Abstract
Pain is inadequately relieved by escalating doses of a strong opioid analgesic such as morphine in up to 25% of patients with cancer-related severe pain complicated by a neuropathic (nerve damage) component. Hence, there is an unmet medical need for research on novel painkiller strategies. In the present work, we used supercritical fluid polymer encapsulation to develop sustained-release poly(lactic-co-glycolic acid) (PLGA) biodegradable microparticles containing the analgesic adjuvant drug ketamine, for injection by the intrathecal route. Using this approach with a range of PLGA co-polymers, drug loading was in the range 10–60%, with encapsulation efficiency (EE) of 60–100%. Particles were mainly in the size range 20–45 µm and were produced in the absence of organic solvents and surfactants/emulsifiers. Investigation of the ketamine release profiles from these PLGA-based microparticles in vitro showed that release took place over varying periods in the range 0.5–4.0 weeks. Of the polymers assessed, the ester end-capped PLGA5050DLG-1.5E gave the best-controlled release profile with drug loading at 10%.
1. Introduction
Unrelenting severe cancer-related pain, particularly that complicated by a neuropathic (nerve damage) component, is difficult to alleviate by escalating doses of strong opioid analgesics such as morphine given alone or in combination with adjuvant analgesic agents by conventional systemic dosing routes [1]. Although cancer can be a terminal disease, there should be no reason to deny a patient the opportunity to live productively and free of pain [2]. Therefore, the goals of pain control should be to optimize the patient’s comfort and function whilst avoiding unnecessary adverse effects from medications [3].
The intrathecal (i.t.) dosing route enables logarithmic scale reductions in the analgesic/adjuvant doses needed to produce adequate pain relief because this route bypasses the systemic metabolism and delivers the drugs near to the receptors/ion channels that transduce pain relief in the spinal cord [4,5]. Importantly, there is some evidence that, in the postoperative setting, adding ketamine to an opioid in patient-controlled analgesia has a beneficial effect on pain relief, is morphine-sparing, and produces less postoperative nausea and vomiting compared with equi-effective opioid analgesic administration alone [6,7]. The efficacy of ketamine alone as an analgesic is still under debate, especially for indications such as chronic pain [8]. When used as an analgesic, sub-anaesthetic doses of ketamine are administered [9]. In rodents, co-injection of ketamine with a strong opioid analgesic attenuated the development of analgesic tolerance and enhanced pain relief [10]. Together, these findings suggest that i.t. co-administration of a strong opioid analgesic with low-dose ketamine in close proximity to their target receptors/ion channels in the spinal cord may produce satisfactory analgesia.
As implantable i.t. delivery devices are associated with a range of catheter-related problems in up to 25% of patients [11] and the surgery is highly invasive, it is clear that new therapeutic approaches are needed. One such strategy is the development of sustained-release biodegradable microparticles for i.t. injection with the potential to restore satisfactory analgesia in patients who would otherwise suffer from intractable cancer-related pain despite the administration of escalating doses of a strong opioid analgesic by conventional oral or parenteral dosing routes [12]. However, successful microencapsulation of analgesic/adjuvant drugs of interest must overcome the challenges of poor drug loading, unacceptably high initial burst release, inadequate sustained-release period, and contamination by unacceptable organic solvent residues.
Polymeric particles are widely used in the field of drug delivery [12,13,14]. The most appropriate carriers for sustained drug delivery are slowly degrading polymers. For example, poly(lactic-co-glycolic) acid (PLGA) and poly(lactic acid) (PLA) are biocompatible, biodegradable polymers that have been approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for use in systemic drug delivery systems [13]. Of these, PLGA-based drug-loaded microparticles, commonly prepared by a water-in-oil-in-water (w/o/w) double-emulsion method or an oil-in-water (o/w) single-emulsion method, hold promise because of their biodegradability and successful application in a range of systemically administered drug formulations [14,15].
With the traditional methods of sustained-release microparticle formulation, large quantities of potentially toxic organic solvents and/or surfactants/emulsifiers are used, possibly leading to unacceptable levels of residual impurities that necessitate the use of further purification steps to ensure their removal [16]. In contrast, supercritical CO2 (scCO2) technology provides a means to prepare particles that are environmentally benign and produce particles that are free from organic solvent residues after processing [17]. The method is flexible and allows control of the composition of the polymer mixtures to control the rates of release of the encapsulated molecules [18]. In particular, this technology provides a clean and reliable method for the production of particles containing therapeutic molecules such as ketamine.
The use of scCO2 to create drug-loaded particles is of particular interest [19] because of its relatively mild conditions (7.4 MPa and 31 °C), allowing the processing of thermally sensitive substances [20,21]. By varying the temperature and pressure, the properties of scCO2 can be changed, enabling it to act as a solvent, antisolvent, solute, or drying agent [22]. Consequently, different particle characteristics can be designed by adjusting the scCO2 processing conditions including temperature, pressure, contact time, diffusion processes, and depressurization conditions [23,24,25].
The precise method of processing using scCO2 depends upon the respective solubilities of the polymer carrier and the drug in the solvent (scCO2) [26]. Howdle and his team reported a method for the production of hGH-loaded polyester particles and demonstrated controlled release in a simian model [27]. In other work, vaccines for tetanus toxoid (TT) were incorporated into PLA particles using scCO2 processing [28]. A single injection of the polymer-TT particles into mice produced similar antibody titres as multiple injections of a commercial alum-adsorbed TT vaccine [28]. These results demonstrate the potential for the preparation of organic solvent-free particles loaded with the molecules of interest.
The simple CriticalMix process utilizes scCO2 to encapsulate active pharmaceutical ingredients (APIs) within a biodegradable polymer matrix for sustained-release applications [26,27,29]. Hence, the aim of our present study was to microencapsulate ketamine into biodegradable polymers to produce sustained-release microparticle formulations for potential i.t. co-injection with strong opioid analgesic-loaded microparticles so as to potentially achieve prolonged periods of satisfactory pain relief.
2. Materials and Methods
2.1. Materials
Various poly(lactic-co-glycolic acid) (PLGA) biodegradable polymers (50:50), (75:25), including 5050DLG-1.5E (5050-1.5E), RG503, RG504, RG7525S, 9010DLG-4.5E (9010-4.5E), 5050DLG-1A (5050-1A), 5050DLG-2A (5050-2A), 5050DLG-4A (5050-4A), 7525DLG-4A (5050-4A), and poly(lactic-acid) (PLA)-2.5A and R202S, were purchased from Evonik Health care (Birmingham, Al, USA). The physicochemical characteristics of these polymers are listed in Table 1. Ketamine HCl powder (98–99%) was from Sigma-Aldrich (Haverhill, UK). Food-grade CO2 was from BOC (Rotherham, UK).

Table 1.
Physicochemical properties of the PLGA and PLA polymers.
2.2. Methods
2.2.1. Preparation of Ketamine-Loaded Polymeric Microparticles
Firstly, ketamine HCl was converted to the corresponding free base by adjusting the pH to ~10 using 2 M NaOH added dropwise according to our previous work (Han et al., 2015). The CritcalMix scCO2 process was optimised on the basis of that described previously [29]. For each of the polymer powders listed in Table 1, a weighed amount was mixed with ketamine in varying ratios (Table 2), and each mixture was loaded into a pressure vessel, pressurised with CO2 and heated to 40 °C at a pressure of 14 MPa. Once the desired temperature was reached, the mixture of CO2/polymer/drug inside the vessel was mixed using mechanical stirring. Liquefaction of the polymer by scCO2 enabled the drug to be mixed into the polymer. Approximately 0.5–1.0 h later heating was stopped, and the polymer/ketamine/scCO2 mixture was allowed to cool to below 25 °C before the CO2 was slowly vented, depressurised through a disk nozzle with a 0.6 mm orifice (unheated), and the polymer solidified to entrap ketamine and form microparticles containing ketamine. Cooling took place over a period of one hour with pressure decreasing from 14 MPa scCO2 to 7.5 MPa (~1000 psi). The microparticles were collected and ground with a pestle and mortar and then sieved through a 45 µm sieve. The final products were stored refrigerated at a mean (±SD) temperature of 5(±3) °C and protected from light in a desiccator.

Table 2.
Ketamine-loaded microparticles—formulations and incorporation efficiency.
2.2.2. Determination of Drug Incorporation Efficiency
Accurately weighed amounts (~10 mg; n = 3) of the ketamine-loaded polymeric microparticles (Table 1) were dissolved in acetonitrile. The ketamine concentrations were quantified using HPLC (Agilent 1200 series) with UV detection at 280 nm. A gradient HPLC method was used for determining the drug loading (DL) using a Waters XBridge BEH C18 column: 3.5 µm, 3.0 × 150 mm. The calibration range was 5–800 µg/mL. The mean (±SD) correlation coefficients for ketamine calibration curves were 0.9998 (±0.0001).
Drug incorporation efficiency, expressed as actual drug loading (% w/w), and encapsulation efficiency (EE % w/w) were calculated using Equations (1) and (2), respectively. The individual values for three replicate determinations and their mean values are reported.
Drug loading (%) = 100 × mass of drug in microparticles/weight of microparticles
EE (%) = 100 × mass of drug in microparticles/mass of drug in microparticles theoretically
2.2.3. Determination of In Vitro Drug Release
Accurately weighed ~20 mg samples of ketamine-loaded polymeric microparticles (n = 3) were suspended in 1mL of PBS and transferred to dialysis tubes (SnakeSkin™ Dialysis Tubing, 3.5K MWCO). Each dialysis tube was sealed, placed into a capped container containing 20mL PBS, and then placed into an incubator maintained at 37.5 °C and shaken horizontally at an oscillating frequency of 120 min−1. Sampling time points were 3 and 24 h, 3, 7, 14, 21, and 28 days. At each time point, a 1 mL aliquot of buffer was taken for analysis and replaced with fresh buffer. The 1 mL samples (n = 3) were filtered using a 0.2 µm nylon syringe filter into a vial for HPLC analysis of the ketamine concentrations.
An isocratic HPLC method was optimised for determining the ketamine release profile using a C18 column: 3.5 µm, 3.0 × 150 mm. The calibration range was 5–800 µg/mL. The mean (±SD) correlation coefficients for ketamine calibration curves were R2 > 0.9999.
2.3. Morphology and Particle Size
Morphological evaluation of the sustained-release ketamine-loaded PLGA microparticles was performed using scanning electron microscopy (Jeol IT300, JEOL Ltd., Tokyo, Japan) to determine shape and surface morphology. The microparticles were sputter-coated with platinum using an Auto Smart Coater (JFC-1300, JEOL Ltd., Tokyo, Japan) before examination using scanning electron microscopy.
2.4. Data Analysis
The data are presented as mean ± standard deviation (SD) or standard error of the mean (SEM) (±SD/SEM). Non-linear regression was used to calculate in vitro drug release using GraphPad PrismTM v7.03 (GraphPad, San Diego, CA, USA). The one-way and two-way ANOVA followed by the Bonferroni test were used for comparing the means of burst release for the various formulation trials herein. The statistical significance criterion was p ≤ 0.05. To investigate the ketamine release kinetics, in vitro drug release data were fitted to various kinetic models, and regression analysis was performed for the selected formulations. The data were plotted as cumulative percentage drug release versus time for the zero-order model, square root of time for the Higuchi model, and log time for the Korsmeyer–Peppas model [30,31,32].
3. Results
3.1. Successful Encapsulation of Ketamine
A total of 112 trials with various PLGA co-polymers individually, as PLGA blends, or as PLGA blended with PLA were conducted. Our focus was upon minimisation of the burst and optimisation of the release profile over 24 h. Thus, we varied temperature, pressure, contact time, stirring rate of the scCO2/polymer/drug solution, as well as depressurization conditions including CO2 venting rate and venting temperature to deliver the optimal formulation. Ketamine as a free base is stable under supercritical CO2 conditions, as no degradation was observed when processed under CriticalMix conditions (data not shown). Ketamine loading was controlled in the range of 10–60% (Figure 1 and Table 2). The recovery of ketamine from the microparticle formulations was in the range 60–100% (mean of 80%) for all trials. Details of the selected 19 formulations of ketamine-loaded microparticles produced herein are summarized in Table 2, including the mean (±SD) actual drug loading (n = 3) and EE.
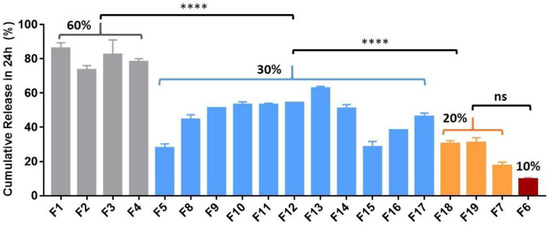
Figure 1.
Mean (±SEM) in vitro ketamine percentage of cumulative release in 24 h for a range of formulations (n = 3). Reducing the ketamine payload from 60% to 30% and from 30% to 10–20% reduced the burst effect (****, p < 0.0001). One-way ANOVA followed by the Bonferroni test.
3.2. In Vitro Release Profiles
For all formulations of the ketamine-loaded microparticles there was a higher initial release phase when the drug loading was increased (Figure 1), followed by a slower nearly zero-order release lasting for up to 28 days (Figure 2, Figure 3 and Figure 4).
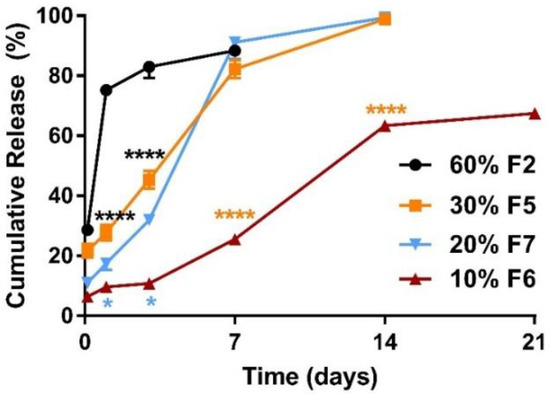
Figure 2.
Comparison of mean (±SEM) in vitro ketamine release profiles between microparticle formulations (n = 3) with ketamine loading at 60% and 30%, 20% and 10%. The 24 h burst release decreased significantly (****, p < 0.0001) from 74.6% (● F2, 60% ketamine and 40% PLGA5050-1.5E) to 27.7% (■ F5, 30% ketamine and 70% PLGA5050-1.5E). Reducing the ketamine load from 30% to 20% and 10% using the same polymer, PLGA5050-1.5E, enabled the polymer matrix to have better control over ketamine release. Sustained release continued for 14 days when ketamine loading was 30% (■ F5), whereas it lasted more than 21 days at 10% ketamine loading (▼ F6). The 24 h burst release was reduced significantly from 27.7% to 9.0% (*, p < 0.05) for microparticles at 10% ketamine loading when compared with 30% ketamine loading. Ketamine loading at 20% (▲) and 30% (■) did not differ significantly (p > 0.05) in terms of release duration, but burst release was reduced from 27.7% to 17.5%. Two-way ANOVA followed by the Bonferroni test. F, Formulation.
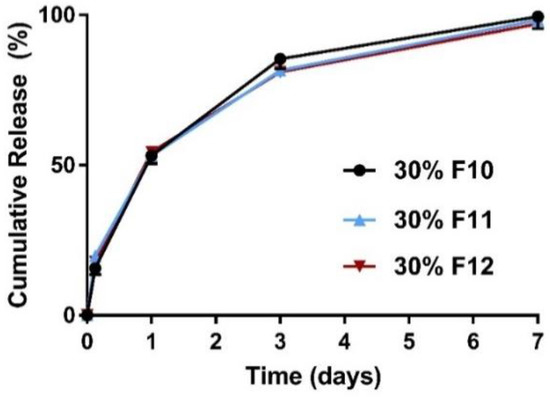
Figure 3.
Mean (±SEM) in vitro ketamine release profiles did not differ significantly (p > 0.05) between the three acid end-capped polymers assessed (n = 3). A maximum of one-week sustained release was achieved in vitro. The cumulative release in 24 h was ~55%, >80% in three days, and 100% in one week for formulations 10–12, respectively. F, Formulation; F10, PLGA5050-1A (50%) and PLGA7525-4A (20%); F11, PLGA5050-2A (70%); F12, PLGA5050-1A (70%).
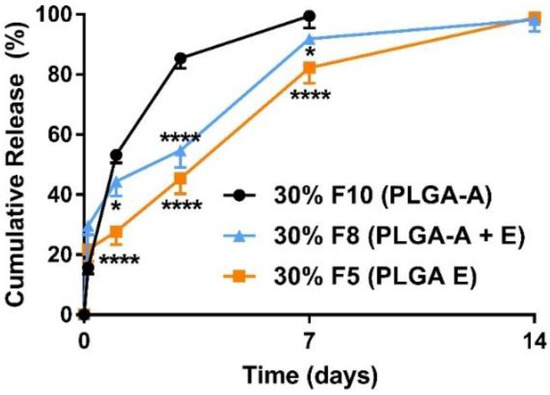
Figure 4.
Mean (±SEM) in vitro ketamine release profiles varied for polymers (n = 3) with different end groups. Incorporation of a low-MW ester end-capped PLGA (▲ F8) with acid end-capped PLGA reduced the rate of release significantly (*, p < 0.05; ****, p < 0.0001) when compared with a blend of acid end-capped PLGAs (● F10, 50% of PLGA5050-1A and 20% of PLGA7525-4A). The 24 h burst release decreased from 53.2% for F10 to 44.4% for F8, and 100% release was achieved over seven days for both formulations. The low-MW ester end-capped PLGA itself improved the release profile (■ F5) compared with a blend of acid end-capped PLGAs (F10). The 24 h burst release decreased significantly (****, p < 0.0001) from 53.2% for F10 to 33.2% for F5, and 100% of ketamine was released in two weeks. Two-way ANOVA followed by the Bonferroni tests. F, Formulation.
3.2.1. Reducing the Ketamine Payload Reduced the Burst Release of Ketamine
When the ketamine payload exceeded 60%, all the ketamine-loaded microparticles formulated with various polymer compositions (high MW vs. low MW) performed similarly in vitro. The 24 h burst release values were over 70% for all trials (e.g., formulations (F) 1–4 in Figure 1). Therefore, the ketamine payload was reduced to 30%, which enabled a greater portion of the microparticle composition to be polymer-controlled and resulted in a reduction of the burst release of ~40% (from ~70% down to ~30%) (Figure 1 and Figure 2). A 14-day sustained release profile was achieved (Figure 2) for F5 (30% ketamine +70% PLGA5050-1.5E) together with a significantly reduced 24 h burst release (****, p < 0.0001) when compared with the corresponding parameters for F2 (60% ketamine + 40% PLGA5050-1.5E) using the same polymer PLGA5050-1.5E (Figure 2).
For microparticles formed using the polymer PLGA5050-1.5E, a further reduction in the ketamine loading from 30% to 10% enabled the polymer matrix to have better control over the release profile (Figure 2). Sustained release was extended for more than 21 days for F6 (10% ketamine + 90% PLGA5050-1.5E), and the 24 h burst release was reduced significantly (p < 0.0001) when loading was reduced from ~30% to ~10% compared with F5 (30% ketamine + 70% PLGA5050-1.5E). The release profile for microparticles loaded with 20% ketamine (F7, 20% ketamine + 80% PLGA5050-1.5E) did not differ significantly when compared with 30% ketamine-loaded microparticles (F5) (p > 0.05) (Figure 2), although the 24 h burst release was reduced from 27.7% to 17.5%.
3.2.2. Ester End-Capped Polymers Are Better at Controlling the Release of Ketamine Compared with Acid End-Capped Polymers
The polymers used in this study were PLGAs and PLAs with varying molecular weights (MW) and end-group functionalities. The physicochemical properties of the polymers used in this study are summarized in Table 1. Particles formulated using acid end-capped polymers had a high burst release, that is, ~55% release within the initial 24 h and >80% release within three days (Figure 3). Together, our data show that for acid end-capped PLGAs, changing the MW/viscosity and possibly the particle matrix had little influence on the rate of release. Our data show a maximum of one-week release in vitro with acid end-capped PLGA polymers (Figure 3). Additionally, varying the ratios of the PLGA5050-1A and the PLGA7525-4A polymers had little influence on the release profiles. High-MW acid end-capped polymers did not perform significantly better than low-MW acid end-capped polymers. Incorporation of a low-MW ester end-capped polymer PLGA5050-1.5E (50%) into PLGA7525-4A (20%) reduced the rate of ketamine release and extended the release over seven days (F8 in Figure 4). Ester end-capped polymers enabled ketamine-loaded microparticles to be formed with release extended to 14 days and more than 21 days (F5, F6, in Figure 2).
3.2.3. In Vitro Drug Release Kinetics
For the kinetic studies, the following plots were made: % drug release versus time (zero-order kinetic model); % drug release versus square root of time (Higuchi model); log % drug release versus log time (Korsmeyer–Peppas model). All plots are given in the supplementary materials (Figures S1–S3), and the results are summarized in Table 3. Most data fit well to the Higuchi model and the Korsmeyer–Peppas model (R2 > 0.9000). The PLGA5050-1.5E-based F6 of 10% DL showed the best fit (R2 = 0.9481) of the observed data to the zero-order model.

Table 3.
Fit parameters of the kinetic model for the release of ketamine-loaded PLGA5050-1.5E-based microparticles.
3.3. Morphology and Particle Size
Visual inspection of the scanning electron micrograph images of F6 (the best performing sustained-release profile herein) in Figure 5 show that the microparticles were random in shape without significant aggregation and/or porosity and were in the size range of 20–45 μm. There was no clear change in particle morphology after six months storage at 2–8 °C (Figure 5B).
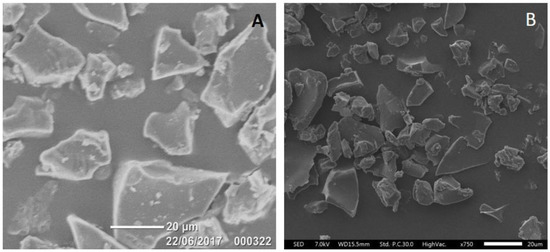
Figure 5.
SEM micrographs illustrating the morphology and size of sustained-release ketamine-loaded microparticles (Formulation 6: 10% Ketamine + 90% PLGA5050-1.5E) stored at 2–8 °C for one month (A) and 6 months (B).
4. Discussion
In the present study, we demonstrate for the first time that the microencapsulation of ketamine using supercritical CO2 technology resulted in microparticles that were solvent- and surfactant-free once CO2 was converted from the liquid to the gaseous state and vented to the atmosphere [22]. Carbon dioxide is relatively inert, non-toxic, and environmentally friendly when compared with organic solvents and emulsifying agents used in classical double-emulsion microparticle formulation methods [16]. As CO2 has a relatively low critical point of 7.38 MPa at 31.1 °C, drug-loaded microparticles can be processed under ambient conditions [33]. Our ketamine data herein highlight the applicability of scCO2 for the incorporation of small bioactive molecules into a range of polymer matrices to achieve the desired release profiles. Importantly, ketamine loading of these polymeric microparticle formulations could be controlled in the range of 10–60%, with EE in the range of 60–100%. For microparticles with ketamine loading in the range of 10–30%, the microencapsulated payloads were 1.5–4.0-fold higher (Table 2) than those achieved in our previous work (6.8%) using a w/o/w double emulsion method (Han et al., 2015). The particle sizes were mainly 20–45 µm (Figure 5), which was within our target size range of 20–60 µm (Han et al., 2015), and in vitro ketamine release was observed over a period of 0.5–4.0 weeks (Figure 2, Figure 3 and Figure 4).
A greater proportion of polymer than encapsulated payload is required to achieve prolonged release. Our findings show that for ketamine, microparticle loading up to a maximum of 30% produced prolonged-release microparticles. Reducing the ketamine payload from 60% to 30% and then to 10% progressively reduced the burst release effect. However, on the flip side, reducing the encapsulated drug payload might adversely affect the requirement of achieving a therapeutic response in vivo (60–100 µg per day), but this remains to be assessed. Our finding that the ketamine release profile was prolonged by increasing the percentage of polymer in the formulation is aligned with work by others [34] whereby the polymer/encapsulated drug ratio was an important factor in controlling the rate of release. Herein, we found that to achieve prolonged release over 21 to 28 days, the polymer-to-microencapsulated ketamine ratio should be 70:30 or 90:10.
Although higher drug loading led to more extensive burst release, the level of drug loading had little effect on the release rate after 24 h, even when the drug loading was as high as 60%. Drug release from PLGA microparticles is underpinned by three factors, namely, external diffusion, internal mass transfer, and polymer degradation [35]. On the basis of our present results, the external diffusion and internal mass transfer likely determine the extent of ketamine release during the first 24 h, and polymer degradation is the main factor controlling release thereafter. Our data extend the findings of Cai et al. [36] and Sah et al. [37] who showed that the concentration gradient does not influence encapsulated drug release until the onset of polymer degradation. In previous work by others using a double-emulsion method, a relatively high drug loading produced a particulate dispersion characterized by increased microparticle porosity rather than the desired molecular dispersion [36,38,39]. This underscores our present finding that higher ketamine loading led to a greater burst release. Another explanation is that some ketamine may have precipitated on the surface of the polymer rather than being incorporated into the polymer matrix [25], causing a relatively high apparent burst drug release.
Our present data show that the use of this supercritical CO2 approach with the ester end-capped PLGA5050-1.5E resulted in microparticles with superior burst and sustained-release properties when compared with those produced using a range of PLGAs and PLAs either individually or in various blends. This could be due to the low glass transition temperature of PLGA5050-1.5E that enabled drug-loaded particles to be formed relatively quickly (Tg = 28.3 °C). Additionally, when the temperature of the scCO2 process is above that of the polymer Tg, this allows higher CO2 sorption and swelling to enhance diffusion of the CO2/ketamine solution into the polymer matrix [40]. Microporosity of the PLGA5050-1.5E-based microparticles is likely to be the major factor aiding the diffusion of ketamine out of the CriticalMix microparticles, causing burst release as the larger area exposed for hydration facilitates rapid diffusion [41]. This is despite the fact that PLGA5050-1.5E has a relatively low inherent viscosity, a property conducive to the formation of particles with smaller pores or without pores. This topic has not been adequately addressed in published studies to date.
Our present data also show that hydrophobic ester end-capped PLGAs are superior at controlling release compared with acid end-capped PLGAs, with the latter showing high burst ketamine release at ~55%, with 100% release within one week (Figure 3) but without adversely affecting the drug incorporation efficiency (Table 2). Our findings for ketamine are in contrast to work by others [36,42] using a classical emulsion method to microencapsulate large molecules (dextran and bovine serum albumin, respectively), whereby the hydrophilic acid end-capped PLGA RG503H (PLGA5050, MW 24–38 kDa, IV 0.32–0.44 dL/g, acid end-capped) led to a high drug loading and sustained release (~4 weeks). For the more hydrophobic RG502 (PLGA5050, MW 7–17 kDa, IV 0.16–0.24 dL/g, ester end-capped), these large molecules were trapped in the matrix and kept in the solid state because of the delayed hydration process [36]. In the current study, it is likely that the delayed hydration process of ester end-capped PLGAs [43] influenced the rate of polymer hydration in vitro and subsequently facilitated the release of ketamine out of the microparticles. This is in agreement with the findings by others that the encapsulation of weakly basic drugs into PLGA can accelerate the biodegradation of PLGA [44,45]. Additionally, the acids may also produce local lowering of the pH [35] to accelerate ketamine release. Therefore, to ensure that the release of the free base forms of small weakly basic molecules, such as ketamine, can be controlled for a longer period, acid end-capped polymers should be avoided.
For ketamine-release kinetics, the high correlation coefficients for the Higuchi and Korsmeyer–Peppas models suggest a Fickian diffusion mechanism for most formulations [30,31,32]. The lowest burst release profile of F6 (10% DL, PLGA5050-1.5E based) fitted well to the zero-order model but not well to the Korsmeyer–Peppas model (R2 = 0.8330, n = 0.4824), suggesting that Fickian diffusion may play a critical role in the burst release.
Previous work by others has demonstrated that the drug release profile could be tailored by altering the molar ratio of the different MW PLGA polymers used in the depot formulation [46]. It was found that PLGA blends generally had intermediate properties when compared with pure polymer formulations [46], suggesting that blends of low-, medium-, and high-MW polymers may form dense and less porous microparticles, enabling a better control of sustained-release properties. However, our data show that burst release was not improved as anticipated for these blended formulations. An alternative approach is to include excipients which swell on contact with water, such as poloxamer 407 (Koliphor F407) [47,48], with the aim of filling the exposed microparticle pores and slow the rate of encapsulated payload diffusion. Thus, polymers/excipients, which become molten at physiological temperature closing up the pores and thereby reducing the overall surface area exposed [49], should be beneficial for extended-release formulations [50]. Hence, future work aimed at the investigation of combinations of PLGA5050-1.5E with various swelling agents to reduce ketamine burst release may be beneficial in further increasing ketamine loading without compromising the desired zero-order sustained release profile.
5. Conclusions
The preparation of ketamine-encapsulated microparticles was successfully achieved using supercritical fluid polymer encapsulation with EE in the range 60–100% in the absence of surfactants and potentially toxic organic solvents. A sustained-release profile extending for ≥21 days was achieved using ester end-capped PLGA5050-1.5E at drug loading of 10% in vitro. It is clear from our findings that payload solubility in scCO2 as well as CO2 sorption and swelling of the polymer vary with temperature, pressure, contact time, stirring rate of the scCO2/polymer/drug solution, as well as depressurization conditions including CO2 venting rate and venting temperature. By systematically varying these parameters and by selecting PLGAs with different physicochemical properties (end groups, viscosity, etc.), small molecule-loaded microparticles with the desired release rate can be produced using supercritical fluid polymer encapsulation instead of organic solvents and emulsifiers that are difficult and costly to remove.
Supplementary Materials
The following are available online at http://www.mdpi.com/1999-4923/10/4/264/s1, Figure S1: Drug release kinetics plots of F2, F5, F6 and F7. Figure S2: Drug release kinetics plots of F10, F11 and F12. Figure S3: Drug release kinetics plots of F14, F15 and F17. Figure S4: DSC curves of PLGA5050-1.5E before and after loading with ketamine. The curves displayed above are the 2nd heating. Heating rate is 10 °C/min from 0 to 300 °C. Figure S5: FTIR spectra of PLGA5050-1.5E before and after loading with ketamine.
Author Contributions
Conceptualization, M.T.S., A.K.W., F.Y.H and S.M.H.; methodology, A.K.H., S.M.H. and A.S.-A.; formal analysis, F.Y.H.; investigation, M.T.S., A.K.W., F.Y.H., A.S.-A. and S.M.H.; resources, S.M.H., A.N., A.K.W. and M.T.S.; data curation, A.S.-A., A.N., C.Z. and F.Y.H.; writing—original draft preparation, F.Y.H.; writing—review and editing, F.Y.H., M.T.S., S.M.H., A.N. and A.K.W.; project administration, F.Y.H., M.T.S. and A.K.W.; funding acquisition, M.T.S. and A.K.W.
Funding
This research was funded by a National Health and Medical Research Council (NHMRC) Grant [APP1107723]. Financial support from the Australian Research Council (LE0775684, LE110100028, LE110100033, LE140100087, LE160100168) is also acknowledged.
Acknowledgments
The authors acknowledge the Queensland Government Smart State Research Facilities Programme for supporting CIPDD research infrastructure. CIPDD is also supported by Therapeutic Innovation Australia (TIA). TIA is supported by the Australian Government through the National Collaborative Research Infrastructure Strategy (NCRIS) program. The particles were produced at Critical Pharmaceuticals Limited, Nottingham, United Kingdom. The authors also acknowledge the use of the equipment managed by the Australian National Fabrication Facility (ANFF-Q) at The University of Queensland. We thank Tushar Kumeria from the School of Pharmacy, University of Queensland, for his useful inputs on release kinetics and models.
Conflicts of Interest
The authors have no conflicts of interest to declare in association with this work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Paice, J.A.; Betty, F. The Management of Cancer Pain. CA Cancer J. Clin. 2011, 61, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Aboawja, M. Patterns of Cancer Diagnoses Referred to Palliative Medicine in a Tertiaryhospital in Dammam-Saudi Arabia. J. Nurs. Healthcare 2017, 2, 1–4. [Google Scholar]
- Nersesyan, H.; Slavin, K.V. Current Aproach to Cancer Pain Management: Availability and Implications of Different Treatment Options. Ther. Clin. Risk Manag. 2007, 3, 381–400. [Google Scholar] [PubMed]
- Stearns, L.; Boortz-Marx, R.; Du Pen, S.; Friehs, G.; Gordon, M.; Halyard, M.; Herbst, L.; Kiser, J. Intrathecal Drug Delivery for the Management of Cancer Pain: A Multidisciplinary Consensus of Best Clinical Practices. J. Support. Oncol. 2005, 3, 399–408. [Google Scholar]
- Bhatia, G.; Lau, M.E.; Koury, K.M.; Gulur, P. Intrathecal Drug Delivery (ITDD) Systems for Cancer Pain. F1000Res 2013, 2, 96. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Wong, C.S.; Chang, J.Y.; Ho, S.T. Intrathecal Ketamine Reduces Morphine Requirements in Patients with Terminal Cancer Pain. Can. J. Anaesth. 1996, 43, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.K.; Bijur, P.E.; Meyer, R.H.; Kenny, M.K.; Solorzano, C.; Gallagher, E.J. Safety and Efficacy of Hydromorphone as an Analgesic Alternative to Morphine in Acute Pain: A Randomized Clinical Trial. Ann. Emerg. Med. 2006, 48, 164–172. [Google Scholar] [CrossRef]
- Jonkman, K.; van de Donk, T.; Dahan, A. Ketamine for Cancer Pain: What Is the Evidence? Curr. Opin. Support Palliat Care 2017, 11, 88–92. [Google Scholar] [CrossRef]
- Jonkman, K.; Dahan, A.; van de Donk, T.; Aarts, L.; Niesters, M.; van Velzen, M. Ketamine for Pain. F1000Res 2017, 6. [Google Scholar] [CrossRef]
- Miyamoto, H.; Saito, Y.; Kirihara, Y.; Hara, K.; Sakura, S.; Kosaka, Y. Spinal Coadministration of Ketamine Reduces the Development of Tolerance to Visceral as Well as Somatic Antinociception During Spinal Morphine Infusion. Anesth. Analg. 2000, 90, 136–141. [Google Scholar] [CrossRef]
- Markman, J.D.; Philip, A. Interventional Approaches to Pain Management. Med. Clin. North Am. 2007, 91, 271. [Google Scholar] [CrossRef] [PubMed]
- Han, F.Y.; Thurecht, K.J.; Lam, A.L.; Whittaker, A.K.; Smith, M.T. Novel Polymeric Bioerodable Microparticles for Prolonged-Release Intrathecal Delivery of Analgesic Agents for Relief of Intractable Cancer-Related Pain. J. Pharm. Sci. 2015, 104, 2334–2344. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; le Breton, A.; Préat, V. Plga-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable Plga-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.F.; Randolph, T.W. Process Variable Implications for Residual Solvent Removal and Polymer Morphology in the Formation of Gentamycin-Loaded Poly(l-Lactide) Microparticles. Pharm. Res. 1998, 15, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of Materials for Regenerative Medicine Using Supercritical Fluid Technology. Bioconj. Chem. 2015, 26, 1159–1171. [Google Scholar] [CrossRef]
- Kelly, C.A.; Naylor, A.; Illum, L.; Shakesheff, K.M.; Howdle, S.M. Supercritical CO2: A Clean and Low Temperature Approach to Blending Pdlla and Peg. Adv. Funct. Mater. 2012, 22, 1684–1691. [Google Scholar] [CrossRef]
- Tabernero, A.; del Valle, E.M.M.; Galan, M.A. Supercritical Fluids for Pharmaceutical Particle Engineering: Methods, Basic Fundamentals and Modelling. Chem. Eng. Process. 2012, 60, 9–25. [Google Scholar] [CrossRef]
- Del Valle, E.M.M.; Galan, M.A. Supercritical Fluid Technique for Particle Engineering: Drug Delivery Applications. Rev. Chem. Eng. 2005, 21, 33–69. [Google Scholar]
- Howdle, S.M.; Watson, M.S.; Whitaker, M.J.; Popov, V.K.; Davies, M.C.; Mandel, F.S.; Wang, J.D.; Shakesheff, K.M. Supercritical Fluid Mixing: Preparation of Thermally Sensitive Polymer Composites Containing Bioactive Materials. Chem. Commun. 2001, 1, 109–110. [Google Scholar] [CrossRef]
- Nuchuchua, O.; Nejadnik, M.R.; Goulooze, S.C.; Lješković, N.J.; Every, H.A.; Jiskoot, W. Characterization of Drug Delivery Particles Produced by Supercritical Carbon Dioxide Technologies. J. Supercrit. Fluids 2017, 128, 244–262. [Google Scholar] [CrossRef]
- Türk, M.; Bolten, D. Polymorphic Properties of Micronized Mefenamic Acid, Nabumetone, Paracetamol and Tolbutamide Produced by Rapid Expansion of Supercritical Solutions (RESS). J. Supercrit. Fluids 2016, 116, 239–250. [Google Scholar] [CrossRef]
- Kiran, E. Supercritical Fluids and Polymers—The Year in Review-2014. J. Supercrit. Fluids 2016, 110, 126–153. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug Loading of Polymer Implants by Supercritical CO2 Assisted Impregnation: A Review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Woods, H.M.; Silva, M.M.C.G.; Nouvel, C.; Shakesheff, K.M.; Howdle, S.M. Materials Processing in Supercritical Carbon Dioxide: Surfactants, Polymers and Biomaterials. J. Mater. Chem. 2004, 14, 1663–1678. [Google Scholar] [CrossRef]
- Jordan, F.; Naylor, A.; Kelly, C.A.; Howdle, S.M.; Lewis, A.; Illum, L. Sustained Release Hgh Microsphere Formulation Produced by a Novel Supercritical Fluid Technology: In Vivo Studies. J. Control. Release 2010, 141, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, A.; van Hooff, P.; Durrant, L.G.; Spendlove, I.; Howdle, S.M.; Woods, H.M.; Whitaker, M.J.; Davies, O.R.; Naylor, A.; Lewis, A.L.; et al. Single Shot Tetanus Vaccine Manufactured by a Supercritical Fluid Encapsulation Technology. Int. J. Pharm. 2011, 413, 147–154. [Google Scholar] [CrossRef]
- Whitaker, M.J.; Hao, J.Y.; Davies, O.R.; Serhatkulu, G.; Stolnik-Trenkic, S.; Howdle, S.M.; Shakesheff, K.M. The Production of Protein-Loaded Microparticles by Supercritical Fluid Enhanced Mixing and Spraying. J. Control. Release 2005, 101, 85–92. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Poloniae Pharm. 2010, 67, 217–223. [Google Scholar]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Nikolaos, A.P. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Ginty, P.J.; Whitaker, M.J.; Shakesheff, K.M.; Howdle, S.M. Drug Delivery Goes Supercritical. Mater. Today 2005, 8, 42–48. [Google Scholar] [CrossRef]
- Kilicarslan, M.; Baykara, T. The Effect of the Drug/Polymer Ratio on the Properties of the Verapamil HCl Loaded Microspheres. Int. J. Pharm. 2003, 252, 99–109. [Google Scholar] [CrossRef]
- Siepmann, J.; Elkharraz, K.; Siepmann, F.; Klose, D. How Autocatalysis Accelerates Drug Release from PLGA-Based Microparticles: A Quantitative Treatment. Biomacromolecules 2005, 6, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Mao, S.; Germershaus, O.; Schaper, A.; Rytting, E.; Chen, D.; Kissel, T. Influence of Morphology and Drug Distribution on the Release Process of Fitc-Dextran-Loaded Microspheres Prepared with Different Types of Plga. J. Microencapsul. 2009, 26, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Sah, H.K.; Toddywala, R.; Chien, Y.W. The Influence of Biodegradable Microcapsule Formulations on the Controlled-Release of a Protein. J. Control. Release 1994, 30, 201–211. [Google Scholar] [CrossRef]
- Rivera, P.A.; Martinez-Oharriz, M.C.; Rubio, M.; Irache, J.M.; Espuelas, S. Fluconazole Encapsulation in PLGA Microspheres by Spray-Drying. J. Microencapsul. 2004, 21, 203–211. [Google Scholar] [CrossRef]
- Le Corre, P.; Rytting, J.H.; Gajan, V.; Chevanne, F.; le Verge, R. In Vitro Controlled Release Kinetics of Local Anaesthetics from Poly(d,l-Lactide) and Poly(Lactide-co-Glycolide) Microspheres. J. Microencapsul. 1997, 14, 243–255. [Google Scholar] [CrossRef]
- Guney, O.; Akgerman, A. Synthesis of Controlled-Release Products in Supercritical Medium. Aiche J. 2002, 48, 856–866. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Hora, M.S.; Rana, R.K.; Nunberg, J.H.; Tice, T.R.; Gilley, R.M.; Hudson, M.E. Release of Human Serum-Albumin from Poly(Lactide-co-Glycolide) Microspheres. Pharm. Res. 1990, 7, 1190–1194. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. Control of Encapsulation Efficiency and Initial Burst in Polymeric Microparticle Systems. Arch Pharm. Res. 2004, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Pitt, C.G. The Acceleration of Degradation-Controlled Drug Delivery from Polyester Microspheres. J. Control. Release 1989, 8, 259–265. [Google Scholar] [CrossRef]
- D’Souza, S.; Faraj, J.A.; Dorati, R.; DeLuca, P.P. Enhanced Degradation of Lactide-co-Glycolide Polymer with Basic Nucleophilic Drugs. Adv. Pharm. 2015, 10. [Google Scholar] [CrossRef]
- Solorio, L.; Olear, A.M.; Hamilton, J.I.; Patel, R.B.; Beiswenger, A.C.; Wallace, J.E.; Zhou, H.Y.; Exner, A.A. Noninvasive Characterization of the Effect of Varying PLGA Molecular Weight Blends on in Situ Forming Implant Behavior Using Ultrasound Imaging. Theranostics 2012, 2, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling Properties of Purified Poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef] [PubMed]
- Csaba, N.; Caamano, P.; Sanchez, A.; Dominguez, F.; Alonso, M.J. PLGA:Poloxamer and PLGA:Poloxamine Blend Nanoparticles: New Carriers for Gene Delivery. Biomacromolecules 2005, 6, 271–278. [Google Scholar] [CrossRef]
- Kang, J.C.; Schwendeman, S.P. Pore Closing and Opening in Biodegradable Polymers and Their Effect on the Controlled Release of Proteins. Mol. Pharm. 2007, 4, 104–118. [Google Scholar] [CrossRef]
- Al-Tahami, K.; Singh, J. Smart Polymer Based Delivery Systems for Peptides and Proteins. Recent Patents Drug Deliv. Formul. 2007, 1, 65–71. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).