Trends in Clinico-Epidemiological Profile and Outcomes of Patients with HIV-Associated Cryptococcal Meningitis in Shanghai, China, 2013–2023
Abstract
1. Introduction
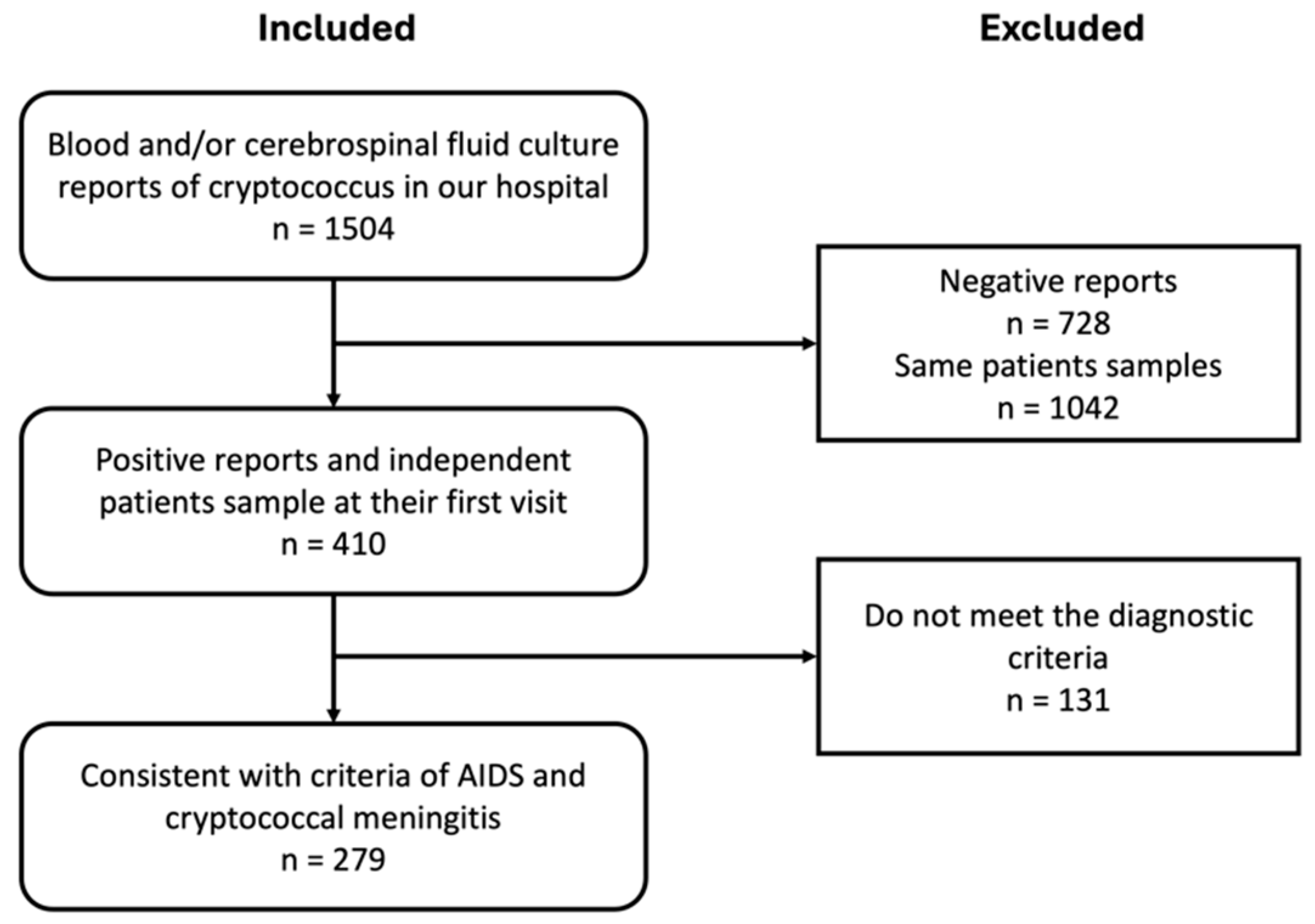
2. Materials and Methods
2.1. Study Subjects
2.2. Information Gathering
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
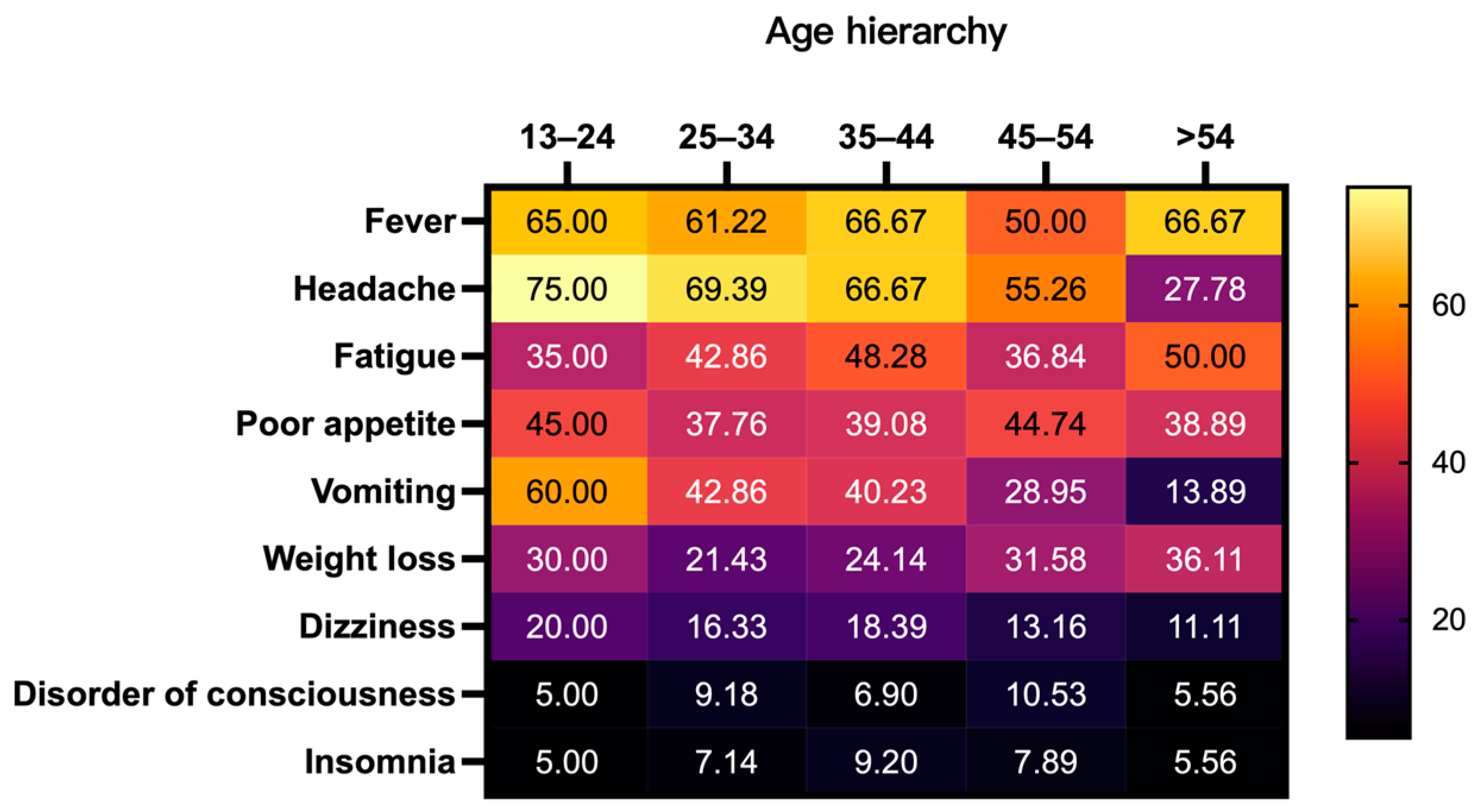
3.2. Initial Clinical Presentation of CM Patients
3.3. Univariate Analysis of Prognosis Factors
3.4. Multivariate Logistic Regression Analysis of Prognostic Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The Global Burden of HIV-Associated Cryptococcal Infection in Adults in 2020: A Modelling Analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [PubMed]
- Julien, C.; Heath, C.H.; Roberts, M.B.; Lane, R.J.; Spelman, T.; Smibert, O.C.; Longhitano, A.; Morrissey, O.; Nield, B.; Tripathy, M.; et al. Current Epidemiology and Clinical Features of Cryptococcus Infection in Patients without Human Immunodeficiency Virus: A Multicenter Study in 46 Hospitals in Australia and New Zealand. Clin. Infect. Dis. 2023, 77, 976–986. [Google Scholar]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 10 January 2024).
- Liu, J.; Dong, R.; Zhang, H.; Yao, S.; Liu, J.; Yang, L.; Fan, L.; Su, X.; Wang, A.; Su, Z.; et al. Clinical Characteristics, Treatment, and Outcome of Low-Risk Non-HIV-Associated Cryptococcal Meningitis: A Retrospective Cohort Study. Med. Mycol. 2023, 61, myad122. [Google Scholar]
- Liu, Y.; Kang, M.; Wu, S.-Y.; Ma, Y.; Chen, Z.-X.; Xie, Y.; Tang, J.-T. Different Characteristics of Cryptococcal Meningitis between Hiv-Infected and HIV-Uninfected Patients in the Southwest of China. Med. Mycol. 2016, 55, 255–261. [Google Scholar]
- Su, X.; Li, W.; Liu, J.; Wang, Y.; Liu, J.; Xu, X.; Yang, L.; Xia, H.; Jiang, Y.; Peng, F. Comparison of Features and Outcomes between HIV-Negative Patients with Cryptococcus Gattii Meningitis and Cryptococcus Neoformans Meningitis in South China. Mycoses 2022, 65, 887–896. [Google Scholar]
- Li, Y.; Zou, M.; Yin, J.; Liu, Z.; Lu, B. Microbiological, Epidemiological, and Clinical Characteristics of Patients with Cryptococcal Meningitis at a Tertiary Hospital in China: A 6-Year Retrospective Analysis. Front. Microbiol. 2020, 11, 1837. [Google Scholar]
- Tugume, L.; Rhein, J.; Hullsiek, K.H.; Mpoza, E.; Kiggundu, R.; Ssebambulidde, K.; Schutz, C.; Taseera, K.; Williams, A.D.; Abassi, M.; et al. HIV-Associated Cryptococcal Meningitis Occurring at Relatively Higher Cd4 Counts. J. Infect. Dis. 2018, 219, 877–883. [Google Scholar]
- Chen, M.; Xu, N.; Xu, J. Cryptococcus Neoformans Meningitis Cases among China’s HIV-Infected Population May Have Been Severely under-Reported. Mycopathologia 2020, 185, 971–974. [Google Scholar] [PubMed]
- Acquired Immunodeficiency Syndrome and Hepatitis C Professional Group, Society of Infectious Diseases, and Chinese Medical Association; Chinese Center for Disease Control and Prevention. Chinese Guidelines for Diagnosis and Treatment of Human Immunodeficiency Virus Infection/Acquired Immunodeficiency Syndrome (2024 Edition). Chin. J. Infect. Dis. 2024, E01–E48. Available online: https://rs.yiigle.com/cmaid/1501644 (accessed on 22 July 2024).
- Centers for Disease Control and Prevention. HIV Surveillance Report: Diagnoses of HIV Infection in the United States and Dependent Areas. 2021. Available online: https://stacks.cdc.gov/view/cdc/149071/cdc_149071_DS1.pdf (accessed on 12 December 2023).
- Clinical and Laboratory Standards Institute (CLSI). Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 4th ed.; CLSI standard M27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global Burden of Disease of HIV-Associated Cryptococcal Meningitis: An Updated Analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar]
- Zhu, L.-P.; Wu, J.-Q.; Xu, B.; Ou, X.-T.; Zhang, Q.-Q.; Weng, X.-H. Cryptococcal Meningitis in Non-HIV-Infected Patients in a Chinese Tertiary Care Hospital, 1997–2007. Med. Mycol. 2010, 48, 570–579. [Google Scholar]
- Tugume, L.; Sebambulidde, K.; Kasibante, J.; Ellis, J.; Wake, R.M.; Gakuru, J.; Lawrence, D.S.; Abassi, M.; Rajasingham, R.; Meya, D.B.; et al. Cryptococcal Meningitis. Nat. Rev. Dis. Primers 2023, 9, 62. [Google Scholar]
- Williamson, P.R.; Joseph, N.; Jarvis, A.A.; Panackal, M.C.; Fisher, S.F.; Molloy, A.L.; Thomas, S. Harrison. Cryptococcal Meningitis: Epidemiology, Immunology, Diagnosis and Therapy. Nat. Rev. Neurol. 2017, 13, 13–24. [Google Scholar] [PubMed]
- Chang, C.C.; Thomas, S.; Harrison, T.A.; Bicanic, M.C.; Tania, C.S.; Adilia, W.; Ferry, H.; Andrej, S.; Rita, O.; Nelesh, P.; et al. Global Guideline for the Diagnosis and Management of Cryptococcosis: An Initiative of the Ecmm and Isham in Cooperation with the Asm. Lancet Infect. Dis. 2024. [Google Scholar]
- Liao, C.-H.; Chi, C.-Y.; Wang, Y.-J.; Tseng, S.W.; Chou, C.-H.; Ho, C.M.; Lin, P.-C.; Ho, M.W.; Wang, J.H. Different Presentations and Outcomes between HIV-Infected and HIV-Uninfected Patients with Cryptococcal Meningitis. J. Microbiol. Immunol. Infect. 2012, 45, 296–304. [Google Scholar]
- Chen, S.C.-A.; Meyer, W.; Sorrell, T.C. Cryptococcus gattii Infections. Clin. Microbiol. Rev. 2014, 27, 980–1024. [Google Scholar] [PubMed]
- Tenforde, M.W.; Shapiro, E.A.; Rouse, B.; Jarvis, J.N.; Li, T.; Eshun-Wilson, I.; Ford, N. Treatment for HIV-Associated Cryptococcal Meningitis. Cochrane Database Syst. Rev. 2018, 2018, CD005647. [Google Scholar]
- Wu, L.; Xiao, J.; Song, Y.; Gao, G.; Zhao, H. The Clinical Characteristics and Outcome of Cryptococcal Meningitis with Aids in a Tertiary Hospital in China: An Observational Cohort Study. BMC Infect. Dis. 2020, 20, 912. [Google Scholar]
- Góralska, K.; Blaszkowska, J.; Dzikowiec, M. Neuroinfections Caused by Fungi. Infection 2018, 46, 443–459. [Google Scholar]
- Qu, J.; Jiang, J.; Lv, X. The Utility of Cerebrospinal Fluid White Cell Count during the Prognostic Assessment for Cryptococcal Meningitis Patients: A Retrospective Study. BMC Infect. Dis. 2020, 20, 571. [Google Scholar]
- Gerard, Y.D.; Hober, M.; Assicot, S.; Alfandari, F.; Ajana, J.M.; Bourez, C.; Chidiac, Y.; Mouton, C.; Wattre, B.P. Procalcitonin as a Marker of Bacterial Sepsis in Patients Infected with HIV-1. J. Infect. 1997, 35, 41–46. [Google Scholar]
- Tokman, S.; Barnett, C.F.; Jarlsberg, L.G.; Taub, P.R.; Boon, S.D.; Davis, J.L.; Cattamanchi, A.; Worodria, W.; Maisel, A.; Huang, L.; et al. Procalcitonin Predicts Mortality in HIV-Infected Ugandan Adults with Lower Respiratory Tract Infections. Respirology 2014, 19, 382–388. [Google Scholar] [PubMed]
- Wang, Q.; Handan, Z.; Yong, T.; Jiaying, Q.; Minghan, Z.; Lijun, X. Aspartate Aminotransferase/Platelet Ratio Index Upon Admission Predicts 24-Week Mortality in Patients with HIV-Associated Talaromyces marneffei. Open Forum Infect. Dis. 2023, 10, ofad593. [Google Scholar] [PubMed]
- Phatlhane, D.V.; Ipp, H.; Erasmus, R.T.; Zemlin, A.E. Evaluating the Use of Procalcitonin in an Asymptomatic, HIV-Infected Antiretroviral Therapy-Naïve, South African Cohort. Clin. Chem. Lab. Med. (CCLM) 2016, 54, 501–508. [Google Scholar] [PubMed]
- Wu, S.-Y.; Kang, M.; Liu, Y.; Chen, Z.-X.; Xiao, Y.-L.; He, C.; Ma, Y. Molecular Epidemiology and Antifungal Susceptibilities of Cryptococcus Species Isolates from Hiv and Non-Hiv Patients in Southwest China. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 287–295. [Google Scholar] [PubMed]
- Bandalizadeh, Z.; Shokohi, T.; Badali, H.; Abastabar, M.; Babamahmoudi, F.; Davoodi, L.; Mardani, M.; Javanian, M.; Cheraghmakani, H.; Sepidgar, A.A.; et al. Molecular Epidemiology and Antifungal Susceptibility Profiles of Clinical Cryptococcus Neoformans/Cryptococcus Gattii Species Complex. J. Med. Microbiol. 2020, 69, 72–81. [Google Scholar] [PubMed]
- Bassetti, M.A.; Vena, E.; Bouza, M.; Peghin, P.; Muñoz, E.; Righi, F.; Pea, M.; Lackner, C.; Flörl, L. Antifungal Susceptibility Testing in Candida, Aspergillus and Cryptococcus Infections: Are the Mics Useful for Clinicians? Clin. Microbiol. Infect. 2020, 26, 1024–1033. [Google Scholar]
- Chen, Y.-C.; Chang, T.-Y.; Liu, J.W.; Chen, F.-J.; Chien, C.-C.; Lee, C.H.; Lu, C.H. Increasing Trend of Fluconazole-Non-Susceptible Cryptococcus Neoformans in Patients with Invasive Cryptococcosis: A 12-Year Longitudinal Study. BMC Infect. Dis. 2015, 15, 277. [Google Scholar]
| Parameters | Years | Subtotal | Spearman | p Values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | |||||
| Gender | |||||||||||||||
| Male | 23 (9.35%) | 27 (10.98%) | 29 (11.79%) | 31 (12.6%) | 25 (10.16%) | 26 (10.57%) | 19 (7.72%) | 26 (10.57%) | 23 (9.35%) | 12 (4.88%) | 5 (2.03%) | 246 | 0.055 | 0.362 † | |
| Female | 3 (9.09%) | 2 (6.06%) | 2 (6.06%) | 6 (18.18%) | 3 (9.09%) | 3 (9.09%) | 4 (12.12%) | 4 (12.12%) | 3 (9.09%) | 1 (3.03%) | 2 (6.06%) | 33 | |||
| Age (Years) | |||||||||||||||
| <13 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 | 0.051 | 0.658 ‡ | |
| 13–24 | 1 (5%) | 4 (20%) | 2 (10%) | 3 (15%) | 3 (15%) | 1 (5%) | 1 (5%) | 0 (0%) | 3 (15%) | 1 (5%) | 1 (5%) | 20 | |||
| 25–34 | 9 (9.18%) | 10 (10.2%) | 12 (12.24%) | 14 (14.29%) | 12 (12.24%) | −8 (8.16%) | 7 (7.14%) | 13 (13.27%) | 9 (9.18%) | 3 (3.06%) | 1 (1.02%) | 98 | |||
| 35–44 | 7 (8.05%) | 9 (10.34%) | 12 (13.79%) | 10 (11.49%) | 5 (5.75%) | 16 (18.39%) | 3 (3.45%) | 7 (8.05%) | 8 (9.2%) | 6 (6.9%) | 4 (4.6%) | 87 | |||
| 45–54 | 7 (18.42%) | 3 (7.89%) | 3 (7.89%) | 2 (5.26%) | 5 (13.16%) | 3 (7.89%) | 9 (23.68%) | 2 (5.26%) | 2 (5.26%) | 2 (5.26%) | 0 (0%) | 38 | |||
| >54 | 2 (5.56%) | 3 (8.33%) | 2 (5.56%) | 8 (22.22%) | 3 (8.33%) | 1 (2.78%) | 3 (8.33%) | 8 (22.22%) | 4 (11.11%) | 1 (2.78%) | 1 (2.78%) | 36 | |||
| ART initiation | |||||||||||||||
| Yes | 4 (13.79%) | 3 (10.34%) | 6 (20.69%) | 5 (17.24%) | 3 (10.34%) | 3 (10.34%) | 0 (0%) | 2 (6.9%) | 2 (6.9%) | 0 (0%) | 1 (3.45%) | 29 | −0.120 | 0.051 † | |
| No | 22 (8.8%) | 26 (10.4%) | 25 (10%) | 32 (12.8%) | 25 (10%) | 26 (10.4%) | 23 (9.2%) | 28 (11.2%) | 24 (9.6%) | 13 (5.2%) | 6 (2.4%) | 250 | |||
| Outcomes | |||||||||||||||
| Survival | 22 (8.8%) | 26 (10.4%) | 27 (10.8%) | 32 (12.8%) | 26 (10.4%) | 27 (10.8%) | 21 (8.4%) | 26 (10.4%) | 24 (9.6%) | 12 (4.8%) | 7 (2.8%) | 250 | −0.066 | 0.266 † | |
| Death | 4 (13.79%) | 3 (10.34%) | 4 (13.79%) | 5 (17.24%) | 2 (6.9%) | 2 (6.9%) | 2 (6.9%) | 4 (13.79%) | 2 (6.9%) | 1 (3.45%) | 0 (0%) | 29 | |||
| Identified pathogens § | |||||||||||||||
| EBV | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2.38%) | 8 (19.05%) | 6 (14.29%) | 11 (26.19%) | 12 (28.57%) | 4 (9.52%) | 0 (0%) | 42 | 0.396 | <0.001 †*** | |
| CMV | 1 (3.33%) | 9 (30%) | 5 (16.67%) | 2 (6.67%) | 2 (6.67%) | 1 (3.33%) | 0 (0%) | 3 (10%) | 5 (16.67%) | 1 (3.33%) | 1 (3.33%) | 30 | −0.055 | 0.439 † | |
| HBV | 5 (29.41%) | 3 (17.65%) | 2 (11.76%) | 3 (17.65%) | 0 (0%) | 2 (11.76%) | 0 (0%) | 0 (0%) | 2 (11.76%) | 0 (0%) | 0 (0%) | 17 | −0.170 | 0.005 †** | |
| Treponema pallidum | 0 (0%) | 0 (0%) | 2 (15.38%) | 0 (0%) | 4 (30.77%) | 1 (7.69%) | 1 (7.69%) | 0 (0%) | 3 (23.08%) | 2 (15.38%) | 0 (0%) | 13 | 0.106 | 0.086 † | |
| Days from illness onset to final diagnosis (Days) | 15 ± 2 | 14 ± 1 | 18 ± 2 | 17 ± 2 | 18 ± 3 | 15 ± 2 | 16 ± 3 | 23 ± 4 | 19 ± 3 | 22 ± 8 | 14 ± 3 | 18 ± 1 | 0.035 | 0.903 ‡ | |
| Hospitalization time (day) | 70 ± 8 | 62 ± 6 | 48 ± 5 | 41 ± 4 | 54 ± 6 | 50 ± 6 | 36 ± 4 | 50 ± 5 | 45 ± 6 | 37 ± 7 | 35 ± 2 | 49 ± 2 | −0.242 | 0.003 ‡** | |
| CD4 count at admission (cell/μL) | 35.7 ± 10.3 | 25.1 ± 3.8 | 31.3 ± 8.1 | 34.7 ± 6.3 | 31.9 ± 6.9 | 25.5 ± 13.9 | 25.8 ± 3.8 | 21.7 ± 3.4 | 32.5 ± 9 | 29.5 ± 8.4 | 16.9 ± 5.1 | 29.2 ± 2.5 | −0.055 | 0.125 ‡ | |
| Parameters | Outcome | p Value | Spearman | ||
|---|---|---|---|---|---|
| Survival | Death | ||||
| Age (year) | 37 (30–45) | 39 (29–52) | 0.694 † | 0.024 | |
| Gender | Male | 222 (90.24%) | 24 (9.76%) | 0.360 § | 0.057 |
| BDG (pg/mL) | 25.63 (1–166.3) | 58.04 (1–236.7) | 0.307 † | 0.067 | |
| WBC in CSF (10^6 cell/μL) | 4 (0.8–12) | 0.5 (0.2–4) | 0.031 †* | −0.129 | |
| RBC in CSF (10^6 cell/μL) | 0 (0–2.8) | 0 (0–2) | 0.763 † | −0.018 | |
| Chloride in CSF (mmol/L) | 119.15 (116–123) | 120 (113.45–124) | 0.990 † | 0.001 | |
| Glucose in CSF (mmol/L) | 2.52 (1.85–3.03) | 2.31 (1.51–3.52) | 0.963 † | −0.003 | |
| Protein in CSF (mg/L) | 417.55 (219.7–629.5) | 396 (237.6–752) | 0.516 † | 0.039 | |
| CSF opening pressure | 270 (160–355) | 315 (207.5–400) | 0.128 † | 0.092 | |
| CD3 count (cell/μL) | 343 (216.67–567.66) | 271 (178–443.5) | 0.048 †* | −0.122 | |
| CD4 count (cell/μL) | 18 (8.11–36) | 13 (6.74–24) | 0.084 † | −0.107 | |
| CD8 count (cell/μL) | 290 (185.5–494) | 228 (145.5–389.75) | 0.066 † | −0.113 | |
| CD4/CD8 | 0.06 (0.03–0.11) | 0.05 (0.03–0.10) | 0.609 † | −0.031 | |
| WBC Count (10^9 cell/μL) | 4.51 (3.19–6.35) | 4.38 (3.61–6.92) | 0.567 † | 0.034 | |
| HIV Viral Load (log10 × copy/mL) | 5.02 (4.61–5.52) | 5.34 (4.85–5.76) | 0.151 † | 0.101 | |
| PCT (ng/mL) | 0.07 (0.04–0.17) | 0.15 (0.13–1.24) | <0.001 †*** | 0.275 | |
| ESR (mm/h) | 44 (23–68.5) | 37 (18–89) | 0.631 † | −0.032 | |
| EBV | Positive(+) | 38 (90.48%) | 4 (9.52%) | 0.841 ‡ | −0.012 |
| CMV | Positive(+) | 24 (80%) | 6 (20%) | 0.131 ‡ | 0.109 |
| HBV | Positive(+) | 14 (82.35%) | 3 (17.65%) | 0.548 ‡ | 0.061 |
| ART initiation | Yes | 29 (100%) | 0 (0%) | 0.054 § | −0.116 |
| Days from illness onset to final diagnosis (day) | 14 (10–21) | 15 (9–28) | 0.496 † | 0.042 | |
| Parameters | Outcome | Subtotal | OR | p Value | ||
|---|---|---|---|---|---|---|
| Survival | Death | |||||
| 5-FC | ||||||
| WT (MIC ≤ 8) | 247 (89.82%) | 28 (10.18%) | 275 | 2.923 | 0.357 † | |
| NWT (MIC > 8) | 3 (75%) | 1 (25%) | 4 | |||
| AmB | ||||||
| WT (MIC ≤ 0.5) | 239 (89.51%) | 28 (10.49%) | 267 | 0.777 | 1.000 † | |
| NWT (MIC > 0.5) | 11 (91.67%) | 1 (8.33%) | 12 | |||
| FCZ | ||||||
| WT (MIC ≤ 8) | 238 (89.81%) | 27 (10.19%) | 265 | 1.467 | 0.646 † | |
| NWT (MIC > 8) | 12 (85.71%) | 2 (14.29%) | 14 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Song, W.; Liu, L.; Qi, T.; Wang, Z.; Tang, Y.; Sun, J.; Xu, S.; Yang, J.; Wang, J.; et al. Trends in Clinico-Epidemiological Profile and Outcomes of Patients with HIV-Associated Cryptococcal Meningitis in Shanghai, China, 2013–2023. Viruses 2024, 16, 1333. https://doi.org/10.3390/v16081333
Zhao Z, Song W, Liu L, Qi T, Wang Z, Tang Y, Sun J, Xu S, Yang J, Wang J, et al. Trends in Clinico-Epidemiological Profile and Outcomes of Patients with HIV-Associated Cryptococcal Meningitis in Shanghai, China, 2013–2023. Viruses. 2024; 16(8):1333. https://doi.org/10.3390/v16081333
Chicago/Turabian StyleZhao, Zihui, Wei Song, Li Liu, Tangkai Qi, Zhenyan Wang, Yang Tang, Jianjun Sun, Shuibao Xu, Junyang Yang, Jiangrong Wang, and et al. 2024. "Trends in Clinico-Epidemiological Profile and Outcomes of Patients with HIV-Associated Cryptococcal Meningitis in Shanghai, China, 2013–2023" Viruses 16, no. 8: 1333. https://doi.org/10.3390/v16081333
APA StyleZhao, Z., Song, W., Liu, L., Qi, T., Wang, Z., Tang, Y., Sun, J., Xu, S., Yang, J., Wang, J., Chen, J., Zhang, R., & Shen, Y. (2024). Trends in Clinico-Epidemiological Profile and Outcomes of Patients with HIV-Associated Cryptococcal Meningitis in Shanghai, China, 2013–2023. Viruses, 16(8), 1333. https://doi.org/10.3390/v16081333






