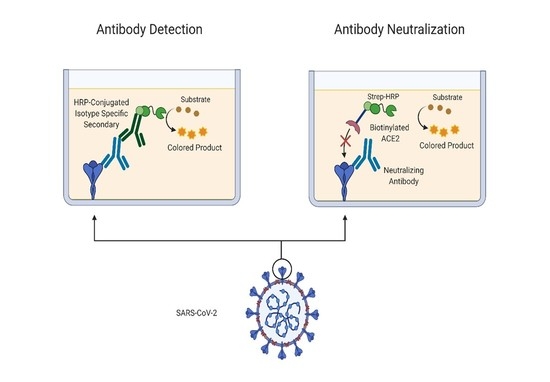
Dynamic Assay for Profiling Anti-SARS-CoV-2 Antibodies and Their ACE2/Spike RBD Neutralization Capacity
Abstract
:1. Introduction
2. Materials and Methods
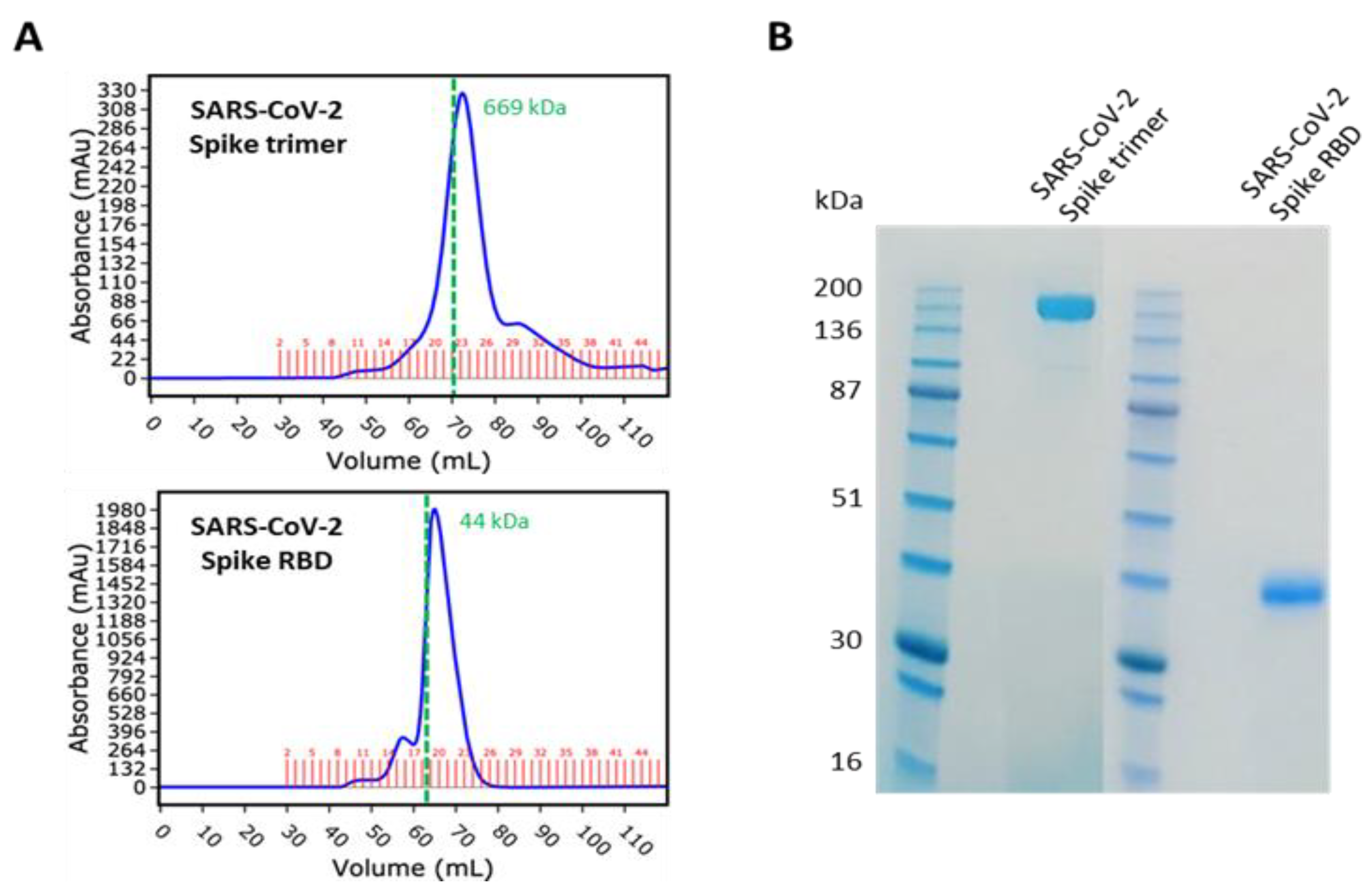
2.1. Recombinant Proteins
2.2. Human Samples
2.3. ELISA
2.4. In Vitro ACE2 Binding Assay
2.5. Pseudovirus Infection Assay
2.6. Statistical Analysis
3. Results
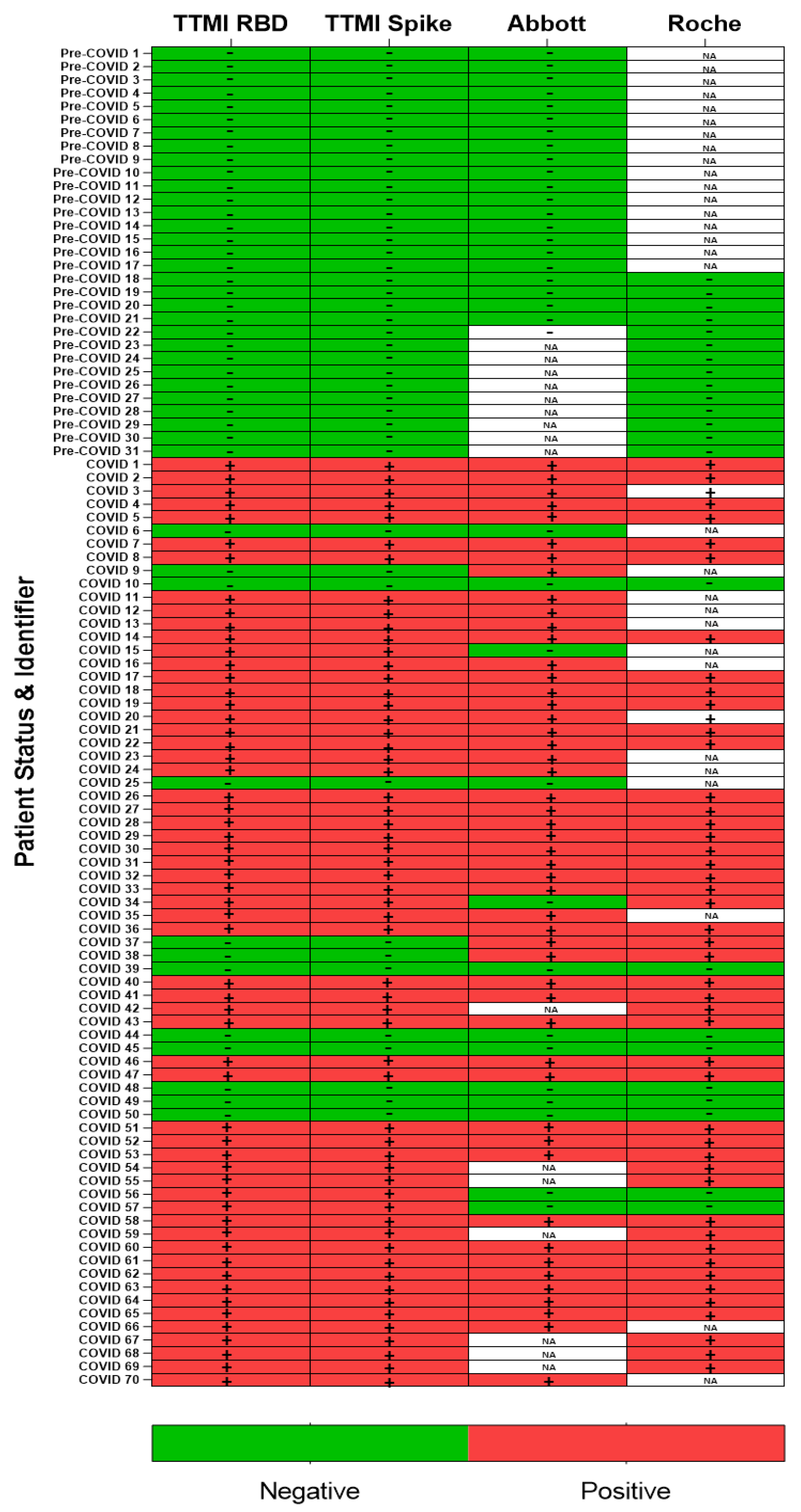
3.1. A SARS-CoV-2 Serological Assay Based on the Spike Protein
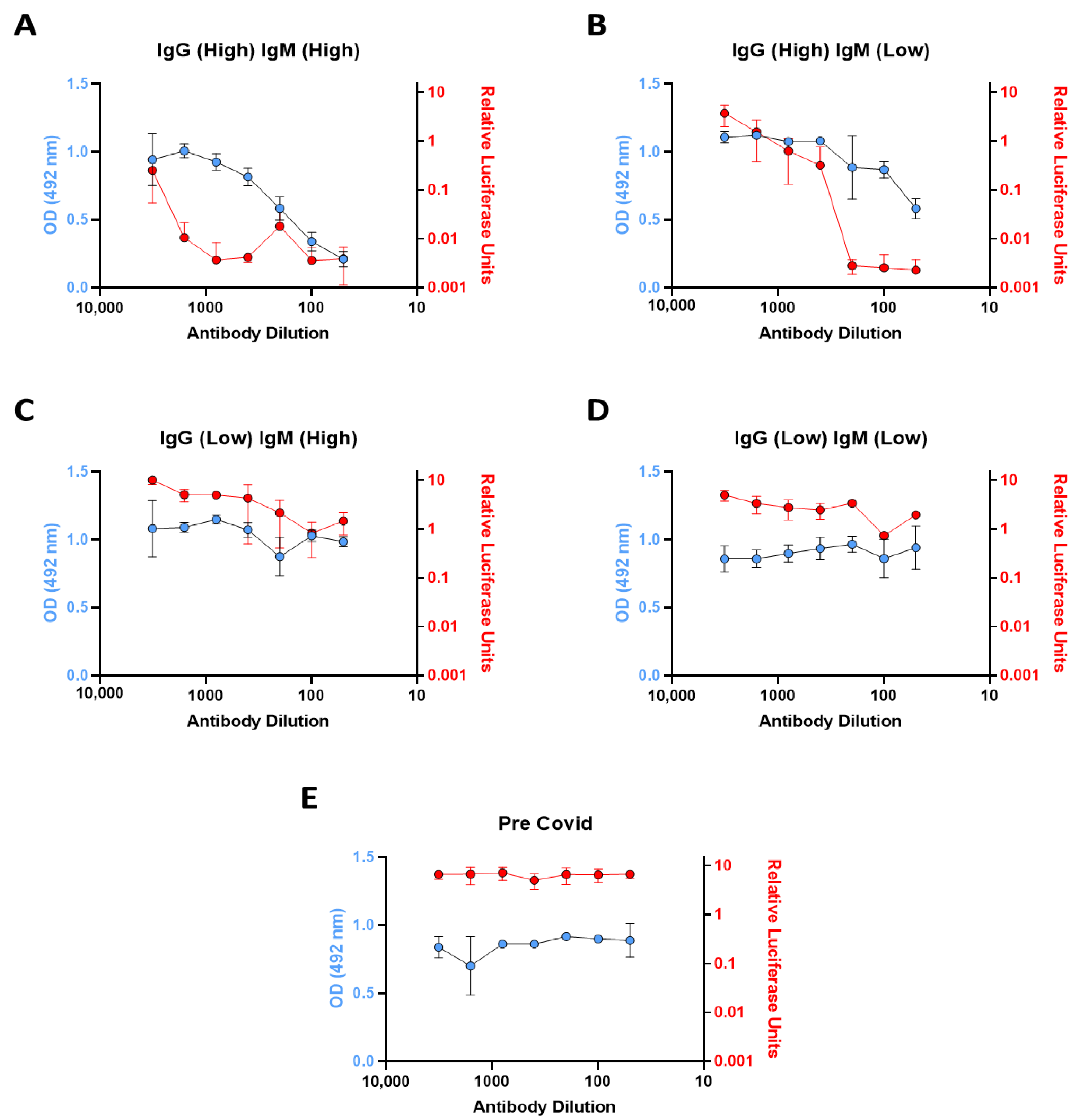
3.2. In Vitro ACE2 Binding Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 24 May 2021).
- U.S. Food & Drug Administration. EUA Authorized Serology Test Performance. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance (accessed on 21 May 2021).
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Hanson, Q.M.; Wilson, K.M.; Shen, M.; Itkin, Z.; Eastman, R.T.; Shinn, P.; Hall, M.D. Targeting ACE2–RBD Interaction as a Platform for COVID-19 Therapeutics: Development and Drug-Repurposing Screen of an AlphaLISA Proximity Assay. ACS Pharmacol. Transl. Sci. 2020, 3, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Thapa, M.; Lei, T.; Ahmed, S.M.S.; Adelsberg, D.C.; Carreno, J.M.; Strohmeier, S.; Schmitz, A.J.; Zafar, S.; Zhou, J.Q.; et al. The plasmablast response to SARS-CoV-2 mRNA vaccination is dominated by non-neutralizing antibodies that target both the NTD and the RBD. medRxiv 2021. [Google Scholar] [CrossRef]
- Conceicao, C.; Thakur, N.; Human, S.; Kelly, J.T.; Logan, L.; Bialy, D.; Bhat, S.; Stevenson-Leggett, P.; Zagrajek, A.K.; Hollinghurst, P.; et al. The SARS-CoV-2 Spike protein has a broad tropism for mammalian ACE2 proteins. PLoS Biol. 2020, 18, e3001016. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, D.; Tan, J.; Jiang, K.; Hernandez, M.M.; Fabre, S.; Amanat, F.; Teo, C.; Arunkumar, G.A.; McMahon, M.; Capuano, C.; et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature 2021, 590, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Kweon, O.J.; Lim, Y.K.; Kim, H.R.; Kim, M.C.; Choi, S.H.; Chung, J.W.; Lee, M.K. Antibody kinetics and serologic profiles of SARS-CoV-2 infection using two serologic assays. PLoS ONE 2020, 15, e0240395. [Google Scholar] [CrossRef] [PubMed]
- Roche Diagnostics. Elecsys® Anti-SARS-CoV-2. Available online: https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html (accessed on 10 May 2021).
- Abbott. SARS-CoV-2 IgG Assay. Available online: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2 (accessed on 10 May 2021).
- Public Health England. Evaluation of Roche Elecsys Anti-SARS-CoV-2 Serology Assay for the Detection of Anti-SARS-CoV-2 Antibodies. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/891598/Evaluation_of_Roche_Elecsys_anti_SARS_CoV_2_PHE_200610_v8.1_FINAL.pdf (accessed on 10 May 2021).
- Public Health England. Evaluation of the Abbott SARS-CoV-2 IgG for the Detection of Anti-SARS-CoV-2 Antibodies. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/890566/Evaluation_of_Abbott_SARS_CoV_2_IgG_PHE.pdf (accessed on 10 May 2021).
- Chao, Y.X.; Rotzschke, O.; Tan, E.K. The role of IgA in COVID-19. Brain Behav. Immun. 2020, 87, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.S.; Zimmerman, M.G.; Kauffman, R.C.; Mantus, G.; Linderman, S.L.; Hudson, W.H.; Vanderheiden, A.; Nyhoff, L.; Davis, C.W.; Adekunle, O.; et al. Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. Cell Rep. Med. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Chen, Y.; Tong, X.; Li, Y.; Gu, B.; Yan, J.; Liu, Y.; Shen, H.; Huang, R.; Wu, C. A comprehensive, longitudinal analysis of humoral responses specific to four recombinant antigens of SARS-CoV-2 in severe and non-severe COVID-19 patients. PLoS Pathog. 2020, 16, e1008796. [Google Scholar] [CrossRef]
- Patil, H.P.; Rane, P.S.; Shrivastava, S.; Palkar, S.; Lalwani, S.; Mishra, A.C.; Arankalle, V.A. Antibody (IgA, IgG, and IgG Subtype) Responses to SARS-CoV-2 in Severe and Nonsevere COVID-19 Patients. Viral Immunol. 2021, 34, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jia, T.; Chen, J.; Zeng, S.; Qiu, Z.; Wu, S.; Li, X.; Lei, Y.; Wang, X.; Wu, W.; et al. The Characterization of Disease Severity Associated IgG Subclasses Response in COVID-19 Patients. Front. Immunol. 2021, 12, 632814. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Trimmer-Smith, L.; et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun. 2020, 11, 4704. [Google Scholar] [CrossRef]
- Deng, W.; Bao, L.; Liu, J.; Xiao, C.; Liu, J.; Xue, J.; Lv, Q.; Qi, F.; Gao, H.; Yu, P.; et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020, 369, 818–823. [Google Scholar] [CrossRef] [PubMed]

| Test | Positive | Negative | N | Odds Ratio | 95% CI | p |
|---|---|---|---|---|---|---|
| Abbott | 50 | 34 | 84 | 0.9516 | 0.5226 to 1.729 | >0.9999 |
| RBD/Spike | 51 | 33 | 84 | |||
| Roche | 46 | 23 | 69 | 1.000 | 0.4840 to 2.066 | >0.9999 |
| RBD/Spike | 46 | 23 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phelan, T.; Dunne, J.; Conlon, N.; Cheallaigh, C.N.; Abbott, W.M.; Faba-Rodriguez, R.; Amanat, F.; Krammer, F.; Little, M.A.; Hughes, G.; et al. Dynamic Assay for Profiling Anti-SARS-CoV-2 Antibodies and Their ACE2/Spike RBD Neutralization Capacity. Viruses 2021, 13, 1371. https://doi.org/10.3390/v13071371
Phelan T, Dunne J, Conlon N, Cheallaigh CN, Abbott WM, Faba-Rodriguez R, Amanat F, Krammer F, Little MA, Hughes G, et al. Dynamic Assay for Profiling Anti-SARS-CoV-2 Antibodies and Their ACE2/Spike RBD Neutralization Capacity. Viruses. 2021; 13(7):1371. https://doi.org/10.3390/v13071371
Chicago/Turabian StylePhelan, Thomas, Jean Dunne, Niall Conlon, Clíona Ní Cheallaigh, W. Mark Abbott, Raquel Faba-Rodriguez, Fatima Amanat, Florian Krammer, Mark A. Little, Gerry Hughes, and et al. 2021. "Dynamic Assay for Profiling Anti-SARS-CoV-2 Antibodies and Their ACE2/Spike RBD Neutralization Capacity" Viruses 13, no. 7: 1371. https://doi.org/10.3390/v13071371
APA StylePhelan, T., Dunne, J., Conlon, N., Cheallaigh, C. N., Abbott, W. M., Faba-Rodriguez, R., Amanat, F., Krammer, F., Little, M. A., Hughes, G., Bergin, C., Kerr, C., Sundaresan, S., Long, A., McCormack, W., & Brady, G. (2021). Dynamic Assay for Profiling Anti-SARS-CoV-2 Antibodies and Their ACE2/Spike RBD Neutralization Capacity. Viruses, 13(7), 1371. https://doi.org/10.3390/v13071371