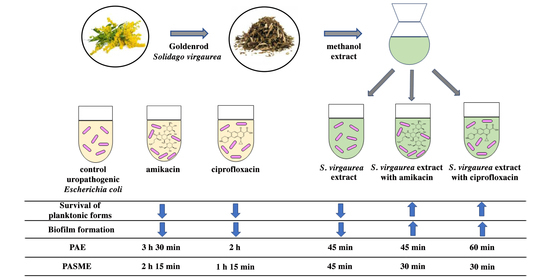
Is it Worth Combining Solidago virgaurea Extract and Antibiotics against Uropathogenic Escherichia coli rods? An In Vitro Model Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Antimicrobial Agents
2.3. Plant Material
2.4. UHPLC-DAD-ESI-MS Analysis
2.5. Quantitative Analysis
2.6. Minimum Inhibitory Concentration (MIC) Determination
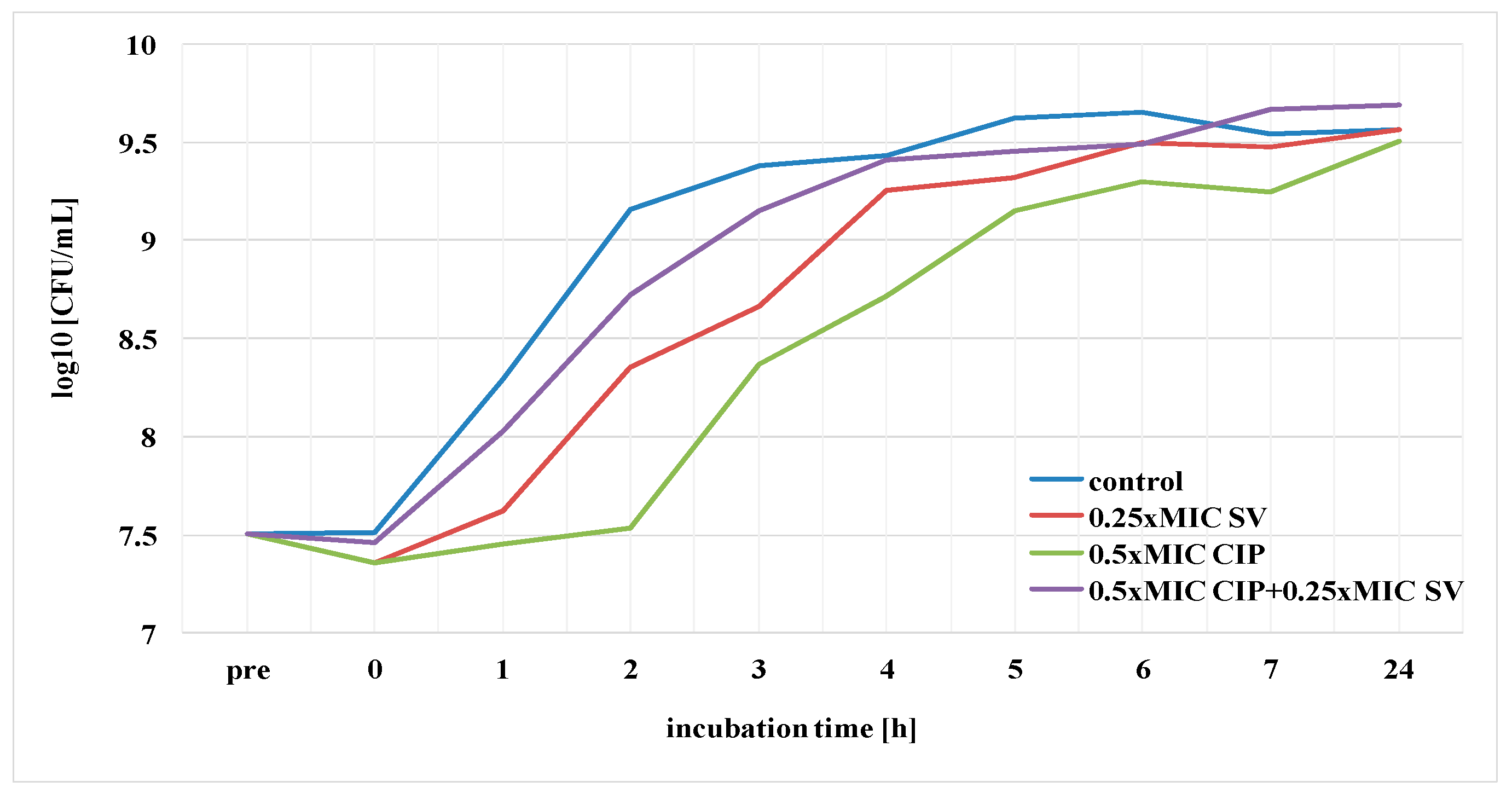
2.7. Effect of S. virgaurea Extract, Antibiotics and Their Combinations on Bacterial Survival
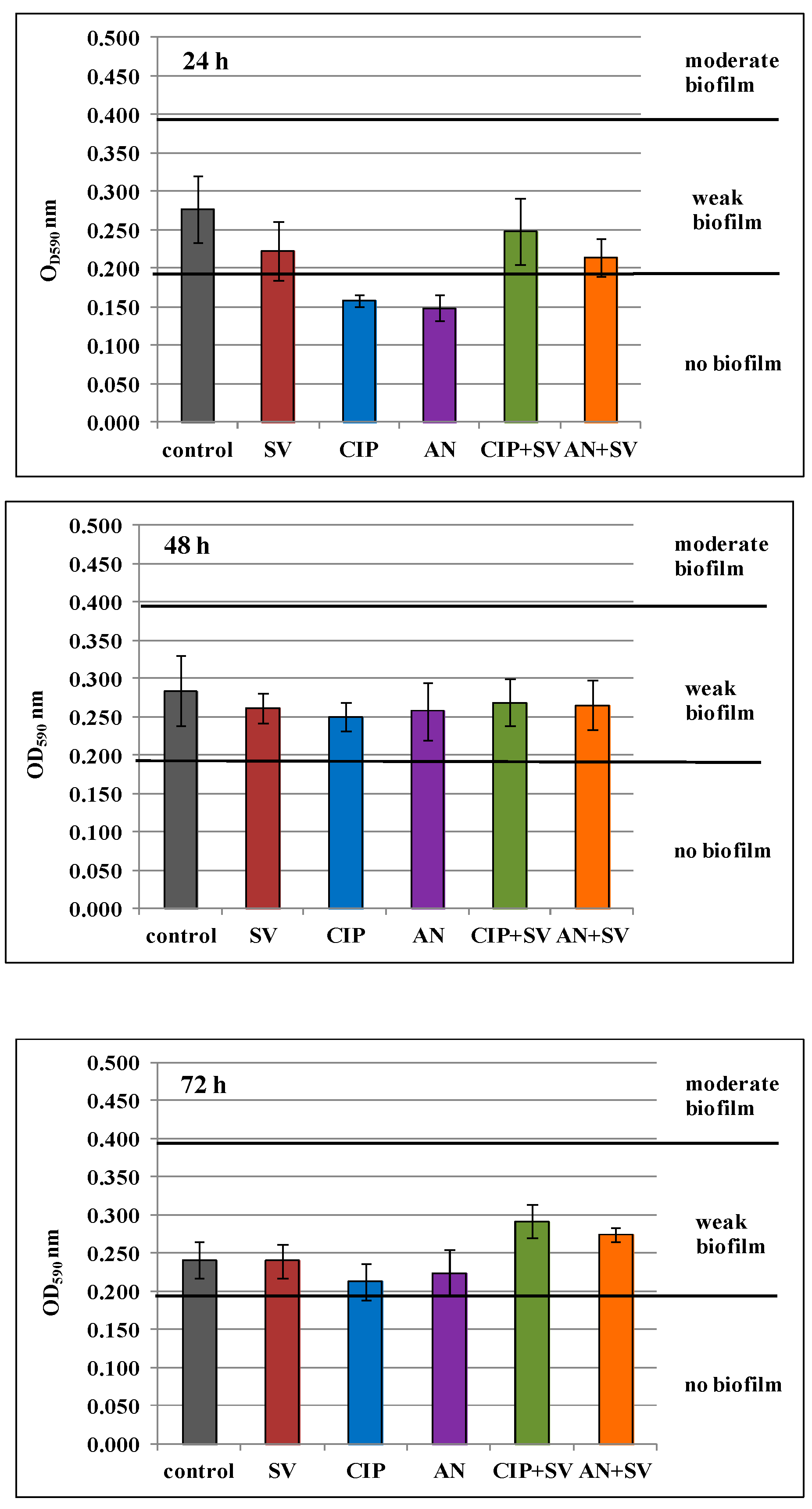
2.8. Effect of S. virgaurea Extract, Antibiotics and Their Combinations on Biofilm Production
2.9. Postantibiotic Effect (PAE) and Postantibiotic Sub-MIC Effect (PASME) in the Presence of S. virgaurea Extract
2.10. Statistical Analysis
3. Results
3.1. Qualitative and Quantitative Analysis of S. virgaurea Extract
3.2. Minimum Inhibitory Concentrations (MICs) of Antibiotics and S. virgaurea Extract
3.3. Effect of S. virgaurea Extract, Antibiotics and Their Combinations on Bacterial Survival
3.4. Effect of S. virgaurea Extract, Antibiotics and Their Combinations on Biofilm Production
3.5. Postantibiotic Effect (PAE) and Postantibiotic Sub-MIC Effect (PASME) in the Presence of S. virgaurea Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roslon, W.; Osinska, E.; Mazur, K.; Geszprych, A. Chemical characteristics of European goldenrod (Solidago virgaurea L. subsp. virgaurea) from natural sites in Central and Eeastern Poland. Acta Sci. Pol. 2014, 13, 55–65. [Google Scholar]
- Woźniak, D.; Ślusarczyk, S.; Domaradzki, K.; Dryś, A.; Matkowski, A. Comparison of polyphenol profile and antimutagenic and antioxidant activities in two species used as source of Solidaginis herba—goldenrod. Chem. Biodivers. 2018, 15, e1800023. [Google Scholar] [CrossRef]
- Metzner, J.; Hirschelmann, R.; Hiller, K. Antiphlogistic and analgesic effects of leiocarposide, a phenolic bisglucoside of Solidago virgaurea L. Pharmazie 1984, 39, 869–870. [Google Scholar]
- Thiem, B.; Wesołowska, M.; Skrzypczak, L.; Budzianowski, J. Phenolic compounds in two Solidago L. species from in vitro culture. Acta Pol. Pharm. 2001, 58, 277–281. [Google Scholar]
- European Medicines Agency. Assessment Report on Solidago virgaurea L. Herba; European Medicines Agency: London, UK, 2008. [Google Scholar]
- Motaal, A.A.; Ezzat, S.M.; Tadros, M.G.; El-Askary, H.I. In vivo anti-inflammatory activity of caffeoylquinic acid derivatives from Solidago virgaurea in rats. Pharm. Biol. 2016, 54, 2864–2870. [Google Scholar] [CrossRef]
- Choi, S.Z.; Choi, S.U.; Lee, K.R. Pytochemical constituents of the aerial parts from Solidago virga-aurea var. gigantea. Arch. Pharmacal. Res. 2004, 27, 164–168. [Google Scholar] [CrossRef]
- Pietta, P.; Gardana, C.; Mauri, P.; Zecca, L. High-performance liquid chromatographic analysis of flavonol glycosides of Solidago virgaurea. J. Chromatogr. A 1991, 558, 296–301. [Google Scholar] [CrossRef]
- Dobjanschi, L.; Fritea, L.; Patay, E.B.; Tamas, M. Comparative study of the morphological and phytochemical characterization of Romanian Solidago species. Pak. J. Pharm. Sci. 2019, 32, 1571–1579. [Google Scholar] [PubMed]
- Chodera, A.; Dabrowska, K.; Sloderbach, A.; Skrzypczak, L.; Budzianowski, J. Effect of flavonoid fractions of Solidago virgaurea L. on diuresis and levels of electrolytes. Acta Pol. Pharm. 1991, 48, 35–37. [Google Scholar] [PubMed]
- Bader, G.; Binder, K.; Hiller, K.; Ziegler-Böhme, H. The antifungal action of triterpene saponins of Solidago virgaurea L. Die Pharmcol. 1987, 42, 140. [Google Scholar]
- Dobjanschi, L.; Zdrinca, M.; Muresan, M.; Vicas, S.; Antonescu, A. The thin layer chromatography analysis of saponins belonging to Solidago species. Fasc. Prot. Mediu. 2013, 21, 56–60. [Google Scholar]
- Tkachev, A.V.; Korolyuk, E.A.; Letchamo, W. Volatile Oil-Bearing Flora of Siberia VIII: Essential Oil Composition and Antimicrobial Activity ofWild Solidago virgaurea L. from the Russian Altai. J. Essent. Oil. Res. 2006, 18, 46–50. [Google Scholar] [CrossRef]
- Kalemba, D. Constituents of the essential oil of Solidago virgaurea L. Flavour Fragr. J. 1998, 13, 373–376. [Google Scholar] [CrossRef]
- Kalemba, D.; Thiem, B. Constituents of the essential oils of four micropropagated Solidago species. Flavour. Fragr. J. 2004, 19, 40–43. [Google Scholar] [CrossRef]
- Starks, C.M.; Williams, R.B.; Goering, M.G.; O’Neil-Johnson, M.; Norman, V.L.; Hu, J.-F.; Garo, E.; Hough, G.W.; Rice, S.M.; Eldridge, G.R. Antibacterial clerodane diterpenes from goldenrod (Solidago virgaurea). Phytochemistry 2010, 71, 104–109. [Google Scholar] [CrossRef]
- Pychenkova, P.A. Dynamics of the amount and characteristics of the polysaccharides of Solidago virgaurea. Chem. Nat. Compd. 1987, 23, 246–247. [Google Scholar] [CrossRef]
- Lam, J. Polyacetylenes of Solidago virgaurea: Their seasonal variation and NMR long-range spin coupling constants. Phytochemistry 1971, 10, 647–653. [Google Scholar] [CrossRef]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A review of its ethnomedicinal uses, phytochemistry, and pharmacological activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Ott, P.G.; Häbe, T.T.; Darcsi, A.; Böszörményi, A.; Alberti, Á.; Krüzselyi, D.; Csontos, P.; Béni, S.; Morlock, G.E. Effect-directed discovery of bioactive compounds followed by highly targeted characterization, isolation and identification, exemplarily shown for Solidago virgaurea. Anal. Chem. 2016, 88, 8202–8209. [Google Scholar] [CrossRef]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Carmen Chifiriuc, M.; Lazar, V. Microbial biofilms in urinary tract infections and prostatitis: Etiology, pathogenicity, and combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob Resist. Infect. Control. 2019, 8, 76. [Google Scholar] [CrossRef]
- Kot, B. Antibiotic resistance among uropathogenic Escherichia coli. Pol. J. Microbiol. 2019, 68, 403–415. [Google Scholar] [CrossRef]
- Lai, P.-J.; Ng, E.-V.; Yang, S.-K.; Moo, C.-L.; Low, W.Y.; Yap, P.S.-X.; Lim, S.-H.E.; Lai, K.-S. Transcriptomic analysis of multi-drug resistant Escherichia coli K-12 strain in response to Lavandula angustifolia essential oil. 3 Biotechnology 2020, 10, 313. [Google Scholar] [CrossRef]
- Scazzocchio, F.; Mondì, L.; Ammendolia, M.G.; Goldoni, P.; Comanducci, A.; Marazzato, M.; Conte, M.P.; Rinaldi, F.; Crestoni, M.E.; Fraschetti, C.; et al. Coriander (Coriandrum sativum) essential oil: Effect on multidrug resistant uropathogenic Escherichia coli. Nat. Prod. Commun. 2017, 12, 623–626. [Google Scholar] [CrossRef]
- Fadli, M.; Bollab, J.M.; Mezriouia, N.E.; Pagès, J.M.; Hassania, L. First evidence of antibacterial and synergistic effects of Thymus riatarum essential oil with conventional antibiotics. Ind. Crop. Prod. 2014, 61, 370–376. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilic, B.S.; Miladinovic, L.C.; Kocic, B.D.; Ciric, V.M.; Stankov-Jovanovic, V.P.; Cvetkovic, O.G. Antibacterial activity of Thymus pulegioides essential oil and its synergistic potential with antibiotics: A chemometric approach. In Recent Progress in Medicinal Plants, Essential Oils III and Phytopharmacology; Govil, J.N., Bhattacharya, S., Eds.; Studium Press LLC: Houston, TX, USA, 2013; pp. 101–136. [Google Scholar]
- Eyler, R.F.; Shvets, K. Clinical pharmacology of antibiotics. Clin. J. Am. Soc. Nephrol. 2019, 14, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Thiem, B.; Goślińska, O. Antimicrobial activity of Solidago virgaurea L. from in vitro cultures. Fitoterapia 2002, 73, 514–516. [Google Scholar] [CrossRef]
- Demir, H.; Acik, L.; Bali, E.B.; Koc, L.Y.; Kaynak, G. Antioxidant and antimicrobial activities of Solidago virgaurea extracts. Afr. J. Biotech. 2009, 8, 274–279. [Google Scholar]
- Anžlovar, S.; Koce, J.D. Antibacterial and antifungal activity of aqueous and organic extracts from indigenous and invasive species of goldenrod (Solidago spp.) grown in Slovenia. Phyton 2014, 54, 135–147. [Google Scholar]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid. Based Complement. Alternat. Med. 2014, 253875, 1–9. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Di Bonaventura, G.; Djukic, S.; Cirkovic, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Gleńsk, M.; Tichaczek-Goska, D.; Środa-Pomianek, K.; Włodarczyk, M.; Wesolowski, C.A.; Wojnicz, D. Differing antibacterial and antibiofilm properties of Polypodium vulgare L. Rhizome aqueous extract and one of its purified active ingredients—Osladin. J. Herb Med. 2019, 17-18, 100261. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Ginestra, G.; Mandalari, G.; Furneri, P.M.; Bisignano, G. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine 2010, 17, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C.; Smith, M.; Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines, 4th ed.; Pharmaceutical Press: London, UK, 2013; p. 813. [Google Scholar]
- Basavegowda, N.; Patra, J.K.; Baek, K.-H. Essential oils and mono/bi/tri-metallic nanocomposites as alternative sources of antimicrobial agents to combat multidrug-resistant pathogenic microorganisms: An overview. Molecules 2020, 25, 1058. [Google Scholar] [CrossRef]
- Moustafa, M.M.; Mohamed, Z.K.; Gheeth, D.M.; Salah, M.G. Antimicrobial potential of various essential oils against multidrug-resistant uropathogenic Escherichia coli. Int. J. Progress. Sci. Technol. 2019, 16, 198–203. [Google Scholar]
- Nabti, L.Z.; Sahli, F.; Laouar, H.; Olowo-okere, A.; Wandjou, J.G.N.; Maggi, F. Chemical composition and antibacterial activity of essential oils from the Algerian endemic Origanum glandulosum Desf. against multidrug-resistant uropathogenic E. coli isolates. Antibiotics 2020, 9, 29. [Google Scholar] [CrossRef]
- Le, N.T.; Donadu, M.G.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, D.; Sanna, G.; Marchetti, M.; Usai, M.; et al. Biological activities of essential oil extracted from leaves of Atalantia sessiflora Guillauminin Vietnam. J. Infect. Dev. Ctries 2020, 14, 1054–1064. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Pistelli, L.; Mancianti, F. Antimicrobial activity of five essential oils against bacteria and fungi responsible for urinary tract infections. Molecules 2018, 23, 1668. [Google Scholar] [CrossRef]
- Okusu, H.; Ma, D.; Nikaido, H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 1996, 178, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Fadli, M.; Chevalier, J.; Saad, A.; Mezrioui, N.E.; Hassani, L.; Pagès, J.M. Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2011, 38, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Ay, E.; Bayraktar, S.; Colak, S.; Kaya, D.A. Effects of Nigella sativa L.’s essential oils on multi drug resistant Escherichia coli isolates. Acta Microbiol. Bulg. 2018, 34, 32–36. [Google Scholar]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.A.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yañéz-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.A.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on the phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Smith, A.H.; Zoetendal, E.; Mackie, R.I. Bacterial mechanisms to overcome inhibitory effects of dietary tannins. Microb. Ecol. 2005, 50, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Rafael, L.; Teresinha, N.; Moritz, J.C.; Maria, I.G.; Dalmarco Eduardo, M.; Fröde Tânia, S. Evaluation of antimicrobial and antiplatelet aggregation effects of Solidago chilensis Meyen. Int. J. Green Pharm. 2009, 3, 35–39. [Google Scholar]
- Kemperman, R.A.; Bolca, S.; Roger, L.C.; Vaughan, E.E. Novel approaches for analyzing gut microbes and dietary polyphenols: Challenges and opportunities. Microbiology 2010, 156, 3224–3231. [Google Scholar] [CrossRef]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef] [PubMed]
- Aboulmagd, E.; Al-Mohammed, H.I.; Al-Badry, S. Synergism and postantibiotic effect of green tea extract and imipenem against methicillin-resistant Staphylococcus aureus. Microbiol. J. 2011, 1, 89–96. [Google Scholar] [CrossRef]
- Braga, L.C.; Leite, A.A.M.; Xavier, K.G.S.; Takahashi, J.A.; Bemquerer, M.P.; Chartone-Souze, E.; Nascimento, A.M.A. Synergic interaction between pomegranate extract and antibiotics against Staphylococcus aureus. Can. J. Microbiol. 2005, 51, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Hussin, W.A.; El-Sayed, W.M. Synergic interactions between selected botanical extracts and tetracycline against Gram positive and Gram negative bacteria. J. Biol. Sci. 2011, 11, 433–441. [Google Scholar] [CrossRef]
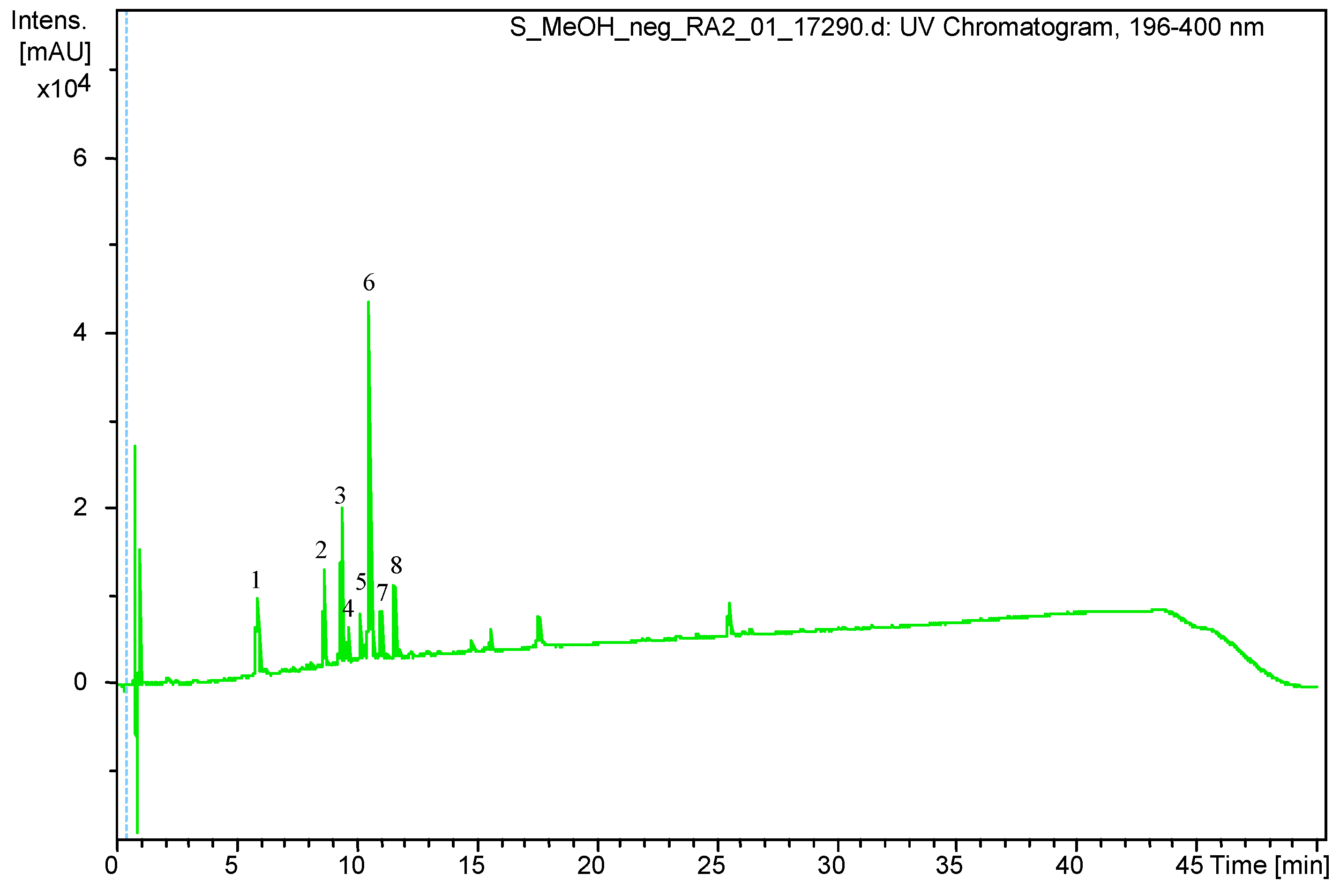




| Peak Number. | Retention Time (Rt) | (M – H) (m/z) | Identification |
|---|---|---|---|
| 1 | 5.9 | 353.0878 | Chlorogenic acid |
| 2 | 8.6 | 613.1767 | Leiocarposide |
| 3 | 9.4 | 609.1460 | Rutin |
| 4 | 9.7 | 463.0881 | Hyperoside/Isoquercitrin |
| 5 | 10.2 | 593.1502 | Kaempferol 3-robinobioside/ Kaempferol 3-rutinoside |
| 6 | 10.6 | 515.1192 | Dicaffeoylquinic acid isomer |
| 7 | 11.1 | 515.1185 | Dicaffeoylquinic acid isomer |
| 8 | 11.6 | 349.0934 | Unknown |
| Sample | T (h) | C (h) | PAE = T−C (h) |
|---|---|---|---|
| 0.25× MIC SV | 2 h | 1 h 15 min | 45 min |
| 1× MIC CIP | 3 h 15 min | 1 h 15 min | 2 h |
| 1× MIC CIP + 0.25× MIC SV | 2 h | 1 h 15 min | 45 min |
| 1× MIC AN | 4 h 45 min | 1 h 15 min | 3 h 30 min |
| 1× MIC AN + 0.25× MIC SV | 2 h 15 min | 1 h 15 min | 1 h |
| Sample | T (h) | C (h) | PASME = T−C (h) |
|---|---|---|---|
| 0.25× MIC SV | 2 h | 1 h 15 min | 45 min |
| 0.5× MIC CIP | 3 h | 1 h 15 min | 1 h 45 min |
| 0.5× MIC CIP + 0.25× MIC SV | 1 h 45 min | 1 h 15 min | 30 min |
| 0.5× MIC AN | 3 h 30 min | 1 h 15 min | 2 h 15 min |
| 0.5× MIC AN + 0.25× MIC SV | 1 h 45 min | 1 h 15 min | 30 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojnicz, D.; Tichaczek-Goska, D.; Gleńsk, M.; Hendrich, A.B. Is it Worth Combining Solidago virgaurea Extract and Antibiotics against Uropathogenic Escherichia coli rods? An In Vitro Model Study. Pharmaceutics 2021, 13, 573. https://doi.org/10.3390/pharmaceutics13040573
Wojnicz D, Tichaczek-Goska D, Gleńsk M, Hendrich AB. Is it Worth Combining Solidago virgaurea Extract and Antibiotics against Uropathogenic Escherichia coli rods? An In Vitro Model Study. Pharmaceutics. 2021; 13(4):573. https://doi.org/10.3390/pharmaceutics13040573
Chicago/Turabian StyleWojnicz, Dorota, Dorota Tichaczek-Goska, Michał Gleńsk, and Andrzej B. Hendrich. 2021. "Is it Worth Combining Solidago virgaurea Extract and Antibiotics against Uropathogenic Escherichia coli rods? An In Vitro Model Study" Pharmaceutics 13, no. 4: 573. https://doi.org/10.3390/pharmaceutics13040573
APA StyleWojnicz, D., Tichaczek-Goska, D., Gleńsk, M., & Hendrich, A. B. (2021). Is it Worth Combining Solidago virgaurea Extract and Antibiotics against Uropathogenic Escherichia coli rods? An In Vitro Model Study. Pharmaceutics, 13(4), 573. https://doi.org/10.3390/pharmaceutics13040573