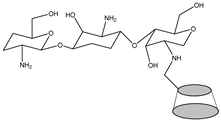
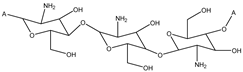
Abstract
Current antiviral therapy research is focused on developing dosage forms that enable highly effective drug delivery, providing a selective effect in the organism, lower risk of adverse effects, a lower dose of active pharmaceutical ingredients, and minimal toxicity. In this article, antiviral drugs and the mechanisms of their action are summarized at the beginning as a prerequisite background to develop relevant drug delivery/carrier systems for them, classified and briefly discussed subsequently. Many of the recent studies aim at different types of synthetic, semisynthetic, and natural polymers serving as a favorable matrix for the antiviral drug carrier. Besides a wider view of different antiviral delivery systems, this review focuses on advances in antiviral drug delivery systems based on chitosan (CS) and derivatized CS carriers. CS and its derivatives are evaluated concerning methods of their preparation, their basic characteristics and properties, approaches to the incorporation of an antiviral drug in the CS polymer as well as CS nanoparticulate systems, and their recent biomedical applications in the context of actual antiviral therapy. The degree of development (i.e., research study, in vitro/ex vivo/in vivo preclinical testing), as well as benefits and limitations of CS polymer and CS nanoparticulate drug delivery systems, are reported for particular viral diseases and corresponding antivirotics.
1. Introduction
Among all the disease-causing agents in humans, viruses are the most notorious, active, and important [1]. Infections by viruses in humans cause millions of deaths around the globe and are accountable for human diseases such as HIV/AIDS, hepatitis, influenza, herpes simplex, common cold, etc. The upsurge in chronic viral infections such as HIV, HCV, HBV, etc., and the emergence of new viruses such as severe acute respiratory syndrome coronavirus (SARS-CoV, causing SARS) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, causing COVID-19), emphasize the growing need for novel strategies to develop antiviral agents. The majority of antiviral drugs are small molecules with diverse roles in clinical use. The large molecules include proteins (interferons, monoclonal antibodies), peptides, and oligonucleotides. Most of these antiviral drugs target the virus’s cellular machinery, while very few of them target the host cells/cellular mechanisms [2]. The advances in antiviral drug discoveries were discussed in the review of Saxena et al. [1]. Menendez-Arias and Delgado [3] focused their very recent review on the latest updates in antiretroviral therapy.
Clinical use of the currently available antiviral drugs is limited by various factors such as toxic side effects and possible viral latency [1]. There are also some general limitations inherent in antiviral chemotherapy. First, the more selective the antiviral drug, the narrower its antiviral activity spectrum. Second, since antiviral drugs target steps in virus replication, the latent phases characteristic of some viral (i.e., herpesviral) infections are not amenable to chemotherapy. This is particularly relevant for herpesvirus and retrovirus infections. Thus, eradication of latent virus infections is not feasible currently. Third, antiviral drug treatment should be started early, before irreversible tissue damage occurs. Such timely treatment is not possible without an early and accurate diagnosis, which is difficult for many viral infections (such as infections of the respiratory tract). Fourth, perhaps inevitably for a specific antimicrobial agent, there is the risk of the emergence of drug-resistant virus strains [1,4].
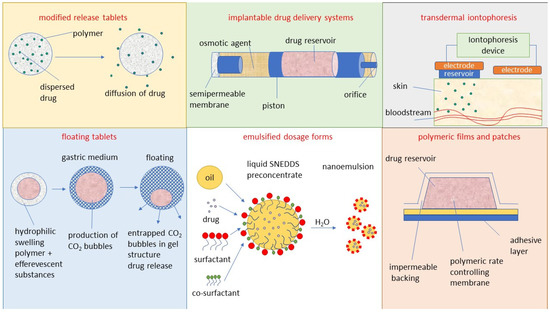
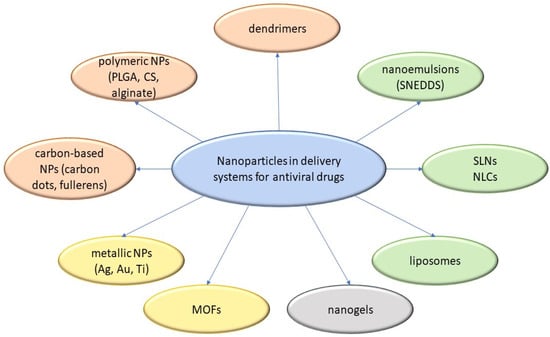
One of the major challenges associated with antiviral drugs is bioavailability, affected by a drug’s ability to be absorbed by the gastrointestinal tract. [5]. An approach oriented toward the development of novel drug delivery systems can be used to achieve efficacious therapy by improving the design, formulation, and delivery of existing antiviral agents. Innovative dosage forms help to decrease toxicity and dosage and may enhance the stability, specificity, and efficiency of antivirals [6]. Sharma et al. and Durai [6,7], in their review papers, discussed the different approaches to drug delivery of antiviral and antiretroviral drugs. Currently, among the most frequently studied systems for antiviral drug delivery are the following: modified-release forms such as depot tablets, multi-unit particulate system tablets [8], floating systems, implants [9], films, micro- and nanoemulsions, and drug carriers such as lipid nanoparticles (NPs) as liposomes [10] or ethosomes [11], solid lipid NPs [12], carbon-based NPs [13] and polymeric NPs [14].
NPs can be defined as submicron colloidal drug carrier systems ranging in size between 10 and 100 nm. Advanced nanoparticulate drugs are more suitable than conventional medicines in terms of site-specific targeting capabilities, sustained and controlled release, increased absorption rates and bioavailability, and improved stability of therapeutic agents. The NP size, hydrophobicity, modified surface, high surface-to-volume ratio, and surface charge are the essential factors that control the targeting capabilities, help in reducing the drug dose and frequency of administration, leading to reduced toxicity and side effects of drugs, and thus improve patient compliance [15,16]. Recently, the FDA published its guidance on “drug products, including biological products, that contain nanomaterials” [17], in doing so establishing the attributes of nanomaterials. The average particle size, particle size distribution (PSD), shape, morphology, surface charge, and concentration, among others, are mentioned as mandatory attributes to be determined [18].
Delshadi et al. [19] discussed, in their review, the ways to overcome the limitations of current antivirals by creating nanoparticulate drug delivery systems. In addition, Maus et al. [5] and Lembo et al. [20] discussed various types of NPs as effective carriers for antiviral drugs. Wang et al. [21] focused on the biosafe nanomaterials used for antiviral therapy and discussed the options for the design of antiviral drugs in the future.
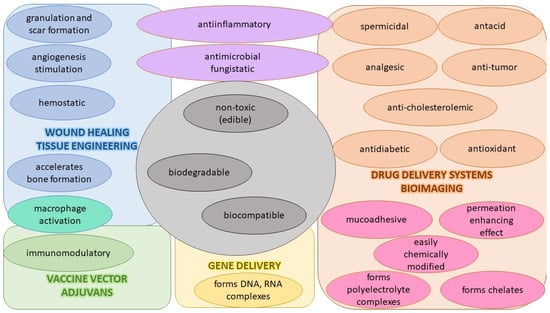
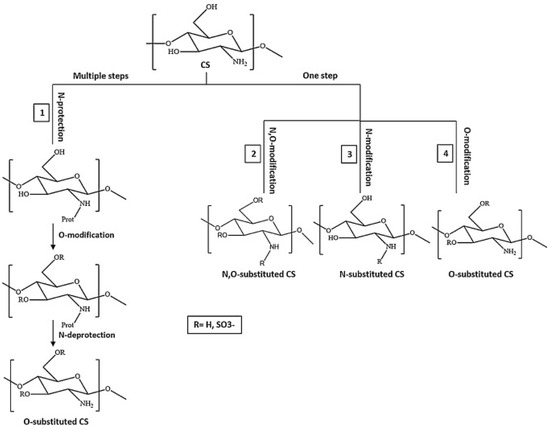
Currently, polymers are being widely used for pharmaceutical applications. Polymeric nanosystems have unique physicochemical characteristics such as prolonged blood circulation time, reduced adverse effects, ability to protect therapeutic agents from degradation, and increased stability. They also increase bioavailability of drugs, can be easily chemically modified, provide controlled drug release, and have high efficacy [22]. One of the most frequently used polymers is CS due to its beneficial properties such as biodegradability, biocompatibility, the ability for wound healing, antimicrobial activity, and, most importantly, mucoadhesiveness, making it a good carrier material in drug delivery systems [23]. CS, being positively charged and engaged in interaction with the mucus membrane, is capable of opening the tight junctions between cells, thus promoting passage through the mucosal cells and enhancing drug permeation [24]. Although CS possesses many functional properties, it has also several limitations such as high hydrophilicity, low ductility, a high degree of swelling, and low thermal stability. A major limiting factor is its poor water solubility. CS is insoluble at a physiological pH (pH 7.4) and ineffective as an absorption enhancer, which interferes with its biomedical application. Improving the solubility of CS is a crucial factor for judicious use in a multitude of applications, which is achieved through chemical modification of CS. Chemical derivatives of CS have received increasing interest over the past decade due to their advocated chemical, biological, and functional advantages over unmodified CS based on their solubility, gelling properties, hydrophobic derivatives with amphiphilic character, capacity to harness self-assembling nanostructures and chemical conjugates with an assortment of bioactive and therapeutic molecules, improved biocompatibility, and enhanced properties for complexing biomolecules (e.g., DNA, RNA) [16]. Classification of the CS derivatives and their methods of preparation, along with the improvement of properties and applications, was offered in recent reviews by Mikušová and Mikuš [16] and Negm et al. [25]. Wang et al. [26] discussed CS derivatives and their applications in biomedicine and Patrulea et al. [27] in wound healing.
The present review paper informs about updates in the area of CS and its role in current pharmaceutical systems for antivirotics. Before discussing the strategies in the development of effective CS dosage forms (Section 4), various antiviral therapy approaches are described (including current antivirals and their mechanisms of action (Section 2), current antiviral delivery approaches, and dosage forms (Section 3)). Then, the article continues with a comprehensive review of CS and its derivatives relevant to antivirotics’ formulation, along with the current state of CS-based polymeric (Section 4.1) as well as NP (Section 4.2) systems proposed for antiviral therapy, either composed of CS derivatives possessing antiviral properties or CS NPs loaded with antivirotics. The advantages and limitations of various CS-based delivery systems for antivirotics are discussed, along with their potential for practical implementation.
5. Conclusions
This review integrates several key aspects relevant to the development of progressive dosage forms and delivery systems for antivirals. Firstly, it provides a comprehensive view of currently used antivirals classified according to the mechanism of action in the organism. Based on this background, new findings concerning innovative dosage forms of 2nd and 3rd generations are discussed. Within these innovations, chitosan-based antivirals and antiviral delivery systems resp. carriers are highlighted. Chitosan and its derivatives are presented as highly potent polymeric as well as nanoparticulate systems accomplishing various important goals in modern antiviral therapy.
It can be summarized there is great interest in developing new sophisticated antiviral systems due to the many limitations of current antiviral therapies and the growing viral pandemics. It is important to have effective means of preventing viral transmission and treating a viral infection in humans and also animals. Although many antivirals are already available, their efficacy is often limited because of factors such as poor solubility, low permeability, poor bioavailability, untargeted release, toxicity, adverse side effects, and antiviral resistance. The development of a new antiviral drug is time-consuming and expensive; therefore, the research is oriented toward innovative dosage forms of known antivirals. Many of their limitations can be overcome using advanced antiviral delivery systems constructed on biodegradable polymers (native as well as derivatized) and corresponding nanotechnology principles.
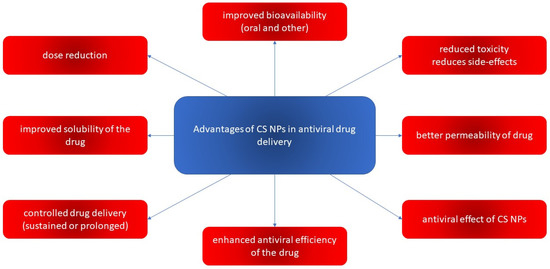
In this context, CS NPs are one of the most popular biopolymer-based NPs used for targeting the delivery of antivirals. They provide high drug encapsulation efficiency. They are often used to create controlled-release drug delivery systems (prolonged or sustained) capable of delivering the drug for longer, which maintains a stable level of the drug in the circulation, as well as reducing the dosing, side effects, and toxicity. CS NPs also have a synergic effect when combined with antiviral drugs and the necessary amount of drug can be reduced. This leads to reduced toxicity, which is another key reason for creating CS NPs. Pharmaceutical vehicles based on CS NPs can be capable of enhancing microbicide performance for better protection from sexually transmitted diseases. A very important reason to use CS NPs as an antiviral drug carrier is improved bioavailability. Oral bioavailability is increased because an antiviral drug encapsulated in CS NPs is protected against gastric degradation, and CS increases uptake by opening the tight junctions in the intestinal epithelium. CS NPs can increase permeation and thus improve the topical, nasal, or vaginal bioavailability of antivirals. Some CS derivates and combinations of CS with other materials can improve the solubility of low-soluble antiviral drugs, thus enhancing their bioavailability and reducing toxicity and dosing. Notable is how CS NPs consisting of specific CS derivates (such as carboxyalkylated, sulfated, quaternized, oligosaccharides) can possess antiviral activity themselves.
Although the use of commercial CS products in drug delivery (at a research or clinical level) could be limited, to some extent, by its structural variability among batches (depending on the CS source and its preparation), the benefits will undoubtedly accelerate its implementation in innovative dosage forms and carrier systems for antivirals, to achieve effective therapy for viral diseases.
Author Contributions
Conceptualization, P.M. and V.M.; writing—original draft preparation, D.Ž. and V.M.; writing—review and editing, V.M. and P.M.; supervision, P.M.; funding acquisition, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by APVV, grant number APVV-15-0585, and by VEGA, grant number VEGA 1/0514/22.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors.
Acknowledgments
This work was supported by the Toxicological and Antidoping Center at the Faculty of Pharmacy, Comenius University Bratislava, and the Library and Editorial Fund of the Faculty of Pharmacy, Comenius University Bratislava.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saxena, S.K.; Mishra, N.; Saxena, R. Advances in antiviral drug discovery and development: Part I: Advancements in antiviral drug discovery. Future Virol. 2009, 4, 101–107. [Google Scholar] [CrossRef]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef]
- Menéndez-Arias, L.; Delgado, R. Update and latest advances in antiretroviral therapy. Trends Pharmacol. Sci. 2022, 43, 6–29. [Google Scholar] [CrossRef]
- De Clercq, E. Chemotherapy of Viral Infections. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Maus, A.; Strait, L.; Zhu, D. Nanoparticles as delivery vehicles for antiviral therapeutic drugs. J. Eng. Regen. Med. 2021, 2, 31–46. [Google Scholar] [CrossRef]
- Sharma, P.; Chawla, A.; Arora, S.; Pawar, P. Novel drug delivery approaches on antiviral and antiretroviral agents. J. Adv. Pharm. Tech. Res. 2012, 3, 147–159. [Google Scholar] [CrossRef]
- Durai, R. Drug delivery approaches of an antiviral drug: A comprehensive review. Asian J. Pharm. 2015, 9, 1–12. [Google Scholar] [CrossRef]
- Čierna, M.; Mučaji, P.; Špaglová, M.; Čuchorová, M.; Macho, O. Chitosan and Sodium Alginate Implementation as Pharmaceutical Excipients in Multiple-Unit Particulate Systems. Polymers 2022, 14, 2822. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Ramvignesh, T.R.; Sundaramoorthy, K.; Ayyappan, T.; Vetrichelvan, T. Research Design and evaluation of novel ophthalmic delivery system of acyclovir for herpes simplex infection. J. Pharm. Biol. Chem. Scien. 2011, 2, 803–814. [Google Scholar]
- Sanna, V.; Satta, S.; Hsiai, T.; Sechi, M. Development of targeted nanoparticles loaded with antiviral drugs for SARS-CoV-2 inhibition. Eur. J. Med. Chem. 2022, 231, 114121. [Google Scholar] [CrossRef]
- Jain, S.; Tiwary, A.K.; Sapra, B.; Jain, N.K. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech 2007, 8, E111. [Google Scholar] [CrossRef]
- Makwana, V.; Jain, R.; Patel, K.; Nivsarkar, M.; Joshi, A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. Int. J. Pharmacol. 2015, 495, 439–446. [Google Scholar] [CrossRef]
- Dostalova, S.; Moulick, A.; Milosavljevic, V.; Guran, R.; Kominkova, M.; Cihalova, K.; Heger, Z.; Blazkova, L.; Kopel, P.; Hynek, D.; et al. Antiviral activity of fullerene C60 nanocrystals modified with derivatives of anionic antimicrobial peptide maximin H5. Chem. Mon. 2016, 147, 905–918. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- FDA/CDER. Guidance for Industry, Drug Products, Including Biological Products, That Contain Nanomaterials; Center for Drug Evaluation and Research: Maryland, MD, USA, 2022. [Google Scholar]
- Marques, C.; Maurizi, L.; Borchard, G.; Jordan, O. Characterization Challenges of Self-Assembled Polymer-SPIONs Nanoparticles: Benefits of Orthogonal Methods. Int. J. Mol. Sci. 2022, 23, 16124. [Google Scholar] [CrossRef] [PubMed]
- Delshadi, R.; Bahrami, A.; McClements, D.J.; Moore, M.D.; Williams, L. Development of nanoparticle-delivery systems for antiviral agents: A review. J. Control. Release Off J. Control. Release Soc. 2021, 331, 30–44. [Google Scholar] [CrossRef]
- Lembo, D.; Donalisio, M.; Civra, A.; Argenziano, M.; Cavalli, R. Nanomedicine formulations for the delivery of antiviral drugs: A promising solution for the treatment of viral infections. Expert Opin. Drug Deliv. 2018, 15, 93–114. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Cao, L.; Ge, K. Constructive strategies for drug delivery systems in antivirus disease therapy by safety materials. Biosaf. Health 2022, 4, 61–170. [Google Scholar] [CrossRef]
- Yang, D. Application of Nanotechnology in the COVID-19 Pandemic. Int. J. Nanomed. 2021, 16, 623–649. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badr, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. Part B 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Khan, A.H.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002620. [Google Scholar] [CrossRef]
- Matthews, T.; Salgo, M.; Greenberg, M.; Chung, J.; DeMasi, R.; Bolognesi, D. Enfuvirtide: The first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat. Rev. Drug Discov. 2004, 3, 215–225. [Google Scholar] [CrossRef] [PubMed]
- LiverTox. Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Kaushik, I.; Ramachandran, S.; Prasad, S.; Srivastava, S.K. Drug rechanneling: A novel paradigm for cancer treatment. Semin. Cancer Biol. 2021, 68, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Scholar, E. Idoxuridine. In XPharm Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–5. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Suita, K.; Ohnuki, Y.; Mototani, Y.; Ishikawa, M.; Ito, A.; Nariyama, M.; Morii, A.; Kiyomoto, K.; Tsunoda, M.; et al. Vidarabine, an anti-herpes agent, prevents occlusal-disharmony-induced cardiac dysfunction in mice. J. Physiol. Sci. 2022, 72, 2. [Google Scholar] [CrossRef]
- Taylor, M.; Gerriets, V. Acyclovir. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Shehata, H. Drugs and Drug Therapy. In Basic Science in Obstetrics and Gynaecology, 4th ed.; Bennett, P., Williamson, C., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2010; pp. 259–277. [Google Scholar] [CrossRef]
- Hoth, A.B.; Rawls, N.; Shorr, R.I. (Eds.) Drugs for the Geriatric Patient; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–115. [Google Scholar] [CrossRef]
- Mondal, D. Cidofovir. In XPharm Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–5. [Google Scholar] [CrossRef]
- Foscarnet. Meyler’s Side Effects of Drugs, 16th ed.; Aronson, J.K., Ed.; Elsevier: Oxford, UK, 2016; pp. 450–451. [Google Scholar] [CrossRef]
- Villemin, D.; Moreau, B.; Bar, N. MCR under Microwave Irradiation: Synthesis in Water of New 2-Amino-bis(2-phosphonoacetic) Acids. Organics 2021, 2, 98–106. [Google Scholar] [CrossRef]
- Bule, M.; Khan, F.; Niaz, K. Antivirals: Past, Present and Future. In Recent Advances in Animal Virology; Malik, Y.S., Singh, R.K., Yadav, M.P., Eds.; Springer: Singapore, 2019; pp. 425–446. [Google Scholar] [CrossRef]
- Waller, D.G.; Sampson, A.P. (Eds.) Chemotherapy of Infections. In Medical Pharmacology and Therapeutics, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 581–629. [Google Scholar] [CrossRef]
- Aliyu, S. Viral, Fungal, Protozoal and Helminthic Infections. In Clinical Pharmacology, 12th ed.; Bennett, P.N., Brown, M.J., Sharma, P., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2012; pp. 221–247. [Google Scholar] [CrossRef]
- Yee, J.; Preuss, C.V. Efavirenz. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Paintsil, E.; Cheng, Y.C. Antiviral Agents. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 223–257. [Google Scholar] [CrossRef]
- Eckhardt, B.J.; Gulick, R.M. Drugs for HIV Infection. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1293–1308. [Google Scholar] [CrossRef]
- Taylor, K.; Fritz, K.; Parmar, M. Lamivudine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Anderson, P.L.; Kakuda, T.N.; Lichtenstein, K.A. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin. Infect. Dis. 2004, 38, 743–753. [Google Scholar] [CrossRef]
- de Vries-Sluijs, T.E.M.S.; Reijnders, J.G.P.; Hansen, B.E.; Zaaijer, H.L.; Prins, J.M.; Pas, S.D.; Schutten, M.; Hoepelman, A.I.M.; Richter, C.; Mulder, J.W.; et al. Long-term therapy with tenofovir is effective for patients co-infected with human immunodeficiency virus and hepatitis B virus. Gastroenterology 2010, 139, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. (Ed.) Adefovir. In Meylers Side Effects of Drugs, 6th ed.; Elsevier: Oxford, UK, 2016; p. 72. [Google Scholar] [CrossRef]
- Rehman, N.; Nguyen, H. Nevirapine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Scott, L.J.; Perry, C.M. Delavirdine: A review of its use in HIV infection. Drugs 2000, 60, 1411–1444. [Google Scholar] [CrossRef] [PubMed]
- Mallayasamy, S.; Penzak, S.R. Pharmacogenomic Considerations in the Treatment of HIV Infection. In Pharmacogenomics, 2nd ed.; Lam, Y.W.F., Scott, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 227–245. [Google Scholar] [CrossRef]
- Bodiwala, H.S.; Sabde, S.; Gupta, P.; Mukherjee, R.; Kumar, R.; Garg, P.; Bhutani, K.K.; Mitra, D.; Singh, I.P. Design and synthesis of caffeoyl-anilides as portmanteau inhibitors of HIV-1 integrase and CCR5. Bioorg. Med. Chem. Lett. 2011, 19, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Chu, Y.; Wang, Y. HIV protease inhibitors: A review of molecular selectivity and toxicity. HIV/AIDS—Res. Palliat. Care 2015, 7, 95–104. [Google Scholar] [CrossRef]
- Pires, D.; Valente, S.; Calado, M.; Mandal, M.; Azevedo-Pereira, M.; Anes, E. Repurposing Saquinavir for Host-Directed Therapy to Control Mycobacterium Tuberculosis Infection. Front. Immunol. 2021, 12, 647728. [Google Scholar] [CrossRef]
- Pollak, E.B.; Parmar, M. Indinavir. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Markland, W.; Rao, B.G.; Parsons, J.D.; Black, J.; Zuchowski, L.; Tisdale, M.; Tung, R. Structural and Kinetic Analyses of the Protease from an Amprenavir-Resistant Human Immunodeficiency Virus Type 1 Mutant Rendered Resistant to Saquinavir and Resensitized to Amprenavir. J. Virol. 2000, 74, 7636–7641. [Google Scholar] [CrossRef]
- Chapman, T.M.; Plosker, G.L.; Perry, C.M. Fosamprenavir: A review of its use in the management of antiretroviral therapy-naive patients with HIV infection. Drugs 2004, 64, 2101–2124. [Google Scholar] [CrossRef] [PubMed]
- Subeha, M.R.; Telleria, C.M. The Anti-Cancer Properties of the HIV Protease Inhibitor Nelfinavir. Cancers 2020, 12, 3437. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Innao, V.; Allegra, A.G.; Pulvirenti, N.; Pugliese, M.; Musolino, C. Antitumorigenic action of nelfinavir: Effects on multiple myeloma and hematologic malignancies (Review). Oncol. Rep. 2020, 43, 1729–1736. [Google Scholar] [CrossRef]
- Information on COVID-19 Treatment, Prevention and Research. COVID-19 Treat Guidel n.d. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 29 January 2023).
- Hung, I.F.-N.; Lung, K.-C.; Tso, E.Y.-K.; Liu, R.; Chung, T.W.-H.; Chu, M.-Y.; Ng, Y.-Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiong, N.; Li, C.; Gong, Y.; Liu, L.; Yang, H.; Tan, X.; Jiang, N.; Zong, Q.; Wang, J.; et al. Efficacy of ribavirin and interferon-α therapy for hospitalized patients with COVID-19: A multicenter, retrospective cohort study. Int. J. Infect. Dis. 2021, 104, 641–648. [Google Scholar] [CrossRef] [PubMed]
- McHutchison, J.G.; Dev, A.T. Future trends in managing hepatitis C. Gastroenterol. Clin. N. Am. 2004, 33, 51–61. [Google Scholar] [CrossRef]
- Lin, C.; Yeh, L.-T.; Vitarella, D.; Hong, Z. Viramidine, a prodrug of ribavirin, shows better liver-targeting properties and safety profiles than ribavirin in animals. Antivir. Chem. Chemother. 2003, 14, 145–152. [Google Scholar] [CrossRef]
- Zarrouk, K.; Piret, J.; Boivin, G. Herpesvirus DNA polymerases: Structures, functions and inhibitors. Virus Res. 2017, 234, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Yan, H.; Wang, J.; Liu, K.; Liu, B.; Shi, Y. Current scenario on non-nucleoside reverse transcriptase inhibitors (2018-present). Arab. J. Chem. 2022, 15, 104378. [Google Scholar] [CrossRef]
- Gill, M.S.A.; Hassan, S.S.; Ahemad, N. Evolution of HIV-1 reverse transcriptase and integrase dual inhibitors: Recent advances and developments. Eur. J. Med. Chem. 2019, 179, 423–448. [Google Scholar] [CrossRef] [PubMed]
- Vardanyan, R.; Hruby, V. Antiviral Drugs. In Synthesis of Best-Seller Drugs; Vardanyan, R., Hruby, V., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 687–736. [Google Scholar] [CrossRef]
- Kim, J.; De Jesus, O. Medication Routes of Administration. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Poole, C.L.; James, S.H. Antiviral Therapies for Herpesviruses: Current Agents and New Directions. Clin. Ther. 2018, 40, 1282–1298. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, T.; Song, J.; Pu, D.; He, D.; Li, J.; Yang, J.; Li, K.; Zhong, C.; Zhang, J. Antiviral Drug Delivery System for Enhanced Bioactivity, Better Metabolism and Pharmacokinetic Characteristics. Int. J. Nanomed. 2021, 16, 4959–4984. [Google Scholar] [CrossRef] [PubMed]
- Richelsen, R.K.B.; Jensen, S.B.; Nielsen, H. Incidence and predictors of intravenous acyclovir-induced nephrotoxicity. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1965–1971. [Google Scholar] [CrossRef]
- Gonda, I. Systemic delivery of drugs to humans via inhalation. J. Int. Soc. Aerosols Med. 2006, 19, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Quiñones-Mateu, M.E.; Das, S.C. Inhaled therapy for COVID-19: Considerations of drugs, formulations and devices. Int. J. Pharm. 2022, 624, 122042. [Google Scholar] [CrossRef]
- Razonable, R.R. Antiviral Drugs for Viruses Other Than Human Immunodeficiency Virus. Mayo Clin. Proc. 2011, 86, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-Y.; Chen, Y.; Huang, Y.-Y.; Cheng, C.-M. Transdermal drug delivery systems for fighting common viral infectious diseases. Drug Deliv. Transl. Res. 2021, 11, 1498–1508. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Kim, J.; Yeom, M.; Lee, T.; Kim, H.-O.; Na, W.; Kang, A.; Lim, J.-W.; Park, G.; Park, C.; Song, D.; et al. Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 2020, 18, 54. [Google Scholar] [CrossRef]
- Muñoz, A.; Sigwalt, D.; Illescas, B.M.; Luczkowiak, J.; Rodríguez-Pérez, L.; Nierengarten, I.; Holler, M.; Remy, J.-S.; Buffet, K.; Vincent, S.P.; et al. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nat. Chem. 2016, 8, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Swaminathan, S.; Donalisio, M.; Civra, A.; Pastero, L.; Aquilano, D.; Vavia, P.; Trotta, F.; Cavalli, R. Encapsulation of Acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int. J. Pharm. 2013, 443, 262–272. [Google Scholar] [CrossRef]
- Cautela, M.P.; Moshe, H.; Sosnik, A.; Sarmento, B.; das Neves, J. Composite films for vaginal delivery of tenofovir disoproxil fumarate and emtricitabine. Eur. J. Pharm. Biopharm. 2019, 138, 3–10. [Google Scholar] [CrossRef]
- Kandeel, M.; Al-Taher, A.; Park, B.K.; Kwon, H.-J.; Al-Nazawi, M. A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. J. Med. Virol. 2020, 92, 1665–1670. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2009, 9, 172–178. [Google Scholar] [CrossRef]
- Reolon, J.B.; Brustolin, M.; Accarini, T.; Viçozzi, G.P.; Sari, M.H.M.; Bender, E.A.; Haas, S.E.; Brum, M.C.S.; Gündel, A.; Colomé, L.M. Co-encapsulation of acyclovir and curcumin into microparticles improves the physicochemical characteristics and potentiates in vitro antiviral action: Influence of the polymeric composition. Eur. J. Pharm. Sci. 2019, 131, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Manyarara, T.E.; Khoza, S.; Dube, A.; Maponga, C.C. Formulation and characterization of a paediatric nanoemulsion dosage form with modified oral drug delivery system for improved dissolution rate of nevirapine. MRS Adv. 2018, 3, 2203. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Alruwaili, N.K.; Alhakamy, N.A.; Abualsunun, W.A.; Bakhaidar, R.B.; Bahmdan, R.H.; Rizg, W.Y.; et al. Oral gel loaded with penciclovir-lavender oil nanoemulsion to enhance bioavailability and alleviate pain associated with herpes labialis. Drug Deliv. 2021, 28, 1043–1054. [Google Scholar] [CrossRef]
- Jha, M. Modified release formulations to achieve the quality target product profile (QTPP). Int. J. Pharm. Sci. Res. 2012, 3, 2376. [Google Scholar]
- Karpe, M.; Mali, N.; Kadam, V. Formulation Development and Evaluation of Acyclovir Orally Disintegrating Tablets. J. Appl. Pharm. Sci. 2012, 2, 101–105. [Google Scholar]
- Gröning, R.; Berntgen, M.; Georgarakis, M. Acyclovir serum concentrations following peroral administration of magnetic depot tablets and the influence of extracorporal magnets to control gastrointestinal transit. Eur. J. Pharm. Biopharm. 1998, 46, 285–291. [Google Scholar] [CrossRef]
- Zielenkiewicz, W.; Koźbiał, M.; Golankiewicz, B.; Poznanski, J. Enhancement of aqueous solubility of tricyclic acyclovir derivatives by their complexation with hydroxypropyl-β-cyclodextrin. J. Therm. Anal. Calorim. 2010, 101, 555–560. [Google Scholar] [CrossRef]
- Bencini, M.; Ranucci, E.; Ferruti, P.; Trotta, F.; Donalisio, M.; Cornaglia, M.; Lembo, D.; Cavalli, R. Preparation and in vitro evaluation of the antiviral activity of the Acyclovir complex of a beta-cyclodextrin/poly(amidoamine) copolymer. J. Control. Release 2008, 126, 17–25. [Google Scholar] [CrossRef]
- Chaudhari, K.D.; Nimbalwar, M.G.; Singhal, N.S.; Panchale, W.A.; Manwar, J.V.; Bakal, R.L. Comprehensive review on characterizations and application of gastro-retentive floating drug delivery system. GSC Adv. Res. Rev. 2021, 7, 35–44. [Google Scholar] [CrossRef]
- Ahmed, F.J.; Drabu, S.; Khatri, S.; Babu, S. Formulation and evaluation of acyclovir capsules. Int. J. Drug Dev. Res. 2011, 3, 162–167. [Google Scholar]
- Ahmed, F.; Drabu, S.; Khatri, S.; Babu, S. Development and evaluation of floating matrix tablets of acyclovir. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 547–553. [Google Scholar]
- Vinodbhai, P.K.; Gohel, D.M.C.; Parikh, D.R.K.; Bariya, D.S.; Suthar, R.N. Sustained release floating microspheres of acyclovir: Formulation, optimization, characterization and in vitro evaluation. Int. J. Drug Dev. Res. 2011, 3, 242–251. [Google Scholar]
- Singhal, P.; Kumar, K.; Pandey, M.; Saraf, S.A. Evaluation of acyclovir loaded oil entrapped calcium alginate beads prepared by ionotropic gelation method. Int. J. Chem. Technol. Res. 2010, 2, 2076–2085. [Google Scholar]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release 2014, 181, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Pillai, J. Nanostructures for the Engineering of Cells, Tissues and Organs. In Implantable Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 473–511. [Google Scholar]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 12, 1379. [Google Scholar] [CrossRef]
- Dash, A.K.; Cudworth, G.C. Therapeutic applications of implantable drug delivery systems. J. Pharmacol. Toxicol. Methods 1998, 40, 1–12. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Alam, A.; Gamal, S.; Naggar, V. Formulation and evaluation of acyclovir ophthalmic inserts. Asian J. Pharm. Sci. 2008, 3, 58–67. [Google Scholar]
- Kerur, S.; Dangari, P.; Deshpande, P. Controlled release polymeric ocular inserts for delivery of acyclovir. Turk. J. Pharm. Sci. 2010, 7, 75–90. [Google Scholar]
- Khan, S.; Ali, A.; Singhavi, D.; Yeole, P. Controlled ocular delivery of acyclovir through rate controlling ocular insert of Eudragit: A technical note. AAPS PharmSciTech 2008, 9, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.B.; Dandagi, P.; Udupa, N.; Gopal, S.V.; Jain, S.S.; Vasanth, S.G. Controlled release polymeric ocular delivery of acyclovir. Pharm. Dev. Technol. 2010, 15, 369–378. [Google Scholar] [CrossRef]
- Han, T.; Das, D.B. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: A review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328. [Google Scholar] [CrossRef]
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.J.; Woolfson, A.D. Transdermal Delivery Applications. In Microneedle-Mediated Transdermal and Intradermal Drug Delivery; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 1–19. [Google Scholar] [CrossRef]
- Kretsos, K.; Kasting, G.B. A Geometrical Model of Dermal Capillary Clearance. Math. Biosci. 2007, 208, 430–453. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Prausnitz, M.R.; Mitragotri, S. Micro-Scale Devices for Transdermal Drug Delivery. Int. J. Pharm. 2008, 364, 227–236. [Google Scholar] [CrossRef]
- Tuan-Mahmood, T.; McCrudden, M.T.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for Intradermal and Transdermal Drug Delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Ita, K. Transdermal Drug Delivery: Progress and Challenges. J. Drug Deliv. Sci. Technol. 2014, 24, 245–250. [Google Scholar] [CrossRef]
- Lashmar, U.T.; Manger, J. Investigation into the potential for iontophoresis facilitated transdermal delivery of acyclovir. Int. J. Pharm. 1994, 111, 73–82. [Google Scholar] [CrossRef]
- Gonsho, A.; Imanidis, G.; Vogt, P.; Kern, E.R.; Tsuge, H.; Su, M.-H.; Choi, S.-H.; Higuchi, W.I. Controlled (trans) dermal delivery of an antiviral agent (acyclovir). I: An in vivo animal model for efficacy evaluation in cutaneous HSV-1 infections. Int. J. Pharm. 1990, 65, 183–194. [Google Scholar] [CrossRef]
- Saxena, A.; Tewari, G.; Saraf, S.A. Formulation and evaluation of mucoadhesive buccal patch of acyclovir utilizing inclusion phenomenon. Braz. J. Pharm. Sci. 2011, 47, 887–897. [Google Scholar] [CrossRef]
- Tallury, P.; Alimohammadi, N.; Kalachandra, S. Poly(ethylene-co-vinyl acetate) copolymer matrix for delivery of chlorhexidine and acyclovir drugs for use in the oral environment: Effect of drug combination, copolymer composition and coating on the drug release rate. Dent. Mater. 2007, 23, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Majithiya, R.J.; Umrethia, M.L.; Murthy, R.S.R. Design and development of microemulsion drug delivery system of acyclovir for improvement of oral bioavailability. AAPS PharmSciTech 2006, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Shishu; Rajan, S.; Kamalpreet. Development of novel microemulsion-based topical formulations of acyclovir for the treatment of cutaneous herpetic infections. AAPS PharmSciTech 2009, 10, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Tamura, M.; Tahara, Y.; Kamiya, N.; Goto, M. Ionic liquid-in-oil microemulsion as a potential carrier of sparingly soluble drug: Characterization and cytotoxicity evaluation. Int. J. Pharm. 2010, 400, 243–250. [Google Scholar] [CrossRef]
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid Based Vesicular Drug Delivery Systems. Adv. Pharm. 2014, 2014, 574673. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J. An overview of functional nanoparticles as novel emerging antiviral therapeutic agents. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110924. [Google Scholar] [CrossRef]
- Attia, I.A.; El-Gizawy, S.A.; Fouda, M.A.; Donia, A.M. Influence of a niosomal formulation on the oral bioavailability of acyclovir in rabbits. AAPS PharmSciTech 2007, 8, E106. [Google Scholar] [CrossRef]
- Alsarra, I.A.; Hamed, A.Y.; Alanazi, F.K. Acyclovir liposomes for intranasal systemic delivery: Development and pharmacokinetics evaluation. Drug Deliv. 2008, 15, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Chetoni, P.; Rossi, S.; Burgalassi, S.; Monti, D.; Mariotti, S.; Saettone, M.F. Comparison of Liposome-Encapsulated Acyclovir with Acyclovir Ointment: Ocular Pharmacokinetics in Rabbits. J. Ocul. Pharmacol. Ther. 2004, 20, 169–177. [Google Scholar] [CrossRef]
- Garg, M.; Jain, N.K. Reduced hematopoietic toxicity, enhanced cellular uptake and altered pharmacokinetics of azidothymidine loaded galactosylated liposomes. J. Drug Target. 2006, 14, 1–11. [Google Scholar] [CrossRef]
- Javan, F.; Vatanara, A.; Azadmanesh, K.; Nabi-Meibodi, M.; Shakouri, M. Encapsulation of ritonavir in solid lipid nanoparticles: In-vitro anti-HIV-1 activity using lentiviral particles. J. Pharm. Pharmacol. 2017, 69, 1002–1009. [Google Scholar] [CrossRef]
- Seyfoddin, A.; Al-Kassas, R. Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev. Ind. Pharm. 2013, 39, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int. J. Pharm. 2020, 579, 119181. [Google Scholar] [CrossRef]
- Perret, F.; Duffour, M.; Chevalier, Y.; Parrot-Lopez, H. Design, synthesis, and in vitro evaluation of new amphiphilic cyclodextrin-based nanoparticles for the incorporation and controlled release of acyclovir. Eur. J. Pharm. Biopharm. 2013, 83, 25–32. [Google Scholar] [CrossRef]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Bono, N.; Pennetta, C.; Bellucci, M.C.; Sganappa, A.; Malloggi, C.; Tedeschi, G.; Candiani, G.; Volonterio, A. Role of Generation on Successful DNA Delivery of PAMAM–(Guanidino)Neomycin Conjugates. ACS Omega 2019, 4, 6796–6807. [Google Scholar] [CrossRef]
- Kawano, Y.; Jordan, O.; Hanawa, T.; Borchard, G.; Patrulea, V. Are Antimicrobial Peptide Dendrimers an Escape from ESKAPE? Adv. Wound Care 2020, 9, 378–395. [Google Scholar] [CrossRef]
- Heitz, M.; Kwok, A.; Eggimann, G.A.; Hollfelder, F.; Darbre, T.; Reymond, J.L. Peptide Dendrimer–Lipid Conjugates as DNA and siRNA Transfection Reagents: Role of Charge Distribution Across Generations. Chimia 2017, 71, 220–225. [Google Scholar] [CrossRef]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef]
- Bourne, N.; Stanberry, L.R.; Kern, E.R.; Holan, G.; Matthews, B.; Bernstein, D.I. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 2000, 44, 2471–2474. [Google Scholar] [CrossRef]
- Oka, H.; Onaga, T.; Koyama, T.; Guo, C.-T.; Suzuki, Y.; Esumi, Y.; Hatano, K.; Terunuma, D.; Matsuoka, K. Sialyl alpha(2-->3) lactose clusters using carbosilane dendrimer core scaffolds as influenza hemagglutinin blockers. Bioorg. Med. Chem. Lett. 2008, 18, 4405–4408. [Google Scholar] [CrossRef] [PubMed]
- Witvrouw, M.; Fikkert, V.; Pluymers, W.; Matthews, B.; Mardel, K.; Schols, D.; Raff, J.; Debyser, Z.; De Clercq, E.; Holan, G.; et al. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol. Pharmacol. 2000, 58, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.L.; Sidwell, R.W.; Gage, T.L.; Okleberry, K.M.; Matthews, B.; Holan, G. Anti-Respiratory Syncytial Virus Activity of Dendrimer Polyanions. Antivir. Res. 1997, 2, A88. [Google Scholar] [CrossRef]
- Rodríguez-Izquierdo, I.; Natalia, C.; García, F.; Muñoz-Fernandez, M.D.L. G2-S16 sulfonate dendrimer as new therapy for treatment failure in HIV-1 entry inhibitors. Nanomedicine 2019, 14, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yoshida, D.; Kanamoto, T.; Nakashima, H.; Uryu, T.; Yoshida, T. Sulfated oligosaccharide cluster with polylysine core scaffold as a new anti-HIV dendrimer. Carbohydr. Polym. 2010, 80, 1111–1115. [Google Scholar] [CrossRef]
- Borges, A.R.; Wieczorek, L.; Johnson, B.; Benesi, A.J.; Brown, B.K.; Kensinger, R.D.; Krebs, F.C.; Wigdahl, B.; Blumenthal, R.; Puri, A.; et al. Multivalent dendrimeric compounds containing carbohydrates expressed on immune cells inhibit infection by primary isolates of HIV-1. Virology 2010, 408, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Doménech, R.; Abian, O.; Bocanegra, R.; Correa, J.; Sousa-Herves, A.; Riguera, R.; Mateu, M.G.; Fernandez-Megia, E.; Velázquez-Campoy, A.; Neira, J.L. Dendrimers as Potential Inhibitors of the Dimerization of the Capsid Protein of HIV-1. Biomacromolecules 2010, 11, 2069–2078. [Google Scholar] [CrossRef]
- Stella, B.; Arpicco, S.; Rocco, F.; Burgalassi, S.; Nicosia, N.; Tampucci, S.; Chetoni, P.; Cattel, L. Nonpolymeric nanoassemblies for ocular administration of acyclovir: Pharmacokinetic evaluation in rabbits. Eur. J. Pharm. Biopharm. 2012, 80, 39–45. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Guo, M.; Xu, T.; Wang, C.; Zhao, M.; Wang, H.; Chen, T.; Zhu, B. The inhibition of H1N1 influenza virus-induced apoptosis by silver nanoparticles functionalized with zanamivir. RSC Adv. 2017, 7, 742–750. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Ramanathan, R.; Jiang, Y.; Read, B.; Golan-Paz, S.; Woodrow, K. Biophysical characterization of small molecule antiviral-loaded nanolipogels for HIV-1 chemoprophylaxis and topical mucosal application. Acta Biomater. 2016, 36, 122–131. [Google Scholar] [CrossRef]
- Jansen, C.; Tran-Cong, N.M.; Schlüsener, C.; Schmitz, A.; Proksch, P.; Janiak, C. MOF@chitosan Composites with Potential Antifouling Properties for Open-Environment Applications of Metal-Organic Frameworks. Solids 2022, 3, 35–54. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Zhou, H.-C.J.; Kitagawa, S. Metal-organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal-organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef]
- Taddei, M. When defects turn into virtues: The curious case of zirconium-based metal-organic frameworks. Coord. Chem. Rev. 2017, 343, 1–24. [Google Scholar] [CrossRef]
- Keskin, S.; Kızılel, S. Biomedical Applications of Metal Organic Frameworks. Ind. Eng. Chem. Res. 2011, 50, 1799–1812. [Google Scholar] [CrossRef]
- Agostoni, V.; Chalati, T.; Horcajada, P.; Willaime, H.; Anand, R.; Semiramoth, N.; Baati, T.; Hall, S.; Maurin, G.; Chacun, H.; et al. Towards an improved anti-HIV activity of NRTI via metal-organic frameworks nanoparticles. Adv. Health Mater. 2013, 2, 1630–1637. [Google Scholar] [CrossRef]
- Akbari, M.; Ghasemzadeh, M.A.; Fadaeian, M. Synthesis and Application of ZIF-8 MOF Incorporated in a TiO2@Chitosan Nanocomposite as a Strong Nanocarrier for the Drug Delivery of Acyclovir. Chem. Sel. 2020, 5, 14564–14571. [Google Scholar] [CrossRef]
- Franklyne, J.S.; Gopinath, P.M.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsions: The rising star of antiviral therapeutics and nanodelivery system-current status and prospects. Curr. Opin. Colloid. Interface Sci. 2021, 54, 101458. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Elahimehr, Z.; Mahboobian, M.M. Acyclovir-Loaded Nanoemulsions: Preparation, Characterization and Irritancy Studies for Ophthalmic Delivery. Curr. Eye Res. 2021, 46, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Nemade, S.M.; Kakad, S.P.; Kshirsagar, S.J.; Padole, T.R. Development of nanoemulsion of antiviral drug for brain targeting in the treatment of neuro-AIDS. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Cherniakov, I.; Domb, A.J.; Hoffman, A. Self-nano-emulsifying drug delivery systems: An update of the biopharmaceutical aspects. Expert Opin. Drug Deliv. 2015, 12, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N.; Rogge, M.C.; Boddy, A.W.; Berger, B.M. Effect of food and a monoglyceride emulsion formulation on danazol bioavailability. J. Clin. Pharmacol. 1993, 33, 381–386. [Google Scholar] [CrossRef]
- Shahba, A.A.-W.; Mohsin, K.; Alanazi, F.K. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: Design, optimization, and in-vitro assessment. AAPS PharmSciTech 2012, 13, 967–977. [Google Scholar] [CrossRef]
- Khan, A.A.; Akhtar, S.; Yadav, Y.; Akhtar, A.; Alelwani, W.; Bannunah, A.M.; Mahmood, S. Lopinavir-Loaded Self-Nanoemulsifying Drug Delivery System for Enhanced Solubility: Development, Characterisation and Caco-2 Cell Uptake. Curr. Drug Deliv. 2022. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Mahmoud, M.G.; Em El Kady, E.; Asker, M.S. Chitin, Chitosan and Glucan, Properties and Applications. World J. Agri. Soil Sci. 2019, 3, 1–19. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid. Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Karava, A.; Lazaridou, M.; Nanaki, S.; Michailidou, G.; Christodoulou, E.; Kostoglou, M.; Iatrou, H.; Bikiaris, D.N. Chitosan Derivatives with Mucoadhesive and Antimicrobial Properties for Simultaneous Nanoencapsulation and Extended Ocular Release Formulations of Dexamethasone and Chloramphenicol Drugs. Pharmaceutics 2020, 12, 594. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef]
- Alqahtani, F.; Aleanizy, F.; El Tahir, E.; Alhabib, H.; Alsaif, R.; Shazly, G.; AlQahtani, H.; Alsarra, I.; Mahdavi, J. Antibacterial Activity of Chitosan Nanoparticles Against Pathogenic N. gonorrhoea. Int. J. Nanomed. 2020, 15, 7877–7887. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Khan, J.A.; Rabbani, F.; Latif, U.; Arfan, M.; Yameen, M.A. Biocompatible biodegradable polymeric antibacterial nanoparticles for enhancing the effects of a third-generation cephalosporin against resistant bacteria. J. Med. Microbiol. 2017, 66, 318–327. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications—A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar] [CrossRef]
- Nwe, N.; Furuike, T.; Tamura, H. Isolation and characterization of chitin and chitosan from marine origin. Adv. Food Nutr. Res. 2014, 72, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jaber, N.; Al-Remawi, M.; Al-Akayleh, F.; Al-Muhtaseb, N.; Al-Adham, I.S.I.; Collier, P.J. A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID-19. J. Appl. Microbiol. 2022, 132, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. In Sustainable Agriculture Reviews; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 35, pp. 49–123. [Google Scholar] [CrossRef]
- Sashiwa, H.; Aiba, S. Chemically modified chitin and chitosan as biomaterials. Progr. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
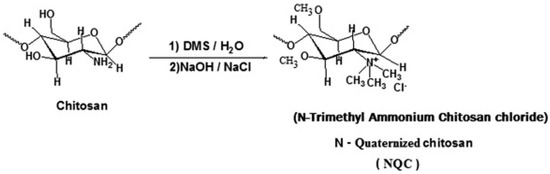
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef]
- Tan, R.S.L.; Hassandarvish, P.; Chee, C.F.; Chan, L.W.; Wong, T.W. Chitosan and its derivatives as polymeric anti-viral therapeutics and potential anti-SARS-CoV-2 nanomedicine. Carbohydr. Polym. 2022, 290, 119500. [Google Scholar] [CrossRef]
- Pathak, K.; Misra, S.; Sehgal, A.; Singh, S.; Bungau, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical Applications of Quaternized Chitosan. Polymers 2021, 13, 2514. [Google Scholar] [CrossRef]
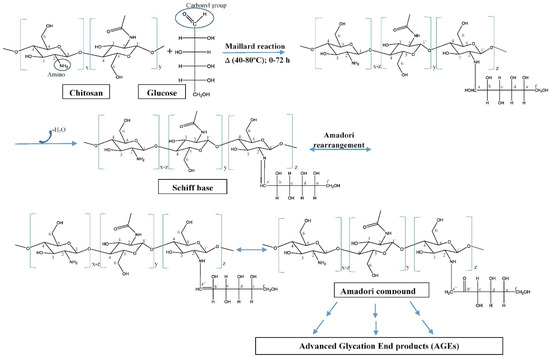
- Chung, Y.-C.; Yeh, J.-Y.; Tsai, C.-F. Antibacterial Characteristics and Activity of Water-Soluble Chitosan Derivatives Prepared by the Maillard Reaction. Molecules 2011, 16, 8504–8514. [Google Scholar] [CrossRef]
- Park, I.K.; Yang, J.; Jeong, H.J.; Bom, H.S.; Harada, I.; Akaike, T.; Kim, S.; Cho, C. Galactosylated chitosan as a synthetic extracellular matrix for hepatocytes attachment. Biomaterials 2003, 24, 2331–2337. [Google Scholar] [CrossRef]
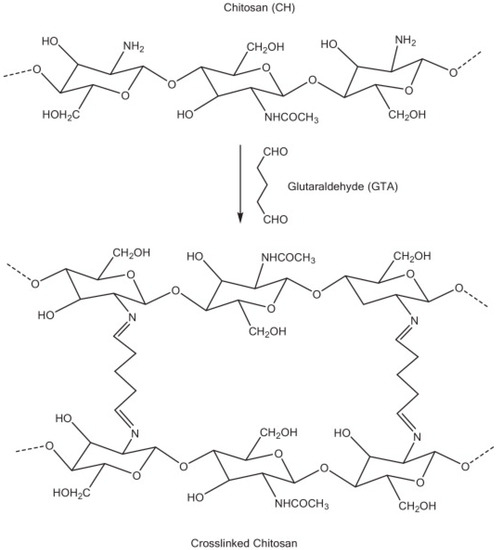
- Monteiro, O.A.; Airoldi, C. Some studies of crosslinking chitosan-glutaraldehyde interaction in a homogeneous system. Int. J. Biol. Macromol. 1999, 26, 119–128. [Google Scholar] [CrossRef]
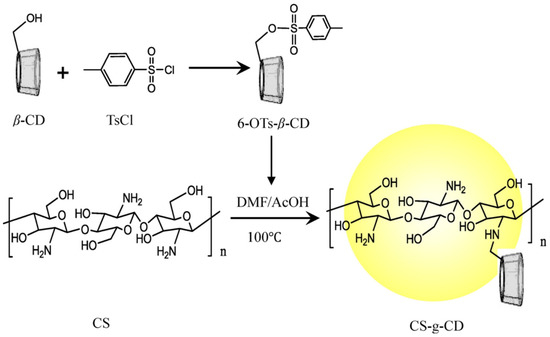
- Ding, W.-Y.; Zheng, S.-D.; Qin, Y.; Yu, F.; Bai, J.-W.; Cui, W.-Q.; Yu, T.; Chen, X.-R.; Bello-Onaghise, G.; Li, Y.-H. Chitosan Grafted With β-Cyclodextrin: Synthesis, Characterization, Antimicrobial Activity, and Role as Absorbefacient and Solubilizer. Front. Chem. 2019, 6, 657. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Biswas, S.; Ghosh, T. Preparation and Evaluation of Silymarin β-cyclodextrin Molecular Inclusion Complexes. J. Young Pharm. 2011, 3, 205–210. [Google Scholar] [CrossRef]
- El-Tahlawy, K.; Gaffar, M.A.; El-Rafie, S. Novel method for preparation of β-cyclodextrin/grafted chitosan and it’s application. Carbohydr. Polym. 2006, 3, 385–392. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Hastarini, E.; Ayudiarti, D.L. Addition of Chitosan Oligomers to Improve Bread Texture. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012034. [Google Scholar] [CrossRef]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef]
- Poecheim, J.; Patrulea, V.; Reichert, C.; Borchard, G. Characterization of pDNA-TMC Nanoparticle Interaction and Stability. Curr. Drug Deliv. 2016, 13, 301–308. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Jeuniaux, C.; Gooday, G.W. (Eds.) International Conference on Chitin and Chitosan; Plenum Press: Senigallia, Italy, 1986; pp. 303–306. [Google Scholar] [CrossRef]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S.; Liu, J. Synthesis, characterization, and antibacterial activity of N,O-quaternary ammonium chitosan. Carbohydr. Res. 2011, 346, 2445–2450. [Google Scholar] [CrossRef]
- Seong, H.S.; Whang, H.S.; Ko, S.W. Synthesis of a Quaternary Ammonium Derivative of Chito-oligosaccharide as Antimicrobial Agent for Cellulosic Fibers. J. Appl. Polym. Sci. 2000, 76, 2009–2015. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Abu Elella, M.H.; Sabaa, M.W. Synthesis, characterization and applications of N-quaternized chitosan/poly(vinyl alcohol) hydrogels. Int. J. Biol. Macromol. 2015, 80, 149–161. [Google Scholar] [CrossRef]
- Doncel-Pérez, E.; Aranaz, I.; Bastida, A.; Revuelta, J.; Camacho, C.; Acosta, N.; Garrido, L.; Civera, C.; García-Junceda, E.; Heras, A.; et al. Synthesis, physicochemical characterization and biological evaluation of chitosan sulfate as heparan sulfate mimics. Carbohydr. Polym. 2018, 191, 225–233. [Google Scholar] [CrossRef]
- Revuelta, J.; Fraile, I.; Monterrey, D.T.; Peña, N.; Benito-Arenas, R.; Bastida, A.; Fernández-Mayoralas, A.; García-Junceda, E. Heparanized chitosans: Towards the third generation of chitinous biomaterials. Mater. Horiz. 2021, 8, 2596–2614. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, H.; Capitani, F.; Krichen, F.; Mantovani, V.; Amor, I.B.; Galeotti, F.; Maccari, F.; Nedjar, N.; Volpi, N.; et al. Studies on European eel skin sulfated glycosaminoglycans: Recovery, structural characterization and anticoagulant activity. Int. J. Biol. Macromol. 2018, 115, 891–899. [Google Scholar] [CrossRef]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Krichen, F.; Ghlissi, Z.; Amor, I.B.; Sayari, N.; Kallel, R.; Gargouri, J.; Sahnoun, Z.; Boudawara, T.; Bougatef, A. In vitro and in vivo anti-coagulant activity and toxicological studies of marine sulfated glycosaminoglycans. Exp. Toxicol. Pathol. 2017, 69, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Campelo, C.S.; Chevallier, P.; Vaz, J.M.; Vieira, R.S.; Mantovani, D. Sulfonated chitosan and dopamine based coatings for metallic implants in contact with blood. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 682–691. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, J.; Chen, R.; Cao, L.; Tang, W.; Lin, D.; Wang, J.; Liu, C. Enhancement of VEGF-Mediated Angiogenesis by 2-N,6-O-Sulfated Chitosan-Coated Hierarchical PLGA Scaffolds. ACS Appl. Mater. Interfaces 2015, 7, 9982–9990. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, Y.; Wang, H.; Yuan, L.; Tan, M.; Shi, X.; Lyu, Z.; Liu, Y.; Chen, H. 6-O-Sulfated Chitosan Promoting the Neural Differentiation of Mouse Embryonic Stem Cells. ACS Appl. Mater. Interfaces 2014, 6, 20043–20050. [Google Scholar] [CrossRef]
- Nishimura, S.-I.; Kai, H.; Shinada, K.; Yoshida, T.; Tokura, S.; Kurita, K.; Nakashima, H.; Yamamoto, N.; Uryu, T. Regioselective syntheses of sulfated polysaccharides: Specific anti-HIV-1 activity of novel chitin sulfates. Carbohydr. Res. 1998, 306, 427–433. [Google Scholar] [CrossRef]
- Vikhoreva, G.; Bannikova, G.; Stolbushkina, P.; Panov, A.; Drozd, N.; Makarov, V.; Varlamov, V.; Gal’Braikh, L. Preparation and anticoagulant activity of a low-molecular-weight sulfated chitosan. Carbohydr. Polym. 2005, 62, 327–332. [Google Scholar] [CrossRef]
- Huang, R.; Du, Y.; Zheng, L.; Liu, H.; Fan, L. A new approach to chemically modified chitosan sulfates and study of their influences on the inhibition of Escherichia coli and Staphylococcus aureus growth. React. Funct. Polym. 2004, 59, 41–51. [Google Scholar] [CrossRef]
- Zhang, K.; Helm, J.; Peschel, D.; Groth, T.; Fischer, S. NMR and FT Raman characterisation of regioselectively sulfated chitosan regarding the distribution of sulfate groups and the degree of substitution. Polymer 2010, 51, 4698–4705. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, J.; Wang, J.; Yao, W.; Liu, C.; Chen, J.; Cao, X. Enhanced bioactivity of bone morphogenetic protein-2 with low dose of 2-N, 6-O-sulfated chitosan in vitro and in vivo. Biomaterials 2009, 30, 1715–1724. [Google Scholar] [CrossRef]
- Wang, R.; Huang, J.; Wei, M.; Zeng, X. The synergy of 6-O-sulfation and N- or 3-O-sulfation of chitosan is required for efficient inhibition of P-selectin-mediated human melanoma A375 cell adhesion. Biosci. Biotechnol. Biochem. 2010, 74, 1697–1700. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, F.; Karagozlu, M.Z.; Pyun, S.-Y.; Kim, S.-K. Sulfation of chitosan oligomers enhances their anti-adipogenic effect in 3T3-L1 adipocytes. Carbohydr. Polym. 2011, 86, 666–671. [Google Scholar] [CrossRef]
- Bianculli, R.H.; Mase, J.D.; Schulz, M.D. Antiviral polymers: Past approaches and future possibilities. Macromolecules 2020, 53, 9158–9186. [Google Scholar] [CrossRef]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Ito, M.; Baba, M.; Sato, A.; Pauwels, R.; De Clercq, E.; Shigeta, S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antivir. Res. 1987, 7, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and sulfated chitosan derivatives for biomedical applications: A review. Carbohydr. Polym. 2018, 202, 382–396. [Google Scholar] [CrossRef]
- Sacco, P.; Cok, M.; Scognamiglio, F.; Pizzolitto, C.; Vecchies, F.; Marfoglia, A.; Marsich, E.; Donati, I. Glycosylated-Chitosan Derivatives: A Systematic Review. Molecules 2020, 25, 1534. [Google Scholar] [CrossRef]
- Yalpani, M.; Hall, L.D. Some chemical and analytical aspects of polysaccharide modifications. III. Formation of branched-chain, soluble chitosan derivatives. Macromolecules 1984, 17, 272–281. [Google Scholar] [CrossRef]
- Donati, I.; Stredanska, S.; Silvestrini, G.; Vetere, A.; Marcon, P.; Marsich, E.; Mozetic, P.; Gamini, A.; Paoletti, S.; Vittur, F. The aggregation of pig articular chondrocyte and synthesis of extracellular matrix by a lactose-modified chitosan. Biomaterials 2005, 26, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Ilina, A.V.; Varlamov, V.P. Galactosylated derivatives of low-molecular-weight chitosan: Obtaining and properties. Appl. Biochem. Microbiol. 2007, 43, 73–77. [Google Scholar] [CrossRef]
- Chung, T.W.; Yang, J.; Akaike, T.; Cho, K.Y.; Nah, J.W.; Kim, S.I.; Cho, C.S. Preparation of alginate/galactosylated chitosan scaffold for hepatocyte attachment. Biomaterials 2002, 23, 2827–2834. [Google Scholar] [CrossRef]
- Ruiz Matute, A.I.; Cardelle-Cobas, A.; García-Bermejo, A.B.; Montilla, A.; Olano, A.; Corzo, N. Synthesis, characterization and functional properties of galactosylated derivatives of chitosan through amide formation. Food Hydrocoll. 2013, 33, 245–255. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated Foods, Chemistry of Browning Reactions in Model Systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Gullón, B.; Montenegro, M.I.; Ruiz-Matute, A.I.; Cardelle-Cobas, A.; Corzo, N.; Pintado, M.E. Synthesis, optimization and structural characterization of a chitosan–glucose derivative obtained by the Maillard reaction. Carbohydr. Polym. 2016, 137, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, P.; Gao, G.F.; Cheng, S. Carbohydrate-functionalized chitosan fiber for influenza virus capture. Biomacromolecules 2011, 12, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Concilio, S.; Cardea, S.; De Marco, I.; Reverchon, E. Complete glutaraldehyde elimination during chitosan hydrogel drying by SC-CO2 processing. J. Supercrit. Fluids 2015, 103, 70–76. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, X.; Wang, Y.; Zhang, X.; Tong, Z. Comparison of chitosan/starch composite film properties before and after cross-linking. Int. J. Biol. Macromol. 2013, 52, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. New way to crosslink chitosan in aqueous solution. Eur. Polym. J. 2010, 46, 1537–1544. [Google Scholar] [CrossRef]
- Jain, H.; Jain, V.; Jain, S.K.; Khangar, P.K. Development and evaluation of acyclovir loaded chitosan microspheres and cross linked with glutaraldehyde. J. Drug Deliv. Ther. 2021, 11, 110–114. [Google Scholar] [CrossRef]
- Machín, R.; Isasi, J.R.; Vélaz, I. β-Cyclodextrin hydrogels as potential drug delivery systems. Carbohydr. Polym. 2012, 87, 2024–2030. [Google Scholar] [CrossRef]
- Jiang, X.; Qi, Y.; Wang, S.; Tian, X. New amphoteric flocculant containing beta-cyclodextrin, synthesis, characterization and decolorization properties. J. Hazard. Mater. 2010, 173, 298–304. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrin-based hydrogels toward improved wound dressings. Crit. Rev. Biotechnol. 2014, 34, 328–337. [Google Scholar] [CrossRef]
- Yuan, Z.; Ye, Y.; Gao, F.; Yuan, H.; Lan, M.; Lou, K.; Wang, W. Chitosan-graft-β-cyclodextrin nanoparticles as a carrier for controlled drug release. Int. J. Pharm. 2013, 446, 191–198. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Alqahtani, M.S.; Mahmood, A.; Barkat, K.; Khan, M.T.; Tulain, U.R.; Rashid, A. β-cyclodextrin chitosan-based hydrogels with tunable pH-responsive properties for controlled release of acyclovir: Design, characterization, safety, and pharmacokinetic evaluation. Drug Deliv. 2021, 28, 1093–1108. [Google Scholar] [CrossRef]
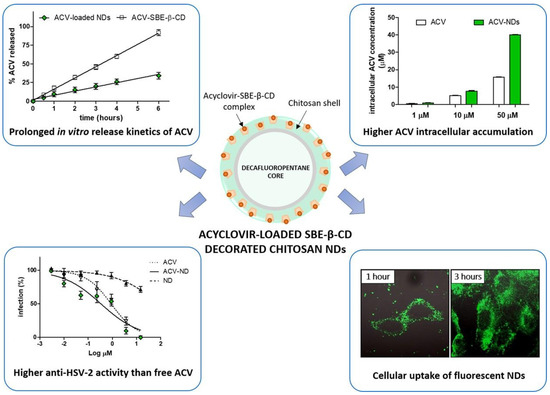
- Donalisio, M.; Argenziano, M.; Rittà, M.; Bastiancich, C.; Civra, A.; Lembo, D.; Cavalli, R. Acyclovir-loaded sulfobutyl ether-β-cyclodextrin decorated chitosan nanodroplets for the local treatment of HSV-2 infections. Int. J. Pharm. 2020, 587, 119676. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. Oxf. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Bonin, M.; Sreekumar, S.; Cord-Landwehr, S.; Moerschbacher, B.M. Preparation of Defined Chitosan Oligosaccharides Using Chitin Deacetylases. Int. J. Mol. Sci. 2020, 21, 7835. [Google Scholar] [CrossRef]
- Benchamas, G.; Huang, G.; Huang, S.; Huang, H. Preparation and biological activities of chitosan oligosaccharides. Trends Food Sci. Technol. 2021, 107, 38–44. [Google Scholar] [CrossRef]
- Liang, Z.; Gong, T.; Sun, X.; Tang, J.Z.; Zhang, Z. Chitosan oligomers as drug carriers for renal delivery of zidovudine. Carbohydr. Polym. 2012, 87, 2284–2290. [Google Scholar] [CrossRef]
- Klausner, E.A.; Zhang, Z.; Chapman, R.L.; Multack, R.F.; Volin, M.V. Ultrapure chitosan oligomers as carriers for corneal gene transfer. Biomaterials 2010, 31, 1814–1820. [Google Scholar] [CrossRef]
- Charelli, L.E.; de Mattos, G.C.; de Jesus Sousa-Batista, A.; Pinto, J.C.; Balbino, T.A. Polymeric nanoparticles as therapeutic agents against coronavirus disease. J. Nanoparticle Res. Interdiscip Forum Nanoscale Sci. Technol. 2022, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Chatterjee, A.; Roy, D.; Ghorai, S.; Pan, D.; Pramanik, S.K.; Chakraborty, N.; Ganguly, J. Chitosan/benzyloxy-benzaldehyde modified ZnO nano template having optimized and distinct antiviral potency to human cytomegalovirus. Carbohydr. Polym. 2022, 278, 118965. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ensinas, A.; Verrier, B.; Primard, C.; Cuvillier, A.; Champier, G.; Paul, S.; Delair, T. Zinc-Stabilized Chitosan-Chondroitin Sulfate Nanocomplexes for HIV-1 Infection Inhibition Application. Mol. Pharm. 2016, 13, 3279–3291. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Sofy, A.R.; Hmed, A.A.; Abd El Haliem, N.F.; Zein, M.A.-E.; Elshaarawy, R.F.M. Polyphosphonium-oligochitosans decorated with nanosilver as new prospective inhibitors for common human enteric viruses. Carbohydr. Polym. 2019, 226, 115261. [Google Scholar] [CrossRef]
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Dabrowska, A.; Botwina, P.; Obloza, M.; Liu, K.; Liu, D.; Guo, X.; et al. HTCC as a Polymeric Inhibitor of SARS-CoV-2 and MERS-CoV. J. Virol. 2021, 95, e01622-20. [Google Scholar] [CrossRef]
- Pyrc, K.; Milewska, A.; Duran, E.B.; Botwina, P.; Lopes, R.; Arenas-Pinto, A.; Badr, M.; Mellor, R.; Kalber, T.; Fernandez-Reyes, D.; et al. SARS-CoV-2 inhibition in human airway epithelial cells using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray. BioRxiv 2020. [Google Scholar] [CrossRef]
- Iacob, A.-T.; Drăgan, M.; Ghețu, N.; Pieptu, D.; Vasile, C.; Buron, F.; Routier, S.; Giusca, S.E.; Caruntu, I.-D.; Profire, L. Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives. Polymers 2018, 10, 607. [Google Scholar] [CrossRef]
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Du, Y.-Z.; Yuan, H.; Zhang, X.-G.; Miao, J.; Cui, F.-D.; Hu, F.-Q. Synthesis of lamivudine stearate and antiviral activity of stearic acid-g-chitosan oligosaccharide polymeric micelles delivery system. Eur. J. Pharm. Sci. 2010, 41, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Wang, Y.-Y.; Qiu, W.-Y.; Wu, J.-Y. Construction and characterization of nanosized curdlan sulfate/chitosan polyelectrolyte complex toward drug release of zidovudine. Carbohydr. Polym. 2017, 174, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kohzadi, S.; Najmoddin, N.; Baharifar, H.; Shabani, M. Functionalized SPION immobilized on graphene-oxide: Anticancer and antiviral study. Diam. Relat. Mater. 2022, 127, 109149. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, S.A.; Abdel-SAlam, A.I.; Moatasim, Y.; Gomaa, M.R.; Gomaa, M.R.; Abdel Fattah, N.F.; Emam, M.H.; Ali, F.; ElShehaby, H.A.; Ragab, E.A.; et al. Antiviral activity of chitosan nanoparticles encapsulating silymarin (Sil–CNPs) against SARS-CoV-2 (in silico and in vitro study). RSC Adv. 2022, 12, 15775–15786. [Google Scholar] [CrossRef]
- Szymańska, E.; Wojasiński, M.; Dąbrowska, J.; Krzyżowska, M.; Nowicka, M.; Ciach, T.; Winnicka, K. Chitosan-poly(ethylene oxide) nanofibrous mat as a vaginal platform for tenofovir disoproxyl fumarate—The effect of vaginal pH on drug carrier performance. Int. J. Biol. Macromol. Part A 2022, 222, 856–867. [Google Scholar] [CrossRef]
- Shao, C.; Yu, Z.; Luo, T.; Zhou, B.; Song, Q.; Li, Z.; Yu, X.; Jiang, S.; Zhou, Y.; Dong, W.; et al. Chitosan-Coated Selenium Nanoparticles Attenuate PRRSV Replication and ROS/JNK-Mediated Apoptosis in vitro. Int. J. Nanomed. 2022, 17, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Rehman, N.; Park, T.J.; Basit, M.A. Antiviral role of nanomaterials: A material scientist’s perspective. RSC Adv. 2023, 13, 47–79. [Google Scholar] [CrossRef]
- Rahman, S.S.; Ahmed, A.B. Preparation, characterization and statistical optimization of Nevirapine loaded chitosan nanoparticle for vaginal delivery. J. Pharm. Sci. 2019, 11, 348–358. [Google Scholar]
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.K.; Janowska, I.; El-Kemary, M. Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Comparative Nanofabrication of PLGA-Chitosan-PEG Systems Employing Microfluidics and Emulsification Solvent Evaporation Techniques. Polymers 2020, 12, 1882. [Google Scholar] [CrossRef] [PubMed]
- Krishna Sailaja, A.; Amareshwar, P. Preparation of bovine serum albumin loaded chitosan nanoparticles using reverse micelle method. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 837–846. [Google Scholar]
- Kumari, G.D.; Raksha, G.; Deepak, K.; Anjana, G.; Mary, C.S. A Review on Chitosan Nanoparticle as a Drug delivery system. Asian J. Pharm. Res. 2020, 10, 299–306. [Google Scholar] [CrossRef]
- Gérard, L.; Salmon-Céron, D. Pharmacology and clinical use of foscarnet. Int. J. Antimicrob. Agents 1995, 5, 209–217. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef]
- Li, X.; Chan, W.K. Transport, metabolism and elimination mechanisms of anti-HIV agents. Adv. Drug Deliv. Rev. 1999, 39, 81–103. [Google Scholar] [CrossRef]
- Kim, A.E.; Dintaman, J.M.; Waddell, D.S.; Silverman, J.A. Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J. Pharmacol. Exp. Ther. 1998, 286, 1439–1445. [Google Scholar] [PubMed]
- Kim, R.B.; Fromm, M.F.; Wandel, C.; Leake, B.; Wood, A.J.; Roden, D.M.; Wilkinson, G.R. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J. Clin. Investig. 1998, 101, 289–294. [Google Scholar] [CrossRef]
- Ramana, L.N.; Sharma, S.; Sethuraman, S.; Ranga, U.; Krishnan, U.M. Evaluation of chitosan nanoformulations as potent anti-HIV therapeutic systems. Biochim. Biophys. Acta 2014, 1840, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, V.H.B. In-vivo pharmacokinetic studies of Dolutegravir loaded spray dried Chitosan nanoparticles as milk admixture for paediatrics infected with HIV. Sci. Rep. 2022, 12, 13907. [Google Scholar] [CrossRef]
- Global Health Sector Strategy on Sexually Transmitted Infections. Available online: https://www.who.int/publications-detail-redirect/WHO-RHR-16.09 (accessed on 29 January 2023).
- Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Carine, T.; Wee Toong, T.; Yi, N.J.; Win Yi, L. Promising Drug Delivery Approaches to Treat Microbial Infections in the Vagina: A Recent Update. Polymers 2020, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Krishnan, S.; Hii, Y.S.; Pan, S.; Chan, Y.S.; Acquah, C.; Danquah, M.K.; Rodrigues, J. Synthesis approach-dependent antiviral properties of silver nanoparticles and nanocomposites. J. Nanostruct. Chem. 2022, 12, 809–831. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Marsich, E.; Donati, I.; Paoletti, S. Silver Nanocomposites and Their Biomedical Applications. In Nanotechnologies for the Life Sciences; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef]
- Ege, D.; Kamali, A.R.; Boccaccini, A.R. Graphene Oxide/Polymer-Based Biomaterials. Adv. Eng. Mater. 2017, 19, 1700627. [Google Scholar] [CrossRef]
- Pichon, C.; Billiet, L.; Midoux, P. Chemical vectors for gene delivery: Uptake and intracellular trafficking. Curr. Opin. Biotechnol. 2010, 21, 640–645. [Google Scholar] [CrossRef]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Nuchuchua, O.; Saesoo, S.; Gonil, P.; Chaleawlert-Umpon, S.; Pimpha, N.; Sramala, I.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. A comparison of spacer on water-soluble cyclodextrin grafted chitosan inclusion complex as carrier of eugenol to mucosae. Carbohydr. Polym. 2013, 92, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Belgamwar, A.; Khan, S.; Yeole, P. Intranasal chitosan-g-HPβCD nanoparticles of efavirenz for the CNS targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Čalija, B.; Milić, J.; Cekić, N.; Krajišnik, D.; Daniels, R.; Savić, S. Chitosan oligosaccharide as prospective cross-linking agent for naproxen-loaded Ca-alginate microparticles with improved pH sensitivity. Drug Dev. Ind. Pharm. 2013, 39, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xu, Y.; Liang, W.; Leung, G.P.H.; Cheung, K.-H.; Zheng, C.; Chen, F.; Lam, J. DNA-loaded chitosan oligosaccharide nanoparticles with enhanced permeability across Calu-3 cells. J. Drug Target. 2013, 21, 474–486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).