 pandemic, and received emergency use authorization (EUA) from the FDA for the pre-exposure prophylaxis, post-exposure prophylaxis, and/or treatment of COVID-19. Although highly effective against early SARS-CoV-2 variants, these products are not currently authorized in the United States due to the emergence and widespread circulation of variants that are resistant to neutralization through these mAbs in cell culture [65,66,67,68,69,70,71]. However, if future variants emerge that are susceptible to these products, their authorization status may change. Refer to the FDA website for updated information on the status of EUAs for mAbs and other COVID-19 therapeutics [72].
In addition to the approved and previously authorized mAbs and those directed against SARS-CoV-2, many other mAbs were or are under development that target existing and emerging diseases [8,73,74,75].
Historically, therapeutic mAbs were derived from immunized mice or rats and engineered as chimeric (V regions from the original mAb expressed with a human constant regions) or humanized (CDRs from the original mAb grafted on to a human V region backbone) mAbs to reduce the immunogenicity due the “foreignness” of rodent mAbs in humans. Currently, most mAbs are of human origin, being derived from either “humanized” mice or other species that express human germline V(D)J region genes or from phage display libraries generated from human donor lymphocytes. However, many antiviral mAbs are isolated directly from previously infected patients [6,7,8,9]. Regardless of the source, many considerations inform the selection and engineering of candidate mAbs.
Engineering of mAbs
Most mAbs developed for viral diseases are, firstly, selected for their ability to neutralize virus entry. However, Fc effector functions play a major role in the immune system’s response to infectious diseases [19]. For mAbs, the contribution of Fc effector functions to disease protection were demonstrated in non-clinical studies for several viruses, including Ebola virus [16], HIV-1 [17,76,77], influenza [78], SARS-COV-2 [79], and Rift Valley fever virus [80]. However, ADE of infection or disease is a possible negative consequence of FcγR binding [21,81]. Therefore, depending on what is known about specific viral diseases, different approaches can be used to engineer the Fc region of mAbs to either enhance or diminish FcγR binding. Amino acid residues were identified in the IgG Fc region that contact the complement component C1q; FcγRs; or the neonatal Fc receptor (FcRn), which is responsible for the long half-life of IgG [82,83]. Substitutions can be engineered at these residues to alter Fc effector functions or extend the half-life of a mAb, which allows less frequent dosing [84]. The half-life of mAbs can also be extended through engineering the Fab region to alter its pH-dependent antigen binding properties, which, upon internalization, leads to antigen dissociation in acidic endosomes and subsequent degradation in lysosomes, while the unbound mAb is recycled back into circulation [85,86]. When combined with Fc modifications to extend antibody half-life, this type of engineering approach can greatly reduce antigen concentrations in plasma [87].
In addition to Fc engineering, there is a better understanding of specific Fc glycan structures and their association with different effector functions, e.g., afucosylated mAbs, which have better antibody dependent cellular cytotoxicity (ADCC) compared to highly fucosylated antibodies, while galactosylation is associated with complement dependent cytotoxicity (CDC) and can influence ADCC activity [88]. Furthermore, mAbs produced in cell culture have considerable heterogeneity in glycosylation patterns. Therefore, cell lines were engineered to produce mAbs with up to 100% afucosylation to enhance ADCC activity [82,89]. The understanding of the relationship between antibody glycan structures and Fc effector functions is ongoing, and additional strategies may be developed to further engineer mAb glycan structures. For example, the effect of galactosylation on ADCC activity may depend on the specific linkage of the galactose monosaccharide [90]. Fc effector functions can be reduced through introducing substitutions at the glycosylation site (N297) in the CH2 domain to prevent the addition of a glycan [91,92], thus providing another glycoengineering approach for antiviral mAbs.
Development of mAb Combinations
Three of the four approved monoclonal antiviral products are single mAbs; however, the anti-Ebola virus mAb cocktail of atoltivimab, maftivimab, odesivimab-ebgn was the first fixed dose co-formulated mAb combination product approved by the FDA. Many other mAbs are used in combination to treat viral diseases and for other indications, but only a few to date are co-formulated [93]. The advantage of antibody cocktails over a single mAb is that they might be less susceptible to escape, depending on the different targeted epitopes. As seen for the anti-SARS-CoV-2 mAb combinations previously authorized for the prophylaxis or treatment of COVID-19, they all target the SARS-CoV-2 receptor binding domain of the SARS-CoV-2 spike protein, but have little neutralization activity against current variants. MAbs that target regions outside the receptor binding domain could neutralize virus or mediate Fc effector functions and might be less susceptible to escape. For example, a recent report demonstrated that mAbs targeting the conserved fusion peptide region adjacent to the S2′ cleavage site of the spike protein are broadly neutralizing against betacoronaviruses [94].
2.2. Advantages and Disadvantages of Polyclonal and Monoclonal Antibodies
There are advantages and disadvantages when selecting a product for treatment or prophylaxis of viral diseases, some of which are summarized in Table 3. For approved products, the choice is often based on which products are available for a specific viral disease. For example, currently only SpIG products are approved in the United States to treat rabies, CMV, HBV, varicella or vaccinia, whereas only mAb therapies are approved to prevent or treat RSV, HIV-1, and EBOV disease. There are other considerations that also play a role in the development or deployment of antibody therapies in an infectious disease setting. Although resistance to polyclonal antibodies is reported [95], polyclonal antiviral products are less likely to result in treatment-emergent resistance or the formation of an antibody response to the treatment (anti-drug antibodies), whereas both issues are a larger concern for mAb products. On the other hand, given the relative ease of engineering, development, and production of mAbs, they are well suited for rapid development, especially in an emerging infectious disease setting. Both types of products can have drawbacks that include the potential to interfere with the immune response to the vaccine or natural infection, as well as specific diagnostics, and the potential to result in enhanced infection or disease, as already described. Despite these limitations, the benefit-to-risk ratio for these approved products is favorable, as demonstrated in clinical trials and through routine clinical use in viral disease settings.

Table 3.
Advantages and disadvantages of specific polyclonal and monoclonal antibodies for prophylaxis or treatment of viral diseases.
5. Future Directions and Conclusions
As the applications of antiviral antibody therapies expand, there are several areas that can benefit from further development. The international standardization of assays and reagents (e.g., viruses and cell lines) for measuring antibody activity could help address the variability in potency often observed for the same antibody in different assays or laboratories (e.g., 10–100-fold range in EC50 values for anti-SARS-CoV-2 mAbs) [220]. In addition, more work is needed to better understand the role of Fc effector functions in viral diseases using cell culture, animal models, and clinical studies. For example, it is not known if less understood mechanisms, such as antibody-dependent cellular trogocytosis, which has been demonstrated predominantly for mAbs developed for oncology indications [245], may also be contributors to antiviral activity. Trogocytosis is shown for some anti-HIV [246,247,248] and anti-SARS-CoV-2 [249] mAbs, but additional studies are needed to understand its contribution to overall activity of these mAbs and how broad a mechanism it may be across viral diseases. Furthermore, pre-clinical assays are being developed that are more physiologically relevant, especially potency assays that capture multiple functions of the antibody. For example, organ-on-a-chip and microphysiological systems can incorporate multiple cell types, including immune cells; simulate blood flow and organ perfusion; and provide data that serves as a bridge between standard cell culture assays and clinical studies [250]. Although still early in development and not commonly used in regulatory applications, such technologies are expected to become increasingly powerful and more widely used. These systems can also help address ethical concerns and societal pressures to replace, reduce, and refine animal research, and they have the potential to provide information that is more predictive of clinical efficacy.
Another area with unharnessed potential, especially for SpIG therapies, is the selection of specific glycosylation signatures to modulate downstream immune responses [251]. These strategies were proposed for use in the setting of autoimmune disease, but they could also potentially be applied to viral diseases. When combined with other novel technologies, such as the production of recombinant IG preparations [252], such methods have the potential to result in antiviral antibody preparations with improved properties.
Production of SpIG from convalescent plasma in a pandemic setting remains time-consuming and challenging. Convalescent plasma is often the earliest available antibody-containing treatment that could be effective for prevention of severe disease. Advances in technologies that can be used to rapidly and inexpensively select donations containing high titers of neutralizing antibodies from among thousands of donations are needed both for direct use of convalescent plasma and manufacturing of SpIG. Biosensor-based methods that reliably measure neutralizing potency in plasma donations and products, and that can be used in a low biocontainment (BSL-2) setting, are promising. In addition to improved donor screening, technical advances in manufacturing that would maximize the yield of SpIG during a pandemic could include affinity matrices or changes in manufacturing steps to allow virus-specific IgM to copurify with IgG.
For mAbs, large volumes of product are usually infused intravenously over several hours. The length of infusion time may depend on the amount of mAb needed per body weight and whether a patient is experiencing infusion-related reactions typical of mAbs. One strategy that was previously used to address administration barriers was co-formulating a high concentration of the mAb with recombinant human hyaluronidase [253]. This enzyme degrades hyaluronic acid in the extracellular matrix, facilitating rapid delivery of large volume subcutaneous injections and increasing the bioavailability of the product. This outcome was previously accomplished with several mAbs for oncology, including the combination of rituximab, trastuzumab, daratumumab or trastuzumab, and pertuzumab with recombinant human hyaluronidase [254]. This approach is being studied with an anti-HIV-1 mAb (clinicaltrials.gov #NCT03538626 and [208,255]). These formulations provide more convenient dosing for patients, but must be supported with adequate non-clinical and clinical safety data.
Other developments for anti-viral mAbs include bispecific antibodies [256], single domain antibodies derived from camelids [257], and other scaffold proteins, such as DARPIns [258] and Adnectins [259], which are engineered in the loop regions between more structured regions of the core domain to mimic antibody CDRs. Some of these technologies may be able to target epitopes that are difficult for traditional antibodies to recognize. Furthermore, these novel constructs may be more cost effective to manufacture than mAb cocktails, and lower doses may be as effective as higher doses of a mAb cocktail. However, clinical studies are needed to determine efficacy and safety and to see if there are issues, such as immunogenicity, related to these novel products.
Whether alone or in combination, antiviral antibody therapies can provide important prophylaxis and treatment options to help relieve the burden of viral diseases. This space is rapidly evolving, and, as more experience is gained through successful clinical applications, the products of the future have the potential to overcome many of the challenges we describe, while continuing to fulfill the promise of safety and effectiveness.
Author Contributions
Conceptualization, writing and editing—all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
References
- Armitage, C. The high burden of infectious disease. Nature 2021, 598, S9. [Google Scholar] [CrossRef]
- Parra, D.; Takizawa, F.; Sunyer, J.O. Evolution of B Cell Immunity. Annu. Rev. Anim. Biosci. 2013, 1, 65–97. [Google Scholar] [CrossRef] [PubMed]
- CBER. Science and the Regulation of Biological Products. Available online: https://www.fda.gov/about-fda/histories-product-regulation/science-and-regulation-biological-products (accessed on 3 May 2023).
- Chen, W.C.; Murawsky, C.M. Strategies for Generating Diverse Antibody Repertoires Using Transgenic Animals Expressing Human Antibodies. Front. Immunol. 2018, 9, 460. [Google Scholar] [CrossRef]
- Sheehan, J.; Marasco, W.A. Phage and Yeast Display. Microbiol. Spectr. 2015, 3, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Wrammert, J.; Smith, K.; Miller, J.; Langley, W.A.; Kokko, K.; Larsen, C.; Zheng, N.-Y.; Mays, I.; Garman, L.; Helms, C.; et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008, 453, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Karuna, S.T.; Corey, L. Broadly Neutralizing Antibodies for HIV Prevention. Annu. Rev. Med. 2020, 71, 329–346. [Google Scholar] [CrossRef]
- Corti, D.; Lanzavecchia, A. Efficient Methods to Isolate Human Monoclonal Antibodies from Memory B Cells and Plasma Cells. Microbiol. Spectr. 2014, 2, 129–139. [Google Scholar] [CrossRef]
- Corti, D.; Misasi, J.; Mulangu, S.; Stanley, D.A.; Kanekiyo, M.; Wollen, S.; Ploquin, A.; Doria-Rose, N.A.; Staupe, R.P.; Bailey, M.; et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science 2016, 351, 1339–1342. [Google Scholar] [CrossRef]
- Marasco, W.A.; Sui, J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat. Biotechnol. 2007, 25, 1421–1434. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daëron, M. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Trkola, A. Humoral immunity to HIV-1: Neutralization and beyond. J. Intern. Med. 2007, 262, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Klasse, P.J. Neutralization of Virus Infectivity by Antibodies: Old Problems in New Perspectives. Adv. Biol. 2014, 2014, 157895. [Google Scholar] [CrossRef] [PubMed]
- Reading, S.A.; Dimmock, N.J. Neutralization of animal virus infectivity by antibody. Arch. Virol. 2007, 152, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Gunn, B.M.; Yu, W.-H.; Karim, M.M.; Brannan, J.M.; Herbert, A.S.; Wec, A.Z.; Halfmann, P.J.; Fusco, M.L.; Schendel, S.L.; Gangavarapu, K.; et al. A Role for Fc Function in Therapeutic Monoclonal Antibody-Mediated Protection against Ebola Virus. Cell Host Microbe 2018, 24, 221–233.e5. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Klein, F.; Pietzsch, J.; Seaman, M.S.; Nussenzweig, M.C.; Ravetch, J.V. Broadly Neutralizing Anti-HIV-1 Antibodies Require Fc Effector Functions for In Vivo Activity. Cell 2014, 158, 1243–1253. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Palese, P.; Wilson, P.C.; Ravetch, J.V. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J. Clin. Investig. 2016, 126, 605–610. [Google Scholar] [CrossRef]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2017, 18, 46–61. [Google Scholar] [CrossRef]
- Phelps, M.; Balazs, A.B. Contribution to HIV Prevention and Treatment by Antibody-Mediated Effector Function and Advances in Broadly Neutralizing Antibody Delivery by Vectored Immunoprophylaxis. Front. Immunol. 2021, 12, 734304. [Google Scholar] [CrossRef]
- Taylor, A.; Foo, S.-S.; Bruzzone, R.; Dinh, L.V.; King, N.J.C.; Mahalingam, S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015, 268, 340–364. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengue Antibody-Dependent Enhancement: Knowns and Unknowns. Microbiol. Spectr. 2014, 2, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Singh, G.; Acklin, J.; Lee, S.; Duehr, J.; Chokola, A.; Frere, J.; Hoffman, K.W.; Foster, G.A.; Krysztof, D.; et al. Dengue Virus Immunity Increases Zika Virus-Induced Damage during Pregnancy. Immunity 2019, 50, 751–762.e5. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Saiz, J.-C.; de Oya, N.J. Antibody-Dependent Enhancement and Zika: Real Threat or Phantom Menace? Front. Cell. Infect. Microbiol. 2018, 8, 44. [Google Scholar] [CrossRef]
- Ramadhany, R.; Hirai, I.; Sasaki, T.; Ono, K.-I.; Ramasoota, P.; Ikuta, K.; Kurosu, T. Antibody with an engineered Fc region as a therapeutic agent against dengue virus infection. Antivir. Res. 2015, 124, 61–68. [Google Scholar] [CrossRef]
- Kotaki, T.; Kurosu, T.; Grinyo-Escuer, A.; Davidson, E.; Churrotin, S.; Okabayashi, T.; Puiprom, O.; Mulyatno, K.C.; Sucipto, T.H.; Doranz, B.J.; et al. An affinity-matured human monoclonal antibody targeting fusion loop epitope of dengue virus with in vivo therapeutic potency. Sci. Rep. 2021, 11, 12987. [Google Scholar] [CrossRef]
- Lu, J.; Chen, L.; Du, P.; Guo, J.; Wang, X.; Jiang, Y.; Yu, Y.; Wang, R.; Yang, Z. A human monoclonal antibody to neutralize all four serotypes of dengue virus derived from patients at the convalescent phase of infection. Virology 2022, 576, 74–82. [Google Scholar] [CrossRef]
- Pinto, A.K.; Hassert, M.; Han, X.; Barker, D.; Carnelley, T.; Branche, E.; Steffen, T.L.; Stone, E.T.; Geerling, E.; Viramontes, K.M.; et al. The Ability of Zika virus Intravenous Immunoglobulin to Protect from or Enhance Zika Virus Disease. Front. Immunol. 2021, 12, 717425. [Google Scholar] [CrossRef]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef]
- Negron, C.; Fang, J.; McPherson, M.J.; Stine, W.B., Jr.; McCluskey, A.J. Separating clinical antibodies from repertoire antibodies, a path to in silico developability assessment. mAbs 2022, 14, 2080628. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.S.; Hoopes, E.M.; Falk, A.C.; Moore, D.J. A human IgM enriched immunoglobulin preparation, Pentaglobin, reverses autoimmune diabetes without immune suppression in NOD mice. Sci. Rep. 2022, 12, 11731. [Google Scholar] [CrossRef] [PubMed]
- Isa, M.B.; Martínez, L.C.; Ferreyra, L.J.; Giordano, M.O.; Barril, P.A.; Massachessi, G.; Nates, S.V. Measles Virus–Specific IgG4 Antibody Titer as a Serologic Marker of Post-vaccinal Immune Response. Viral Immunol. 2006, 19, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Siekman, S.L.; Pongracz, T.; Wang, W.; Nouta, J.; Kremsner, P.G.; da Silva-Neto, P.V.; Esen, M.; Kreidenweiss, A.; Held, J.; Trapé, Á.A.; et al. The IgG glycome of SARS-CoV-2 infected individuals reflects disease course and severity. Front. Immunol. 2022, 13, 993354. [Google Scholar] [CrossRef]
- Gardner, C.L.; Sun, C.; Luke, T.; Raviprakash, K.; Wu, H.; Jiao, J.-A.; Sullivan, E.; Reed, D.S.; Ryman, K.D.; Klimstra, W.B. Antibody Preparations from Human Transchromosomic Cows Exhibit Prophylactic and Therapeutic Efficacy against Venezuelan Equine Encephalitis Virus. J. Virol. 2017, 91, e00226-17. [Google Scholar] [CrossRef]
- Saied, A.A.; Nascimento, M.S.L.; Rangel, A.H.D.N.; Skowron, K.; Grudlewska-Buda, K.; Dhama, K.; Shah, J.; Abdeen, A.; El-Mayet, F.S.; Ahmed, H.; et al. Transchromosomic bovines-derived broadly neutralizing antibodies as potent biotherapeutics to counter important emerging viral pathogens with a special focus on SARS-CoV-2, MERS-CoV, Ebola, Zika, HIV-1, and influenza A virus. J. Med. Virol. 2022, 94, 4599–4610. [Google Scholar] [CrossRef]
- Cohn, E.J.; Strong, L.E.; Hughes, W.L.; Mulford, D.J.; Ashworth, J.N.; Melin, M.; Taylor, H.L. Preparation and Properties of Serum and Plasma Proteins. IV. A System for the Separation into Fractions of the Protein and Lipoprotein Components of Biological Tissues and Fluids. J. Am. Chem. Soc. 1946, 68, 459–475. [Google Scholar] [CrossRef]
- Oncley, J.L.; Melin, M.; Richert, D.A.; Cameron, J.W.; Gross, P.M. The Separation of the Antibodies, Isoagglutinins, Prothrombin, Plasminogen and β1-Lipoprotein into Subfractions of Human Plasma. J. Am. Chem. Soc. 1949, 71, 541–550. [Google Scholar] [CrossRef]
- Lebing, W.; Remington, K.M.; Schreiner, C.; Paul, H.-I. Properties of a new intravenous immunoglobulin (IGIV-C, 10%) produced by virus inactivation with caprylate and column chromatography. Vox Sang. 2003, 84, 193–201. [Google Scholar] [CrossRef]
- CytoGam Prescribing Information. Available online: https://www.accessdata.fda.gov/spl/data/2a40733c-106b-41cf-94f0-f10a03180ac8/2a40733c-106b-41cf-94f0-f10a03180ac8.xml (accessed on 3 May 2023).
- Vandeberg, P.; Cruz, M.; Diez, J.M.; Merritt, W.K.; Santos, B.; Trukawinski, S.; Wellhouse, A.; Jose, M.; Willis, T. Production of anti-SARS-CoV-2 hyperimmune globulin from convalescent plasma. Transfusion 2021, 61, 1705–1709. [Google Scholar] [CrossRef]
- Burnouf, T.; Gathof, B.; Bloch, E.M.; Bazin, R.; de Angelis, V.; Patidar, G.K.; Rastvorceva, R.M.G.; Oreh, A.; Goel, R.; Rahimi-Levene, N.; et al. Production and Quality Assurance of Human Polyclonal Hyperimmune Immunoglobulins against SARS-CoV-2. Transfus. Med. Rev. 2022, 36, 125–132. [Google Scholar] [CrossRef] [PubMed]
- HyperRAB Prescribing Information. Available online: https://www.accessdata.fda.gov/spl/data/f993778d-01fb-4670-af67-a0e08d6b258b/f993778d-01fb-4670-af67-a0e08d6b258b.xml (accessed on 3 May 2023).
- Imogam Prescribing Information. Available online: https://www.accessdata.fda.gov/spl/data/8026005f-7587-47fe-bb78-ec6247a3434b/8026005f-7587-47fe-bb78-ec6247a3434b.xml (accessed on 3 May 2023).
- Kedrab Prescribing Information. Available online: https://www.accessdata.fda.gov/spl/data/5e5c130a-693b-47f9-b44a-3d8f9cde3f98/5e5c130a-693b-47f9-b44a-3d8f9cde3f98.xml (accessed on 3 May 2023).
- VariZIG Prescribing Information. Available online: https://www.accessdata.fda.gov/spl/data/272379b7-f0e7-4560-8d79-3fd0024c3010/272379b7-f0e7-4560-8d79-3fd0024c3010.xml (accessed on 3 May 2023).
- Levin, M.J.; Duchon, J.M.; Swamy, G.K.; Gershon, A.A. Varicella zoster immune globulin (VARIZIG) administration up to 10 days after varicella exposure in pregnant women, immunocompromised participants, and infants: Varicella outcomes and safety results from a large, open-label, expanded-access program. PLoS ONE 2019, 14, e0217749. [Google Scholar] [CrossRef] [PubMed]
- Vaccinia Immune Globulin Prescribing Information. Available online: https://www.fda.gov/media/78174/download (accessed on 3 May 2023).
- Centers for Disease Control and Prevention. Household transmission of vaccinia virus from contact with a military smallpox vaccinee—Illinois and Indiana, 2007. MMWR Morb. Mortal. Wkly. Rep. 2007, 56, 478–481. [Google Scholar]
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 532–536. [Google Scholar]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients—Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef]
- GamaSTAN Prescribing Information. Available online: https://www.accessdata.fda.gov/spl/data/38a323af-7c25-42d1-9c29-532ef61999b8/38a323af-7c25-42d1-9c29-532ef61999b8.xml (accessed on 3 May 2023).
- HyperHEP B Prescribing Information. Available online: https://www.accessdata.fda.gov/spl/data/391b2218-8a15-4e5e-8717-aa49efcc2210/391b2218-8a15-4e5e-8717-aa49efcc2210.xml (accessed on 3 May 2023).
- Nabi-HB Prescribing Information. Available online: https://www.accessdata.fda.gov/spl/data/ee1560c0-18e1-b617-e053-2a95a90aa1af/ee1560c0-18e1-b617-e053-2a95a90aa1af.xml (accessed on 3 May 2023).
- HepaGAM B Prescribing Information. Available online: https://www.accessdata.fda.gov/spl/data/56525de0-f47d-11eb-85b4-0800200c9a66/56525de0-f47d-11eb-85b4-0800200c9a66.xml (accessed on 3 May 2023).
- Te, H.; Doucette, K. Viral hepatitis: Guidelines by the American Society of Transplantation Infectious Disease Community of Practice. Clin. Transplant. 2019, 33, e13514. [Google Scholar] [CrossRef]
- FDA. Letter to Immune Globulin (Human) Licensed Manufacturers: Option to Lower Lot Release Specification for Required Measles Antibody Potency Testing. Available online: https://www.fda.gov/media/118428/download (accessed on 3 May 2023).
- Stauft, C.B.; Tegenge, M.; Khurana, S.; Lee, Y.; Selvaraj, P.; Golding, H.; Wang, T.; Golding, B. Pharmacokinetics and Efficacy of Human Hyperimmune Intravenous Immunoglobulin Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Adult Syrian Hamsters. Clin. Infect. Dis. 2021, 75, e459–e465. [Google Scholar] [CrossRef]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Mbaya, O.T.; Mukumbayi, P.; Mulangu, S. Review: Insights on Current FDA-Approved Monoclonal Antibodies against Ebola Virus Infection. Front. Immunol. 2021, 12, 721328. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Cots, M.B.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- de Melo, G.D.; Hellert, J.; Gupta, R.; Corti, D.; Bourhy, H. Monoclonal antibodies against rabies: Current uses in prophylaxis and in therapy. Curr. Opin. Virol. 2022, 53, 101204. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Crescioli, S.; Chenoweth, A.; Visweswaraiah, J.; Reichert, J.M. Antibodies to watch in 2023. mAbs 2022, 15, 2153410. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2021, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 Leads to Widespread Escape from Neutralizing Antibody Responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Sheward, D.J.; Kim, C.; Fischbach, J.; Sato, K.; Muschiol, S.; Ehling, R.A.; Björkström, N.K.; Hedestam, G.B.K.; Reddy, S.T.; Albert, J.; et al. Omicron sublineage BA.2.75.2 exhibits extensive escape from neutralising antibodies. Lancet Infect. Dis. 2022, 22, 1538–1540. [Google Scholar] [CrossRef]
- Holland, T.L.; Ginde, A.A.; Paredes, R.; Murray, T.A.; Engen, N.; Grandits, G.; Vekstein, A.; Ivey, N.; Mourad, A.; Sandkovsky, U.; et al. Tixagevimab–cilgavimab for treatment of patients hospitalised with COVID-19: A randomised, double-blind, phase 3 trial. Lancet Respir. Med. 2022, 10, 972–984. [Google Scholar] [CrossRef]
- Imai, M.; Ito, M.; Kiso, M.; Yamayoshi, S.; Uraki, R.; Fukushi, S.; Watanabe, S.; Suzuki, T.; Maeda, K.; Sakai-Tagawa, Y.; et al. Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB. N. Engl. J. Med. 2023, 388, 89–91. [Google Scholar] [CrossRef]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19) EUA Information. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs (accessed on 17 February 2023).
- Dibo, M.; Battocchio, E.C.; Dos Santos Souza, L.M.; Da Silva, M.D.V.; Banin-Hirata, B.K.; Sapla, M.M.; Marinello, P.; Rocha, S.P.D.; Faccin-Galhardi, L.C. Antibody Therapy for the Control of Viral Diseases: An Update. Curr. Pharm. Biotechnol. 2019, 20, 1108–1121. [Google Scholar] [CrossRef]
- Hastie, K.M.; Cross, R.W.; Harkins, S.S.; Zandonatti, M.A.; Koval, A.P.; Heinrich, M.L.; Rowland, M.M.; Robinson, J.E.; Geisbert, T.W.; Garry, R.F.; et al. Convergent Structures Illuminate Features for Germline Antibody Binding and Pan-Lassa Virus Neutralization. Cell 2019, 178, 1004–1015.e14. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Buck, T.; Zandonatti, M.; Yin, J.; Moon-Walker, A.; Fang, J.; Koval, A.; Heinrich, M.L.; Rowland, M.M.; Avalos, R.D.; et al. A cocktail of protective antibodies subverts the dense glycan shield of Lassa virus. Sci. Transl. Med. 2022, 14, eabq0991. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.K. Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology 2013, 142, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Asokan, M.; Dias, J.; Liu, C.; Maximova, A.; Ernste, K.; Pegu, A.; McKee, K.; Shi, W.; Chen, X.; Almasri, C.; et al. Fc-mediated effector function contributes to the in vivo antiviral effect of an HIV neutralizing antibody. Proc. Natl. Acad. Sci. USA 2020, 117, 18754–18763. [Google Scholar] [CrossRef] [PubMed]
- Vanderven, H.A.; Kent, S. The protective potential of Fc-mediated antibody functions against influenza virus and other viral pathogens. Immunol. Cell Biol. 2020, 98, 253–263. [Google Scholar] [CrossRef]
- Zhang, A.; Stacey, H.D.; D’Agostino, M.R.; Tugg, Y.; Marzok, A.; Miller, M.S. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat. Rev. Immunol. 2022, 1–16. [Google Scholar] [CrossRef]
- Cartwright, H.N.; Barbeau, D.J.; McElroy, A.K. Isotype-Specific Fc Effector Functions Enhance Antibody-Mediated Rift Valley Fever Virus Protection In Vivo. Msphere 2021, 6, e0055621. [Google Scholar] [CrossRef]
- Smatti, M.K.; Al Thani, A.A.; Yassine, H.M. Viral-Induced Enhanced Disease Illness. Front. Microbiol. 2018, 9, 2991. [Google Scholar] [CrossRef]
- Almagro, J.C.; Daniels-Wells, T.R.; Perez-Tapia, S.M.; Penichet, M.L. Progress and Challenges in the Design and Clinical Development of Antibodies for Cancer Therapy. Front. Immunol. 2018, 8, 1751. [Google Scholar] [CrossRef]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions—Improving Antibodies for Cancer Treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef]
- Ko, S.; Jo, M.; Jung, S.T. Recent Achievements and Challenges in Prolonging the Serum Half-Lives of Therapeutic IgG Antibodies Through Fc Engineering. Biodrugs 2021, 35, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Ishii, S.; Tachibana, T.; Maeda, A.; Higuchi, Y.; Shimaoka, S.; Moriyama, C.; Watanabe, T.; Takubo, R.; Doi, Y.; et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol. 2010, 28, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Mimoto, F.; Hattori, K. pH-dependent antigen-binding antibodies as a novel therapeutic modality. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2014, 1844, 1943–1950. [Google Scholar] [CrossRef]
- Igawa, T.; Maeda, A.; Haraya, K.; Tachibana, T.; Iwayanagi, Y.; Mimoto, F.; Higuchi, Y.; Ishii, S.; Tamba, S.; Hironiwa, N.; et al. Engineered Monoclonal Antibody with Novel Antigen-Sweeping Activity In Vivo. PLoS ONE 2013, 8, e63236. [Google Scholar] [CrossRef] [PubMed]
- Reusch, D.; Tejada, M.L. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 2015, 25, 1325–1334. [Google Scholar] [CrossRef]
- Golay, J.; Andrea, A.E.; Cattaneo, I. Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Front. Immunol. 2022, 13, 929895. [Google Scholar] [CrossRef]
- Hatfield, G.; Tepliakova, L.; Gingras, G.; Stalker, A.; Li, X.; Aubin, Y.; Tam, R.Y. Specific location of galactosylation in an afucosylated antiviral monoclonal antibody affects its FcγRIIIA binding affinity. Front. Immunol. 2022, 13, 972168. [Google Scholar] [CrossRef]
- Tao, M.H.; Morrison, S.L. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J. Immunol. 1989, 143, 2595–2601. [Google Scholar] [CrossRef]
- Bolt, S.; Routledge, E.; Lloyd, I.; Chatenoud, L.; Pope, H.; Gorman, S.D.; Clark, M.; Waldmann, H. The generation of a humanized, non-mitogenic CD3 monoclonal antibody which retains in vitro immunosuppressive properties. Eur. J. Immunol. 1993, 23, 403–411. [Google Scholar] [CrossRef]
- Liu, D.; Shameem, M. Antiviral monoclonal antibody cocktails as a modern weapon in combating pandemics. Ther. Deliv. 2022, 13, 67–69. [Google Scholar] [CrossRef]
- Dacon, C.; Tucker, C.; Peng, L.; Lee, C.-C.D.; Lin, T.-H.; Yuan, M.; Cong, Y.; Wang, L.; Purser, L.; Williams, J.K.; et al. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science 2022, 377, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, S.; Virata, M.L.; Yan, H.; Zhong, L.; Deng, L.; Xu, Y.; He, Y.; Struble, E.; Zhang, P. Multiple epitopes of hepatitis B virus surface antigen targeted by human plasma-derived immunoglobulins coincide with clinically observed escape mutations. J. Med. Virol. 2021, 94, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research OoPQ. Potency Assay Considerations for Monoclonal Antibodies and Other Therapeutic Proteins Targeting Viral Pathogens, Guidance for Industry (Draft). Available online: https://www.fda.gov/media/165746/download (accessed on 3 May 2023).
- Jiang, X.-R.; Song, A.; Bergelson, S.; Arroll, T.; Parekh, B.; May, K.; Chung, S.; Strouse, R.; Mire-Sluis, A.; Schenerman, M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat. Rev. Drug Discov. 2011, 10, 101–111. [Google Scholar] [CrossRef]
- Schmidt, F.; Weisblum, Y.; Muecksch, F.; Hoffmann, H.-H.; Michailidis, E.; Lorenzi, J.C.; Mendoza, P.; Rutkowska, M.; Bednarski, E.; Gaebler, C.; et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020, 217, e20201181. [Google Scholar] [CrossRef]
- Clapham, P.R. Vesicular Stomatitis Virus Pseudotypes of Retroviruses. Methods Mol. Biol. 2003, 8, 95–102. [Google Scholar] [CrossRef]
- Kim, Y.; Zheng, X.; Eschke, K.; Chaudhry, M.Z.; Bertoglio, F.; Tomić, A.; Krmpotić, A.; Hoffmann, M.; Bar-On, Y.; Boehme, J.; et al. MCMV-based vaccine vectors expressing full-length viral proteins provide long-term humoral immune protection upon a single-shot vaccination. Cell. Mol. Immunol. 2022, 19, 234–244. [Google Scholar] [CrossRef]
- Racine, T.; Kobinger, G.P.; Arts, E.J. Development of an HIV vaccine using a vesicular stomatitis virus vector expressing designer HIV-1 envelope glycoproteins to enhance humoral responses. AIDS Res. Ther. 2017, 14, 55. [Google Scholar] [CrossRef]
- Takada, A.; Feldmann, H.; Stroeher, U.; Bray, M.; Watanabe, S.; Ito, H.; McGregor, M.; Kawaoka, Y. Identification of Protective Epitopes on Ebola Virus Glycoprotein at the Single Amino Acid Level by Using Recombinant Vesicular Stomatitis Viruses. J. Virol. 2003, 77, 1069–1074. [Google Scholar] [CrossRef]
- Takada, A.; Robison, C.; Goto, H.; Sanchez, A.; Murti, K.G.; Whitt, M.A.; Kawaoka, Y. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 1997, 94, 14764–14769. [Google Scholar] [CrossRef]
- Bannert, N.; Farzan, M.; Friend, D.S.; Ochi, H.; Price, K.S.; Sodroski, J.; Boyce, J.A. Human Mast Cell Progenitors Can Be Infected by Macrophagetropic Human Immunodeficiency Virus Type 1 and Retain Virus with Maturation In Vitro. J. Virol. 2001, 75, 10808–10814. [Google Scholar] [CrossRef]
- Connor, R.I.; Chen, B.K.; Choe, S.; Landau, N.R. Vpr Is Required for Efficient Replication of Human Immunodeficiency Virus Type-1 in Mononuclear Phagocytes. Virology 1995, 206, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Freed, E.O.; Englund, G.; Martin, M.A. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J. Virol. 1995, 69, 3949–3954. [Google Scholar] [CrossRef] [PubMed]
- Louder, M.K.; Sambor, A.; Chertova, E.; Hunte, T.; Barrett, S.; Ojong, F.; Sanders-Buell, E.; Zolla-Pazner, S.; McCutchan, F.E.; Roser, J.D.; et al. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology 2005, 339, 226–238. [Google Scholar] [CrossRef]
- Lundquist, C.A.; Zhou, J.; Aiken, C. Nef Stimulates Human Immunodeficiency Virus Type 1 Replication in Primary T Cells by Enhancing Virion-Associated gp120 Levels: Coreceptor-Dependent Requirement for Nef in Viral Replication. J. Virol. 2004, 78, 6287–6296. [Google Scholar] [CrossRef] [PubMed]
- Sarzotti-Kelsoe, M.; Daniell, X.; Todd, C.A.; Bilska, M.; Martelli, A.; LaBranche, C.; Perez, L.G.; Ochsenbauer, C.; Kappes, J.C.; Rountree, W.; et al. Optimization and validation of a neutralizing antibody assay for HIV-1 in A3R5 cells. J. Immunol. Methods 2014, 409, 147–160. [Google Scholar] [CrossRef]
- Matsuura, Y.; Tania, H.; Suzukic, K.; Someyab, T.K.; Suzukib, R.; Aizakib, H.; Ishiib, K.; Moriishi, K.; Robison, C.S.; Whitt, M.A.; et al. Characterization of Pseudotype VSV Possessing HCV Envelope Proteins. Virology 2001, 286, 263–275. [Google Scholar] [CrossRef]
- Renelt, S.; Schult-Dietrich, P.; Baldauf, H.-M.; Stein, S.; Kann, G.; Bickel, M.; Kielland-Kaisen, U.; Bonig, H.; Marschalek, R.; Rieger, M.A.; et al. HIV-1 Infection of Long-Lived Hematopoietic Precursors In Vitro and In Vivo. Cells 2022, 11, 2968. [Google Scholar] [CrossRef]
- Riepler, L.; Rössler, A.; Falch, A.; Volland, A.; Borena, W.; Von Laer, D.; Kimpel, J. Comparison of Four SARS-CoV-2 Neutralization Assays. Vaccines 2020, 9, 13. [Google Scholar] [CrossRef]
- Chikere, K.; Webb, N.E.; Chou, T.; Borm, K.; Sterjovski, J.; Gorry, P.R.; Lee, B. Distinct HIV-1 entry phenotypes are associated with transmission, subtype specificity, and resistance to broadly neutralizing antibodies. Retrovirology 2014, 11, 48. [Google Scholar] [CrossRef]
- Mann, A.M.; Rusert, P.; Berlinger, L.; Kuster, H.; Günthard, H.F.; Trkola, A. HIV sensitivity to neutralization is determined by target and virus producer cell properties. Aids 2009, 23, 1659–1667. [Google Scholar] [CrossRef]
- Miyamoto, F.; Kawaji, K.; Oishi, S.; Fujii, N.; Kaku, M.; Kodama, E.N. Anti-HIV-1 activity determined by β-galactosidase activity in the multinuclear activation of an indicator assay is comparable with that by a conventional focus counting method. Antivir. Chem. Chemother. 2015, 24, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Spenlehauera, C.; Gordon, C.A.; Trkolac, A.; Moore, J.P. A Luciferase-Reporter Gene-Expressing T-Cell Line Facilitates Neutralization and Drug-Sensitivity Assays That Use Either R5 or X4 Strains of Human Immunodeficiency Virus Type 1. Virology 2001, 280, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Sarzotti-Kelsoe, M.; Bailer, R.T.; Turk, E.; Lin, C.-L.; Bilska, M.; Greene, K.M.; Gao, H.; Todd, C.A.; Ozaki, D.A.; Seaman, M.S.; et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J. Immunol. Methods 2014, 409, 131–146. [Google Scholar] [CrossRef]
- Bentley, E.M.; Mather, S.T.; Temperton, N.J. The use of pseudotypes to study viruses, virus sero-epidemiology and vaccination. Vaccine 2015, 33, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Comas-Garcia, M.; Colunga-Saucedo, M.; Rosales-Mendoza, S. The Role of Virus-Like Particles in Medical Biotechnology. Mol. Pharm. 2020, 17, 4407–4420. [Google Scholar] [CrossRef]
- Du, R.; Cui, Q.; Caffrey, M.; Rong, L. Ebola Virus Entry Inhibitors. Adv. Exp. Med. Biol. 2022, 1366, 155–170. [Google Scholar] [CrossRef]
- Kaku, Y.; Noguchi, A.; Marsh, G.A.; Barr, J.A.; Okutani, A.; Hotta, K.; Bazartseren, B.; Fukushi, S.; Broder, C.C.; Yamada, A.; et al. Second generation of pseudotype-based serum neutralization assay for Nipah virus antibodies: Sensitive and high-throughput analysis utilizing secreted alkaline phosphatase. J. Virol. Methods 2012, 179, 226–232. [Google Scholar] [CrossRef]
- Kaku, Y.; Noguchi, A.; Marsh, G.A.; McEachern, J.A.; Okutani, A.; Hotta, K.; Bazartseren, B.; Fukushi, S.; Broder, C.C.; Yamada, A.; et al. A neutralization test for specific detection of Nipah virus antibodies using pseudotyped vesicular stomatitis virus expressing green fluorescent protein. J. Virol. Methods 2009, 160, 7–13. [Google Scholar] [CrossRef]
- Khetawat, D.; Broder, C.C. A Functional Henipavirus Envelope Glycoprotein Pseudotyped Lentivirus Assay System. Virol. J. 2010, 7, 312. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef]
- Rudometova, N.B.; Shcherbakov, D.N.; Rudometov, A.P.; Ilyichev, A.A.; Karpenko, L.I. Model systems of human immunodef iciency virus (HIV-1) for in vitro eff icacy assessment of candidate vaccines and drugs against HIV-1. Vavilov J. Genet. Breed. 2022, 26, 214–221. [Google Scholar] [CrossRef]
- Steeds, K.; Hall, Y.; Slack, G.S.; Longet, S.; Strecker, T.; Fehling, S.K.; Wright, E.; Bore, J.A.; Koundouno, F.R.; Konde, M.K.; et al. Pseudotyping of VSV with Ebola virus glycoprotein is superior to HIV-1 for the assessment of neutralising antibodies. Sci. Rep. 2020, 10, 14289. [Google Scholar] [CrossRef] [PubMed]
- Steffen, I.; Simmons, G. Pseudotyping Viral Vectors with Emerging Virus Envelope Proteins. Curr. Gene Ther. 2016, 16, 47–55. [Google Scholar] [CrossRef]
- Wang, B.; Meng, X.-J. Structural and molecular biology of hepatitis E virus. Comput. Struct. Biotechnol. J. 2021, 19, 1907–1916. [Google Scholar] [CrossRef]
- Ryu, W. Molecular Virology of Human Pathogenic Viruses, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 19; pp. 263–275. [Google Scholar] [CrossRef]
- Gasmi, M.; Glynn, J.; Jin, M.-J.; Jolly, D.J.; Yee, J.-K.; Chen, S.-T. Requirements for Efficient Production and Transduction of Human Immunodeficiency Virus Type 1-Based Vectors. J. Virol. 1999, 73, 1828–1834. [Google Scholar] [CrossRef]
- Salmon, P.; Trono, D. Lentiviral Vectors for the Gene Therapy of Lympho-Hematological Disorders. Curr. Top. Microbiol. Immunol. 2002, 261, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.A.; Greene, K.M.; Yu, X.; Ozaki, D.A.; Gao, H.; Huang, Y.; Wang, M.; Li, G.; Brown, R.; Wood, B.; et al. Development and implementation of an international proficiency testing program for a neutralizing antibody assay for HIV-1 in TZM-bl cells. J. Immunol. Methods 2012, 375, 57–67. [Google Scholar] [CrossRef]
- Wei, X.; Decker, J.M.; Liu, H.; Zhang, Z.; Arani, R.B.; Kilby, J.M.; Saag, M.S.; Wu, X.; Shaw, G.M.; Kappes, J.C. Emergence of Resistant Human Immunodeficiency Virus Type 1 in Patients Receiving Fusion Inhibitor (T-20) Monotherapy. Antimicrob. Agents Chemother. 2002, 46, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Seaman, M.S.; Janes, H.; Hawkins, N.; Grandpre, L.E.; Devoy, C.; Giri, A.; Coffey, R.T.; Harris, L.; Wood, B.; Daniels, M.G.; et al. Tiered Categorization of a Diverse Panel of HIV-1 Env Pseudoviruses for Assessment of Neutralizing Antibodies. J. Virol. 2010, 84, 1439–1452. [Google Scholar] [CrossRef]
- Hoenen, T. Minigenome Systems for Filoviruses. Methods Mol Biol. 2018, 1604, 237–245. [Google Scholar] [CrossRef]
- Cavrois, M.; De Noronha, C.; Greene, W.C. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 2002, 20, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bentsman, G.; Potash, M.J.; Volsky, D.J. Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC Neurosci. 2007, 8, 31. [Google Scholar] [CrossRef]
- Saeed, M.F.; Kolokoltsov, A.A.; Davey, R.A. Novel, rapid assay for measuring entry of diverse enveloped viruses, including HIV and rabies. J. Virol. Methods 2006, 135, 143–150. [Google Scholar] [CrossRef]
- Tobiume, M.; Lineberger, J.E.; Lundquist, C.A.; Miller, M.D.; Aiken, C. Nef Does Not Affect the Efficiency of Human Immunodeficiency Virus Type 1 Fusion with Target Cells. J. Virol. 2003, 77, 10645–10650. [Google Scholar] [CrossRef]
- Tscherne, D.M.; Manicassamy, B.; García-Sastre, A. An enzymatic virus-like particle assay for sensitive detection of virus entry. J. Virol. Methods 2010, 163, 336–343. [Google Scholar] [CrossRef]
- Wyma, D.J.; Jiang, J.; Shi, J.; Zhou, J.; Lineberger, J.E.; Miller, M.D.; Aiken, C. Coupling of Human Immunodeficiency Virus Type 1 Fusion to Virion Maturation: A Novel Role of the gp41 Cytoplasmic Tail. J. Virol. 2004, 78, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.; Han, M.; Woottum, M.; Bracq, L.; Bouchet, J.; Xie, M.; Benichou, S. Virus-Mediated Cell-Cell Fusion. Int. J. Mol. Sci. 2020, 21, 9644. [Google Scholar] [CrossRef] [PubMed]
- Bossart, K.N.; Broder, C.C. Viral Glycoprotein-Mediated Cell Fusion Assays Using Vaccinia Virus Vectors. Methods Mol. Biol. 2004, 269, 309–331. [Google Scholar] [CrossRef]
- Moulard, M.; Phogat, S.K.; Shu, Y.; Labrijn, A.F.; Xiao, X.; Binley, J.M.; Zhang, M.-Y.; Sidorov, I.A.; Broder, C.C.; Robinson, J.; et al. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120–CD4–CCR5 complexes. Proc. Natl. Acad. Sci. USA 2002, 99, 6913–6918. [Google Scholar] [CrossRef]
- Saw, W.T.; Matsuda, Z.; Eisenberg, R.J.; Cohen, G.H.; Atanasiu, D. Using a split luciferase assay (SLA) to measure the kinetics of cell–cell fusion mediated by herpes simplex virus glycoproteins. Methods 2015, 90, 68–75. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Nguyen, L.N.T.; Adkins, J.L.; Schank, M.; Khanal, S.; Dang, X.; Cao, D.; Thakuri, B.K.C.; Lu, Z.; et al. Blockade of SARS-CoV-2 spike protein-mediated cell–cell fusion using COVID-19 convalescent plasma. Sci. Rep. 2021, 11, 5558. [Google Scholar] [CrossRef]
- Oguntuyo, K.Y.; Stevens, C.S.; Hung, C.T.; Ikegame, S.; Acklin, J.A.; Kowdle, S.S.; Carmichael, J.C.; Chiu, H.-P.; Azarm, K.D.; Haas, G.D.; et al. Quantifying Absolute Neutralization Titers against SARS-CoV-2 by a Standardized Virus Neutralization Assay Allows for Cross-Cohort Comparisons of COVID-19 Sera. MBio 2021, 12, e02492-20. [Google Scholar] [CrossRef]
- Collins, F.S.; Woodcock, J.; Graham, B.S.; Arvin, A.; Bieniasz, P.; Ho, D.; Alter, G.; Nussenzweig, M.; Burton, D.; Tavel, J.; et al. Therapeutic Neutralizing Monoclonal Antibodies: Report of a Summit Sponsored by Operation Warp Speed and the National Institutes of Health. Available online: https://www.nih.gov/sites/default/files/research-training/initiatives/activ/20200909-mAb-summit-pub.pdf (accessed on 17 May 2023).
- Li, Y.; O’Dell, S.; Walker, L.M.; Wu, X.; Guenaga, J.; Feng, Y.; Schmidt, S.D.; McKee, K.; Louder, M.K.; Ledgerwood, J.E.; et al. Mechanism of Neutralization by the Broadly Neutralizing HIV-1 Monoclonal Antibody VRC01. J. Virol. 2011, 85, 8954–8967. [Google Scholar] [CrossRef]
- Lorenzi, J.C.C.; Mendoza, P.; Cohen, Y.Z.; Nogueira, L.; Lavine, C.; Sapiente, J.; Wiatr, M.; Mugo, N.R.; Mujugira, A.; Delany, S.; et al. Neutralizing Activity of Broadly Neutralizing Anti-HIV-1 Antibodies against Primary African Isolates. J. Virol. 2021, 95, e01909-20. [Google Scholar] [CrossRef]
- Sanders, D.A. No false start for novel pseudotyped vectors. Curr. Opin. Biotechnol. 2002, 13, 437–442. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Huang, W.; Li, X.; Wang, Y. Current status on the development of pseudoviruses for enveloped viruses. Rev. Med. Virol. 2017, 28, e1963. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.E.; Zhang, X.; Case, J.B.; Winkler, E.S.; Liu, Y.; VanBlargan, L.A.; Liu, J.; Errico, J.M.; Xie, X.; Suryadevara, N.; et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 2021, 27, 717–726. [Google Scholar] [CrossRef]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef]
- Dong, J.; Zost, S.J.; Greaney, A.J.; Starr, T.N.; Dingens, A.S.; Chen, E.C.; Chen, R.E.; Case, J.B.; Sutton, R.E.; Gilchuk, P.; et al. Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail. Nat. Microbiol. 2021, 6, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Pollett, S.D.; Neerukonda, S.N.; Wang, W.; Wang, R.; Vassell, R.; Epsi, N.J.; Fries, A.C.; Agan, B.K.; Lindholm, D.A.; et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster–elicited serum but evades most convalescent serum and therapeutic antibodies. Sci. Transl. Med. 2022, 14, eabn8543. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef]
- Yamasoba, D.; Kimura, I.; Nasser, H.; Morioka, Y.; Nao, N.; Ito, J.; Uriu, K.; Tsuda, M.; Zahradnik, J.; Shirakawa, K.; et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike. Cell 2022, 185, 2103–2115.e19. [Google Scholar] [CrossRef]
- Farrell, A.G.; Dadonaite, B.; Greaney, A.J.; Eguia, R.; Loes, A.N.; Franko, N.M.; Logue, J.; Carreño, J.M.; Abbad, A.; Chu, H.Y.; et al. Receptor-Binding Domain (RBD) Antibodies Contribute More to SARS-CoV-2 Neutralization When Target Cells Express High Levels of ACE2. Viruses 2022, 14, 2061. [Google Scholar] [CrossRef] [PubMed]
- Lempp, F.A.; Soriaga, L.B.; Montiel-Ruiz, M.; Benigni, F.; Noack, J.; Park, Y.-J.; Bianchi, S.; Walls, A.C.; Bowen, J.E.; Zhou, J.; et al. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature 2021, 598, 342–347. [Google Scholar] [CrossRef]
- Van Blargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022, 28, 490–495. [Google Scholar] [CrossRef] [PubMed]
- FDA. Integrated Review Application Number 761172. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/761172Orig1s000IntegratedR.pdf (accessed on 17 February 2023).
- FDA. Multi-Discipline Review, Application Number 761169. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/761169Orig1s000MultidisciplineR.pdf (accessed on 17 February 2023).
- Hsieh, Y.-T.; Aggarwal, P.; Cirelli, D.; Gu, L.; Surowy, T.; Mozier, N.M. Characterization of FcγRIIIA effector cells used in in vitro ADCC bioassay: Comparison of primary NK cells with engineered NK-92 and Jurkat T cells. J. Immunol. Methods 2017, 441, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Parekh, B.S.; Berger, E.; Sibley, S.; Cahya, S.; Xiao, L.; LaCerte, M.A.; Vaillancourt, P.; Wooden, S.; Gately, D. Development and validation of an antibody-dependent cell-mediated cytotoxicity-reporter gene assay. mAbs 2012, 4, 310–318. [Google Scholar] [CrossRef]
- de Taeye, S.W.; Rispens, T.; Vidarsson, G. The Ligands for Human IgG and Their Effector Functions. Antibodies 2019, 8, 30. [Google Scholar] [CrossRef]
- Goncalvez, A.P.; Engle, R.E.; St Claire, M.; Purcell, R.H.; Lai, C.-J. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA 2007, 104, 9422–9427. [Google Scholar] [CrossRef]
- Huang, X.; Yue, Y.; Li, D.; Zhao, Y.; Qiu, L.; Chen, J.; Pan, Y.; Xi, J.; Wang, X.; Sun, Q.; et al. Antibody-dependent enhancement of dengue virus infection inhibits RLR-mediated Type-I IFN-independent signalling through upregulation of cellular autophagy. Sci. Rep. 2016, 6, 22303. [Google Scholar] [CrossRef]
- Littaua, R.; Kurane, I.; Ennis, F.A. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. 1990, 144, 3183–3186. J. Immunol. [CrossRef]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef]
- Shafer, R.W.; Chou, S. Mechanisms of Resistance to Antiviral Agents. Man. Clin. Microbiol. 2015, 111, 1894–1912. [Google Scholar] [CrossRef]
- Vere Hodge, A.; Field, H.J. General Mechanisms of Antiviral Resistance. Genet. Evol. Infect. Dis. 2011, 339–362. [Google Scholar] [CrossRef]
- FDA. Antiviral Product Development—Conducting and Submitting Virology Studies to the Agency, Guidance for Industry. Available online: https://www.fda.gov/media/71223/download (accessed on 2 May 2023).
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M.; et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 2020, 369, 1014–1018. [Google Scholar] [CrossRef]
- Copin, R.; Baum, A.; Wloga, E.; Pascal, K.E.; Giordano, S.; Fulton, B.O.; Zhou, A.; Negron, N.; Lanza, K.; Chan, N.; et al. The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies. Cell 2021, 184, 3949–3961.e11. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Addetia, A.; Hannon, W.W.; Choudhary, M.C.; Dingens, A.S.; Li, J.Z.; Bloom, J.D. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science 2021, 371, 850–854. [Google Scholar] [CrossRef]
- Weisblum, Y.; Schmidt, F.; Zhang, F.; DaSilva, J.; Poston, D.; Lorenzi, J.C.; Muecksch, F.; Rutkowska, M.; Hoffmann, H.-H.; Michailidis, E.; et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 2020, 9, e61312. [Google Scholar] [CrossRef] [PubMed]
- Ibalizumab Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761065lbl.pdf (accessed on 2 May 2023).
- Ruiz, S.I.; Zumbrun, E.E.; Nalca, A. Animal Models of Human Viral Diseases. Anim. Model. Study Hum. Dis. 2017, 853–901. [Google Scholar]
- Beddingfield, B.J.; Maness, N.J.; Fears, A.C.; Rappaport, J.; Aye, P.P.; Russell-Lodrigue, K.; Doyle-Meyers, L.A.; Blair, R.V.; Carias, A.M.; Madden, P.J.; et al. Effective Prophylaxis of COVID-19 in Rhesus Macaques Using a Combination of Two Parenterally-Administered SARS-CoV-2 Neutralizing Antibodies. Front. Cell. Infect. Microbiol. 2021, 11, 753444. [Google Scholar] [CrossRef]
- Haagmans, B.L.; Noack, D.; Okba, N.M.A.; Li, W.; Wang, C.; Bestebroer, T.; de Vries, R.; Herfst, S.; de Meulder, D.; Verveer, E.; et al. SARS-CoV-2 Neutralizing Human Antibodies Protect against Lower Respiratory Tract Disease in a Hamster Model. J. Infect. Dis. 2021, 223, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Barker, D.; Lew, J.; Manoharan, V.; van Kessel, J.; Haupt, R.; Toth, D.; Frieman, M.; Falzarano, D.; Kodihalli, S. Efficacy of COVID-HIGIV in animal models of SARS-CoV-2 infection. Sci. Rep. 2022, 12, 16956. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ryu, D.-K.; Lee, J.; Kim, Y.-I.; Seo, J.-M.; Kim, Y.-G.; Jeong, J.-H.; Kim, M.; Kim, J.-I.; Kim, P.; et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021, 12, 288. [Google Scholar] [CrossRef]
- Maisonnasse, P.; Aldon, Y.; Marc, A.; Marlin, R.; Dereuddre-Bosquet, N.; Kuzmina, N.A.; Freyn, A.W.; Snitselaar, J.L.; Gonçalves, A.; Caniels, T.G.; et al. COVA1-18 neutralizing antibody protects against SARS-CoV-2 in three preclinical models. Nat. Commun. 2021, 12, 6097. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.S.; Gilchuk, P.; Yu, J.; Bailey, A.L.; Chen, R.E.; Chong, Z.; Zost, S.J.; Jang, H.; Huang, Y.; Allen, J.D.; et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell 2021, 184, 1804–1820.e16. [Google Scholar] [CrossRef]
- Yadav, P.D.; Mendiratta, S.K.; Mohandas, S.; Singh, A.K.; Abraham, P.; Shete, A.; Bandyopadhyay, S.; Kumar, S.; Parikh, A.; Kalita, P.; et al. ZRC3308 Monoclonal Antibody Cocktail Shows Protective Efficacy in Syrian Hamsters against SARS-CoV-2 Infection. Viruses 2021, 13, 2424. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef]
- FDA. S6(R1) Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals, Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/s6r1-preclinical-safety-evaluation-biotechnology-derived-pharmaceuticals (accessed on 17 February 2023).
- Mahmood, I.; Tegenge, M.A. Prediction of tissue concentrations of monoclonal antibodies in mice from plasma concentrations. Regul. Toxicol. Pharmacol. 2018, 97, 57–62. [Google Scholar] [CrossRef]
- Keeler, S.P.; Fox, J.M. Requirement of Fc-Fc Gamma Receptor Interaction for Antibody-Based Protection against Emerging Virus Infections. Viruses 2021, 13, 1037. [Google Scholar] [CrossRef]
- Schmaljohn, A.L.; Orlandi, C.; Lewis, G.K. Deciphering Fc-mediated Antiviral Antibody Functions in Animal Models. Front. Immunol. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Robbie, G.J.; Criste, R.; Dall’Acqua, W.F.; Jensen, K.; Patel, N.K.; Losonsky, G.A.; Griffin, M.P. A Novel Investigational Fc-Modified Humanized Monoclonal Antibody, Motavizumab-YTE, Has an Extended Half-Life in Healthy Adults. Antimicrob. Agents Chemother. 2013, 57, 6147–6153. [Google Scholar] [CrossRef]
- Smith, P.; Di Lillo, D.J.; Bournazos, S.; Li, F.; Ravetch, J.V. Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc. Natl. Acad. Sci. USA 2012, 109, 6181–6186. [Google Scholar] [CrossRef] [PubMed]
- Proetzel, G.; Roopenian, D.C. Humanized FcRn mouse models for evaluating pharmacokinetics of human IgG antibodies. Methods 2013, 65, 148–153. [Google Scholar] [CrossRef]
- FDA. Product Development under the Animal Rule, Guidance for Industry. Available online: https://www.fda.gov/media/88625/download (accessed on 17 February 2023).
- FDA. Animal Rule Approvals. Available online: https://www.fda.gov/drugs/nda-and-bla-approvals/animal-rule-approvals (accessed on 17 February 2023).
- Sharp, J.C.; Fletcher, W.B. Experience of anti-vaccinia immunoglobulin in the United Kingdom. Lancet 1973, 301, 656–659. [Google Scholar] [CrossRef]
- Bahmanyar, M.; Fayaz, A.; Nour-Salehi, S.; Mohammadi, M.; Koprowski, H. Successful protection of humans exposed to rabies infection. Postexposure treatment with the new human diploid cell rabies vaccine and antirabies serum. JAMA 1976, 236, 2751–2754. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.P.; Weng, M.K.; Hofmeister, M.G.; Moore, K.L.; Doshani, M.; Kamili, S.; Koneru, A.; Haber, P.; Hagan, L.; Romero, J.R.; et al. Prevention of Hepatitis A Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR. Recomm. Rep. 2020, 69, 1–38. [Google Scholar] [CrossRef]
- Fisher, R.W.; Reed, J.L.; Snoy, P.J.; Mikolajczyk, M.G.; Bray, M.; Scott, D.E.; Kennedy, M.C. Postexposure Prevention of Progressive Vaccinia in SCID Mice Treated with Vaccinia Immune Globulin. Clin. Vaccine Immunol. 2011, 18, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.; Wright, M.E. Progressive Vaccinia. Clin. Infect. Dis. 2003, 36, 766–774. [Google Scholar] [CrossRef]
- FDA. REGEN-COV (Casirivimab and Imdevimab). Available online: https://www.fda.gov/media/145611/download (accessed on 17 February 2023).
- FDA. Bamlanivimab and Etesevimab. Available online: https://www.fda.gov/media/145802/download (accessed on 17 February 2023).
- FDA. EVUSHELD™ (Tixagevimab Co-Packaged with Cilgavimab). Available online: https://www.fda.gov/media/154701/download (accessed on 17 February 2023).
- Ejemel, M.; Smith, T.G.; Greenberg, L.; Carson, W.C.; Lowe, D.; Yang, Y.; Jackson, F.R.; Morgan, C.N.; Martin, B.E.; Kling, C.; et al. A cocktail of human monoclonal antibodies broadly neutralizes North American rabies virus variants as a promising candidate for rabies post-exposure prophylaxis. Sci. Rep. 2022, 12, 9403. [Google Scholar] [CrossRef]
- Koszalka, P.; Subbarao, K.; Baz, M. Preclinical and clinical developments for combination treatment of influenza. PLoS Pathog. 2022, 18, e1010481. [Google Scholar] [CrossRef]
- Maertens, J.; Logan, A.C.; Jang, J.; Long, G.; Tang, J.-L.; Hwang, W.Y.K.; Koh, L.P.; Chemaly, R.; Gerbitz, A.; Winkler, J.; et al. Phase 2 Study of Anti-Human Cytomegalovirus Monoclonal Antibodies for Prophylaxis in Hematopoietic Cell Transplantation. Antimicrob. Agents Chemother. 2020, 64, e02467-19. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, S.; Garrett, N.; Baxter, C.; Karim, Q.A.; Karim, S.S.A. Clinical Trials of Broadly Neutralizing Monoclonal Antibodies for Human Immunodeficiency Virus Prevention: A Review. J. Infect. Dis. 2020, 223, 370–380. [Google Scholar] [CrossRef] [PubMed]
- FDA. Codevelopment of Two or More New Investigational Drugs for Use in Combination, Guidance for Industry. Available online: https://www.fda.gov/media/80100/download (accessed on 17 February 2023).
- Verrier, F.; Nádas, A.; Gorny, M.K.; Zolla-Pazner, S. Additive Effects Characterize the Interaction of Antibodies Involved in Neutralization of the Primary Dualtropic Human Immunodeficiency Virus Type 1 Isolate 89.6. J. Virol. 2001, 75, 9177–9186. [Google Scholar] [CrossRef]
- Keck, Z.-Y.; Girard-Blanc, C.; Wang, W.; Lau, P.; Zuiani, A.; Rey, F.A.; Krey, T.; Diamond, M.S.; Foung, S.K.H. Antibody Response to Hypervariable Region 1 Interferes with Broadly Neutralizing Antibodies to Hepatitis C Virus. J. Virol. 2016, 90, 3112–3122. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, M.C.; Kinchen, V.J.; Wasilewski, L.N.; Flyak, A.I.; Ray, S.C.; Crowe, J.E.; Bailey, J.R. Synergistic anti-HCV broadly neutralizing human monoclonal antibodies with independent mechanisms. Proc. Natl. Acad. Sci. USA 2018, 115, E82–E91. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Katinger, H.; Posner, M.R.; Cavacini, L.; Zolla-Pazner, S.; Gorny, M.K.; Sodroski, J.; Chou, T.-C.; Baba, T.W.; Ruprecht, R.M. Synergistic Neutralization of Simian-Human Immunodeficiency Virus SHIV-vpu + by Triple and Quadruple Combinations of Human Monoclonal Antibodies and High-Titer Anti-Human Immunodeficiency Virus Type 1 Immunoglobulins. J. Virol. 1998, 72, 3235–3240. [Google Scholar] [CrossRef]
- Miglietta, R.; Pastori, C.; Venuti, A.; Ochsenbauer, C.; Lopalco, L. Synergy in monoclonal antibody neutralization of HIV-1 pseudoviruses and infectious molecular clones. J. Transl. Med. 2014, 12, 346. [Google Scholar] [CrossRef]
- Ter Meulen, J.; Van Den Brink, E.N.; Poon, L.L.M.; Marissen, W.E.; Leung, C.S.W.; Cox, F.; Cheung, C.Y.; Bakker, A.Q.; Bogaards, J.A.; van Deventer, E.; et al. Human Monoclonal Antibody Combination against SARS Coronavirus: Synergy and Coverage of Escape Mutants. PLoS Med. 2006, 3, e237. [Google Scholar] [CrossRef]
- Zhong, L.; Haynes, L.; Struble, E.B.; Tamin, A.; Virata-Theimer, M.L.; Zhang, P. Antibody-mediated synergy and interference in the neutralization of SARS-CoV at an epitope cluster on the spike protein. Biochem. Biophys. Res. Commun. 2009, 390, 1056–1060. [Google Scholar] [CrossRef]
- Patel, H.D.; Nikitin, P.; Gesner, T.; Lin, J.J.; Barkan, D.T.; Ciferri, C.; Carfi, A.; Yazdi, T.A.; Skewes-Cox, P.; Wiedmann, B.; et al. In Vitro Characterization of Human Cytomegalovirus-Targeting Therapeutic Monoclonal Antibodies LJP538 and LJP539. Antimicrob. Agents Chemother. 2016, 60, 4961–4971. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination with Etesevimab on Viral Load in Patients with Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.A.; Shapiro, M.B.; Haigwood, N.L.; Hessell, A.J. Advancing HIV Broadly Neutralizing Antibodies: From Discovery to the Clinic. Front. Public Health 2021, 9, 690017. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Peacock, T.P.; Harvey, W.T.; Hughes, J.; Wright, D.W.; Willett, B.J.; Thomson, E.; Gupta, R.K.; Peacock, S.J.; Robertson, D.L.; et al. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat. Rev. Microbiol. 2023, 21, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Julg, B.; Stephenson, K.E.; Wagh, K.; Tan, S.C.; Zash, R.; Walsh, S.; Ansel, J.; Kanjilal, D.; Nkolola, J.; Walker-Sperling, V.E.K.; et al. Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: A phase 1 clinical trial. Nat. Med. 2022, 28, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.E.Z.; Seah, S.G.K.; Chye, D.H.; Massey, S.; Torres, M.; Lim, A.P.C.; Wong, S.K.K.; Neo, J.J.Y.; Wong, P.S.; Lim, J.H.; et al. The Fc-mediated effector functions of a potent SARS-CoV-2 neutralizing antibody, SC31, isolated from an early convalescent COVID-19 patient, are essential for the optimal therapeutic efficacy of the antibody. PLoS ONE 2021, 16, e0253487. [Google Scholar] [CrossRef]
- Schäfer, A.; Muecksch, F.; Lorenzi, J.C.; Leist, S.R.; Cipolla, M.; Bournazos, S.; Schmidt, F.; Maison, R.M.; Gazumyan, A.; Martinez, D.R.; et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J. Exp. Med. 2021, 218, e20201993. [Google Scholar] [CrossRef]
- Yamin, R.; Jones, A.T.; Hoffmann, H.-H.; Schäfer, A.; Kao, K.S.; Francis, R.L.; Sheahan, T.P.; Baric, R.S.; Rice, C.M.; Ravetch, J.V.; et al. Fc-engineered antibody therapeutics with improved anti-SARS-CoV-2 efficacy. Nature 2021, 599, 465–470. [Google Scholar] [CrossRef]
- Chigutsa, E.; Jordie, E.; Riggs, M.; Nirula, A.; Elmokadem, A.; Knab, T.; Chien, J.Y. A Quantitative Modeling and Simulation Framework to Support Candidate and Dose Selection of Anti-SARS-CoV-2 Monoclonal Antibodies to Advance Bamlanivimab into a First-in-Human Clinical Trial. Clin. Pharmacol. Ther. 2021, 111, 595–604. [Google Scholar] [CrossRef]
- Chigutsa, E.; O’brien, L.; Ferguson-Sells, L.; Long, A.; Chien, J. Population Pharmacokinetics and Pharmacodynamics of the Neutralizing Antibodies Bamlanivimab and Etesevimab in Patients with Mild to Moderate COVID-19 Infection. Clin. Pharmacol. Ther. 2021, 110, 1302–1310. [Google Scholar] [CrossRef]
- Magyarics, Z.; Leslie, F.; Bartko, J.; Rouha, H.; Luperchio, S.; Schörgenhofer, C.; Schwameis, M.; Derhaschnig, U.; Lagler, H.; Stiebellehner, L.; et al. Randomized, Double-Blind, Placebo-Controlled, Single-Ascending-Dose Study of the Penetration of a Monoclonal Antibody Combination (ASN100) Targeting Staphylococcus aureus Cytotoxins in the Lung Epithelial Lining Fluid of Healthy Volunteers. Antimicrob. Agents Chemother. 2019, 63, e00350-19. [Google Scholar] [CrossRef]
- Nehls, J.; Businger, R.; Hoffmann, M.; Brinkmann, C.; Fehrenbacher, B.; Schaller, M.; Maurer, B.; Schönfeld, C.; Kramer, D.; Hailfinger, S.; et al. Release of Immunomodulatory Ebola Virus Glycoprotein-Containing Microvesicles Is Suppressed by Tetherin in a Species-Specific Manner. Cell Rep. 2019, 26, 1841–1853.e6. [Google Scholar] [CrossRef] [PubMed]
- Rydell, G.E.; Prakash, K.; Norder, H.; Lindh, M. Hepatitis B surface antigen on subviral particles reduces the neutralizing effect of anti-HBs antibodies on hepatitis B viral particles in vitro. Virology 2017, 509, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Deming, D.J.; Patel, N.; McCarthy, M.P.; Mishra, L.; Shapiro, A.M.; Suzich, J.A. Potential for Palivizumab Interference with Commercially Available Antibody–antigen Based Respiratory Syncytial Virus Diagnostic Assays. Pediatr. Infect. Dis. J. 2013, 32, 1144–1146. [Google Scholar] [CrossRef] [PubMed]
- General Chapter: USP. General Tests and Assays, Biological Tests and Assays, <85> Bacterial Endotoxins Test. The United States Pharmacopeia—National Formulary, Rockville, MD. Available online: https://doi.usp.org/USPNF/USPNF_M98830_02_01.html (accessed on 17 May 2023).
- Vir Biothechnology, Inc. Press Release, 11 June 2022. Available online: https://investors.vir.bio/news-releases/news-release-details/vir-biotechnology-presents-new-data-evaluating-potential-vir-0 (accessed on 17 February 2023).
- Vir Biothechnology, Inc. Press Release, 25 June 22. Available online: https://investors.vir.bio/news-releases/news-release-details/vir-biotechnology-announces-new-clinical-data-its-broad (accessed on 22 February 2022).
- Xiao, F.; Fofana, I.; Thumann, C.; Mailly, L.; Alles, R.; Robinet, E.; Meyer, N.; Schaeffer, M.; Habersetzer, F.; Doffoël, M.; et al. Synergy of entry inhibitors with direct-acting antivirals uncovers novel combinations for prevention and treatment of hepatitis C. Gut 2015, 64, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Caskey, M.; Klein, F.; Lorenzi, J.C.C.; Seaman, M.S.; West, A.P., Jr.; Buckley, N.; Kremer, G.; Nogueira, L.; Braunschweig, M.; Scheid, J.F.; et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 2015, 522, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Mouquet, H.; Scharf, L.; Euler, Z.; Liu, Y.; Eden, C.; Scheid, J.F.; Halper-Stromberg, A.; Gnanapragasam, P.N.P.; Spencer, D.I.R.; Seaman, M.S.; et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. USA 2012, 109, E3268–E3277. [Google Scholar] [CrossRef] [PubMed]
- Clinicalinfo. Available online: https://clinicalinfo.hiv.gov/en/drugs (accessed on 3 May 2023).
- Kiso, M.; Yamayoshi, S.; Kawaoka, Y. Triple combination therapy of favipiravir plus two monoclonal antibodies eradicates influenza virus from nude mice. Commun. Biol. 2020, 3, 219. [Google Scholar] [CrossRef]
- Cross, R.W.; Bornholdt, Z.A.; Prasad, A.N.; Borisevich, V.; Agans, K.N.; Deer, D.J.; Abelson, D.M.; Kim, D.H.; Shestowsky, W.S.; Campbell, L.A.; et al. Combination therapy protects macaques against advanced Marburg virus disease. Nat. Commun. 2021, 12, 1891. [Google Scholar] [CrossRef]
- Cross, R.W.; Bornholdt, Z.A.; Prasad, A.N.; Woolsey, C.; Borisevich, V.; Agans, K.N.; Deer, D.J.; Abelson, D.M.; Kim, D.H.; Shestowsky, W.S.; et al. Combination therapy with remdesivir and monoclonal antibodies protects nonhuman primates against advanced Sudan virus disease. J. Clin. Investig. 2022, 7, e159090. [Google Scholar] [CrossRef]
- Nakamura, G.; Chai, N.; Park, S.; Chiang, N.; Lin, Z.; Chiu, H.; Fong, R.; Yan, D.; Kim, J.; Zhang, J.; et al. An In Vivo Human-Plasmablast Enrichment Technique Allows Rapid Identification of Therapeutic Influenza a Antibodies. Cell Host Microbe 2013, 14, 93–103. [Google Scholar] [CrossRef]
- Paules, C.I.; Lakdawala, S.; McAuliffe, J.M.; Paskel, M.; Vogel, L.; Kallewaard, N.L.; Zhu, Q.; Subbarao, K. The Hemagglutinin A Stem Antibody MEDI8852 Prevents and Controls Disease and Limits Transmission of Pandemic Influenza Viruses. J. Infect. Dis. 2017, 216, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Tharakaraman, K.; Subramanian, V.; Viswanathan, K.; Sloan, S.; Yen, H.-L.; Barnard, D.L.; Leung, Y.H.C.; Szretter, K.J.; Koch, T.J.; Delaney, J.C.; et al. A broadly neutralizing human monoclonal antibody is effective against H7N9. Proc. Natl. Acad. Sci. USA 2015, 112, 10890–10895. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.S.; Choi, J.-A.; Kim, P.; Ryu, D.-K.; Yang, E.; Son, D.; Shin, J.; Park, H.; Lee, S.; Lee, H.; et al. Broader neutralization of CT-P27 against influenza A subtypes by combining two human monoclonal antibodies. PLoS ONE 2020, 15, e0236172. [Google Scholar] [CrossRef] [PubMed]
- Lindorfer, M.A.; Taylor, R.P. FcγR-Mediated Trogocytosis 2.0: Revisiting History Gives Rise to a Unifying Hypothesis. Antibodies 2022, 11, 45. [Google Scholar] [CrossRef]
- Massanella, M.; Puigdomènech, I.; Cabrera, C.; Fernandez-Figueras, M.T.; Aucher, A.; Gaibelet, G.; Hudrisier, D.; García, E.; Bofill, M.; Clotet, B.; et al. Antigp41 antibodies fail to block early events of virological synapses but inhibit HIV spread between T cells. Aids 2009, 23, 183–188. [Google Scholar] [CrossRef]
- Richardson, S.I.; Crowther, C.; Mkhize, N.N.; Morris, L. Measuring the ability of HIV-specific antibodies to mediate trogocytosis. J. Immunol. Methods 2018, 463, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Kramski, M.; Parsons, M.S.; Stratov, I.; Kent, S.J. HIV-specific antibody immunity mediated through NK cells and monocytes. Curr. HIV Res. 2013, 11, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Shiakolas, A.R.; Kramer, K.J.; Wrapp, D.; Richardson, S.I.; Schäfer, A.; Wall, S.; Wang, N.; Janowska, K.; Pilewski, K.A.; Venkat, R.; et al. Cross-reactive coronavirus antibodies with diverse epitope specificities and Fc effector functions. Cell Rep. Med. 2021, 2, 100313. [Google Scholar] [CrossRef]
- Low, L.A.; Sutherland, M.; Lumelsky, N.; Selimovic, S.; Lundberg, M.S.; Tagle, D.A. Organs-on-a-Chip. Adv. Exp. Med. Biol. 2020, 1230, 27–42. [Google Scholar] [CrossRef]
- Washburn, N.; Schwab, I.; Ortiz, D.; Bhatnagar, N.; Lansing, J.C.; Medeiros, A.; Tyler, S.; Mekala, D.; Cochran, E.; Sarvaiya, H.; et al. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc. Natl. Acad. Sci. USA 2015, 112, E1297–E1306. [Google Scholar] [CrossRef]
- Keating, S.M.; Mizrahi, R.A.; Adams, M.S.; Asensio, M.A.; Benzie, E.; Carter, K.P.; Chiang, Y.; Edgar, R.C.; Gautam, B.K.; Gras, A.; et al. Generation of recombinant hyperimmune globulins from diverse B-cell repertoires. Nat. Biotechnol. 2021, 39, 989–999. [Google Scholar] [CrossRef]
- Frost, G.I. Recombinant human hyaluronidase (rHuPH20): An enabling platform for subcutaneous drug and fluid administration. Expert Opin. Drug Deliv. 2007, 4, 427–440. [Google Scholar] [CrossRef]
- Pitiot, A.; Heuzé-Vourc’h, N.; Sécher, T. Alternative Routes of Administration for Therapeutic Antibodies—State of the Art. Antibodies 2022, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, S.; Garrett, N.; Karim, Q.A.; Zuma, N.Y.; Capparelli, E.; Baxter, C.; Gengiah, T.; Archary, D.; Samsunder, N.; Rose, N.D.; et al. Assessing the safety and pharmacokinetics of the anti-HIV monoclonal antibody CAP256V2LS alone and in combination with VRC07-523LS and PGT121 in South African women: Study protocol for the first-in-human CAPRISA 012B phase I clinical trial. BMJ Open 2020, 10, e042247. [Google Scholar] [CrossRef]
- Nyakatura, E.K.; Soare, A.Y.; Lai, J.R. Bispecific antibodies for viral immunotherapy. Hum. Vaccines Immunother. 2017, 13, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Sroga, P.; Safronetz, D.; Stein, D.R. Nanobodies: A new approach for the diagnosis and treatment of viral infectious diseases. Futur. Virol. 2020, 15, 195–205. [Google Scholar] [CrossRef]
- Walser, M.; Mayor, J.; Rothenberger, S. Designed Ankyrin Repeat Proteins: A New Class of Viral Entry Inhibitors. Viruses 2022, 14, 2242. [Google Scholar] [CrossRef] [PubMed]
- Wensel, D.; Sun, Y.; Davis, J.; Li, Z.; Zhang, S.; McDonagh, T.; Fabrizio, D.; Cockett, M.; Krystal, M. A Novel gp41-Binding Adnectin with Potent Anti-HIV Activity Is Highly Synergistic when Linked to a CD4-Binding Adnectin. J. Virol. 2018, 92, e00421-18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).