Human Herpes Virus-6 (HHV-6) Reactivation after Hematopoietic Cell Transplant and Chimeric Antigen Receptor (CAR)- T Cell Therapy: A Shifting Landscape
Abstract
1. Introduction
2. Epidemiology of HHV-6B after Allogeneic HCT
2.1. Incidence, Viral Kinetics and Risk Factors for HHV-6B
2.2. An Update for the Current Clinical Practice Landscape
2.3. Clinical Manifestations and Diagnostic Pitfalls
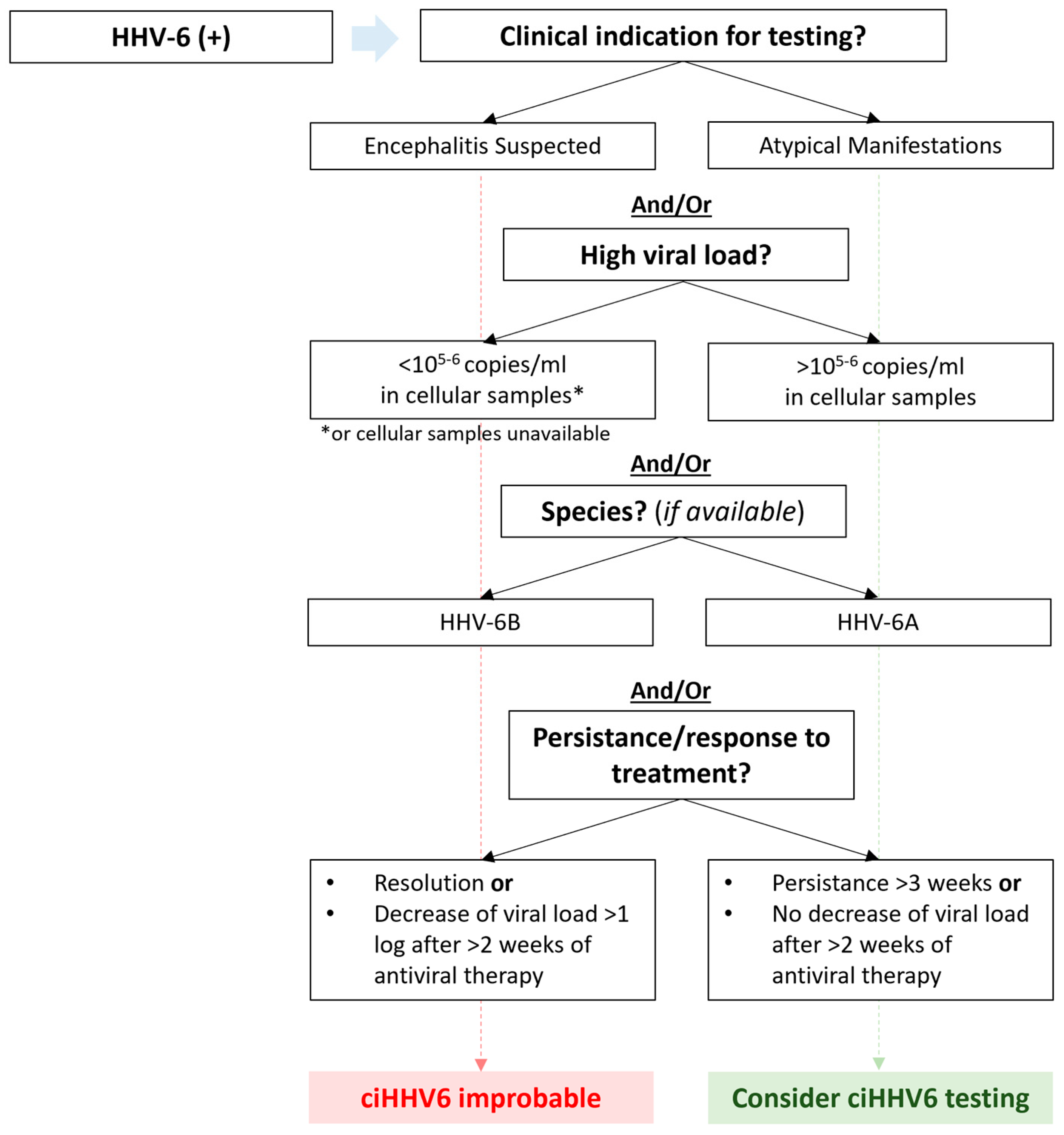
2.4. Chromosomally Integrated HHV-6
3. Epidemiology of HHV-6B after Autologous HCT
4. Epidemiology of HHV-6B after CAR-T Cell Therapy
5. Diagnostic Strategies
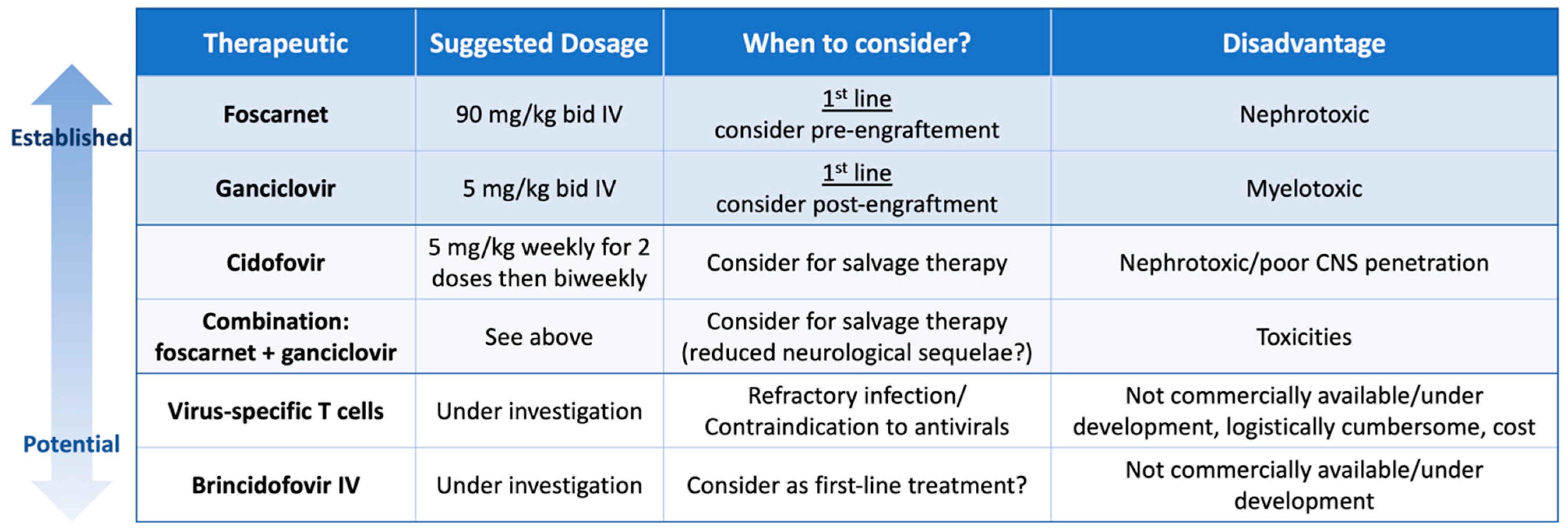
6. Treatment Strategies
7. Virus-Specific T cells
8. Discussion and Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zerr, D.M.; Meier, A.S.; Selke, S.S.; Frenkel, L.M.; Huang, M.-L.; Wald, A.; Rhoads, M.P.; Nguy, L.; Bornemann, R.; Morrow, R.A.; et al. A Population-Based Study of Primary Human Herpesvirus 6 Infection. N. Engl. J. Med. 2005, 352, 768–776. [Google Scholar] [CrossRef]
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Human Herpesviruses 6A, 6B, and 7. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Ward, K.N.; Hill, J.A.; Hubacek, P.; de la Camara, R.; Crocchiolo, R.; Einsele, H.; Navarro, D.; Robin, C.; Cordonnier, C.; Ljungman, P. Guidelines from the 2017 European Conference on Infections in Leukaemia for Management of HHV-6 Infection in Patients with Hematologic Malignancies and after Hematopoietic Stem Cell Transplantation. Haematologica 2019, 104, 2155–2163. [Google Scholar] [CrossRef]
- Hill, J.A.; Koo, S.; Guzman Suarez, B.B.; Ho, V.T.; Cutler, C.; Koreth, J.; Armand, P.; Alyea, E.P., 3rd; Baden, L.R.; Antin, J.H.; et al. Cord-Blood Hematopoietic Stem Cell Transplant Confers an Increased Risk for Human Herpesvirus-6-Associated Acute Limbic Encephalitis: A Cohort Analysis. Biol. Blood Marrow Transplant. 2012, 18, 1638–1648. [Google Scholar] [CrossRef]
- Zerr, D.M.; Boeckh, M.; Delaney, C.; Martin, P.J.; Xie, H.; Adler, A.L.; Huang, M.-L.; Corey, L.; Leisenring, W.M. HHV-6 Reactivation and Associated Sequelae after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2012, 18, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Oshima, K.; Ikebe, T.; Takano, K.; Kanamori, H.; Kondo, T.; Ueda, Y.; Mori, T.; Hashimoto, H.; Ogawa, H.; et al. Clinical Characteristics and Outcome of Human Herpesvirus-6 Encephalitis after Allogeneic Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant. 2017, 52, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Magaret, A.S.; Hall-Sedlak, R.; Mikhaylova, A.; Huang, M.-L.; Sandmaier, B.M.; Hansen, J.A.; Jerome, K.R.; Zerr, D.M.; Boeckh, M. Outcomes of Hematopoietic Cell Transplantation Using Donors or Recipients with Inherited Chromosomally Integrated HHV-6. Blood 2017, 130, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Heldman, M.R.; Job, C.; Maalouf, J.; Morris, J.; Xie, H.; Davis, C.; Stevens-Ayers, T.; Huang, M.L.; Jerome, K.R.; Fann, J.R.; et al. Association of Inherited Chromosomally Integrated Human Herpesvirus 6 with Neurologic Symptoms and Management after Allogeneic Hematopoietic Cell Transplantation. Transplant. Cell Ther. 2021, 27, 795.e1–795.e8. [Google Scholar] [CrossRef] [PubMed]
- Aimola, G.; Beythien, G.; Aswad, A.; Kaufer, B.B. Current Understanding of Human Herpesvirus 6 (HHV-6) Chromosomal Integration. Antivir. Res. 2020, 176, 104720. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Serada, S.; Kawabata, A.; Ota, M.; Hayashi, E.; Naka, T.; Yamanishi, K.; Mori, Y. CD134 Is a Cellular Receptor Specific for Human Herpesvirus-6B Entry. Proc. Natl. Acad. Sci. USA 2013, 110, 9096–9099. [Google Scholar] [CrossRef] [PubMed]
- Saviola, A.J.; Zimmermann, C.; Mariani, M.P.; Signorelli, S.A.; Gerrard, D.L.; Boyd, J.R.; Wight, D.J.; Morissette, G.; Gravel, A.; Dubuc, I.; et al. Chromatin Profiles of Chromosomally Integrated Human Herpesvirus. Front. Microbiol. 2019, 10, 456369. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Mayer, B.T.; Xie, H.; Leisenring, W.M.; Huang, M.-L.; Stevens-Ayers, T.; Milano, F.; Delaney, C.; Sorror, M.L.; Sandmaier, B.M.; et al. The Cumulative Burden of Double-Stranded DNA Virus Detection after Allogeneic HCT Is Associated with Increased Mortality. Blood 2017, 129, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Zerr, D.M.; Corey, L.; Kim, H.W.; Huang, M.-L.; Nguy, L.; Boeckh, M. Clinical Outcomes of Human Herpesvirus 6 Reactivation after Hematopoietic Stem Cell Transplantation. Clin. Infect. Dis. 2005, 40, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Kim, S.J.; Lee, Y.J.; Burack, D.; Nichols, P.; Maloy, M.; Perales, M.A.; Giralt, S.A.; Jakubowski, A.A.; Papanicolaou, G.A. Co-Infections by Double-Stranded DNA Viruses after Ex Vivo T Cell-Depleted, CD34+ Selected Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Perruccio, K.; Sisinni, L.; Perez-Martinez, A.; Valentin, J.; Capolsini, I.; Massei, M.S.; Caniglia, M.; Cesaro, S. High Incidence of Early Human Herpesvirus-6 Infection in Children Undergoing Haploidentical Manipulated Stem Cell Transplantation for Hematologic Malignancies. Biol. Blood Marrow Transplant. 2018, 24, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L.; Dahi, P.B.; Zheng, J.; Devlin, S.M.; Lubin, M.; Gonzales, A.M.; Giralt, S.A.; Perales, M.A.; Papadopoulos, E.B.; Ponce, D.M.; et al. Frequent Human Herpesvirus-6 Viremia but Low Incidence of Encephalitis in Double-Unit Cord Blood Recipients Transplanted without Antithymocyte Globulin. Biol. Blood Marrow Transplant. 2014, 20, 787–793. [Google Scholar] [CrossRef]
- Ogata, M.; Satou, T.; Kadota, J.; Saito, N.; Yoshida, T.; Okumura, H.; Ueki, T.; Nagafuji, K.; Kako, S.; Uoshima, N.; et al. Human Herpesvirus 6 (HHV-6) Reactivation and HHV-6 Encephalitis after Allogeneic Hematopoietic Cell Transplantation: A Multicenter, Prospective Study. Clin. Infect. Dis. 2013, 57, 671–681. [Google Scholar] [CrossRef]
- Toomey, D.; Phan, T.L.; Phan, T.; Hill, J.A.; Zerr, D.M. Viral Encephalitis after Hematopoietic Cell Transplantation: A Systematic Review. Transplant. Cell Ther. 2023, 29, 636.e1–636.e9. [Google Scholar] [CrossRef]
- Abidi, M.Z.; Hari, P.; Chen, M.; Kim, S.; Battiwala, M.; Dahi, P.B.; Diaz, M.A.; Gale, R.P.; Ganguly, S.; Gergis, U.; et al. Virus Detection in the Cerebrospinal Fluid of Hematopoietic Stem Cell Transplant Recipients Is Associated with Poor Patient Outcomes: A CIBMTR Contemporary Longitudinal Study. Bone Marrow Transplant. 2019, 54, 1354–1360. [Google Scholar] [CrossRef]
- Noviello, M.; Lorentino, F.; Xue, E.; Racca, S.; Furnari, G.; Valtolina, V.; Campodonico, E.; Dvir, R.; Lupo-Stanghellini, M.T.; Giglio, F.; et al. Human Herpesvirus 6-Specific T-Cell Immunity in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Blood Adv. 2023, 7, 5446–5457. [Google Scholar] [CrossRef]
- Hill, J.A.; Mayer, B.T.; Xie, H.; Leisenring, W.M.; Huang, M.-L.; Stevens-Ayers, T.; Milano, F.; Delaney, C.; Jerome, K.R.; Zerr, D.M.; et al. Kinetics of Double-Stranded DNA Viremia After Allogeneic Hematopoietic Cell Transplantation. Clin. Infect. Dis. 2018, 66, 368–375. [Google Scholar] [CrossRef]
- Hill, J.A.; Vande Vusse, L.K.; Xie, H.; Chung, E.L.; Yeung, C.C.S.; Seo, S.; Stevens-Ayers, T.; Fisher, C.E.; Huang, M.-L.; Stewart, F.M.; et al. Human Herpesvirus 6B and Lower Respiratory Tract Disease After Hematopoietic Cell Transplantation. J. Clin. Oncol. 2019, 37, 2670–2681. [Google Scholar] [CrossRef]
- D’Souza, A.; Fretham, C.; Lee, S.J.; Arora, M.; Brunner, J.; Chhabra, S.; Devine, S.; Eapen, M.; Hamadani, M.; Hari, P.; et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol. Blood Marrow Transplant. 2020, 26, e177–e182. [Google Scholar] [CrossRef]
- Singh, A.; Dandoy, C.E.; Chen, M.; Kim, S.; Mulroney, C.M.; Kharfan-Dabaja, M.A.; Ganguly, S.; Maziarz, R.T.; Kanakry, C.G.; Kanakry, J.A.; et al. Post-Transplantation Cyclophosphamide Is Associated with an Increase in Non-Cytomegalovirus Herpesvirus Infections in Patients with Acute Leukemia and Myelodysplastic Syndrome. Transplant. Cell Ther. 2022, 28, 48.e1–48.e10. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R.; Abid, M.B.; Auletta, J.J.; Bashey, A.; Beitinjaneh, A.; Castillo, P.; Chemaly, R.F.; Chen, M.; Ciurea, S.; Dandoy, C.E.; et al. Posttransplant Cyclophosphamide Is Associated with Increased Cytomegalovirus Infection: A CIBMTR Analysis. Blood 2021, 137, 3291–3305. [Google Scholar] [CrossRef] [PubMed]
- Khimani, F.; Ranspach, P.; Elmariah, H.; Kim, J.; Whiting, J.; Nishihori, T.; Locke, F.L.; Perez Perez, A.; Dean, E.; Mishra, A.; et al. Increased Infections and Delayed CD4+ T Cell but Faster B Cell Immune Reconstitution after Post-Transplantation Cyclophosphamide Compared to Conventional GVHD Prophylaxis in Allogeneic Transplantation. Transplant. Cell Ther. 2021, 27, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Little, J.S.; Duléry, R.; Shapiro, R.M.; Aleissa, M.M.; Prockop, S.E.; Koreth, J.; Ritz, J.; Antin, J.H.; Cutler, C.; Nikiforow, S.; et al. Opportunistic Infections in Patients Receiving Post-Transplantation Cyclophosphamide: Impact of Haploidentical versus Unrelated Donor Allograft. Transplant. Cell Ther. 2024, 30, 233.e1–233.e14. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Stern, A.; Karantoni, E.; Nawar, T.; Han, G.; Zavras, P.; Dumke, H.; Cho, C.; Tamari, R.; Shaffer, B.; et al. Impact of Letermovir Primary Cytomegalovirus Prophylaxis on 1-Year Mortality After Allogeneic Hematopoietic Cell Transplantation: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, 75, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Sassine, J.; Khawaja, F.; Shigle, T.L.; Handy, V.; Foolad, F.; Aitken, S.L.; Jiang, Y.; Champlin, R.; Shpall, E.; Rezvani, K.; et al. Refractory and Resistant Cytomegalovirus After Hematopoietic Cell Transplant in the Letermovir Primary Prophylaxis Era. Clin. Infect. Dis. 2021, 73, 1346–1354. [Google Scholar] [CrossRef]
- Ljungman, P.; Dahl, H.; Xu, Y.-H.; Larsson, K.; Brytting, M.; Linde, A. Effectiveness of Ganciclovir against Human Herpesvirus-6 Excreted in Saliva in Stem Cell Transplant Recipients. Bone Marrow Transplant. 2007, 39, 497–499. [Google Scholar] [CrossRef]
- Ogata, M.; Takano, K.; Moriuchi, Y.; Kondo, T.; Ueki, T.; Nakano, N.; Mori, T.; Uoshima, N.; Nagafuji, K.; Yamasaki, S.; et al. Effects of Prophylactic Foscarnet on Human Herpesvirus-6 Reactivation and Encephalitis in Cord Blood Transplant Recipients: A Prospective Multicenter Trial with an Historical Control Group. Biol. Blood Marrow Transplant. 2018, 24, 1264–1273. [Google Scholar] [CrossRef]
- Hill, J.A.; Nichols, W.G.; Marty, F.M.; Papanicolaou, G.A.; Brundage, T.M.; Lanier, R.; Zerr, D.M.; Boeckh, M.J. Oral Brincidofovir Decreases the Incidence of HHV-6B Viremia after Allogeneic HCT. Blood 2020, 135, 1447–1451. [Google Scholar] [CrossRef]
- Kampouri, E.; Zamora, D.; Kiem, E.S.; Liu, W.; Ibrahimi, S.; Blazevic, R.L.; Lovas, E.A.; Kimball, L.E.; Huang, M.L.; Jerome, K.R.; et al. Human Herpesvirus-6 Reactivation and Disease after Allogeneic Haematopoietic Cell Transplantation in the Era of Letermovir for Cytomegalovirus Prophylaxis. Clin. Microbiol. Infect. 2023, 29, 1450.e1–1450.e7. [Google Scholar] [CrossRef]
- Admiraal, R.; de Koning, C.C.H.; Lindemans, C.A.; Bierings, M.B.; Wensing, A.M.J.; Versluys, A.B.; Wolfs, T.F.W.; Nierkens, S.; Boelens, J.J. Viral Reactivations and Associated Outcomes in the Context of Immune Reconstitution after Pediatric Hematopoietic Cell Transplantation. J. Allergy Clin. Immunol. 2017, 140, 1643–1650. [Google Scholar] [CrossRef]
- Wainwright, M.S.; Martin, P.L.; Morse, R.P.; Lacaze, M.; Provenzale, J.M.; Edward Coleman, R.; Morgan, M.A.; Hulette, C.; Kurtzberg, J.; Bushnell, C.; et al. Human Herpesvirus 6 Limbic Encephalitis after Stem Cell Transplantation. Ann. Neurol. 2001, 50, 612–619. [Google Scholar] [CrossRef]
- Seeley, W.W.; Marty, F.M.; Holmes, T.M.; Upchurch, K.; Soiffer, R.J.; Antin, J.H.; Baden, L.R.; Bromfield, E.B. Post-Transplant Acute Limbic Encephalitis: Clinical Features and Relationship to HHV6. Neurology 2007, 69, 156–165. [Google Scholar] [CrossRef]
- Handley, G. Current Role of Prospective Monitoring and Preemptive and Prophylactic Therapy for Human Herpesvirus 6 after Allogeneic Stem Cell Transplantation. Open Forum Infect. Dis. 2022, 9, ofac398. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Boeckh, M.J.; Sedlak, R.H.; Jerome, K.R.; Zerr, D.M. Human Herpesvirus 6 Can Be Detected in Cerebrospinal Fluid without Associated Symptoms after Allogeneic Hematopoietic Cell Transplantation. J. Clin. Virol. 2014, 61, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Handley, G.; Hasbun, R.; Okhuysen, P. Human Herpesvirus 6 and Central Nervous System Disease in Oncology Patients: A Retrospective Case Series and Literature Review. J. Clin. Virol. 2021, 136, 104740. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Satou, T.; Kawano, R.; Goto, K.; Ikewaki, J.; Kohno, K.; Ando, T.; Miyazaki, Y.; Ohtsuka, E.; Saburi, Y.; et al. Plasma HHV-6 Viral Load-Guided Preemptive Therapy against HHV-6 Encephalopathy after Allogeneic Stem Cell Transplantation: A Prospective Evaluation. Bone Marrow Transplant. 2008, 41, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.L.; Carlin, K.; Ljungman, P.; Politikos, I.; Boussiotis, V.; Boeckh, M.; Shaffer, M.L.; Zerr, D.M. Human Herpesvirus-6B Reactivation Is a Risk Factor for Grades II to IV Acute Graft-versus-Host Disease after Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Biol. Blood Marrow Transplant. 2018, 24, 2324–2336. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Myerson, D.; Sedlak, R.H.; Jerome, K.R.; Zerr, D.M. Hepatitis Due to Human Herpesvirus 6B after Hematopoietic Cell Transplantation and a Review of the Literature. Transpl. Infect. Dis. 2014, 16, 477–483. [Google Scholar] [CrossRef]
- Seo, S.; Renaud, C.; Kuypers, J.M.; Chiu, C.Y.; Huang, M.-L.; Samayoa, E.; Xie, H.; Yu, G.; Fisher, C.E.; Gooley, T.A.; et al. Idiopathic Pneumonia Syndrome after Hematopoietic Cell Transplantation: Evidence of Occult Infectious Etiologies. Blood 2015, 125, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Lee, Y.J.; Vande Vusse, L.K.; Xie, H.; Chung, E.L.; Waghmare, A.; Cheng, G.S.; Zhu, H.; Huang, M.L.; Hill, G.R.; et al. HHV-6B Detection and Host Gene Expression Implicate HHV-6B as Pulmonary Pathogen after Hematopoietic Cell Transplant. Nat. Commun. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Wang, F.Z.; Larsson, K.; Linde, A.; Ljungman, P. Human Herpesvirus 6 Infection and Cytomegalovirus-Specific Lymphoproliferative Responses in Allogeneic Stem Cell Transplant Recipients. Bone Marrow Transplant. 2002, 30, 521–526. [Google Scholar] [CrossRef]
- Humar, A.; Malkan, G.; Moussa, G.; Greig, P.; Levy, G.; Mazzulli, T. Human Herpesvirus-6 Is Associated with Cytomegalovirus Reactivation in Liver Transplant Recipients. J. Infect. Dis. 2000, 181, 1450–1453. [Google Scholar] [CrossRef]
- Dewin, D.R.; Catusse, J.; Gompels, U.A. Identification and Characterization of U83A Viral Chemokine, a Broad and Potent β-Chemokine Agonist for Human CCRs with Unique Selectivity and Inhibition by Spliced Isoform. J. Immunol. 2006, 176, 544–556. [Google Scholar] [CrossRef]
- Tormo, N.; Solano, C.; de la Cámara, R.; Garcia-Noblejas, A.; Cardeñoso, L.; Clari, M.Á.; Nieto, J.; López, J.; Hernández-Boluda, J.C.; Remigia, M.J.; et al. An Assessment of the Effect of Human Herpesvirus-6 Replication on Active Cytomegalovirus Infection after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2010, 16, 653–661. [Google Scholar] [CrossRef]
- Pellett, P.E.; Ablashi, D.V.; Ambros, P.F.; Agut, H.; Caserta, M.T.; Descamps, V.; Flamand, L.; Gautheret-Dejean, A.; Hall, C.B.; Kamble, R.T.; et al. Chromosomally Integrated Human Herpesvirus 6: Questions and Answers. Rev. Med. Virol. 2012, 22, 144. [Google Scholar] [CrossRef]
- Ward, K.N.; Leong, H.N.; Nacheva, E.P.; Howard, J.; Atkinson, C.E.; Davies, N.W.S.; Griffiths, P.D.; Clark, D.A. Human Herpesvirus 6 Chromosomal Integration in Immunocompetent Patients Results in High Levels of Viral DNA in Blood, Sera, and Hair Follicles. J. Clin. Microbiol. 2006, 44, 1571. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Watanabe, K.; Ohye, T.; Suzuki, K.; Matsubara, T.; Shimizu, N.; Kurahashi, H.; Yoshikawa, T.; Katano, H.; Inoue, N.; et al. Molecular and Virological Evidence of Viral Activation From Chromosomally Integrated Human Herpesvirus 6A in a Patient with X-Linked Severe Combined Immunodeficiency. Clin. Infect. Dis. 2014, 59, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; de la Camara, R.; Cordonnier, C.; Einsele, H.; Engelhard, D.; Reusser, P.; Styczynski, J.; Ward, K. Management of CMV, HHV-6, HHV-7 and Kaposi-Sarcoma Herpesvirus (HHV-8) Infections in Patients with Hematological Malignancies and after SCT. Bone Marrow Transplant. 2008, 42, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Inazawa, N.; Hori, T.; Nojima, M.; Saito, M.; Igarashi, K.; Yamamoto, M.; Shimizu, N.; Yoto, Y.; Tsutsumi, H. Virus Reactivations after Autologous Hematopoietic Stem Cell Transplantation Detected by Multiplex PCR Assay. J. Med. Virol. 2017, 89, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, M.P.; Rybka, W.B.; Stewart, J.A.; Patton, J.L.; Stamey, F.R.; Elsawy, M.; Pellett, P.E.; Armstrong, J.A. Human Herpesvirus 6: Infection and Disease Following Autologous and Allogeneic Bone Marrow Transplantation. Blood 1996, 87, 5341–5354. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Tanaka-Taya, K.; Hara, J.; Fujisaki, H.; Matsuda, Y.; Ohta, H.; Osugi, Y.; Okada, S.; Yamanishi, K. Inverse Relationship between Human Herpesvirus-6 and -7 Detection after Allogeneic and Autologous Stem Cell Transplantation. Bone Marrow Transplant. 2001, 27, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Imbert-Marcille, B.M.; Tang, X.W.; Lepelletier, D.; Besse, B.; Moreau, P.; Billaudel, S.; Milpied, N. Human Herpesvirus 6 Infection after Autologous or Allogeneic Stem Cell Transplantation: A Single-Center Prospective Longitudinal Study of 92 Patients. Clin. Infect. Dis. 2000, 31, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Shargian-Alon, L.; Yahav, D.; Rozovski, U.; Dovrat, S.; Amitai, I.; Sela-Navon, M.; Pasvolsky, O.; Raanani, P.; Yeshurun, M. Human Herpes Virus 6 Reactivation Following Autologous Hematopoietic Cell Transplantation—A Single-Center Experience. Leuk. Lymphoma 2019, 60, 2230–2236. [Google Scholar] [CrossRef]
- Colombier, M.A.; Amorim, S.; Salmona, M.; Thieblemont, C.; Legoff, J.; Lafaurie, M. HHV-6 Reactivation as a Cause of Fever in Autologous Hematopoietic Stem Cell Transplant Recipients. J. Infect. 2017, 75, 155–159. [Google Scholar] [CrossRef]
- Horowitz, N.; Oren, I.; Lavi, N.; Zuckerman, T.; Benyamini, N.; Kra-Oz, Z.; Held, V.; Avivi, I. New Rising Infection: Human Herpesvirus 6 Is Frequent in Myeloma Patients Undergoing Autologous Stem Cell Transplantation after Induction Therapy with Bortezomib. Bone Marrow Res. 2012, 2012, 409765. [Google Scholar] [CrossRef]
- Baird, J.H.; Epstein, D.J.; Tamaresis, J.S.; Ehlinger, Z.; Spiegel, J.Y.; Craig, J.; Claire, G.K.; Frank, M.J.; Muffly, L.; Shiraz, P.; et al. Immune Reconstitution and Infectious Complications Following Axicabtagene Ciloleucel Therapy for Large B-Cell Lymphoma. Blood Adv. 2021, 5, 143–155. [Google Scholar] [CrossRef]
- Rejeski, K.; Perez Perez, A.; Sesques, P.; Hoster, E.; Berger, C.S.; Jentzsch, L.; Mougiakakos, D.; Frölich, L.; Ackermann, J.; Buecklein, V.; et al. CAR-HEMATOTOX: A Model for CAR T-Cell Related Hematological Toxicity in Relapsed/Refractory Large B-Cell Lymphoma. Blood 2021, 138, 2499–2513. [Google Scholar] [CrossRef]
- Kampouri, E.; Ibrahimi, S.S.; Xie, H.; Wong, E.R.; Hecht, J.B.; Sekhon, M.K.; Vo, A.; Stevens-Ayers, T.L.; Green, D.J.; Gauthier, J.; et al. CMV Reactivation and CMV-Specific Cell-Mediated Immunity after Chimeric Antigen Receptor T-Cell Therapy. Clin. Infect. Dis. 2023, ciad708. [Google Scholar] [CrossRef]
- Beyar-Katz, O.; Kikozashvili, N.; Bar On, Y.; Amit, O.; Perry, C.; Avivi, I.; Gold, R.; Herishanu, Y.; Benyamini, N.; Duek, A.; et al. Characteristics and Recognition of Early Infections in Patients Treated with Commercial Anti-CD19 CAR-T Cells. Eur. J. Haematol. 2021, 108, 52–60. [Google Scholar] [CrossRef]
- Spanjaart, A.M.; van der Valk, F.M.; van Rooijen, G.; Brouwer, M.C.; Kersten, M.J. Confused about Confusion. N. Engl. J. Med. 2022, 386, 80–87. [Google Scholar] [CrossRef]
- Shah, M.; Kuhnl, A.; Shields, G.; Sudhanva, M.; Metaxa, V.; Wong, S.; Yallop, D.; Patten, P.; Inam, S.; Hockings, C.; et al. Human Herpesvirus 6 Encephalitis Following Axicabtagene Ciloleucel Treatment for Refractory Diffuse Large B Cell Lymphoma. Hemasphere 2021, 5, e535. [Google Scholar] [CrossRef] [PubMed]
- Rebechi, M.T.; Bork, J.T.; Riedel, D.J. HHV-6 Encephalitis After Chimeric Antigen Receptor T-Cell Therapy (CAR-T): 2 Case Reports and a Brief Review of the Literature. Open Forum Infect. Dis. 2021, 8, ofab470. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma (ZUMA-1): A Single-Arm, Multicentre, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Handley, G.; Khawaja, F.; Kondapi, D.S.; Lee, H.J.; Kaufman, G.P.; Neelapu, S.S.; Fayad, L.E.; Tummala, S.; Chi, L.; Strati, P.; et al. Human Herpesvirus 6 Myelitis after Chimeric Antigen Receptor T-Cell Therapy. Int. J. Infect. Dis. 2021, 112, 327–329. [Google Scholar] [CrossRef]
- Grant, S.J.; Grimshaw, A.A.; Silberstein, J.; Murdaugh, D.; Wildes, T.M.; Rosko, A.E.; Giri, S. Clinical Presentation, Risk Factors, and Outcomes of Immune Effector Cell-Associated Neurotoxicity Syndrome Following Chimeric Antigen Receptor T Cell Therapy: A Systematic Review. Transplant. Cell Ther. 2022, 28, 294–302. [Google Scholar] [CrossRef]
- Kampouri, E.; Kiem, E.; Vo, A.; Liu, W.L.; Chalal, C.; Basom, R.S.; Davis, C.; Turtle, C.J.; Shadman, M.; Till, B.; et al. 2711. Viral Encephalitis after Chimeric Antigen Receptor (CAR)-Modified T-Cell Therapy: A Retrospective Cohort Study. Open Forum Infect. Dis. 2023, 10, ofad500.2322. [Google Scholar] [CrossRef]
- Rejeski, K.; Subklewe, M.; Aljurf, M.; Bachy, E.; Balduzzi, A.C.; Barba, P.; Bruno, B.; Benjamin, R.; Carrabba, M.G.; Chabannon, C.; et al. Immune Effector Cell-Associated Hematotoxicity (ICAHT): EHA/EBMT Consensus Grading and Best Practice Recommendations. Blood 2023, 142, 865–877. [Google Scholar] [CrossRef]
- Kampouri, E.; Ibrahimi, S.; Hecht, J.; Keane-Candib, J.; Xie, H.; Stevens-Ayers, T.; Gauthier, J.; Maloney, D.; Huang, M.-L.; Jerome, K.; et al. CMV and HHV-6 after Chimeric Antigen Receptor (CAR)-T-Cell Immunotherapy for B-Cell Malignancies: A Prospective Study. Transplant. Cell Ther. 2023, 29, S210–S211. [Google Scholar] [CrossRef]
- Lareau, C.A.; Yin, Y.; Maurer, K.; Sandor, K.D.; Daniel, B.; Yagnik, G.; Peña, J.; Crawford, J.C.; Spanjaart, A.M.; Gutierrez, J.C.; et al. Latent Human Herpesvirus 6 Is Reactivated in CAR T Cells. Nature 2023, 623, 608–615. [Google Scholar] [CrossRef] [PubMed]
- De Pagter, P.J.; Schuurman, R.; De Vos, N.M.; Mackay, W.; Van Loon, A.M. Multicenter External Quality Assessment of Molecular Methods for Detection of Human Herpesvirus 6. J. Clin. Microbiol. 2010, 48, 2536–2540. [Google Scholar] [CrossRef]
- WHO/BS/2017.2321 Collaborative Study to Establish the 1st WHO IS for Human Herpes Virus 6B (HHV-6B) DNA for Nucleic Acid Amplification Technique (NAT)-Based Assays. Available online: https://www.who.int/publications/m/item/WHO-BS-2017.2321 (accessed on 31 January 2024).
- Hill, J.A. Human Herpesvirus 6 in Transplant Recipients: An Update on Diagnostic and Treatment Strategies. Curr. Opin. Infect. Dis. 2019, 32, 584–590. [Google Scholar] [CrossRef]
- Sedlak, R.H.; Cook, L.; Huang, M.-L.; Magaret, A.; Zerr, D.M.; Boeckh, M.; Jerome, K.R. Identification of Chromosomally Integrated Human Herpesvirus 6 by Droplet Digital PCR. Clin. Chem. 2014, 60, 765–772. [Google Scholar] [CrossRef]
- Vellucci, A.; Leibovitch, E.C.; Jacobson, S. Using Droplet Digital PCR to Detect Coinfection of Human Herpesviruses 6A and 6B (HHV-6A and HHV-6B) in Clinical Samples. Methods Mol. Biol. 2018, 1768, 99–109. [Google Scholar] [CrossRef]
- Sedlak, R.H.; Hill, J.A.; Nguyen, T.; Cho, M.; Levin, G.; Cook, L.; Huang, M.L.; Flamand, L.; Zerr, D.M.; Boeckh, M.; et al. Detection of Human Herpesvirus 6B (HHV-6B) Reactivation in Hematopoietic Cell Transplant Recipients with Inherited Chromosomally Integrated HHV-6A by Droplet Digital PCR. J. Clin. Microbiol. 2016, 54, 1223–1227. [Google Scholar] [CrossRef]
- Prichard, M.N.; Whitley, R.J. The Development of New Therapies for Human Herpesvirus 6. Curr. Opin. Virol. 2014, 9, 148–153. [Google Scholar] [CrossRef]
- Akhyani, N.; Fotheringham, J.; Yao, K.; Rashti, F.; Jacobson, S. Efficacy of Antiviral Compounds in Human Herpesvirus-6-Infected Glial Cells. J. Neurovirol 2006, 12, 284–293. [Google Scholar] [CrossRef]
- Toomey, D.; Phan, T.L.; Nguyen, V.; Phan, T.T.; Ogata, M. Retrospective Case Analysis of Antiviral Therapies for HHV-6 Encephalitis after Hematopoietic Stem Cell Transplantation. Transpl. Infect. Dis. 2021, 23, e13443. [Google Scholar] [CrossRef]
- Ogata, M.; Uchida, N.; Fukuda, T.; Ikegame, K.; Kamimura, T.; Onizuka, M.; Kato, K.; Kobayashi, H.; Sasahara, Y.; Sawa, M.; et al. Clinical Practice Recommendations for the Diagnosis and Management of Human Herpesvirus-6B Encephalitis after Allogeneic Hematopoietic Stem Cell Transplantation: The Japan Society for Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2020, 55, 1004–1013. [Google Scholar] [CrossRef]
- Olson, A.L.; Politikos, I.; Brunstein, C.; Milano, F.; Barker, J.; Hill, J.A. Guidelines for Infection Prophylaxis, Monitoring and Therapy in Cord Blood Transplantation. Transplant. Cell Ther. 2021, 27, 359–362. [Google Scholar] [CrossRef]
- Ogata, M.; Satou, T.; Inoue, Y.; Takano, K.; Ikebe, T.; Ando, T.; Ikewaki, J.; Kohno, K.; Nishida, A.; Saburi, M.; et al. Foscarnet against Human Herpesvirus (HHV)-6 Reactivation after Allo-SCT: Breakthrough HHV-6 Encephalitis Following Antiviral Prophylaxis. Bone Marrow Transplant. 2013, 48, 257–264. [Google Scholar] [CrossRef]
- Ishiyama, K.; Katagiri, T.; Hoshino, T.; Yoshida, T.; Yamaguchi, M.; Nakao, S. Preemptive Therapy of Human Herpesvirus-6 Encephalitis with Foscarnet Sodium for High-Risk Patients after Hematopoietic SCT. Bone Marrow Transplant. 2011, 46, 863–869. [Google Scholar] [CrossRef]
- Ishiyama, K.; Katagiri, T.; Ohata, K.; Hosokawa, K.; Kondo, Y.; Yamazaki, H.; Takami, A.; Nakao, S. Safety of Pre-Engraftment Prophylactic Foscarnet Administration after Allogeneic Stem Cell Transplantation. Transpl. Infect. Dis. 2012, 14, 33–39. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, L.; Xue, M.; Liu, J.; Guo, Z. Low-Dose Foscarnet Preemptive Therapy for Cytomegalovirus Viremia after Haploidentical Bone Marrow Transplantation. Biol. Blood Marrow Transplant. 2009, 15, 519–520. [Google Scholar] [CrossRef][Green Version]
- El Jurdi, N.; Rogosheske, J.; DeFor, T.; Bejanyan, N.; Arora, M.; Bachanova, V.; Betts, B.; He, F.; Holtan, S.; Janakiram, M.; et al. Prophylactic Foscarnet for Human Herpesvirus 6: Effect on Hematopoietic Engraftment after Reduced-Intensity Conditioning Umbilical Cord Blood Transplantation. Transplant. Cell Ther. 2021, 27, 84.e1–84.e5. [Google Scholar] [CrossRef]
- Vittayawacharin, P.; E’Leimat, G.; Lee, B.J.; Griffin, S.; Doh, J.; Nam, H.; Blodget, E.; Jeyakumar, D.; Kongtim, P.; Ciurea, S.O. Once-Daily Foscarnet Is Effective for Human Herpesvirus 6 Reactivation after Hematopoietic Stem Cell Transplantation. Transplant. Cell Ther. 2023, 29, 397.e1–397.e6. [Google Scholar] [CrossRef]
- Walti, C.S.; Stuehler, C.; Palianina, D.; Khanna, N. Immunocompromised Host Section: Adoptive T-Cell Therapy for DsDNA Viruses in Allogeneic Hematopoietic Cell Transplant Recipients. Curr. Opin. Infect. Dis. 2022, 35, 302–311. [Google Scholar] [CrossRef]
- Green, A.; Rubinstein, J.D.; Grimley, M.; Pfeiffer, T. Virus-Specific T Cells for the Treatment of Systemic Infections Following Allogeneic Hematopoietic Cell and Solid Organ Transplantation. J. Pediatr. Infect. Dis. Soc. 2024, 13, S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Tzannou, I.; Papadopoulou, A.; Naik, S.; Leung, K.; Martinez, C.A.; Ramos, C.A.; Carrum, G.; Sasa, G.; Lulla, P.; Watanabe, A.; et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2017, 35, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Tzannou, I.; Wu, M.; Ramos, C.; Sasa, G.; Martinez, C.; Lulla, P.; Krance, R.A.; Scherer, L.; Ruderfer, D.; et al. Posoleucel, an Allogeneic, Off-the-Shelf Multivirus-Specific T-Cell Therapy, for the Treatment of Refractory Viral Infections in the Post-HCT Setting. Clin. Cancer Res. 2023, 29, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, S.S.; Young, J.-A.H.; Schuster, M.W.; Yared, J.A.; Myers, G.D.; Matzko, M.; Adnan, S.; Hill, J.A.; Bansal, R. Final Clinical Outcomes from a Phase 2 Trial of Posoleucel, an Off-the-Shelf, Multivirus-Specific T-Cell Therapy, for the Prevention of Clinically Significant Viral Infections Post-HCT. Blood 2022, 140, 878–879. [Google Scholar] [CrossRef]
- AlloVir Provides Updates on Phase 3 Clinical Development Program for Posoleucel, an Allogeneic Virus-Specific T Cell Therapy|AlloVir, Inc. Available online: https://ir.allovir.com/news-releases/news-release-details/allovir-provides-updates-phase-3-clinical-development-program (accessed on 12 February 2024).
- Zaki Salahuddin, S.; Ablashi, D.V.; Markham, P.D.; Josephs, S.F.; Sturzenegger, S.; Kaplan, M.; Halligan, G.; Biberfeld, P.; Wong-Staal, F.; Kramarsky, B.; et al. Isolation of a New Virus, HBLV, in Patients with Lymphoproliferative Disorders. Science (1979) 1986, 234, 596–601. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kampouri, E.; Handley, G.; Hill, J.A. Human Herpes Virus-6 (HHV-6) Reactivation after Hematopoietic Cell Transplant and Chimeric Antigen Receptor (CAR)- T Cell Therapy: A Shifting Landscape. Viruses 2024, 16, 498. https://doi.org/10.3390/v16040498
Kampouri E, Handley G, Hill JA. Human Herpes Virus-6 (HHV-6) Reactivation after Hematopoietic Cell Transplant and Chimeric Antigen Receptor (CAR)- T Cell Therapy: A Shifting Landscape. Viruses. 2024; 16(4):498. https://doi.org/10.3390/v16040498
Chicago/Turabian StyleKampouri, Eleftheria, Guy Handley, and Joshua A. Hill. 2024. "Human Herpes Virus-6 (HHV-6) Reactivation after Hematopoietic Cell Transplant and Chimeric Antigen Receptor (CAR)- T Cell Therapy: A Shifting Landscape" Viruses 16, no. 4: 498. https://doi.org/10.3390/v16040498
APA StyleKampouri, E., Handley, G., & Hill, J. A. (2024). Human Herpes Virus-6 (HHV-6) Reactivation after Hematopoietic Cell Transplant and Chimeric Antigen Receptor (CAR)- T Cell Therapy: A Shifting Landscape. Viruses, 16(4), 498. https://doi.org/10.3390/v16040498





