Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials
Abstract
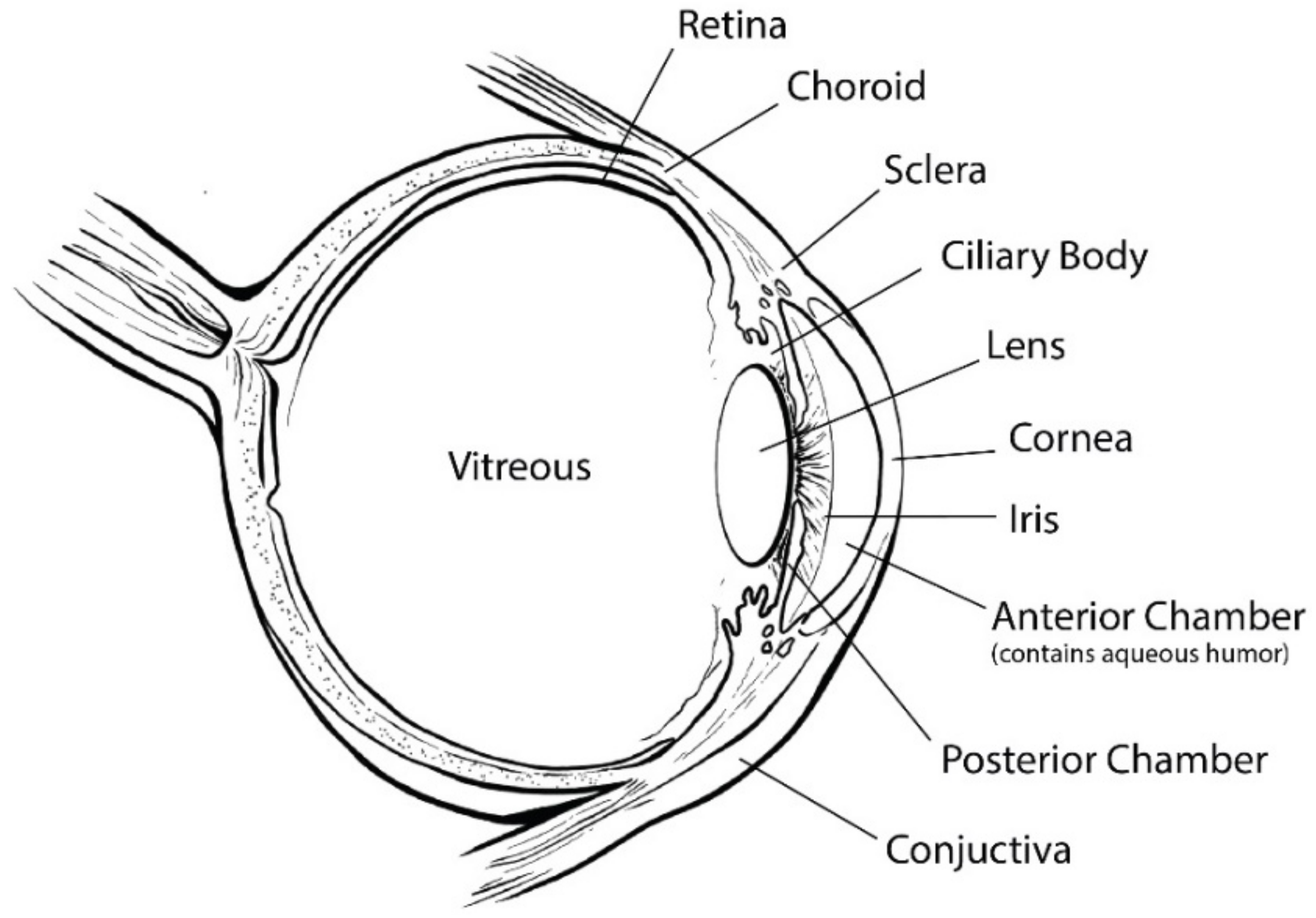
1. Introduction
2. Nonbiodegradable Implants
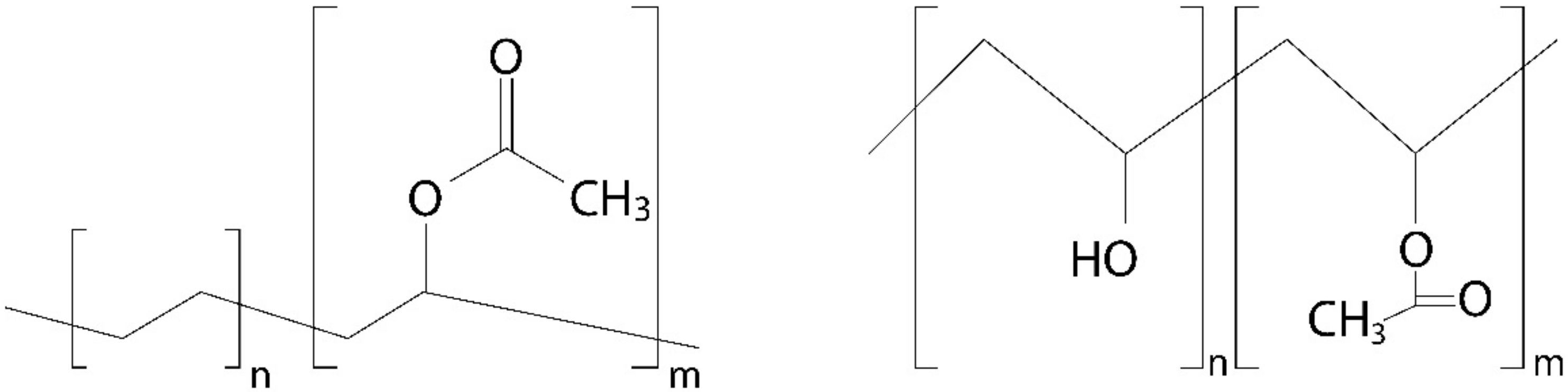
3. EVA-PVA
4. PS
5. PU
6. Materials Trends in NBI
7. Biodegradable Implants
8. PCL
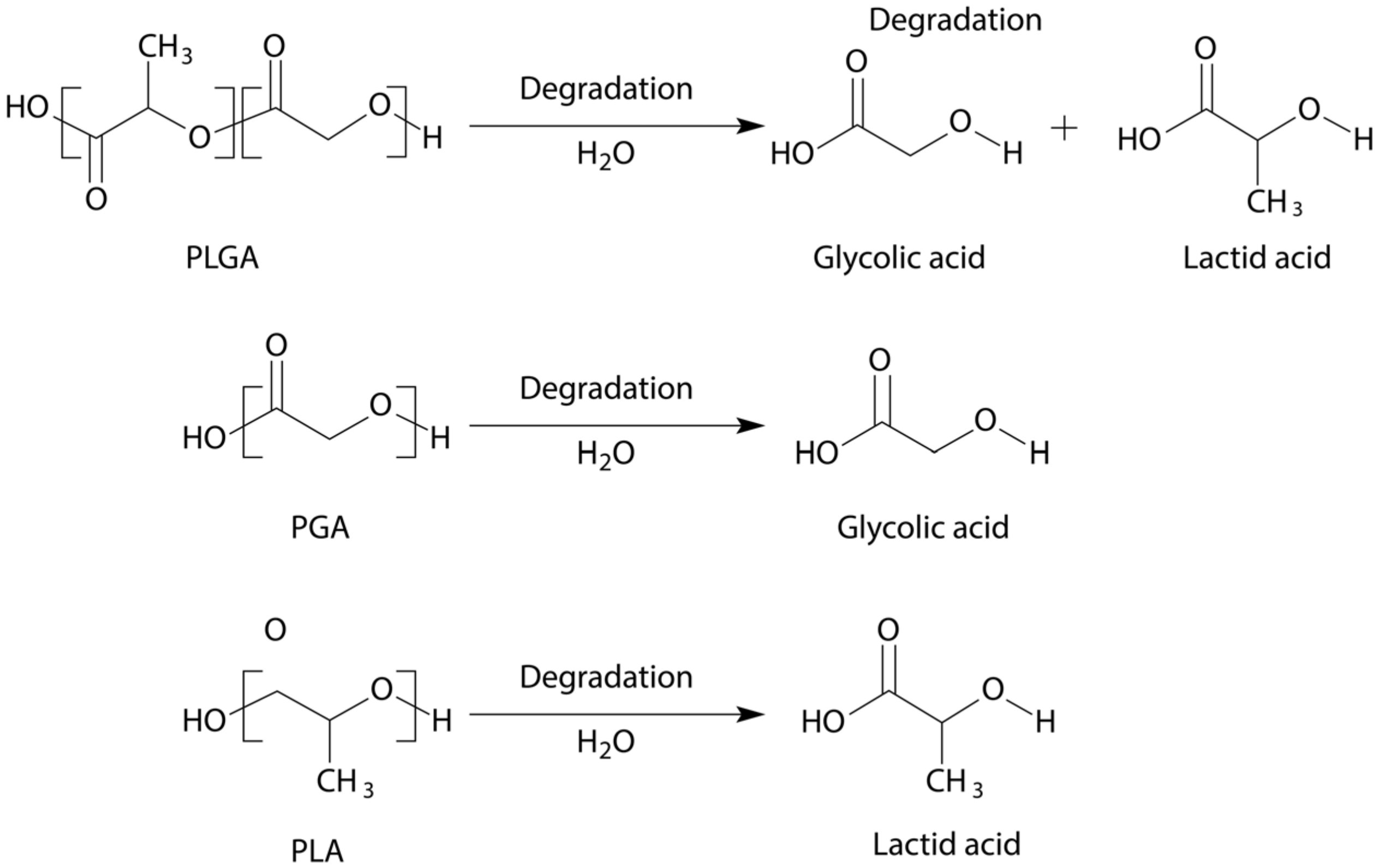
9. PLA, PGA, PLGA
10. Materials Trends in BI
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sonali, N.; Moreno, M.; Nirmal, J.; Fernandez, A.A.; Venkatraman, S.; Agrawal, R. Protein delivery to the back of the eye: Barriers, carriers and stability of anti-VEGF proteins. Drug Discov. Today 2017, 22, 416–423. [Google Scholar] [CrossRef]
- Rupenthal, I.D. Drug-device combination approaches for delivery to the eye. Curr. Opin. Pharmacol. 2017, 36, 44–51. [Google Scholar] [CrossRef]
- Gote, V.; Pal, D. Ocular implants in the clinic and under clinical investigation for ocular disoreders. EC Ophthalmol. 2019, 10, 660–666. [Google Scholar]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control Release 2017, 248, 96–116. [Google Scholar] [CrossRef]
- Raghava, S.; Hammond, M.; Kompella, U.B. Periocular routes for retinal drug delivery. Expert Opin. Drug Deliv. 2004, 1, 99–114. [Google Scholar] [CrossRef]
- Urtti, A.; Salminen, L.; Miinalainen, O. Systemic absorption of ocular pilocarpine is modified by polymer matrices. Int. J. Pharm. 1985, 23, 147–161. [Google Scholar] [CrossRef]
- Kaji, H.; Nagal, N.; Nishizawa, M.; Abe, T. Drug delivery devices for retinal diseases. Adv. Drug Deliv. Rev. 2018, 15, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Agrahari, V.; Mandal, A.; Pal, D.; Mitra, A.K. How are we improving the delivery to back of the eye? Advances and challenges of novel therapeutic approaches. Expert Opin. Drug Deliv. 2017, 14, 1145–1162. [Google Scholar] [CrossRef]
- Lee, S.S.; Hughes, P.; Ross, A.D.; Robinson, M.R. Biodegradable implants for sustained drug release in the eye. Pharm. Res. 2010, 27, 2043–2053. [Google Scholar] [CrossRef]
- Masadeh, R.; Obaidat, R.; Alsmadi, M.; Altaani, B.; Khanfar, M.; Alshyab, R.; Qaoud, M. Technical insight into biodegradable polymers used in implants. Jordan J. Pharm. Sci. 2018, 11, 133–160. [Google Scholar]
- Tamboli, V.; Mishra, G.P.; Mitra, A.K. Biodegradable polymers for ocular drug delivery. In Advances in Ocular Drug Delivery; Mitra, A.K., Ed.; Research Signpost: Kerala, India, 2012; pp. 65–86. [Google Scholar]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control Release 2014, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, A.; Joshi, M.; Christoforidis, J. Drug delivery implants in the treatment of vitreous inflammation. Mediat. Inflamm. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Sanborn, G.E.; Anand, R.; Torti, R.E.; Nightingale, S.D.; Cal, S.X.; Yates, B.; Ashton, P.; Smith, T. Sustained-release Ganciclovir therapy for treatment of Cytomegalovirus Retinitis. Arch. Ophtalmol. 1992, 110, 188–195. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y.; Kimura, H.; Sakurai, E.; Tabata, Y. Drug delivery from ocular implants. Expert Opin. Drug Deliv. 2006, 3, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.P.; Singh, R.P.; Sears, J.E.; Lowder, C.Y.; Kaiser, P.K. Evaluation of fluocinolone acetonide sustained release implant (Retisert) dissociation during implant removal and exchange surgery. Am. J. Ophthalmol. 2012, 154, 969–973. [Google Scholar] [CrossRef]
- Martin, D.F.; Parks, D.J.; Mellow, S.D.; Ferris, F.L.; Walton, R.C.; Remaley, N.A.; Chew, E.Y.; Ashton, P.; Davis, M.D.; Nussenblatt, R.B. Treament of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant: A randomized controlled clinical trial. Arch. Ophtalmol. 1994, 112, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D. The biocompatibility of implant materials. In Host Response to Bomaterials; Badylak, S.F., Ed.; Academic Press: Oxford, UK, 2015; pp. 37–51. [Google Scholar]
- Wang, K.; Deng, Q. The thermal and mechanical properties of poly(ethylene-co-vinyl acetate) random copolymers (PEVA) and its covalently crosslinked analogues (cPEVA). Polymers 2019, 11, 1055. [Google Scholar] [CrossRef]
- Chalykh, A.E.; Stepanneko, V.Y.; Scherbina, A.A.; Balashova, E.G. Adhesive proeprties fo ehtylene and vinyl acetate copolymers. Polym. Sci. Ser. D 2009, 2, 8–15. [Google Scholar] [CrossRef]
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. J. Control Release 2017, 28, 284–295. [Google Scholar] [CrossRef]
- Almeida, A.; Possemiers, S.; Boone, M.N.; De Beer, T.; Quinten, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur. J. Pharm. Biopharm. 2011, 77, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Rudnik, E. Properties and applications. In Compostable Polymer Materials; Elsevier: London, UK, 2019. [Google Scholar]
- Gohil, J.M.; Bhattacharya, A.; Ray, P. Studies on the crosslinking of poly(vinyl alcohol). J. Polym. Res. 2006, 13, 161–169. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartiage and orthopedic applications. J. Biomed. Mater. Res. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Smith, T.J.; Pearson, P.A.; Blandford, D.L.; Brown, J.D.; Goins, K.A.; Hollins, J.L.; Schmeisser, E.T.; Glavinos, P.; Baldwin, L.B.; Ashton, P. Intravitreal sustained-release ganciclovir. Arch. Ophtalmol. 1992, 110, 255–258. [Google Scholar] [CrossRef]
- Musch, D.C.; Martin, D.F.; Gordon, J.F.; Davis, M.D.; Kuppermann, B.D.; Group, G.I.S. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. N. Engl. J. Med. 1997, 337, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Brem, H.; Tapper, D. Biocompatibility of polymeric delivery systems for macromolecules. J. Biomed. Mater. Res. 1981, 15, 267–277. [Google Scholar] [CrossRef]
- Bourges, J.L.; Bloque, C.; Thomas, A.; Froussart, F.; Bochot, A.; Azan, F.; Gurny, R.; Ben-Ezra, D.; Behar-Cohen, F. Intraocular implants for extended drug delivery: Therapeutic applications. Adv. Drug Deliv. Rev. 2006, 58, 1182–1202. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.J.; Kolodner, H.; Morgan, K.S.; Escapini Jr, H.; Sevel, D.; Kaufman, H.E. Polyvinyl alcohol as a protective coating on intraocular lenses. Arch. Ophtalmol. 1980, 98, 1840–1842. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y. Medical Devices for the treatment of eye diseases. Drug Deliv. 2010, 197, 469–489. [Google Scholar]
- Jaffe, G.J.; Martin, D.F.; Callanan, D.G.; Pearson, P.A.; Levy, B.; Comstock, T.; Group, F.A.U.S. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis: Thirty-four-week results of a multicenter randomized clinical study. Ophthalmology 2006, 113, 1020–1027. [Google Scholar] [CrossRef]
- Jaffe, G.J.; McCallum, R.M.; Brandchaud, B.; Skalak, C.; Butuner, Z.; Ashton, P. Long term follow up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology 2005, 112, 1192–1198. [Google Scholar] [CrossRef]
- Haghjou, N.; Soheilian, M.; Abdekhodaie, M.J. Sustained release intraocular drug delivery devices for treatment of uveitis. J. Ophthalmic Vis. Res. 2011, 6, 317–329. [Google Scholar]
- Schwartz, G.S.; Flynn Jr, H.W. Fluocinolone acetonide implantable device for diabetic retinopathy. Curr. Pharm. Biotechnol. 2011, 12, 347–351. [Google Scholar] [CrossRef]
- Brumm, M.V.; Nguyen, Q.D. Fluocinolone acetonide intravitreal sustained release device—A new addition to the armamentarium of uveitic management. Int. J. Nanomed. 2007, 2, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.S. ILUVIEN® technology in the treatment of center-involving diabetic macular edema: A review of the literature. Ther. Deliv. 2018, 9, 547–556. [Google Scholar] [CrossRef]
- Brady, C.J.; Villanti, A.C.; Law, H.A.; Rahimy, E.; Reddy, R.; Sieving, P.C.; Garg, S.J.; Tang, J. Corticosteroid implants for chronic non-infectious uveitis. Cochrane Database Syst. Rev. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Mruthyunjaya, P.; Jaffe, G.J. Medidur insert technology. Retin. Phys. 2007, 4, 25–28. [Google Scholar]
- Molokhia, S.A.; Sant, H.; Simonis, J.; Bishop, C.J.; Burr, R.M.; Gale, B.K.; Ambati, B.K. The capsule drug device: Novel approach for drug delivery to the eye. Vision Res. 2010, 50, 680–685. [Google Scholar] [CrossRef]
- Kuperman, B.D.; Loewenstein, A. Drug delivery to the posterior segment of the eye. Dev. Ophthalmol. 2010, 47, 59–72. [Google Scholar]
- Kane, F.E.; Burdan, J.; Cutino, A.; Green, K.E. Iluvien (TM): A new sustained delivery technology for posterior eye disease. Expert Opin. Drug Deliv. 2008, 5, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Hafiz, G.; Shah, S.M.; Bloom, S.; Brown, D.M.; Bsquets, M.; Ciulla, T.A.; Feiner, L.; Sabates, N.B.; Billman, K.; et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology 2010, 117, 1393–1399. [Google Scholar] [CrossRef]
- Massa, H.; Nagar, A.M.; Vergados, A.; Dadoukis, P.; Patra, S.; Panos, G.D. Intravitreal fluocinolone acetonide implant (ILUVIEN®) for diabetic macular edema: A literature review. J. Int. Med. Res. 2019, 47, 31–43. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable polymeric drug devices: Classification, manufacture, materials and clinical applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.; Ho, C.M. Surface molecular property modifications for poly(dimethylsiloxane) (PDMS) based microfluidic devices. Miicrofluid Nanofluid 2009, 7, 291–306. [Google Scholar] [CrossRef]
- Pinto, S.; Alves, P.; Matos, C.M.; Santos, A.C.; Rodrigues, L.R.; Teixiera, J.A.; Gil, M.H. Poly (dimethyl siloxane) surface modification by low pressure plasma to imrove its characteristics towards biomedical applications. Coll. Surf. B 2010, 81, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Brook, M.A.; Shearwood, H. Silicone elastomers for reduced protein adsorption. Biomaterials 2004, 25, 2273–2282. [Google Scholar] [CrossRef]
- Rahimi, A.; Mashak, A. Review on rubbers in medicine: Natural, silicone and polyurethane rubbers. Plast. Rubber Compos. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Lee, J.H.; Pidaparti, R.M.; Atkinson, G.M.; Moorthy, R.S. Design of an implantable device for ocular drug delivery. J. Drug Deliv. 2012, 2012, 1–8. [Google Scholar]
- Teoh, S.H.; Tang, Z.G.; Hastings, G.W. Thermoplastic polymers in biomedical applications: Structures, properties and processing. In Handbook of Biomaterial Properties; Black, J., Hastings, G., Eds.; Springer: Boston, MA, USA, 1998; pp. 270–301. [Google Scholar]
- Subramaniam, A.; Sethuraman, S. Biomedical applications of nondegradable polymers. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2014; pp. 301–308. [Google Scholar]
- Da Silva, G.R.; Da Silva Cunha, A., Jr.; Behar-Cohen, F.; Ayres, E.; Orefice, R.L. Biodegradation of polyurethanes and nanocomposites to non-cytotoxic degradation products. Polym. Degrad. Stab. 2010, 95, 491–499. [Google Scholar] [CrossRef]
- Krasowska, K.; Heimokswa, A.; Rutkowska, M. Environmental degradability of polyurethanes. In Thermoplastic Elastomers—Synthesis and Applications; IntechOpen: London, UK, 2015; pp. 75–94. [Google Scholar] [CrossRef]
- Zhang, X.; Battiston, K.G.; McBane, J.E.; Matheson, L.A.; Labow, R.S.; Santerre, J.P. Design of biodegradable polyurethanes and the interactions of the polymers nad their degradation by-products within in vitro and in vivo environments. In Advances in Polyurethane Biomaterials; Cooper, S.L., Guan, J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 75–114. [Google Scholar] [CrossRef]
- Da Silva, G.R.; Da Silva Cunha, A., Jr.; Behar-Cohen, F.; Ayres, E.; Orefice, R.L. Biodegradable polyurethane nanocomposites containing dexamethasone for ocular route. Mater. Sci. Eng. C 2011, 31, 414–422. [Google Scholar] [CrossRef]
- Pino, F.C.H.; Da Silva Cunha, A., Jr.; Orefice, R.L.; Ayres, E.; Andrade, S.P.; Dias, C.; Lima, L.; Moura, S.A.L.; da Silva, G.R. Controlled release of triamcinolone actonide from polyurethane implantable devices: Application for inhibition of inflammatory-angiogenesis. J. Mater. Sci. Mater. Med. 2012, 23, 1431–1445. [Google Scholar] [CrossRef]
- Silva Paula, J.; Coimbra Ribeiro, V.R.; Chahud, F.; Cannellini, R.; Monteiro, T.C.; de Lima Gomes, E.C.; Sol Reinach, P.; Veronese Rodrigues, M.L.; Silva-Cunha, A. Bevacizumab-loaded polyurethane subconjunctival implants: Effects on experimental glaucoma filtration surgery. J. Ocul. Pharmacol. Ther. 2013, 29, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.H. Development of implantable medical devices: From an engineering perspective. Int. Neurourol. J. 2013, 17, 98–106. [Google Scholar] [CrossRef]
- Testi, I.; Pavesio, C. Preliminary evaluation of YUTIQTM (fluocinolone acetonide intravitreal implant 0.18 mg) in posterior uveitis. Ther. Deliv. 2019, 10, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Banker, A.S.; Pavesio, C.; Merrill, P. Emerging treatments ofr non-infectious uveitis. US Opthalmic Rev. 2018, 11, 81–86. [Google Scholar] [CrossRef]
- Ins, E.P. A Guide to Administering YUTIQ. Available online: https://yutiq.com/downloads/US-YUT-1900111%20Injection%20Brochure_single%20pages.pdf (accessed on 10 March 2021).
- Cai, C.X.; Skalak, C.; Keenan, R.T.; Grewal, D.S.; Jaffe, G.J. Time to disease recurrence in noninfectious uveitis following long-acting injectable fluocinolone acetonide implant. Graefe’s Arch. Clin. Exp. 2020, 258, 1023–1030. [Google Scholar] [CrossRef]
- Georgiev, A.; Dimov, D.; Spassova, E.; Assa, J.; Dineff, P.; Danev, G. Chemical and physical properties of polyimides: Biomedical and engineering applications. In Higher Performance Polymers—Polyimides Based—From Chemistry to Applications; Abadie, M., Ed.; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Constantin, C.P.; Aflori, M.; Damian, R.F.; Rusu, R.D. Biocompatibility of polyimides: A mini review. Materials 2019, 12, 3166. [Google Scholar] [CrossRef] [PubMed]
- Comprehensive Guide on Polyimide. Available online: https://omnexus.specialchem.com/selection-guide/polyimide-pi-plastic (accessed on 4 September 2020).
- Lee, D.J. Intraocular implants for the treatment of autoimmune uveitis. J. Funct. Biomater. 2015, 6, 650–666. [Google Scholar] [CrossRef]
- Kotwal, V.B.; Saifee, M.; Inamdar, N.; Bishe, K. Biodegradable polymers: Which, when and why. Indian J. Pharm. Sci. 2007, 69, 616–625. [Google Scholar]
- Christoforidis, J.B.; Chang, S.; Jiang, A.; Wang, J.; Cebulla, C.M. Intravitreal devices for the treatment of vitreous inflammation. Mediat. Inflamm. 2012, 2012, 126463–126471. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, properties, and applications. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–36. [Google Scholar] [CrossRef]
- Lance, K.D.; Çgood, S.D.; çmendes, T.S.; Ishikiriyama, M.; Chew, P.; Estes, S.; Yamada, K.; Mudumba, S.; Bhisitkul, R.B.; Desai, T.A. In vitro and in vivo sustained zero-order delivery of rapamycin (Sirolimus) from a biodegradable intraocular device. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7331–7337. [Google Scholar] [CrossRef]
- Kim, J.; Kudisch, K.J.; Mudumba, S.; Asada, H.; Aya-Shibuya, E.; Bhisitkul, R.B.; Desai, T.A. Biocompatibility and pharmacokinetic analysis of an intracameral polycaprolactone drug delivery implant for glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4341–4346. [Google Scholar] [CrossRef]
- Bernards, D.A.; Bhisitkul, R.B.; Wynn, P.; Steedman, M.R.; Lee, O.T.; Wong, F.; Thoongsuwan, S.; Desai, T.A. Ocular biocompatibility and structural integrity of micro-and nanostructured poly(caprolactone) films. J. Ocul. Pharmacol. Ther. 2013, 29, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mei, L.; Song, C.; Cui, X.M.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implants. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Polymer Data Handbook; Oxford University Press: Oxford, UK, 1999; p. 1012.
- Schlesinger, E.B.; Bernards, D.A.; Chen, H.H.; Feindt, J.; Cao, J.; Dix, D.; Romano, C.; Bhisitkul, R.B.; Desai, T.A. Device design methodology and formulation of a protein therapeutic for sustained release intraocular delivery. Bioeng. Transl. Med. 2018, 4, 152–163. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, S.; Li, J.; Nan, K.; Lan, B.; Jin, Y.; Chen, H.; Cheng, L. Sustained release of triamcinolone actonide from an episcleral plaque of multilayered poly-e-caprolactone matrix. Acta Biomater. 2014, 10, 126–133. [Google Scholar] [CrossRef]
- Borhani, H.; Peyman, G.A.; Rahimy, M.H.; Thompson, H. Suppression of experimental proliferative vitreoretinopathy by sustained intraocular delivery of 5-FU. Int. Ophtalmol. 1995, 19, 43–49. [Google Scholar] [CrossRef]
- Beeley, N.R.F.; Rossi, J.V.; Mello-Filho, P.A.A.; Mahmoud, M.I.E.; Fujii, G.Y.; De Juan, E.; Varner, S.E. Fabrication, implantaion, elution, and retrieval of a steroid-loaded polycaprolactone subretinal implant. J. Biomed. Mater. Res. A 2005, 73, 437–444. [Google Scholar] [CrossRef]
- Silva-Cunha, A.; Ligorio Fialho, S.; Naud, M.C.; Behar-Cohen, F. Poly-E-caprolactone intravitreous devices: An In vivo study. Physiol. Pharmacol. 2009, 50, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Shi, W.; Yuan, G.; Xie, L.; Wang, S.; Lin, P. Intravitreal implantation of the biodegradable cyclosporin A drug delivery system for experimental chronic uveitis. Graefe’s Arch. Clin. Exp. Ophtalmol. 2006, 244, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, T.; Xie, L.; Wang, S. FK506 in a biodegradable glycolide-co-clatide-co-caprolactone polymer for prolongation of corneal allograft survival. Curr. Eye Res. 2005, 30, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Boia, R.; Dias, P.A.N.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; de Sousa, H.C.; Ambrosio, A.F.; Braga, M.E.M.; et al. Porous poly(e-caprolactone) implants: A novel strategy for efficient intraocular drug delivery. J. Control Release 2019, 316, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Rawas-Qalaji, M.; Williams, C.A. Adances in ocular drug delivery. Curr. Eye Res. 2011, 37, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamada, H. The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J. Appl. Polym. Sci. 2006, 101, 1816–1825. [Google Scholar] [CrossRef]
- Bourges, J.L.; Gautier, S.E.; Delie, F.; Bejjani, R.A.; Jeanny, J.C.; Gurny, R.; Ezra, D.B.; Behar-Cohen, F.F. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Retina 2003, 44, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Kimura, H.; Tabata, Y.; Ogura, Y. Biodegradable scleral plugs for vitreoretinal drug delivery. Adv. Drug Deliv. Rev. 2001, 52, 25–36. [Google Scholar] [CrossRef]
- Okabe, J.; Kimura, H.; Kunou, N.; Okabe, K.; Kato, A.; Ogura, Y. Biodegradable intrascleral implant for ssutained introcular delivery of betamethasone phosphate. Investig. Ophthalmol. Vis. Sci. 2003, 44, 740–744. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lim, J.O.; Kim, H.K.; Kim, S.Y.; Shin, J.P. A novel design of one-side coated biodegradable intrascleral implant for the sustained release of triamcinolone acetonide. Eur. J. Pharm. Biopharm. 2008, 70, 179–186. [Google Scholar] [CrossRef]
- Shin, J.P.; Park, Y.C.; Oh, J.H.; Lee, J.W.; Kim, Y.M.; Lim, J.O.; Kim, S.Y. Biodegradable intrascleral implant of triamcinolone acetonide in experimental uveitis. J. Ocul. Pharmacol. Ther. 2009, 25, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zambon, J.P.; De Sá Barreto, L.S.; Nakamura, A.N.S.E.; Duailibi, S.; Leite, K.; Magalhaes, R.S.; Orlando, G.; Ross, C.L.; Almeida, F.G. Histological changes induced by polyglycolic-acid (PGA) scaffolds seeded with autologous adipose or muscle-derived stem cells when implanted on rabbit bladder. Organogenesis 2014, 10, 278–288. [Google Scholar] [CrossRef]
- Muniswamy, V.J.; Raval, N.; Gondaliya, P.; Tambe, V.; Kalia, K.; Tekade, R.K. Dendrimer-cationized-albumin encrusted polymeric nanoparticle improves penetration and anticancer activity of doxorubicin. Int. J. Pharm. 2018, 555, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Manickavasagam, D.; Oyewumi, M.O. Critical assessment of implantable drug delivery devices in glaucoma management. J. Drug Deliv. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Sharma, A.K.; Arya, A.; Sahoo, P.K.; Majumdar, D.K. Overview of biopolymers as carriers of antiphlogistic agents for treatment of diverse ocular inflammations. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 1, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic-acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Souza, M.C.M.; Fialho, S.L.; Souza, P.A.F.; Fulgencio, G.O.; Da Silva, G.R.; Silva-Cunha, A. Tacrolimus-loaded PLGA implants: In vivo release and ocular toxicity. Curr. Eye Res. 2014, 39, 99–102. [Google Scholar] [CrossRef]
- Peng, Y.; Ang, M.; Foo, S.; Lee, W.S.; Ma, Z.; Venkatraman, S.S.; Wong, T.T. Biocompatibility and biodegradation studies of subconjunctival implants in rabbit eyes. PLoS ONE 2011, 6, e22057. [Google Scholar] [CrossRef] [PubMed]
- Chennamaneni, S.R.; Mamalis, C.; Archer, B.; Oakey, Z.; Ambati, B.K. Development of a novel bioerodible dexamethasone implant for uveitis and postoperative cataract inflammation. J. Control Release 2013, 167, 53–59. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. In-situ forming PLGA implants for intraocular dexamethasone delivery. Int. J. Pharm. 2018, 548, 337–348. [Google Scholar] [CrossRef]
- Callanan, D.G.; Gupta, S.; Boyer, D.S.; Ciulla, T.A.; Singer, M.A.; Kuppermann, B.D.; Liu, C.C.; Li, X.Y.; Hollander, D.A.; Schiffman, R.M.; et al. Dexamethasone intravitreal implant in combination with laser photocoaguation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013, 120, 1843–1851. [Google Scholar] [CrossRef]
- Bansal, R.; Bansal, P.; Kulkarni, P.; Gupta, V.K.; Sharma, A.K.; Gupta, A. Wandering Ozurdex Implant. J. Ophthalmic Inflamm. Infect. 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Querques, L.; Querques, G.; Lattanzio, R.; Gigante, S.R.; Del Turco, C.; Corradetti, G.; Cascavilla, M.L.; Bandello, F. Repeated intravitreal dexamethasone implant (Ozurdex) for retinal vein occlusion. Ophthalmologica 2013, 229, 21–25. [Google Scholar] [CrossRef]
- Haller, J.A.; Bandello, F.; Belfort, R., Jr.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.H.; Jacques, M.L.; Jiao, J.; et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010, 117, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Lowder, C.; Belfort, R.; Lightman, S.; Foster, C.S.; Robinson, M.R.; Schiffman, R.M.; Li, X.Y.; Cui, H.; Whitcup, S.M. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch. Ophtalmol. 2011, 129, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Seah, S.K.; Husain, R.; Gazzard, G.; Lim, M.C.; Hoh, S.T.; Oen, F.T.; Aung, T. Use of surodex in phacotrabeculectomy surgery. Am. J. Ophthalmol. 2005, 139, 927–928. [Google Scholar] [CrossRef]
- Tan, D.T.; Chee, S.P.; Lim, L.; Lim, A.S. Randomized clinical trial of a new dexamethasone delivery system (Surodex) for treatment of post-cataract surgery inflammation. Ophthalmology 1999, 106, 223–231. [Google Scholar] [CrossRef]
- Silva, G.; Fialho, S.L.; Siqueira, R.C.; Jorge, R.; Da Silva Cunha, A. Implants as drug delivery devices for the treatment of eye diseases. Braz. J. Pharm. Sci. 2010, 46, 585–595. [Google Scholar] [CrossRef]
- Kuno, N.; Fujii, S. Ocular drug delivery systems fo the posterior segment: A review. Retina Today 2012, May, 54–59. [Google Scholar]
- Jeong, J.; Bae, S.H.; Min, K.S.; Seo, J.M.; Chung, H.; Kim, S.J. A miniaturized eye-conformable, and longterm reliable retinal prosthesis using monolithic fabrication of liquid crystal polymer (LCP). IEEE Trans. Biomed. 2015, 62, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Blanco-Fernández, B.; Puga, A.M.; Concheiro, A. Cross linked ionic polysaccharides for stimuli-sensistive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y.A. Polysaccharides in ocular drug delivery. Pharmaceutics 2020, 12, 22. [Google Scholar] [CrossRef]
- De Campos, A.M.; Diebold, Y.; Carvalho, E.L.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles as new ocular drug delivery systems: In vitro stability, in vivo fate, and cellular toxicity. Pharm. Res. 2004, 21, 803–810. [Google Scholar] [CrossRef]
- Manna, S.; Augsburger, J.J.; Correa, Z.M.; Landero, J.A.; Banerjee, R.K. Development of chitosan and polylactic acid based methotrexate intravitreal micro-implants to treat primary intraocular lymphoma: An in vitro study. J. Biomech. Eng. 2014, 136, 021018. [Google Scholar] [CrossRef]
- Manna, S.; Donnell, A.M.; Kaval, N.; Al-Rjoub, M.F.; Augsburger, J.J.; Benerjee, R.K. Improved design and characterization of PLGA/PLA-coated chitosan based micro-implants for controlled release of hydrophilic drugs. Int. J. Pharm. 2018, 547, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Varshochian, R.; Rafiee-Tehrani, M.; Dorkoosh, F.A.; Khoshayand, M.R.; Dinarvand, R. Ocular implant containing bevacizumab-loaded chitosan nanoparticles intended for choroidal neovascularization treatment. J. Biomed. Mater. Res. 2018, 106, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, E.; Vandervelden, C.; Fakhari, A.; Qian, J.; Berkland, C.; Gehrke, S.H. Design of hollow hyaluronic acid cylinders for sustained intravitreal protein delivery. J. Pharm. Sci. 2018, 107, 2354–2365. [Google Scholar] [CrossRef] [PubMed]
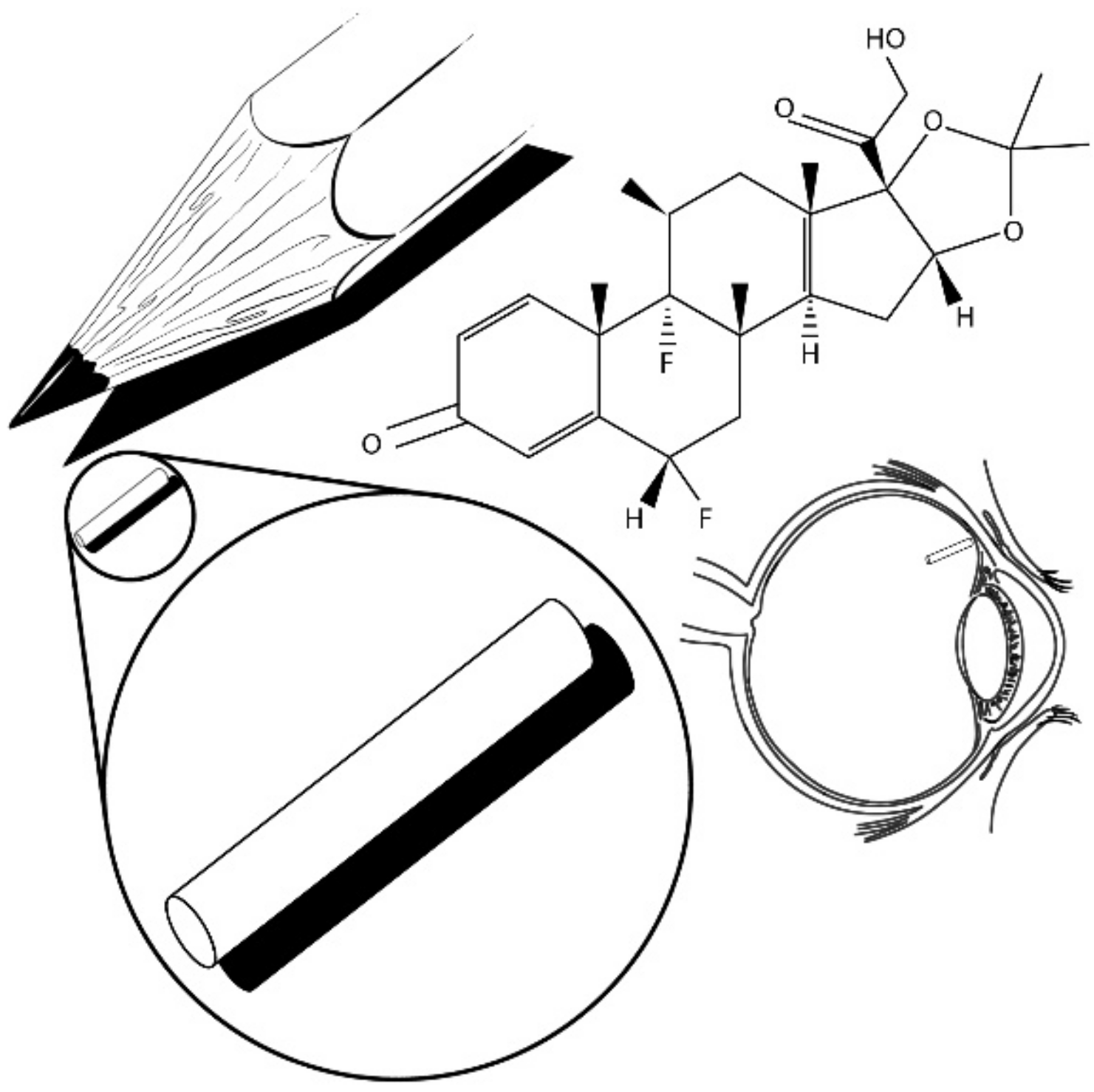
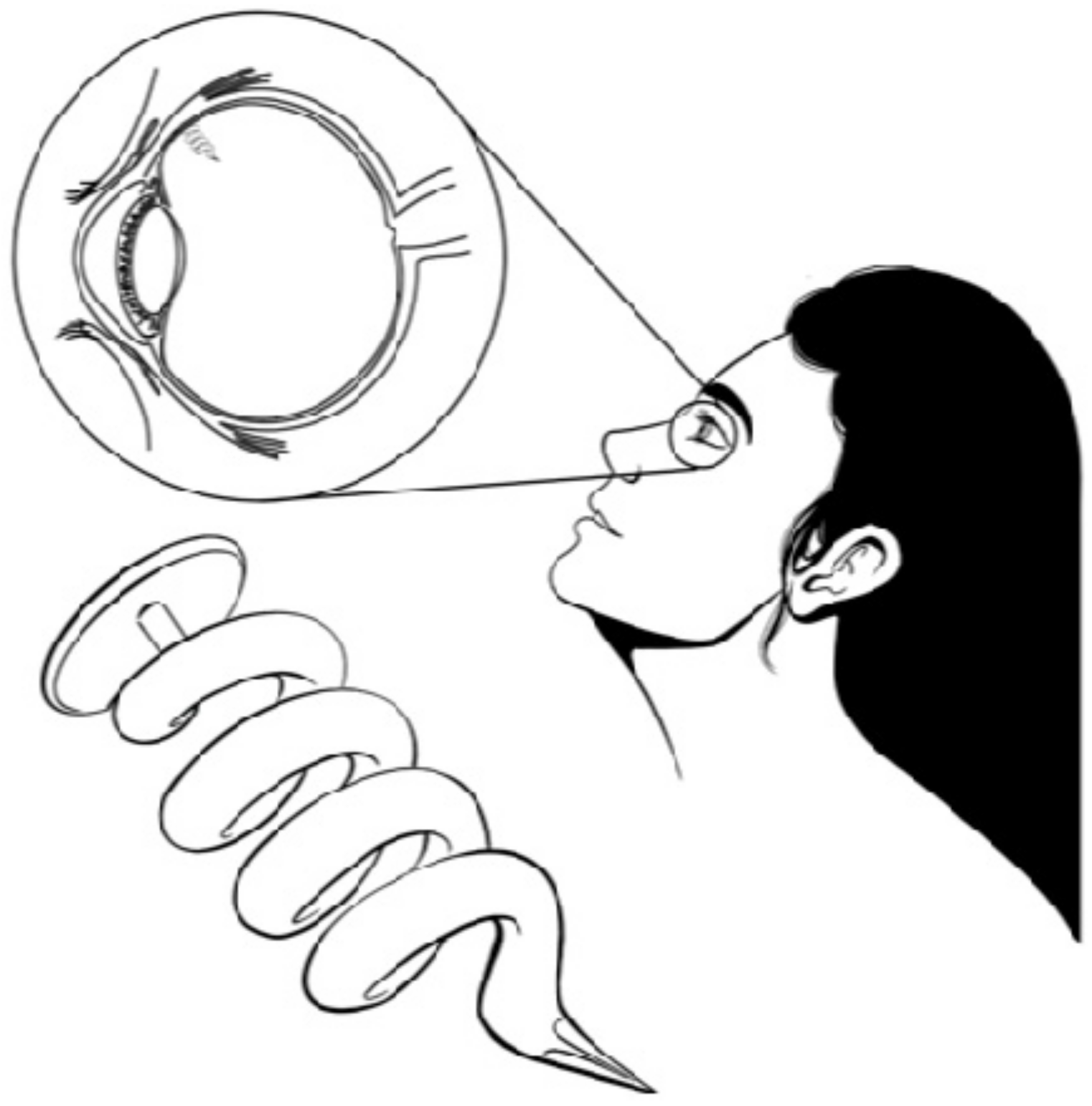
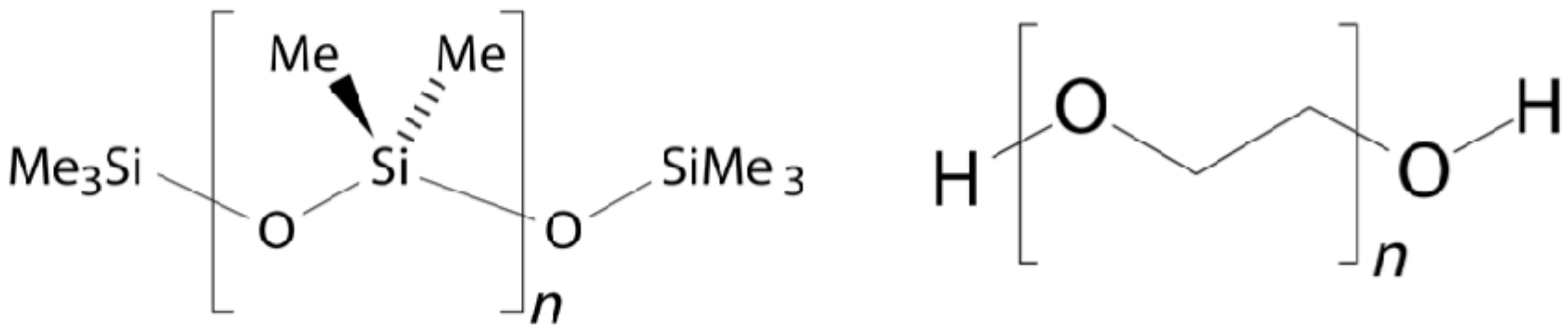
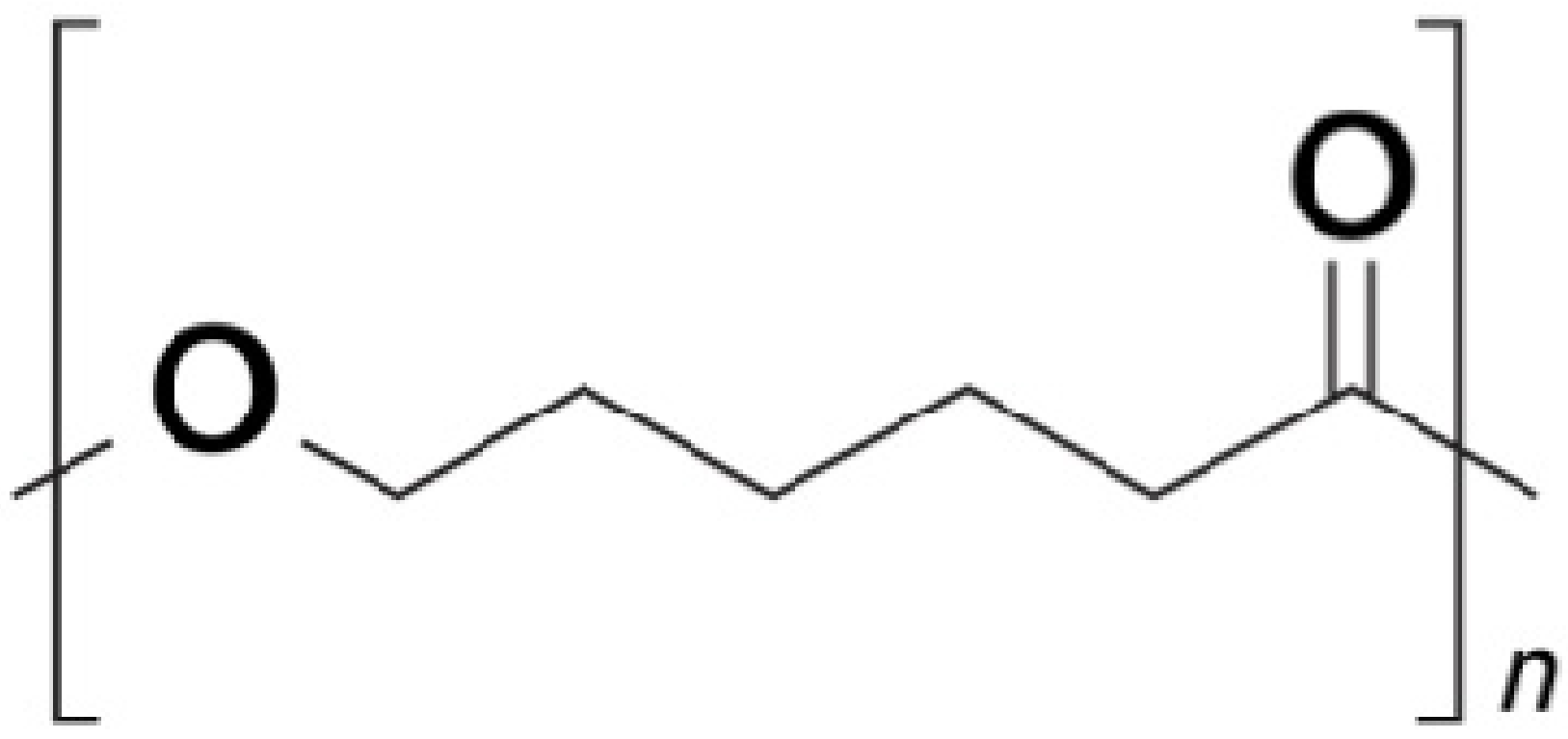
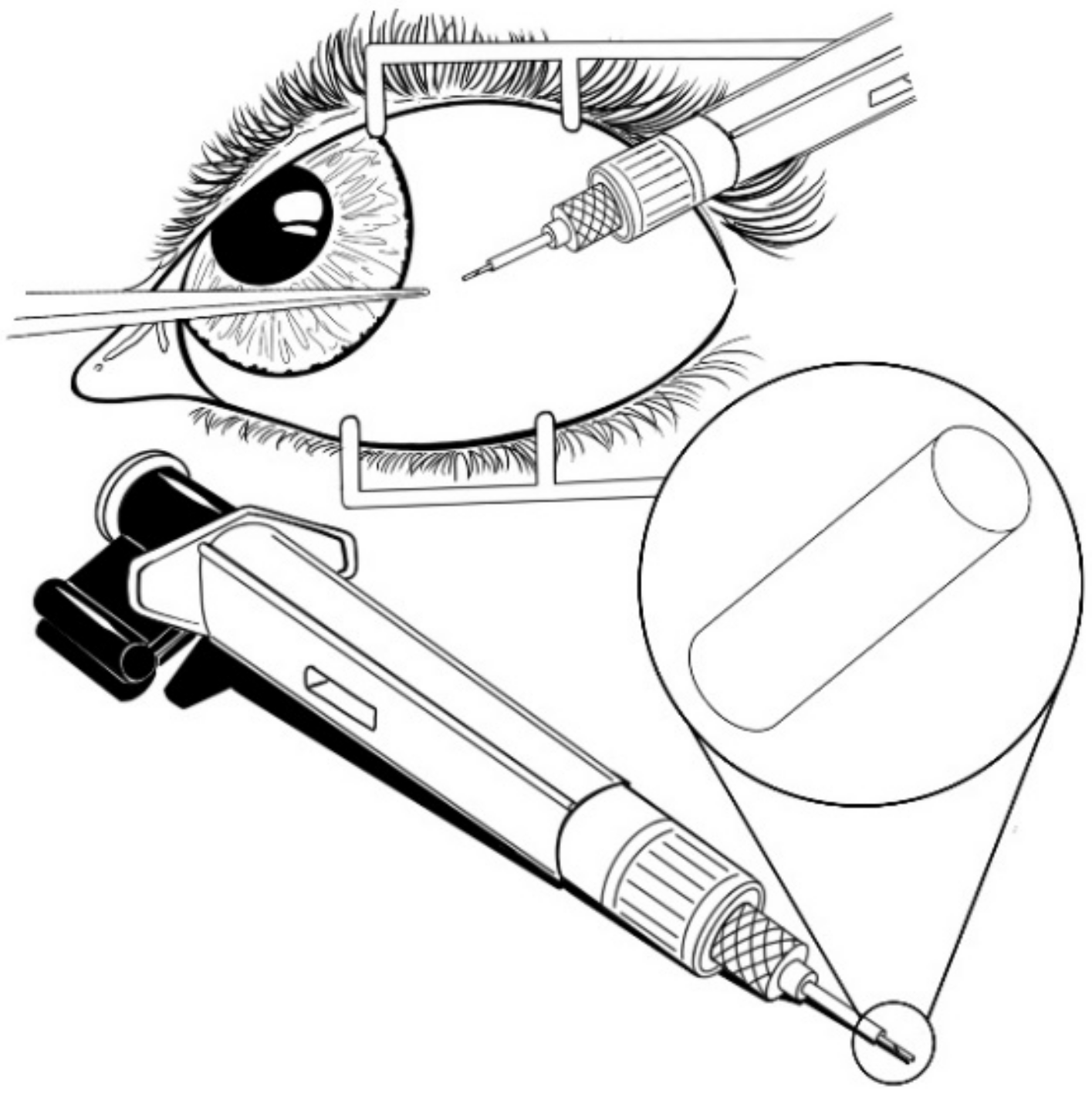
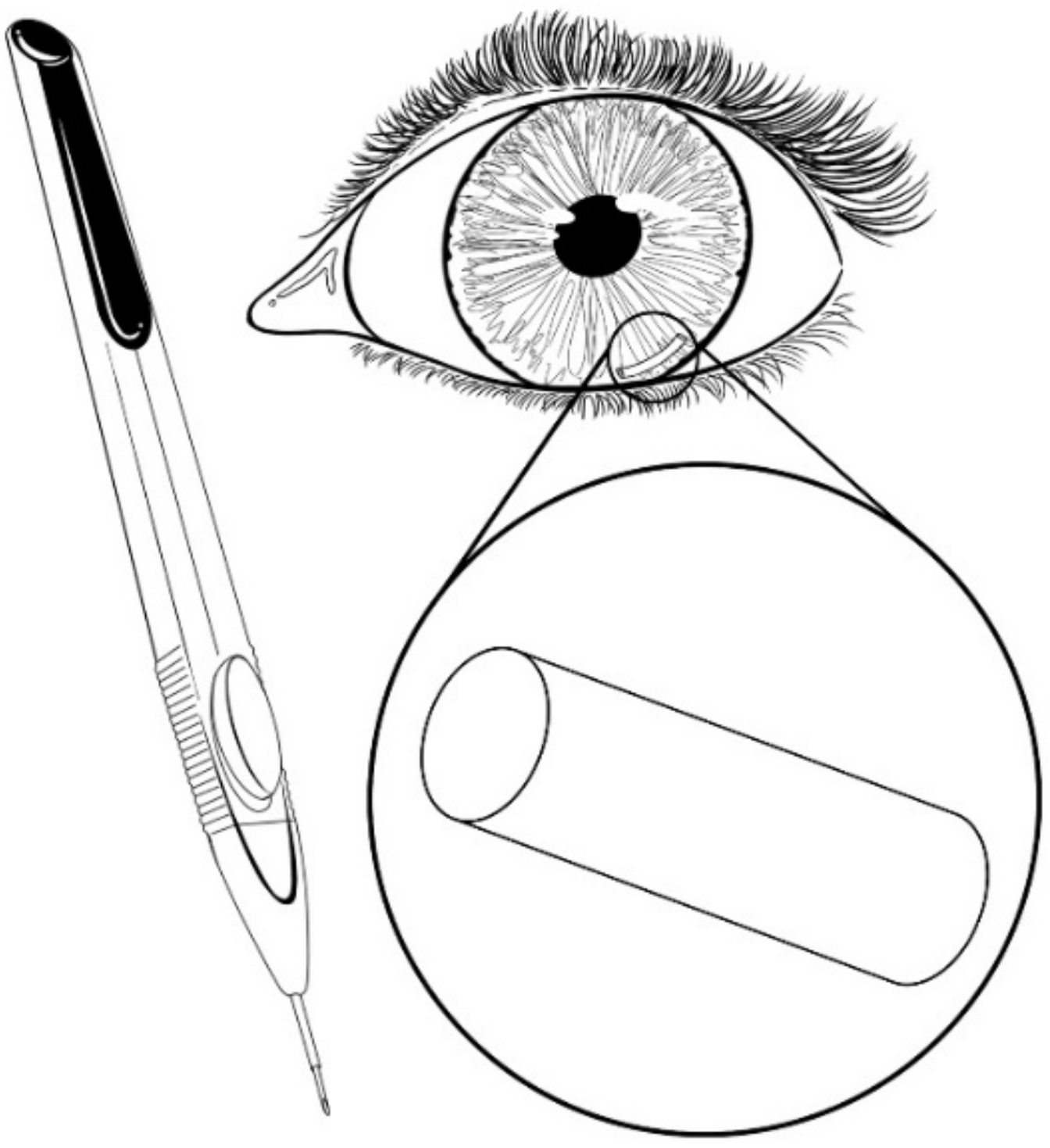
| Sample a | VA (wt%) b | DOC c |
|---|---|---|
| PEVA11 | 11 | 45.7 ± 0.7 |
| PEVA20 | 20 | 36.7 ± 0.8 |
| PEVA31 | 31 | 27.6 ± 0.7 |
| PEVA35 | 35 | 13.1 ± 0.7 |
| PEVA44 | 44 | 8.4 ± 0.8 |
| Sample a | VA (wt%) b | Tm,p c (°C) |
|---|---|---|
| EVA7 | 7 | 103 |
| EVA14 | 14 | 97 |
| EVA20 | 20 | 70 |
| PEVA29 | 29 | 60 |
| EVA30 | 30 | 60 |
| EVA40 | 40 | 25 |
| Segment | Effect |
|---|---|
| PEG | Increases solubility and hydrolytic degradation |
| PTCM | Contributes to maintain mechanical properties for long periods |
| PCL | Improves hydrolytic degradation |
| GAE | Reduces enzymatic biodegradation rate |
| PCN | Provides high tensile strength, yields relatively low pro-inflammatory degradation products |
| HEMA | Confers cross-linking functionality to the polymer |
| DTH | Contributes to peptide degradation by products |
| ISO | Enhances biological activity |
| Property | Value |
|---|---|
| Elongation at break | 90% |
| Flexibility | 2.48–4.10 GPa |
| Young Modulus | 1.3–4.0 GPa |
| Density | 1.31–1.43 g/cm3 |
| Glass transition temperature | 250–340 °C |
| Property | Units | Value or Condition |
|---|---|---|
| Molar mass (of repeat unit) | g·mol−1 | 114 |
| Weight average molar mass (Mw) | g·mol−1 | 74,000 |
| Number average molar mass (Mn) | g·mol−1 | 25,000 |
| Intrinsic viscosity | cm3·g−1 | 0.9 |
| Physical state | - | Semicrystalline |
| Solvents | - | Dimethylacetamide, benzene, chloroform |
| Degree of crystallinity | % | 69 |
| Glass transition temperature | K | 201 |
| Melting temperature | K | 331 |
| Heat of fusion | kJ·mol−1 | 8.9 |
| Materials | Design of the Device | Drug | Role of the Materials | References |
|---|---|---|---|---|
| EVA-PVA | Reservoir surrounded by a membrane | Glancicovir | EVA: membrane to limit the surface area for the permeability of the medication PVA: frame that regulates the rate of drug permeability | [8,9,14,15,16,17,18] |
| PVA-Silicone | Tablet geometry | FA | PVA: suture tab and coating Silicone: coating | [16,17,33,34,35,36,37,38,39] |
| PVA | Cylinder | FA | PVA: caps to regulate drug release rate | [40] |
| PVA-PMMA-Silicone | Ring | Avastin | PVA: polymeric carrier to control release and stability of the drug PMMA: reservoir material Silicone: check valves for refilling | [41] |
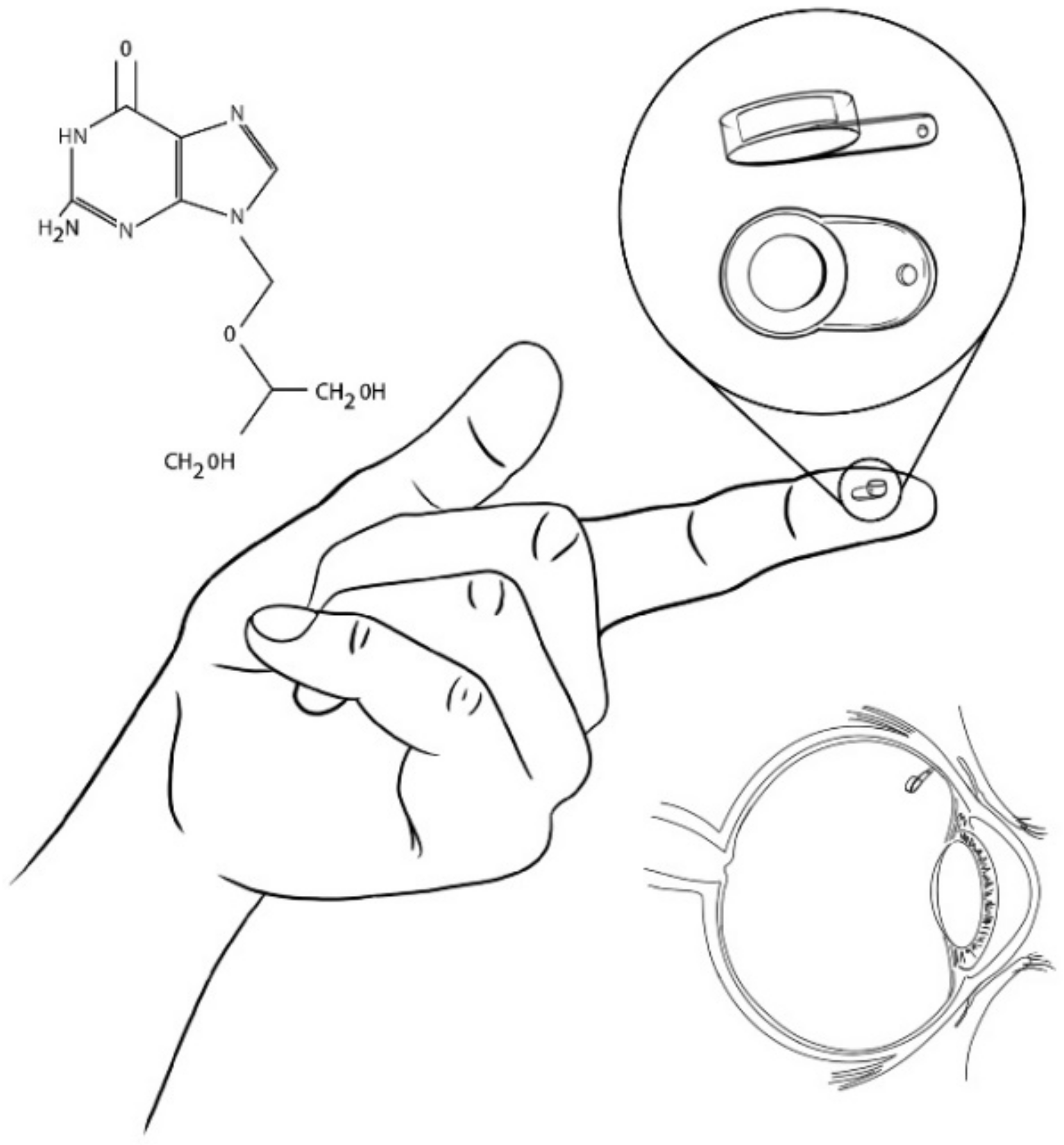
| Titanium-PVA-EVA | Helix | TA | Titanium-PVA-EVA: reservoir | [14,42] |
| PU-CNPs-PCL | DXM | PU: base material CNPs: as additive to modify mechanical properties PCL: as soft segment to improve polymer degradation, biocompatibility and stability | [57] | |
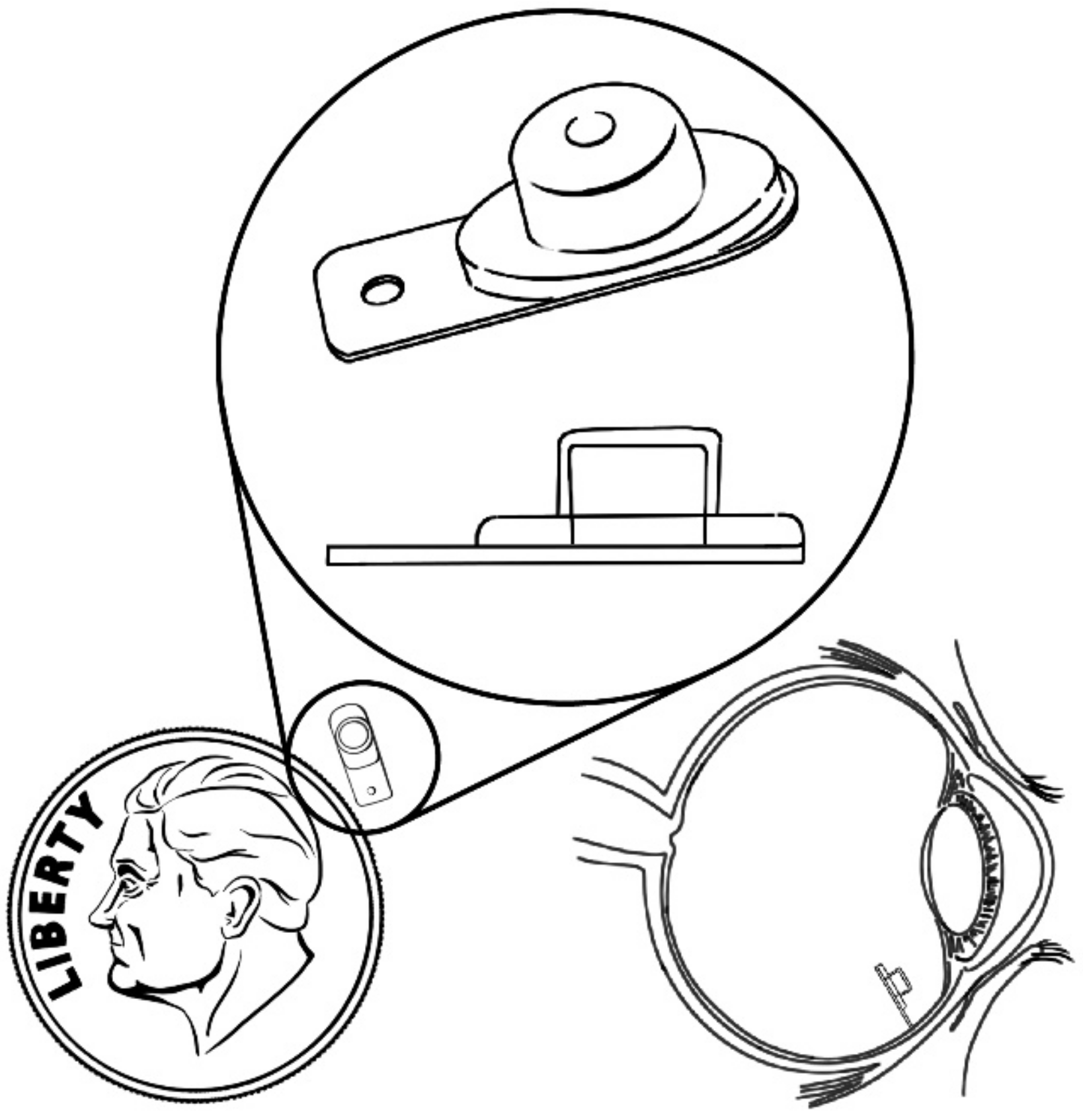
| PI-PVA-Silicone | Tubular | - | PI: base material PVA: membrane Silicone: adhesive plug | [63] |
| PCL | - | TA, 5-FU, DXM | PCL: carrier | [80,81,82] |
| PLA | Disc | TA | PLA: base material | [92] |
| PLGA-HPMC | Rod | DXM | PLGA-HPMC: base material | [35,108,109,110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701. https://doi.org/10.3390/pharmaceutics13050701
García-Estrada P, García-Bon MA, López-Naranjo EJ, Basaldúa-Pérez DN, Santos A, Navarro-Partida J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics. 2021; 13(5):701. https://doi.org/10.3390/pharmaceutics13050701
Chicago/Turabian StyleGarcía-Estrada, Paulina, Miguel A. García-Bon, Edgar J. López-Naranjo, Dulce N. Basaldúa-Pérez, Arturo Santos, and Jose Navarro-Partida. 2021. "Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials" Pharmaceutics 13, no. 5: 701. https://doi.org/10.3390/pharmaceutics13050701
APA StyleGarcía-Estrada, P., García-Bon, M. A., López-Naranjo, E. J., Basaldúa-Pérez, D. N., Santos, A., & Navarro-Partida, J. (2021). Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics, 13(5), 701. https://doi.org/10.3390/pharmaceutics13050701






