Abstract
Aedes aegypti and Aedes albopictus are the vectors of important arboviruses: dengue fever, chikungunya, Zika, and yellow fever. Female mosquitoes acquire arboviruses by feeding on the infected host blood, thus being able to transmit it to their offspring. The intrinsic ability of a vector to infect itself and transmit a pathogen is known as vector competence. Several factors influence the susceptibility of these females to be infected by these arboviruses, such as the activation of the innate immune system through the Toll, immunodeficiency (Imd), JAK-STAT pathways, and the interference of specific antiviral response pathways of RNAi. It is also believed that the presence of non-pathogenic microorganisms in the microbiota of these arthropods could influence this immune response, as it provides a baseline activation of the innate immune system, which may generate resistance against arboviruses. In addition, this microbiome has direct action against arboviruses, mainly due to the ability of Wolbachia spp. to block viral genome replication, added to the competition for resources within the mosquito organism. Despite major advances in the area, studies are still needed to evaluate the microbiota profiles of Aedes spp. and their vector competence, as well as further exploration of the individual roles of microbiome components in activating the innate immune system.
Keywords:
mosquito vectors; Aedes; microbiota; vector borne diseases; arboviruses; vector competence 1. Introduction
Arthropod-borne virus (arbovirus) is a non-taxonomic designation for viruses that can replicate in both vertebrate hosts and arthropod vectors [1,2]. There are >500 recognized arboviruses worldwide distributed into seven families: Togaviridae, Flaviviridae, Bunyaviridae, Reoviridae, Rhabdoviridae, Orthomyxoviridae, and Asfarviridae. About 150 of these, mainly from the genera Flavivirus spp. and Alphavirus spp., are known to cause severe human illness [3,4]. The most prevalent arboviral diseases in the last four decades—namely, dengue fever (DENV; 100 million symptomatic cases/year), chikungunya virus (CHIKV; 693,000 cases/year), Zika virus (ZIKV; 500,000 cases/year), and yellow fever (YFV; 130,000 cases/year)—represent an important cause of morbidity and mortality in developing countries. Accordingly, they also pose a substantial economic burden for health systems, particularly in tropical and subtropical regions [5,6].
The past 50 years have witnessed a dramatic re-emergence of epidemic arboviral diseases, mostly resulting from the modern world triad: urbanization, globalization, and international mobility [7,8]. Re-emergence is not an exclusive characteristic of arboviruses; however, their rapid and geographically extensive dispersal constitutes an important difference from other pathogens [9]. The ongoing geographical expansion of the DENV pandemic in the tropics and subtropics, along with the explosive onsets of CHIKV and, more recently, ZIKV infection-associated neurological disorders and neonatal malformations in Latin America, have all highlighted the need for an active fight to prevent the viral perpetuation and the emergence of outbreaks [10,11,12].
Arboviruses usually have a short incubation period ranging from 3 to 10 days, and most infected individuals remain asymptomatic or present mild symptoms [13]. Dengue is a potentially more serious infection due to its hemorrhagic component that can lead to circulatory collapse [13]. This disease is currently present in more than 100 tropical and subtropical countries, with 40% of the world’s population at risk of acquiring the disease [14]. The 2014 Zika epidemic stands out, a period in which several countries, mainly in South America, were affected [15]. Zika disease is characterized by arthralgia, myalgia, rash, headache, fever, and enlarged lymph nodes and is usually self-limiting. However, it has complications such as the demyelinating polyneuropathy Guillain-Barré Syndrome and Congenital Zika Syndrome, the only virus capable of such in the Flaviviridae family [13]. Chikungunya is an acute infection in which the main symptoms are high fever and severe polyarthralgia. It has a mortality rate of about 0.1%, and the elderly population is the most affected. The disease can also manifest itself through more protracted symptoms, such as chronic polyarthralgia, neuropathic pain and mood swings, which can significantly impair the patient’s quality of life. CHIKV virus can be transmitted vertically during birth, resulting in congenital encephalitis in half of the cases [13,16].
Due to the lack of vaccines against arboviruses licensed at this time, the battle against vector-borne diseases mostly relies on vector control [12]. DENV, CHIKV, ZIKV and YFV are predominantly transmitted horizontally to humans through the bite of infected female mosquitoes Aedes aegypti and A. albopictus in sporadic outbreaks over an interval of years or annually in a cyclical, seasonal pattern [17,18,19]. Aedes aegypti are small, dark mosquitoes with white markings on their legs and a wide distribution worldwide, especially in tropical and subtropical regions with environmental disturbances [20,21]. On the other hand, the likely secondary vector of arboviruses, A. albopictus, are known to colonize all five continents [22]. Despite this extensive geographic distribution, the transmission of mosquito-borne arboviruses requires high densities of competent vectors, a high vector survival rate, and frequent contact between vectors and susceptible vertebrate hosts [23]. When taken together, these factors constitute vector capacity, which, alongside vector competence—i.e., the intrinsic ability of a mosquito population to become infected with a particular pathogen and transmit it to susceptible hosts [24]—determines the efficiency of a vector population to transmit a pathogen under natural conditions.
In this sense, a multitude of microbes that colonize different organs and tissues of mosquitoes, including the intestine, salivary glands, and reproductive tissues, seem to play a pivotal role in the vectorial ability to transmit viral pathogens [25]. High-performance sequencing and metagenomic analyses have advanced our understanding of the composition and functionality of the microbiota of Aedes spp. [26,27]. Recent studies have highlighted that the mosquito microbiota can modulate the susceptibility to arboviral infection, both through the production of antiviral proteins and the activation of the mosquito’s innate immune system [28]. Dissecting these mechanisms underlying vector competence constitutes an important field in the development of effective strategies to control the spread of arboviral epidemic diseases. In this study, we aim to explore the role of the microbiome on the vector competence of Aedes spp. and to discuss the novelties and perspectives in this area.
2. Methods
This comprehensive review aimed to investigate the role of the microbiome of Aedes spp. in vector competence. We performed a search of PubMed/MEDLINE, Virtual Health Library (BVS), and Latin American and Caribbean Health Sciences Literature (LILACS) databases until February 2023. Medical Subject Headings (MeSH) index terms and free-text words were combined for search strategy development. Search terms included ‘Aedes [Mesh]’, ‘Microbiota [Mesh]’, ‘Microbiome’, ‘Microbial Community’, ‘Microbial Community Composition’, ‘Microbial Community Structure’, and ‘vector competence’. Boolean operators (AND, OR) were also used to narrow or broaden the search as required. The titles and abstracts of the articles were analyzed, and studies that answered the research question were included. Subsequently, we described the main findings of the retrieved studies and their limitations and pointed out prospects in the research field.
3. Aedes spp.: Understanding the Vector Interaction with Arboviruses
For mosquito-borne arboviruses, under natural conditions, a susceptible adult female becomes infected by taking a blood meal from a viremic host [28]. Upon ingestion, arboviruses must infect and replicate in midgut epithelial cells, thereby overcoming the midgut infection barrier (MIB), the host’s potent innate immune responses, finally facing the effects of the luminal and sometimes internal microbiota [29,30]. Subsequently, they must escape, disseminate and invade the salivary gland when the vector is able to transmit the arbovirus by a bite to a new host [31]. On the other hand, arboviruses can also be maternally transmitted by infected females to their offspring through the infection of germinal tissues [32].
The MIB in refractory mosquitoes could be attributed to multiple factors, including (1) a lack of adequate cell surface receptors for the pathogens to initiate infection; (2) virus filtration by peritrophic matrix; (3) its inactivation by midgut digestive enzymes; (4) strong immune responses against pathogen replication; or (5) effects of luminal and sometimes internal microbiota [33]. Indeed, the first step in the responses of Aedes spp. against arboviruses occurs on the surface of the midgut epithelium itself [34]. Nevertheless, pattern recognition by specialized receptors is the first step in orchestrating robust innate immune responses. Upon viral-patterns recognition, pathogen-recognition receptors (PRRs) trigger and regulate distinct downstream complex immune signaling pathways such as Toll, the immune deficiency (Imd), the Janus kinase/signal transducers, and activators of transcription (JAK-STAT), and the RNA interference (RNAi) specific antiviral response pathways [35]. After this interaction, humoral and cellular responses take place, inducing both antimicrobial peptides (AMPs) expression and hemocyte-mediated immune responses, such as phagocytosis, nodulation, and encapsulation, respectively [36,37].
The Toll pathway is recognized for its importance in the immune response to Gram-positive bacteria, fungi, and especially DENV in mosquitoes [38]. Recognition of arbovirus-related PAMPs by PRRs triggers proteolytic cascades that lead to the cleavage of the cytokine Späetzle. In a Späetzle -cleavage-dependent manner, Toll transmembrane receptor activation drives MyD88, Tube, and Pelle-mediated intracellular signaling pathway. It thereby culminates in phosphorylation and subsequent proteasomal degradation of Cactus, which binds to the NF-κB-like transcription factor Dorsal (Rel1). Finally, Cactus degradation allows the translocation of Rel1 to the nucleus and subsequent expression of AMPs and other immune effectors [39,40]. The transcription of innate immunity genes encoding AMPs is also highly IMD-dependent. In contrast to the Toll, the Imd pathway is also activated during Gram-negative bacterial infection, and its induction is known to lead to the degradation of the negative regulator Caspar. This process promotes the translocation of Relish 2 (Rel2) to the nucleus, which activates IMD-regulated AMPs transcription [41,42]. Similarly, the JAK-STAT pathway is suggested to play an essential role in antiviral defense in mosquitoes [43]. Finally, it is important to mention that, despite not being a classical immune signaling pathway, mosquito RNAi is the major innate immune pathway controlling antiviral response and, thereby, arbovirus infection and transmission. The RNAi machinery is triggered when dsRNA enters a cell and results in the degradation of cognate viral mRNA, thus inhibiting viral replication and promoting viral clearance [43,44].
These common pathways between immune responses to arboviruses and other microorganisms help to explain why increasing evidence also highlights microbiota as an important exponent of mosquito-arbovirus interactions. It appears that the presence of the microbiome induces a state of basal activation of the innate immune system of Aedes spp. that would be associated with modulation of susceptibility to arbovirus infection [45,46]. Figure 1 outlines this potential three-way interaction, which will be the subject of further discussion below.
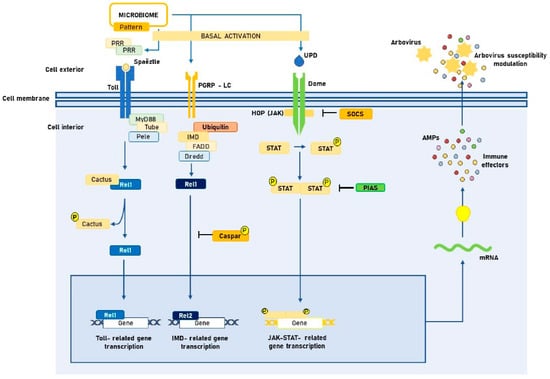
Figure 1.
Tripartite interaction between Aedes spp. midgut microbiota, innate immune signaling pathways and arboviruses. PRR: Pattern recognition receptors. PAMP: Pathogen-associated molecular patterns. JAK: Janus kinase. STAT: Signal transducers and activators of transcription. UPD: Unpaired peptide ligand. PGRP-LC: Peptidoglycan recognition proteins. AMP: Antimicrobial peptide. FADD: Fas-associated death domain. SOCS: Suppressors of cytokine signaling. PIAS: Protein inhibitor of activated STAT. IMD: Immune deficiency. Created with Microsoft PowerPoint, version 16.16.24.
4. Formation and Composition of the Microbiome
The formation of the microbiome begins in the early developmental stages of Aedes spp., since through vertical transmission, eggs inherit microorganisms from their progenitors [47]. Already in the larval stage, there is a strong interaction with the developing water, where the symbiosis occurs primarily through feeding [48,49]. Factors such as geographic location, physicochemical characteristics of the water, larval population density, and food availability are the main determinants of the microbiome in this first moment and may promote or inhibit the colonization of the larval organism [50]. Dickson et al. recently attempted to identify if habitat-related differences in bacterial communities in larval development sites could mediate environmental variation in vector-borne pathogen transmission. The authors showed that exposure to different bacteria during larval development could influence the variation in adult traits in A. aegypti. More interestingly, it also appears to be the potential transmission mechanism of medically relevant human pathogens [51]. In turn, Wang et al. found that the microbiota of A. albopictus is strongly determined by its larval stage habitat and physiological status (e.g., developmental stage) [52].
During adulthood, both intrinsic and extrinsic host factors can cause changes in the microbiota. Among the extrinsic factors, geographic location and food availability remain important influencers. Mosquitoes collected from different regions of the world show significant taxonomic variation in terms of microorganisms, suggesting that the microbiota has native characteristics, which may partly explain the differences in the incidence of arboviruses around the globe [26]. However, these differences are not as significant at the phylum level, and greater variability exists at lower taxonomic levels [48]. This may support the idea that what occurs is the sum of a “core microbiome” composed of similar Operational Taxonomic Units (OTUs), regardless of geographic location, with locally acquired microorganisms. This resulting microbiome is key to understanding and controlling the transmission of arboviruses by mosquitoes of the genus Aedes spp. [25].
As for the factors intrinsic to the host, we can highlight mating and feeding type. A study by Díaz et al. analyzed the reproductive tract of Aedes aegypti and Aedes albopictus females using metabarcoding of the 16S rRNA, where it was possible to observe that blood feeding and mating cause changes in the relative populations of the microbiome components in order to favor the metabolic pathways associated with egg laying [53]. In addition, it was observed that sugar fermentation increases the number of anaerobic microorganisms [48]. Oliveira et al. also demonstrated that mosquitoes fed only sugar produce a greater amount of reactive oxygen species (ROS) when compared to those fed only blood. The low availability of ROS is conducive to the expansion of bacterial levels, causing higher mortality [54]. Finally, blood consumption of hosts treated with antibiotics causes a decrease in the number of bacteria present in the microbiome. This impairs blood digestion and, consequently, egg laying by females [48].
The arthropod microbiome is composed of several microorganisms, of which we will highlight bacteria, fungi, and viruses. Other forms of microorganisms, such as helminths, have also been described [25], but further studies are needed to clarify which ones and how they act in the organism of these insects. Table 1 summarizes the results of the most important studies on the microbiome composition of Aedes spp.

Table 1.
Findings of the studies on the microbiome composition of Aedes spp.
4.1. Bacteria
Bacteria are widely found in various tissues of Aedes spp. mosquitoes and constitute more than 99% of the components of the microbiome [25]. In a recent study, Lin et al. collected mosquito samples reared under the same laboratory conditions and compared the microbial composition of the midgut and entire bodies of A. aegypti and A. albopictus from Southern China using 16S rRNA gene sequencing. The authors found that microbes in the entire bodies of both male and female A. aegypti mainly included seven genera: Leptothrix spp., Methylobacterium spp., Enterobacter spp., Methylotenera spp., uncultured bacteria, Escherichia-Shigella spp., and Sphingomonas spp. No microbe was absolutely dominant [56]. Conversely, the bacterial composition of A. albopictus is concentrated in a few genera, such as Wolbachia spp. and Bacillus spp.. Another work carried out in the Kandy district, Sri Lanka, showed that the microbiota found in the larval intestine of A. aegypti was composed of bacteria of the genus Bacillariophyta spp., Cyanobacteria/Cyanophyta spp., and Ochrophyta spp. in its majority, followed by Charophyta spp. and Euglenozoa spp. in a lower frequency. However, the midgut contents of A. albopictus belonged mainly to Chlorophyta spp., and less frequently Cyanobacteria/Cyanophyta spp., Bacillariophyta spp., Euglenozoa spp., Ochrophyta spp., and Charophyta spp. [65]. A survey in Panama revealed the cultivable bacterial species in the midgut of adult females of A. aegypti were six distinct genera: Asaia spp., Aeromonas spp., Enterobacter spp., Paenibacillus spp., Proteus spp., and Comamonas spp. [45]. In Brazil, the main bacteria isolated from the midgut of A. aegypti were Acinetobacter spp., Aeromonas spp., Cedecea spp., Cellulosimicrobium spp., Elizabethkingia spp., Enterobacter spp., Lysinibacillus spp., Pantoea spp., Pseudomonas spp., Serratia spp. and Staphylococcus spp., 72% of them Gram-negative [55]. Thus, it is possible to notice a great variability of genera that infect Aedes spp., despite some of these being identified in more than one study.
Indeed, the available studies on the establishment of a core midgut microbiome profile of Aedes spp. are somewhat divergent, even within the same species. Nascimento et al. aimed to describe the culture-dependent native microbiota associated with the female A. aegypti (strain PP-Campos) through 16S rRNA gene sequencing. Their results revealed eleven isolates from the native bacterial community of A. aegypti (strain PP-Campos)—Acinetobacter spp., Aeromonas spp., Cedecea spp., Cellulosimicrobium spp., Elizabethkingia spp., Enterobacter spp., Lysinibacillus spp., Pantoea spp., Pseudomonas spp., Serratia spp., and Staphylococcus spp. [55]. In another Brazilian study, David et al. aimed to profile the midgut microbial diversity throughout the A. aegypti lifespan and address possible determinants of microbiota structure in adult females collected in the northwest of Rio de Janeiro city (RJ). In turn, these authors suggested that A. aegypti harbors lifelong stable core microbiota mostly composed of the genera Pseudomonas spp., Acinetobacter spp., Aeromonas spp., and Stenotrophomonas spp. and the families Oxalobacteraceae, Enterobacteriaceae, and Comamonadaceae [28]. Regarding A. albopictus, Rosso et al. highlighted that A. albopictus collected in Italy had lower diversity and different microbiota composition compared to samples collected in France and Vietnam. Their results suggest that the mosquito possesses a core microbiota consisting mainly of the genus Pseudomonas spp. [59]. Minard et al. counter these findings by demonstrating that all mosquitoes collected from different sites have a bacterial microbiota dominated by a single taxon, Wolbachia pipientis. However, the authors note that the diversity index values likely underestimate the true diversity of the mosquito microbiota due to the high abundance of Wolbachia spp. sequences [63]. These studies indicate that when it comes to wild mosquitoes, the composition of adult midgut microbiota between different strains derived from distinct geographic populations of Aedes spp. is markedly distinct.
The bacterial composition of the microbiome changes significantly throughout the developmental stages of the mosquito. During metamorphosis, approximately 90% of the bacteria present in the digestive tract are shed [66]. As described above, factors such as vector competence, blood feeding, and infection with different microorganisms cause changes in the redox state of arthropod metabolism and, consequently, constant changes in the mosquito microbiota [25,26]. In addition to arboviruses, these changes can favor other public health harms. Hyde et al. used 16S rRNA gene sequencing to analyze larvae and adults of Aedes aegypti reared in colonies, finding the presence of antibiotic-resistant bacterial OTUs, mainly beta-lactamases [67]. The fact that mosquitoes are laboratory cultured, not captured from the wild, and yet are colonized by resistant bacteria raises the question of what role Aedes spp. may play in increasing bacterial resistance.
Bacteria can also be used as biomarkers of infection. The presence of the genus Serratia spp. in A. aegypti is more common in DENV and CHIKV endemic regions [48]. Upon infection, there is an interaction between the bacterial P40 polypeptide and the viral prohibitin protein and the degradation of mucins attached to the intestinal membrane, reducing its protection [25]. Thus, Serratia spp. is thought to facilitate arbovirus infection [68,69].
4.2. Virus
Recent advances in metagenomic next-generation sequencing (mNGS) have also led to the discovery of a variety of RNA viruses associated with hematophagous insects [70]. The Aedes spp. virome is known to be formed by arboviruses and, in greater proportion, by insect-specific viruses (ISVs) [25]. ISVs are a non-taxonomic designation for viruses that appear to be restricted to insects due to their inability to replicate in vertebrate hosts. To date, ISVs have been identified as members of the families Flaviviridae, Togaviridae, Peribunyaviridae, Phenuiviridae, Rhabdoviridae, Mesoniviridae, Tymoviridae, Birnaviridae, Nodaviridae, Reoviridae, Parvoviridae, Iridoviridae, Permutotetraviridae, Iflaviridae, Orthomyxoviridae, and Totiviridae [71,72,73]. Because of their natural association with arthropods, these viruses can be considered exclusive members of viral communities of insects (virome) and are not considered pathogens [25]. In fact, studies have shown that ISVs of adult A. aegypti and A. albopictus may play a critical role in regulating viral invasion by generating resistance to infection against other arboviruses [74].
ISVS coexist with mosquitoes for long periods of time and are passed to offspring through vertical transmission. Recent research shows similar ancestral genetic traits between ISVs and arboviruses, as well as the presence of viral RNA in the transcriptome of Aedes spp. mosquitoes, which may mean that the mosquito plays a role in the mutation and evolution of ISVs to arboviruses [25]. Metagenomic studies show a high prevalence of the Phasi Charoen-like virus (PCLV) in A. aegypti from various parts of the globe, which may indicate the coevolution of the virus with the African ancestor of Aedes spp. [47,75]. Further investigations are needed to clarify if and how ISVs evolved from infecting only arthropods to vertebrates.
4.3. Fungi
Few fungal species have been identified so far as components of the microbiome of Aedes spp. [48]. A study developed by Zouache et al. characterized the fungal microbiota of A. aegypti larvae taken from their natural habitat. The main species found belonged to the order Pleosporales, Trichosphaeriales, Eurotiale and Capnodiales (phylum Ascomycota); and Tremellales, Polyporales and Wallemiales (phylum Basidiomycota) [50]. Another study by Tawidian et al. analyzed fungal communities associated with the larvae and developmental site of A. albopictus mosquitoes using high-throughput sequencing of the internal transcribed spacer 2 meta barcode markers. The identified species belonged to the phyla Ascomycota (the filamentous Aspergillus spp., Beauveria spp., Cladosporium spp., Penicillium spp. and Trichoderma spp., and the yeast Candida spp.) and yeasts of the phylum Basidiomycota (Cryptococcus spp., Rhodosporidium spp., Rhodotorula spp., and Trichosporon spp.), similar by 44% at the genus level to other previous studies [76]. This similarity at the phylum level between the studies may support the idea of a “core microbiome”.
The presence of eukaryotes can affect the organism in different ways. The species P. citrinum can inhibit the development of A. aegypti eggs through the production of mycotoxins [50]. Talaromyces spp., on the other hand, is able to suppress trypsin enzyme expression in the insect midgut, facilitating arbovirus infection [25]. Fungi can also limit bacterial growth through the production of antibiotic substances, directly influencing the microbiota [50]. However, few studies have focused on the role of fungi as parasites or components of the Aedes spp. microbiome, making their impact unclear.
5. The Role of the Microbiome on Vector Competence
The effects of the microbiome on vector competence are complex and not fully understood. To date, three main hypotheses of microbiota-driven mechanisms for modulating Aedes spp. vector competence are highlighted: (1) innate immune system basal activation [45,46]; (2) direct antiviral activity [77]; and (3) resource competition [78]. Figure 2 represents each of these mechanisms separately. Thus, we hereby discuss the main findings regarding these possibilities.
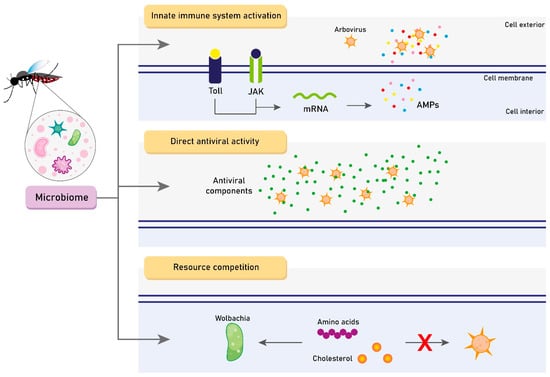
Figure 2.
The three main hypotheses of microbiome-driven mechanisms for modulating Aedes spp. vector competence. JAK: Janus kinase. AMP: Antimicrobial peptide. Created with Adobe Illustrator.
5.1. Innate Immune System Activation
Xi et al. performed an interesting comparison between midgut samples of conventionally bred mosquitoes and aseptic mosquitoes in which the microbiome was depleted. These authors found that aseptic A. aegypti demonstrated lower levels of several Toll pathway-regulated AMPs (defensin, cecropin, attacin, and gambacin) and had higher midgut DENV2 titers. These findings support the hypothesis that the mosquito microbiota stimulates a certain basal level of immune gene expression through the activation of the Toll pathway, which, in turn, mediates the antiviral activity [38]. Another study has accordingly suggested that Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway in A. aegypti, which also leads to the production of AMPs that inhibit DENV-2 [79]. This reinforces the idea that Wolbachia is capable of inhibiting arbovirus infection of Aedes spp. It, therefore, highlights the potential of using the microbiome as a vector control strategy, which can fundamentally change the course of the fight against vector-borne diseases [79,80]. Finally, a study conducted by Ramirez et al. showed the reintroduction of bacterial isolates via a sugar meal into the midgut of A. aegypti mosquitoes resulted in a significant reduction in DENV infection in the case of one bacterial isolate, Proteus spp. Prsp_P. These authors emphasize the hypothesis that these bacteria may either indirectly exert an anti-arbovirus effect by boosting basal immunity [45]. Likewise, Terradas et al. showed that while Wolbachia infection induces genes in the Toll, JAK/STAT, and RNAi pathways, only reduced expression of RNAi leads to a rebound of dengue virus loads in A. aegypti-Wolbachia-infected cells [81]. However, the magnitude of the effect explained less than 10% of the total DENV load. In this sense, the authors found that these bacteria, by inducing microRNA (miRNA) production, suppress the expression of essential genes during viral genome methylation [81]. Their important analysis sheds light on the mosquito as a holobiont unit, in which the mosquito, its midgut microflora, and DENV are involved in complex reciprocal tripartite interactions [45,81].
5.2. Direct Antiviral Activity
Immune modulation is not the only mechanism through which Wolbachia influences the vector competence of Aedes spp. A study by Rainey et al. might suggest that Wolbachia-mediated pathogen blocking occurs early in infection before the inducible immune responses’ onset. It is currently accepted that these bacteria may block viral genome replication early in infection without a transcriptional response by endosymbiont or host small RNA pathways [82]. Concurrently, other reports suggested that Chromobacterium Csp_P reduces DENV infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogenic activities, mainly through Csp_P—produced stable bioactive factors with transmission-blocking and therapeutic potential. These properties highlighted its potential for the development of arbovirus control strategies [83]. Lastly, Joyce et al. also demonstrated that isolated from the A. albopictus midgut—namely, Pseudomonas rhodesiae, Enterobacter ludwigii, and Vagococcus salmoninarium—have been shown to directly inhibit the La Crosse virus independently of the mosquito immune system [84]. All these findings support the possibility that the microbiota exerts an important direct antiviral effect, mainly through the secretion of antiviral components.
5.3. Resource Competition
Lu et al. recently performed a study to understand why Wolbachia induces DENV resistance in transinfected A. aegypti mosquitoes, but not in A. albopictus. Apparently, Wolbachia density in the midgut, fat body, and salivary gland of A. albopictus is 80-, 18-, and 24-fold less than that of A. aegypti, respectively [78]. These results suggest that these bacteria induce density-dependent inhibition of DENV in mosquito cells, which could correlate to Wolbachia-arbovirus competition for limited resources. For example, intracellular cholesterol and amino acids are required by both viruses and Wolbachia. Thereby, the competition between them could lead to metabolite depletion, cellular stress, and subsequent inhibition of arbovirus replication—as observed in Wolbachia-DENV and Wolbachia-CHIKV co-infections [85,86,87,88].
6. Conclusions
Understanding the mechanisms underlying vector competence is an important area for the development of effective strategies to control the outbreak of arboviral epidemic diseases. In this study, we argue for the central role of the microbiome in modulating the intrinsic ability of a mosquito population to become infected with a particular pathogen and transmit it to susceptible hosts. Although powerful sequencing and metagenomic analyses have advanced, our understanding of the composition and functionality of the microbiota of Aedes spp. and our knowledge of its direct impact on vector competence remains limited. Three main hypotheses for these microbiota-driven mechanisms are currently highlighted: (1) innate immune system basal activation; (2) direct antiviral activity; and (3) resource competition. However, to date, no study has been able to identify the diverse microbiota profiles in Aedes spp. populations and correlate them with vector competence. The individual mechanisms of the microbiome components for basal activation of the mosquito immune system or direct antiviral activity have also not been fully elucidated. These limitations preclude the establishment of definitive conclusions. On the other hand, innovative high-throughput sequencing methods, such as RNA sequencing (RNA-seq), are now widely used in the transcriptomics field for applications like gene expression profiling. Alongside the advancing of our knowledge in microbiomics, the potential discovery of vector-related hub genes and transcripts could provide us with new insights into the tripartite interaction between Aedes spp. midgut microbiota, innate immune signaling pathways, and arboviruses. Future studies should focus on answering these questions.
Author Contributions
Q.R.F., A.F.N., I.S.B., F.F.B.L., J.O.d.S.N., L.A.F. and M.N.M. discussed the concept, designed, and wrote the manuscript. F.F.B.L. and Q.R.F. produced the content and illustration of the figures. L.S.d.B.A., F.K.B. and F.F.d.M. reviewed and made suggestions for the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The first author—Q.R.F.—is supported by the Permanecer Program of the Federal University of Bahia, Brazil (Project 23909 Plan 46046). The second author—F.F.B.L.—is supported by the Scientific Initiation Scholarship Programme (PIBIC) of Bahia State Research Support Foundation, FAPESB, Brazil (N°BOL 18252022). The corresponding authors–F.F.M.—is a CNPq Research Productivity Fellow (PQ).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Arthropod-Borne and Rodent-Borne Viral Diseases. Report of a WHO Scientific Group; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1985; No. 719; pp. 1–116.
- Madewell, Z.J. Arboviruses and Their Vectors. South. Med. J. 2020, 113, 520–523. [Google Scholar] [CrossRef]
- Hubálek, Z.; Rudolf, I.; Nowotny, N. Arboviruses Pathogenic for Domestic and Wild Animals. Adv. Virus Res. 2014, 89, 201–275. [Google Scholar] [PubMed]
- Franklinos, L.H.V.; Jones, K.E.; Redding, D.W.; Abubakar, I. The Effect of Global Change on Mosquito-Borne Disease. Lancet Infect. Dis. 2019, 19, e302–e312. [Google Scholar] [CrossRef] [PubMed]
- Lima-Camara, T.N. Emerging Arboviruses and Public Health Challenges in Brazil. Rev. Saúde Pública 2016, 50, 36. [Google Scholar] [CrossRef]
- Gubler, D.J. The Global Threat of Emergent/Re-Emergent Vector-Borne Diseases. In Vector Biology, Ecology and Control; Springer: Berlin/Heidelberg, Germany, 2010; pp. 39–62. [Google Scholar]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic Arboviral Diseases: Priorities for Research and Public Health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging Arboviruses: Why Today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R. Arboviruses: A Family on the Move. Adv. Exp. Med. Biol. 2018, 1062, 1–10. [Google Scholar]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The Emergence of Arthropod-Borne Viral Diseases: A Global Prospective on Dengue, Chikungunya and Zika Fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef]
- Kleber de Oliveira, W.; Cortez-Escalante, J.; De Oliveira, W.T.G.H.; do Carmo, G.M.I.; Henriques, C.M.P.; Coelho, G.E.; Araújo de França, G.V. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- Leta, S.; Beyene, T.J.; De Clercq, E.M.; Amenu, K.; Kraemer, M.U.G.; Revie, C.W. Global Risk Mapping for Major Diseases Transmitted by Aedes Aegypti and Aedes Albopictus. Int. J. Infect. Dis. 2018, 67, 25–35. [Google Scholar] [CrossRef]
- Weaver, S.C.; Charlier, C.; Vasilakis, N.; Lecuit, M. Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu. Rev. Med. 2018, 69, 395–408. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, Z.; Wen, Z.; Liu, Y.; Zeng, C.; Xiao, D.; Ou, M.; Han, Y.; Huang, S.; Liu, D.; et al. Global Epidemiology of Dengue Outbreaks in 1990–2015: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2017, 7, 317. [Google Scholar] [CrossRef]
- Chang, C.; Ortiz, K.; Ansari, A.; Gershwin, M.E. The Zika Outbreak of the 21st Century. J. Autoimmun. 2016, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vairo, F.; Haider, N.; Kock, R.; Ntoumi, F.; Ippolito, G.; Zumla, A. Chikungunya: Epidemiology, Pathogenesis, Clinical Features, Management, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1003–1025. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and A. albopictus. Elife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.T.d.O.; de Oliveira Mota, M.T.; Terzian, A.C.; Silva, M.L.C.; Estofolete, C.; Nogueira, M.L. Mosquito-Transmitted Viruses—the Great Brazilian Challenge. Braz. J. Microbiol. 2016, 47, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Mosquito Life Cycle: Aedes aegypti. Available online: https://www.cdc.gov/dengue/resources/factSheets/MosquitoLifecycleFINAL.pdf (accessed on 10 January 2023).
- Espinal, M.A.; Andrus, J.K.; Jauregui, B.; Waterman, S.H.; Morens, D.M.; Santos, J.I.; Horstick, O.; Francis, L.A.; Olson, D. Emerging and Reemerging Aedes-Transmitted Arbovirus Infections in the Region of the Americas: Implications for Health Policy. Am. J. Public Health 2019, 109, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes Albopictus, an Arbovirus Vector: From the Darkness to the Light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Viglietta, M.; Bellone, R.; Blisnick, A.A.; Failloux, A.-B. Vector Specificity of Arbovirus Transmission. Front. Microbiol. 2021, 12, 3446. [Google Scholar] [CrossRef]
- Degallier, N.; Teixeira, J.M.S.; Chaib, A.d.J.M.; Barbosa, H.F.; Rios, J.A. Estudos Experimentais Sobre Competência Vetorial de Aedes Aegypti E Aedes Albopictus Para Os Vírus Da Dengue E Febre Amarela. Inf. Epidemiol. SUS 2001, 10, 9–11. [Google Scholar]
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito Microbiota and Implications for Disease Control. Trends Parasitol. 2020, 36, 98–111. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, D.; Muñoz, M.; Ramírez, J.D. Aedes Aegypti and A. albopictus Microbiome/virome: New Strategies for Controlling Arboviral Transmission? Parasit. Vectors 2022, 15, 1–13. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes Aegypti Vector Competence Studies: A Review. Infect. Genet. Evol. 2019, 67, 191. [Google Scholar] [CrossRef]
- Dada, N.; Jupatanakul, N.; Minard, G.; Short, S.M.; Akorli, J.; Villegas, L.M. Considerations for Mosquito Microbiome Research from the Mosquito Microbiome Consortium. Microbiome 2021, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- David, M.R.; Santos, L.M.B.D.; Vicente, A.C.P.; Maciel-de-Freitas, R. Effects of Environment, Dietary Regime and Ageing on the Dengue Vector Microbiota: Evidence of a Core Microbiota throughout Aedes Aegypti Lifespan. Memórias Inst. Oswaldo Cruz 2016, 111, 577–587. [Google Scholar] [CrossRef]
- Carrington, L.B.; Simmons, C.P. Human to Mosquito Transmission of Dengue Viruses. Front. Immunol. 2014, 5, 290. [Google Scholar] [CrossRef] [PubMed]
- Azar, S.R.; Weaver, S.C. Vector Competence: What Has Zika Virus Taught Us? Viruses 2019, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Ciota, A.T. Dissecting Vectorial Capacity for Mosquito-Borne Viruses. Curr. Opin. Virol. 2015, 15, 112–118. [Google Scholar] [CrossRef]
- Teixeira, A.F.; de Brito, B.B.; Correia, T.M.L.; Viana, A.I.S.; Carvalho, J.C.; da Silva, F.A.F.; Santos, M.L.C.; da Silveira, E.A.; Neto, H.P.G.; da Silva, N.M.P.; et al. Simultaneous Circulation of Zakat, Dengue, and Chikungunya Viruses and Their Vertical Co-Transmission among Aedes aegypti. Acta Trop. 2021, 215, 105819. [Google Scholar] [CrossRef]
- Franz, A.W.E.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, P.; Sirisena, P.; Dubey, S.K.; Kumar, R.; Shrinet, J.; Sunil, S. Mosquito Innate Immunity. Insects 2018, 9, 95. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, R.; Zhang, J. The Diversity of Pattern Recognition Receptors (PRRs) Involved with Insect Defense against Pathogens. Curr. Opin. Insect Sci. 2019, 33, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Satyavathi, V.V.; Minz, A.; Nagaraju, J. Nodulation: An Unexplored Cellular Defense Mechanism in Insects. Cell. Signal. 2014, 26, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Browne, N.; Heelan, M.; Kavanagh, K. An Analysis of the Structural and Functional Similarities of Insect Hemocytes and Mammalian Phagocytes. Virulence 2013, 4, 597–603. [Google Scholar] [CrossRef]
- Xi, Z.; Ramirez, J.L.; Dimopoulos, G. The Aedes aegypti Toll Pathway Controls Dengue Virus Infection. PLoS Pathog. 2008, 4, e1000098. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Dimopoulos, G. The Toll Immune Signaling Pathway Control Conserved Anti-Dengue Defenses across Diverse A. aegypti Strains and against Multiple Dengue Virus Serotypes. Dev. Comp. Immunol. 2010, 34, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.-M.; Hoffmann, J.A. Pillars Article: The Dorsoventral Regulatory Gene Cassette spätzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults. Cell. 1996. 86: 973–983. J. Immunol. 2012, 188, 5210–5220. [Google Scholar]
- Ramirez, J.L.; Muturi, E.J.; Barletta, A.B.F.; Rooney, A.P. The Aedes aegypti IMD Pathway Is a Critical Component of the Mosquito Antifungal Immune Response. Dev. Comp. Immunol. 2019, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meister, S.; Kanzok, S.M.; Zheng, X.-L.; Luna, C.; Li, T.-R.; Hoa, N.T.; Clayton, J.R.; White, K.P.; Kafatos, F.C.; Christophides, G.K.; et al. Immune Signaling Pathways Regulating Bacterial and Malaria Parasite Infection of the Mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2005, 102, 11420–11425. [Google Scholar] [CrossRef]
- Blair, C.D. Mosquito RNAi Is the Major Innate Immune Pathway Controlling Arbovirus Infection and Transmission. Future Microbiol. 2011, 6, 265–277. [Google Scholar] [CrossRef]
- Balakrishna Pillai, A.; Nagarajan, U.; Mitra, A.; Krishnan, U.; Rajendran, S.; Hoti, S.L.; Mishra, R.K. RNA Interference in Mosquito: Understanding Immune Responses, Double-Stranded RNA Delivery Systems and Potential Applications in Vector Control. Insect Mol. Biol. 2017, 26, 127–139. [Google Scholar]
- Ramirez, J.L.; Souza-Neto, J.; Cosme, R.T.; Rovira, J.; Ortiz, A.; Pascale, J.M.; Dimopoulos, G. Reciprocal Tripartite Interactions between the Aedes Aegypti Midgut Microbiota, Innate Immune System and Dengue Virus Influences Vector Competence. PLoS Negl. Trop. Dis. 2012, 6, e1561. [Google Scholar]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The Endosymbiotic Bacterium Wolbachia Induces Resistance to Dengue Virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar]
- Ramos-Nino, M.E.; Fitzpatrick, D.M.; Tighe, S.; Eckstrom, K.M.; Hattaway, L.M.; Hsueh, A.N.; Stone, D.M.; Dragon, J.; Cheetham, S. High Prevalence of Phasi Charoen-like Virus from Wild-Caught Aedes aegypti in Grenada, W.I. as Revealed by Metagenomic Analysis. PLoS ONE 2020, 15, e0227998. [Google Scholar]
- Scolari, F.; Casiraghi, M.; Bonizzoni, M. Aedes spp. and Their Microbiota: A Review. Front. Microbiol. 2019, 10, 2036. [Google Scholar] [CrossRef]
- MacLeod, H.J.; Dimopoulos, G.; Short, S.M. Larval Diet Abundance Influences Size and Composition of the Midgut Microbiota of Aedes aegypti Mosquitoes. Front. Microbiol. 2021, 12, 645362. [Google Scholar]
- Zouache, K.; Martin, E.; Rahola, N.; Gangue, M.F.; Minard, G.; Dubost, A.; Van, V.T.; Dickson, L.; Ayala, D.; Lambrechts, L.; et al. Larval Habitat Determines the Bacterial and Fungal Microbiota of the Mosquito Vector Aedes aegypti. FEMS Microbiol. Ecol. 2022, 98, fiac016. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.B.; Jiolle, D.; Minard, G.; Moltini-Conclois, I.; Volant, S.; Ghozlane, A.; Bouchier, C.; Ayala, D.; Paupy, C.; Moro, C.V.; et al. Carryover Effects of Larval Exposure to Different Environmental Bacteria Drive Adult Trait Variation in a Mosquito Vector. Sci. Adv. 2017, 3, e1700585. [Google Scholar] [PubMed]
- Wang, X.; Liu, T.; Wu, Y.; Zhong, D.; Zhou, G.; Su, X.; Xu, J.; Sotero, C.F.; Sadruddin, A.A.; Wu, K.; et al. Bacterial Microbiota Assemblage in Aedes albopictus Mosquitoes and Its Impacts on Larval Development. Mol. Ecol. 2018, 27, 2972–2985. [Google Scholar]
- Díaz, S.; Camargo, C.; Avila, F.W. Characterization of the Reproductive Tract Bacterial Microbiota of Virgin, Mated, and Blood-Fed Aedes aegypti and Aedes albopictus Females. Parasit. Vectors 2021, 14, 1–12. [Google Scholar]
- Oliveira, J.H.M.; Gonçalves, R.L.S.; Lara, F.A.; Dias, F.A.; Gandara, A.C.P.; Menna-Barreto, R.F.S.; Edwards, M.C.; Laurindo, F.R.M.; Silva-Neto, M.A.C.; Sorgine, M.H.F.; et al. Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of Aedes aegypti and Allows Proliferation of Intestinal Microbiota. PLoS Pathog. 2011, 7, e1001320. [Google Scholar] [CrossRef]
- do Nascimento, R.M.; Campolina, T.B.; Chaves, B.A.; Delgado, J.L.F.; Godoy, R.S.M.; Pimenta, P.F.P.; Secundino, N.F.C. The Influence of Culture-Dependent Native Microbiota in Zika Virus Infection in Aedes Aegypti. Parasit. Vectors 2022, 15, 57. [Google Scholar]
- Lin, D.; Zheng, X.; Sanogo, B.; Ding, T.; Sun, X.; Wu, Z. Bacterial Composition of Midgut and Entire Body of Laboratory Colonies of Aedes aegypti and Aedes albopictus from Southern China. Parasit. Vectors 2021, 14, 586. [Google Scholar] [PubMed]
- Hegde, S.; Khanipov, K.; Albayrak, L.; Golovko, G.; Pimenova, M.; Saldaña, M.A.; Rojas, M.M.; Hornett, E.A.; Motl, G.C.; Fredregill, C.L.; et al. Microbiome Interaction Networks and Community Structure from Laboratory-Reared and Field-Collected Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus Mosquito Vectors. Front. Microbiol. 2018, 9, 2160. [Google Scholar]
- Villegas, L.E.M.; Campolina, T.B.; Barnabe, N.R.; Orfano, A.S.; Chaves, B.A.; Norris, D.E.; Pimenta, P.F.P.; Secundino, N.F.C. Zika Virus Infection Modulates the Bacterial Diversity Associated with Aedes aegypti as Revealed by Metagenomic Analysis. PLoS ONE 2018, 13, e0190352. [Google Scholar]
- Rosso, F.; Tagliapietra, V.; Albanese, D.; Pindo, M.; Baldacchino, F.; Arnoldi, D.; Donati, C.; Rizzoli, A. Reduced Diversity of Gut Microbiota in Two Aedes Mosquitoes Species in Areas of Recent Invasion. Sci. Rep. 2018, 8, 16091. [Google Scholar] [CrossRef]
- Mancini, M.V.; Damiani, C.; Accoti, A.; Tallarita, M.; Nunzi, E.; Cappelli, A.; Bozic, J.; Catanzani, R.; Rossi, P.; Valzano, M.; et al. Estimating Bacteria Diversity in Different Organs of Nine Species of Mosquito by next Generation Sequencing. BMC Microbiol. 2018, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Audsley, M.D.; Seleznev, A.; Albert Joubert, D.; Woolfit, M.; O’Neill, S.L.; McGraw, E.A. Wolbachia infection Alters the Relative Abundance of Resident Bacteria in adult Aedes aegypti mosquitoes, but Not Larvae. Mol. Ecol. 2018, 27, 297–309. [Google Scholar] [PubMed]
- Audsley, M.D.; Ye, Y.H.; McGraw, E.A. The Microbiome Composition of Aedes aegypti Is Not Critical for Wolbachia-Mediated Inhibition of Dengue Virus. PLOS Negl. Trop. Dis. 2017, 11, e0005426. [Google Scholar]
- Minard, G.; Tran, F.-H.; Dubost, A.; Tran-Van, V.; Mavingui, P.; Moro, C.V. Pyrosequencing 16S rRNA Genes of Bacteria Associated with Wild Tiger Mosquito Aedes albopictus: A Pilot Study. Front. Cell. Infect. Microbiol. 2014, 4, 59. [Google Scholar] [CrossRef]
- Coon, K.L.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Mosquitoes Rely on Their Gut Microbiota for Development. Mol. Ecol. 2014, 23, 2727. [Google Scholar] [PubMed]
- Ranasinghe, H.A.K.; Amarasinghe, L.D. Naturally Occurring Microbiota in Dengue Vector Mosquito Breeding Habitats and Their Use as Diet Organisms by Developing Larvae in the Kandy District, Sri Lanka. BioMed Res. Int. 2020, 2020, 5830604. [Google Scholar] [PubMed]
- Frankel-Bricker, J.; Buerki, S.; Feris, K.P.; White, M.M. Influences of a Prolific Gut Fungus (Zancudomyces culisetae) on Larval and Adult Mosquito (Aedes aegypti)-Associated Microbiota. Appl. Environ. Microbiol. 2020, 86, e02334-19. [Google Scholar]
- Hyde, J.; Gorham, C.; Brackney, D.E.; Steven, B. Antibiotic Resistant Bacteria and Commensal Fungi Are Common and Conserved in the Mosquito Microbiome. PLoS ONE 2019, 14, e0218907. [Google Scholar]
- Apte-Deshpande, A.; Paingankar, M.; Gokhale, M.D.; Deobagkar, D.N. Serratia odorifera a Midgut Inhabitant of Aedes aegypti Mosquito Enhances Its Susceptibility to Dengue-2 Virus. PLoS ONE 2012, 7, e40401. [Google Scholar]
- Apte-Deshpande, A.D.; Paingankar, M.S.; Gokhale, M.D.; Deobagkar, D.N. Serratia odorifera Mediated Enhancement in Susceptibility of Aedes Aegypti for Chikungunya Virus. Indian J. Med. Res. 2014, 139, 762. [Google Scholar]
- Bolling, B.G.; Weaver, S.C.; Tesh, R.B.; Vasilakis, N. Insect-Specific Virus Discovery: Significance for the Arbovirus Community. Viruses 2015, 7, 4911–4928. [Google Scholar]
- Carvalho, V.L.; Long, M.T. Insect-Specific Viruses: An Overview and Their Relationship to Arboviruses of Concern to Humans and Animals. Virology 2021, 557, 34–43. [Google Scholar] [PubMed]
- Agboli, E.; Leggewie, M.; Altinli, M.; Schnettler, E. Mosquito-Specific Viruses-Transmission and Interaction. Viruses 2019, 11, 873. [Google Scholar]
- Roundy, C.M.; Azar, S.R.; Rossi, S.L.; Weaver, S.C.; Vasilakis, N. Insect-Specific Viruses: A Historical Overview and Recent Developments. Adv. Virus Res. 2017, 98, 119–146. [Google Scholar]
- Öhlund, P.; Lundén, H.; Blomström, A.-L. Insect-Specific Virus Evolution and Potential Effects on Vector Competence. Virus Genes 2019, 55, 127–137. [Google Scholar]
- Calisher, C.H.; Higgs, S. The Discovery of Arthropod-Specific Viruses in Hematophagous Arthropods: An Open Door to Understanding the Mechanisms of Arbovirus and Arthropod Evolution? Annu. Rev. Entomol. 2018, 63, 87–103. [Google Scholar] [PubMed]
- Tawidian, P.; Coon, K.L.; Jumpponen, A.; Cohnstaedt, L.W.; Michel, K. Host-Environment Interplay Shapes Fungal Diversity in Mosquitoes. mSphere 2021, 6, e00646-21. [Google Scholar] [PubMed]
- Ramirez, J.L.; Short, S.M.; Bahia, A.C.; Saraiva, R.G.; Dong, Y.; Kang, S.; Tripathi, A.; Mlambo, G.; Dimopoulos, G. Chromobacterium Csp_P Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and In Vitro Anti-Pathogen Activities. PLoS Pathog. 2014, 10, e1004398. [Google Scholar]
- Lu, P.; Bian, G.; Pan, X.; Xi, Z. Wolbachia Induces Density-Dependent Inhibition to Dengue Virus in Mosquito Cells. PLoS Negl. Trop. Dis. 2012, 6, e1754. [Google Scholar]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia Induces Reactive Oxygen Species (ROS)-Dependent Activation of the Toll Pathway to Control Dengue Virus in the Mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2012, 109, E23–E31. [Google Scholar]
- Iturbe-Ormaetxe, I.; Walker, T.; O’ Neill, S.L. Wolbachia and the Biological Control of Mosquito-borne Disease. EMBO Rep. 2011, 12, 508–518. [Google Scholar] [PubMed]
- Terradas, G.; Albert Joubert, D.; McGraw, E.A. The RNAi Pathway Plays a Small Part in Wolbachia-Mediated Blocking of Dengue Virus in Mosquito Cells. Sci. Rep. 2017, 7, srep43847. [Google Scholar]
- Rainey, S.M.; Martinez, J.; McFarlane, M.; Juneja, P.; Sarkies, P.; Lulla, A.; Schnettler, E.; Varjak, M.; Merits, A.; Miska, E.A.; et al. Wolbachia Blocks Viral Genome Replication Early in Infection without a Transcriptional Response by the Endosymbiont or Host Small RNA Pathways. PLOS Pathog. 2016, 12, e1005536. [Google Scholar]
- Saraiva, R.G.; Fang, J.; Kang, S.; Angleró-Rodríguez, Y.I.; Dong, Y.; Dimopoulos, G. Aminopeptidase Secreted by Chromobacterium sp. Panama Inhibits Dengue Virus Infection by Degrading the E Protein. PLoS Negl. Trop. Dis. 2018, 12, e0006443. [Google Scholar]
- Joyce, J.D.; Nogueira, J.R.; Bales, A.A.; Pittman, K.E.; Anderson, J.R. Interactions Between La Crosse Virus and Bacteria Isolated From the Digestive Tract of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 389–394. [Google Scholar] [PubMed]
- Caragata, E.P.; Rancès, E.; O’Neill, S.L.; McGraw, E.A. Competition for Amino Acids Between Wolbachia and the Mosquito Host, Aedes aegypti. Microb. Ecol. 2014, 67, 205–218. [Google Scholar] [PubMed]
- Lindsey, A.; Bhattacharya, T.; Newton, I.; Hardy, R. Conflict in the Intracellular Lives of Endosymbionts and Viruses: A Mechanistic Look at Wolbachia-Mediated Pathogen-Blocking. Viruses 2018, 10, 141. [Google Scholar] [PubMed]
- Sinkins, S.P. Wolbachia and Arbovirus Inhibition in Mosquitoes. Future Microbiol. 2013, 8, 1249–1256. [Google Scholar]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).