In-Line and Off-Line Monitoring of Skin Penetration Profiles Using Confocal Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gel Formulations
2.3. Preparation of Porcine Ear Skin
2.4. Incubation of Dermatomed Porcine Ear Skin
2.5. Confocal Raman Spectroscopy Analysis of Skin Penetrations
2.6. Spectral Data Analysis
2.7. Characterization of Penetration Profiles
2.8. Statistical Data Analysis
3. Results and Discussion
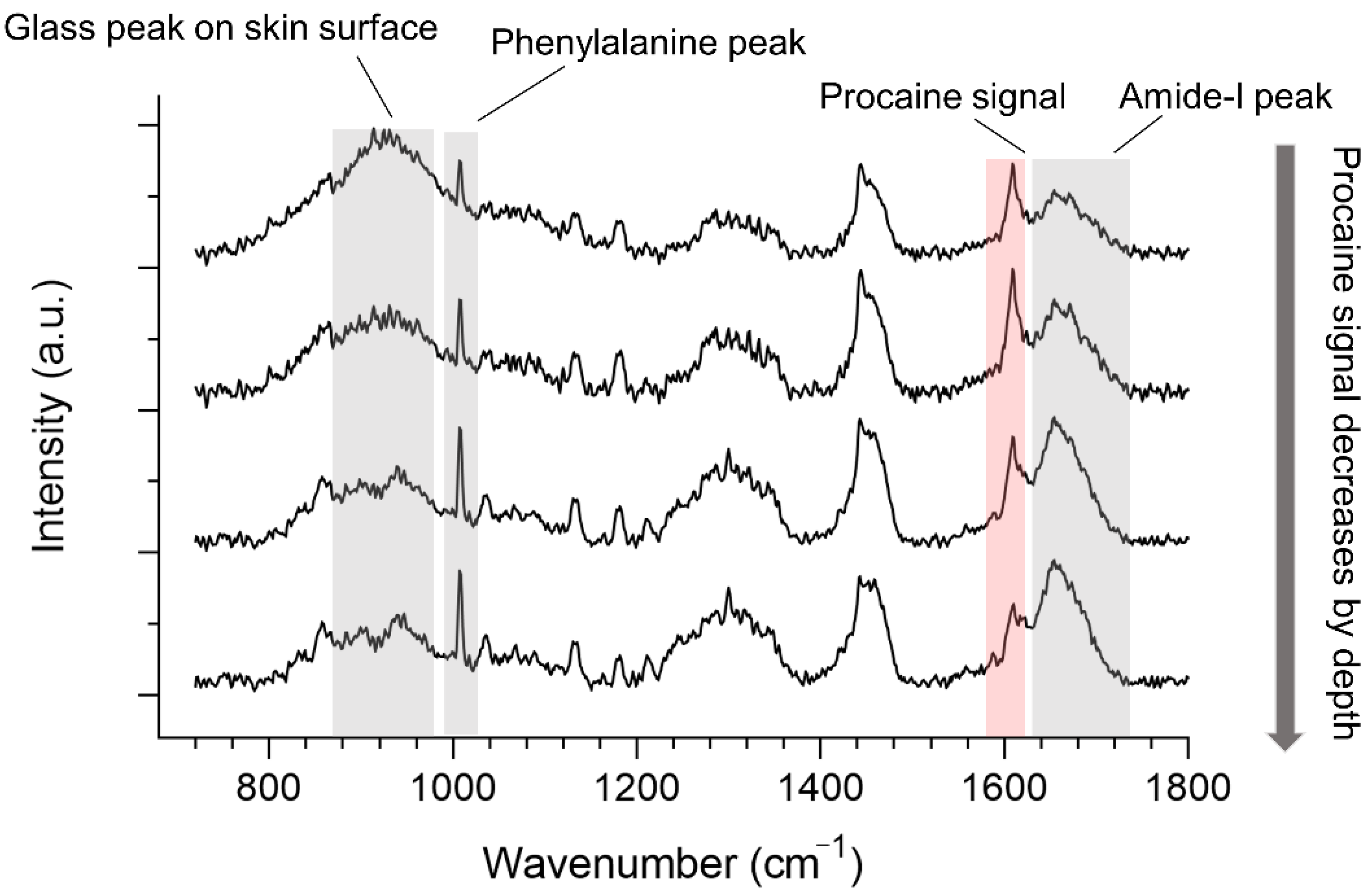
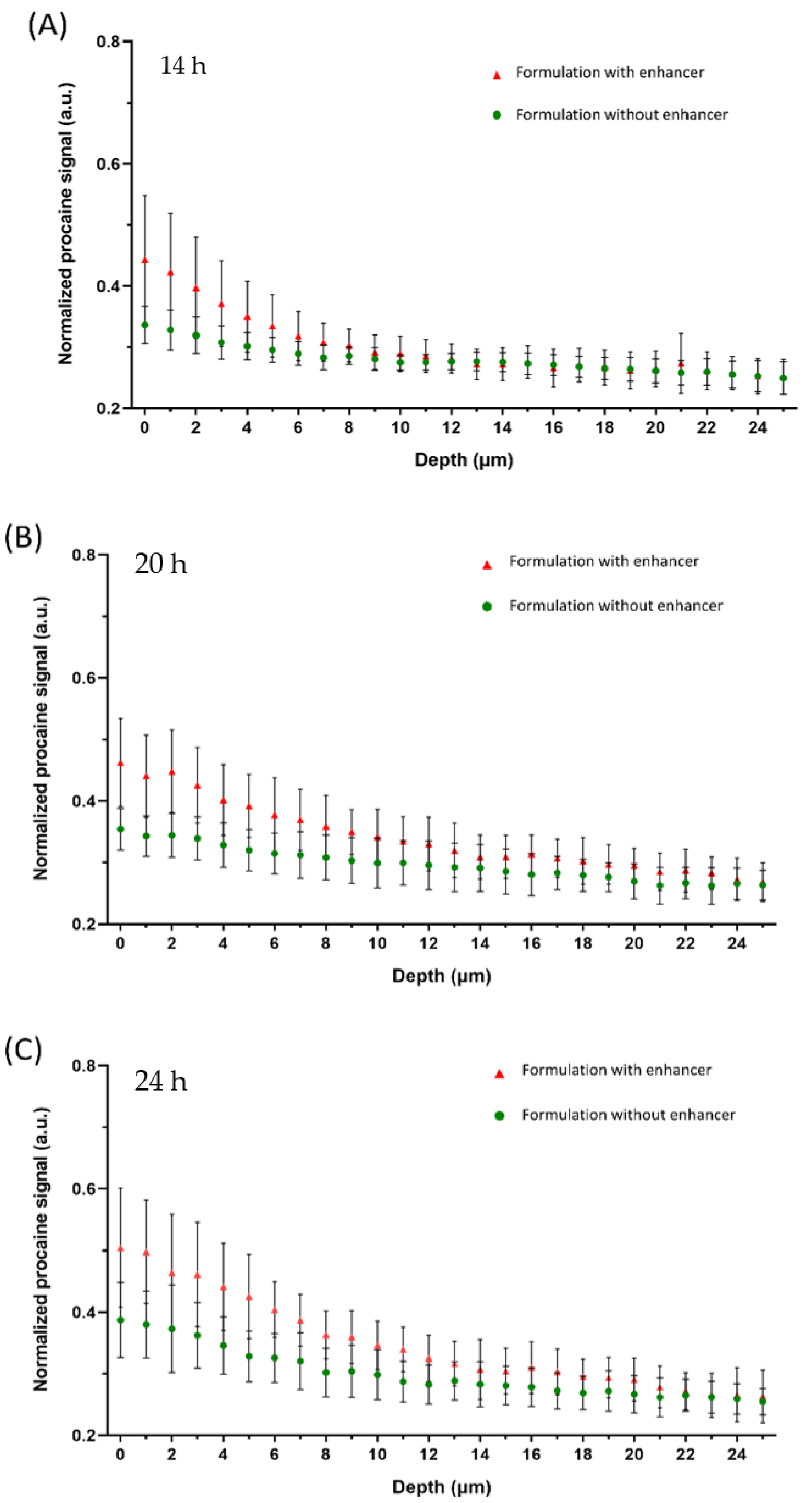
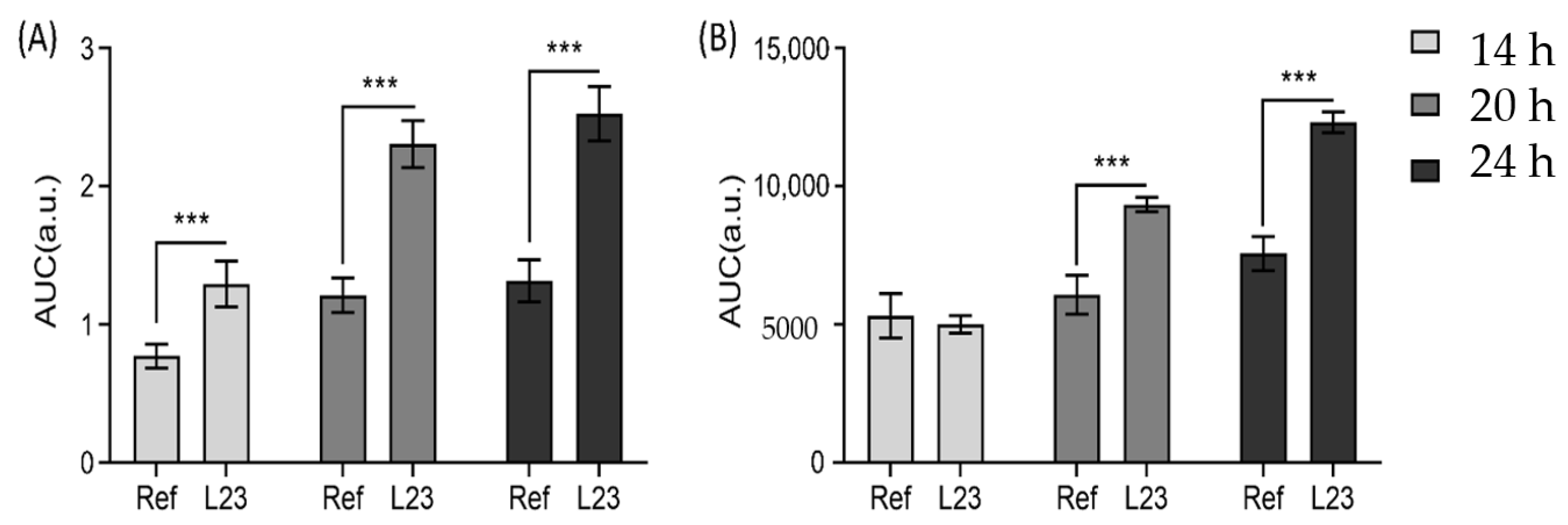
3.1. Off-Line Monitoring of Drug Penetration
3.2. In-Line Monitoring Drug Penetration
3.3. In-Line and Off-Line Comparisons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choe, C.; Choe, S.; Schleusener, J.; Lademann, J.; Darvin, M.E. Modified normalization method in in vivo stratum corneum analysis using confocal Raman microscopy to compensate nonhomogeneous distribution of keratin. J. Raman Spectrosc. 2019, 50, 5596. [Google Scholar] [CrossRef]
- Björklund, S.; Engblom, J.; Thuresson, K.; Sparr, E. A water gradient can be used to regulate drug transport across skin. J. Control. Release 2010, 143, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Ramadan, W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloids Surfaces B Biointerfaces 2010, 75, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Lunter, D.; Daniels, R. Measuring skin penetration by confocal Raman microscopy (CRM): Correlation to results from conventional experiments. In Proceedings of the Medical Imaging 2016: Biomedical Applications in Molecular, Structural, and Functional Imaging, San Diego, CA, USA, 27 February–3 March 2016; Volume 9788, p. 978829. [Google Scholar]
- Lunter, D.J. How Confocal Is Confocal Raman Microspectroscopy on the Skin? Impact of Microscope Configuration and Sample Preparation on Penetration Depth Profiles. Ski. Pharmacol. Physiol. 2016, 29, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Lunter, D. Determination of skin penetration profiles by confocal Raman microspectroscopy: Statistical evaluation of optimal microscope configuration. J. Raman Spectrosc. 2016, 48, 152–160. [Google Scholar] [CrossRef]
- Lunter, D.; Daniels, R. Confocal Raman microscopic investigation of the effectiveness of penetration enhancers for procaine delivery to the skin. J. Biomed. Opt. 2014, 19, 126015. [Google Scholar] [CrossRef]
- Miloudi, L.; Bonnier, F.; Tfayli, A.; Yvergnaux, F.; Byrne, H.J.; Chourpa, I.; Munnier, E. Confocal Raman spectroscopic imaging for in vitro monitoring of active ingredient penetration and distribution in reconstructed human epidermis model. J. Biophotonics 2017, 11, e201700221. [Google Scholar] [CrossRef]
- Ascencio, S.M.; Choe, C.; Meinke, M.C.; Müller, R.H.; Maksimov, G.V.; Wigger-Alberti, W.; Lademann, J.; Darvin, M.E. Confocal Raman microscopy and multivariate statistical analysis for determination of different penetration abilities of caffeine and propylene glycol applied simultaneously in a mixture on porcine skin ex vivo. Eur. J. Pharm. Biopharm. 2016, 104, 51–58. [Google Scholar] [CrossRef]
- Dos Santos, L.; Tippavajhala, V.K.; Mendes, T.D.O.; Da Silva, M.G.P.; Fávero, P.P.; Soto, C.A.T.; Martin, A.A. Evaluation of penetration process into young and elderly skin using confocal Raman spectroscopy. Vib. Spectrosc. 2019, 100, 123–130. [Google Scholar] [CrossRef]
- Dos Santos, L.; Sousa, M.P.J.; Azoia, N.G.; Cavaco-Paulo, A.M.; Martin, A.A.; Favero, P.P. In vivo confocal Raman spectroscopy and molecular dynamics analysis of penetration of retinyl acetate into stratum corneum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 174, 279–285. [Google Scholar] [CrossRef]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Auböck, J.; Wolzt, M.; Valenta, C. In vitro vs. in vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Ski. Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Tfayli, A.; Guillard, E.; Manfait, M.; Baillet-Guffroy, A. Raman spectroscopy: Feasibility of in vivo survey of stratum corneum lipids, effect of natural aging. Eur. J. Dermatol. EJD 2012, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Caspers, P.J.; Bruining, H.A.; Puppels, G.J.; Lucassen, G.W.; Carter, E.A. In Vivo Confocal Raman Microspectroscopy of the Skin: Noninvasive Determination of Molecular Concentration Profiles. J. Investig. Dermatol. 2001, 116, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Albèr, C.; Brandner, B.; Björklund, S.; Billsten, P.; Corkery, R.; Engblom, J. Effects of water gradients and use of urea on skin ultrastructure evaluated by confocal Raman microspectroscopy. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2470–2478. [Google Scholar] [CrossRef]
- Ilić, T.; Pantelić, I.; Lunter, D.; Đorđević, S.; Marković, B.; Ranković, D.; Daniels, R.; Savić, S. Critical quality attributes, in vitro release and correlated in vitro skin permeation—in vivo tape stripping collective data for demonstrating therapeutic (non)equivalence of topical semisolids: A case study of “ready-to-use” vehicles. Int. J. Pharm. 2017, 528, 253–267. [Google Scholar] [CrossRef]
- Escobar-Chavez, J.J.; Merino-Sanjuán, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The Tape-Stripping Technique as a Method for Drug Quantification in Skin. J. Pharm. Pharm. Sci. 2008, 11, 104–130. [Google Scholar] [CrossRef]
- Vyumvuhore, R.; Michael-Jubeli, R.; Verzeaux, L.; Boudier, D.; Le Guillou, M.; Bordes, S.; Libong, D.; Tfayli, A.; Manfait, M.; Closs, B. Lipid organization in xerosis: The key of the problem? Int. J. Cosmet. Sci. 2018, 40, 549–554. [Google Scholar] [CrossRef]
- Nagelreiter, C.; Raffeiner, S.; Geyerhofer, C.; Klang, V.; Valenta, C. Influence of drug content, type of semi-solid vehicle and rheological properties on the skin penetration of the model drug fludrocortisone acetate. Int. J. Pharm. 2013, 448, 305–312. [Google Scholar] [CrossRef]
- Zhang, Z.; Lunter, D. Confocal Raman microspectroscopy as an alternative to differential scanning calorimetry to detect the impact of emulsifiers and formulations on stratum corneum lipid conformation. Eur. J. Pharm. Sci. 2018, 121, 1–8. [Google Scholar] [CrossRef]
- Zhang, Z.; Lunter, D. Confocal Raman microspectroscopy as an alternative method to investigate the extraction of lipids from stratum corneum by emulsifiers and formulations. Eur. J. Pharm. Biopharm. 2018, 127, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Halper, M.; Pribyl, R.; Baurecht, D.; Valenta, C. Distribution of phospholipid based formulations in the skin investigated by combined ATR-FTIR and tape stripping experiments. Int. J. Pharm. 2017, 519, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, M.; Holper, E.; Baurecht, D.; Valenta, C. Monitoring the Distribution of Surfactants in the Stratum Corneum by Combined ATR-FTIR and Tape-Stripping Experiments. Ski. Pharmacol. Physiol. 2015, 28, 167–175. [Google Scholar] [CrossRef]
- Hoppel, M.; Baurecht, D.; Holper, E.; Mahrhauser, D.; Valenta, C. Validation of the combined ATR-FTIR/tape stripping technique for monitoring the distribution of surfactants in the stratum corneum. Int. J. Pharm. 2014, 472, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Geneidi, A.S.; Guy, R.H. Uptake of Microemulsion Components into the Stratum Corneum and Their Molecular Effects on Skin Barrier Function. Mol. Pharm. 2010, 7, 1266–1273. [Google Scholar] [CrossRef]
- Belsey, N.A.; Garrett, N.L.; Contreras-Rojas, L.R.; Pickup-Gerlaugh, A.J.; Price, G.J.; Moger, J.; Guy, R.H. Evaluation of drug delivery to intact and porated skin by coherent Raman scattering and fluorescence microscopies. J. Control. Release 2014, 174, 37–42. [Google Scholar] [CrossRef]
- Krombholz, R.; Lunter, D. A New Method for In-Situ Skin Penetration Analysis by Confocal Raman Microscopy. Molecules 2020, 25, 4222. [Google Scholar] [CrossRef]
- Shin, S.-C.; Cho, C.-W.; Yang, K.-H. Development of lidocaine gels for enhanced local anesthetic action. Int. J. Pharm. 2004, 287, 73–78. [Google Scholar] [CrossRef]
- Liu, Y.; Lunter, D. Systematic Investigation of the Effect of Non-Ionic Emulsifiers on Skin by Confocal Raman Spectroscopy—A Comprehensive Lipid Analysis. Pharmaceutics 2020, 12, 223. [Google Scholar] [CrossRef]
- Liu, Y.; Lunter, D.J. Tracking heavy-water-incorporated confocal Raman spectroscopy for evaluating the effects of PEGylated emulsifiers on skin barrier. J. Biophotonics 2020, 13, e202000286. [Google Scholar] [CrossRef]
- Binder, L.; Valenta, C.; Lunter, D. Determination of skin penetration profiles by confocal Raman microspectroscopy: Evaluation of interindividual variability and interlab comparability. J. Raman Spectrosc. 2020, 51, 1037–1043. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, C.; Shen, Y.; Chen, Z.; Silveira, E.S.; Zhang, L.; Wei, M.; Liu, C.; De Sena-Tomas, C.; Targoff, K.; et al. Optical imaging of metabolic dynamics in animals. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Franzen, L.; Vidlářová, L.; Kostka, K.-H.; Schaefer, U.F.; Windbergs, M. Freeze-drying as a preserving preparation technique forin vitrotesting of human skin. Exp. Dermatol. 2013, 22, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.F.; Craig, D.Q.M.; Hadgraft, J.; Lane, M.E. The application of ATR-FTIR spectroscopy and multivariate data analysis to study drug crystallisation in the stratum corneum. Eur. J. Pharm. Biopharm. 2017, 111, 16–25. [Google Scholar] [CrossRef]



| Formulation | Hydroxypropyl Methylcellulose | Poloxamer 407 | L23 | Procaine-HCl | Water |
|---|---|---|---|---|---|
| A | 0.2 | 2.0 | 0 | 1.0 | 6.8 |
| B | 0.2 | 2.0 | 0.5 | 1.0 | 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krombholz, R.; Liu, Y.; Lunter, D.J. In-Line and Off-Line Monitoring of Skin Penetration Profiles Using Confocal Raman Spectroscopy. Pharmaceutics 2021, 13, 67. https://doi.org/10.3390/pharmaceutics13010067
Krombholz R, Liu Y, Lunter DJ. In-Line and Off-Line Monitoring of Skin Penetration Profiles Using Confocal Raman Spectroscopy. Pharmaceutics. 2021; 13(1):67. https://doi.org/10.3390/pharmaceutics13010067
Chicago/Turabian StyleKrombholz, Richard, Yali Liu, and Dominique Jasmin Lunter. 2021. "In-Line and Off-Line Monitoring of Skin Penetration Profiles Using Confocal Raman Spectroscopy" Pharmaceutics 13, no. 1: 67. https://doi.org/10.3390/pharmaceutics13010067
APA StyleKrombholz, R., Liu, Y., & Lunter, D. J. (2021). In-Line and Off-Line Monitoring of Skin Penetration Profiles Using Confocal Raman Spectroscopy. Pharmaceutics, 13(1), 67. https://doi.org/10.3390/pharmaceutics13010067






