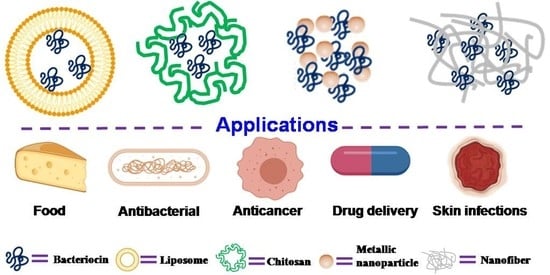
Potential Novel Food-Related and Biomedical Applications of Nanomaterials Combined with Bacteriocins
Abstract
1. Introduction
2. Different Bacteriocins and Their Spectrum
3. Stability of Bacteriocins
4. Advantages and Limitations of Bacteriocins
5. Bacteriocin–Nanomaterial Combination
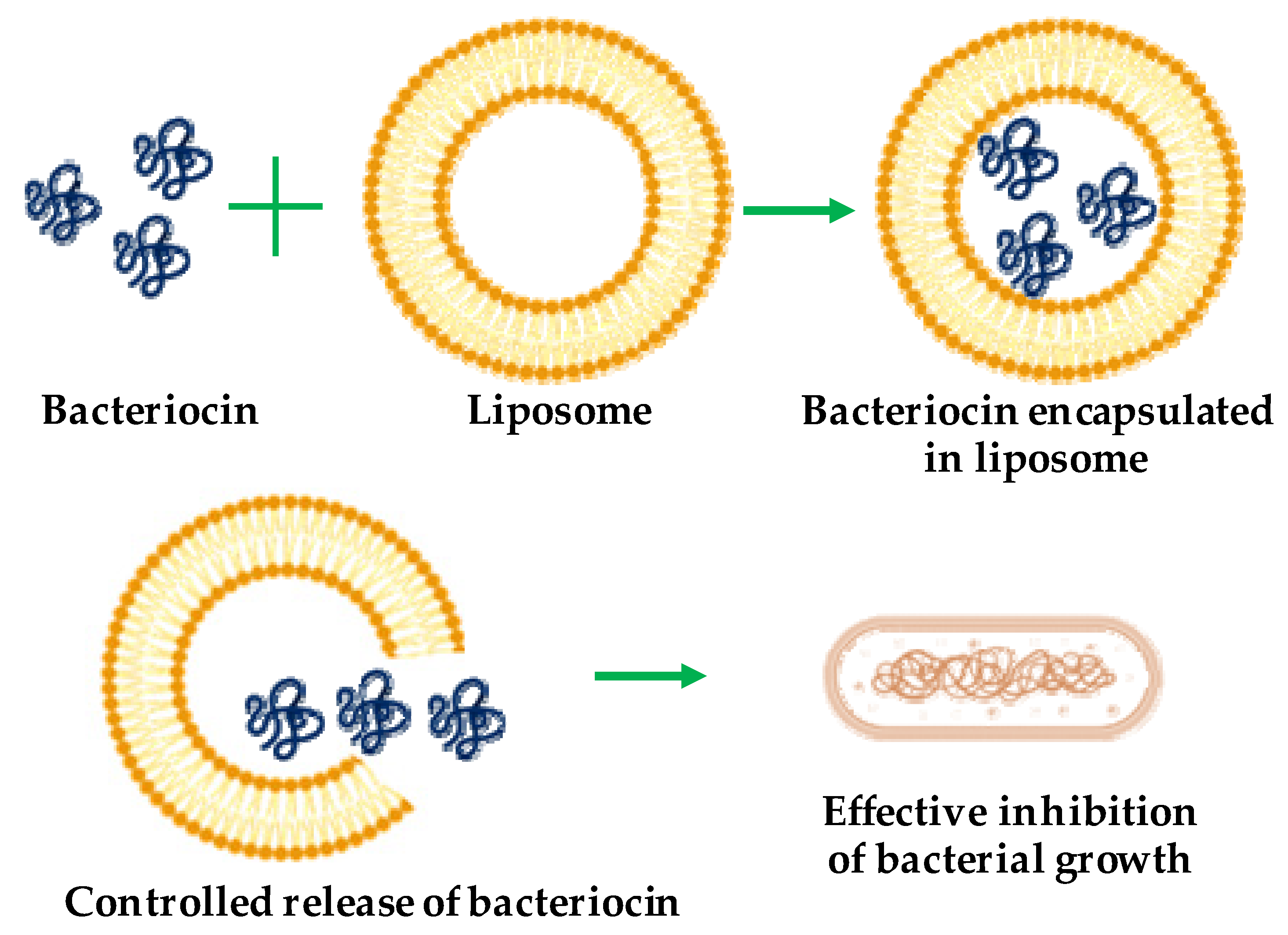
5.1. Liposomes
5.2. Chitosan
5.3. Metallic Nanoparticles
5.4. Nanofibers
5.5. Other Nanoplatforms
6. Applications
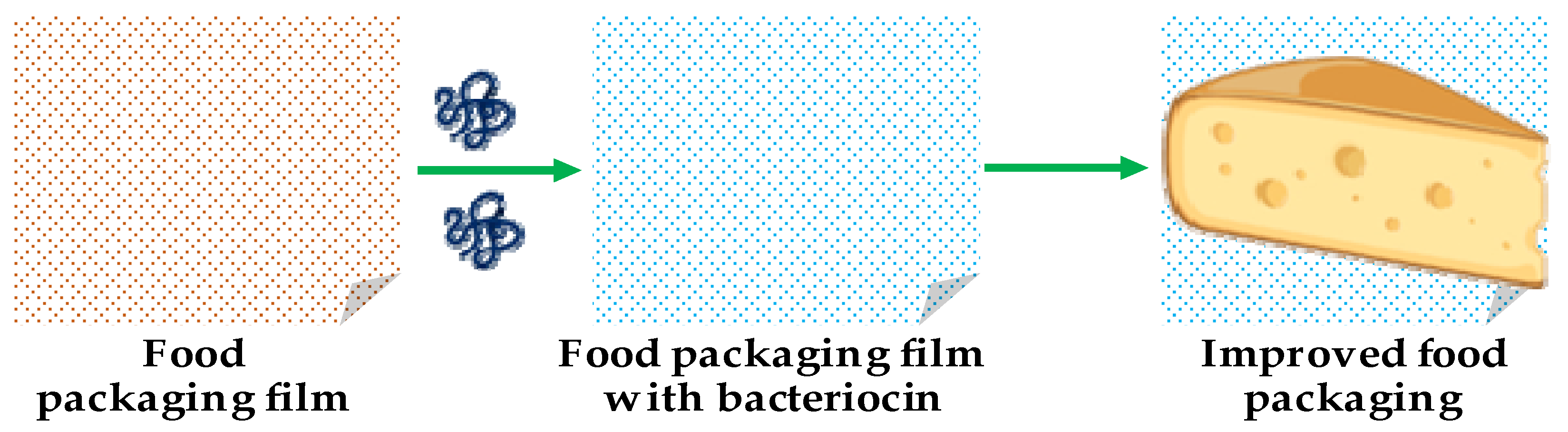
6.1. Food Applications
6.2. Antibacterial Activity
6.3. Anticancer Activity
6.4. Other Biomedical Applications
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Kjos, M.; Nes, I.F.; Diep, D.B.; Lotfipour, F. Natural antimicrobial peptides from bacteria: Characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012, 113, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R. Bacteriocins of lactic acid bacteria. Biochimie 1988, 70, 337–349. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A. Bacteriocin from LAB for medical and health applications. In Beneficial Microorganisms in Medical and Health Applications. Microbiology Monographs; Liong, M.T., Ed.; Springer: Cham, Switzerland, 2015; Volume 28, pp. 199–221. [Google Scholar]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; O’Connor, R.; Cotter, P.D.; Ross, R.P.; Hill, C. In Vitro activities of nisin and nisin derivatives alone and in combination with antibiotics against Staphylococcus biofilms. Front. Microbiol. 2016, 7, 508. [Google Scholar] [CrossRef] [PubMed]
- Vukomanović, M.; Žunič, V.; Kunej, Š.; Jančar, B.; Jeverica, S.; Podlipec, R.; Suvorov, D. Nano-engineering the antimicrobial spectrum of lantibiotics: Activity of nisin against gram negative bacteria. Sci. Rep. 2017, 7, 4324. [Google Scholar] [CrossRef]
- Baindara, P.; Korpole, S.; Grover, V. Bacteriocins: Perspective for the development of novel anticancer drugs. Appl. Microbiol. Biotechnol. 2018, 102, 10393–10408. [Google Scholar] [CrossRef]
- Fahim, H.A.; Khairalla, A.S.; El-Gendy, A.O. Nanotechnology: A valuable strategy to improve bacteriocin formulations. Front. Microbiol. 2016, 7, 1385. [Google Scholar] [CrossRef]
- Radaic, A.; de Jesus, M.B.; Kapila, Y.L. Bacterial anti-microbial peptides and nano-sized drug delivery systems: The state of the art toward improved bacteriocins. J. Control. Release 2020, 321, 100–118. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.-s. Nanomaterials as delivery vehicles and components of new strategies to combat bacterial infections: Advantages and limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Lee, S.; Lee, Y.; Kim, S.; Kim, K.-s. A new nano-platform of erythromycin combined with Ag nano-particle ZnO nano-structure against methicillin-resistant Staphylococcus aureus. Pharmaceutics 2020, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- Micenková, L.; Bosák, J.; Kucera, J.; Hrala, M.; Dolejšová, T.; Šedo, O.; Linke, D.; Fišer, R.; Šmajs, D. Colicin Z, a structurally and functionally novel colicin type that selectively kills enteroinvasive Escherichia coli and Shigella strains. Sci. Rep. 2019, 9, 11127. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, L.J.; Deringer, J.R.; Brayton, K.A.; Sawant, A.A.; Besser, T.E.; Call, D.R. Characterization of a novel microcin that kills enterohemorrhagic Escherichia coli O157:H7 and O26. Appl. Environ. Microbiol. 2012, 78, 6592–6599. [Google Scholar] [CrossRef]
- Sidhu, P.K.; Nehra, K. Bacteriocin-nanoconjugates as emerging compounds for enhancing antimicrobial activity of bacteriocins. J. King Saud Univ. Sci. 2019, 31, 758–767. [Google Scholar] [CrossRef]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front Microbiol. 2014, 5, 241. [Google Scholar]
- Li, Q.; Montalban-Lopez, M.; Kuipers, O.P. Increasing the antimicrobial activity of nisin-based Lantibiotics against Gram-Negative pathogens. Appl. Environ. Microbial. 2018, 84, e00052-18. [Google Scholar] [CrossRef]
- Weishaupt, R.; Heuberger, L.; Siqueira, G.; Gutt, B.; Zimmermann, T.; Maniura-Weber, K.; Salentinig, S.; Faccio, G. Enhanced antimicrobial activity and structural transitions of a nanofibrillated cellulose-nisin biocomposite suspension. ACS Appl. Mater. Interfaces 2018, 10, 20170–20181. [Google Scholar] [CrossRef]
- Holcapkova, P.; Raskova, Z.K.; Hrabalikova, M.; Salakova, A.; Drbohlav, J.; Sedlarik, V. Isolation and thermal stabilization of bacteriocin nisin derived from whey for antimicrobial modifications of polymers. Int. J. Polym. Sci. 2017, 2017, 3072582. [Google Scholar] [CrossRef]
- Wang, G. Post-translational modifications of natural antimicrobial peptides and strategies for peptide engineering. Curr. Biotechnol. 2012, 1, 72–79. [Google Scholar] [CrossRef]
- Lagedroste, M.; Reiners, J.; Smits, S.H.J.; Schmitt, L. Impact of the nisin modification machinery on the transport kinetics of NisT. Sci. Rep. 2020, 10, 12295. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhu, L.; Wang, Z.; Zhan, X. Enhanced stability of the bactericidal activity of nisin through conjugation with gellan gum. Int. J. Biol. Macromol. 2020, 148, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Bagde, P.; Vigneshwaran, N. Improving the stability of bacteriocin extracted from Enterococcus faecium by immobilization onto cellulose nanocrystals. Carbohydr. Polym. 2019, 209, 172–180. [Google Scholar] [CrossRef]
- Fahim, H.A.; El Rouby, W.M.A.; El-Gendy, A.O.; Khairalla, A.S.; Naguib, I.A.; Farghali, A.A. Enhancement of the productivity of the potent bacteriocin avicin A and improvement of its stability using nanotechnology approaches. Sci. Rep. 2017, 7, 10604. [Google Scholar] [CrossRef]
- Da Silva Malheiros, P.; Daroit, D.J.; Brandelli, A. Food applications of liposome-encapsulated antimicrobial peptides. Trends Food Sci. Technol. 2010, 21, 284–292. [Google Scholar] [CrossRef]
- Da Silva Malheiros, P.; Sant’Anna, V.; Utpott, M.; Brandelli, A. Antilisterial activity and stability of nanovesicle-encapsulated antimicrobial peptide P34 in milk. Food Control 2012, 23, 42–47. [Google Scholar] [CrossRef]
- Lee, E.H.; Khan, I.; Oh, D.H. Evaluation of the efficacy of nisin-loaded chitosan nanoparticles against foodborne pathogens in orange juice. J. Food Sci. Technol. 2018, 55, 1127–1133. [Google Scholar] [CrossRef]
- Salmaso, S.; Elvassore, N.; Bertucco, A.; Lante, A.; Caliceti, P. Nisin-loaded poly-L-lactide nano-particles produced by CO2 anti-solvent precipitation for sustained antimicrobial activity. Int. J. Pharm. 2004, 287, 163–173. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocin-antimicrobial synergy: A medical and food perspective. Front. Microbiol. 2017, 8, 1205. [Google Scholar] [CrossRef]
- Sharma, T.K.; Sapra, M.; Chopra, A.; Sharma, R.; Patil, S.D.; Malik, R.K.; Pathania, R.; Navani, N.K. Interaction of bacteriocin-capped slver nanoparticles with food pathogens and their antibacterial effect. Int. J. Green Nanotechnol. 2012, 4, 93–110. [Google Scholar] [CrossRef]
- Joo, N.E.; Ritchie, K.; Kamarajan, P.; Miao, D.; Kapila, Y.L. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC1. Cancer Med. 2012, 1, 295–305. [Google Scholar] [CrossRef]
- Ray Mohapatra, A.; Jeevaratnam, K. Inhibiting bacterial colonization on catheters: Antibacterial and antibiofilm activities of bacteriocins from Lactobacillus plantarum SJ33. J. Glob. Antimicrob. Resist. 2019, 19, 85–92. [Google Scholar] [CrossRef]
- Hegarty, J.W.; Guinane, C.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Bacteriocin production: A relatively unharnessed probiotic trait? F1000Research 2016, 5, 2587. [Google Scholar] [CrossRef]
- Gillor, O.; Etzion, A.; Riley, M.A. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 2008, 81, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2019, 77, 99–113. [Google Scholar] [CrossRef]
- Flynn, J.; Durack, E.; Collins, M.N.; Hudson, S.P. Tuning the strength and swelling of an injectable polysaccharide hydrogel and the subsequent release of a broad spectrum bacteriocin, nisin A. J. Mater. Chem. B. 2020, 8, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Heunis, T.; Bshena, O.; Klumperman, B.; Dicks, L. Release of bacteriocins from nanofibers prepared with combinations of poly(d,l-lactide) (PDLLA) and poly(ethylene oxide) (PEO). Int. J. Mol. Sci. 2011, 12, 2158–2173. [Google Scholar] [CrossRef]
- Woraprayote, W.; Pumpuang, L.; Tosukhowong, A.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Antimicrobial biodegradable food packaging impregnated with Bacteriocin 7293 for control of pathogenic bacteria in pangasius fish fillets. LWT 2018, 89, 427–433. [Google Scholar] [CrossRef]
- Bastos Mdo, C.; Coelho, M.L.; Santos, O.C. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiology 2015, 161, 683–700. [Google Scholar] [CrossRef]
- Dicks, L.M.T.; van Staden, A.D.P.; Klumperman, B. Bacteriocins and nanotechnology. In Functionalized Nanomaterials for the Management of Microbial Infection; Boukherroub, R., Szunerits, S., Drider, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 267–288. [Google Scholar]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Lujan, H.; Griffin, W.C.; Taube, J.H.; Sayes, C.M. Synthesis and characterization of nanometer-sized liposomes for encapsulation and microRNA transfer to breast cancer cells. Int. J. Nanomed. 2019, 14, 5159–5173. [Google Scholar] [CrossRef] [PubMed]
- Tsumori, H.; Shimizu, Y.; Nagatoshi, K.; Sakurai, Y.; Yamakami, K. Prospects for liposome-encapsulated nisin in the prevention of dental caries. In Interface Oral Health Science 2014; Sasaki, K., Suzuki, O., Takahashi, N., Eds.; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Benech, R.O.; Kheadr, E.E.; Lacroix, C.; Fliss, I. Antibacterial activities of nisin Z encapsulated in liposomes or produced in situ by mixed culture during cheddar cheese ripening. Appl. Environ. Microbiol. 2002, 68, 5607–5619. [Google Scholar] [CrossRef] [PubMed]
- Were, L.M.; Bruce, B.; Davidson, P.M.; Weiss, J. Encapsulation of nisin and lysozyme in liposomes enhances efficacy against Listeria monocytogenes. J. Food Prot. 2004, 67, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.M.; Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Characterization of antimicrobial-bearing liposomes by ζ-potential, vesicle size, and encapsulation efficiency. Food Biophys. 2007, 2, 1–9. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innov. Food Sci. Emerg. Technol. 2016, 36, 287–293. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Jafari, S.M.; Williams, L. Nanoencapsulated nisin: An engineered natural antimicrobial system for the food industry. Trends Food Sci. Technol. 2019, 94, 20–31. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of chitosan and its derivatives for biomedical applications in the central nervous system. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Krishnappa, S.; Debnath, S.; Jayaprakash, C. Biocompatible chitosan nanoparticles incorporated bacteriocin (CSNps-B) preparation for the controlled release and improved anti-bacterial activity against food borne pathogenic bacteria Listeria monocytogenes. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 625–631. [Google Scholar]
- Alishahi, A. Antibacterial effect of chitosan nanoparticle loaded with nisin for the prolonged effect. J. Food Saf. 2014, 34, 111–118. [Google Scholar] [CrossRef]
- Divsalar, E.; Tajik, H.; Moradi, M.; Forough, M.; Lotfi, M.; Kuswandi, B. Characterization of cellulosic paper coated with chitosan-zinc oxide nanocomposite containing nisin and its application in packaging of UF cheese. Int. J. Biol. Macromol. 2018, 109, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Klouj, A.; Revol-Junelles, A.M.; Desobry, S. Controlled release of nisin from HPMC, sodium caseinate, poly-lactic acid and chitosan for active packaging applications. J. Food Eng. 2014, 143, 178–185. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.S. Recent advances in nanomaterial-based wound-healing therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Kim, K.-s. Antibacterial potential of Ni-doped zinc oxide nanostructure: Comparatively more effective against Gram-negative bacteria including multidrug resistant strains. RSC Adv. 2020, 10, 1232–1242. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, S.; Kim, K.-s. A nontoxic biocompatible nanocomposite comprising black phosphorus with Au–ɣ-Fe2O3 nanoparticles. RSC Adv. 2020, 10, 16162–16167. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Kim, K.-s. Easy one-pot low-temperature synthesized Ag-ZnO nanoparticles and their activity against clinical isolates of methicillin-resistant Staphylococcus aureus. Front. Bioeng. Biotechnol. 2020, 8, 216. [Google Scholar] [CrossRef]
- Pandit, R.; Rai, M.; Santos, C.A. Enhanced antimicrobial activity of the food-protecting nisin peptide by bioconjugation with silver nanoparticles. Environ. Chem. Lett. 2017, 15, 443–452. [Google Scholar] [CrossRef]
- Preet, S.; Pandey, S.K.; Kaur, K.; Chauhan, S.; Saini, A. Gold nanoparticles assisted co-delivery of nisin and doxorubicin against murine skin cancer. J. Drug Deliv. Sci. Technol. 2019, 53, 101147. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Torres, N.I.; Noll, K.S.; Xu, S.; Li, J.; Huang, Q.; Sinko, P.J.; Wachsman, M.B.; Chikindas, M.L. Safety, formulation, and in vitro antiviral activity of the antimicrobial peptide subtilosin against herpes simplex virus type 1. Probiotics Antimicrob. Proteins 2013, 5, 26–35. [Google Scholar] [CrossRef]
- Ahire, J.J.; Dicks, L.M. Nisin incorporated with 2,3-Dihydroxybenzoic acid in nanofibers inhibits biofilm formation by a methicillin-resistant strain of Staphylococcus aureus. Probiotics Antimicrob. Proteins. 2015, 7, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T.; Shabbir, S.; Noor, T.; Imran, M. Antimicrobial and antibiofilm potential of bacteriocin loaded nano-vesicles functionalized with rhamnolipids against foodborne pathogens. LWT. 2019, 116, 108583. [Google Scholar] [CrossRef]
- Breukink, E.; Ganz, P.; de Kruijff, B.; Seelig, J. Binding of Nisin Z to bilayer vesicles as determined with isothermal titration calorimetry. Biochemistry 2000, 39, 10247–10254. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Kumar, V.; Singh, B.; Tiwari, S.K. Phospholipid/Polydiacetylene vesicle-based colorimetric assay for high-throughput screening of bacteriocins and halocins. Appl. Biochem. Biotechnol. 2017, 182, 142–154. [Google Scholar] [CrossRef]
- Prombutara, P.; Kulwatthanasal, Y.; Supaka, N.; Sramala, I.; Chareonpornwattana, S. Production of nisin-loaded solid lipid nanoparticles for sustained antimicrobial activity. Food Control. 2012, 24, 184–190. [Google Scholar] [CrossRef]
- Bi, L.; Yang, L.; Narsimhan, G.; Bhunia, A.K.; Yao, Y. Designing carbohydrate nanoparticles for prolonged efficacy of antimicrobial peptide. J. Control Release 2011, 150, 150–156. [Google Scholar] [CrossRef]
- Niaz, T.; Shabbir, S.; Noor, T.; Rahman, A.; Bokhari, H.; Imran, M. Potential of polymer stabilized nano-liposomes to enhance antimicrobial activity of nisin Z against foodborne pathogens. LWT 2018, 96, 98–110. [Google Scholar] [CrossRef]
- Zou, Y.; Lee, H.Y.; Seo, Y.C.; Ahn, J. Enhanced antimicrobial activity of nisin-loaded liposomal nanoparticles against foodborne pathogens. J. Food Sci. 2012, 77, M165–M170. [Google Scholar] [CrossRef]
- Boelter, J.F.; Brandelli, A. Innovative bionanocomposite films of edible proteins containing liposome-encapsulated nisin and halloysite nanoclay. Colloids Surf. B. Biointerfaces 2016, 145, 740–747. [Google Scholar] [CrossRef]
- Imran, M.; Revol-Junelles, A.-M.; Paris, C.; Guedon, E.; Linder, M.; Desobry, S. Liposomal nanodelivery systems using soy and marine lecithin to encapsulate food biopreservative nisin. LWT Food Sci. Technol. 2015, 62, 341–349. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Oh, D.-H. Evaluation of nisin-loaded chitosan-monomethyl fumaric acid nanoparticles as a direct food additive. Carbohydr. Polym. 2018, 184, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hui, G.; Liu, W.; Feng, H.; Li, J.; Gao, Y. Effects of chitosan combined with nisin treatment on storage quality of large yellow croaker (Pseudosciaena crocea). Food Chem. 2016, 203, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.K.; Elsabagh, R.; Trinetta, V. Evaluation of novel synergistic antimicrobial activity of nisin, lysozyme, EDTA nanoparticles, and/or ZnO nanoparticles to control foodborne pathogens on minced beef. Food Control. 2018, 92, 249–254. [Google Scholar] [CrossRef]
- Song, Z.; Yuan, Y.; Niu, C.; Dai, L.; Wei, J.; Yue, T. Iron oxide nanoparticles functionalized with nisin for rapid inhibition and separation of Alicyclobacillus spp. RSC Adv. 2017, 7, 6712–6719. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Ramachandran, S.; Shiamala, G.A. Combined effect of bacteriocin with gold nanoparticles against food spoiling bacteria—An approach for food packaging material preparation. Int. Food Res. J. 2013, 20, 1909–1912. [Google Scholar]
- Saini, S.; Sillard, C.; Belgacem, M.N.; Bras, J. Nisin anchored cellulose nanofibers for long term antimicrobial active food packaging. RSC Adv. 2016, 6, 12422–12430. [Google Scholar] [CrossRef]
- Soto, K.M.; Hernández-Iturriaga, M.; Loarca-Piña, G.; Luna-Bárcenas, G.; Mendoza, S. Antimicrobial effect of nisin electrospun amaranth: Pullulan nanofibers in apple juice and fresh cheese. Int. J. Food Microbiol. 2019, 295, 25–32. [Google Scholar] [CrossRef]
- Cui, H.; Changzhu, L.; Juan, W.; Lin, L. Improving anti-listeria activity of cheese packaging via nanofiber containing nisin-loaded nanoparticles. LWT-Food Sci. Technol. 2017, 81, 233–242. [Google Scholar] [CrossRef]
- Chopra, M.; Kaur, P.; Bernela, M.; Thakur, R. Surfactant assisted nisin loaded chitosan-carageenan nanocapsule synthesis for controlling food pathogens. Food Control 2014, 37, 158–164. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Zhao, X.; Li, L.; Chen, M.; Cheng, J.; Liu, J.; Li, X. Antibacterial activity and cytotoxicity of novel silkworm-like nisin@PEGylated MoS2. Colloids Surf. B. Biointerfaces 2019, 183, 110491. [Google Scholar] [CrossRef] [PubMed]
- Malheiros, P.d.S.; Sant’Anna, V.; Barbosa, M.d.S.; Brandelli, A.; Franco, B.D. Effect of liposome-encapsulated nisin and bacteriocin-like substance P34 on Listeria monocytogenes growth in Minas frescal cheese. Int. J. Food. Microbiol. 2012, 156, 272–277. [Google Scholar] [CrossRef] [PubMed]
- García-Toledo, J.A.; Torrestiana-Sánchez, B.; Martínez-Sánchez, C.E.; Tejero-Andrade, J.M.; García-Bórquez, A.; Mendoza-García, P.G. Nanoencapsulation of a bacteriocin from Pediococcus acidilactici ITV26 by microfluidization. Food. Bioprocess Technol. 2019, 12, 88–97. [Google Scholar] [CrossRef]
- Zohri, M.; Alavidjeh, M.S.; Haririan, I.; Ardestani, M.S.; Ebrahimi, S.E.; Sani, H.T.; Sadjadi, S.K. A comparative study between the antibacterial effect of nisin and nisin-loaded chitosan/alginate nanoparticles on the growth of Staphylococcus aureus in raw and pasteurized milk samples. Probiotics Antimicrob. Proteins. 2010, 2, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Sherman, S.; Filocamo, S.; Steckl, A.J. Long-term antimicrobial effect of nisin released from electrospun triaxial fiber membranes. Acta Biomater. 2017, 53, 242–249. [Google Scholar] [CrossRef]
- Goudarzi, F.; Asadi, A.; Afsharpour, M.; Jamadi, R.H. In Vitro characterization and evaluation of the cytotoxicity effects of nisin and nisin-loaded PLA-PEG-PLA nanoparticles on Gastrointestinal (AGS and KYSE-30), Hepatic (HepG2) and Blood (K562) Cancer Cell Lines. AAPS PharmSciTech 2018, 19, 1554–1566. [Google Scholar] [CrossRef]
- Heunis, T.D.; Smith, C.; Dicks, L.M. Evaluation of a nisin-eluting nanofiber scaffold to treat Staphylococcus aureus-induced skin infections in mice. Antimicrob. Agents Chemother. 2013, 57, 3928–3935. [Google Scholar] [CrossRef]
- Mossallam, S.F.; Amer, E.I.; Diab, R.G. Potentiated anti-microsporidial activity of Lactobacillus acidophilus CH1 bacteriocin using gold nanoparticles. Exp. Parasitol. 2014, 144, 14–21. [Google Scholar] [CrossRef]
- Gruskiene, R.; Krivorotova, T.; Staneviciene, R.; Ratautas, D.; Serviene, E.; Sereikaite, J. Preparation and characterization of iron oxide magnetic nanoparticles functionalized by nisin. Colloids Surf. B. Biointerfaces 2018, 169, 126–134. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Balay, D.; Hu, Y.; McMullen, L.M.; Gänzle, M.G. Effect of chitosan, and bacteriocin—Producing Carnobacterium maltaromaticum on survival of Escherichia coli and Salmonella Typhimurium on beef. Int. J. Food Microbiol. 2019, 290, 68–75. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]

| Nano-Combination with Bacteriocin | Effect | Reference |
|---|---|---|
| Nisin Z with nanoliposome | Synergistically inhibited the growth of food-borne pathogens | [71] |
| Nisin into phosphatidylcholine nanoliposomes | Inhibited the microbial growth in milk | [49] |
| Nisin-loaded liposomal nanoparticles | Inhibition of two main food-borne pathogens, L. monocytogenes and S. aureus | [72] |
| Liposome-encapsulated nisin | Active food packaging | [73] |
| Liposome-encapsulated nisin | Synergistically act as excellent bio-preservative | [74] |
| Nisin-loaded chitosan-monomethyl fumaric acid nanoparticles | Excellent as a nanocarrier for food-grade antimicrobial agents and potential alternative food preservative in beverages | [75] |
| Chitosan with nisin | Better preservation of large yellow croaker | [76] |
| Nisin, lysozyme, EDTA nanoparticles, and/or ZnO | Inhibited the growth of food-borne pathogens in minced beef | [77] |
| Iron oxide nanoparticles functionalized with nisin | Inhibited the growth of food borne pathogens | [78] |
| Bacteriocin with gold nanoparticles | Synergistic growth inhibition against food spoiling microorganisms | [79] |
| Nisin-anchored cellulose nanofibers | Active food packaging | [80] |
| Nisin into amaranth-protein-isolate:pullulan (API:PUL) nanofibers | Sustained release and enhanced antimicrobial activity against food-borne pathogens in fresh cheese and apple juice | [81] |
| Nisin into nanofibers | Prevent the growth of L. monocytogenes in packaged cheese | [82] |
| Nano-Combination with Bacteriocin | Effect | Reference |
|---|---|---|
| Nisin@PEGylated MoS2 | Antibacterial activity against both gram-positive and -negative bacteria with low toxicity | [84] |
| Liposome-encapsulated nisin | Excellent stability and antimicrobial activity L. monocytogenes | [85] |
| Liposome-encapsulated nisin | Prevention of dental caries | [45] |
| Pediocin-encapsulated liposomes | Antilisterial activity | [86] |
| Chitosan nanoparticles loaded with nisin | Two-fold higher antimicrobial activity than that of nisin alone | [87] |
| Chitosan nanoconjugates loaded with bacteriocins | Synergistic antimicrobial activity against L. monocytogenes | [53] |
| Bacteriocin-Ag nanoparticles | Synergistic antimicrobial activity | [32,61] |
| Bacteriocin-Au nanoparticles | Synergistic antimicrobial activity | [79] |
| Plantaricin 423 and bacteriocin ST4SA into nanofibers | The combination retained its 88% original antimicrobial activity at 37 °C | [60] |
| Nisin electrospun into triaxial fiber membranes | Sustained release of nisin for antimicrobial activity | [88] |
| Nisin-loaded PLA-PEG-PLA nanoparticles | Showed cytotoxic effects on different cancer cell lines | [89] |
| Au nanoparticles with nisin | Delivery of the drug with anticancer activity against murine skin cancer | [62] |
| Nisin-eluting nanofiber scaffold | Excellent wound healing activity against S. aureus-induced skin infections in mice | [90] |
| Bacteriocin with gold nanoparticles | Showed excellent antimicrosporidial activity | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naskar, A.; Kim, K.-s. Potential Novel Food-Related and Biomedical Applications of Nanomaterials Combined with Bacteriocins. Pharmaceutics 2021, 13, 86. https://doi.org/10.3390/pharmaceutics13010086
Naskar A, Kim K-s. Potential Novel Food-Related and Biomedical Applications of Nanomaterials Combined with Bacteriocins. Pharmaceutics. 2021; 13(1):86. https://doi.org/10.3390/pharmaceutics13010086
Chicago/Turabian StyleNaskar, Atanu, and Kwang-sun Kim. 2021. "Potential Novel Food-Related and Biomedical Applications of Nanomaterials Combined with Bacteriocins" Pharmaceutics 13, no. 1: 86. https://doi.org/10.3390/pharmaceutics13010086
APA StyleNaskar, A., & Kim, K.-s. (2021). Potential Novel Food-Related and Biomedical Applications of Nanomaterials Combined with Bacteriocins. Pharmaceutics, 13(1), 86. https://doi.org/10.3390/pharmaceutics13010086