Abstract
Objectives: Cytomegalovirus (CMV) infection is a significant health concern affecting numerous expectant mothers across the globe. CMV is the leading cause of health problems and developmental delays among infected infants. Notably, this study examines CMV infection in pregnancy, its management, prevention mechanisms, and treatment options. Methods: Specifically, information from the Cochrane Library, PUBMED, Wiley Online, Science Direct, and Taylor Francis databases were reviewed along with additional records identified through the register, the Google Scholar search engine. Based on the search, 21 articles were identified for systematic review. Results: A total of six randomized controlled trials (RCTs) were utilized for a meta-analytic review. As heterogeneity was substantial, the random effects model was used for meta-analysis. Utilizing the random-effects model, the restricted maximum likelihood (REML) approach, the estimate of effect size (d = −0.479, 95% CI = −0.977 to 0.019, p = 0.060) suggests the results are not statistically significant, so it cannot be inferred that the prevention methods used were effective, despite an inverse relationship between treatment and number of infected cases. The findings indicated that several techniques are used to prevent, diagnose, and manage CMV infection during pregnancy, including proper hygiene, ultrasound examination (US), magnetic resonance imaging (MRI), amniocentesis, viremia, hyperimmunoglobulin (HIG), and valacyclovir (VACV). Conclusions: The current review has significant implications for addressing CMV infection in pregnancy. Specifically, it provides valuable findings on contemporary management interventions to prevent and treat CMV infection among expectant mothers. Therefore, it allows relevant stakeholders to address these critical health concerns and understand the effectiveness of the proposed prevention and treatment options.
1. Introduction
Cytomegalovirus (CMV) is the most common congenital infection worldwide. Annually, approximately 0.5–2.5% of newborns are born with congenital cytomegalovirus infection (cCMV), making the disease a global healthcare problem [1,2]. cCMV infection is the most typical cause of non-genetic hearing loss and neurologic disabilities, affecting 8–21% of infected children [3]. Even if children are born without signs of infection, about 20% will develop neurologic sequelae such as cerebral palsy, intellectual disability, epileptic seizures, and congenital, inflammatory eye disease [4]. In addition, 25 percent of these asymptomatic children develop late-onset hearing loss by the age of 4. cCMV may also be a significant contributor to antenatal stillbirth [5]. Therefore, the consequences of congenital CMV infection place a very substantial burden on health systems worldwide.
The virus is transmitted through direct or indirect contact with human secretions, such as urine, saliva, vaginal secretions, semen, breast milk, and blood products, and transplanted organs. Virus secretion is the longest in primary infection and it is the leading cause of congenital infection. As an immuno-incompetent organism, the fetus is particularly vulnerable to the consequences of intrauterine infection. Maternal–fetal transmission after primary maternal CMV infection occurs at a rate about 30% during first trimester of pregnancy, increasing over 70% at the third trimester [6]. The risk of health consequences for the fetus is highest when the virus is transmitted to the mother during the preconceptional period. Likewise, health challenges can occur if transmission occurs within the first trimester of pregnancy, reaching up to 30–40%, and decreases significantly with each trimester. Contrarily, the possibility of virus transmission via the placenta behaves inversely and is lowest in the first weeks of pregnancy and highest in the 3rd trimester. Therefore, determining the optimal prevention method is vital to reducing the risk of fetal infection. There is a limited number of worldwide guidelines for CMV screening in pregnant mothers; therefore, congenital diseases remain undetected in many asymptomatic children at birth until symptoms appear later in life [7,8,9]. This feature complicates the establishment of a clear link between disease symptoms and the verification of a congenital CMV infection. There is a growing knowledge pool of the mechanisms of fetal damage, expectations of pregnant patients, especially in the case of proven intrauterine infection, and the data from studies on antiviral drugs and immunoglobulins. Such developments necessitate addressing the possibility of prophylactic measures and pharmacological treatment, which can reduce adverse consequences for the fetus. Data on the use of antiviral drugs in pregnancy are growing. In addition, physicians have attempted immunomodulatory treatment with immunoglobulins.
The evidence favoring pharmacological interventions for CMV infections is increasingly growing. For women with compromised immune systems or organ transplant recipients, the approved antiviral drugs for the infection are ganciclovir, valacyclovir, cidofovir, and foscarnet [4]. An example of their use is when physicians use valacyclovir and gancyclovir for the treatment and prevention of congenital CMV infection. Valacyclovir acts against CMV’s DNA polymerase when utilized at a high dose. Shahar-Nissan et al.’s study revealed that valacyclovir effectively minimizes the rate of CMV infection among infants following primary maternal infection during early pregnancy. They argued that the early treatment of pregnant women might help avoid pregnancy termination or the delivery of infected infants [10]. Despite the outcome of this study on the efficacy of valacyclovir, there are no official CMV treatments during pregnancy. Likewise, effective measures to prevent maternal CMV infections and transmission to children are lacking. The existing guidelines recommend that physicians provide antenatal therapy as a research protocol treatment [7,8,9]. The vaccine to prevent this infection is unavailable, and treatment alternatives in pregnancy are limited. Pregnant women whose previous offspring attend day nurseries or kindergartens, nursery or kindergarten teachers, and medical staff in pediatric hospitals are at risk of contracting primary infections during pregnancy. Advances in CMV infection prevention and treatment are a priority globally. Literature data still indicate a low level of awareness of the risks associated with cCMV infection and the primary prevention options before and during pregnancy among both patients and healthcare providers [11,12,13,14,15]. Educational interventions and proper hygiene are effective measures to avoid CMV infection in pregnant women.
Caring for preschoolers while pregnant is a high risk; therefore, preventing maternal infection of CMV is necessary [1]. As with the treatment, there is no approved vaccine to prevent CMV infection among pregnant women. Health professionals recommend hygiene measures to avoid exchanging body fluids and prevent maternal contamination [1]. Prioritizing proper hygiene can help in preventing infection in pregnancy; education intervention is the most significant alternative strategy in minimizing the risk of the disease [4,16]. Enlightening expectant women on CMV infection and proper hygiene is a practical approach to preventing such infection among them.
The current review and meta-analysis highlight cytomegalovirus infection in pregnancy, covering its management and prevention options. The study prioritized articles discussing CMV infection among pregnant women. Given that CMV infection in pregnancy is an alarming problem, it is worth exploring ways to manage, prevent, and control it.
2. Methods
2.1. Eligibility Criteria
The preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) underpin the current review [17]. The protocol of this systematic review is registered in OSF Registries: https://doi.org/10.17605/OSF.IO/QFDRX (accessed on 14 August 2023). Studies associated with therapeutic interventions to manage, prevent, and treat congenital CMV infection in the population under investigation were included in the review. The focus in the selection of studies was on those investigating pregnant women and fetuses infected with CMV. Studies on general populations such as adult men and non-pregnant women were excluded from this review. Additionally, studies performed on patients with immunosuppression factors were omitted. This review prioritizes RCTs and cohort studies which were subsequently meta-analyzed. Review articles and case reports were excluded from the study. Finally, the study duration or publication date was limited to the years 2002–2022. The studies selected for the analysis were restricted to English, therefore, studies published in languages other than English were not considered in this review.
2.2. Information Sources
The primary information sources were from the following online literature databases: PUBMED, Wiley Online, Science Direct, Cochrane Library, and Taylor Francis Online, as well as sources of grey literature. Specifically, the Google scholar search engine was also used to access relevant and valuable information.
2.3. Search Strategy
The article search was conducted electronically on the following databases: PUBMED, Wiley Online, Science Direct, Cochrane Library, Taylor Francis Online. Applicable terms and keywords related to cytomegalovirus infection and pregnancy were used in this study. As for the grey literature, non-published studies, such as theses and dissertations, were analyzed to retrieve valuable information. The primary keywords included cytomegalovirus, CMV, pregnancy, and congenital.
2.4. Selection Process
The relevant studies were selected based on the inclusion and exclusion criteria. Articles written in English, full-text information, and relevant content based on their titles and abstracts were reviewed. The screening process was stepwise and followed an elaborate scanning procedure. The selected studies were screened, focusing on their abstracts and titles to determine their relevance or eligibility based on the inclusion criteria. Next, the full text of the selected studies was examined.
2.5. Data Collection Process
The review followed PRISMA guidelines [18].
2.6. Data Items
In the current review, the data items included pregnant women, therapeutic interventions to manage or prevent CMV infections, and treatment options for infection in pregnancy.
2.7. Study Risk of Bias Assessment
Assessing the risk of bias using the GRADE table will ensure that the selected studies are the most suitable for addressing the issue under review. Specifically, several domains of bias will be analyzed, including confounding factors, selection of participants in the study, intervention classification, missing information, and deviations from planned interventions. These domains will be classified based on the risk of biased judgment. Thus, the studies included in the review will be ranked following the overall bias risk.
2.8. Synthesis Methods
The selected studies for inclusion in this review were synthesized based on the type of intervention used. Specifically, various interventions were reported based on specificity, sensitivity analysis, and evaluation of the results measured concerning CMV infection. The study population was based on the diagnosis of CMV, including infection during pregnancy, abnormalities identified during an ultrasound scan, and infection at birth. During the synthesis of the findings, the primary outcomes involved the frequency of interventions for infection management during pregnancy. At the same time, the secondary outcomes were infants with or without cCMV, and with or without symptoms. The method of meta-analysis utilized was random-effects analysis using the Restricted Maximum Likelihood (REML) method [19]. As the variation was deemed important to be included in the uncertainty estimation of the regression coefficient estimates, fixed-effects models were ruled out as excess residual variance do not affect the computation of uncertainty estimates in fixed-effects models. The random-effects model generalizes the fixed-effect model, integrating components of variation between studies within the effect size, uncertain parameters, and estimates increasing residual variance [19].
3. Results
3.1. Study Selection
An electronic database and grey literature search generated 4074 articles after eliminating duplicates. Title or abstract screening resulted in the exclusion of 3997 journals that did not contain relevant information. In addition, the remaining 37 articles were screened on full-text review, leaving 21 studies that met the set inclusion criteria for the systematic review and 6 that met the criteria for the meta-analytic review. The excluded studies failed to meet the eligibility criteria. Researchers exclude studies for several reasons, including unsuitable study population or intervention. In this regard, 6 studies were included in the current systematic review and meta-analysis to explore CMV infection in pregnancy in terms of its management, prevention, and treatment options. The following PRISMA flowchart describes the study selection process for this review (Figure 1).
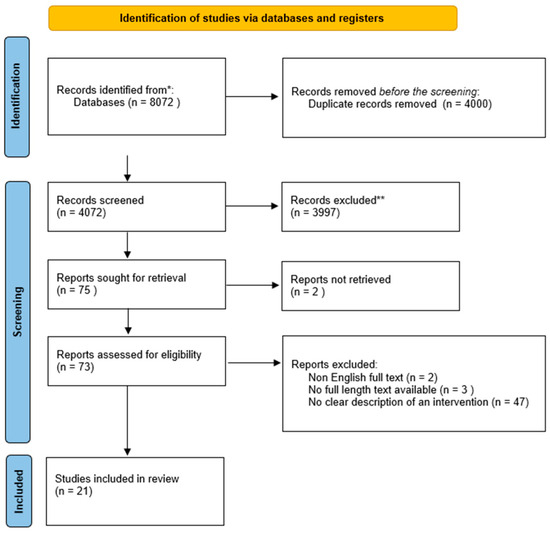
Figure 1.
Systematic review flow chart. Note: * PUBMED, Wiley Online, Science Direct, Cochrane Library, Taylor Francis Online, and Google Scholar; ** out of the theme.
3.2. Study Characteristics
The studies had specific characteristics, including the author, year of publication, study design, and management interventions before and after prenatal diagnosis (Table 1).

Table 1.
Study characteristics.
3.3. Risk of Bias in Studies
As these were randomized studies, the risk of bias was considerably low for the meta-analysis. For the systematic review, the cohort studies carried a high risk for selection biases, performance bias, and detection bias (Table 2).

Table 2.
GRADE–the risk of bias.
3.4. Results Synthesis
The key themes isolated based on an analysis of the RCT and cohort studies were prevention (primary and secondary), intervention, or management. The studies, overall, yielded important results. The synthesis of the literature review examined prevention utilizing hygiene as a form of primary prevention. Secondary prevention involved administration of hyper-immunoglobulin or valacyclovir prior to the confirmation of infection and before performing the amniocentesis. While secondary prevention carries a high risk of infection, it should be necessary to wait at least six weeks after presumed maternal infection to confirm or exclude maternal–fetal CMV transmission. Management and intervention, on the other hand, involves treatment with HIG and/or VACV, monitoring with ultrasound (US), and magnetic resonance imaging (MRI), and cordocentesis with platelet evaluation for inhibited hematopoiesis and impaired bone marrow.
3.5. Prevention
3.5.1. Primary Prevention
Prevention of primary nature involves imparting hygiene information to mothers to prevent CMV in mothers and infants. A cohort study by Revello et al. involved imparting hygiene information to mothers in an intervention group (n = 331) as opposed to a control group (n = 315) who had no such intervention. Hygiene information was shared among pregnant women vulnerable to CMV infection due to occupational or personal reasons. The study involved testing CMV-seronegative mothers at the time of screening for maternal serum for fetus aneuploidy at 11 or 12 weeks of gestational age and giving hygiene recommendations before prosectively testing for CMV until delivery. While self-report measures were used to assess the acceptance of the hygiene recommendations as a secondary outcome, CMV seroconversion was the primary outcome. Researchers in the study found that in the intervention group (n = 381) who received hygiene counseling and CMV data, 13 women underwent miscarriage at 12–18 gestational weeks, and four of these women were not tested further. Another nine women remained CMV seronegative after a year following study completion. Around 37 women were not followed up, while 2 women seroconverted at 12–18 gestational weeks and 2 between 18 weeks and delivery. Only 4 (n = 331) women seroconverted after the completion of the study (1.2%, 95% CI 0.3–3.1). CMV-specific low avidity IgM and IgG in 5 women suggested detection of primary CMV during the first trimester after testing at 11–12 gestational weeks. In contrast, the comparison group involved 315 CMV seronegative women, wherein 24 mothers had undergone seroconversion (7.6%, 95% CI 4.9–11.1). Retrospectively, 4 additional women were diagnosed with primary CMV infection acquired in the first trimester of gestation due to CMV-specific IgM and IgG of low avidity. Therefore, the seroconversion rates were significantly lower for the intervention group than the control group (Δ = 6.4%, 95% CI 3.2–9.6, p < 0.001). Vulnerable pregnant women receiving CMV information showed lower risk,1.2:7.6, for acquiring CMV infection than uniformed mothers (crude odds ratio = 0.15, 95% CI 0.05–0.43). Regarding neonates infected in the intervention compared to the comparison group, of 28 neonates, 11 of them were infected in all, with 3 in the intervention group as opposed to 8 in the control group. Regarding the secondary outcome, hygiene information acquisition, 80 percent of the respondents followed recommendations either always (14%) or often (66%). A total of 93% of the respondents agreed that the CMV hygiene counseling and information was worth suggesting to mothers vulnerable to CMV infection. Therefore, the study effectively established that a primary prevention strategy could be effective in identifying and providing required CMV information and hygiene counseling to lower maternal CMV rates and congenital CMV infections [3].
3.5.2. Secondary Prevention
Cohort studies and RCTs have established the efficacy of HIG, and CMV vaccinations, for preventing congenital CMV. Pass et al., in a phase 2 RCT study, evaluated the role of vaccine prevention strategies where three doses of recombinant CMV envelope glycoprotein E with MF59 adjuvant were compared with a placebo at three different times, namely 0, 1, and 6 months. In this RCT study, mothers were randomly assigned to either the treatment (n = 234) or control condition (n = 230) and after one year of follow-up, 49 confirmed infections were reported, with 18 in the vaccine group as opposed to 31 in the placebo group [22]. Within a 42-month period the vaccine group was more likely to be infection-free compared to the placebo group (p < 0.001) using Kaplan-Meier analysis. Pass et al. found infection rate estimates per 100 person years had a vaccine effectiveness rate of 50% (95% CI 7–73%). Additionally, one CMV case was noted among the vaccine group, as opposed to three infections in the placebo group. The study provided support for the use of a glycoprotein E for neutralizing antibodies, and such antibodies could play a powerful role in enhancing the protective effect of the vaccine [22].
Kagan et al. carried out a cohort study, where pregnant mothers (n = 40) were studied, with median gestational age at first presentation being an average of 9.6 weeks and ranging from 5.1 to 14.3 weeks. In the HIG treatment group, HIG doses were administered between one and six times for each patient. CMV-IgG showed periodic fluctuations on a bi-weekly period. In contrast, IgG avidity, after an initial increase after the first administration of HIG, remained stable throughout the treatment period. Maternal to fetal transmission before amniocentesis was noted in a single case, or 2.5%. Two additional subjects experienced late gestation transmission. All three cases with mother–fetus transmission brought transmission rates to 7.5% (95% CI 1.6–20.4%) in over 40 cases. Infected neonates showed no symptoms at birth. Matched historically, the control group covered 180 pregnant mothers. In this group, 38 transmissions (95% CI, 26.2–45.0%) took place, which was significantly higher (35.2%) than the HIG treatment group [31].
In another cohort study, Kagan et al. examined the effect of HIG on CMV prevention and detection. The study involved pregnant mothers (n = 149) and fetuses (n = 153), with median maternal age pegged at 32 years and weight at 65 kg. The median gestational age at referral was 9.4 weeks. Median anti-CMV IgG levels were 5.7 U/mL, while the anti-CMV IgM index was 2.5%, while 22.3% was the CMV IgG avidity. Amniocentesis was done at a median gestational age of 20.4 weeks. In 143 cases, or 93.5%, the fetuses were not infected, while 10 cases were marked by CMV maternal–fetal infection [38].
Nigro and Adler conducted a cohort study that also established the use of HIG as a CMV prevention therapy. Mothers (n = 304) and their infants (n = 281) participated in this study, where a follow-up was performed for 173 uninfected and 106 infected cases over four years. A total of 157 women were provided two doses of 200IU per kg of HIG per infusion. The researchers found an increase of 1.8 times the rate of congenital infection aOR = 5.2, p < 0.0001) and 1.8 times increase in maternal viral DNAemia prior to HIG administration (aOR = 3.0, p = 0.002). Abnormal ultrasounds were noted (aOR = 59, p = 0.0002). A diagnosis of material infection by seroconversion by avidity was present (aOR = 3.3, p = 0.007). The absence of HIG and abnormal ultrasounds were predictive of symptoms (p = 0.001). Long-term sequelae were predicted in those not receiving HIG (aOR = 13.2, p = 0.001). Abnormal ultrasounds were noted (OR = 7.6, p < 0.003) along with early maternal gestation (OR = 0.9, p = 0.017) [33].
In Devlieger et al.’s study, 4800 individuals were randomly assigned to the treatment arm, wherein 52 were seroconverted (median GA: 24 [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] weeks) and 45 were followed up. In this study, 4735 randomly chosen patients constituted the control group, from which 42 were seroconverted, 34 followed up (providing data for 28 infants), and 8 chose Cytotect off-label. In the control group cCMV rates were 13 out of 28 neonates (46.4% [CI 27.51–66.13]), as opposed to 16 out of 45 newborns (35.6% [CI 21.87–51.22]) (p = 0.46) in the treatment group [34].
Faure-Bardon et al., in their study, found that of 310 identified CMV-maternal primary infection (MPI) cases, 269 underwent amniocentesis, of which 65 accepted treatments using VACV. This longitudinal case–control cohort study serologically screened pregnant mothers between 2009 and 2020. For untreated cases, 65 mothers were selected as controls matched for periconceptional infections and gestational age at amniocentesis. Additionally, the researchers initiated VACV at 12.71 median gestational age and median duration of treatment being 35 days. Multivariate logistic regression showed that fetal infection in the treated group was lower (OR 0.318, 95% CI 0.120–0.841). Therefore, the acceptability, benefit, and tolerance of the VACV as a secondary prevention strategy was established [36].
In another related study by Faure-Bardon et al., the focus was on PCR by CVS as a means of evaluating the feasibility of the viral genome in the case of maternal primary infection with CMV during early pregnancy. In 37 pregnancies where CVS was performed, CMV PCR was positive in only 3 cases and negative in the remaining ones. CMV-PCR following amniocentesis at a median gestational range of 17.6 weeks was positive for the three cases which were also positive post CVS. Out of 34 patients with negative findings following CVS, amniocentesis excluded infection of the fetus in 31 cases, and confirmed it in the remaining three [35].
3.5.3. Therapy of Fetal Infection
HIG has been widely used to manage CMV maternal fetal transmission post the contraction of CMV by the mothers. In a cohort study, Nigro et al. examined the effect of passive immunization on maternal–fetal CMV transmission. Of the 31 mothers in the study, one gave birth to an infant diagnosed with cCMV. HIG was linked to lower CMV incidences, with an adjusted odds ratio of 0.02, p < 0.001. Nigro et al. also utilized a control group of 14 mothers to assess the effect of the absence of HIG with 50% of the mothers (n = 7) not receiving HIG. Among the prevention group (n = 37 mothers) who received HIG, 6 of 37 (16%) had infants diagnosed with CMV than 19 of 47 mothers (40%) not receiving HIG. Specifically, HIG was associated with increased specific CMV-IgG concentrations, limited natural killer cells, lowered HLA DR + cells and raised avidity, signaling no adverse side effects [20]. Regarding the cohort studies, the key patterns of the results suggest that interventions, on average, were effective in preventing maternal–fetal transmission of CMV. Buxmann et al. administered prenatal CMV-HIG after the diagnosis of primary maternal CMV infection. A total of 115 doses were administered (n = 40 mothers and 6 fetuses). The treatment group comprised 4 mothers, while the multinomial group included 38 mothers and their 39 infants [23]. Intravenous CMV-HIG was administered in the treatment group under certain conditions. In the treatment group, three children emerged CMV positive, remaining asymptomatic at birth and at follow-ups, while one was symptomatic. In the multinomial group, 37 women and two infants received in utero CMV-HIG. In the multinomial group, 9 children in tested positive for cCMV, including TOP. In addition, 8 children in the multinomial group showed cCMV infection and were asymptomatic at birth and follow-up. No severe side effects occurred and HIG was tolerated well in those to whom it was administered. A smaller risk for intrauterine CMV maternal–fetus transmission was noted following the HIG administration. However, according to Buxmann et al., prenatal cCMV was not reversed after the HIG [23].
In contrast, a cohort study by Visentin et al. demonstrated ample proof of the positive impact of HIG in CMV prevention or for the associated adverse outcomes. Women with early primary CMV (n = 592) were part of the study, wherein 446 women were tested using amniocentesis and 92 fetuses were detected as having CMV, while 24 mothers underwent TOP, HIG was administered to 31 women. In contrast, a control group of 37 mothers received no treatment. Additionally, fetuses were matched on maternal age and the detection of pathology in the ultrasound and CMV load. Ultimately, 4 infants in the treatment condition (13%; 95% CI 1–25%) and 16 infants of untreated mothers (43%; 95% CI, 27–59%) presented with adverse outcomes (p < 0.001 by the 2-tailed Fisher exact test) [24].
Roxby et al. analyzed data for 141 infants (n = 148 mothers), compared results using ITT (intention to treat) analysis, and found differences between trial groups regarding susceptibility to CMV. Infant and mother characteristics at baseline were comparable. CMV infection rates between trial groups, however, did not differ significantly with 47 out of 71 newborns (66 percent) receiving valacyclovir (500 mg/twice a day) being infected and 46 as compared to 70 infants (66%) receiving a placebo diagnosed with CMV. The researchers did not note differences in CMV median time. In over 92% of the breast milk samples, CMV infection was discovered. The study, therefore, reported negative findings [25].
Revello et al.’s research revealed in the HIG (intervention) group, the rate of infant infection was 30% (18 fetuses, n = 61 mothers), but in the placebo group, it was 44% (27 fetuses, n = 62 mothers), highlighting the difference of around 14 percentage points (95% CI -3 to 31; p > 0.05) [26].
Nigro et al., in a cohort study of 351 mothers and 358 infants at a mean gestational birth age of 38.2 weeks, found multiple HIG doses of anywhere from one to eight were linked to increasing birth weight (p = 0.0006) and delivery-linked gestational age (p = 0.014). Infants without symptoms and those linked to multiple HIG maternal doses were associated with prevention of such fetal infections as CMV [27].
Delle Chiaie et al. retrospectively evaluated data from 50 females including three twin pregnancies. The mothers received 2 or more HIG infusions in the dose of 200 U/kg. The gestational median age at the time of CMV diagnosis in mothers was 13 weeks. Mothers received 142 doses of HIG, which were tolerated well. The pre-term birth rate was 4.2% in single pregnancies. CMV-linked sequelae in infants with cCMV was 10.5%. HIG application was favorably associated with a milder cCMV infection clinical course [28]. In contrast to the earlier finding by Buxmann et al., a milder course of the disease was noted following the administration of the HIG.
In contrast, in another cohort study, Minsart et al. administered CMV-specific HIG to16 women for suspected CMV during pregnancy while 55 mothers did not have any specific treatment and used bivariate analysis to show that HIG treatment did not considerably lower CMV or post-natal infections in the considerable treatment and prophylactic groups [29]. In contrast, a cohort study by Blazquez-Gamero et al. examined mother–infant pairs with a median gestation birth age of 39 weeks (interquartile range: 38–40) and two premature cases. Of 30 cases using amniocentesis, 21 fetuses showed a CMV PCR positive status (70%), one fetus with positive PCR results was injected an HIG dose and presented with CMV-PCR negative status showing no symptoms at 12 months. A total of 24 infants were infected at birth and 16 showed no CMV sequelae at 12 months. A clinical assessment was performed during the neonatal period and 12 months later in 16 and 15 infants, respectively. A total of 50% were symptomatic at birth and 4 in 15 showed losses of hearing at 12 months, while 3 were impaired neurologically. High sequelae of odds risk were noted for fetus before HIG administration, as suggested by ultrasonography. In contrast, in the prevention group, (n = 17), 7 fetuses, or 41%, were affected by CMV, while of 19 fetuses, 18 were diagnosed with cCMV and 8 showed CMV abnormalities in fetal ultrasonography before the HIG was administered [30]. In another cohort study, Seidel et al. also evaluated the role of HIG as a CMV management therapy. In a cohort of 46 mothers, 11 intrauterine infections were confirmed, which can be translated into transmission rate of 23.9%. In the control group, the transmission rate was 39.9%, marking a significant difference (p = 0.026). Hughes et al. demonstrated that the primary event outcome occurrence was noted in 46 fetuses or neonates (n = 203 mothers) (22.7%) in the hyperimmune globulin (intervention) group and in 37 fetuses (n = 191 mothers) (19.4%) in the placebo group (RR = 1.17; 95% CI 0.80–1.72; p > 0.05) [37]. A total of 10.3% and 5.4% infants were delivered with birthweight lower than 5th percentile in the intervention and control group, respectively (RR = 1.92; 95% CI 0.92–3.99) [32].
Another cohort study evaluated the effect of valacyclovir (8 g/day) on maternal–fetal transmission of CMV. Jacquemard et al. found that viral loads in fetal blood lowered following valaciclovir treatment within a span of one to 12 weeks (p = 0.02). A total of 20 pregnancies and 21 fetuses were treated at week 28, on average, spanning a range of 22–34 weeks for the duration, on average, of seven weeks (range 1–12 weeks). While 10 infants were developed normally between one and five years of age, six out of seven cases involved medical termination of pregnancy (TOP) due to cerebral lesions, while one fetus did not survive. In the control group not receiving valacyclovir treatment (n = 24), 14 or 58.3% had adverse outcomes such as TOP, CMV infection or fetal demise while the remaining 10 were healthy during the follow-up [21]. Shahar-Nissan et al., in their randomized double-blind placebo-controlled study, found that valacyclovir (2 × 4 g/day) therapy reduced fetal CMV infection rates after maternal primary infection early in pregnancy or periconceptual time. For the final analysis, 45 patients in the valacyclovir group and 45 in the placebo group were taken. The results indicated a 71% reduction in infection (11% in valacyclovir group vs. 30% in placebo; p = 0.027; OR 0.29, 95% CI 0.09–0.9) A majority of the RCTs showed the effectiveness of CMV prevention and treatment therapies such as valacyclovir and HIG, as well as passive vaccination [10].
3.6. Meta-Analysis
Utilizing the random effects model REML approach, the estimate of effect size (d = −0.479, 95% CI = −0.977–0.019, p = 0.060) suggests the results are not statistically significant, as shown in Table 3. Meta-analysis results suggested that overall conclusions based on the review of RCTs were not statistically significant. Two RCTs were outside the 95% confidence interval, suggesting that the results were not reliable for these studies, while the remaining 4 studies showed conflicting evidence. In addition, two studies suggested that the treatment group reported better CMV prevention and detection, while studies to the right of the effect line suggested that the outcome of interest occurred more frequently in the treatment or the control group.

Table 3.
Random Effects Model REML approach.
3.7. Forest Plot
The study results show the effect estimates from individual studies, while diamonds demonstrate pooled results, as seen in Figure 2. Longer lines are associated with wider confidential intervals, pointing to low reliability of such study results, as noted in studies by Shahar-Nissan et al., Devlieger et al., and Revello et al. [10,26,34]. In contrast, the studies by Hughes et al., Pass et al. and Roxby et al. show higher reliability [22,25,37]. Studies to the left of the vertical line such as Pass et al. and Shahar-Nissan et al. showed that outcome of interest (CMV infection) occurred less frequently in the intervention than the control group, which Revello et al. also demonstrated to some extent [10,22,26]. In the study by Roxby et al., there were limited differences between the treatment and control group, demonstrating no particular effect of the CMV infection prevention intervention in the study [25]. On the other hand, Hughes et al. show the outcome of interest occurred more frequently in the treatment compared to the control group place on the right side of the vertical line [37]. The study by Devlieger et al. showed that the difference between the treatment and control group on the outcome of interest was not wide although CMV infections occurred less frequently in the intervention than the comparison or control group [34].
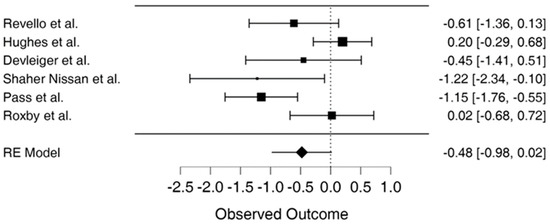
Figure 2.
Forest plot for meta-analysis [3,10,22,25,34,37].
3.8. Publication Bias
The funnel plot demonstrates limited publication bias as the funnel plot is not completely asymmetrical, with two studies located on either side of the effect line and one study falling outside the 95% confidence interval on each side of the effect line, as seen in Figure 3 showing the funnel plot.
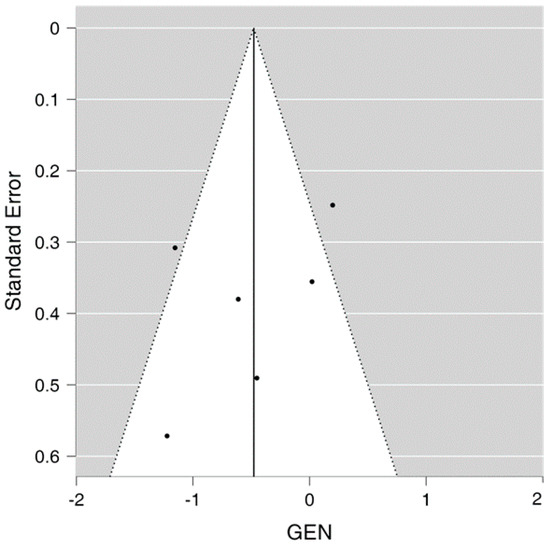
Figure 3.
Funnel plot for the meta-analysis.
3.9. Sensitivity Analysis
The sensitivity analysis indicates that using a random-effects model produces disparate results compared to the fixed-effects model. As there was an inverse association between treatment and the number of infected patients, the results showed an estimate of −0.381 (95% CI = −0.662–0.101, p = 0.008), indicating that the preventative strategies used were effective. The results of the meta-analysis should be interpreted with caution.
3.10. Heterogeneity
The 12 percentage or heterogeneity estimate suggests substantial heterogeneity at 64.83%. The studies are not homogenous, necessitating the use of the random effects model (Table 4).

Table 4.
Residual Heterogeneity Estimates.
4. Discussion
Primary CMV infection during pregnancies poses a significant risk of fetal transmission with the possibility of severe sequelae. Although there is no globally approved approach to prevent CMV infections, several mechanisms have been suggested and researched to determine their efficacy in preventing or treating these infections among expectant mothers. In other words, no suitable treatment option has been approved to address CMV infection in pregnancy. The current review and meta-analysis examined CMV infection in pregnancy, focusing on its prevention, management, and treatment options.
Early examination of the possibility of infection during prenatal diagnosis can help prevent cases of CMV in pregnancy. Amniocentesis is a method to withdraw amniotic fluid from the uterine cavity through a trans-abdominal approach using a needle [39]. Specifically, it is an invasive approach for the prenatal diagnosis of several pregnancy-related conditions [40]. The procedure can be done from at least 6–8 weeks post proven seroconversion or within 20–21 weeks of pregnancy otherwise. Amniocentesis allows for the confirmation or exclusion of the presence of the virus in the amniotic fluid and assess the viral load of CMV DNA in it [39]. Therefore, it is one of the practical approaches for detecting or excluding CMV infections during pregnancy.
HIG is also one of the approaches that have been suggested to prevent or minimize CMV infection vertical transmission. In other words, HIG is one of the interventions that can be utilized successfully in preventing maternal–fetal transmission. Furthermore, it has been proposed to treat primary CMV infection in pregnancy to minimize the risk of intrauterine viral transmission and morbidity associated with such a condition. Using HIG in pregnant women with primary CMV infection has proven safe and effective in preventing congenital disease using a high dosages. HIG use in pregnant women after CMV primary infection may not significantly reduce the infection rate; however, it is safe and can have favorable outcomes on the infected fetuses’ symptoms and sequelae. Accordingly, the risk of long-term sequelae in fetuses in the absence of US abnormalities before HIG is low, making it an appropriate option in handling infected fetuses. Detailed ultrasound evaluation of fetuses is recommended with special emphasis on looking for fetal infection-related changes in the placenta, fetal nervous system (detail fetal neurosonography assessment) and inflammation-related changes in other fetal organs. Likewise, valacyclovir is a potential congenital CMV preventive measure that helps to minimize vertical transmission. Specifically, it has been suggested as a practical approach in symptomatic CMV-infected fetuses to mitigate the risk of severe sequelae.
Additionally, based on the analysis of previous studies, the available treatment option for CMV infections during pregnancy include valacyclovir and HIG. US and MRI are valuable approaches to predicting the outcome of infected fetuses. They help in identifying lesions with poor prognoses. With the US technique, fetal abnormalities like intrauterine growth restrictions indicate infection [41]. The US scan method is non-invasive. In addition, it suggests symptomatic fetal infection, especially if the CMV infection has been confirmed by amniocentesis. Preventive treatment is effective in inhibiting viral transmission after maternal CMV infection. Specifically, this treatment is most effective, especially when started immediately after detecting presumed maternal contamination. Therefore, fetal transmission reduction is crucial among mothers infected during the early stages of their pregnancy.
Generally, the articles reviewed in the current study focused on the prevention and treatment options for CMV infection in pregnancy. The studies highlighted the various approaches and interventions to prevent or treat CMV infection among pregnant women. These techniques include HIG, serial monitoring, and valacyclovir. Despite the extensive research on the prevention and treatment of CMV infections among this population, there is no universally approved method to protect pregnant women from CMV infections. However, the meta-analysis also found these treatment and prevention strategies impacted the infection rate, in that there was an inverse relationship between treatment, prevention, and infection rates, as per the estimate of effect sizes, yet the results were not statistically significant.
Overall, the review focused on obtaining a global overview of the existing management, prevention, and treatment practices for CMV infections during pregnancy. However, the main limitation of this review is the heterogeneity in study design. Therefore, future reviews should focus on studies with homogeneous designs to yield the intended outcomes. The outcomes of this review will provide positive insights into the management of CMV infection; thus, significantly contributing to the existing body of knowledge.
5. Conclusions
The result of the systematic review suggests HIG as a form of CMV intervention for prevention and treatment of the viral maternal-to-fetal infection were effective, with cohort studies among the reviewed research showing HIG was linked to lower chances of CMV disease. HIG was associated with increased CMV-specific IgG concentrations, lowered natural killer cells, lowered HLA-DR + cells and raised avidity and normal development of infants following maternal CMV infections. Adverse outcomes, such as TOP, were avoided using HIG therapy.
However, while the systematic review suggests the treatment was effective when interventions such as hygiene prevention, valacyclovir and HIG were used, meta-analysis involving analysis of the effect of HIG and valacyclovir suggested mixed or inconclusive findings. The results of this study reveal the complex effect of HIG, valacyclovir, passive vaccination, and hygiene promotion as interventions for preventing or treating congenital CMV and preventing maternal–fetal viral transmission.
Author Contributions
Conceptualization, M.R.-K.; methodology, M.R.-K. software, M.R.-K. validation, H.H., D.B., formal analysis, M.R.-K., J.G., H.H., M.M.-W., M.S., A.G., A.K., J.S., W.G., D.B., R.J., M.G., W.K.; investigation, M.R.-K.; writing—original draft preparation, M.R.-K. writing—review and editing, J.G., H.H., M.M.-W., M.S., A.G., A.K., J.S., W.G., D.B., R.J., M.G., W.K.; visualization, M.R.-K.; supervision, H.H., D.B.; funding acquisition, M.R.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available upon the reasonable request.
Conflicts of Interest
The authors report there are no competing interest to declare.
References
- Leruez-Ville, M.; Foulon, I.; Pass, R.; Ville, Y. Cytomegalovirus Infection during Pregnancy: State of the Science. Am. J. Obstet. Gynecol. 2020, 223, 330–349. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Banay, R.F.; Hart, J.E.; Laden, F. A Review of the Health Benefits of Greenness. Curr. Epidemiol. Rep. 2015, 2, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Tibaldi, C.; Masuelli, G.; Frisina, V.; Sacchi, A.; Furione, M.; Arossa, A.; Spinillo, A.; Klersy, C.; Ceccarelli, M.; et al. Prevention of Primary Cytomegalovirus Infection in Pregnancy. EBioMedicine 2015, 2, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Navti, O.B.; Al-Belushi, M.; Konje, J.C. Cytomegalovirus Infection in Pregnancy—An Update. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 216–222. [Google Scholar] [CrossRef]
- Walker, S.P.; Palma-Dias, R.; Wood, E.M.; Shekleton, P.; Giles, M.L. Cytomegalovirus in Pregnancy: To Screen or Not to Screen. BMC Pregnancy Childbirth 2013, 13, 96. [Google Scholar] [CrossRef]
- Kagan, K.O.; Sonek, J.; Hamprecht, K. Antenatal Treatment Options for Primary Cytomegalovirus Infections. Curr. Opin. Obs. Gynecol. 2018, 30, 355–360. [Google Scholar] [CrossRef]
- Boucoiran, I.; Yudin, M.; Poliquin, V.; Caddy, S.; Gantt, S.; Castillo, E. Guideline No. 420: Cytomegalovirus Infection in Pregnancy. J. Obstet. Gynaecol. Can. 2021, 43, 893–908. [Google Scholar] [CrossRef]
- Boucoiran, I.; Yudin, M.; Poliquin, V.; Caddy, S.; Gantt, S.; Castillo, E. Corrigendum to “Guideline No. 420: Cytomegalovirus Infection in Pregnancy” [Journal of Obstetrics and Gynaecology Canada 43/7 (2021) 893-908]. J. Obs. Gynaecol. Can. 2021, 43, 1466. [Google Scholar] [CrossRef]
- Khalil, A.; Heath, P.; Jones, C.; Soe, A.; Ville, Y.G. Congenital Cytomegalovirus Infection: Update on Treatment. BJOG Int. J. Obstet. Gynaecol. 2018, 125, e1–e11. [Google Scholar] [CrossRef]
- Shahar-Nissan, K.; Pardo, J.; Peled, O.; Krause, I.; Bilavsky, E.; Wiznitzer, A.; Hadar, E.; Amir, J. Valaciclovir to Prevent Vertical Transmission of Cytomegalovirus after Maternal Primary Infection during Pregnancy: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2020, 396, 779–785. [Google Scholar] [CrossRef]
- Cordier, A.G.; Guitton, S.; Vauloup-Fellous, C.; Grangeot-Keros, L.; Benachi, A.; Picone, O. Awareness and Knowledge of Congenital Cytomegalovirus Infection among Health Care Providers in France. J. Clin. Virol. 2012, 55, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Cordier, A.G.; Guitton, S.; Vauloup-Fellous, C.; Grangeot-Keros, L.; Ayoubi, J.M.; Benachi, A.; Picone, O. Awareness of Cytomegalovirus Infection among Pregnant Women in France. J. Clin. Virol. 2012, 53, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Willame, A.; Blanchard-Rohner, G.; Combescure, C.; Irion, O.; Posfay-Barbe, K.; Martinez de Tejada, B. Awareness of Cytomegalovirus Infection among Pregnant Women in Geneva, Switzerland: A Cross-Sectional Study. Int. J. Environ. Res. Public. Health 2015, 12, 15285–15297. [Google Scholar] [CrossRef] [PubMed]
- Pereboom, M.T.R.; Manniën, J.; Spelten, E.R.; Hutton, E.K.; Schellevis, F.G. Maternal Cytomegalovirus Infection Prevention: The Role of Dutch Primary Care Midwives. Midwifery 2014, 30, 1196–1201. [Google Scholar] [CrossRef]
- Pereboom, M.T.R.; Manniën, J.; Spelten, E.R.; Schellevis, F.G.; Hutton, E.K. Observational Study to Assess Pregnant Women’s Knowledge and Behaviour to Prevent Toxoplasmosis, Listeriosis and Cytomegalovirus. BMC Pregnancy Childbirth 2013, 13, 98. [Google Scholar] [CrossRef]
- Adler, S.P.; Marshall, B. Cytomegalovirus Infections. Pediatr. Rev. 2007, 28, 92–100. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Cooper, H.; Hedges, L.V.; Valentine, J.C. Potentials and Limitations of Research Synthesis. In The Handbook of Research Synthesis and Meta-Analysis; Russell Sage Foundation: Manhattan, NY, USA, 2019; pp. 517–525. [Google Scholar]
- Nigro, G.; Adler, S.P.; La Torre, R.; Best, A.M. Passive Immunization during Pregnancy for Congenital Cytomegalovirus Infection. N. Engl. J. Med. 2005, 353, 1350–1362. [Google Scholar] [CrossRef]
- Jacquemard, F.; Yamamoto, M.; Costa, J.-M.; Romand, S.; Jaqz-Aigrain, E.; Dejean, A.; Daffos, F.; Ville, Y. Maternal Administration of Valaciclovir in Symptomatic Intrauterine Cytomegalovirus Infection. BJOG Int. J. Obstet. Gynaecol. 2007, 114, 1113–1121. [Google Scholar] [CrossRef]
- Pass, R.F.; Zhang, C.; Evans, A.; Simpson, T.; Andrews, W.; Huang, M.-L.; Corey, L.; Hill, J.; Davis, E.; Flanigan, C.; et al. Vaccine Prevention of Maternal Cytomegalovirus Infection. N. Engl. J. Med. 2009, 360, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Buxmann, H.; Stackelberg, O.M.v.; Schlößer, R.L.; Enders, G.; Gonser, M.; Meyer-Wittkopf, M.; Hamprecht, K.; Enders, M. Use of Cytomegalovirus Hyperimmunoglobulin for Prevention of Congenital Cytomegalovirus Disease: A Retrospective Analysis. J. Perinat. Med. 2012, 40, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Visentin, S.; Manara, R.; Milanese, L.; Da Roit, A.; Forner, G.; Salviato, E.; Citton, V.; Magno, F.M.; Orzan, E.; Morando, C.; et al. Early Primary Cytomegalovirus Infection in Pregnancy: Maternal Hyperimmunoglobulin Therapy Improves Outcomes Among Infants at 1 Year of Age. Clin. Infect. Dis. 2012, 55, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Roxby, A.C.; Atkinson, C.; Ásbjörnsdóttir, K.; Farquhar, C.; Kiarie, J.N.; Drake, A.L.; Wald, A.; Boeckh, M.; Richardson, B.; Emery, V.; et al. Maternal Valacyclovir and Infant Cytomegalovirus Acquisition: A Randomized Controlled Trial among HIV-Infected Women. PLoS ONE 2014, 9, e87855. [Google Scholar] [CrossRef]
- Revello, M.G.; Lazzarotto, T.; Guerra, B.; Spinillo, A.; Ferrazzi, E.; Kustermann, A.; Guaschino, S.; Vergani, P.; Todros, T.; Frusca, T.; et al. A Randomized Trial of Hyperimmune Globulin to Prevent Congenital Cytomegalovirus. N. Engl. J. Med. 2014, 370, 1316–1326. [Google Scholar] [CrossRef]
- Nigro, G.; Capretti, I.; Manganello, A.-M.; Best, A.M.; Adler, S.P. Primary Maternal Cytomegalovirus Infections during Pregnancy: Association of CMV Hyperimmune Globulin with Gestational Age at Birth and Birth Weight. J. Matern.-Fetal Neonatal Med. 2015, 28, 168–171. [Google Scholar] [CrossRef]
- Chiaie, L.D.; Neuberger, P.; Vochem, M.; Lihs, A.; Karck, U.; Enders, M. No Evidence of Obstetrical Adverse Events after Hyperimmune Globulin Application for Primary Cytomegalovirus Infection in Pregnancy: Experience from a Single Centre. Arch. Gynecol. Obs. 2018, 297, 1389–1395. [Google Scholar] [CrossRef]
- Minsart, A.-F.; Smiljkovic, M.; Renaud, C.; Gagné, M.-P.; Lamarre, V.; Kakkar, F.; Boucher, M.; Boucoiran, I. Use of Cytomegalovirus-Specific Hyperimmunoglobulins in Pregnancy: A Retrospective Cohort. J. Obstet. Gynaecol. Can. 2018, 40, 1409–1416. [Google Scholar] [CrossRef]
- Blázquez-Gamero, D.; Galindo Izquierdo, A.; Del Rosal, T.; Baquero-Artigao, F.; Izquierdo Méndez, N.; Soriano-Ramos, M.; Rojo Conejo, P.; González-Tomé, M.I.; García-Burguillo, A.; Pérez Pérez, N.; et al. Prevention and Treatment of Fetal Cytomegalovirus Infection with Cytomegalovirus Hyperimmune Globulin: A Multicenter Study in Madrid. J. Matern.-Fetal Neonatal Med. 2019, 32, 617–625. [Google Scholar] [CrossRef]
- Kagan, K.O.; Enders, M.; Schampera, M.S.; Baeumel, E.; Hoopmann, M.; Geipel, A.; Berg, C.; Goelz, R.; De Catte, L.; Wallwiener, D.; et al. Prevention of Maternal-Fetal Transmission of Cytomegalovirus after Primary Maternal Infection in the First Trimester by Biweekly Hyperimmunoglobulin Administration. Ultrasound Obs. Gynecol. 2019, 53, 383–389. [Google Scholar] [CrossRef]
- Seidel, V.; Hackelöer, M.; Rancourt, R.C.; Henrich, W.; Siedentopf, J.-P. Fetal and Maternal Outcome after Hyperimmunoglobulin Administration for Prevention of Maternal–Fetal Transmission of Cytomegalovirus during Pregnancy: Retrospective Cohort Analysis. Arch. Gynecol. Obs. 2020, 302, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Adler, S.P.; Congenital Cytomegalic Disease Collaborating Group. High-Dose Cytomegalovirus (CMV) Hyperimmune Globulin and Maternal CMV DNAemia Independently Predict Infant Outcome in Pregnant Women With a Primary CMV Infection. Clin. Infect. Dis. 2020, 71, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Devlieger, R.; Buxmann, H.; Nigro, G.; Enders, M.; Jückstock, J.; Siklós, P.; Wartenberg-Demand, A.; Schüttrumpf, J.; Schütze, J.; Rippel, N.; et al. Serial Monitoring and Hyperimmunoglobulin versus Standard of Care to Prevent Congenital Cytomegalovirus Infection: A Phase III Randomized Trial. FDT 2021, 48, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Faure-Bardon, V.; Fourgeaud, J.; Guilleminot, T.; Magny, J.-F.; Salomon, L.J.; Bernard, J.-P.; Leruez-Ville, M.; Ville, Y. First-Trimester Diagnosis of Congenital Cytomegalovirus Infection after Maternal Primary Infection in Early Pregnancy: Feasibility Study of Viral Genome Amplification by PCR on Chorionic Villi Obtained by CVS. Ultrasound Obstet. Gynecol. 2021, 57, 568–572. [Google Scholar] [CrossRef]
- Faure-Bardon, V.; Fourgeaud, J.; Stirnemann, J.; Leruez-Ville, M.; Ville, Y. Secondary Prevention of Congenital Cytomegalovirus Infection with Valacyclovir Following Maternal Primary Infection in Early Pregnancy. Ultrasound Obstet. Gynecol. 2021, 58, 576–581. [Google Scholar] [CrossRef]
- Hughes, B.L.; Clifton, R.G.; Rouse, D.J.; Saade, G.R.; Dinsmoor, M.J.; Reddy, U.M.; Pass, R.; Allard, D.; Mallett, G.; Fette, L.M.; et al. A Trial of Hyperimmune Globulin to Prevent Congenital Cytomegalovirus Infection. N. Engl. J. Med. 2021, 385, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kagan, K.O.; Enders, M.; Hoopmann, M.; Geipel, A.; Simonini, C.; Berg, C.; Gottschalk, I.; Faschingbauer, F.; Schneider, M.O.; Ganzenmueller, T.; et al. Outcome of Pregnancies with Recent Primary Cytomegalovirus Infection in First Trimester Treated with Hyperimmunoglobulin: Observational Study. Ultrasound Obstet. Gynecol. 2021, 57, 560–567. [Google Scholar] [CrossRef]
- Cruz-Lemini, M.; Parra-Saavedra, M.; Borobio, V.; Bennasar, M.; Goncé, A.; Martínez, J.; Borrell, A. How to Perform an Amniocentesis. Ultrasound Obstet. Gynecol. 2014, 44, 727–731. [Google Scholar] [CrossRef]
- Antsaklis, P.; Antsaklis, A.; Theodora, M. Invasive Prenatal Diagnosis: Amniocentesis. Donald Sch. J. Ultrasound Obstet. Gynecol. 2015, 9, 307–313. [Google Scholar] [CrossRef]
- Naing, Z.W.; Scott, G.M.; Shand, A.; Hamilton, S.T.; van Zuylen, W.J.; Basha, J.; Hall, B.; Craig, M.E.; Rawlinson, W.D. Congenital Cytomegalovirus Infection in Pregnancy: A Review of Prevalence, Clinical Features, Diagnosis and Prevention. Aust. New Zealand J. Obstet. Gynaecol. 2016, 56, 9–18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).


