Abstract
There is increasing research interest in the ORF3 accessory protein of PEDV as a critical element for viral virulence. Here, wild type ORF3 (ORF3wt) gene was constructed in pEGFP-C1 vector. Additionally, two truncation mutants, ORF3-N (1-98 amino acids [aa]) and ORF3-C (99-224 aa) were inserted in the same vector. Results of ORF3 expression revealed early cytoplasmic localization but 12 h after transfection, ORF3 accumulated around the nucleus, especially ORF3-N. This caused chromosome condensation and morphological distortion that culminated in cell death. In comparison with the native cells expressing GFP alone, ORF3wt-induced lethality was 6.61% above baseline while ORF3- C expression resulted in moderate increase in cell death (0.64%). ORF3-N was affected the most with 220.32% increased lethality. It was, therefore, inferred that the ORF3 gene encodes a protein that causes nuclear damage, distorts cell morphology and leads to cell death. Furthermore, the role of the protein could be inherent in the N-terminal domain, which consists of the transmembrane domains. These findings underpin the importance of ORF3 gene expression in the host and are rudimental insights for further exploration into the mechanistic interactions of ORF3 and the host, as well as a possible role in pathogenesis in PEDV and other coronaviruses.
1. Introduction
Porcine epidemic diarrhea virus (PEDV) is the causative agent of a devastating swine enteric disease that presents with watery diarrhea, vomiting anorexia and severe dehydration among sows [1]. Since its early documentation in Europe, PEDV has spread to other parts of the globe with outbreak cases reported across many other countries in Southeast Asia as well as the Americas [2,3,4,5,6]. PEDV infects swine populations of all age groups with up to 100% mortality reported, especially among neonatal piglets, causing significant economic losses in the industry [7,8].
PEDV belongs to the coronaviridae family in the order Nidovirales [9]. Members of this group have a large, single-stranded RNA genome (approximately 28–30 kilobases) of positive polarity. The PEDV genome consists of five open reading frames (ORFs) downstream of ORF1, four of which code for structural proteins, including spike (S), membrane (M), small membrane (sM) and nucleocapsid (N) in order. The fifth is ORF3 located between S and M genes [10,11]. It is part of a group of unique small proteins encoded in coronaviruses termed as accessory proteins and believed to interfere with host immune response [12].
Adaptation of PEDV to culture results in mutations of the ORF3 gene. This has been associated with attenuation of the virus [13,14,15]. Additionally, mutations in ORF3 as well as spike genes resulting from increased passages contributed to reduced proliferation and pathogenesis of PEDV [16]. It has been demonstrated that the ORF3 gene of the prototype PEDV encodes an ion channel consisting of four transmembrane domains that are critical for virus production [17]. However, the laboratory strains are predicted to consist of transmembrane 1 and 2 between amino acids 40 and 98 but lack in transmembrane 3 and 4 [17,18]. This ORF3 protein aggregates in the cytoplasm, particularly in the endoplasmic reticulum (ER), inducing autophagy through endoplasmic stress [19]. Of note, ORF3-protein prolongs the S-phase of a mitotic cell [20].
Despite increasing evidence that implicates the ORF3 protein in virulence of PEDV, Kristen–Burmann et al. [21] suggest that ORF3 importance in pathogenicity is modest. To address such gaps in the knowledge of the ORF3 protein, there is a need to conduct continued investigations of the gene as well as the encoded protein. The current study reports critical cellular observations made in PEDV-ORF3wt/N/C-expressing cells. The analysis sheds light into the possible interactions of the ORF3 protein with the host cell.
2. Materials and Methods
2.1. Cell Culture
Mycoplasma-free cell-lines obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) were employed in the study. These included African green monkey kidney cells, Vero (ATCC CCL-81), human embryonic kidney (HEK) 293T/17cells (ATCC CRL-11268), and mouse embryonic fibroblasts, NIH/3T3 (ATCC CRL-1658). Cell culture media and supplements were sourced from Thermo Fisher Scientific, Waltham, MA, USA. Cell culture protocols were described by ATCC with modifications. Briefly, minimum essential medium (MEM) supplemented with 5% of fetal bovine serum (FBS) and antibiotics (100 U penicillin and 100 µg streptomycin per milliliter, (Invitrogen) was used to culture Vero cells. For 293 T and NIH 3T3 cells, Dubbelco’s minimum essential medium with the above supplements was used. All cells were maintained at 37 °C, 5% CO2.
For plasmid cloning, Escherichia coli (E. coli) DH5 alpha cells were sourced (Invitrogen, CA, USA). Yeast extract and tryprone for bacteria media preparation were purchased from Intron Biotechnology.
2.2. Propagation of Cell-Adapted Pedv (Kpedv-9 Strain)
The cell-adapted strain of the Korean PEDV isolate, KPEDV-9, was maintained in the infectious diseases laboratory at Chungnam National University. For the purposes of obtaining the ORF3 gene, the virus was cultured in Vero cells as described, with some modifications [22]. Briefly, KPEDV-9 was inoculated into confluent Vero cells at a M.O.I. ≥ 1 and maintained at 37 °C, 5% CO2 for 1 h. Inoculum was removed and cultured in serum-free MEM at 37 °C, 5% CO2 for 48 h. The supernatant was harvested and stored at −70 °C. Progeny virions trapped in intracellular vesicles were released by repeated freeze-thawing. This was then centrifuged at 2000× g for 10 min at 4 °C and the cell debri free supernatant was collected. Virus concentration and partial purification were performed by ultracentrifugation under a 20% sucrose cushion at 28,000 rpm for 3.5 h. The pellet was re-suspended in 10 mM phosphate-buffered saline (PBS, pH 7.4) and stored at –70 °C.
2.3. ORF3 cDNA Synthesis
PEDV genomic RNA was extracted using the Ribo_spin vRDTM kit following manufacturer’s instructions (GeneAll Biotechnology, Seoul, Republic of Korea). Reverse transcription ensued using SuperScriptTM III First-Strand Synthesis System as directed (Thermo Fisher Scientific, Waltham, MA, USA). Briefly, RNA was incubated together with Oligo (dT) primers and assorted deoxyribonuleotide triphosphates for 5 min at 65 °C to denature secondary structures. Next, reverse transcription of the first strand template was performed at 50 °C for 50 min before terminating the reaction at 85 °C for 5 min. Finally, reaction was chilled on ice before RNase treatment at 37 °C for 20 min.
2.4. Polymerase Chain Reaction (PCR)
ORF3, membrane and nuleocapsid genes were each amplified by PCR in a 50 μL mixture containing 100 ng of PEDV cDNA, gene specific primers of 1 μL (10 pM) each (Table 1), 2.5 μL (10 mM) of dNTPs, 5 μL of 10× PCR buffer (100 mM Tris–HCl (pH 8.8 at 25 °C), and 2.5 U of native pfu DNA polymerase enzyme (Solgent, Daejeon, Republic of Korea). The reaction was carried out using the following reaction cycles (Biometra TPersonal, Gottingen, Germany): initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation, and primer annealing and fragment sunthesis at 72 °C. The amplification product was analyzed by electrophoresis on 1% of ethidium bromide-stained agarose gel.

Table 1.
Primer sequences used for amplification of various PEDV genes and fragments.
2.5. Plasmid Constructs
ORF3, membrane, and nucleocapsid gene sequence were inserted into 5′ Bgl II/3′ Kpn I sites of pEGFP-C1 (Clonetech, CA, USA). The membrane and nucleocapsid proteins were used for comparative analysis of expression patterns against ORF3. Furthermore, the ORF3 gene was truncated following nucleotide analysis by the Simple Modular Architecture Research Tool (SMART) [23,24] as previously described [18]. Briefly, ORF3 was truncated into two fragments: N-term consisting of first 98 amino acids, (aa), and C-term, aa 99-224. In this model, two predicted transmembrane domains were in ORF3-N while ORF3-C had no transmembrane region. The sequences were designated as ORF3wt (wild type), ORF3-N (N-terminal domain) and ORF3-C (C-terminal domain). These mutants were amplified by PCR using fragment specific primers (Table 1) and cloned into Bgl II and Kpn I sites of pEGFP-C1 for expression.
2.6. Sequencing
All PCR-amplified sequences were verified by sequencing (Solgent, Daejeon, Republic of Korea) using universal pEGFP-C1 primers (Table 2).

Table 2.
Primer sequences used for sequencing of ORF3wt and ORF3-C/ORF3-N.
2.7. Expression of Orf3/Mutants, Membrane and Nucleocapsid
ORF3wt, ORF3-N and ORF3-C/pEGFP-C1 constructs were separately transfected in HEK-293T as well as NIH 3T3 cells. Expression of ORF3/mutant proteins was analyzed alongside GFP in an empty vector as a reference for transfection efficiency. Both HEK-293T and NIH 3T3 cell types were seeded in a six-well plate at a density of 2.5 × 105/mL of the medium and incubated for 24 h at 37 °C and 5% CO2. The pEGFP-C1 empty vector (3 µg) as well as constructs containing ORF3wt, ORF3-N, ORF3-C, membrane or nucleocapsid were separately transfected into HEK-293T cells by the calcium phosphate method as described [25]. For NIH3T3 cells, ORF3wt/mutant constructs and pEGFP-C1 empty vector were independently lipo-transfected using Fugene HD (Roche, Basel, Switzerland), following the manufacturer’s instructions. Analysis of expression was performed in a time range of 6 h to 24 h post-transfection and visualized by GFP florescence at X40 microscopic magnification (Leica DM IL, Wetzlar, Germany).
2.8. Dapi Staining
Investigations of ORF3 impact on cellular DNA were performed by DAPI staining (Sigma-Aldrich, Saint Louis, MO, USA) as described [26]. Briefly, at 6, 12, 24, 36 and 42 h post-transfection, the cells were washed with 1× PBS and fixed with 5% of formaldehyde for 10 min. Cells were washed with 1× PBS and permeabilized with 0.1% Nonidet P-40 for 10 min. Cells were incubated with DAPI dissolved in 1× PBS at a final concentration of 1 µg/mL for 10 min in the dark. All above procedures were carried out at room temperature. Visualization was by florescence microscopy (Leica DM IL).
2.9. Cell Cytotoxicity Assay
Expression of ORF3wt and mutants caused cell death. Cell lethality was evaluated by three replicate counts of dead cells floating in media using a haemocytometer (Abcam, Cambridge, England) without staining. The metabolic activities of ORF3wt/mutant-transfected cells were evaluated by MTT (3-[4,5-dimethythiazol-2-yl]-2,5-diphenyl tetrazolium bromide) reduction assay using the commercial Cyto XTM kit (LPS Solution, Daejeon, Republic of Korea). HEK-293T cells were seeded (2.5 × 105 cells/mL) in a six-well plate and transfected after 24 h with 3 µg of empty vector or pEGFP-C1 containing inserts of ORF3wt, ORF3-N or ORF3-C. At 24 h post-transfection, the cells were trypsin-treated and seeded at 10,000 per well density in a 96-well plate then incubated at 37 °C in 5% of CO2. Metabolic activity assays were carried out in a time range of 30 to 60 h. Briefly, cells were treated with 10 µL of cyto X solution for 4 h, after which optical density values were taken at 450 nm (Tecan Sunrise Remote, Mannedorf, Switzerland). As the positive control, cells were treated with 50 mM of hydrogen peroxide (H2O2), to induce oxidative stress, for 4 h. while the native cells (negative control) were treated with the transfection reagent alone.
3. Results
3.1. Orf3 Protein Localization Shifts from the Cytoplasm to the Nucleus
Florescence microscopy of HEK-293T cells expressing GFP alone or in fusion with ORF3wt or its truncated mutants showed that the ORF3wt/ORF3-N/ORF3-C proteins had initial expression in the cytosol (6 h post-transfection). However, ORF3wt and ORF3-N began to aggregate around the nucleus as observed from 12 h of transfection and continued afterwards. Similarly, ORF3-C demonstrated nuclear localization but to a lesser extent. On the other hand, GFP protein expressed and remained in the cytoplasm (Figure 1).
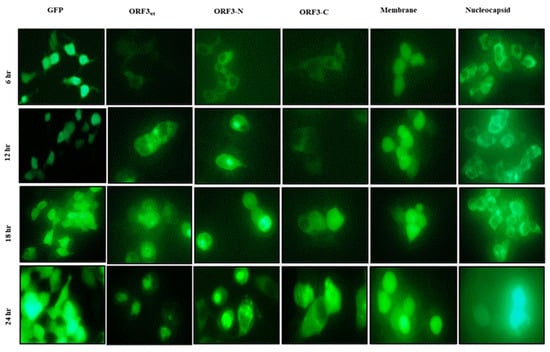
Figure 1.
U.V. microscopy of HEK-293T cells. HEK-293T cells expressing GFP protein alone or GFP fused with ORF3wt/ ORF3-N/ORF3-C/ membrane/nucleocapsid proteins. GFP protein alone was examined at ×200 magnification due to high expression efficiency and glow. The PEDV proteins were analyzed at ×400 magnification. Early (6 h) cytoplasmic localization of ORF3/ ORF3-N proteins was observed in the cytoplasm but at 12 h, these proteins exhibited accumulation around the nucleus, proceeding to aggregate in the nucleus at 24 h. ORF3-C had relatively lower nuclear accumulation relative to ORF3wt and ORF3-N. The GFP, membrane and nucleocapsid proteins were observed in the cytosol with no shift to the nucleus.
3.2. Orf3 Protein Causes Nuclear Condensation and Morphological Distortion
Analyses of expression of the various ORF3 proteins by U.V. microscopy revealed that ORF3 reduced the expression efficiency of GFP. Furthermore, ORF3/mutant-expressing cells had distorted morphology (Figure 2A). Analyses of DAPI-stained HEK-293T and NIH 3T3 cells revealed ORF3wt and that its truncated mutants condensed the nucleus following accumulation of these proteins in the nucleus. Merging of U.V. and DAPI images suggested that ORF3 proteins had sequestered the nuclei of host cells. Furthermore, the nuclear condensation was associated with cell rounding and shrinking. From morphological observation of the cells, ORF3-N exhibited more cellular damage than ORF3wt and ORF3-C in order, especially apparent in the HEK-293T cells. While GFP alone also appeared to be present in the nucleus, no adverse effect was observed on the nuclei or morphology of the cells (Figure 2 and Figure 3).
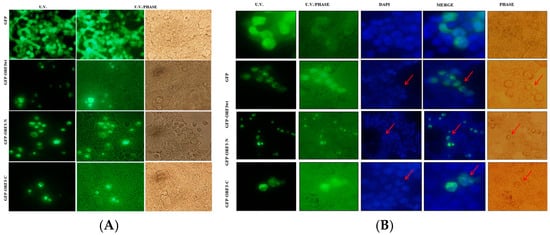
Figure 2.
ORF3 expression causes morphological and nucleus distortion. Microscope images were taken at ×200 magnification. In (A), about 90% of HEK-293T live cells expressing GFP alone had a normal morphology. Transfection with ORF3wt/ORF3-N/ORF3-C-fused GFP proteins had reduced transfection efficiency. The expression of ORF3 or truncated mutants resulted in shape distortion (rounding). ORF3-C effect on cells was low compared to the ORF3 and wild type. In (B), fixed DAPI-stained HEK-293T cells expressing GFP protein alone or GFP fused with ORF3wt/ ORF3-N/ORF3-C proteins were analyzed. U.V. microscopy revealed GFP florescence; ORF3wt/ORF3-N/ORF3-C-fused GFP proteins exhibited nuclear localization. DAPI stain revealed nuclei condensation in GFP-ORF3/mutant-expressing cells but not in cells expressing GFP alone. The merged images show that ORF3/mutant proteins sequester the nucleic material. The U.V./phase and phase images together reveal that the GFP-fluorescing cells are morphologically distorted (rounding) where fused with ORF3wt/ORF3-N/ORF3-C but not in GFP alone. However, ORF3-C causes lesser distortion in comparison with ORF3wt and ORF3-N. Arrows point to the condensed nuclei (DAPI) or rounded cells (phase). GFP control also appears to be present in the nucleus but the DAPI-stained nucleus remained well rounded and no morphology anomalies were observed in phase images.
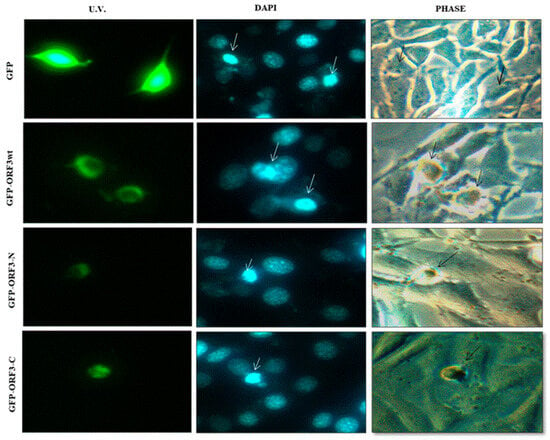
Figure 3.
DAPI stained NIH 3T3. Fixed DAPI stained NIH 3T3 cells expressing GFP protein alone or GFP fused with ORF3wt/ ORF3-N/ORF3-C proteins. U.V. microscopy reveals GFP florescence in the cytoplasm; GFP-ORF3wt/ORF3-N/ORF3-C exhibits nuclear localization. DAPI stain reveals nucleic appearance in GFP-ORF3/mutant-expressing cells comparable to GFP-expressing cells (shown by white arrows). ORF3wt / ORF3-N and ORF3-C expression has associated morphological distortion evidenced in the phase images (shown by black arrows). NIH 3T3 cells expressing GFP alone are not morphologically distorted.
3.3. Orf3 Proteins Induced Cell Mortality in Expressed Cells
After 24 h post-transfection of the HEK-293T cells, fluorescence microscopy revealed that some ORFwt, ORF3-N and ORF3-C-expressing cells were detached from substratum, indicating death. The floating cells were still fluorescing green. In contrast, cell death did not occur with the expression of GFP alone. Similarly to observations on cell damage, ORF3-N was associated with the most cell lethality followed by ORFwt and ORF3-C in order. The mean number of dead cells was determined at about 2.46 × 104 for HEK-293T/ORFwt, 7.38 × 104 for HEK-293T/ORF3-N, and 2.3 × 104 for HEK-293T cells alone as well as HEK-293T/GFP and HEK-293T/ORF3-C cells. Relative to the native cells, ORF3wt increased cell death by 6.61%, ORF3-N caused 220.32% increased lethality, and ORF3-C had 0.64% more dead cells (Figure 4).
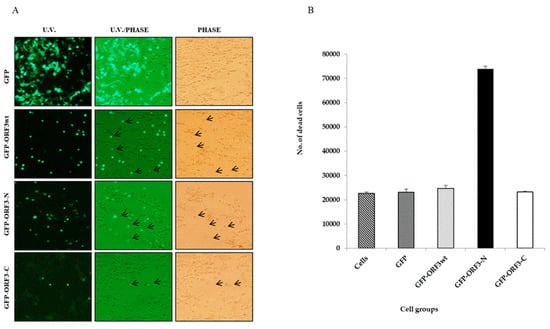
Figure 4.
ORF3 expression has associated cell lethality. (A). Observation of GFP- and GFP-tagged ORF3wt or mutants under U.V. microscope. U.V. and light together (U.V./light) and under light microscope (phase). Green flourescing cells were observed floating in media of ORF3wt and ORF3-N-expressing HEK-293T cells. Arrows point to floating cells which were considered as dead. (B). Value of means of counted dead cells from each cell group. Cell counting was performed twice per cell group using a hemocytometer and reported as mean ± SD (standard deviation). Results of ORF3-C-expressing cells were comparable to GFP alone but the detached cells were increased where ORF3wt or ORF3-N was expressed with ORF3-N causing the greatest impact to cells.
3.4. Orf3 Down-Regulated Cell Metabolism
Analyses of mitochondrial succinate dehydrogenase by cell treatment with a reducible chromogenic substrate, MTT, showed that the O.D450 values were relatively lower in GFP-fused ORF3wt, ORF3-N or ORF3-C-expressing cells than those expressing GFP only, or native cells. In reference to the native cells, at 30 h of expression, ORF3wt had 15% decreased metabolic activity, ORF3-N decreased by 11%, and ORF3-C was lower by 14%. At 48 h, cellular metabolism was determined to be 54% for ORF3wt-expressing cells, 56% for ORF3-N, and 68% for ORF3-C. At 60 h, the metabolic activities recorded were 59%, 64% and 81% for ORF3wt, ORF3-N and ORF3-C-expressing cells, respectively. (Figure 5).
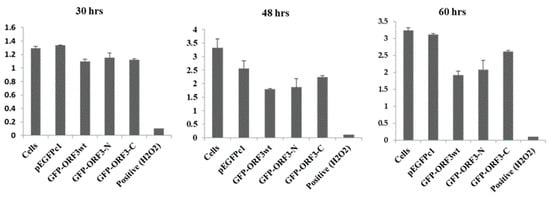
Figure 5.
Quantitative activities of mitochondrial succinate dehydroagenase. MTT assay of 293T cells treated with 50 mM of H2O2 for 4 h (positive); GFP, GFP/ORF3wt, ORF3-N or ORF3-C transfections and O.D.450 values determined at the indicated time points. Cells were treated with transfection reagents without DNA for negative control. Expression of OFR3 proteins negatively impacted on host cell metabolism; ORF3wt had the greatest impact (44% average reduction) followed by ORF3-N (average 30%) and ORF3-C (average 22%). The positive control was for the validation of the MTT assay. The values are reported as means of three replicates ± SD.
4. Discussion
The product encoded by the ORF3 gene of PEDV has been speculated to be important for pathogenicity [12]. We have previously reported that the ORF3 protein suffers host inhibition [18], as independently observed [27]. On the contrary, PEDV structural genes of membrane and nucleocapsid expressed the respective proteins readily. HEK-293T cells expressing membrane, nucleocapsid, ORF3 or its truncation mutants revealed cytoplasmic localization of the PEDV proteins. This was consistent with other independent investigators [19]. However, unlike membrane and nucleocapsid, ORF3/mutant proteins’ cytosolic expression was transitory with observations of aggregation in the nucleus as from 12 h onwards.
Microscopic examination of DAPI-stained DNA demonstrated nuclear condensation and disintegration. Though merged images of the green florescent and blue DAPI-stained images did not show complete overlap of the two, the blue and green co-appeared within the nuclear space. This led to a postulation that the ORF3 protein may actively interact with nuclear membrane. Some members of rhabdoviruses can exemplify this imagination albeit in reverse manner. These nucleorhabdoviruses display a nucleocapsid-coated granular matrix, which buds at the inner nuclear membrane, and accumulate in the perinuclear space [28]. It is known that the PEDV-ORF3 protein traffics to and accumulates in the Golgi area via the exocytic pathway. Moreover, the C-terminal consists of an essential motif for transport from the ER without which the ORF3 protein is retained in the ER as well as ER-to-Golgi intermediate compartment [29]. This could explain our observations of ORF3 translocation from the cytoplasm and accumulation around the nucleus. Furthermore, proteomic analyses have demonstrated that the PEDV-ORF3 protein interacts with host proteins mainly in the endo-lysosomal organelles with consequent interference of immune signaling [30,31].
On the other hand, GFP also appeared in the nucleus. Indeed, GFP has been shown to escape nuclear membrane blockage due to its small size (27 kDa). The nuclear pore complex facilitates entry of small molecules into the nucleus and GFP has been found to diffuse bi-directionally via this portal [32]. However, GFP was shown to neither affect the function of co-expressed proteins nor cell viability by Marshall et al. [33]. Other independent investigators documented the suitability of GFP fusion proteins in the study of subcellular localization as well as transient expression assays, revealing that the larger molecules could not penetrate the nuclear envelop by simple diffusion. In particular, the GFP-DETI protein had limited nuclear localization, demonstrating that GFP cannot transport a fusion protein to the nucleus. The nuclear localization of GFP-fused proteins is only possible by active or facilitated transport. As such, GFP-COP1 localized to discrete subnuclear particles and the GFP did not affect the function of COP1 consistent with Marshall et al. [34].
Although PEDV-ORF3 protein has been reported to have a limited role in pathogenesis [21], our findings suggest the contrary. ORF3wt and ORF3-N, in particular, caused nuclear condensation, morphological distortion and cell death. Though a cell count may not discriminate a viable floating cell from a dead one, the marked difference in the count in ORF3wt and ORF3-N should be of curious interest. Similarly, ORF3 protein was reported to cause endoplasmic stress that resulted in the disruption of ER and cell membrane, leading to cell death [19]. While nuclear condensation is likely to occur from apoptosis, Zou et al. [19] demonstrated that the ORF3-protein does not trigger apoptosis. Thus, ORF3-protein perceivably causes necrotic cell death, which could be critical for causing intestinal lesions.
To investigate the probable cause of cell death, metabolic activity was assayed. ORF3wt and ORF3-N in particular down-regulated host metabolism, and caused nuclear condensation, morphological distortion and cell death. Similarly, ORF3 protein was independently reported to cause endoplasmic stress that resulted in the disruption of ER and cell membrane, leading to cell death [19]. While nuclear condensation is likely to occur from apoptosis, Zou et al. [19] demonstrated that ORF3-protein does not trigger apoptosis. Thus, ORF3-protein perceivably causes necrotic cell death, which could be critical for causing intestinal lesions. It is not clear how ORF3-protein down-regulated metabolism but with endoplasmic stress, cell physiology could be sub-optimized.
5. Conclusions
It is noteworthy that while our findings attribute importance to the N-terminal domain, and the circulating ORF3 gene is shown to have four transmembrane domains [17]. These together form an important ion channel. Transmembrane 3 and 4 occur in the C-terminal of our model, and are lost upon culture adaptation, reducing ion channel activity and the pathogenicity of the virus. Our results attribute the ORF3 impact to the transmembrane domains in ORF3-N. The impact of ORF3wt which invariably has the transmembrane was not as high. This can be explained by inhibited expression of the whole gene (18) rather than diverged pattern of function. Taken together, we conclude that the function of ORF3-protein is inherent in its transmembrane domains and that all the four transmembrane domains are critical for activity and virulence.
Author Contributions
Conceptualization, H.-J.S. and J.-R.R.; methodology, N.A.K., J.-Y.Y. and E.-S.P.; software, N.A.K. and J.-Y.Y.; validation, H.-J.S. and J.-R.R.; formal analysis, H.-J.S., G.F. and J.-R.R.; investigation, N.A.K., J.-Y.Y. and E.-S.P.; resources, H.-J.S. and J.-R.R.; data curation, N.A.K., J.-E.Y. and J.-Y.Y.; laboratory analysis, N.A.K., E.-S.P., J.-Y.Y. and J.-R.R.; writing—original draft preparation, N.A.K.; writing—review and editing, N.A.K., H.-J.S.,G.F. and J.-R.R.; visualization, N.A.K., J.-Y.Y. and E.-S.P.; supervision, H.-J.S. and J.-R.R.; project administration, H.-J.S. and J.-R.R.; funding acquisition, H.-J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, RS-2023-NR076878.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be provided on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pensaert, M.B.; de Bouck, P. A New Coronavirus-like Particle Associated with Diarrhea in Swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; He, Q. New Variants of Porcine Epidemic Diarrhea Virus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1350–1353. [Google Scholar] [CrossRef]
- Kweon, C.H.; Kwon, B.J.; Jung, T.S.; Kee, Y.J.; Hur, D.H.; Hwang, E.K.; An, S.H. Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Korean J. Vet. Res. 1993, 33, 249–254. [Google Scholar]
- Song, D.; Park, B. Porcine epidemic diarrhoea virus: A comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 2012, 44, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Raizman, E.; Snider, T.; Goyal, S.; Torremorell, M.; Perez, A.M. Emergence of Porcine Epidemic Diarrhoea in North America. 2014. Focus On, 9. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/07414a27-7f92-4cc7-86fb-fc0ef7332d5e/content (accessed on 15 March 2022).
- Russell, L.E.; Polo, J.; Meeker, D. The Canadian 2014 porcine epidemic diarrhoea virus outbreak: Important risk factors that were not considered in the epidemiological investigation could change the conclusions. Transbound. Emerg. Dis. 2020, 67, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.L.; Yu, L.Y.; Liu, J. Development and evaluation of enzyme-linked immunosorbent assay based on recombinant nucleocapsid protein for detection of porcine epidemic diarrhea (PEDV) antibodies. Vet. Mic. 2007, 123, 86–92. [Google Scholar] [CrossRef]
- Antas, M.; Woźniakowski, G. Current status of porcine epidemic diarrhoea (PED) in European pigs. J. Vet. Res. 2019, 63, 465–470. [Google Scholar] [CrossRef]
- Cavanagh, D. Nidovirales: A new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997, 142, 629–633. [Google Scholar]
- Bridgen, A.; Kocherhans, R.; Tobler, K.; Carvajal, A.; Ackermann, M. Further analysis of the genome of porcine epidemic diarrhoea virus. In Coronaviruses Arteriviruses; Springer: Boston, MA, USA, 1998; pp. 781–786. [Google Scholar] [CrossRef]
- Kocherhans, R.; Bridgen, A.; Ackermann, M.; Tobler, K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 2001, 23, 137–144. [Google Scholar] [CrossRef]
- Brian, D.A.; Baric, R.S. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 2005, 287, 1–30. [Google Scholar] [CrossRef]
- Bernasconi, C.; Guscetti, F.; Utiger, A.; Van Reeth, K.; Ackermann, M.; Pospischil, A. Experimental infections of gnotobiotic piglets with a cell culture adapted porcine epidemic diarrhoea virus: Clinical, histopathological and immunohistochemical findings. In Immunobiology of Viral Infections; Schwyzer, M., Ackermann, M., Eds.; Fondation Marcel Merieux: Lyon, France, 1995; pp. 542–546. [Google Scholar]
- Song, D.S.; Yang, J.S.; Oh, J.S.; Han, J.H.; Park, B.K. Differentiation of a Vero Cell Adapted Porcine Epidemic Diarrhea Virus from Korean Field Strains by Restriction Fragment Length Polymorphism Analysis of ORF 3. Vaccine 2003, 21, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Moon, H.J.; Luo, Y.; Kim, H.K.; Kim, E.M.; Yang, J.S.; Song, D.S.; Kang, B.K.; Lee, C.S.; Park, B.K. Cloning and Further Sequence Analysis of the ORF3 Gene of Wild-and Attenuated-Type Porcine Epidemic Diarrhea Viruses. Virus Genes 2008, 36, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Jermsutjarit, P.; Mebumroong, S.; Watcharavongtip, P.; Lin, H.; Tantituvanont, A.; Kaeoket, K.; Piñeyro, P.; Nilubol, D. Evolution and virulence of porcine epidemic diarrhea virus following in vitro and in vivo propagation. Sci. Rep. 2024, 14, 12279. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lu, W.; Chen, J.; Xie, S.; Shi, H.; Hsu, H.; Yu, W.; Xu, K.; Bian, C.; Fischer, W.B.; et al. PEDV ORF3 Encodes an Ion Channel Protein and Regulates Virus Production. FEBS Lett. 2012, 586, 384–391. [Google Scholar] [CrossRef]
- Kamau, N.; Yu, J.; Park, E.; Rho, J.; Hong, E.; Shin, H. Strenuous expression of porcine epidemic diarrhea virus ORF3 protein suggests host resistance. Vet. Mic. 2024, 110193. [Google Scholar] [CrossRef]
- Zou, D.; Xu, J.; Duan, X.; Xu, X.; Li, P.; Cheng, L.; Zheng, L.; Li, X.; Zhang, Y.; Wang, X.; et al. Porcine Epidemic Diarrhea Virus ORF3 Protein Causes Endoplasmic Reticulum Stress to Facilitate Autophagy. Vet. Microbiol. 2019, 235, 209–219. [Google Scholar] [CrossRef]
- Ye, S.; Li, Z.; Chen, F.; Li, W.; Guo, X.; Hu, H.; He, Q. Porcine epidemic diarrhea virus ORF3 gene prolongs S-phase, facilitates formation of vesicles and promotes the proliferation of attenuated PEDV. Virus Genes 2015, 51, 385–392. [Google Scholar] [CrossRef]
- Kristen-Burmann, C.; Rogger, P.; Veiga, I.B.; Riebesehl, S.; Rappe, J.; Ebert, N.; Sautter, C.A.; Kelly, J.N.; Stalder, H.; Ehmann, R.; et al. Reverse genetic assessment of the roles played by the spike protein and ORF3 in porcine epidemic diarrhea virus pathogenicity. J. Virol. 2023, 97, e01964-22. [Google Scholar] [CrossRef]
- Hofmann, M.; Wyler, R. Propagation of the Virus of Porcine Epidemic Diarrhea in Cell Culture. J. Clin. Microbiol. 1988, 26, 2235–2239. [Google Scholar] [CrossRef]
- Schultz, J.; Milpetz, F.; Bork, P.; Ponting, C.P. SMART, a Simple Modular Architecture Research Tool: Identification of Signaling Domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5857–5864. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent Updates to the Protein Domain Annotation Resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef]
- Graham, F.L.; van der Eb, A.J. A New Technique for the Assay of Infectivity of Human Adenovirus 5 DNA. Virology 1973, 52, 456–467. [Google Scholar] [CrossRef]
- Kapuscinski, J. DAPI: A DNA-specific fluorescent probe. Biotech. Histochem. 1995, 70, 220–233. [Google Scholar] [CrossRef]
- Schmitz, A.; Tobler, K.; Suter, M.; Ackermann, M. Prokaryotic Expression of Porcine Epidemic Diarrhoea Virus ORF3. Adv. Exp. Med. Biol. 1998, 440, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, A.; Lohuis, D.; Dijkstra, J.; Peters, D. Morphogenesis of sonchus yellow net virus in cowpea protoplasts. J. Ultrastruct. Res. 1985, 90, 294–303. [Google Scholar] [CrossRef]
- Si, F.; Chen, B.; Hu, X.; Yu, R.; Dong, S.; Wang, R.; Li, Z. Porcine epidemic diarrhea virus ORF3 protein is transported through the exocytic pathway. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Kaewborisuth, C.; Yingchutrakul, Y.; Roytrakul, S.; Jongkaewwattana, A. Porcine epidemic diarrhea virus (PEDV) ORF3 interactome reveals inhibition of virus replication by cellular VPS36 protein. Viruses 2019, 11, 382. [Google Scholar] [CrossRef]
- Kaewborisuth, C.; Koonpaew, S.; Srisutthisamphan, K.; Viriyakitkosol, R.; Jaru-Ampornpan, P.; Jongkaewwattana, A. PEDV ORF3 independently regulates IκB kinase β-mediated NF-κB and IFN-β promoter activities. Pathogens 2020, 9, 376. [Google Scholar] [CrossRef]
- Wei, X.; Henke, G.; Strübing, C.; Brown, B.; Clapham, E. Real-time imaging of nuclear permeation by EGFP in single intact cells. Biophys. J. 2003, 84, 1317–1327. [Google Scholar] [CrossRef]
- Marshall, J.; Molloy, R.; Moss, W.; Howe, R.; Hughes, E. The jellyfish green fluorescent protein: A new tool for studying ion channel expression and function. Neuron 1995, 14, 211–215. [Google Scholar] [CrossRef]
- Von Arnim, G.; Deng, W.; Stacey, G. Cloning vectors for the expression of green fluorescent protein fusion proteins in transgenic plants. Gene 1998, 221, 35–43. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).