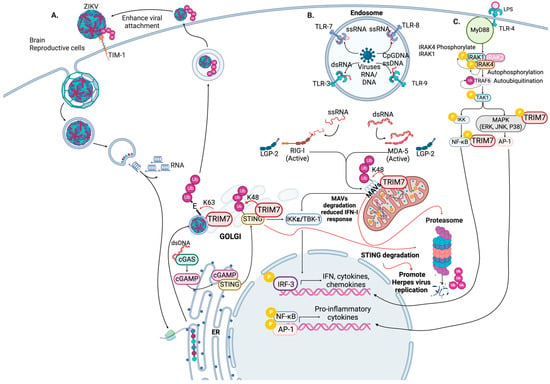
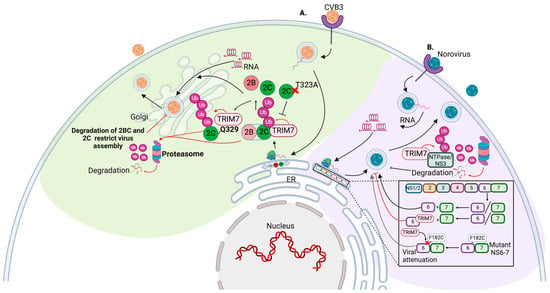
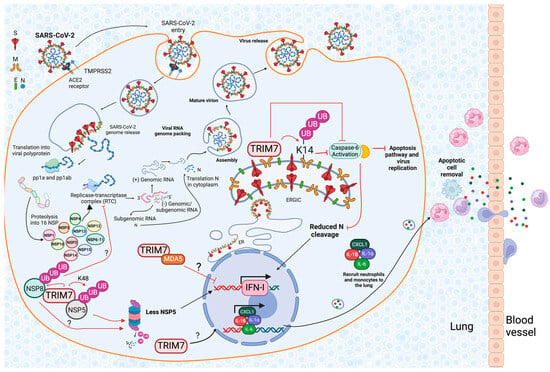
Abstract
The E3 ubiquitin ligase TRIM7 is known to have dual roles during viral infections. Like other TRIM proteins, TRIM7 can regulate the IFN pathway via the regulation of the cytosolic receptors RIG-I or MDA-5, which promote the production of type I interferons (IFN-I) and antiviral immune responses. Alternatively, under certain infectious conditions, TRIM7 can negatively regulate IFN-I signaling, resulting in increased virus replication. A growing body of evidence has also shown that TRIM7 can, in some cases, ubiquitinate viral proteins to promote viral replication and pathogenesis, while in other cases it can promote degradation of viral proteins through the proteasome, reducing virus infection. TRIM7 can also regulate the host inflammatory response and modulate the production of inflammatory cytokines, which can lead to detrimental inflammation. TRIM7 can also protect the host during infection by reducing cellular apoptosis. Here, we discuss the multiple functions of TRIM7 during viral infections and its potential as a therapeutic target.
1. Introduction
Post-translational modifications (PTMs) of proteins, such as ubiquitination, are necessary to regulate the function and stability of a given protein [1]. The ubiquitination process consists of the addition of the small protein ubiquitin (Ub) to the lysine (K) residue of a target protein [2]. Ub itself possesses 7 lysine residues (K6, K11, K27, K29, K33, K48, and K63) and the α amino group of the N-terminal methionine (Met1), where another Ub can be covalently attached to form polyubiquitin chains [3,4,5]. Three enzymes are necessary for the ubiquitination process: the E1-activating enzyme, which activates Ub in an ATP-dependent reaction, the E2-conjugating enzyme, which catalyzes the transfer of the Ub to the target protein, and the E3 ubiquitin ligases which determines the target of ubiquitination [6,7]. The E3-Ub ligases are classified into three types: RING finger, HECT, and RBR types [8]. The RING finger type binds to the E2-conjugating enzyme and the substrate protein, facilitating the transfer of Ub from the E2 to the target protein [9]. This group includes the tripartite motif-containing (TRIM) proteins that have been shown to play dual roles during viral infections [10]. Multiple studies have shown that TRIMs have antiviral activity by promoting Type I Interferon (IFN-I) responses during viral infections or by targeting viral proteins for degradation [2,11,12,13,14,15,16]. However, during some viral infections, TRIM proteins can negatively affect the antiviral response or directly promote virus infection by modifying viral proteins [17,18,19].
The structure of the TRIM proteins encompasses the conserved RBCC domain, which includes a RING E3 ligase domain (R), one or two B-box motifs (B), and a coiled-coil domain [20]. The RING domain is responsible for the interaction with the E2 Ub-conjugating enzymes, and the CC domain promotes oligomerization [20,21], while the C-terminal varies between the different TRIM proteins. The most common domain found is the PRY-SPRY (B30.2), either in combination or individually, and this domain is involved in substrate recognition [10,20]. In this work, we will focus on the E3-Ub ligase TRIM7 and discuss its dual roles during viral infections and its potential as a therapeutic target.
2. TRIM7 Structure and Isoforms
Human TRIM7, also known as RNF90, has an approximate molecular weight of 56 kDa. It was initially identified in a yeast two-hybrid system as a protein that interacts with and activates glycogenin-1 (GNIP) [22]. The structure of TRIM7 consists of the conserved RBCC region, which includes a RING domain, a B-Box domain, and a CC domain. The C-terminal region contains the PRY-SPRY domain (Figure 1A).
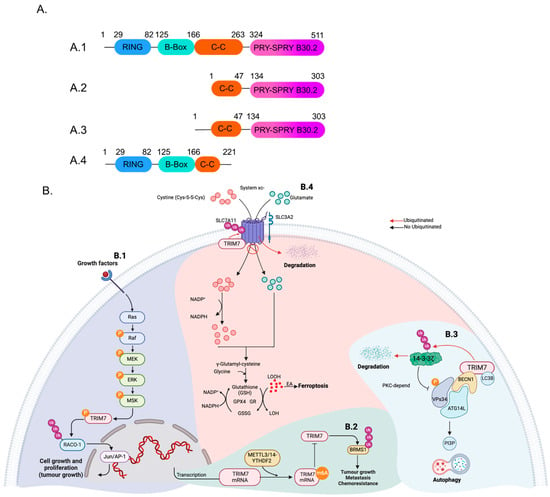
Figure 1.
The pro- and anti-tumoral functions of TRIM7. (A) Depicts the predicted protein architecture encoded by the GNIP (TRIM7) gene in cartoon form. The numbers indicate the approximate demarcation of the different domains for (A.1) GNIP1, (A.2) GNIP2, (A.3) GNIP3, and (A.4) TRIM7 short form. (B.1) The c-Jun/AP-1 transcription factor is crucial for proliferation and apoptosis. MSK1 phosphorylates TRIM7 in response to direct activation by the Ras-Raf-MEK-ERK pathway. This modification stimulates TRIM7 and mediates Lys63-linked ubiquitination of RACO-1, leading to RACO-1 protein stabilization. Consequently, TRIM7 depletion reduces RACO-1 levels and RACO-1-dependent gene expression. (B.2) Modifying TRIM7 mRNA with N6-methyladenosine (m6A) through METTL3/14-YTHDF2 decreases its expression. This regulation enables TRIM7 to control the migration and invasiveness of osteosarcoma cells by ubiquitinating the breast cancer metastasis suppressor (BRMS1). (B.3) TRIM7 can regulate autophagy. Biochemically, GNIP1 binds to BECN1 and LC3B, inducing autophagy by promoting the formation of autophagic protein complexes. Additionally, TRIM7 promotes autophagy progression by mediating the K48-linked ubiquitination of 14-3-3ζ, a negative regulator of autophagy. (B.4) Ferroptosis is induced by the excessive accumulation of lipid hydroperoxides in the cellular membrane. GPX4 regulates lipid peroxidation using GSH to reduce lipid hydroperoxides (LOOH) to lipid alcohols (LOH), suppressing ferroptosis. GSH is oxidized to GSSG in this process. When SLC7A11 is ubiquitinated BY TRIM7, the system xc- is truncated, accumulating LOOH and subsequent ferroptosis, inhibiting gastric cancer progression. GR: glutathione reductase, GPX4: glutathione peroxidase 4, GSH: reduced glutathione, GSSG: oxidized glutathione (glutathione disulfide), LOOH: lipid hydroperoxide, LOH: lipid alcohol, EA: excessive accumulation. Black arrows indicate positive activation of the pathway, and red arrows indicate negative regulation of the pathway by promoting degradation of the protein. Figure was created with BioRender.com.
The GNIP1 gene (also called TRIM7), encodes four spliced isoforms TRIM7/GNIP1 (referred to here as simply TRIM7), GNIP2, GNIP3, and a short form of TRIM7 [23]. Out of these four isoforms, only TRIM7/GNP1 and the short form possess the RING domain typical of the TRIM proteins [23] (Figure 1A). On the other hand, GNP2 and GNP3 are truncated isoforms with a shorter N-terminal domain that includes the CC domain and the conserved C-terminal domain B30.2, which is responsible for the binding with glycogenin-1 (Figure 1A) [23,24]. It is important to note that different names have been used in the literature for different isoforms disregarding the conventional concept that a canonical TRIM should contain the RBCC domain. In some cases, GNIP1 has been used to refer to the full-length gene containing the tripartite motif while TRIM7 is a shorter isoform [25]. Here, we adhere to the common nomenclature, and we refer to TRIM7 as the long isoform containing the RBCC-SPRY domains.
TRIM7 is expressed in several organs in mice, it has been observed that the brain, kidney, liver, lung, heart, muscle, testis, and uterus have high expression of TRIM7 [26,27]. At the cellular level, innate cells such as B cells, macrophages, and dendritic cells (DCs) express high levels of this E3 ligase [27]. TRIM7 is also expressed in human PBMCs, and its expression was downregulated in sepsis patients [28].
The truncated form of TRIM7 has been linked to the reduction of tumor growth by promoting apoptosis [25].
3. TRIM7 in Non-Viral Conditions
TRIM7 has been shown to regulate various cellular processes, including autophagy, cell death, migration, and invasiveness of tumor cells. After activation of the JNK and Ras/MAPK signaling pathway by cell growth factors, TRIM7 is phosphorylated at S107. This phosphorylation enables TRIM7 to ubiquitinate and stabilize RACO-1, allowing the activation of c-Jun/AP-1 to promote the transcription of target genes important in promoting cell survival and proliferation, which increases lung tumor growth progression [29] (Figure 1B). Moreover, TRIM7 was found to be expressed at higher levels in tissues of patients with osteosarcoma, and its increased expression correlates with poor prognosis in these patients; mechanistically, TRIM7 positively regulates cell migration and invasiveness of osteosarcoma cells by ubiquitinating the breast cancer metastasis suppressor (BRMS1) at the lysine K184 [30]. The expression of TRIM7 is regulated at the mRNA level by the N6-methyladenosine (m6A) modification which is mediated by METTL3 and METTL14 (Figure 1B). This modification affects the stability of the mRNA leading to reduced levels of TRIM7 at the protein level. It was observed that samples from osteosarcoma patients showed reduced m6A modification of TRIM7 mRNA, thereby increasing the levels of TRIM7 protein and its pro-tumoral functions [30].
TRIM7 was also shown to induce cancer progression in lung cancer cells by promoting the degradation of 14-3-3 ζ (a negative regulator of Vsp34) to trigger autophagy (Figure 1B). Additionally, TRIM7 can interact with Beclin-1 and LC3B to induce the formation of the autophagosomes and promote autophagy, which can lead to the proliferation and migration of lung cancer cells [31].
Conversely, other studies have demonstrated that TRIM7 is downregulated in samples from gastric cancer (GC) patients. This downregulation is significant as TRIM7 acts as a tumor suppressor, inhibiting the proliferation of GC cells and inducing ferroptosis [32] (Figure 1B). The induction of ferroptosis occurs by the K48 ubiquitination and subsequent degradation of the solute carrier family 7 member 11 (SLC7A11), and is a particularly intriguing aspect of TRIM7’s role in gastric cancer cells [32]. Additionally, TRIM7 negatively regulates the Src-mTORC1-S6K axis in hepatocellular carcinoma cells and ubiquitinates the Src kinase in a K48 manner to promote its degradation. Lower levels of Src kinase reduced activation of both mTORC1 and S6K, leading to reduced proliferation and invasiveness of the hepatocellular carcinoma cells [33]. There is only one report describing the role of TRIM7 in bacterial infections; during L. monocytogenes infection, TRIM7 reduces bacteria burden in mice by promoting K63 ubiquitination of ATG7 and induces autophagy [34].
All of these studies highlight the diverse functions of TRIM7 within the cell, as it regulates essential cellular processes. However, due to it belonging to the TRIM family, extensive research has been conducted on its role in viral infections.
7. Conclusions and Perspectives
Viruses, including highly pathogenic ones like ZIKV and SARS-CoV-2, rely on host cells’ specific factors for successful replication. Among these factors, the Ub system plays a crucial role. It regulates protein degradation, signal transduction, and DNA repair, and it facilitates virus replication by degrading host factors or modifying viral proteins. Our comprehensive review delves into the complexities of this interaction, with a specific focus on the role of the E3-Ub ligase TRIM7 in the ubiquitination process and its potential implications in viral infection. This analysis underscores the importance of understanding the interplay between viruses and the host cell’s regulatory systems, such as the Ub system, in developing effective host-mediated strategies for combating viral infections.
TRIM7, a protein that ubiquitinates specific structural proteins, is critical in virus replication. This non-degradative mechanism is increasingly recognized as a standard feature of emergent viruses from different families. Recent research in SARS-CoV-2 has unveiled that TRIM7’s ubiquitination of M protein may also have an antiviral function, potentially impeding the spread of the virus. This dual role of TRIM7 underscores the complex nature of the immune response to viral infections. Since ubiquitination sites on the membrane protein of SARS-CoV-2 appeared during the pandemic, it could point to a viral escape mechanism counteracting the antiviral effects of TRIM7, which warrants further investigation.
Our broad understanding of the mechanisms by which viruses replicate, particularly the specific steps involved in ubiquitination, is a crucial step toward effective viral infection management. Future studies should aim to identify the precise subcellular compartments in which this process occurs. This knowledge could pave the way for the development of targeted strategies to disrupt viral replication, a promising avenue in the fight against a wide range of viral infections. These insights hold immense promise, instilling a sense of hope for the future of viral infection management.
Author Contributions
M.G.-O. and M.I.G. wrote the manuscript. M.G.-O. and C.A.R.-S. designed and conceptualized the figures. All authors contributed to the editing and improvement of the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the US National Institute of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) grant number R01AI134907 and the Building Interdisciplinary Research Careers in Women’s Health Program (BIRCWH) K12HD052023 awarded to M.I.G.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, J.M.; Hammaren, H.M.; Savitski, M.M.; Baek, S.H. Control of protein stability by post-translational modifications. Nat. Commun. 2023, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Uchil, P.D.; Hinz, A.; Siegel, S.; Coenen-Stass, A.; Pertel, T.; Luban, J.; Mothes, W. TRIM protein-mediated regulation of inflammatory and innate immune signaling and its association with antiretroviral activity. J. Virol. 2013, 87, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Rape, M. The ubiquitin code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef] [PubMed]
- van Tol, S.; Hage, A.; Giraldo, M.I.; Bharaj, P.; Rajsbaum, R. The TRIMendous Role of TRIMs in Virus-Host Interactions. Vaccines 2017, 5, 23. [Google Scholar] [CrossRef]
- Damgaard, R.B. The ubiquitin system: From cell signalling to disease biology and new therapeutic opportunities. Cell Death Differ. 2021, 28, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Hage, A.; Rajsbaum, R. To TRIM or not to TRIM: The balance of host-virus interactions mediated by the ubiquitin system. J. Gen. Virol. 2019, 100, 1641–1662. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.D.; Ritterhoff, T.; Klevit, R.E.; Brzovic, P.S. E2 enzymes: More than just middle men. Cell Res. 2016, 26, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 ubiquitin ligases: Styles, structures and functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Valerdi, K.M.; Hage, A.; van Tol, S.; Rajsbaum, R.; Giraldo, M.I. The Role of the Host Ubiquitin System in Promoting Replication of Emergent Viruses. Viruses 2021, 13, 369. [Google Scholar] [CrossRef]
- Giraldo, M.I.; Hage, A.; van Tol, S.; Rajsbaum, R. TRIM Proteins in Host Defense and Viral Pathogenesis. Curr. Clin. Microbiol. Rep. 2020, 7, 101–114. [Google Scholar] [CrossRef]
- Rajsbaum, R.; Stoye, J.P.; O’Garra, A. Type I interferon-dependent and -independent expression of tripartite motif proteins in immune cells. Eur. J. Immunol. 2008, 38, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Rajsbaum, R.; Versteeg, G.A.; Schmid, S.; Maestre, A.M.; Belicha-Villanueva, A.; Martinez-Romero, C.; Patel, J.R.; Morrison, J.; Pisanelli, G.; Miorin, L.; et al. Unanchored K48-linked polyubiquitin synthesized by the E3-ubiquitin ligase TRIM6 stimulates the interferon-IKKepsilon kinase-mediated antiviral response. Immunity 2014, 40, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, G.A.; Rajsbaum, R.; Sanchez-Aparicio, M.T.; Maestre, A.M.; Valdiviezo, J.; Shi, M.; Inn, K.S.; Fernandez-Sesma, A.; Jung, J.; Garcia-Sastre, A. The E3-ligase TRIM family of proteins regulates signaling pathways triggered by innate immune pattern-recognition receptors. Immunity 2013, 38, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, A.; Kajaste-Rudnitski, A.; Oteiza, A.; Nicora, L.; Towers, G.J.; Mechti, N.; Vicenzi, E. TRIM22 inhibits influenza A virus infection by targeting the viral nucleoprotein for degradation. J. Virol. 2013, 87, 4523–4533. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zou, C.; Wang, X.; Huang, C.; Feng, T.; Pan, W.; Wu, Q.; Wang, P.; Dai, J. Interferon-stimulated TRIM69 interrupts dengue virus replication by ubiquitinating viral nonstructural protein 3. PLoS Pathog. 2018, 14, e1007287. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Wang, S.; Wu, F.; Chen, Z.; Li, C.; Cheng, G.; Qin, F.X. Inhibition of Influenza A Virus Replication by TRIM14 via Its Multifaceted Protein-Protein Interaction With NP. Front. Microbiol. 2019, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Bharaj, P.; Atkins, C.; Luthra, P.; Giraldo, M.I.; Dawes, B.E.; Miorin, L.; Johnson, J.R.; Krogan, N.J.; Basler, C.F.; Freiberg, A.N.; et al. The Host E3-Ubiquitin Ligase TRIM6 Ubiquitinates the Ebola Virus VP35 Protein and Promotes Virus Replication. J. Virol. 2017, 91, e00833-17. [Google Scholar] [CrossRef] [PubMed]
- van Tol, S.; Kalveram, B.; Ilinykh, P.A.; Ronk, A.; Huang, K.; Aguilera-Aguirre, L.; Bharaj, P.; Hage, A.; Atkins, C.; Giraldo, M.I.; et al. Ubiquitination of Ebola virus VP35 at lysine 309 regulates viral transcription and assembly. PLoS Pathog. 2022, 18, e1010532. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Salazar, C.A.; van Tol, S.; Mailhot, O.; Gonzalez-Orozco, M.; Galdino, G.T.; Warren, A.N.; Teruel, N.; Behera, P.; Afreen, K.S.; Zhang, L.; et al. Ebola virus VP35 interacts non-covalently with ubiquitin chains to promote viral replication. PLoS Biol. 2024, 22, e3002544. [Google Scholar] [CrossRef]
- van Gent, M.; Sparrer, K.M.J.; Gack, M.U. TRIM Proteins and Their Roles in Antiviral Host Defenses. Annu. Rev. Virol. 2018, 5, 385–405. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Wu, W.; Zhuo, W.; Liu, W.; Zhang, Y.; Cheng, M.; Chen, Y.G.; Gao, N.; Yu, H.; et al. Structural insights into the TRIM family of ubiquitin E3 ligases. Cell Res. 2014, 24, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Skurat, A.V.; Dietrich, A.D.; Zhai, L.; Roach, P.J. GNIP, a novel protein that binds and activates glycogenin, the self-glucosylating initiator of glycogen biosynthesis. J. Biol. Chem. 2002, 277, 19331–19338. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Dietrich, A.; Skurat, A.V.; Roach, P.J. Structure-function analysis of GNIP, the glycogenin-interacting protein. Arch. Biochem. Biophys. 2004, 421, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Munoz Sosa, C.J.; Issoglio, F.M.; Carrizo, M.E. Crystal structure and mutational analysis of the human TRIM7 B30.2 domain provide insights into the molecular basis of its binding to glycogenin-1. J. Biol. Chem. 2021, 296, 100772. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lu, Z.; Wang, X.; Liu, Y.; Han, T.; Wang, Y.; Wang, T.; Gan, M.; Xie, C.; Wang, J.; et al. E3 ubiquitin ligase TRIM7 negatively regulates NF-kappa B signaling pathway by degrading p65 in lung cancer. Cell Signal 2020, 69, 109543. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.I.; Xia, H.; Aguilera-Aguirre, L.; Hage, A.; van Tol, S.; Shan, C.; Xie, X.; Sturdevant, G.L.; Robertson, S.J.; McNally, K.L.; et al. Envelope protein ubiquitination drives entry and pathogenesis of Zika virus. Nature 2020, 585, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhu, X.; Yang, Z.; Zhang, W.; Sun, Z.; Ji, Q.; Chen, X.; Zhu, J.; Wang, C.; Nie, S. E3 ubiquitin ligase tripartite motif 7 positively regulates the TLR4-mediated immune response via its E3 ligase domain in macrophages. Mol. Immunol. 2019, 109, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Ma, A.; Liu, J.; Zhou, W.; Cao, P.; Chu, T.; Fan, L. Study on the expression of TRIM7 in peripheral blood mononuclear cells of patients with sepsis and its early diagnostic value. BMC Infect. Dis. 2022, 22, 865. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Diefenbacher, M.E.; Mylona, A.; Kassel, O.; Behrens, A. The E3 ubiquitin ligase Trim7 mediates c-Jun/AP-1 activation by Ras signalling. Nat. Commun. 2015, 6, 6782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Z.; Zhu, X.; Qian, G.; Zhou, Y.; Sun, Y.; Yu, W.; Wang, J.; Lu, H.; Lin, F.; et al. N6-Methyladenosine modification of the TRIM7 positively regulates tumorigenesis and chemoresistance in osteosarcoma through ubiquitination of BRMS1. EBioMedicine 2020, 59, 102955. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Y.; Ai, W.; Wang, Y.; Gan, M.; Jiang, Q.; Han, T.; Wang, J.B. GNIP1 functions both as a scaffold protein and an E3 ubiquitin ligase to regulate autophagy in lung cancer. Cell Commun. Signal 2022, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, T.; Zeng, R.; Zhang, K.; Li, B.; Zhu, Z.; Ma, X.; Zhang, Y.; Li, L.; Zhu, J.; et al. The E3 ligase TRIM7 suppresses the tumorigenesis of gastric cancer by targeting SLC7A11. Sci. Rep. 2024, 14, 6655. [Google Scholar] [CrossRef]
- Zhu, L.; Qin, C.; Li, T.; Ma, X.; Qiu, Y.; Lin, Y.; Ma, D.; Qin, Z.; Sun, C.; Shen, X.; et al. The E3 ubiquitin ligase TRIM7 suppressed hepatocellular carcinoma progression by directly targeting Src protein. Cell Death Differ. 2020, 27, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, X.; Huang, Y.; Zhang, Q.; Pei, J.; Wang, Y.; Goren, I.; Ma, S.; Song, Z.; Liu, Y.; et al. TRIM7/RNF90 promotes autophagy via regulation of ATG7 ubiquitination during L. monocytogenes infection. Autophagy 2023, 19, 1844–1862. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, G.; Qin, X.; Huang, Y.; Ren, X.; Sun, J.; Ma, S.; Liu, Y.; Song, D.; Liu, Y.; et al. Negative Regulation of RNF90 on RNA Virus-Triggered Antiviral Immune Responses Targeting MAVS. Front. Immunol. 2021, 12, 730483. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, Y.; Cui, Y.; Song, D.; Zhang, G.; Ma, S.; Liu, Y.; Chen, M.; Chen, F.; Wang, H.; et al. RNF90 negatively regulates cellular antiviral responses by targeting MITA for degradation. PLoS Pathog. 2020, 16, e1008387. [Google Scholar] [CrossRef]
- Gonzalez-Orozco, M.; Tseng, H.C.; Hage, A.; Xia, H.; Behera, P.; Afreen, K.; Penaflor-Tellez, Y.; Giraldo, M.I.; Huante, M.; Puebla-Clark, L.; et al. TRIM7 ubiquitinates SARS-CoV-2 membrane protein to limit apoptosis and viral replication. bioRxiv 2024. [Google Scholar] [CrossRef]
- Li, M.; Yan, J.; Zhu, H.; Guo, C.; Jiang, X.; Gao, Y.; Liu, X.; Jiang, P.; Bai, J. TRIM7 inhibits encephalomyocarditis virus replication by activating interferon-beta signaling pathway. Vet. Microbiol. 2023, 281, 109729. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, Z.; Yi, Z. Identification and characterization of key residues in Zika virus envelope protein for virus assembly and entry. Emerg. Microbes Infect. 2022, 11, 1604–1620. [Google Scholar] [CrossRef]
- Fan, W.; Mar, K.B.; Sari, L.; Gaszek, I.K.; Cheng, Q.; Evers, B.M.; Shelton, J.M.; Wight-Carter, M.; Siegwart, D.J.; Lin, M.M.; et al. TRIM7 inhibits enterovirus replication and promotes emergence of a viral variant with increased pathogenicity. Cell 2021, 184, 3410–3425.e3417. [Google Scholar] [CrossRef] [PubMed]
- Baggen, J.; Thibaut, H.J.; Strating, J.; van Kuppeveld, F.J.M. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018, 16, 368–381. [Google Scholar] [CrossRef]
- Liang, X.; Xiao, J.; Li, X.; Liu, Y.; Lu, Y.; Wen, Y.; Li, Z.; Che, X.; Ma, Y.; Zhang, X.; et al. A C-terminal glutamine recognition mechanism revealed by E3 ligase TRIM7 structures. Nat. Chem. Biol. 2022, 18, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Yan, X.; Zhang, B.; Song, L.; Feng, Q.; Ye, C.; Zhou, Z.; Yang, Z.; Li, Y.; Zhang, Z.; et al. C-terminal glutamine acts as a C-degron targeted by E3 ubiquitin ligase TRIM7. Proc. Natl. Acad. Sci. USA 2022, 119, e2203218119. [Google Scholar] [CrossRef] [PubMed]
- Luptak, J.; Mallery, D.L.; Jahun, A.S.; Albecka, A.; Clift, D.; Ather, O.; Slodkowicz, G.; Goodfellow, I.; James, L.C. TRIM7 Restricts Coxsackievirus and Norovirus Infection by Detecting the C-Terminal Glutamine Generated by 3C Protease Processing. Viruses 2022, 14, 1610. [Google Scholar] [CrossRef] [PubMed]
- Orchard, R.C.; Sullender, M.E.; Dunlap, B.F.; Balce, D.R.; Doench, J.G.; Virgin, H.W. Identification of Antinorovirus Genes in Human Cells Using Genome-Wide CRISPR Activation Screening. J. Virol. 2019, 93, e01324-18. [Google Scholar] [CrossRef] [PubMed]
- Sullender, M.E.; Pierce, L.R.; Annaswamy Srinivas, M.; Crockett, S.L.; Dunlap, B.F.; Rodgers, R.; Schriefer, L.A.; Kennedy, E.A.; Stewart, B.M.; Doench, J.G.; et al. Selective Polyprotein Processing Determines Norovirus Sensitivity to Trim7. J. Virol. 2022, 96, e0070722. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; McDougal, M.B.; Schoggins, J.W. Enterovirus 3C Protease Cleaves TRIM7 To Dampen Its Antiviral Activity. J. Virol. 2022, 96, e0133222. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Guo, J.; Xu, S.; Zhou, J.; Chen, Q.; Tong, X.; Wang, D.; Peng, G.; Fang, L.; et al. SARS-CoV-2 nsp5 Exhibits Stronger Catalytic Activity and Interferon Antagonism than Its SARS-CoV Ortholog. J. Virol. 2022, 96, e0003722. [Google Scholar] [CrossRef]
- Liang, W.; Gu, M.; Zhu, L.; Yan, Z.; Schenten, D.; Herrick, S.; Li, H.; Samrat, S.K.; Zhu, J.; Chen, Y. The main protease of SARS-CoV-2 downregulates innate immunity via a translational repression. Signal Transduct. Target. Ther. 2023, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Aktepe, T.E.; Deerain, J.M.; McAuley, J.L.; Audsley, M.D.; David, C.T.; Purcell, D.F.J.; Urin, V.; Hartmann, R.; Moseley, G.W.; et al. SARS-CoV-2 suppresses IFNbeta production mediated by NSP1, 5, 6, 15, ORF6 and ORF7b but does not suppress the effects of added interferon. PLoS Pathog. 2021, 17, e1009800. [Google Scholar] [CrossRef]
- Zheng, Y.; Deng, J.; Han, L.; Zhuang, M.W.; Xu, Y.; Zhang, J.; Nan, M.L.; Xiao, Y.; Zhan, P.; Liu, X.; et al. SARS-CoV-2 NSP5 and N protein counteract the RIG-I signaling pathway by suppressing the formation of stress granules. Signal Transduct. Target. Ther. 2022, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, C.; Rao, Y.; Ngo, C.; Feng, J.J.; Zhao, J.; Zhang, S.; Wang, T.Y.; Carriere, J.; Savas, A.C.; et al. SARS-CoV-2 Nsp5 Demonstrates Two Distinct Mechanisms Targeting RIG-I and MAVS To Evade the Innate Immune Response. mBio 2021, 12, e0233521. [Google Scholar] [CrossRef]
- Stukalov, A.; Girault, V.; Grass, V.; Karayel, O.; Bergant, V.; Urban, C.; Haas, D.A.; Huang, Y.; Oubraham, L.; Wang, A.; et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature 2021, 594, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Hu, B.; Xiao, H.; Tan, X.; Li, Y.; Tang, K.; Zhang, Y.; Cai, K.; Ding, B. The E3 Ubiquitin Ligase RNF5 Facilitates SARS-CoV-2 Membrane Protein-Mediated Virion Release. mBio 2021, 13, e0316821. [Google Scholar] [CrossRef]
- Chu, H.; Hou, Y.; Yang, D.; Wen, L.; Shuai, H.; Yoon, C.; Shi, J.; Chai, Y.; Yuen, T.T.; Hu, B.; et al. Coronaviruses exploit a host cysteine-aspartic protease for replication. Nature 2022, 609, 785–792. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).