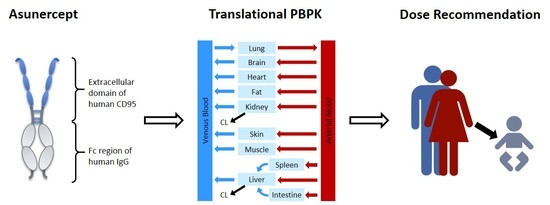
Translational PBPK Modeling of the Protein Therapeutic and CD95L Inhibitor Asunercept to Develop Dose Recommendations for Its First Use in Pediatric Glioblastoma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Study Data
2.2. Software
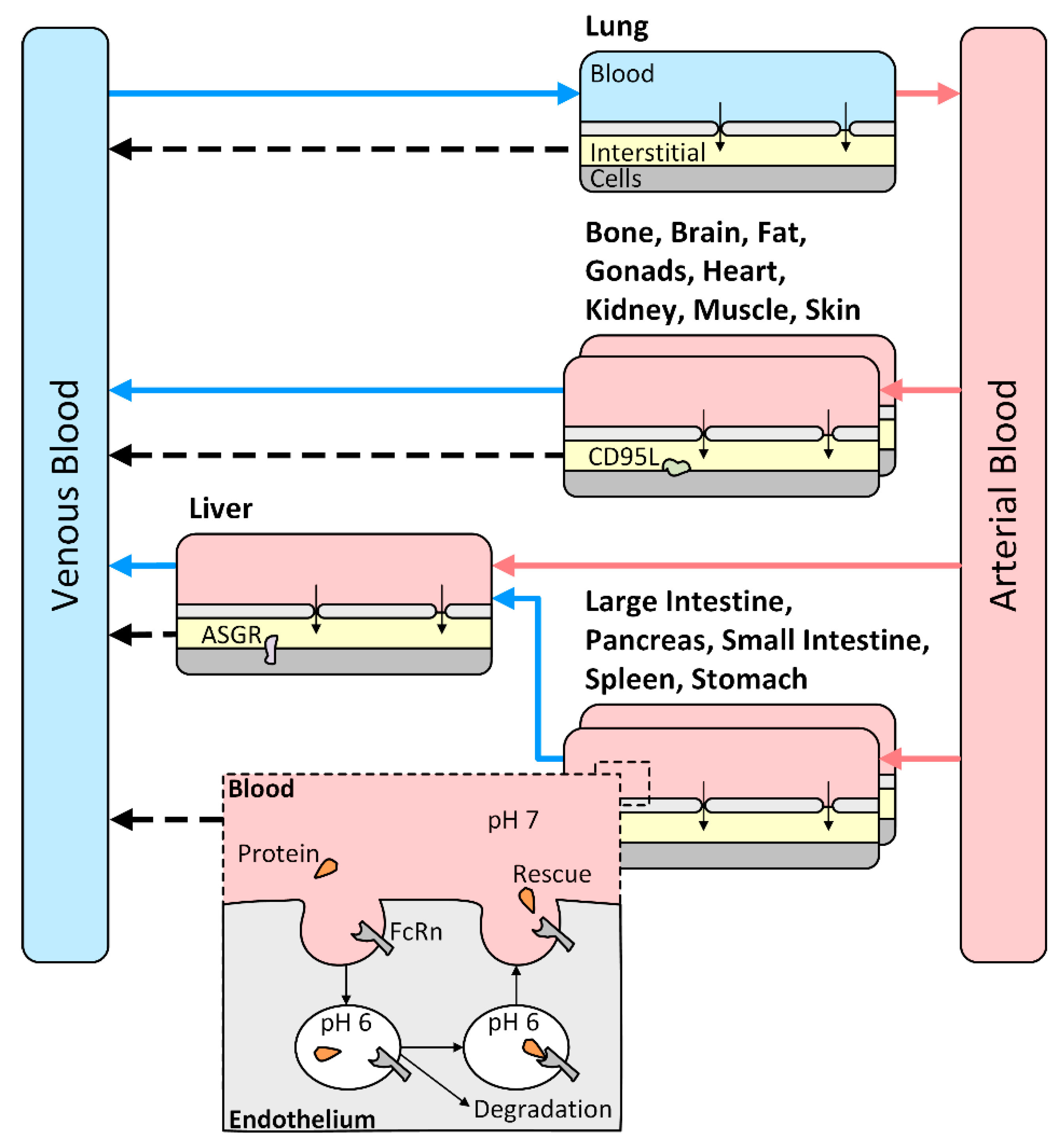
2.3. PBPK Model Building
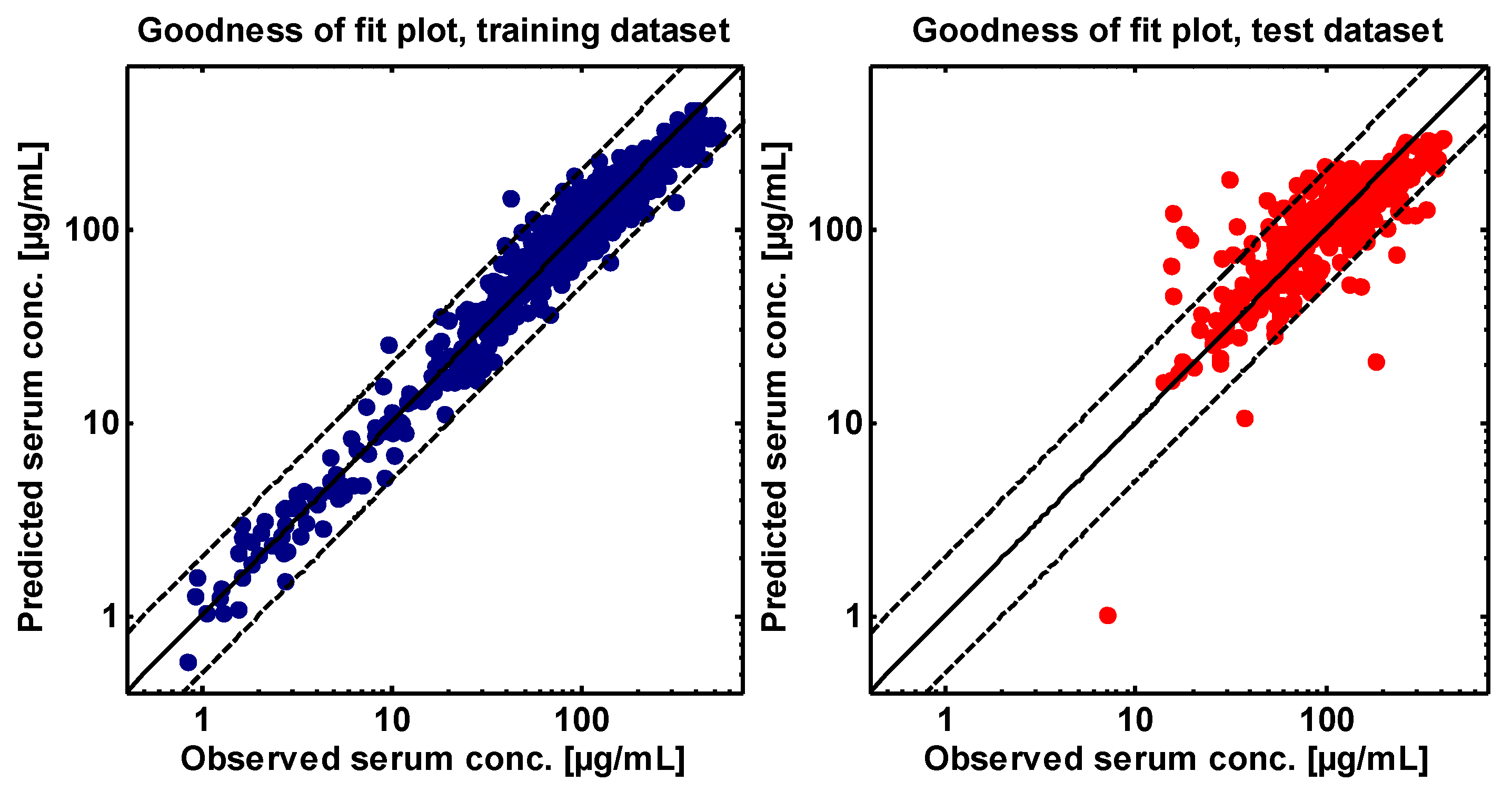
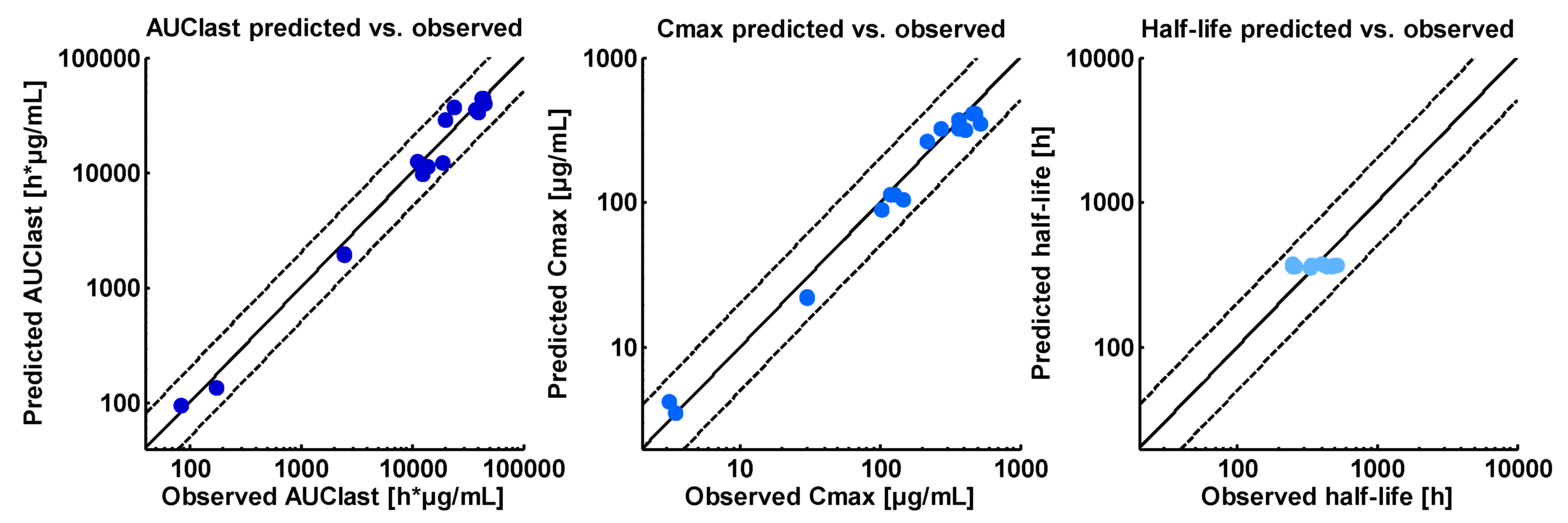
2.4. PBPK Model Evaluation
2.5. Extrapolation to Children
2.6. Prediction of Pediatric Exposure and Development of Dose Recommendations
3. Results
3.1. Asunercept Adult PBPK Model
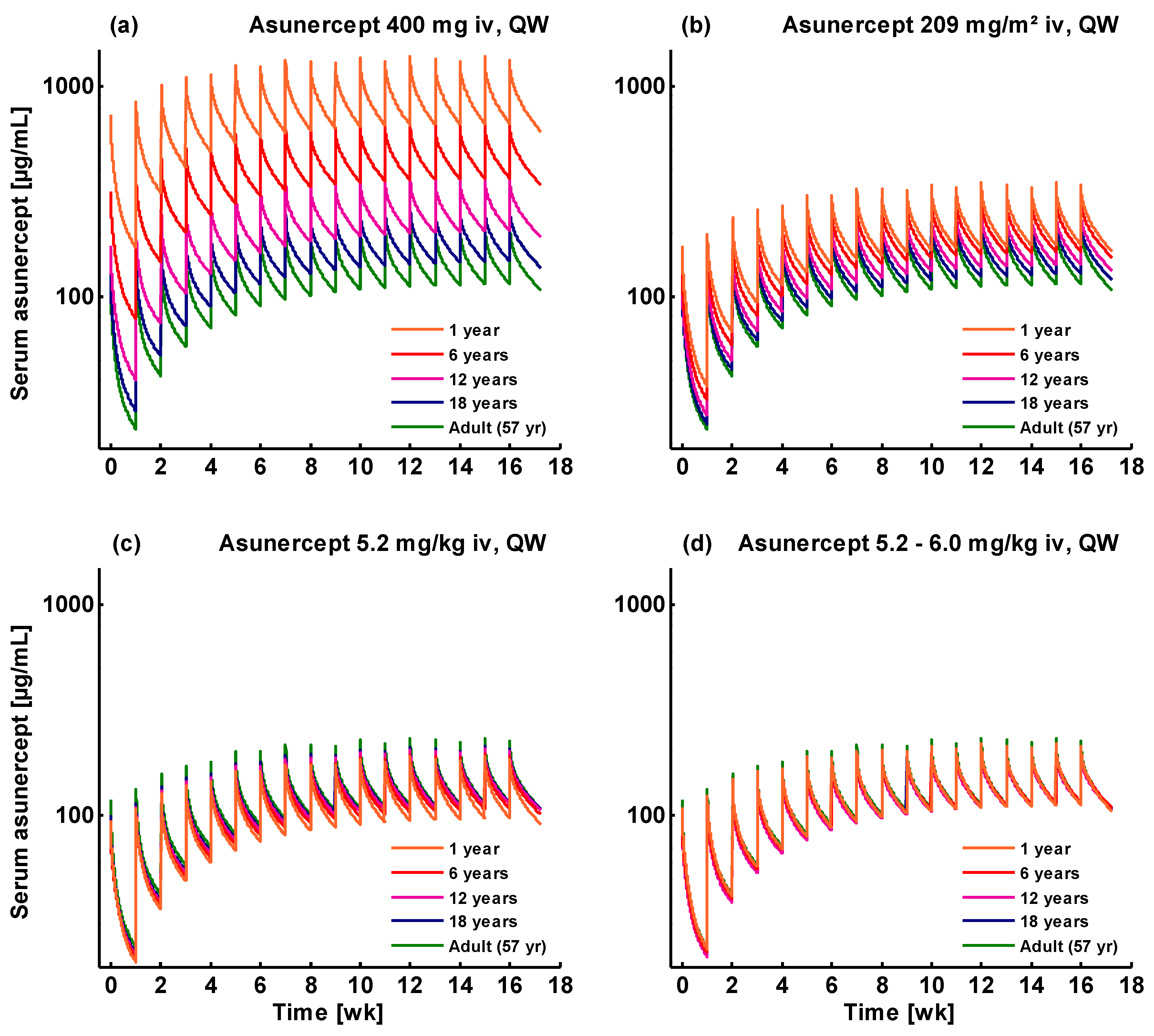
3.2. Pediatric Extrapolation
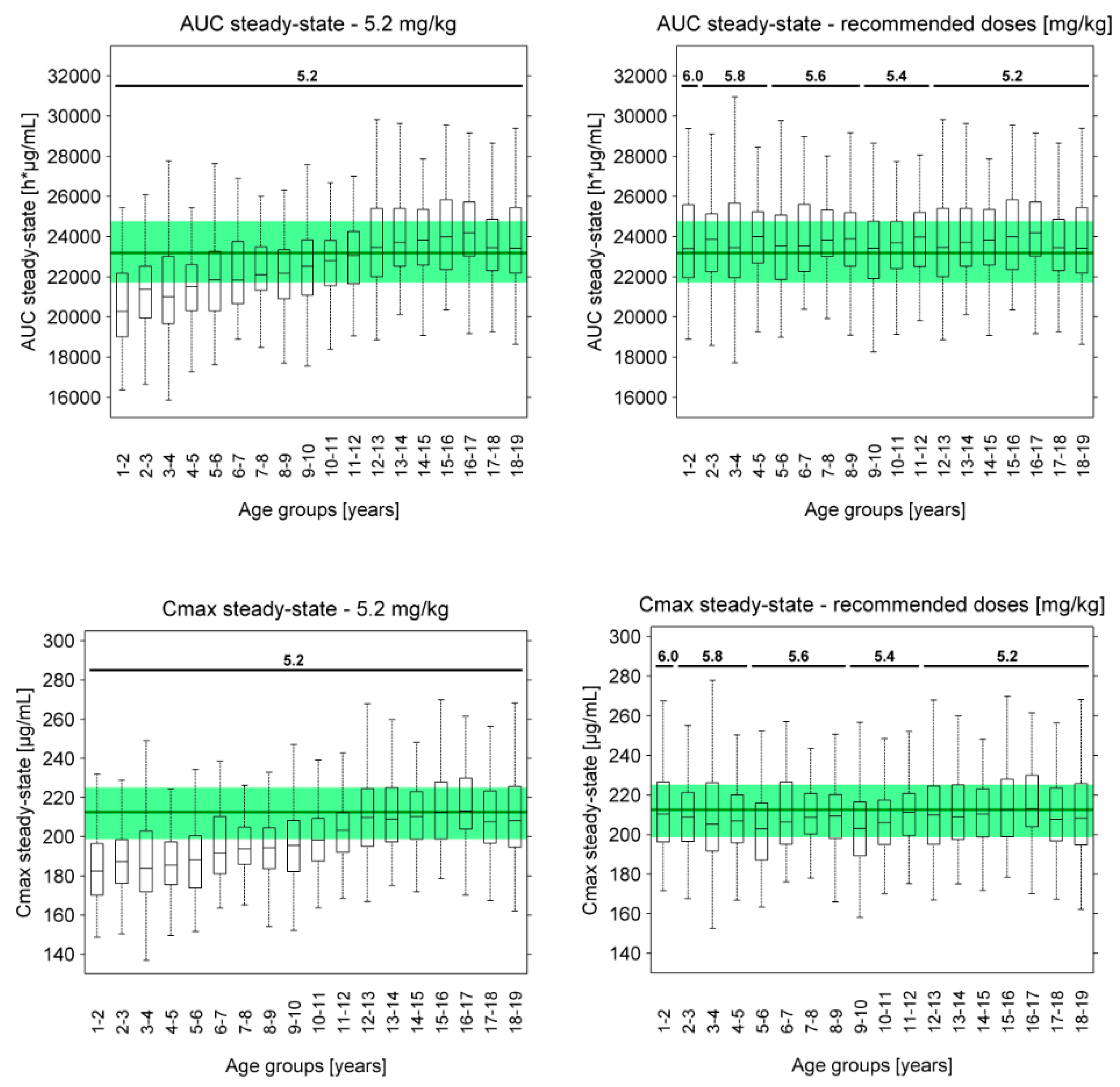
3.3. Pediatric Dose Recommendations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro. Oncol. 2014, 16 (Suppl. 4), iv1–iv63. [Google Scholar] [CrossRef]
- Wick, W.; Fricke, H.; Junge, K.; Kobyakov, G.; Martens, T.; Heese, O.; Wiestler, B.; Schliesser, M.G.; von Deimling, A.; Pichler, J.; et al. A phase II, randomized, study of weekly APG101 + reirradiation versus reirradiation in progressive glioblastoma. Clin. Cancer Res. 2014, 20, 6304–6313. [Google Scholar] [CrossRef] [PubMed]
- Kleber, S.; Sancho-Martinez, I.; Wiestler, B.; Beisel, A.; Gieffers, C.; Hill, O.; Thiemann, M.; Mueller, W.; Sykora, J.; Kuhn, A.; et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 2008, 13, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, P.; Ellert-Miklaszewska, A.; Kwiatkowska, A.; Kaminska, B. Non-apoptotic Fas signaling regulates invasiveness of glioma cells and modulates MMP-2 activity via NFkappaB-TIMP-2 pathway. Cell. Signal. 2010, 22, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.R.; Edginton, A.N. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacomet. Syst. Pharm. 2014, 3, e148. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration—Center for Drug Evaluation and Research. General Clinical Pharmacology Considerations for Pediatric Studies for Drugs and Biological Products. Guidance for Industry. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm425885.pdf (accessed on 23 January 2019).
- European Medicines Agency. Guideline on the Reporting of Physiologically Based Pharmacokinetic (PBPK) Modelling and Simulation. Available online: https://www.ema.europa.eu/documents/scientific-guideline/guideline-reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation_en.pdf (accessed on 23 January 2019).
- Templeton, I.E.; Jones, N.S.; Musib, L. Pediatric dose selection and utility of PBPK in determining dose. AAPS J. 2018. [Google Scholar] [CrossRef]
- Tuettenberg, J.; Seiz, M.; Debatin, K.-M.; Hollburg, W.; von Staden, M.; Thiemann, M.; Hareng, B.; Fricke, H.; Kunz, C. Pharmacokinetics, pharmacodynamics, safety and tolerability of APG101, a CD95-Fc fusion protein, in healthy volunteers and two glioma patients. Int. Immunopharmacol. 2012, 13, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lehr, T.; Hanke, N.; Kunz, C.; Fricke, H. Linking population pharmacokinetics, tumor growth inhibition and survival of recurrent glioblastoma patients treated with asunercept, a Fas ligand inhibitor. Clin. Cancer Res. 2019. in submission. [Google Scholar]
- Open Systems Pharmacology Suite Manual, Version 7.4. Available online: https://github.com/Open-Systems-Pharmacology/OSPSuite.Documentation/blob/master/Open Systems Pharmacology Suite.pdf (accessed on 22 February 2019).
- Valentin, J. Basic anatomical and physiological data for use in radiological protection: Reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals: ICRP Publication 89. Ann. ICRP 2002, 32, 5–265. [Google Scholar]
- Edginton, A.N.; Schmitt, W.; Willmann, S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin. Pharm. 2006, 45, 1013–1034. [Google Scholar] [CrossRef]
- Willmann, S.; Becker, C.; Burghaus, R.; Coboeken, K.; Edginton, A.; Lippert, J.; Siegmund, H.-U.; Thelen, K.; Mück, W. Development of a paediatric population-based model of the pharmacokinetics of rivaroxaban. Clin. Pharm. 2014, 53, 89–102. [Google Scholar] [CrossRef]
- Niederalt, C.; Kuepfer, L.; Solodenko, J.; Eissing, T.; Siegmund, H.-U.; Block, M.; Willmann, S.; Lippert, J. A generic whole body physiologically based pharmacokinetic model for therapeutic proteins in PK-Sim. J. Pharmacokinet. Pharmacodyn. 2018. [Google Scholar] [CrossRef]
- Grewal, P.K.; Uchiyama, S.; Ditto, D.; Varki, N.; Le, D.T.; Nizet, V.; Marth, J.D. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat. Med. 2008, 14, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.L.; van den Berg, C.W.; Bowen, D.J. ASGR1 and ASGR2, the genes that encode the asialoglycoprotein receptor (Ashwell receptor), are expressed in peripheral blood monocytes and show interindividual differences in transcript profile. Mol. Biol. Int. 2012, 2012, 283974. [Google Scholar] [CrossRef]
- Xerri, L.; Devilard, E.; Hassoun, J.; Mawas, C.; Birg, F. Fas ligand is not only expressed in immune privileged human organs but is also coexpressed with Fas in various epithelial tissues. Mol. Pathol. 1997, 50, 87–91. [Google Scholar] [CrossRef]
- Sträter, J.; Walczak, H.; Hasel, C.; Melzner, I.; Leithäuser, F.; Möller, P. CD95 ligand (CD95L) immunohistochemistry: A critical study on 12 antibodies. Cell Death Differ. 2001, 8, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Sträter, J.; Mariani, S.M.; Walczak, H.; Rücker, F.G.; Leithäuser, F.; Krammer, P.H.; Möller, P. CD95 ligand (CD95L) in normal human lymphoid tissues: A subset of plasma cells are prominent producers of CD95L. Am. J. Pathol. 1999, 154, 193–201. [Google Scholar] [CrossRef]
- De Maria, R.; Testa, U.; Luchetti, L.; Zeuner, A.; Stassi, G.; Pelosi, E.; Riccioni, R.; Felli, N.; Samoggia, P.; Peschle, C. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood 1999, 93, 796–803. [Google Scholar] [PubMed]
- García-Moreno, C.; Catalán, M.P.; Ortiz, A.; Alvarez, L.; De la Piedra, C. Modulation of survival in osteoblasts from postmenopausal women. Bone 2004, 35, 170–177. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, K.H.; Choi, J.A.; Lee, J.H.; Kim, H.K.; Won, N.H.; Kim, I. Fas (APO-1/CD95) ligand and Fas expression in renal cell carcinomas: Correlation with the prognostic factors. Arch. Pathol. Lab. Med. 2000, 124, 687–693. [Google Scholar]
- Hamann, K.J.; Dorscheid, D.R.; Ko, F.D.; Conforti, A.E.; Sperling, A.I.; Rabe, K.F.; White, S.R. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am. J. Respir. Cell Mol. Biol. 1998, 19, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, N.; Hastings, E.; Keays, M.; Melnichuk, O.; Tang, Y.A.; Williams, E.; Dylag, M.; Kurbatova, N.; Brandizi, M.; Burdett, T.; et al. ArrayExpress update—Simplifying data submissions. Nucleic Acids Res. 2015, 43, D1113–D1116. [Google Scholar] [CrossRef] [PubMed]
- GeneCards: The Human Gene Database. Protein Expression of Asialoglycoprotein Receptor 1. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=ASGR1&keywords=asgr#expression (accessed on 23 January 2019).
- GeneCards: The Human Gene Database. Protein Expression of Asialoglycoprotein Receptor 2. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=ASGR2&keywords=asgr#expression (accessed on 23 January 2019).
- Collins, J.C.; Stockert, R.J.; Morell, A.G. Asialoglycoprotein receptor expression in murine pregnancy and development. Hepatology 1984, 4, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Petell, J.K.; Doyle, D. Developmental regulation of the hepatocyte receptor for galactose-terminated glycoproteins. Arch. Biochem. Biophys. 1985, 241, 550–560. [Google Scholar] [CrossRef]
- Petell, J.K.; Quaroni, A.; Hong, W.J.; Hixson, D.C.; Amarri, S.; Reif, S.; Bujanover, Y. Alteration in the regulation of plasma membrane glycoproteins of the hepatocyte during ontogeny. Exp. Cell Res. 1990, 187, 299–308. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- GeneCards: The Human Gene Database. Protein Expression of Neonatal Fc Receptor. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=FCGRT&keywords=neonatal,Fc-receptor#expression (accessed on 23 January 2019).
- Zhou, J.; Johnson, J.E.; Ghetie, V.; Ober, R.J.; Ward, E.S. Generation of mutated variants of the human form of the MHC class I-related receptor, FcRn, with increased affinity for mouse immunoglobulin G. J. Mol. Biol. 2003, 332, 901–913. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishii-Watabe, A.; Tada, M.; Kobayashi, T.; Kanayasu-Toyoda, T.; Kawanishi, T.; Yamaguchi, T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: A comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol. 2010, 184, 1968–1976. [Google Scholar] [CrossRef]
- Morell, A.; Terry, W.D.; Waldmann, T.A. Metabolic properties of IgG subclasses in man. J. Clin. Invest. 1970. [Google Scholar] [CrossRef]
- Rath, T.; Baker, K.; Dumont, J.A.; Peters, R.T.; Jiang, H.; Qiao, S.-W.; Lencer, W.I.; Pierce, G.F.; Blumberg, R.S. Fc-fusion proteins and FcRn: Structural insights for longer-lasting and more effective therapeutics. Crit. Rev. Biotechnol. 2015, 35, 235–254. [Google Scholar] [CrossRef]
- Edlund, H.; Melin, J.; Parra-Guillen, Z.P.; Kloft, C. Pharmacokinetics and pharmacokinetic-pharmacodynamic relationships of monoclonal antibodies in children. Clin. Pharm. 2015, 54, 35–80. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I. Pharmacokinetic considerations in designing pediatric studies of proteins, antibodies, and plasma-derived products. Am. J. 2016, 23, e1043–e1056. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Edginton, A. Pediatric physiology in relation to the pharmacokinetics of monoclonal antibodies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Hardiansyah, D.; Ng, C.M. Effects of the FcRn developmental pharmacology on the pharmacokinetics of therapeutic monoclonal IgG antibody in pediatric subjects using minimal physiologically-based pharmacokinetic modelling. MAbs 2018, 10, 1144–1156. [Google Scholar] [CrossRef]
- Tian, Z.; Sutton, B.J.; Zhang, X. Distribution of rat neonatal Fc receptor in the principal organs of neonatal and pubertal rats. J. Recept. Signal Transduct. Res. 2014, 34, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Akilesh, S.; Christianson, G.J.; Roopenian, D.C.; Shaw, A.S. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J. Immunol. 2007, 179, 4580–4588. [Google Scholar] [CrossRef]
- Montoyo, H.P.; Vaccaro, C.; Hafner, M.; Ober, R.J.; Mueller, W.; Ward, E.S. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc. Natl. Acad. Sci. USA 2009, 106, 2788–2793. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Balthasar, J.P. Application of physiologically based pharmacokinetic modeling to predict the effects of FcRn inhibitors in mice, rats, and monkeys. J. Pharm. Sci. 2019, 108, 701–713. [Google Scholar] [CrossRef]
- Li, T.; Balthasar, J.P. Development and evaluation of a physiologically based pharmacokinetic model for predicting the effects of anti-FcRn therapy on the disposition of endogenous IgG in humans. J. Pharm. Sci. 2019, 108, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Xiao, G.; Gan, L.S. Receptor-mediated endocytosis and brain delivery of therapeutic biologics. Int. J. Cell Biol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Rioux, N.; Waters, N.J. Physiologically based pharmacokinetic modeling in pediatric oncology drug development. Drug Metab. Dispos. 2016. [Google Scholar] [CrossRef]
- Grimstein, M.; Yang, Y.; Zhang, X.; Grillo, J.; Huang, S.-M.; Zineh, I.; Wang, Y. Physiologically based pharmacokinetic modeling in regulatory science: An update from the U.S. Food and Drug Administration’s Office of Clinical Pharmacology. J. Pharm. Sci. 2019, 108, 21–25. [Google Scholar] [CrossRef]
- Cole, S.; Hay, J.L.; Luzon, E.; Nordmark, A.; Rusten, I.S. European regulatory perspective on pediatric physiologically based pharmacokinetic models. Int. J. Pharm. 2017, 2, 113–124. [Google Scholar] [CrossRef]

| Dose (mg) | Treatment | n | Sex (% male) | Age (years) | Weight (kg) | Height (cm) | SA Ratio | Study Reference |
|---|---|---|---|---|---|---|---|---|
| 0.2 /kg | iv (1 h), SD | 2 | 100 | 30–41 | 66–86 | 183–187 | 0.27 | APG101_CD_001 |
| 1.0 /kg | iv (1 h), SD | 2 | 100 | 33–44 | 87–89 | 178–184 | 0.34 | APG101_CD_001 |
| 5.0 /kg | iv (1 h), SD | 4 | 100 | 28–33 | 65–94 | 177–185 | 0.34 | APG101_CD_001 |
| 15.0 /kg | iv (1 h), SD | 4 | 100 | 21–40 | 64–84 | 175–183 | 0.34 | APG101_CD_001 |
| 20.0 /kg | iv (1 h), SD | 4 | 100 | 25–44 | 63–82 | 178–188 | 0.34 | APG101_CD_001 |
| 400 | iv (0.5 h), QW | 35 | 62.9 | 23–73 | 50–127 | 151–190 | 0.30–0.57 | APG101_CD_002 |
| Parameter | Value (95% CI) | Unit | Reference | Description |
|---|---|---|---|---|
| MW | 84082.0 | g/mol | APG | Molecular weight |
| fu | 100.0 | % | APG | Fraction unbound in serum |
| Radius (solute) | 4.01 | nm | calculated | Hydrodynamic radius of the drug |
| CD95L Kd | 1.91 (±0.28) | µmol/L | optimized | Dissociation constant |
| CD95L koff | 9.70 (±3.13) | ×10−5 /min | optimized | Dissociation rate constant |
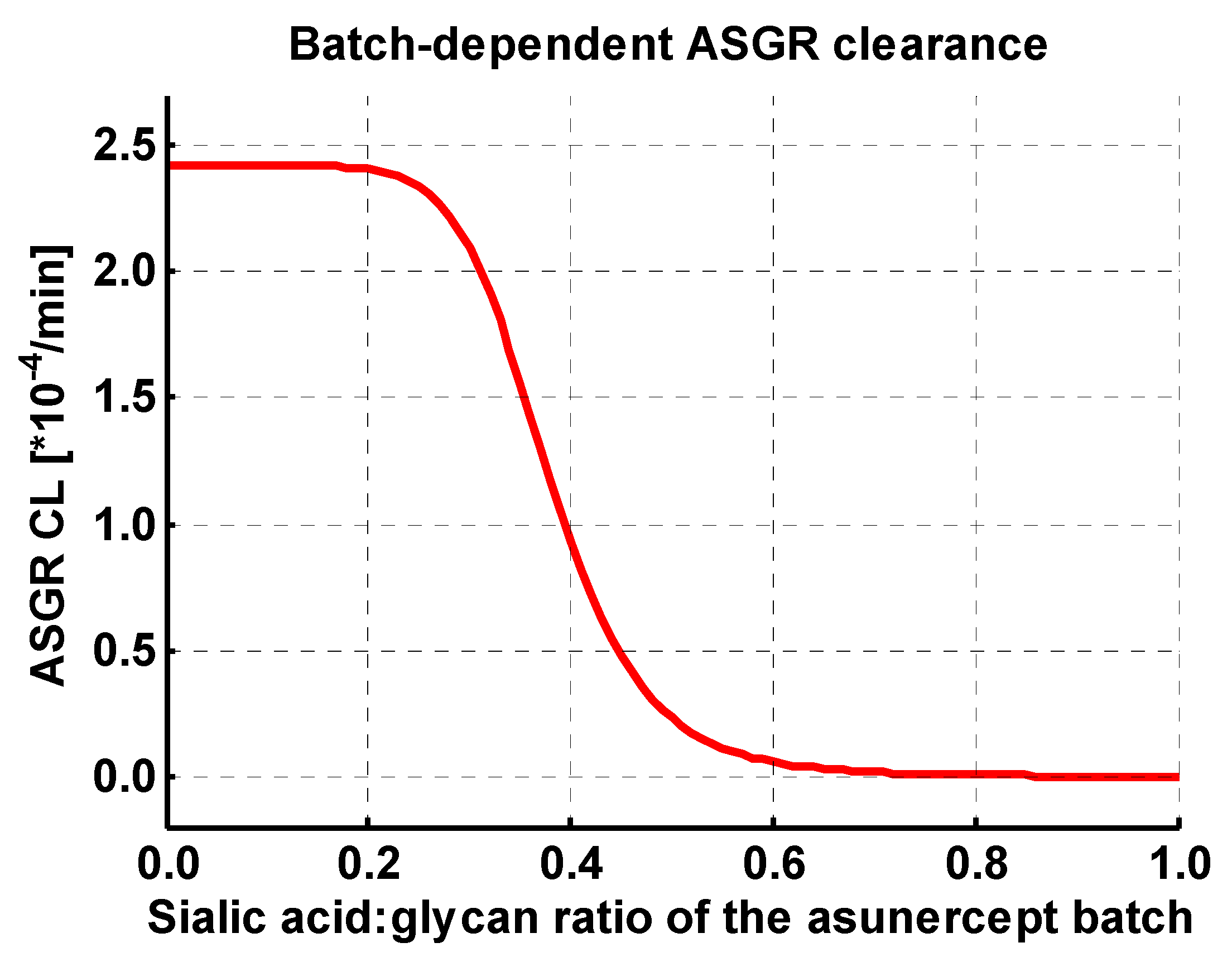
| ASGR CLspec | 2.43 (±2.98) | ×10−4 /min | optimized | Normalized first order clearance |
| FcRn Kd (lysosomal) | 1.73 (±0.16) | µmol/L | optimized | Dissociation constant |
| FcRn Kd (serum) | 999999.0 | µmol/L | [16] | Dissociation constant |
| FcRn kass | 0.87 | L/(µmol*min) | [16] | Association rate constant |
| GFR fraction | 0.0 | - | APG | Fraction of filtered drug reaching the urine |
| Dose [mg/kg] | ID | AUClast Pred [h*µg/mL] | AUClast Obs [h*µg/mL] | AUClast Pred/obs | Cmax Pred [µg/mL] | Cmax Obs [µg/mL] | Cmax Pred/obs | Half-life Pred [h] | Half-life Obs [h] | Half-life Pred/obs |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.2 | 010 | 138 | 174 | 0.79 | 3.5 | 3.5 | 1.01 | - | - | - |
| 0.2 | 011 | 96 | 84 | 1.14 | 4.3 | 3.1 | 1.37 | - | - | - |
| 1.0 | 014 | 1944 | 2464 | 0.79 | 21.9 | 30.1 | 0.73 | 369 | 340 | 1.08 |
| 1.0 | 015 | 1977 | 2471 | 0.80 | 22.3 | 29.9 | 0.75 | 364 | 258 | 1.41 |
| 5.0 | 017 | 12030 | 18780 | 0.64 | 113.4 | 126.1 | 0.90 | 369 | 513 | 0.72 |
| 5.0 | 019 | 11155 | 13817 | 0.81 | 104.2 | 145.0 | 0.72 | 366 | 248 | 1.47 |
| 5.0 | 020 | 9697 | 12431 | 0.78 | 88.8 | 103.7 | 0.86 | 376 | 249 | 1.51 |
| 5.0 | 022 | 12353 | 11246 | 1.10 | 113.5 | 119.6 | 0.95 | 373 | 419 | 0.89 |
| 15.0 | 023 | 34716 | 37101 | 0.94 | 322.6 | 363.3 | 0.89 | 353 | 332 | 1.06 |
| 15.0 | 025 | 37096 | 24014 | 1.54 | 322.3 | 274.8 | 1.17 | 369 | 420 | 0.88 |
| 15.0 | 026 | 33580 | 39658 | 0.85 | 317.9 | 402.9 | 0.79 | 363 | 472 | 0.77 |
| 15.0 | 027 | 28933 | 20119 | 1.44 | 264.7 | 217.8 | 1.22 | 375 | 401 | 0.93 |
| 20.0 | 029 | 40082 | 45555 | 0.88 | 349.0 | 524.4 | 0.67 | 371 | 496 | 0.75 |
| 20.0 | 031 | 43846 | 42466 | 1.03 | 413.1 | 454.2 | 0.91 | 367 | 424 | 0.86 |
| 20.0 | 033 | 43729 | 44423 | 0.98 | 411.9 | 482.4 | 0.85 | 359 | 437 | 0.82 |
| 20.0 | 034 | 40271 | 44046 | 0.91 | 374.1 | 366.9 | 1.02 | 361 | 437 | 0.83 |
| GMFE | 1.22 | 1.20 | 1.24 | |||||||
| GMFE range | 1.02–1.56 | 1.01–1.50 | 1.06–1.51 | |||||||
| Pred/obs within 2-fold | 16/16 | 16/16 | 14/14 | |||||||
| Pediatric Age Group | Recommended Weekly Dose |
|---|---|
| 1–2 years | 6.0 mg/kg |
| 2–5 years | 5.8 mg/kg |
| 5–9 years | 5.6 mg/kg |
| 9–12 years | 5.4 mg/kg |
| >12 years | 5.2 mg/kg |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanke, N.; Kunz, C.; Thiemann, M.; Fricke, H.; Lehr, T. Translational PBPK Modeling of the Protein Therapeutic and CD95L Inhibitor Asunercept to Develop Dose Recommendations for Its First Use in Pediatric Glioblastoma Patients. Pharmaceutics 2019, 11, 152. https://doi.org/10.3390/pharmaceutics11040152
Hanke N, Kunz C, Thiemann M, Fricke H, Lehr T. Translational PBPK Modeling of the Protein Therapeutic and CD95L Inhibitor Asunercept to Develop Dose Recommendations for Its First Use in Pediatric Glioblastoma Patients. Pharmaceutics. 2019; 11(4):152. https://doi.org/10.3390/pharmaceutics11040152
Chicago/Turabian StyleHanke, Nina, Claudia Kunz, Meinolf Thiemann, Harald Fricke, and Thorsten Lehr. 2019. "Translational PBPK Modeling of the Protein Therapeutic and CD95L Inhibitor Asunercept to Develop Dose Recommendations for Its First Use in Pediatric Glioblastoma Patients" Pharmaceutics 11, no. 4: 152. https://doi.org/10.3390/pharmaceutics11040152
APA StyleHanke, N., Kunz, C., Thiemann, M., Fricke, H., & Lehr, T. (2019). Translational PBPK Modeling of the Protein Therapeutic and CD95L Inhibitor Asunercept to Develop Dose Recommendations for Its First Use in Pediatric Glioblastoma Patients. Pharmaceutics, 11(4), 152. https://doi.org/10.3390/pharmaceutics11040152