Disinfectants against African Swine Fever: An Updated Review
Abstract
1. Introduction
2. Results
2.1. Methods for Testing the Virucidal Activity of Disinfectants
2.2. Chemical Compounds Tested against ASFV
2.2.1. Acids
2.2.2. Alkalis
2.2.3. Aldehydes
2.2.4. Chlorine and Chlorine Compounds
2.2.5. Iodine Compounds
2.2.6. Oxidizing Agents
2.2.7. Phenol Compounds
2.2.8. Quaternary Ammonium Compounds
2.2.9. Plant Extracts
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, L.K.; Abrams, C.C.; Bowick, G.; Goatley, L.C.; Kay-Jackson, P.C.; Chapman, D.; Liverani, E.; Nix, R.; Silk, R.; Zhang, F. African Swine Fever Virus Proteins Involved in Evading Host Defence Systems. Vet. Immunol. Immunopathol. 2004, 100, 117–134. [Google Scholar] [CrossRef]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef]
- Andrés, G.; García-Escudero, R.; Simón-Mateo, C.; Viñuela, E. African Swine Fever Virus Is Enveloped by a Two-Membraned Collapsed Cisterna Derived from the Endoplasmic Reticulum. J. Virol. 1998, 72, 8988–9001. [Google Scholar] [CrossRef]
- Breese, S.S.; Stone, S.S.; Deboer, C.J.; Hess, W.R. Electron Microscopy of the Interaction of African Swine Fever Virus with Ferritin-Conjugated Antibody. Virology 1967, 31, 508–513. [Google Scholar] [CrossRef]
- Carrascosa, J.L.; Carazo, J.M.; Carrascosa, A.L.; García, N.; Santisteban, A.; Viñuela, E. General Morphology and Capsid Fine Structure of African Swine Fever Virus Particles. Virology 1984, 132, 160–172. [Google Scholar] [CrossRef]
- Qu, H.; Ge, S.; Zhang, Y.; Wu, X.; Wang, Z. A Systematic Review of Genotypes and Serogroups of African Swine Fever Virus. Virus Genes 2022, 58, 77–87. [Google Scholar] [CrossRef]
- Bastos, A.D.S.; Penrith, M.L.; Crucière, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; Thomson, G.R. Genotyping Field Strains of African Swine Fever Virus by Partial P72 Gene Characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef]
- Lubisi, B.A.; Bastos, A.D.S.; Dwarka, R.M.; Vosloo, W. Molecular Epidemiology of African Swine Fever in East Africa. Arch. Virol. 2005, 150, 2439–2452. [Google Scholar] [CrossRef]
- Boshoff, C.I.; Bastos, A.D.S.; Gerber, L.J.; Vosloo, W. Genetic Characterisation of African Swine Fever Viruses from Outbreaks in Southern Africa (1973–1999). Vet. Microbiol. 2007, 121, 45–55. [Google Scholar] [CrossRef]
- Achenbach, J.E.; Gallardo, C.; Nieto-Pelegrín, E.; Rivera-Arroyo, B.; Degefa-Negi, T.; Arias, M.; Jenberie, S.; Mulisa, D.D.; Gizaw, D.; Gelaye, E.; et al. Identification of a New Genotype of African Swine Fever Virus in Domestic Pigs from Ethiopia. Transbound. Emerg. Dis. 2017, 64, 1393–1404. [Google Scholar] [CrossRef]
- Quembo, C.J.; Jori, F.; Vosloo, W.; Heath, L. Genetic Characterization of African Swine Fever Virus Isolates from Soft Ticks at the Wildlife/Domestic Interface in Mozambique and Identification of a Novel Genotype. Transbound. Emerg. Dis. 2018, 65, 420–431. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Woźniakowski, G. The Unique Genetic Variation within the O174L Gene of Polish Strains of African Swine Fever Virus Facilitates Tracking Virus Origin. Arch. Virol. 2019, 164, 1667–1672. [Google Scholar] [CrossRef]
- Thanh, T.H.T.; Duc, T.A.; Viet, L.D.; Van Tuan, H.; Thi, N.C.; Thi, C.N.; Thi, N.H.; Vu, D.H. Rapid Identification for Serotyping of African Swine Fever Virus Based on the Short Fragment of the EP402R Gene Encoding for CD2-Like Protein. Acta Vet. Brno. 2021, 71, 98–106. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Q.; Lin, P.; Liu, C.; Li, L.; Wu, X.; Chi, T.; Xu, T.; Ge, S.; Liu, Y.; et al. Genome Comparison of African Swine Fever Virus China/2018/AnhuiXCGQ Strain and Related European P72 Genotype II Strains. Transbound. Emerg. Dis. 2019, 66, 1167–1176. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Zhang, X.; He, S.; Chen, Y.; Liu, X.; Guo, C. Genetic Characterization and Variation of African Swine Fever Virus China/GD/2019 Strain in Domestic Pigs. Pathogens 2022, 11, 97. [Google Scholar] [CrossRef]
- Mulumba-Mfumu, L.K.; Saegerman, C.; Dixon, L.K.; Madimba, K.C.; Kazadi, E.; Mukalakata, N.T.; Oura, C.A.L.; Chenais, E.; Masembe, C.; Ståhl, K.; et al. African Swine Fever: Update on Eastern, Central and Southern Africa. Transbound. Emerg. Dis. 2019, 66, 1462–1480. [Google Scholar] [CrossRef]
- Penrith, M.L. History of ‘Swine Fever’ in Southern Africa. J. S. Afr. Vet. Assoc. 2013, 84, 6. [Google Scholar] [CrossRef]
- Njau, E.P.; Machuka, E.M.; Cleaveland, S.; Shirima, G.M.; Kusiluka, L.J.; Okoth, E.A.; Pelle, R. African Swine Fever Virus (ASFV): Biology, Genomics and Genotypes Circulating in Sub-Saharan Africa. Viruses 2021, 13, 2285. [Google Scholar] [CrossRef]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Ståhl, K. Epidemiological Considerations on African Swine Fever in Europe 2014–2018. Porc. Health Manag. 2019, 5, 6. [Google Scholar] [CrossRef]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African Swine Fever Status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef]
- Dixon, L.K.; Sun, H.; Roberts, H. African Swine Fever. Antiviral Res. 2019, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Franzke, K.; Beer, M. African Swine Fever—A Review of Current Knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Animal Disease Information System (ADIS). Available online: https://ec.europa.eu/food/animals/animal-diseases/animal-disease-information-system-adis_en (accessed on 16 March 2022).
- Pereira De Oliveira, R.; Hutet, E.; Lancelot, R.; Paboeuf, F.; Duhayon, M.; Boinas, F.; Pérez de León, A.A.; Filatov, S.; Le Potier, M.F.; Vial, L. Differential Vector Competence of Ornithodoros Soft Ticks for African Swine Fever Virus: What If It Involves More than Just Crossing Organic Barriers in Ticks? Parasites Vectors 2020, 13, 618. [Google Scholar] [CrossRef] [PubMed]
- Jori, F.; Bastos, A.D.S. Role of Wild Suids in the Epidemiology of African Swine Fever. Ecohealth 2009, 6, 296–310. [Google Scholar] [CrossRef]
- Jori, F.; Vial, L.; Penrith, M.L.; Pérez-Sánchez, R.; Etter, E.; Albina, E.; Michaud, V.; Roger, F. Review of the Sylvatic Cycle of African Swine Fever in Sub-Saharan Africa and the Indian Ocean. Virus Res. 2013, 173, 212–227. [Google Scholar] [CrossRef]
- De Lorenzi, G.; Borella, L.; Alborali, G.L.; Prodanov-Radulović, J.; Štukelj, M.; Bellini, S. African Swine Fever: A Review of Cleaning and Disinfection Procedures in Commercial Pig Holdings. Res. Vet. Sci. 2020, 132, 262–267. [Google Scholar] [CrossRef]
- Holl, H.; Youngner, J.S. Virus-Lipid Interactions. II. The Mechanism of Adsorption of Lipophilic Viruses to Water-Insoluble Polar Lipids. Virology 1959, 8, 319–343. [Google Scholar] [CrossRef]
- EN 14675:2015; Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Veterinary Area. CEN/TC 216—Chemical Disinfectants and Antiseptics. Available online: https://standards.iteh.ai/catalog/standards/cen/a4bf27b1-bb63-4574-9c27-89b4fa6eb4f9/en-14675-2015 (accessed on 16 March 2022).
- ASTM E1053-20; Standard Practice to Assess Virucidal Activity of Chemicals Intended for Disinfection of Inanimate, Nonporous Environmental Surfaces. Book of Standards, Developed by Subcommittee: E35.15. 2020. Available online: https://webstore.ansi.org/standards/astm/astme105320 (accessed on 16 March 2022).
- OECD Guidance Document on Quantitative Methods for Evaluating the Activity of Microbicides Used on Hard Non-Porous Surfaces-OECD. Available online: https://www.oecd.org/chemicalsafety/testing/evaluating-the-activity-of-microbicides-used-on-hard-non-porous-surfaces.htm (accessed on 16 March 2022).
- Krug, P.W.; Lee, L.J.; Eslami, A.C.; Larson, C.R.; Rodriguez, L. Chemical Disinfection of High-Consequence Transboundary Animal Disease Viruses on Nonporous Surfaces. Biologicals 2011, 39, 231–235. [Google Scholar] [CrossRef]
- Krug, P.W.; Larson, C.R.; Eslami, A.C.; Rodriguez, L.L. Disinfection of Foot-and-Mouth Disease and African Swine Fever Viruses with Citric Acid and Sodium Hypochlorite on Birch Wood Carriers. Vet. Microbiol. 2012, 156, 96–101. [Google Scholar] [CrossRef]
- Krug, P.W.; Davis, T.; O’Brien, C.; LaRocco, M.; Rodriguez, L.L. Disinfection of Transboundary Animal Disease Viruses on Surfaces Used in Pork Packing Plants. Vet. Microbiol. 2018, 219, 219–225. [Google Scholar] [CrossRef]
- Gebel, J.; Exner, M.; French, G.; Chartier, Y.; Christiansen, B.; Gemein, S.; Goroncy-Bermes, P.; Hartemann, P.; Heudorf, U.; Kramer, A.; et al. The Role of Surface Disinfection in Infection Prevention. GMS Hyg. Infect. Control 2013, 8, Doc10. [Google Scholar] [CrossRef] [PubMed]
- EN 14885:2018; Chemical Disinfectants and Antiseptics—Application of European Standards for Chemical Disinfectants and Antiseptics. CEN/TC 216—Chemical Disinfectants and Antiseptics. Available online: https://standards.iteh.ai/catalog/standards/cen/25a6be49-689b-4ac0-b0fc-0f06f0dadf60/en-14885-2018 (accessed on 16 March 2022).
- Tarka, P.; Nitsch-Osuch, A. Evaluating the Virucidal Activity of Disinfectants According to European Union Standards. Viruses 2021, 13, 534. [Google Scholar] [CrossRef] [PubMed]
- UNI EN 17122:2020; Chemical Disinfectants and Antiseptics—Quantitative Non-Porous Surface Test for the Evaluation of Virucidal Activity of Chemical Disinfectants and Antiseptics Used in the Veterinary Area. CEN/TC 216—Chemical Disinfectants and Antiseptics. Available online: https://store.uni.com/p/UNI1607369/uni-en-171222020-294125/UNI1607369_EEN (accessed on 16 March 2022).
- Gabbert, L.R.; Neilan, J.G.; Rasmussen, M. Recovery and Chemical Disinfection of Foot-and-Mouth Disease and African Swine Fever Viruses from Porous Concrete Surfaces. J. Appl. Microbiol. 2020, 129, 1092–1101. [Google Scholar] [CrossRef]
- Turner, C.; Williams, S.M. Laboratory-Scale Inactivation of African Swine Fever Virus and Swine Vesicular Disease Virus in Pig Slurry. J. Appl. Microbiol. 1999, 87, 148–157. [Google Scholar] [CrossRef]
- Shirai, J.; Kanno, T.; Tsuchiya, Y.; Mitsubayashi, S.; Seki, R. Effects of Chlorine, Iodine, and Quaternary Ammonium Compound Disinfectants on Several Exotic Disease Viruses. J. Vet. Med. Sci. 2000, 62, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.H.; Kim, S.; Kang, Y.E.; Han, B.; Seo, S.J.; Kim, Y.W.; Her, M.; Jeong, W. Virucidal Efficacy of Acidic Electrolyzed Water (Aew) against African Swine Fever Virus and Avian Influenza Virus. J. Vet. Med. Sci. 2021, 83, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sovijit, W.; Taesuji, M.; Rattanamas, K.; Punyadarsaniya, D.; Mamom, T.; Nguyen, H.T.; Ruenphet, S. In Vitro Cytotoxicity and Virucidal Efficacy of Potassium Hydrogen Peroxymonosulfate Compared to Quaternary Ammonium Compound under Various Concentrations, Exposure Times and Temperatures against African Swine Fever Virus. Vet. World 2021, 14, 2936–2940. [Google Scholar] [CrossRef]
- Kalmar, I.D.; Cay, A.B.; Tignon, M. Sensitivity of African Swine Fever Virus (ASFV) to Heat, Alkalinity and Peroxide Treatment in Presence or Absence of Porcine Plasma. Vet. Microbiol. 2018, 219, 144–149. [Google Scholar] [CrossRef]
- Juszkiewicz, M.; Walczak, M.; Mazur-Panasiuk, N.; Woźniakowski, G. Effectiveness of Chemical Compounds Used against African Swine Fever Virus in Commercial Available Disinfectants. Pathogens 2020, 9, 878. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Wang, W.; Sun, Y.; Zhang, J.; Fatima, M.; Jia, X.; Qiu, H.J. Efficient Inactivation of African Swine Fever Virus by Ozonized Water. Vet. Microbiol. 2020, 247, 108796. [Google Scholar] [CrossRef]
- Heckert, R.A.; Best, M.; Jordan, L.T.; Dulac, G.C.; Eddington, D.L.; Sterritt, W.G. Efficacy of Vaporized Hydrogen Peroxide against Exotic Animal Viruses. Appl. Environ. Microbiol. 1997, 63, 3916–3918. [Google Scholar] [CrossRef] [PubMed]
- Juszkiewicz, M.; Walczak, M.; Mazur-Panasiuk, N.; Woźniakowski, G. Virucidal Effect of Chosen Disinfectants against African Swine Fever Virus (ASFV)—Preliminary Studies. Pol. J. Vet. Sci. 2019, 22, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.S.; Hess, W.R. Effects of Some Disinfectants on African Swine Fever Virus. Appl. Microbiol. 1973, 25, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Taesuji, M.; Rattanamas, K.; Punyadarsaniya, D.; Mamom, T.; Nguyen, H.T.; Ruenphet, S. In Vitro Primary Porcine Alveolar Macrophage Cell Toxicity and African Swine Fever Virus Inactivation Using Five Commercially Supply Compound Disinfectants under Various Condition. J. Vet. Med. Sci. 2021, 83, 1800–1804. [Google Scholar] [CrossRef]
- Pan, L.; Luo, R.; Wang, T.; Qi, M.; Wang, B.; Sun, M.; Luo, Y.; Ji, C.; Sun, Y.; Qiu, H.J. Efficient Inactivation of African Swine Fever Virus by a Highly Complexed Iodine. Vet. Microbiol. 2021, 263, 109245. [Google Scholar] [CrossRef]
- Juszkiewicz, M.; Walczak, M.; Woźniakowski, G.; Szczotka-Bochniarz, A. Virucidal Activity of Plant Extracts against African Swine Fever Virus. Pathogens 2021, 10, 1357. [Google Scholar] [CrossRef]
- Haas, B.; Ahl, R.; Böhm, R.; Strauch, D. Inactivation of Viruses in Liquid Manure. Rev. Sci. Tech. 1995, 14, 435–445. [Google Scholar] [CrossRef]
- De Benedictis, P.; Beato, M.S.; Capua, I. Inactivation of Avian Influenza Viruses by Chemical Agents and Physical Conditions: A Review. Zoonoses Public Health 2007, 54, 51–68. [Google Scholar] [CrossRef]
- Animal Health Australia AUSVETPLAN Decontamination Manual v3.2. 2008. Available online: file:///C:/Users/msbeato/Desktop/AVP_Decon_v3.2_2008-1.pdf (accessed on 16 March 2022).
- Cunliffe, H.R.; Blackwell, J.H.; Walker, J.S. Glutaraldehyde Inactivation of Exotic Animal Viruses in Swine Heart Tissue. Appl. Environ. Microbiol. 1979, 37, 1044–1046. [Google Scholar] [CrossRef]
- Maris, P. Modes of Action of Disinfectants. Rev. Sci. Tech. 1995, 14, 47–55. [Google Scholar] [CrossRef]
- Bui, V.N.; Nguyen, K.V.; Pham, N.T.; Bui, A.N.; Dao, T.D.; Nguyen, T.T.; Nguyen, H.T.; Trinh, D.Q.; Inui, K.; Uchiumi, H.; et al. Potential of Electrolyzed Water for Disinfection of Foot-and-Mouth Disease Virus. J. Vet. Med. Sci. 2017, 79, 726–729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Severing, A.L.; Rembe, J.D.; Koester, V.; Stuermer, E.K. Safety and Efficacy Profiles of Different Commercial Sodium Hypochlorite/Hypochlorous Acid Solutions (NaClO/HClO): Antimicrobial Efficacy, Cytotoxic Impact and Physicochemical Parameters in Vitro. J. Antimicrob. Chemother. 2019, 74, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.; Muriana, P.M. Evaluation of Electrolytically-Generated Hypochlorous Acid (‘Electrolyzed Water’) for Sanitation of Meat and Meat-Contact Surfaces. Foods 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- OIE. Terrestrial Animal Health Code: General Provisions; OIE: Paris, France, 2019; Volume 1, ISBN 9789295108851. [Google Scholar]
- FAO. FAO Biosecurity Toolkit. In Portal; FAO: Roma, Italy, 2007; 128p. [Google Scholar]
- Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on Transmissible Animal Diseases and Amending and Repealing Certain Acts in the Area of Animal Health (‘Animal Health Law’) Official Journal of the European Union. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2016.084.01.0001.01.ENG (accessed on 16 March 2022).
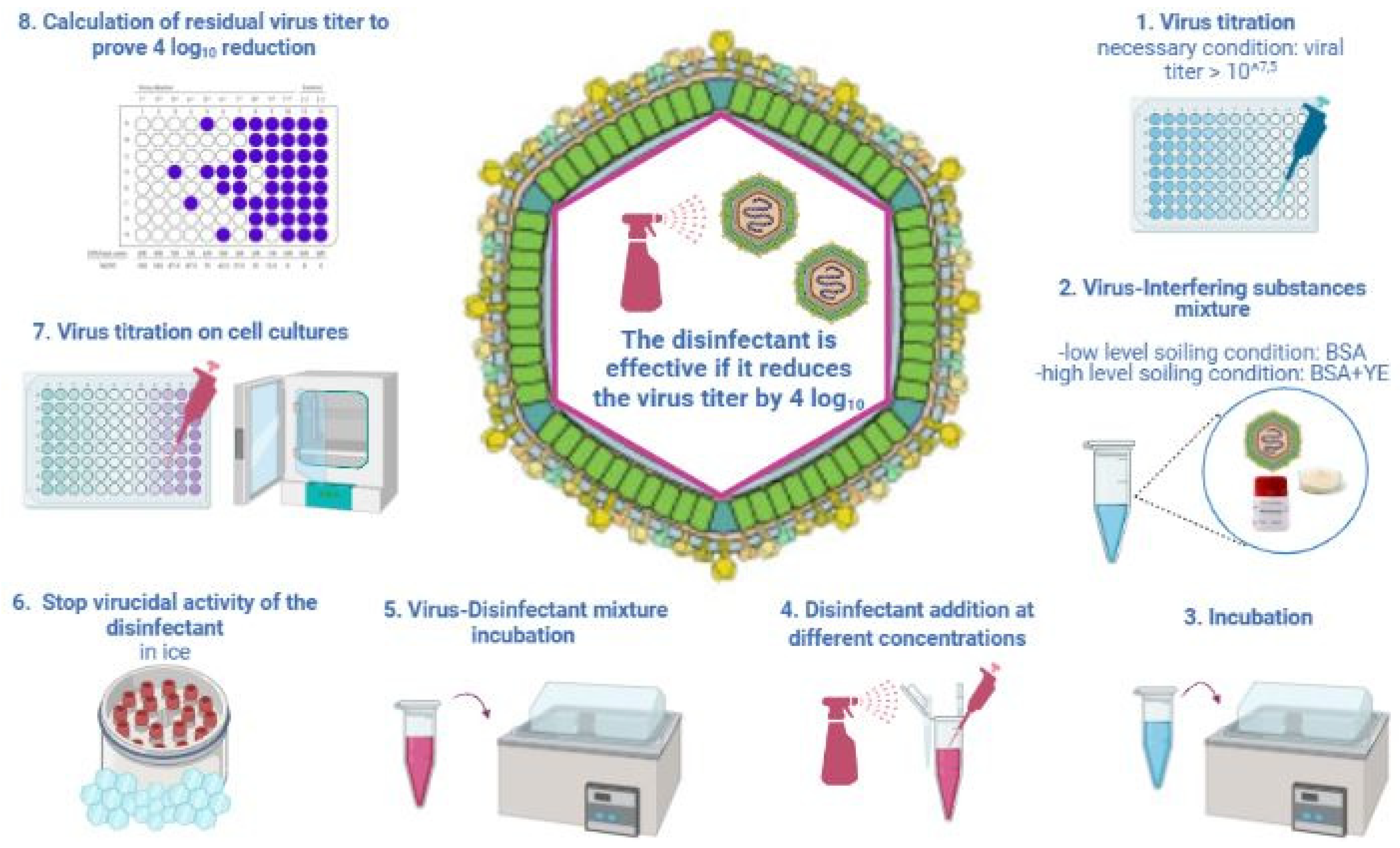
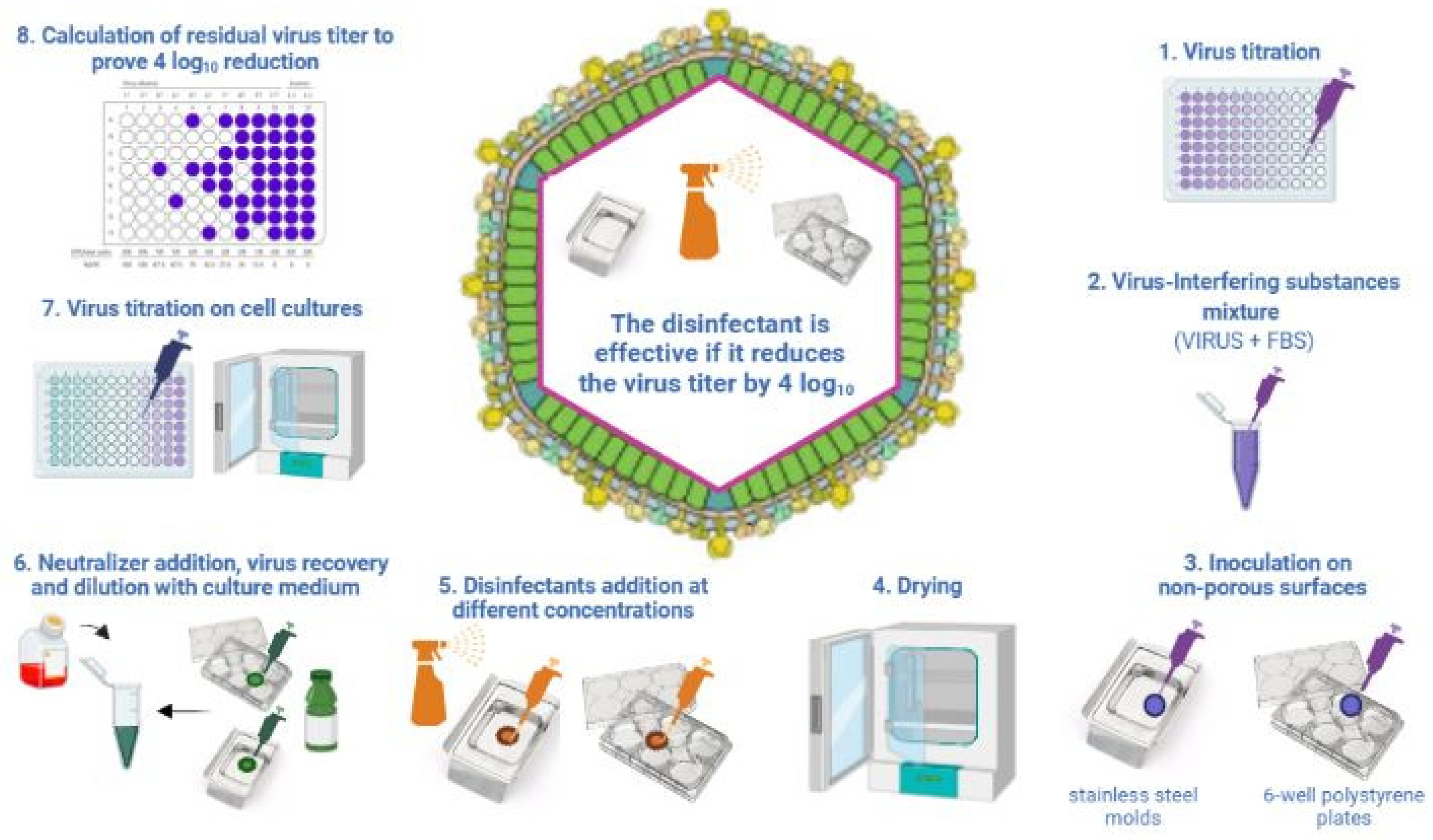
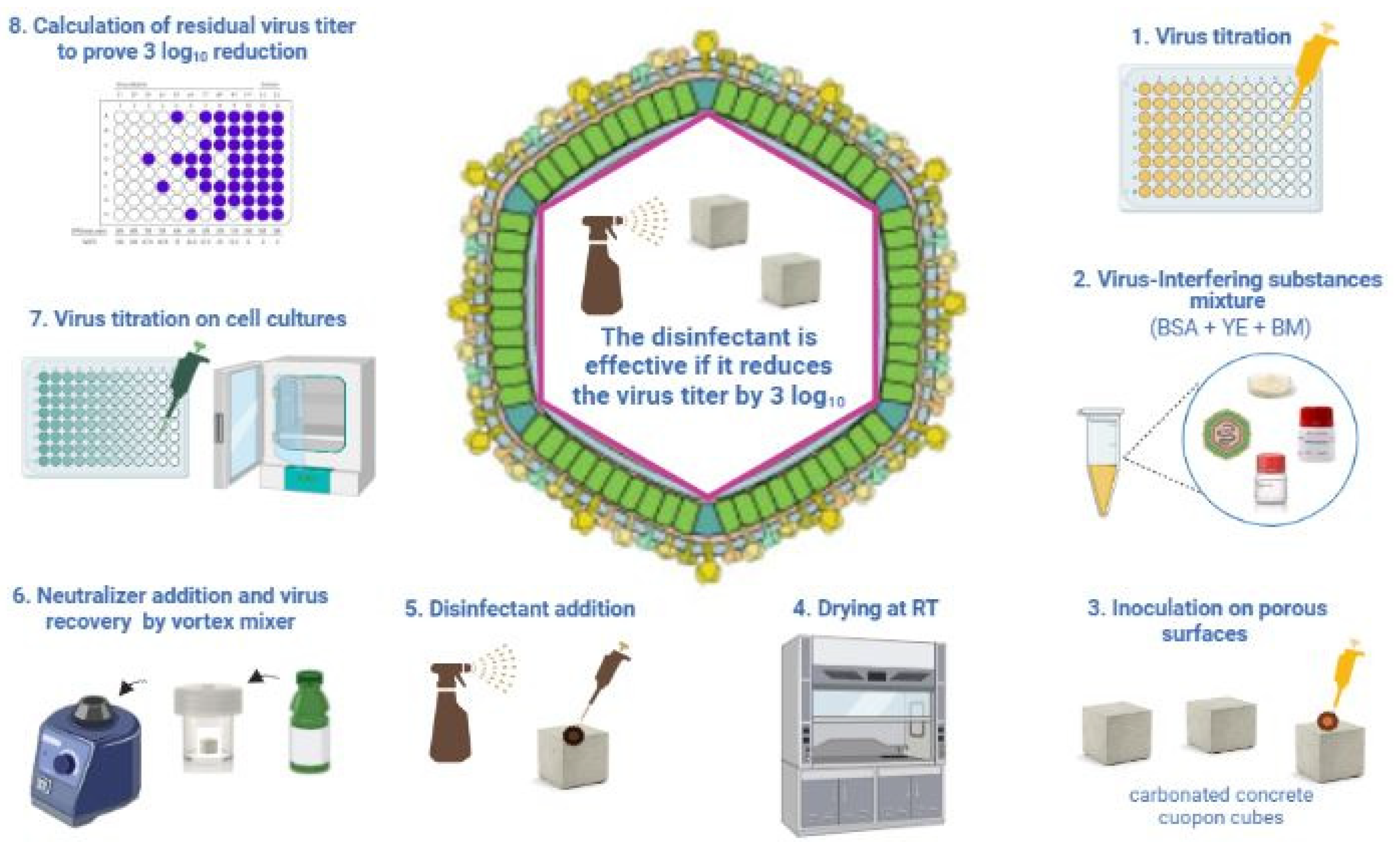
| UNI EN 14675:2015 Quantitative Suspension Test (Phase 2/Step 1) | OECD 2013 Carrier Test (Disks of Stainless Steel) | ASTM E1053-20 Carrier Test (Glass Petri Dishes) | |
|---|---|---|---|
| Virus Volume | 1000 µL | 10 µL | 200 µL |
| Disinfectant Volume | 8000 µL | 50 µL | 2000 µL |
| Ratio | 1:8 | 1:5 | 1:10 |
| Interfering Substances | 1% BSA + 1% YE OR ONLY 0.3% BSA | 5% YE + 5% BSA + 5% BM | NOT REQUIRED |
| Contact Time and Temperature | 30 min ± 10 s + 10 °C ± 1 °C | LABEL INDICATION + 20–25 °C | LABEL INDICATION + 20–25 °C |
| Water | 400 ppm | 338–394 ppm | 400 ppm |
| Virus Titer Reduction | 4 log10 | 3 log10 | 4 log10 |
| Chemical Group/Active Substance | Active Concentration | Contact Time (min) | Temperature (°C) | Virus | Genotype | Conditions | Test Method | Paper | Recommended Use |
|---|---|---|---|---|---|---|---|---|---|
| Alkalis | |||||||||
| Sodium hydroxide | 1% | 5 | 4 | Lilongwe 20/1 | VIII | Pig slurry | Suspension | Turner et al., 1999 [40] | Not efficacious at room temperature. Do not use in the presence of aluminium and derived alloys |
| 2 and 3% | 30 | 10 | BA71V | I | BSA; BSA + YE | UNI EN 14675:2015 | Juszkiewicz et al., 2020 [45] | ||
| Calcium hydroxide | 1% | 5 | 4 | Lilongwe 20/1 | VIII | Pig slurry | Suspension | Turner et al., 1999 [40] | Use for walls and floors |
| Acids | |||||||||
| Acetic acid | 1 | 10 | 22 | BA71V | I | Steel and plastic | ASTM E1053-20 modified | Krug et al., 2011 [32] | |
| 2% | 30 | 10 | BA71V | I | BSA; BSA + YE | UNI EN 14675:2015 | Juszkiewicz et al., 2020 [45] | ||
| Citric acid | 1 and 2% | 10 | 22 | BA71V | I | Plastic | ASTM E1053-20 modified | Krug et al., 2011 [32] | Safe for clothes and body decontamination |
| 1% | 10 | 22 | BA71V | I | Steel | ASTM E1053-20 modified | Krug et al., 2011 [32] | ||
| 2% | 30 | 22 | BA71V | I | Birch wood veneer | ASTM E1053-20 modified | Krug et al., 2012 [33] | ||
| Chlorine Compounds | |||||||||
| Sodium hypoclorite | 500 ppm | 10 | 22 | BA71V | I | Steel and plastic | ASTM E1053-20 modified | Krug et al., 2011 [33] | Effective for most applications, decreased efficacy in presence of organic material. Less stable in warm, sunny conditions above +15 °C. Toxic for eyes and skin |
| 2000 ppm | 30 | 22 | BA71V | I | Birch wood veneer | ASTM E1053-20 modified | Krug et al., 2012 [33] | ||
| 6% | 30 | RT | Lisbon 60 | I | None | Suspension | Shirai et al., 2000 [41] | ||
| 1% | 30 | 10 | BA71V | I | BSA, BSA + YE | UNI EN 14675:2015 | Juszkiewicz et al., 2020 [45] | ||
| Acidic electrolyzed water | 80 ppm | 30 | 4 | BA71V | I | 5% FBS | Suspension | Rhee et al., 2021 [42] | |
| Oxiding Agents | |||||||||
| Ozonized water (O3) | 20 mg/L | 10 | 20–25 | SY 18 | II | None | Suspension | Zhang et al., 2020 [46] | |
| Potassium hydrogen | 600 ppm | 10 | RT | BA71V | I | Steel, plastic, concrete | ASTM E1053-20 modified | Krug et al., 2018 [34] | Use for laboratory equipment. Excellent disinfectant active against all viruses and bacteria. Mildly corrosive for many metals |
| 1/200 | 30 | 20 | VNUA-ASFV-L01/HN/04/19 | II | None | Suspension | Sovijit et al., 2021 [43] | ||
| 1/200 | 30 | 4 and 20 | VNUA-ASFV-L01/HN/04/19 | II | None | Suspension | Sovijit et al., 2021 [43] | ||
| 1% | 30 | 10 | BA71V | I | BSA, BSA + YE | UNI EN 14675:2015 | Juszkiewicz et al., 2020 [45] | ||
| 2 and 5% | 5, 10 | 20–25 | BA71V | I | BSA + YE + BM | OECD 2013 | Gabbert et al., 2020 [39] | ||
| Vaporized hydrogen peroxide | 30% | 30 | 30–40 | Lisbon 61 | I | 5% FBS | Vaporization | Heckert et al., 1997 [47] | Use for laboratory equipment |
| Hydrogen peroxide | 102.6 mM (35% stock solution) | 10 | 48 | Lisbon 60 | I | Plasma | Suspension | Kalmar et al., 2018 [44] | Use for laboratory equipment. Rinse after use |
| Aldehydes | |||||||||
| Glutaraldehyde | 0.1% | 30 | 10 | BA71V | I | BSA + YE | UNI EN 14675:2015 | Juszkiewicz et al., 2019 [48] | Excellent disinfectant effective against all viruses and bacteria. Avoid eye and skin contact |
| 1% | 30 | 10 | BA71V | I | BSA | UNI EN 14675:2015 | Juszkiewicz et al., 2020 [45] | ||
| Phenol Compounds | |||||||||
| Phenol | 1% | 30 | 10 | BA71V | I | BSA + YE | UNI EN 14675:2015 | Juszkiewicz et al., 2020 [45] | Efficacious in the presence of organic material. Rinse after use |
| o-Phenilphenol | 0.5% | 60 | RT | Lisbon 60 | I | None | In vivo test | Stone and Hess 1973 [49] | |
| Quaternary Ammonium Compounds | |||||||||
| Benzalkonium chloride | 1% | 30 | 10 | BA71V | I | BSA | UNI EN 14675:2015 | Juszkiewicz et al., 2020 [45] | Recommended for pesonal use. Do not use with hard water |
| Quaternary ammonium | 1/200 | 1 | 4 | VNUA-ASFV-L01/HN/04/19 | II | None | Suspension | Sovijit et al., 2021 [43] | |
| Didecyldimethylammonium chloride | 10% | 30 | RT | Lisbon 60 | I | None | Suspension | Shirai et al., 2000 [41] | |
| 0.09%-0.0275%/0.1% | 30 | 4 and 20 | VNUA-ASFV-L01/HN/04/19 | II | None | Suspension | Taesuji et al., 2021 [50] | ||
| Quaternary ammonia | 800 ppm | 10 | RT | BA71V | I | Steel, plastic, concrete | ASTM E1053-20 modified | Krug et al., 2018 [34] | |
| Iodine Compounds | |||||||||
| Povidone-iodine (5% iodine content) | 5% | 15 | RT | ASFV pig/HLJ/18 | II | None | Spray | Pan et al., 2021 [51] | |
| Potassium tetraglicine triiodide | 3% | 30 | RT | Lisbon 60 | I | None | Suspension | Shirai et al., 2000 [41] | |
| Plant Extracts | |||||||||
| Peppermint | 30% | 30 | 10 | BA71V | I | BSA; BSA + YE | UNI EN 14675:2015 | Juszkiewicz et al., 2021 [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beato, M.S.; D’Errico, F.; Iscaro, C.; Petrini, S.; Giammarioli, M.; Feliziani, F. Disinfectants against African Swine Fever: An Updated Review. Viruses 2022, 14, 1384. https://doi.org/10.3390/v14071384
Beato MS, D’Errico F, Iscaro C, Petrini S, Giammarioli M, Feliziani F. Disinfectants against African Swine Fever: An Updated Review. Viruses. 2022; 14(7):1384. https://doi.org/10.3390/v14071384
Chicago/Turabian StyleBeato, Maria Serena, Federica D’Errico, Carmen Iscaro, Stefano Petrini, Monica Giammarioli, and Francesco Feliziani. 2022. "Disinfectants against African Swine Fever: An Updated Review" Viruses 14, no. 7: 1384. https://doi.org/10.3390/v14071384
APA StyleBeato, M. S., D’Errico, F., Iscaro, C., Petrini, S., Giammarioli, M., & Feliziani, F. (2022). Disinfectants against African Swine Fever: An Updated Review. Viruses, 14(7), 1384. https://doi.org/10.3390/v14071384







