Abstract
The recent multi-country outbreak of Mpox (Monkeypox disease) constituted a public health emergency. Although animal-to-human transmission is known to be the primary way of transmission, an increasing number of cases transmitted by person-to-person contact have been reported. During the recent Mpox outbreak sexual or intimate contact has been considered the most important way of transmission. However, other routes of transmission must not be ignored. The knowledge of how the Monkeypox Virus (MPXV) spreads is crucial to implement adequate measures to contain the spread of the disease. Therefore, this systematic review aimed to collect scientific data published concerning other implicated sources of infection beyond sexual interaction, such as the involvement of respiratory particles, contact with contaminated surfaces and skin-to-skin contact. The current study was performed using the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Publications analyzing contacts of Mpox index cases and their outcome after contact were included. A total of 7319 person-to-person contacts were surveyed and 273 of them became positive cases. Positive secondary transmission of MPXV was verified after contact with people cohabiting in the same household, with family members, with healthcare workers, or within healthcare facilities, and sexual contact or contact with contaminated surfaces. Using the same cup, sharing the same dishes, and sleeping in the same room or bed were also positively associated with transmission. Five studies showed no evidence of transmission despite contact with surfaces, skin-to-skin contact, or through airway particles within healthcare facilities where containment measures were taken. These records support the case for person-to-person transmission and suggest that other types of contact beyond sexual contact pose a significant risk of acquiring the infection. Further investigation is crucial to elucidate MPXV transmission dynamics, and to implement adequate measures to contain the spread of the infection.
1. Introduction
Mpox (Monkeypox disease) is a zoonotic infectious disease established in humans characterized by rash, fever, skin lesions, lymphadenopathy, headache, upper respiratory symptoms, oral ulcers, vomiting, and conjunctivitis [1]. This disease is caused by the Monkeypox virus (MPXV), a virus that belongs to the genus Orthopoxvirus and the family Poxviridae [2]. Although this virus was originally found in animals, it was firstly identified in humans in 1970 in the Democratic Republic of the Congo [3,4,5]. Historically, MPXV genetic diversity has been classified into two clades [6]. However, a new proposal for MPXV classification has been established into three clades (I, IIa, and IIb). Clade I corresponds to the prior “Congo Basin clade”; while clades IIa and IIb correspond to the “West Africa clade” [7]. The first outbreak of human Mpox outside its endemic region, registered in the USA, was indirectly related to various infected rodent species that were imported from West Africa. This outbreak occurred in prairie dogs living in contact with the rodent’s species imported from Ghana. As a result, the prairie dogs transmitted MPXV to approximately 40 humans. This was the first known outbreak of Mpox outside of Africa [8,9]. In November of 2022, the top 10 countries with reported Mpox cases were USA, Brazil, Spain, France, the United Kingdom, Germany, Colombia, Peru, Mexico, and Canada [10].
Primary animal-to-human infection is assumed to occur when handling Mpox infected animals through direct (touch, bites, or scratch) [11] or indirect contact, although the exact mechanism(s) remains to be defined. The virus is assumed to enter the body through broken skin, respiratory tract, or mucous membranes (eyes, nose, or mouth) [10]. Secondary human-to-human transmission occurs presumably through large respiratory droplets or direct or indirect contact with body fluids, lesion material, and contaminated surfaces or other material, such as clothing or linens [12]. Prolonged contact with patients renders hospital staff and family members at a greater risk of infection [13]. The risk of nosocomial transmission, vertical transmission, and fetal deaths have already been described [14,15]. One health approach is necessary for disease detection, including wildlife surveillance and investigation into animal reservoir.
This systematic review aims to summarize the evidence associated with the human-to-human transmission of the MPXV, and other sources of infection beyond sexual transmission, such as the involvement of respiratory particles, the contact with contaminated surfaces, and skin-to-skin contact.
2. Materials and Methods
2.1. Protocol
This systematic review was conducted based on the recommendations presented in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [16]. The methodology used, including the screening of the title and abstract, full text reading, inclusion/exclusion, and data extraction criteria were accepted by all authors.
2.2. Eligibility Criteria
This systematic review was performed to be an updated review to collect scientific published data concerning other implicated sources of Mpox infection beyond sexual interaction. Articles meeting the following criteria were eligible for inclusion: (1) studies concerning people who contacted with cases of Mpox, and (2) whose outcome after contact was evaluated. We included publications without restrictions regarding the type of classification of the studies. Thus, articles referring to human-to-human contact, letters to the editors, observational studies, and other systematic reviews were included. Furthermore, there were no restrictions on the language or related to the date of publication of the articles. Publications up to the date of 15 April 2023were searched and included. Articles addressing animal-to-human or human-to-animal contact, vertical transmission, reviews, and case reports were excluded.
2.3. Information Sources and Search Strategy
We performed an electronic search on four databases, PubMed, Scopus, Web of Science, and Preprints (date of initial search 1 November 2022; last update 15 April 2023). To ensure that the search was inclusive, keywords (alone or in combination) related to scientific literature concerning Mpox person-to-person transmission were included. For this research, we used the MeSH Browser from the NIH (National Institute of Health) that allows a user to search directly for MeSH terms. The queries used are presented in Supplementary Table S1 and included the terms: “monkeypox”, “transmission”, “transmissions”, “transmissibility”, transmissible”, “clothing”, “clothes”, “contact”, “surface”, “surfaces”, “fomite”, “fomites”, “particle”, “particles”, “sexual”, “mucous”, “contaminated”, “contamination”, “respiratory”, “skin”, “skin-to-skin”, “humans”, and “human”.
2.4. Study Selection
Two independent researchers (P.P. and M.A.C.) selected the articles one by one for inclusion or exclusion based on the title and abstract. The conflicts were solved by a third researcher (C.L.). The full text of the resulting articles was analyzed by two researchers, who selected independently the articles for inclusion. Once more, potential conflicts were discussed and solved by a third author.
An online program (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia) was used to remove duplicates, to conduct the screening of the articles, and to find the ones eligible for full text analysis.
2.5. Data Collection Process
Three authors (P.P., M.A.C. and M.F.M.G.) extracted information independently from the included articles and reached a consensus for data inclusion. Data extraction was conducted using Microsoft Word software and predefined tables. The following information was collected from each included study: author, year of publication, study design, setting data, case definition, type of contact/mode of transmission, index case(s), contacts (possible), secondary cases, conclusion, and limitations.
2.6. Study Quality Assessment
The risk of bias was evaluated using the quality assessment tool from the National Institutes of Health, which allowed us to classify the studies into poor, fair, or good quality depending on the fulfilment of the criteria defined by this tool [17]. This assessment was performed by M.A.C., M.F.M.G., and C.L. Divergences resulting from this process were discussed between the authors.
3. Results
3.1. Number of Retrieved Papers
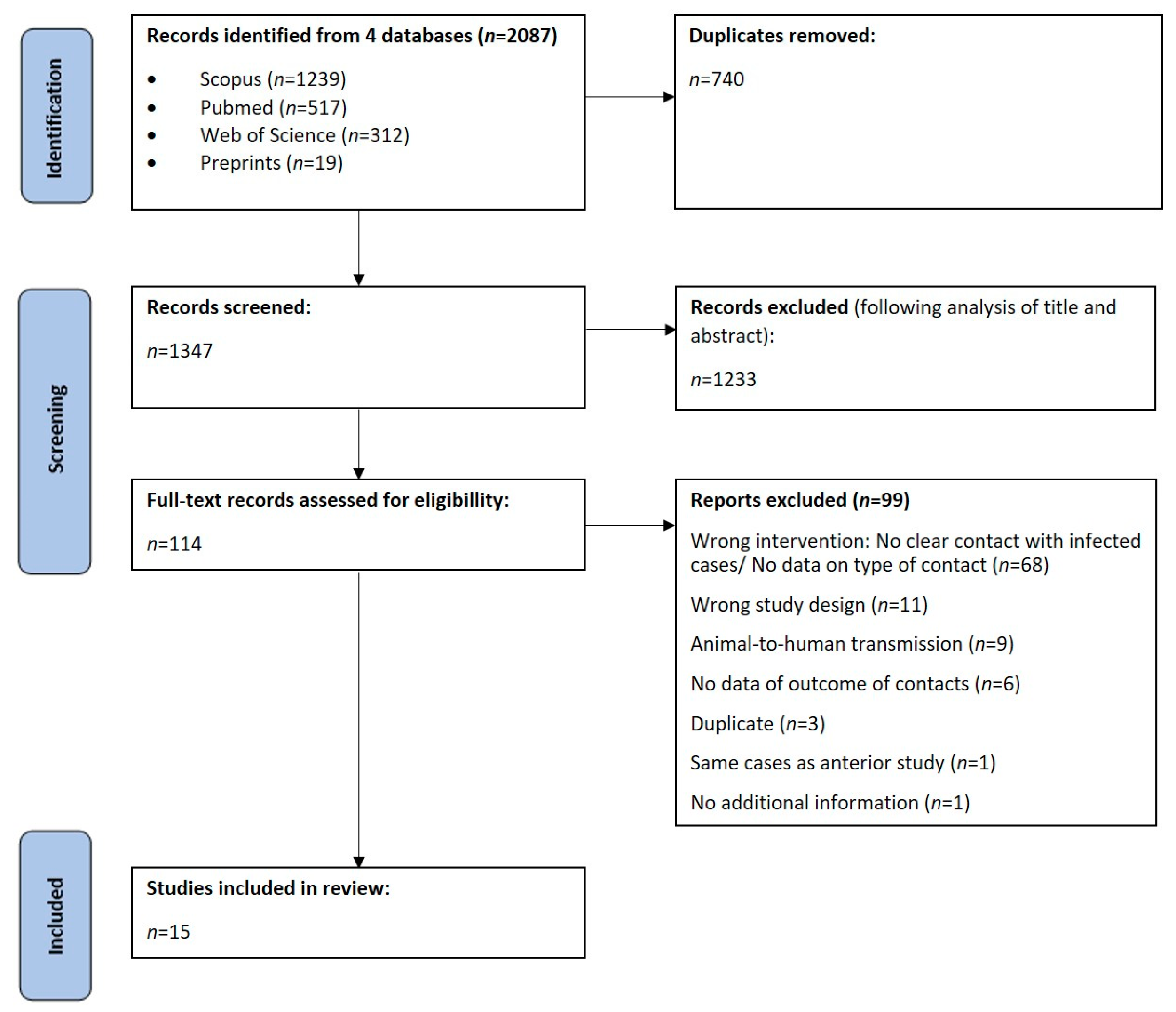
A total of 2087 articles were found in our research on 15 April 2023, 740 of which were duplicated. Out of 1347 articles, 1233 were excluded after analysis of title and abstract and 114 were included for full-text analysis. We further excluded 99 articles considering inclusion and exclusion criteria. A final number of 15 observational studies were included in this systematic review: five retrospective cohorts and ten prospective cohorts. These results are summarized in Figure 1.
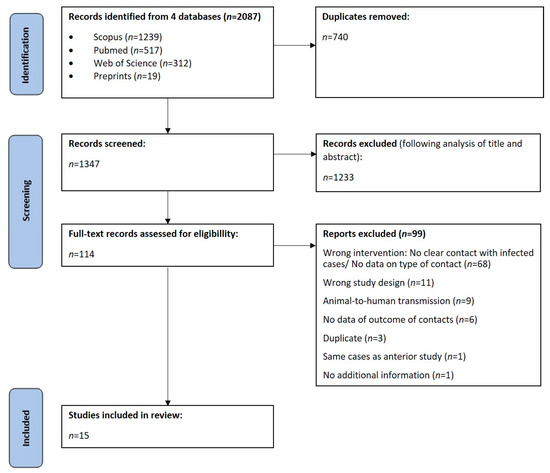
Figure 1.
Flow chart of study selection.
3.2. Study Characteristics
This systematic review included 15 studies [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] (Table 1): seven studies were from Africa [18,19,20,21,23,24,25], six of which from Zaire, now called Democratic Republic of the Congo [18,19,20,21,24], three studies from the USA [22,29,30], one from Singapore [26], and three studies from Europe, one from United Kingdom [27] and two from Spain [28,32], respectively. One study had no reference to the country of origin [31]. Ten studies were published before 2022 [18,19,20,21,22,23,24,25,26,27], and the remaining five were published in 2022 or 2023 [28,29,30,31,32].

Table 1.
Characteristics of the 13 included studies.
3.3. Outcome Assessment and Evidence of Contact with MPXV
The clade of MPXV involved was only known in one study, which was the West African clade [29]. In two studies, the outcome assessment was only clinical [26,27]. Another study does not specify how the outcome was assessed [31]. The other 12 had, at some point, confirmation by serological findings or virus isolation [18,19,20,21,22,23,24,25,28,29,30]. All studies reported person-to-person contact with infected patients. A total of 7319 contacts were surveyed, and 273 of them were considered Mpox positive cases. Seven studies reported household contact [18,19,20,21,23,24,28], six studies reported surface/fomites contact [22,26,28,30,31,32], two of them at a tattoo studio [28,32], two studies reported potential contact with airway particles [22,30] and three reported skin-to-skin contact [22,30,31], two studies reported intrafamilial contact [23,25], one study reported sexual contact [28], one study reported direct contact with the patient or the patient’s surroundings or specimens [26], and one study reported person-to-person but no sexual or skin-to-skin contact [29]. Two studies focused on Mpox transmission on pediatric population [23,28].
3.4. Evidence of Transmission
Ten studies showed positive secondary transmission of MPXV [18,19,20,21,23,24,25,27,28,32]. In these, there was evidence of contact with people cohabiting in the same household [18,19,20,21,23,24,28], with family members [23,25], with healthcare workers [25,27], or within healthcare facilities [23], with contaminated surfaces at a tattoo studio [28,32] and sexual [28]. Drinking from the same cup, eating from the same dish, sleeping in the same room, and sleeping in the same bed were positively associated with transmission [24]. Five studies showed no evidence of transmission [22,26,29,30,31] despite contact with surfaces, skin-to-skin contact, or through airway particles in five healthcare centers [22,26,27,30,31] and one in a prison facility [29].
Data related to the use of mask, gloves, or other protective equipment were available in 7 of the 15 studies [22,23,26,27,29,30,31], 2 of which reported low usage of protective measures [23,29].
3.5. Risk of Bias within and across Studies
A total of six studies were considered to have poor quality [18,19,23,25,28,32], seven studies were considered fair [22,24,26,27,29,30,31], and two studies were considered as good [20,21]. The studies with poor quality consequently resulted in low levels of evidence. Participants were lost to follow-up in some studies and others had a very low population size. The same cases were probably present in different included studies, which could result in duplicate information. The use of gloves and other protective equipment made it somewhat difficult to measure adequately the exposure of contacts. A previous smallpox vaccination history could underestimate the risk of transmission to unvaccinated individuals. Outcomes were not always obtained by PCR techniques, having been evaluated only symptomatically, which meant that outcome measures were not consistently applied within and across studies. Additionally, the studies are not entirely comparable because they occurred in different places, at different moments in time, and they must be analyzed taking these questions into account.
4. Discussion
4.1. Summary of Evidence
Although several studies suggested a household transmission of the virus, the type of contact occurring in the habitation is difficult to understand [18,19,20,21,23,28]. The transmission of MPXV could be a result of skin-to-skin contact, respiratory particles, sharing the same household surfaces or fomites, or intimate contact. For instance, Nolen et al. [24] showed that people who share the same cup and dish, and sleep in the same room or bed are prone to transmitting the virus to others. Such outcome corroborates the assumption that MPXV might be transmitted by surfaces or fomites. Nevertheless, activities such as kissing or laundering clothes showed no correlation with the acquisition of the virus [24]. Although the type of transmission is not clear, these studies reinforce the possibility of person-to-person transmission of MPXV. However, it was reported that people who shared the same space with a confirmed Mpox case in a prison facility in Chicago—despite some of them being offered post-exposure prophylaxis—did not reveal positive transmission after contact with the index patient [29]. None of the exposed reported sexual contact with others nor skin-to-skin contact; they only reported washing their clothes in communal showers, sharing personal hygiene items, or sitting on other’s beds. Therefore, additional data on the type of contact between people who live in the same household are needed.
Some studies showed no evidence of transmission of the virus after exposure of healthcare workers to infected patients through potentially contaminated surfaces and fomites, airway particles like during aerosol-generating procedures, skin-to-skin contact [22,26,30,31], and through handling patient’s specimens [26]. The use of protective measures like masks, gloves, and medical gowns by healthcare workers while caring for patients with Mpox can protect themselves from infection [22,26,30,31]. Nevertheless, transmission to healthcare workers was documented by Besombes et al. [25], in Central African Republic and to other patients in a hospital in Democratic Republic of Congo by Learned et al. [23]. We highlight the importance of educating people and healthcare personnel to prevent infectious outbreaks by strengthening health centers’ capacity and resources in remote forest areas as well as to learn measures to control the transmission to themselves and others.
Besombes et al. [25] and Learned et al. [23] showed also a positive intrafamilial transmission and a cascade of spread over three and six waves of person-to-person transmission, respectively, which shows the implication of other types of transmission other than sexual contact: from an index patient to his two daughters, two sisters, and one sister-in-law [25] and from at least two contacts in a family that ended up having Mpox confirmed [23]. Person-to-person transmission was also verified in the pediatric population [28], aged 7 months to 17 years old, which was related to possible sexual transmission, transmission via contaminated material in a tattoo and piercing studio, or by contact with their parents. There is evidence that MPXV can be widely spread through contaminated materials at tattoo and piercing studios [28,32], which is a reminder that surfaces must be cleaned after usage at these places.
After the beginning of the recent Mpox outbreak in 2022, a study conducted by Vaughan et al. [33] assessed the current epidemiological situation, based on confirmed cases of Mpox in 36 countries in the European region submitted to the European Surveillance System (TESSy). The authors demonstrated that the majority of MPXV cases were likely to be transmitted sexually. Other cases were described to involve a surface/fomite, non-sexual, and non-healthcare related transmission. However, the contact with clear confirmed cases was not possible in this study given that the analyses performed were based on data submitted to TESSy database.
Regarding the transmission through surfaces, four studies [34,35,36,37] evaluated the presence of MPXV on surfaces in the household [34,35], workplace [36], and a hospital environment [37] after a positive case was in close contact with those surfaces. All studies showed high amounts of detectable virus on surfaces directly touched by a person with Mpox, and the virus could be present either on porous or nonporous surfaces [34,35]. When the specimens were collected by swabbing the surfaces were cultivated on cell cultures (Vero cells), the virus was showed to be viable in some specimens in two studies [34,37] and not viable in any specimen in one study [35]. Morgan et al. [34] stated that the virus viability can be maintained for at least a period of 15 days after the contact. Therefore, these results show that MPXV could infect people through contact with surfaces. Although this hypothesis has not been confirmed yet, Mpox-specific cleaning, maintaining appropriate hand hygiene, and decontamination measures of surfaces should be considered in such situations [35,36]. Nevertheless, additional studies regarding MPXV transmission via contaminated surfaces and objects are crucial since there is still insufficient knowledge on this topic.
Due to a lack of data regarding the transmission of MPXV, it is not clear what has changed concerning the transmission dynamics comparing Mpox cases before and after the outbreak of 2022. Although it has been shown that individuals were more prone to acquire infection following sexual contact, we need more clear evidence that clearly states that.
A recent study by Al-Raeei [38] looked at the contagiousness of MPXV and has given a basic reproduction number related to its transmissibility considering the recent world outbreak. They found that the average R0 number, after evaluating the dynamics of one country of each continent was 1.2810. As R0 is >1, it means that the epidemic is evolving rather than plateauing. The estimated number of R0 is important to know at what stage of outbreak we are in and what can be done to contain it.
4.2. Limitations
This systematic review poses several limitations. Some studies showed low quality or did not mention what type of person-to-person contact played a role in the transmission [18,19,23,25,28]. Moreover, other studies which contained only epidemiological data and lacked information on the type of contact between two infected individuals were excluded, which may bias the results. Additionally, the 2022 outbreak is a recent occurrence and thus, there is still little information, which does not address the knowledge gaps about Mpox transmission dynamics.
There is also an additional risk of bias in establishing the transmission potential of the virus because some patients received post-exposure prophylaxis [29], while others were vaccinated against MPXV [18,19,20,21,22,25,27,29,30]; in other studies, information about previous vaccination was lacking.
Some of the included articles did not confirm a Mpox infection among contacts by detection of MPXV DNA using the PCR technique. That can lead to a bias of the results because it is possible that some infected people might be asymptomatic. Some individuals, especially healthcare workers, used protection like masks and gloves while making contact with a positive case. That probably limited the spread of infection, and the results could be biased [21,26,27,29,30]. People might have made contact in different ways at the same time, thus making it difficult to know which type of contact was responsible for the infection.
5. Conclusions
The recent outbreak of MPXV still is of great concern. Cases of infection have been reported in several countries, worldwide, and the virus transmission initially started as person-to-person contact. This systematic review aimed to clarify the different types of transmission that have been established. The findings of this systematic review support the view that MPXV can be transmitted person-to-person in addition to sexual contact, by piercing and tattooing, contaminated surfaces, objects, and fomites.
Nevertheless, considering the limited studies on Mpox in humans, there is a need for an improvement in the quality of studies and further investigation focusing on understanding the types of transmission of the virus. It is very important to know how individuals can protect themselves from infection and which precautions are needed. Yet, the use of masks is recommended when in contact with any suspect or confirmed case, especially in healthcare facilities. Activities such as frequent hand disinfection and appropriate surface cleaning are widely recommended.
In future investigations, it is necessary to maintain a good follow-up of case contacts and to correctly establish the type of contact between people. In addition, confirmation of an Mpox infection by PCR should be considered in all studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15051074/s1, Table S1: Queries used for database search in PubMed, Scopus, and Web of Science.
Author Contributions
Conceptualization, C.L.; methodology, P.P., M.A.C. and C.L.; formal analysis, P.P., M.A.C., M.F.M.G. and C.L.; investigation, P.P., M.A.C., M.F.M.G. and C.L.; data curation, P.P., M.A.C., M.F.M.G. and C.L.; writing—original draft preparation, P.P., M.A.C. and M.F.M.G.; writing—review and editing, P.P., M.A.C., M.F.M.G., A.G.R. and C.L.; supervision, C.L.; project administration, C.L. and A.G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by NORTE-01-0145-FEDER-000057 (SexHealth & Prostate Cancer, Psychobiological Determinants of Sexual Health in Men with Prostate Cancer), financially supported by the Horizon Europe program and Norte 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the individual post-doctoral research grant offered to M.F.M.G.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jaiswal, V.; Nain, P.; Mukherjee, D.; Joshi, A.; Savaliya, M.; Ishak, A.; Batra, N.; Maroo, D.; Verma, D. Symptomatology, prognosis, and clinical findings of Monkeypox infected patients during COVID-19 era: A systematic-review. Immun. Inflamm. Dis. 2022, 10, e722. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y. The 2022 monkeypox outbreak in nonendemic countries: A review for the emergency department clinician. Clin. Exp. Emerg. Med. 2022, 9, 281–285. [Google Scholar] [CrossRef]
- Chandran, D.; Dhama, K.; Chakraborty, S.; Mohapatra, R.K.; Yatoo, M.I. Monkeypox: An Update on Current Knowledge and Research Advances. J. Exp. Biol. Agric. Sci. 2022, 10, 679–688. [Google Scholar] [CrossRef]
- Breman, J.G.; Steniowski, M.V.; Zanotto, E.; Gromyko, A.I.; Arita, I. Human monkeypox, 1970–1979. Bull. World Health Organ. 1980, 58, 165–185. [Google Scholar]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Huo, S.; Chen, Y.; Lu, R.; Zhang, Z.; Zhang, G.; Zhao, L.; Deng, Y.; Wu, C.; Tan, W. Development of two multiplex real-time PCR assays for simultaneous detection and differentiation of monkeypox virus IIa, IIb, and I clades and the B.1 lineage. Biosaf. Health 2022, 4, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Kong, J.D.; Mahroum, N.; Tsigalou, C.; Khamisy-Farah, R.; Converti, M.; Wu, J. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: A preliminary pooled data analysis and literature review. J. Med. Virol. 2023, 95, e27931. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Chowdary, Y.; Schomogyi, M.; Stevens, J.; Patel, J.; Karem, K.; Fischer, M.; Kuehnert, M.J.; Zaki, S.R.; Paddock, C.D.; et al. Human Monkeypox Infection: A Family Cluster in the Midwestern United States. J. Infect. Dis. 2004, 190, 1833–1840. [Google Scholar] [CrossRef]
- Ligon, B. Monkeypox: A review of the history and emergence in the Western hemisphere. Semin. Pediatr. Infect. Dis. 2004, 15, 280–287. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. 2022 Mpox Outbreak Global Map. Available online: https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html (accessed on 15 April 2023).
- Brown, K.; Leggat, P.A. Human Monkeypox: Current State of Knowledge and Implications for the Future. Trop. Med. Infect. Dis. 2016, 1, 8. [Google Scholar] [CrossRef]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Zachary, K.C.; Shenoy, E.S. Monkeypox transmission following exposure in healthcare facilities in nonendemic settings: Low risk but limited literature. Infect. Control Hosp. Epidemiol. 2022, 43, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Dashraath, P.; Nielsen-Saines, K.; Rimoin, A.; Mattar, C.N.; Panchaud, A.; Baud, D. Monkeypox in pregnancy: Virology, clinical presentation, and obstetric management. Am. J. Obstet. Gynecol. 2022, 227, 849–861.e7. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, F.; Pagani, G.; Buca, D.; Khalil, A. Monkeypox infection in pregnancy: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2023, 5, 100747. [Google Scholar] [CrossRef] [PubMed]
- Liberati, M.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- National Heart Lung and Blood Institute. Study Quality Assessment Tools; NHLBI: Bethesda, MD, USA, 2019.
- Arita, I.; Jezek, Z.; Khodakevich, L.; Ruti, K. Human Monkeypox: A Newly Emerged Orthopoxvirus Zoonosis in the Tropical Rain Forests of Africa. Am. J. Trop. Med. Hyg. 1985, 34, 781–789. [Google Scholar] [CrossRef]
- Jezek, Z.; Arita, I.; Mutombo, M.; Dunn, C.; Nakano, J.; Szczeniowski, M. Four generations of probable person-to-person transmission of human monkeypox. Am. J. Epidemiol. 1986, 123, 1004–1012. [Google Scholar] [CrossRef]
- Jezek, Z.; Marennikova, S.S.; Mutumbo, M.; Nakano, J.H.; Paluku, K.M.; Szczeniowski, M. Human Monkeypox: A Study of 2510 Contacts of 214 Patients. J. Infect. Dis. 1986, 154, 551–555. [Google Scholar] [CrossRef]
- Jezek, Z.; Grab, B.; Szczeniowski, M.V.; Paluku, K.M.; Mutombo, M. Human monkeypox: Secondary attack rates. Bull. World Health Organ. 1988, 66, 465–470. [Google Scholar]
- Fleischauer, A.T.; Kile, J.C.; Davidson, M.; Fischer, M.; Karem, K.L.; Teclaw, R.; Messersmith, H.; Pontones, P.; Beard, B.A.; Braden, Z.H.; et al. Evaluation of Human-to-Human Transmission of Monkeypox from Infected Patients to Health Care Workers. Clin. Infect. Dis. 2005, 40, 689–694. [Google Scholar] [CrossRef]
- Learned, L.A.; Bolanda, J.D.; Li, Y.; Reynolds, M.; Moudzeo, H.; Wassa, D.W.; Libama, F.; Harvey, J.M.; Likos, A.; Formenty, P.; et al. Extended interhuman transmission of monkeypox in a hospital community in the republic of the congo, 2003. Am. J. Trop. Med. Hyg. 2005, 73, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Tamfum, J.-J.M.; Kabamba, J.; Likofata, J.; Katomba, J.; McCollum, A.M.; Monroe, B.; Kalemba, L.; Mukadi, D.; Bomponda, P.L.; et al. Introduction of Monkeypox into a Community and Household: Risk Factors and Zoonotic Reservoirs in the Democratic Republic of the Congo. Am. J. Trop. Med. Hyg. 2015, 93, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Besombes, C.; Gonofio, E.; Konamna, X.; Selekon, B.; Grant, R.; Gessain, A.; Berthet, N.; Manuguerra, J.-C.; Fontanet, A.; Nakouné, E. Intrafamily Transmission of Monkeypox Virus, Central African Republic, 2018. Emerg. Infect. Dis. 2019, 25, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, W.M.; Vasoo, S.; Ho, H.J.A.; Chan, M.; Yeo, T.W.; Manauis, C.M.; Ang, H.; De, P.P.; Ang, B.S.P.; Chow, A.L.P. Monitoring healthcare professionals after monkeypox exposure: Experience from the first case imported to Asia. Infect. Control Hosp. Epidemiol. 2020, 41, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; Aarons, E.; Astbury, J.; Brooks, T.; Chand, M.; Flegg, P.; Hardman, A.; Harper, N.; Jarvis, R.; Mawdsley, S.; et al. Human-to-Human Transmission of Monkeypox Virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020, 26, 782–785. [Google Scholar] [CrossRef]
- Aguilera-Alonso, D.; Alonso-Cadenas, J.A.; Roguera-Sopena, M.; Lorusso, N.; Miguel, L.G.S.; Calvo, C. Monkeypox virus infections in children in Spain during the first months of the 2022 outbreak. Lancet Child Adolesc. Health 2022, 6, e22–e23. [Google Scholar] [CrossRef]
- Hagan, L.M. Monkeypox Case Investigation—Cook County Jail, Chicago, Illinois, July–August 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1271–1277. [Google Scholar] [CrossRef]
- Marshall, K.E.; Barton, M.; Nichols, J.; de Perio, M.A.; Kuhar, D.T.; Spence-Davizon, E.; Barnes, M.; Herlihy, R.K.; Czaja, C.A.; Healthcare Personnel Monitoring Team. Health care personnel exposures to subsequently laboratory-confirmed monkeypox patients—Colorado, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1216–1219. [Google Scholar] [CrossRef]
- Phelippeau, M.; Loison, G.; Rucay, P.; Le Guillou-Guillemette, H.; Ndiaye, D.; Dubée, V.; Legeay, C. Fortuitous diagnosis of monkeypox in a patient hospitalized for several days: Risk assessment and follow-up for exposed healthcare workers. J. Hosp. Infect. 2023, 131, 244–246. [Google Scholar] [CrossRef]
- Viedma-Martinez, M.; Dominguez-Tosso, F.R.; Jimenez-Gallo, D.; Garcia-Palacios, J.; Riera-Tur, L.; Montiel-Quezel, N.; Linares-Barrios, M. MPXV Transmission at a Tattoo Parlor. N. Engl. J. Med. 2023, 388, 92–94. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Cenciarelli, O.; Colombe, S.; de Sousa, L.A.; Fischer, N.; Gossner, C.M.; Pires, J.; Scardina, G.; Aspelund, G.; Avercenko, M.; et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European Region, 7 March to 23 August 2022. Eurosurveillance 2022, 27, 2200620. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.N.; Whitehill, F.; Doty, J.B.; Schulte, J.; Matheny, A.; Stringer, J.; Delaney, L.J.; Esparza, R.; Rao, A.K.; McCollum, A.M. Environmental Persistence of Monkeypox Virus on Surfaces in Household of Person with Travel-Associated Infection, Dallas, Texas, USA, 2021. Emerg. Infect. Dis. 2022, 28, 1982–1989. [Google Scholar] [CrossRef]
- Pfeiffer, J.A. High-contact object and surface contamination in a household of persons with monkeypox virus infection—Utah, June 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.; Gould, S.; Spencer, A.; Onianwa, O.; Furneaux, J.; Grieves, J.; Summers, S.; Crocker-Buqué, T.; Fletcher, T.; Bennett, A.; et al. Monkeypox virus contamination in an office-based workplace environment. J. Hosp. Infect. 2022, 130, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Nörz, D.; Pfefferle, S.; Brehm, T.T.; Franke, G.; Grewe, I.; Knobling, B.; Aepfelbacher, M.; Huber, S.; Klupp, E.M.; Jordan, S.; et al. Evidence of surface contamination in hospital rooms occupied by patients infected with monkeypox, Germany, June 2022. Eurosurveillance 2022, 27, 2200477. [Google Scholar] [CrossRef] [PubMed]
- Al-Raeei, M. The study of human monkeypox disease in 2022 using the epidemic models: Herd immunity and the basic reproduction number case. Ann. Med. Surg. 2023, 85, 316–321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).