Abstract
Monkeypox disease (MPX) is currently considered a global threat after COVID-19. European Medicines Agency (EMA) approved Tecovirimat in capsule dosage form (200 mg) as the first treatment for MPX in January 2022. This article highlights Tecovirimat’s development and patent literature review and is believed to benefit the scientists working on developing MPX treatments. The literature for Tecovirimat was gathered from the website of SIGA Technologies (developer of Tecovirimat), regulatory agencies (EMA, United States Food and Drug Administration (USFDA), and Health Canada), PubMed, and freely accessible clinical/patent databases. Tecovirimat was first recognized as an anti-orthopoxvirus molecule in 2002 and developed by SIGA Technologies. The USFDA and Health Canada have also recently approved Tecovirimat to treat smallpox in 2018 and 2021, respectively. The efficacy of Tecovirimat was verified in infected non-human primates (monkeys) and rabbits under the USFDA’s Animal Rule. Most clinical studies have been done on Tecovirimat’s safety and pharmacokinetic parameters. The patent literature has revealed inventions related to the capsule, injection, suspension, crystalline forms, amorphous form, and drug combinations (Tecovirimat + cidofovir) and process for preparing Tecovirimat. The authors foresee the off-label use of Tecovirimat in the USA and Canada for MPX and other orthopoxvirus infections. The authors also trust that there is immense scope for developing new Tecovirimat-based treatments (new drug combinations with other antivirals) for orthopoxvirus and other viral diseases. Drug interaction studies and drug resistance studies on Tecovirimat are also recommended. Tecovirimat is believed to handle the current MPX outbreak and is a new hope of biosecurity against smallpox or orthopoxvirus-related bioterrorism attack.
1. Introduction
Monkeypox disease (MPX) is currently considered a global threat [1,2,3,4,5,6]. The zoonotic monkeypox virus (MPXV) (Family: Poxviridae; Subfamily: Chordopoxvirinae; Genus: Orthopoxvirus), a double-stranded DNA virus, causes MPX. The MPXV is related to the smallpox variola virus, also an orthopoxvirus [1,3,4,5,6]. The MPXV was first identified and isolated from monkeys in 1958 in Denmark [3,5,6]. Therefore, it is called MPXV. The first confirmed human MPX case was reported in 1970 in the Democratic Republic of Congo [3,4,5,6]. MPX is typical and endemic in African countries, including Cameroon, the Central African Republic, the Democratic Republic of Congo, Liberia, Nigeria, Sierra Leone, and Ghana. The first case of MPX outside Africa was reported in 2003 in the United States [3,4,5,6]. African rodents are thought to be the natural reservoir of MPXV, and MPXV infections have been reported in humans, monkeys, mice, rats, squirrels, and prairie [2,5,6]. In the past, outbreaks of MPX have occurred in the Democratic Republic of Congo (1997 and 2020), African regions (1997), the Central African Republic (2016), Nigeria (2017 and 2018), Cameroon (2018), Singapore (2019), the United Kingdom and Northern Ireland (2021), and the United States of America (2003 and 2021) [7]. In 2021–2022, the outbreak of MPX (about 1285 confirmed cases) had re-emerged in about 28 non-African countries comprising America (Argentina, Canada, Mexico, and the United States of America), Eastern Mediterranean (Morocco and the United Arab Emirates), Europe (Austria, Belgium, Czechia, Denmark, Finland, France, Germany, Hungary, Ireland, Israel, Italy, Latvia, Malta, Netherlands, Norway, Portugal, Slovenia, Spain, Sweden, Switzerland, and the United Kingdom), and Western Pacific (Australia). This outbreak is also present in African countries, with 59 confirmed cases of MPX and more than 1536 suspected cases [8].
The MPXV can be transmitted to humans by direct contact (oral, nasal, intradermal, etc.) with the body fluid, respiratory droplets, or skin lesion of the infected animal. Contact with contaminated fomites can also cause human MPXV infection. Human-to-human transmission is also possible. Therefore, household members and sexual partners are at a greater risk of developing MPX [6]. Two clades of MPXV (Central African or Congo Basin clade and West African clade) have been reported, wherein the Congo Basin clade is more virulent and transmissible than the West African clade [4,6,9]. It is thought that the reported cases of MPX in non-African countries are due to the West African clade of MPXV [4,9]. The MPXV replicates/multiplies at the infection site, causes local inflammation, primary viremia, and spreads to lymph nodes/lymphoid organs, leading to secondary viremia and symptoms of MPX [10]. The symptoms of monkeypox appear within 5–21 days. The symptoms of MPX include fever, chills, headache, muscle ache (myalgia), backache, exhaustion (asthenia), swollen lymph nodes (lymphadenopathy), and rashes (face, mouth, chest, hand, feet, anus, genitals, conjunctivae, etc.) [11,12,13]. MPX diagnosis is challenging because of its similarity to smallpox and chickenpox. Diagnosis of MPX can be made by PCR testing and sequencing analysis. However, the lymphadenopathy at the prodromal stage of MPX is considered an essential feature of MPX to distinguish MPX from smallpox and chickenpox [11,12,13]. If untreated, MPX can cause secondary infections, sepsis, bronchopneumonia, encephalitis, and corneal disease leading to loss of vision and death (3–6% cases) [12]. Some factors that are promoting the rapid MPXV spread across the globe include cessation of smallpox vaccination, increased interaction between humans and animals, increased travel to affected countries, decreased international coordination, difficulty in the diagnosis of MPX, lack of research on monkeypox, and poor availability of MPX treatments (supportive care, vaccine, cidofovir, and Tecovirimat) [14].
In January 2022, the European Medicines Agency (EMA) approved Tecovirimat as the first oral treatment for monkeypox. This article highlights Tecovirimat’s pharmaceutical development, clinical trials, inventions, and patent literature in a single document. The literature for Tecovirimat was gathered from the website of SIGA Technologies (developer of Tecovirimat), regulatory agencies (EMA, United States Food and Drug Administration (USFDA), and Health Canada), PubMed, and freely accessible clinical/patent databases. Tecovirimat, TPOXX, SIGA-246, SIGA246, ST-246, ST246, and SIGA Technologies, alone or in combination with other keywords (smallpox/monkeypox), were used to collect the literature from the sources mentioned earlier.
2. Tecovirimat
2.1. Chemistry
Tecovirimat (Synonyms: TPOXX, SIGA-246, SIGA246, ST-246, ST246; CAS registry numbers: 816458-31-8, 869572-92-9; Molecular Formula: C19H15F3N2O3; Molecular Weight: 376.332; Chemical name: N-[(3aR,4R,4aR,5aS,6S,6aS)-3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6 ethenocycloprop[f]isoindol-2(1H)-yl]-4-(trifluoromethyl)benzamide) is a small tetracyclic acylhydrazide molecule used to treat orthopox viral infection, including monkeypox [15,16,17]. On 6 January 2022, the EMA approved the capsule dosage form (200 mg) of crystalline Tecovirimat monohydrate (Figure 1) (Molecular Formula: C19H15F3N2O3·H2O; Molecular Weight: 394.35; CAS registry number: 1162664-19-8) to treat monkeypox, smallpox, and cowpox, which are caused by viruses of the same family (orthopoxviruses) [16].

Figure 1.
Chemical structure of Tecovirimat monohydrate.
The chemical structure of Tecovirimat has some chiral carbons. Therefore, it exists in many stereoisomeric forms. However, only one stereoisomer is used in the approved dosage form of Tecovirimat (Figure 1) [18,19]. Tecovirimat monohydrate (a non-hygroscopic white crystalline solid) is insoluble in water at a pH range of 2.0–6.5 [19] but demonstrates high permeability in CaCo-2 cells. Accordingly, Tecovirimat monohydrate is classified as a BCS-II drug [18,19]. Tecovirimat exists in different polymorphic forms, including crystalline Tecovirimat monohydrate Form I, amorphous form, hemihydrate Form V, and other crystalline forms. The crystalline Tecovirimat monohydrate Form I is used to develop the marketed dosage form (capsule). Furthermore, the crystalline Tecovirimat monohydrate Form I can be prepared consistently by recrystallization from a mixture of ethyl acetate and water [18].
2.2. Worldwide Approval
Tecovirimat monohydrate has been approved by the EMA [16], USFDA [17], and Health Canada [20,21] to treat smallpox (Table 1). However, only EMA has approved Tecovirimat to treat monkeypox [16].

Table 1.
Rx data of the EMA, USFDA, and Health Canada approved Tecovirimat.
2.3. Development
Tecovirimat was recognized in 2002 as an effective molecule against orthopoxvirus-induced cytopathic effects by a high throughput screening process of 356,240 compounds [25,26]. The chemical structure of Tecovirimat has been provided first time in Patent Cooperation Treaty (PCT) Patent Application Publication Number WO2004112718A2 (previously owned by Viropharma) [27]. The crystalline Tecovirimat monohydrate used as the active ingredient of TPOXX was disclosed in 2011 for the first time in the United States Patent Application Publication number US2011236434A1, which has now been granted as US9339466B2 (SIGA Technologies) [28]. SIGA Technologies have developed TPOXX (capsule and intravenous dosage forms of Tecovirimat) in collaboration with the Biomedical Advances Research and Development Authority (BARDA) of the US Department of Health and Human Services. Tecovirimat was mainly developed to combat smallpox-related bioterror concerns [17,20,21]. SIGA Technologies is also developing Tecovirimat’s oral (liquid) formulations [17]. The development timeline of Tecovirimat is provided in Figure 2 [17,25].
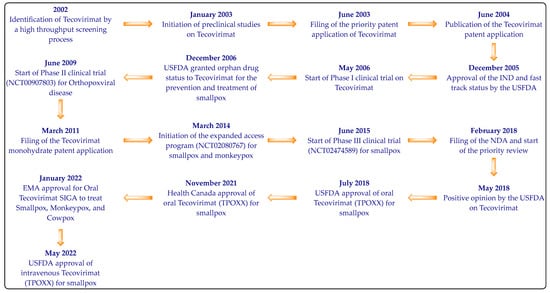
Figure 2.
The development timeline of Tecovirimat.
2.4. Pharmacology
The in vitro antiviral activity-based concentration of Tecovirimat inhibiting the virus-induced cytopathic effect (CPE) by 50% (EC50 in μmol/L) was promising against different orthopoxvirus (Variola = 0.016–0.067; Monkeypox = 0.014–0.039; Rabbitpox = 0.015; Vaccinia = 0.009). It completely inhibited the CPE of the wild-type cowpox virus and extracellular vaccinia virus formation but had little effect on the intracellular formation of the vaccinia virus [17]. The clinical trials in humans with smallpox are neither feasible nor ethical. Therefore, the efficacy of Tecovirimat was verified in infected rabbits and non-human primates (monkeys) under the USFDA’s Animal Rule [17,29,30,31,32,33]. The effectiveness of Tecovirimat in the non-human primate monkeypox model utilizing MPX strain Zaire 79 (V79-I-005) infected cynomolgus monkeys is reported [29,30,31,32]. Tecovirimat (3, 10, 30, and 100 mg/Kg once a day) was administered orally to different groups of infected monkeys after three days of infection (stage of secondary viremia). The treatment duration was 14 days. Tecovirimat administration demonstrated 100% protection to the infected animal and reduced the viral load and lesion formation. The animal not receiving Tecovirimat died or required euthanasia after 33 days of the infection. An equivalent drug concentration level in animals and humans was observed at a dose of 10 mg/Kg/day and 400 mg/day, respectively. This observation suggested an adequate oral dose of 400 mg/day for 14 days to treat monkeypox or smallpox infections in humans [29,30,31,32]. The study of Tecovirimat in the rabbitpox virus model also demonstrated a 90–100% survival rate for rabbits when Tecovirimat (20 mg/Kg, 40 mg/Kg, 80 mg/Kg, and 120 mg/Kg) was administered for 14 days after starting the dose on day four after the infection [30,31,32].
The mechanism of action of Tecovirimat is well explained in the literature [17,25], documents released by SIGA Technologies [20,21], and the approved drug label of TPOXX [23,30,31]. Tecovirimat is an orthopoxvirus-specific VP37 protein inhibitor that inhibits the virus’s systemic spread to other cells [17,20,21,23,25,30,31]. VP37 protein was recognized as a target of Tecovirimat by the genetic mapping of Tecovirimat-resistant mutant viruses [25]. The orthopoxvirus-specific VP37 protein is responsible for forming an envelope around the orthopoxvirus-like MPXV. Creating this envelope is essential for exiting the virus from the cell and spreading the virus to other cells. The mechanism of action of Tecovirimat is also depicted in Figure 3.

Figure 3.
Mechanism of action of Tecovirimat.
The important pharmacological parameters of Tecovirimat are provided in Table 2.

Table 2.
Important pharmacological parameters of Tecovirimat.
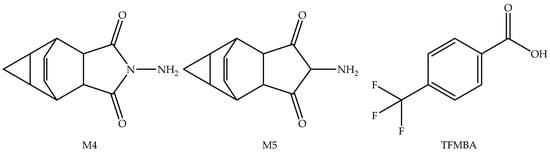
Figure 4.
Pharmacologically inactive metabolites of Tecovirimat.
3. Clinical Studies
The information about past and ongoing clinical studies on Tecovirimat was collected from the clinical database [36], employing different keywords. This search was done on 11 July 2022, and revealed many clinical studies on Tecovirimat (Tecovirimat = 11; SIGA-246 = 10; SIGA246 = 10; ST-246 = 11; ST246 = 11; TPOXX = 11). The overlapping results were removed, and the summary of the finalized 12 studies is provided in Table 3. The readers can open the page of the clinical database [36] and get the complete clinical study data using the National Clinical Trial number (NCT number) for each study mentioned in Table 3.

Table 3.
Summary of the clinical studies of Tecovirimat.
The clinical studies of Tecovirimat cited in Table 3 are related to smallpox (six studies), vaccinia (one study), healthy volunteers (one study), vaccinia (one study), orthopox viral disease (two studies), and monkeypox (two studies). No study has been conducted on children and pregnant women. In summary, the clinical studies of Tecovirimat were mainly related to its pharmacokinetic and safety parameters. Tecovirimat was well tolerated without any serious side effects in the phase I studies (NCT00728689 and NCT00431951). The phase II study (NCT00907803) revealed headache and nausea as Tecovirimat’s most frequent side effects without any serious effect on patients’ wellbeing. The phase III clinical study (NCT02474589) did not identify any safety concerns with the oral administration of Tecovirimat and demonstrated that Tecovirimat’s bioavailability increases up to 50% when taken with food. Our search on PubMed also revealed some pharmacokinetic studies on Tecovirimat [37,38,39,40,41]. The pharmacokinetic parameters of Tecoviramat are now well established and have already been discussed in Table 2. Accordingly, the authors have not elaborated on the studies related to pharmacokinetic parameters of Tecovirimate.
4. Patent Searching and Summary
Tecovirimat’s patents/patent applications were searched on 11 July 2022, through freely available patent databases (Espacenet, Patentscope, and the United States Patent and Trademark Office) [42,43,44,45,46]. A preliminary search employing different keywords and combinations thereof (Tecovirimat; TPOXX; SIGA246; ST-246 + Monkeypox; ST-246 + Smallpox; ST246 + Monkeypox; ST246 + Smallpox; SIGA-246 + Smallpox; SIGA-246 + Monkeypox; SIGA + Smallpox; SIGA + Monkeypox) provided hundreds of hits, which were not possible to discuss in this review. Tecovirimat has been developed by SIGA Technologies and is the marketing authorization holder for Tecovirimat in the USA, Europe, and Canada. Accordingly, the authors are providing a summary of patents/patent applications assigned to SIGA Technologies, including the patents listed in the Orange Book (OB) of the USFDA [47] and other important patents/patent applications explicitly claiming inventions of Tecovirimat (Table 4). It should be noted that most of the patent numbers mentioned in Table 4 belong to the same patent family. Therefore, they have identical authors and titles but different claims, patent numbers, and grant dates.

Table 4.
Summary of the critical patents/patent applications related to Tecovirimat.
5. Discussion
MPX is the currently emerging global outbreak. The MPX is closely related but less fatal than smallpox disease [1,2,3,4,5,6]. Smallpox vaccination is thought to control MPX because the MPXV and smallpox virus are genetically close relatives and share common signs and symptoms. However, smallpox was eradicated long ago, and the routine administration of the smallpox vaccine has not been done by a large part of the global population [17]. This has increased the chances of individuals getting infected with MPX, making it a currently unmet medical need.
In January 2022, the EMA approved the Tecovirimat capsule (200 mg), developed by SIGA Technologies, as the first treatment for MPX [16]. Tecovirimat was mainly invented for treating smallpox because of bioterrorism concerns. The US Strategic National Stockpile has reserved about 2 million doses of Tecovirimat and other vaccines/drugs (JYNNEOS, ACAM2000, cidofovir, and vaccinia immunoglobulin intravenous (VIGIV)) in case of any bioterrorist attack [17]. The USFDA and Health Canada approved Tecovirimat for treating smallpox in 2018 and 2021, respectively, but not MPX [17,20,21]. Based on the EMA approval, the off-label use of Tecovirimat and the post-exposure prevention of MPX is foreseeable in the USA and Canada. These countries may also approve Tecovirimat for MPX shortly. The regulatory approval of Tecovirimat in other MPX-affected countries is also predictable soon. The EMA-, Health Canada-, and USFDA-approved capsules of Tecovirimat contain the crystalline Form I of Tecovirimat monohydrate because of its thermodynamic stability over other polymorphic forms [18,19]. Tecovirimat has been developed under the Animal Rule, which does not require efficacy-based clinical trials in humans for life-threatening, unnatural, and severe diseases. Accordingly, the efficacy of Tecovirimat was established in non-human primates (monkeys) and rabbits [17,29,30,31,32,33]. Tecovirimat also demonstrated high efficacy in different animal models against all recognized pathogenic orthopoxviruses at suggested human doses or its equivalents [81]. This is also evident from the clinical study data (Table 3), wherein most clinical studies were based on Tecovirimat’s pharmacokinetic and safety parameters. The drug–drug interaction, drug–disease interaction, and drug–food interaction studies are essential parameters concerning the safety and efficacy of a drug [82]. The interaction of Tecovirimat with repaglinide and midazolam has been established [30,31,32]. The contraindication of Tecovirimat injection in patients with renal injury is also reported [34]. Tecovirimat with milk, yogurt, applesauce, etc., is also recommended [30,31]. Tecovirimat has not shown any teratogenic effect in mice but showed non-teratogenic adverse events in maternal rabbits [18]. The authors believe that further studies must be done concerning drug interactions and teratogenic effects of Tecovirimat in higher animal models to ensure patient safety. Accordingly, the patient treated with Tecovirimat must be monitored, which may help identify its new benefits/side effect. Particular emphasis must be given while monitoring pregnant/pediatric/geriatric patients, patients suffering from chronic diseases such as diabetes, hypertension, cancer, etc., and patients on polypharmacy treatment. SIGA Technologies collaborates with many organizations to tackle the MPX outbreak [83]. Hopefully, this collaboration will also take care of the points mentioned earlier.
A DNA virus like MPXV has a lower mutation rate, but a mutation in the virus may develop resistance to a drug [2,81]. A nucleotide and frameshift mutation has been reported in the currently circulating strain of MPXV, which may increase its transmissibility [2]. Furthermore, mutations in MPXV are also possible. The authors opine to assess the efficacy of Tecovirimat against the MPXV that demonstrated nucleotide and frameshift mutation. This study may help to understand the possibility of Tecovirimat-resistance development by the MPXV. The case of Tecovirimat resistance among unresponsive patients to Tecovirimat must also be considered [3]. Tecovirimat is an inhibitor of the VP37 protein of orthopoxviruses [17,20,21,23,25,30,31]. The authors believe that drug repurposing-based and artificial intelligence-empowered drug development programs on the existing marketed antiviral drugs may also help to identify VP37 protein inhibitors for further development.
Developing commercially important inventions requires an understanding of existing patented inventions. SIGA Technologies owns many patents related to Tecovirimat (Table 4). These patents claim the marketed pharmaceutical compositions (capsule and intravenous injection) and suspension; method of treating orthopoxvirus infection; different polymorphic forms (crystalline and amorphous); and process for preparing Tecovirimat. SIGA Technologies is also developing an oral suspension formulation of Tecovirimat in collaboration with BARDA [17]. The synergistic drug combination patent application (Tecovirimat + cidofovir) has also been filed [78]. Tecovirimat does not interfere with the DNA replication process of MPXV and is unable to prevent the formation of intracellular MPXV (Figure 3). Accordingly, the authors trust that combining Tecovirimat with existing DNA replication inhibitors may provide synergistic effects. The effect of Tecovirimat may be reduced in immunocompromised patients (HIV/AIDS, leukaemia, lymphoma, generalized malignancies, etc.) [30,31,32]. Combining immunity boosters such as zinc, quercetin, vitamin C, thymoquinone, and other antioxidants [84,85,86] may increase Tecovirimat’s efficacy for MPX among immunocompromised patients. The authors believe that there is an excellent scope for developing more and better combinations of Tecovirimat against orthopoxvirus infections.
6. Conclusions
Tecovirimat is a new hope against the MPX outbreak and biosecurity against smallpox or orthopoxvirus-related bioterrorism attacks. At the time this article was written, Tecovirimat was only approved by the EMA to treat MPX. We expect the regulatory approval of Tecovirimat in other MPX-affected countries shortly. The efficacy of Tecovirimat in MPXV demonstrating nucleotide and frameshift mutation is warranted to assess the possibility of Tecovirimat resistance. Tecovirimat has been established as a safe and effective MPX treatment, but further Tecovirimat interaction-based pharmacological studies are also necessary to provide safe and effective treatment. Finally, the research programs on Tecovirimat-based drug combinations are also suggested to develop better MPX treatments.
Author Contributions
Conceptualization, M.I. and M.A. (Mazen Almehmadi); methodology, M.I., M.A. (Mazen Almehmadi), M.A. (Mamdouh Allahyani), A.A.A. (Ahad Amer Alsaiari) and M.K.; software, M.K.; formal analysis, M.A. (Mazen Almehmadi), M.A. (Mamdouh Allahyani) and A.A.A. (Ahad Amer Alsaiari); investigation, M.K.A., A.S.A., K.H.H., L.I.A., S.S.A., A.N.A. and A.A.A. (Afeefah Awaid Aldhafeeri); resources, M.I., M.A. (Mazen Almehmadi) and M.K.A.; writing—original draft preparation, M.K.A., A.S.A., K.H.H., L.I.A., S.S.A., A.N.A. and A.A.A. (Afeefah Awaid Aldhafeeri); writing—review and editing, M.I., M.A. (Mazen Almehmadi), M.A. (Mamdouh Allahyani), A.A.A. (Ahad Amer Alsaiari) and M.K.; supervision, M.I.; project administration, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All the authors are thankful to their respective institutes for providing support for this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Memariani, M.; Memariani, H. Multinational monkeypox outbreak: What do we know and what should we do? Ir. J. Med. Sci. 2022, 1971, 1–2. [Google Scholar]
- Zhang, Y.; Zhang, J.Y.; Wang, F.S. Monkeypox outbreak: A novel threat after COVID-19? Mil. Med. Res. 2022, 9, 29. [Google Scholar] [CrossRef]
- Yoo, J.H. Once bitten, twice shy: Our attitude towards monkeypox. J. Korean Med. Sci. 2022, 37, e188. [Google Scholar] [CrossRef]
- Out, A.; Ebenso, B.; Walley, J.; Barceló, J.M.; Ochu, C.L. Global human monkeypox outbreak: Atypical presentation demanding urgent public health action. Lancet Microbe 2022, 3, e554–e555. [Google Scholar] [CrossRef]
- Sklenovská, N.; Van, R.M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front. Public Health 2018, 6, 241. [Google Scholar] [CrossRef]
- Moore, M.; Zahra, F. Monkeypox. In StatPearls [Internet]. Treasure Island (FL); StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- World Health Organization. Monkeypox. Available online: https://www.who.int/emergencies/emergency-events/item/monkeypox (accessed on 11 July 2022).
- World Health Organization. Multi-Country Monkeypox Outbreak: Situation Update. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON392 (accessed on 11 July 2022).
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Kabuga, A.I.; El Zowalaty, M.E. A review of the monkeypox virus and a recent outbreak of skin rash disease in Nigeria. J. Med. Virol. 2019, 91, 533–540. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Monkeypox Symptoms. Available online: https://www.cdc.gov/poxvirus/monkeypox/symptoms.html (accessed on 11 July 2022).
- World Health Organization. Monkeypox. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 11 July 2022).
- Brown, K.; Leggat, P.A. Human monkeypox: Current state of knowledge and implications for the Future. Trop. Med. Infect. Dis. 2016, 1, 8. [Google Scholar] [CrossRef]
- Jain, N.; Lansiaux, E.; Simanis, R. The new face of monkeypox virus: An emerging global emergency. New Microbes New Infect. 2022, 47, 100989. [Google Scholar] [CrossRef]
- TPOXX. Available online: https://www.rxlist.com/tpoxx-drug.htm#clinpharm (accessed on 11 July 2022).
- European Medicines Agency. Tecovirimat SIGA. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tecovirimat-siga (accessed on 11 July 2022).
- Hoy, S.M. Tecovirimat: First global approval. Drugs 2018, 78, 1377–1382. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report: Tecovirimat SIGA. Available online: https://www.ema.europa.eu/en/documents/assessment-report/tecovirimat-siga-epar-public-assessment-report_en.pdf (accessed on 11 July 2022).
- Center for Drug Evaluation and Research. Product Quality Review. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/208627Orig1s000ChemR.pdf (accessed on 11 July 2022).
- SIGA Human BioArmor. TPOXX (Tecovirimat) Fact Sheet. Available online: https://www.siga.com/wp-content/uploads/2022/06/TPOXXFactSheet_2022.pdf (accessed on 11 July 2022).
- SIGA Human BioArmor. Smallpox. Available online: https://www.siga.com/wp-content/uploads/2022/06/CorporateBrochure_2022.pdf (accessed on 11 July 2022).
- Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Tecovirimat. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm (accessed on 11 July 2022).
- Product Monograph. TPOXX. Available online: https://pdf.hres.ca/dpd_pm/00063782.PDF (accessed on 11 July 2022).
- Government of Canada. Register of Innovative Drugs. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/register-innovative-drugs.html (accessed on 11 July 2022).
- Grosenbach, D.W.; Jordan, R.; Hruby, D.E. Development of the small-molecule antiviral ST-246 as a smallpox therapeutic. Future Virol. 2011, 6, 653–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, R.; Leeds, J.M.; Tyavanagimatt, S.; Hruby, D.E. Development of ST-246 for treatment of poxvirus infections. Viruses 2010, 2, 2409–2435. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.; Bailey, T.R.; Rippin, S.R. Compounds, Compositions and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. PCT Patent WO2,004,112,718A2, 29 December 2004. [Google Scholar]
- Tyavanagimatt, S.R.; Stone, M.A.C.L.; Weimers, W.C.; Nelson, D.; Bolken, T.C.; Hruby, D.E.; O’neill, M.H.; Sweetapple, G.; Mccloughan, K.A. Polymorphic Forms of ST-246 and Methods of Preparation. U.S. Patent 9,339,466B2, 17 May 2016. [Google Scholar]
- Jordan, R.; Goff, A.; Frimm, A.; Corrado, M.L.; Hensley, L.E.; Byrd, C.M.; Mucker, E.; Shamblin, J.; Bolken, T.C.; Wlazlowski, C.; et al. ST-246 antiviral efficacy in a non-human primate monkeypox model: Determination of the minimal effective dose and human dose justification. Antimicrob. Agents Chemother. 2009, 53, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Highlights of Prescribing Information. TPOXX. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208627s000lbl.pdf (accessed on 11 July 2022).
- Summary of Product Characteristics. Tecovirimat SIGA. Available online: https://www.ema.europa.eu/en/documents/product-information/tecovirimat-siga-epar-product-information_en.pdf (accessed on 11 July 2022).
- Grosenbach, D.W.; Honeychurch, K.; Rose, E.A.; Chinsangaram, J.; Frimm, A.; Maiti, B.; Lovejoy, C.; Meara, I.; Long, P.; Hruby, D.E. Oral tecovirimat for the treatment of smallpox. N. Engl. J. Med. 2018, 379, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pevear, D.C.; Davies, M.H.; Collett, M.S.; Bailey, T.; Rippen, S.; Barone, L.; Burns, C.; Rhodes, G.; Tohan, S.; et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J. Virol. 2005, 79, 13139–13149. [Google Scholar] [CrossRef]
- Highlights of Prescribing Information. TPOXX. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214518s000lbl.pdf (accessed on 11 July 2022).
- SIGA Technologies. FDA Advisory Committee Briefing Document: Tecovirimat for the Treatment of Smallpox Disease. Available online: https://www.fda.gov/media/112808/download (accessed on 11 July 2022).
- U.S. National Library of Medicines. ClinicalTrials Database. Available online: https://www.clinicaltrials.gov/ (accessed on 11 July 2022).
- Chinsangaram, J.; Honeychurch, K.M.; Tyavanagimatt, S.R.; Leeds, J.M.; Bolken, T.C.; Jones, K.F.; Jordan, R.; Marbury, T.; Ruckle, J.; Mee-Lee, D.; et al. Safety and pharmacokinetics of the anti-orthopoxvirus compound ST-246 following a single daily oral dose for 14 days in human volunteers. Antimicrob. Agents Chemother. 2012, 56, 4900–4905. [Google Scholar] [CrossRef]
- Chinsangaram, J.; Honeychurch, K.M.; Tyavanagimatt, S.R.; Bolken, T.C.; Jordan, R.; Jones, K.F.; Marbury, T.; Lichtenstein, I.; Pickens, M.; Corrado, M.; et al. Pharmacokinetic comparison of a single oral dose of polymorph form I versus form V capsules of the antiorthopoxvirus compound ST-246 in human volunteers. Antimicrob. Agents Chemother. 2012, 56, 3582–3586. [Google Scholar] [CrossRef]
- Jordan, R.; Chinsangaram, J.; Bolken, T.C.; Tyavanagimatt, S.R.; Tien, D.; Jones, K.F.; Frimm, A.; Corrado, M.L.; Pickens, M.; Landis, P.; et al. Safety and pharmacokinetics of the antiorthopoxvirus compound ST-246 following repeat oral dosing in healthy adult subjects. Antimicrob. Agents Chemother. 2010, 54, 2560–2566. [Google Scholar] [CrossRef]
- Jordan, R.; Tien, D.; Bolken, T.C.; Jones, K.F.; Tyavanagimatt, S.R.; Strasser, J.; Frimm, A.; Corrado, M.L.; Strome, P.G.; Hruby, D.E. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob. Agents Chemother. 2008, 52, 1721–1727. [Google Scholar] [CrossRef]
- Leeds, J.M.; Fenneteau, F.; Gosselin, N.H.; Mouksassi, M.S.; Kassir, N.; Marier, J.F.; Chen, Y.; Grosenbach, D.; Frimm, A.E.; Honeychurch, K.M.; et al. Pharmacokinetic and pharmacodynamic modeling to determine the dose of ST-246 to protect against smallpox in humans. Antimicrob. Agents Chemother. 2013, 57, 1136–1143. [Google Scholar] [CrossRef]
- Espacenet. Patent Search. Available online: https://worldwide.espacenet.com/patent/search (accessed on 11 July 2022).
- WIPO IP Portal. Patentscope. Available online: https://patentscope.wipo.int/search/en/structuredSearch.jsf (accessed on 11 July 2022).
- Imran, M.; Alshrari, A.S.; Tauseef, M.; Khan, S.A.; Hudu, S.A.; Abida. Mucormycosis medications: A patent review. Expert Opin. Ther. Pat. 2021, 31, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Alshrari, A.S.; Thabet, H.K.; Abida; Bakht, M.A. Synthetic molecules as DprE1 inhibitors: A patent review. Expert Opin. Ther. Pat. 2021, 31, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khan, S.A.; Abida; Alshrari, A.S.; Eltahir, M.M.M.; Alshammari, M.K.; Harshan, A.A.; Alshammari, N.A. Small molecules as kinetoplastid specific proteasome inhibitors for leishmaniasis: A patent review from 1998 to 2021. Expert Opin. Ther. Pat. 2022, 32, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Asdaq, S.M.B.; Khan, S.A.; Unnikrishnan, M.D.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Mohzari, Y.; Alrashed, A.; AlMotairi, M.; et al. Innovations and patent trends in the development of USFDA approved protein kinase inhibitors in the last two decades. Pharmaceuticals 2021, 14, 710. [Google Scholar] [CrossRef] [PubMed]
- Ordan, R.; Bailey, T.R.; Rippin, S.R. Compounds, Compositions and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. U.S. Patent 8,124,643B2, 28 February 2012. [Google Scholar]
- Jordan, R.; Bailey, T.R.; Rippin, S.R. Compounds, Compositions, and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. U.S. Patent 7,737,168B2, 15 June 2010. [Google Scholar]
- Jordan, R.F.; Bailey, T.R.; Rippin, S.R.; Dai, D. Compounds, Compositions and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. U.S. Patent 8,802,714B2, 12 August 2014. [Google Scholar]
- Jordan, R.; Bailey, T.R.; Rippin, S.R.; Dai, D. Compounds, Compositions and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. U.S. Patent 8,530,509B2, 10 September 2013. [Google Scholar]
- Tyavanagimatt, S.R.; Anderson, M.A.C.L.S.; Weimers, W.; Kasi, G.K.; Samuel, N.K.P.; Bolken, T.C.; Hruby, D.E. ST-246 Liquid Formulations and Methods. U.S. Patent 10,576,165B2, 3 March 2020. [Google Scholar]
- Tyavanagimatt, S.R.; Stone, M.A.C.L.; Weimers, W.C.; Kasi, G.K.; Samuel, P.N.K.; Bolken, T.; Hruby, D.E. ST-246 Liquid Formulations. U.S. Patent 9,233,097B2, 12 January 2016. [Google Scholar]
- Tyavanagimatt, S.R.; Anderson, M.A.C.L.S.; Weimers, W.; Kasi, G.K.; Samuel, N.K.P.; Bolken, T.C.; Hruby, D.E. ST-246 Liquid Formulations and Methods. U.S. Patent 9,907,859B2, 6 March 2018. [Google Scholar]
- Jordan, R.F.; Bailey, T.R.; Rippin, S.R.; Dai, D. Chemicals, Compositions, and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. U.S. Patent 8,039,504B2, 18 October 2011. [Google Scholar]
- Jordan, R.; Bailey, T.R.; Rippin, S.R. Compounds, Compositions and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. U.S. Patent 7,956,197B2, 7 June 2011. [Google Scholar]
- Tyavanagimatt, S.R.; Anderson, M.A.C.L.S.; Weimers, W.C.; Nelson, D.; Bolken, T.C.; Hruby, D.E.; O’Neill, M.H.; Sweetapple, G.; McCloughan, K.A. Polymorphic Forms of ST-246 and Methods of Preparation. U.S. Patent 9,744,154B2, 29 August 2017. [Google Scholar]
- Tyavanagimatt, S.R.; Anderson, M.A.C.L.S.; Weimers, W.C.; Nelson, D.; Bolken, T.C.; Hruby, D.E.; O’Neill, M.H.; Sweetapple, G.; McCloughan, K.A. Polymorphic Forms of ST-246 and Methods of Preparation. U.S. Patent 10,045,964B2, 14 August 2018. [Google Scholar]
- Tyavanagimatt, S.R.; Anderson, M.A.C.L.S.; Weimers, W.C.; Nelson, D.; Bolken, T.C.; Hruby, D.E.; O’Neill, M.H.; Sweetapple, G.; McCloughan, K.A. Polymorphic Forms of ST-246 and Methods of Preparation. U.S. Patent 10,406,137B2, 10 September 2019. [Google Scholar]
- Tyavanagimatt, S.R.; Anderson, M.A.C.L.S.; Weimers, W.C.; Nelson, D.; Bolken, T.C.; Hruby, D.E.; O’Neill, M.H.; Sweetapple, G.; McCloughan, K.A. Polymorphic Forms of ST-246 and Methods of Preparation. U.S. Patent 10,933,050B2, 2 March 2021. [Google Scholar]
- Jordan, R.; Bailey, T.R.; Rippin, S.R.; Dai, D. Compounds, Compositions and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. U.S. Patent 9,045,418B2, 2 June 2015. [Google Scholar]
- Tyavanagimatt, S.R.; Anderson, M.A.C.L.S.; Weimers, W.; Kasi, G.K.; Samuel, N.K.P.; Bolken, T.C.; Hruby, D.E. ST-246 Liquid Formulations and Methods. U.S. Patent 10,124,071B2, 13 November 2018. [Google Scholar]
- Tyavanagimatt, S.R.; Anderson, M.A.C.L.S.; Weimers, W.; Kasi, G.K.; Samuel, N.K.P.; Bolken, T.C.; Hruby, D.E. ST-246 Liquid Formulations and Methods. U.S. Patent 10,864,282B2, 15 December 2020. [Google Scholar]
- Tyavanagimatt, S.R.; Reeves, M.; Samuel, N.K.P.; Priebe, S.; Tan, Y.; Hruby, D.E. Rehydration of Micronized Tecovirimat Monohydrate. U.S. Patent 10,716,759B2, 21 July 2020. [Google Scholar]
- Tyavanagimatt, S.R.; Reeves, M.; Samuel, N.K.P.; Priebe, S.; Tan, Y.; Hruby, D.E. Rehydration of Micronized Tecovirimat monohydrate. U.S. Patent 10,406,103B2, 10 September 2019. [Google Scholar]
- Tyavanagimatt, S.R.; Samuel, N.K.P.; Paz, J.; Tan, Y.; Hruby, D.E. Amorphous Tecovirimat Preparation. U.S. Patent 9,670,158B2, 6 June 2017. [Google Scholar]
- Tyavanagimatt, S.R.; Samuel, N.K.P.; Paz, J.; Tan, Y.; Hruby, D.E. Amorphous Tecovirimat Preparation. U.S. Patent 9,889,119B2, 13 February 2018. [Google Scholar]
- Dai, D. Methods of Preparing Tecovirimat. U.S. Patent 10,155,723B2, 18 December 2018. [Google Scholar]
- Dai, D. Methods of Preparing Tecovirimat. U.S. Patent 10,662,155B2, 26 May 2020. [Google Scholar]
- Dai, D. Methods of Preparing Tecovirimat. U.S. Patent 9,546,137B2, 17 January 2017. [Google Scholar]
- Dai, D. Methods of Preparing Tecovirimat. U.S. Patent 9,862,683B2, 9 January 2018. [Google Scholar]
- Jordan, R.F.; Bailey, T.R.; Rippin, S.R.; Dai, D. Chemicals, Compositions, and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. U.S. Patent 7,687,641B2, 30 March 2010. [Google Scholar]
- Dai, D.; Bailey, T.R.; Rippin, S.R.; Jordan, R. Chemicals, Compositions, and Methods for Treatment and Prevention of Orthopoxvirus Infections and Associated Diseases. Australian Patent 2,012,268,859B2, 5 May 2016. [Google Scholar]
- Tyavanagimatt, S.R.; Holt, K.; Tan, Y.; Anderson, M.A.C.L.S.; Hruby, D.E. ST-246 (Tecovirimat Monohydrate) Suspension Formulations. U.S. Patent 2,021,212,987A1, 15 July 2021. [Google Scholar]
- Zhong, W.; Yang, M.; Gong, W.; Wang, Y.; Gao, C.; Zhou, X.; Li, S. Injectable Pharmaceutical Composition of Tecovirimat and Preparation Method Thereof. U.S. Patent 11,369,587B2, 28 June 2022. [Google Scholar]
- Zhong, W.; Yang, M.; Gong, W.; Wang, Y.; Gao, C.; Zhou, X.; Li, S. Oral Pharmaceutical Composition of Tecovirimat and Preparation Method Thereof. U.S. Patent 11,318,115B2, 3 May 2022. [Google Scholar]
- Dai, Q.; Dong, M.; Yu, S.; Peng, B.; Wang, Y.; Zhu, M. Tecovirimat Dry Suspension and Preparation Method Thereof. Chinese Patent 102,406,617B, 28 August 2013. [Google Scholar]
- Almond, M.R.; Painter, G.R. Compounds, Compositions and Methods for the Treatment of Poxvirus Infections. U.S. Patent 8,642,577B2, 4 February 2014. [Google Scholar]
- Szalay, A.A.; Chen, N.; Yu, Y.A. Use of a Chemotherapeutic Agent in the Preparation of a Medicament for Treating or Ameliorating an Adverse Side Effect Associated with Oncolytic Viral Therapy. European Patent 2,202,297B1, 14 May 2014. [Google Scholar]
- Dai, Q.; Dong, M.; Hu, J. Compound ST-246 Containing a Crystal Water, crystal Thereof and Preparation Method Thereof. Chinese Patent 101,445,478B, 6 April 2011. [Google Scholar]
- Russo, A.T.; Grosenbach, D.W.; Chinsangaram, J.; Honeychurch, K.M.; Long, P.G.; Lovejoy, C.; Maiti, B.; Meara, I.; Hruby, D.E. An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. Expert Rev. Anti-Infect. Ther. 2021, 19, 331–344. [Google Scholar] [CrossRef]
- Tran, M.T.; Grillo, J.A. Translation of drug interaction knowledge to actionable labeling. Clin. Pharmacol. Ther. 2019, 105, 1292–1295. [Google Scholar] [CrossRef]
- SIGA Human BioArmor. Press Releases. Available online: https://investor.siga.com/press-releases (accessed on 11 July 2022).
- Imran, M.; Fatima, W.; Alzahrani, A.K.; Suhail, N.; Alshammari, M.K.; Alghitran, A.A.; Alshammari, F.N.; Ghoneim, M.M.; Alshehri, S.; Shakeel, F. Development of therapeutic and prophylactic zinc compositions for use against COVID-19: A glimpse of the trends, inventions, and patents. Nutrients 2022, 14, 1227. [Google Scholar] [CrossRef]
- Imran, M.; Thabet, H.K.; Alaqel, S.I.; Alzahrani, A.R.; Abida; Alshammari, M.K.; Kamal, M.; Diwan, A.; Asdaq, S.M.B.; Alshehri, S. The therapeutic and prophylactic potential of quercetin against COVID-19: An outlook on the clinical studies, inventive compositions, and patent literature. Antioxidants 2022, 11, 876. [Google Scholar] [CrossRef]
- Imran, M.; Khan, S.A.; Abida; Alshammari, M.K.; Alkhaldi, S.M.; Alshammari, F.N.; Kamal, M.; Alam, O.; Asdaq, S.M.B.; Alzahrani, A.K.; et al. Nigella sativa L. and COVID-19: A glance at the anti-COVID-19 chemical constituents, clinical trials, inventions, and patent literature. Molecules 2022, 27, 2750. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).