Abstract
Gene therapy vectors derived from different viral species have become a fixture in biomedicine, both for direct therapeutic intervention and as tools to facilitate cell-based therapies, such as chimeric antigen receptor-based immunotherapies. On the contrary, extracellular vesicles have only recently gained a massive increase in interest and, concomitantly, knowledge in the field has drastically risen. Viral infections and extracellular vesicle biology overlap in many ways, both with pro- and antiviral outcomes. In this review, we take a closer look at these interactions for the most prominent groups of viral vectors (Adenoviral, Adeno-associated and Retro/Lentiviral vectors) and the possible implications of these overlaps for viral vector technology and its biomedical applications.
1. Introduction: Viral Vectors and Extracellular Vesicles
In the last decade, lipid enclosed particles of cellular origin, unable to replicate independent of their cellular source (generally termed extracellular vesicles, EVs) and produced by a wide range of cell types from all kingdoms of life, have attracted significant interest. While earlier believed to be artifacts (“cell dust”) or simple waste products, they are emerging as complex signal transduction vectors with implications for a wealth of physiological and pathological situations. Reviews on the topic are numerous [1,2,3,4,5,6,7,8,9,10]. Extracellular vesicles are produced from eukaryotic, as well as bacterial and archaeal, sources [4]. Transfer of vesicles between cells from different kingdoms is possible and may have provided an evolutionary booster. Different subtypes have been described in various size ranges and with various content types (see Figure 1 and Table 1 for more information). Amongst these, the eukaryotic exosomes seem to carry the strongest promise for biomedical applications. Differentiation between subtypes can be difficult. The immunogenicity of EVs is tunable: from inherently low (when autologous) to ensure safe and efficient delivery, to high by recombinantly displaying antigens in vaccine development strategies [11]. EVs are discussed as a means for enabling gene and drug delivery, vaccine development and novel diagnostic strategies. The latter make use of so-called liquid biopsies. These allow us to gain information on cellular or tissue states from body fluid samples, leading to reduced invasiveness and less mechanical stress at the site of, e.g., a solid tumor [12,13]. Loading of protein to EVs has been achieved, e.g., for catalase to RAW264.7 cell-derived exosomes [14], as well as for cytokines, antibody fragments, RNA binding proteins, vaccine antigens and Cas9 proteins [15]. Targeting of proteins to EV compartments is feasible by using scaffold proteins in fusion proteins [15] or by post-translational modifications [16]. Small molecules, such as the cytostatic taxane paclitaxel, have been loaded into EVs [14] as well as the cytostatic anthracycline doxirubicin [17] or the anti-inflammatory and anti-neoplastic polyphenol curcumin [18]. An interesting aspect of exosomal biomedical application is their platform nature, which allows the implementation of more than one modification on the same physical unit. In one example, exosomes were engineered to display RGD peptide motives (thus targeting αv integrins) on their surfaces and simultaneously be loaded with doxorubicin [17]. In this case, different sites on the EVs are being exploited: soluble elements are delivered to the lumen of EVs and lipophilic factors may be attached to the vesicle membrane. Display of targeting peptides has also been used in conjunction with nucleic acid delivery [19]. EVs were loaded with siRNA by electroporation and for targeting, RVG peptides were attached to the surface via fusion with an exosomal marker protein and were used to deliver siRNA to mouse brains intravenously, indicating the capability of EV delivery systems to cross the blood brain barrier and, specifically, enter neurons, microglia and oligodendrocytes in the brain [19]. Mostly, small non-coding RNA species are used in EV-based delivery strategies [20,21,22]; however, linear DNA was also reported to associate with EVs [23].
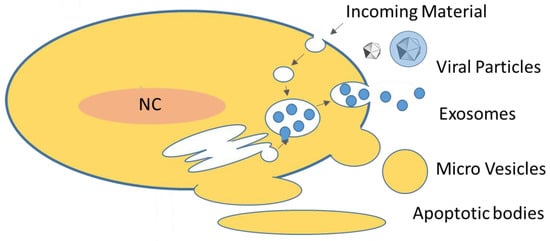
Figure 1.
Overview of extracellular vesicles in eukarya: EVs are either produced by membrane shedding (apoptotic vesicles, micro vesicles) or via the endosomal system (a crude schematic of the endosomal system is indicated). Eukaryotic cells produce mainly exosomes, micro vesicles and, under apoptotic conditions, apoptotic vesicles. Exosomes are generated as intra-luminal vesicles (ILVs) in multi-vesicluar bodies (MVB). Incoming material refers to material taken up by cells, e.g., by endocytosis. Also, EVs are entering cells by these mechanisms. For more details see text and Table 1; modified from [24].
EVs are defined as submicron lipid bilayer enclosed vesicles that are unable to replicate independently. This definition includes enveloped virus (eVI) and already hints at the close ties between VIs and EVs [24,25,26,27,28] (see also Table 1 and Figure 2). Introducing nucleic acid to target-cells and—in the case of wild type (wt) virus—replicating and ensuring expression of viral proteins is at the core of viral life cycles. Over time, due to (co-)evolution and selection of the best adapted means of replication in a specific host, a degree of technical “optimization” has been achieved that artificial or synthetic systems are lacking. Not surprisingly, biomedicine is exploiting the fact: amongst others, members of the families Retroviridae (i.e., Murine leukemia virus, MLV; Human immunodeficiency virus, HIV), Adenoviridae (i.e., adenovirus, AV) and Parvoviridae (i.e., adeno-associated viruses, AAV) have become standard tools as viral vector (VV) systems in biotechnology and biomedicine to facilitate high efficiency gene transfer in research and therapeutic settings. Therapies based on the use of VVs may either make direct use by transduction of diseased cells and redressing pathological situations from cancer to infectious diseases, or VVs may be used as tools to enable cell-based therapies, such as chimeric antigen receptor (CAR) based immune-therapies [29,30]. In addition to delivery functions, viral vectors are also used in oncolytic settings: conditional replication of VVs in tumor cells is exploited to directly attack malignancies and mount a more effective anti-tumor immune response [31,32,33,34].
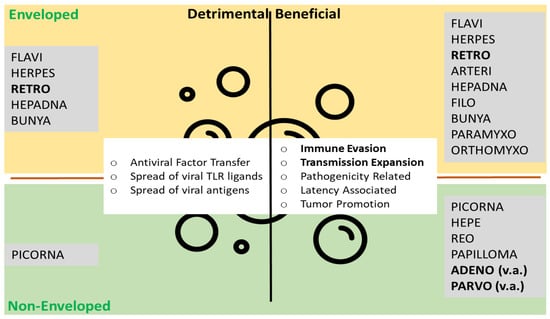
Figure 2.
Extracellular vesicles and viral particles. The top lists enveloped viruses associated with EVs, the bottom non-enveloped viruses. On the left, mechanisms detrimental for viruses (antiviral) are listed, on the right, beneficial mechanisms (proviral). Viral families giving rise to the viral vector systems discussed here in bold. Functions most useful for viral vector systems in bold. TLR toll-like receptor, v.a. vector associated. Modified from Nolte-′t Hoen et al. [26].

Table 1.
Overview of Eukaryotic Extracellular Vesicles.
Table 1.
Overview of Eukaryotic Extracellular Vesicles.
| Source | Type | Vesicle | Diameter (in nm) | Density (in g/mL) | Marker | Functions | Reference |
|---|---|---|---|---|---|---|---|
| Eukarya | Ectosomal | Microvesicles | 100–1000 | n.d. * | Integrins, selectins, CD40 | Intercellular communication, Immunity | [7] |
| Apoptotic bodies | 1000–5000 | 1.16–1.28 | Annexin V, phosphatidylserine | phagocytosis stimulation | |||
| Endosomal | Exosomes | 30–100 | 1.13–1.19 | Alix, Tsg101, tetraspanins (CD81, CD63, CD9), flotillin | Intercellular communication, Immunity | ||
| Virus-associated | Virocell vesicles | n.a. ** | n.a. ** | virus-specific | Transmission | [26,27] | |
| Viral Vesicles | n.a. ** | n.a. ** | virus-specific | Infection support | |||
| Virion Packaging vesicles | n.a. ** | n.a. ** | virus-specific | Infection support | |||
| Virus-Like Particles | n.a. ** | n.a. ** | virus-specific | Infection support | |||
| Infectious viral particles | virus-dependent | 1.1–1.2 for mammalian virus | virus-specific | Virus propagation, Cellular reprogramming |
n.a. ** depends on carrier vesicle type; n.d. * no accounts found in literature; CD cluster of differentiation; ESCRT endosomal sorting complex required for transport; Tsg101 tumor susceptibility gene 101; virocell vesicles (EVs produced by infected cells, with no viral content present, may however contain elements modified by virus activity); viral vesicles (EVs containing viral nucleic acids); virion-packaging vesicles (EVs containing virions i.e., in Hepatitis A and E); virus-like-particles (replication-incompetent virions). Infection support refers to facilitating of viral transmission by non-infectious virus-associated vesicles; modified from Metzner and Zaruba [24].
The advantages and disadvantages of the different viral vector system are well known, and selection based on application type (e.g., ex vivo vs. in vivo, transient vs. stable) allows one to choose the right viral vector for a wide range of pathologies. The advent of practical, less cumbersome gene correction or editing technologies has even enhanced the impact of VV technology further [35]. Viral vectors are definitely past their coming of age and have reached market authorization status and the clinics with treatments such as Kymriah (Lentiviral), Strimvelis, Zalmoxis (both Retroviral), Luxturna and Zolgensma (all AAV-based) [36]. However, issues regarding the safety and efficiency of viral vectors are still pending. EV-based vectors may prove to be a viable alternative [21].
3. Discussion—Impact of EV-VI Interplay on Viral Vector Technology
From the relationship of wt virus with extracellular vesicles or, more specifically, exosomes, we have seen that both pro and antiviral responses are possible and, as a consequence, both a beneficial and a detrimental outcome seems possible in relation to VVs. Effects will depend on the source of EVs (patient, cell culture, media, serum or other body fluids) and on therapy modalities (administration route, necessary titers, etc.). Immunity evasion and transmission range/efficacy are the most promising strategies to apply in improving VV applications (see Table 2 for examples).

Table 2.
Examples for VV-EV interactions.
3.1. Technical Issues
Although it may seem trivial, issues regarding the preparation or analysis of VV preparations probably constitute the strongest influence of EVs on VV technology. EVs play a role as contaminants in viral preparation, and especially for enveloped viruses it can be difficult to properly separate the two. However, potentially loosely considered as non-functional VV particles, they would contribute to an unfavourable total-to-infectious particles ratio, a quality parameter for VV preparations [56]. Thus, efficacy of therapies or interventions may be reduced and greater efforts in purification strategies seem advisable. Downstream, analytical procedures may also be hindered by the presence of EVs in VV preps. Marker distribution is overlapping, and viral protein markers may be found on EVs, potentially leading to a misinterpretation of results and—for quantitative results—an overestimation of vector titers. To overcome such issues, single particle analysis of viral particles will help [56]. When gaining information on single particles, ideally in a multi-parametric fashion, distinguishing, quantifying and sorting is possible. Indeed, a process very similar to flow cytometry, which has been termed flow virometry or nanovariant flow cytometry [57], is gaining more and more momentum. Furthermore, a combination of biological and physical parameters may help to unequivocally identify EVs and VIs. Nanoparticle tracking analysis (NTA) and tunable resistive pulse sensing (TRPS) provide information on titer, diameter and zeta potential (a correlate of surface charge) [56,58]. Both techniques have been used for quality control of VVs and vaccine preparations.
3.2. EVs Inhibiting VVs
For wildtype virus, inhibitory functions mostly come from the EV signal transduction capabilities (i.e., by inducing an antiviral state in EV recipient cells), an issue that seems less severe since, in most cases, replication incompetent viral vectors are used, allowing only a single round of infection. Therefore, the induction of an antiviral state in potential recipient cells may hardly influence efficacy of therapy. However, the same as for wild type virus would apply in the case of replication-competent viral vectors, which may be used e.g., in oncolytic virus strategies [59]. For non-enveloped viruses, effects considered to be positive for wt virus may actually be detrimental in some cases: cloaking by EVs will hide surface characteristics and potentially change (expand) the tropism range, thus increasing off-target effects. Finding countermeasures for such processes will be difficult. General or localized suppression of vesiculation may be feasible; however, a better understanding of mechanisms is necessary to estimate unwanted effects. Overexpression of targeting factors directed to EV biogenesis may help to alleviate the issue and actually prove to be an asset for EV delivery systems [15,16].
3.3. EVs Facilitating VVs
Proviral effects for wt virus mediated by EVs are mostly based on immune evasion or transmission expansion capabilities. VV systems can benefit from such strategies. As suggested by the interactions of wt viruses, such strategies may have evolved most likely naturally as a consequence of virus–host co-evolution.
Immune evasion or manipulation is mostly interesting for in vivo delivery strategies and may indeed be helpful, especially for non-enveloped VVs (AV, AAV). Dominant epitopes can be hidden underneath exosomal cloaks, which will display host, rather than viral, proteins. Examples for this strategy have been tried both in AV and AAV vectors: AAV vectors were found to be more resistant to neutralizing anti-AAV antibodies when associated to EVs [40], and AV-based oncolytic virus was not changed in immunomodulatory capacity when EV associated [32].
Transmission expansion may be of interest for both ex and in vivo approaches. Providing additional anchor points for attachment to the desired target cells may help to increase specificity and efficacy of VV based therapies, especially when using recombinant EVs overexpressing targeting molecules. Indeed, this approach may be extended to immunity modifying strategies by displaying stimulators or inhibitors of specific immune functions. An interesting alternative to genetic manipulation of EV-producing cells is the use of post-exit methods such as Molecular Painting [60,61,62,63,64,65,66] or function-spacer-lipid constructs [67]. In an AV vector system, association of VV to vesicles improved the efficacy of oncolytic virus therapy on tumors with low coxsackie-and-adenovirus (CAR) [34]. In AAV exosome, associated AAV8 vectors were demonstrated to efficiently transduce lymphocytes after systemic delivery [39].
In the case of enveloped viral vectors, help is somewhat limited. Acquiring an envelope structure enabled functions, that may be conferred to non-enveloped viruse by EVs: nonfunctional viral particles may work as an immune decoy (e.g., for Hepatitis B). The envelope may also be used as the primary platform for modification of VVs. However, production capacities may be increased or redirected. Tetraspanins are markers of EVs and have been shown to increase exosome production when over expressed [47]. Interestingly, in an LV vector system, overexpression of the tetraspanin (and exosome marker) CD9 did not increase viral titers but lead to a faster kinetic and an increased efficacy of the vectors [47]. For enveloped viruses, a fine-tuning of production capabilities and functional optimization is feasible by exploiting EV biogenesis, and such strategies may also be adopted for non-enveloped viruses [55].
3.4. Perspectives and Outlook
Clinical trials have likely been impacted by EVs. Outcomes may be a tally of pro- and anti-vector effects, probably leading to an overall sub-optimal performance. Measures for a more regulated handling of EVs have been discussed [68,69]. Thus, addressing the balance of EV and VI in VV-based therapies may be a way to optimize the efficacy and safety of VV-mediated biomedical strategies [70]. Due to persisting issues regarding, e.g., immunogenicity of viral vectors, exosomal vectors may prove to be a more secure alternative to classic VVs, depending on a better understanding of EV biogenesis, sub-classes and functionalities. For technical applications, the controlled generation of hybrids or chimeras may prove beneficial by keeping the viral genetics in place [38] but cloak to help with targeting, immune modulation and qualitative and quantitative production parameters. Such chimeras may be implemented by artificial cloaking, i.e., using liposomes [71] or by different methods of coercing vesicles from cells, such as mechanical slicing of cells to generate cellular vesicles (i.e., by forcing through polymer membranes) [34]. Extension of molecular re-design of EV and VI-based biomedical applications by using metabolic engineering and/or synthetic biology may provide a path to develop cellular factories for optimized EV-based delivery applications. On the other hand, viral proteins may be used as “accessory” proteins in EV preparations to manipulate specific target cell functions. Bi-phasic functionalities, i.e., in a “cloak and dagger” fashion, seem promising, and a specifically designed EV shell may protect the vector not only from external damaging sources, but also from premature activation. Expanding on such strategies will, for example, allow combination therapies for cancer treatments by a single delivery event. Of course, potential improvements will depend on the intended use of the vectors. Vaccines based on viral vector platforms may benefit from the use of prokaryotic vesicles as a means to provide adjuvant effects.
We are only at the start and patterns are only beginning to emerge. However, the issues discussed here definitely warrant further research, as a better understanding of the connex between viral replication and extracellular vesicle biology will drastically improve the biomedical capacities of both strategies.
Author Contributions
Writing—original draft preparation, M.Z., C.M.; writing—review and editing, M.Z., C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Open Access Funding by the University of Veterinary Medicine Vienna.
Conflicts of Interest
The Authors declare no conflict of interest.
References
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Maas, S.L.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2019, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Iraci, N.; Leonardi, T.; Gessler, F.; Vega, B.; Pluchino, S. Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int. J. Mol. Sci. 2016, 17, 171. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Jadli, A.S.; Ballasy, N.; Edalat, P.; Patel, V.B. Inside(sight) of tiny communicator: Exosome biogenesis, secretion, and uptake. Mol. Cell. Biochem. 2020, 467, 77–94. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Ferrantelli, F.; Chiozzini, C.; Manfredi, F.; Giovannelli, A.; Leone, P.; Federico, M. Simultaneous CD8+ T-Cell Immune Response against SARS-Cov-2 S, M, and N Induced by Endogenously Engineered Extracellular Vesicles in Both Spleen and Lungs. Vaccines 2021, 9, 240. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Ribeiro, I.; Jorge, J.; Gonçalves, A.; Sarmento-Ribeiro, A.; Melo, J.; Carreira, I. Liquid Biopsies: Applications for Cancer Diagnosis and Monitoring. Genes 2021, 12, 349. [Google Scholar] [CrossRef]
- Garcia-Romero, N.; Esteban-Rubio, S.; Rackov, G.; Carrión-Navarro, J.; Belda-Iniesta, C.; Ayuso-Sacido, A. Extracellular vesicles compartment in liquid biopsies: Clinical application. Mol. Asp. Med. 2018, 60, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Dooley, K.; McConnell, R.E.; Xu, K.; Lewis, N.D.; Haupt, S.; Youniss, M.R.; Martin, S.; Sia, C.L.; McCoy, C.; Moniz, R.J.; et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol. Ther. 2021, 29, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Atukorala, I.; Mathivanan, S. The Role of Post-Translational Modifications in Targeting Protein Cargo to Extracellular Vesicles. Subcell. Biochem. 2021, 97, 45–60. [Google Scholar] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Martellucci, S.; Orefice, N.S.; Angelucci, A.; Luce, A.; Caraglia, M.; Zappavigna, S. Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy? Int. J. Mol. Sci. 2020, 21, 6486. [Google Scholar] [CrossRef]
- Orefice, N. Development of New Strategies Using Extracellular Vesicles Loaded with Exogenous Nucleic Acid. Pharmaceutics 2020, 12, 705. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef] [Green Version]
- Metzner, C.; Zaruba, M. On the Interplay of Extracellular Vesicles and Viral Infections. Trillium Exctracellular Vesicles 2020, 2, 14–27. [Google Scholar] [CrossRef]
- Badierah, R.A.; Uversky, V.N.; Redwan, E.M. Dancing with Trojan horses: An interplay between the extracellular vesicles and viruses. J. Biomol. Struct. Dyn. 2021, 39, 3034–3060. [Google Scholar] [CrossRef]
- Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular vesicles and viruses: Are they close relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef] [Green Version]
- Forterre, P. The virocell concept and environmental microbiology. ISME J. 2012, 7, 233–236. [Google Scholar] [CrossRef]
- Dogrammatzis, C.; Waisner, H.; Kalamvoki, M. Cloaked Viruses and Viral Factors in Cutting Edge Exosome-Based Therapies. Front. Cell Dev. Biol. 2020, 8, 376. [Google Scholar] [CrossRef]
- Qin, V.M.; D’Souza, C.; Neeson, P.J.; Zhu, J.J. Chimeric Antigen Receptor beyond CAR-T Cells. Cancers 2021, 13, 404. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Saari, H.; Somersalo, P.; Crescenti, D.; Kuryk, L.; Aksela, L.; Capasso, C.; Madetoja, M.; Koskinen, K.; Oksanen, T.; et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J. Control. Release 2018, 283, 223–234. [Google Scholar] [CrossRef]
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Mazzaferro, V.; Ciana, P. Systemic Administration and Targeted Delivery of Immunogenic Oncolytic Adenovirus Encapsulated in Extracellular Vesicles for Cancer Therapies. Viruses 2018, 10, 558. [Google Scholar] [CrossRef] [Green Version]
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Rinner, B.; Cerullo, V.; Yliperttula, M.; Mazzaferro, V.; Ciana, P. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J. Control. Release 2019, 294, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Zhang, H.; Wei, J.; Wu, J. Extracellular Vesicles-Mimetic Encapsulation Improves Oncolytic Viro-Immunotherapy in Tumors With Low Coxsackie and Adenovirus Receptor. Front. Bioeng. Biotechnol. 2020, 8, 574007. [Google Scholar] [CrossRef] [PubMed]
- Tasca, F.; Wang, Q.; Goncalves, M. Adenoviral Vectors Meet Gene Editing: A Rising Partnership for the Genomic Engineering of Human Stem Cells and Their Progeny. Cells 2020, 9, 953. [Google Scholar] [CrossRef]
- Ma, C.-C.; Wang, Z.-L.; Xu, T.; He, Z.-Y.; Wei, Y.-Q. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef] [PubMed]
- Bello-Morales, R.; Praena, B.; De La Nuez, C.; Rejas, M.T.; Guerra, M.; Galán-Ganga, M.; Izquierdo, M.; Calvo, V.; Krummenacher, C.; López-Guerrero, J.A. Role of Microvesicles in the Spread of Herpes Simplex Virus 1 in Oligodendrocytic Cells. J. Virol. 2018, 92, e00088-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saari, H.; Turunen, T.; Lõhmus, A.; Turunen, M.; Jalasvuori, M.; Butcher, S.J.; Ylä-Herttuala, S.; Viitala, T.; Cerullo, V.; Siljander, P.R.M.; et al. Extracellular vesicles provide a capsid-free vector for oncolytic adenoviral DNA delivery. J. Extracell. Vesicles 2020, 9, 1747206. [Google Scholar] [CrossRef]
- Breuer, C.B.; Hanlon, K.S.; Natasan, J.-S.; Volak, A.; Meliani, A.; Mingozzi, F.; Kleinstiver, B.P.; Moon, J.J.; Maguire, C.A. In vivo engineering of lymphocytes after systemic exosome-associated AAV delivery. Sci. Rep. 2020, 10, 4544–4549. [Google Scholar] [CrossRef]
- Maguire, C.A.; Balaj, L.; Sivaraman, S.; Crommentuijn, M.H.; Ericsson, M.; Mincheva-Nilsson, L.; Baranov, V.; Gianni, D.; Tannous, B.A.; Sena-Esteves, M.; et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 2012, 20, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Santiana, M.; Ghosh, S.; Ho, B.A.; Rajasekaran, V.; Du, W.-L.; Mutsafi, Y.; De Jésus-Diaz, D.A.; Sosnovtsev, S.V.; Levenson, E.A.; Parra, G.I.; et al. Vesicle-Cloaked Virus Clusters Are Optimal Units for Inter-organismal Viral Transmission. Cell Host Microbe 2018, 24, 208–220.e8. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-H.; Du, W.; Hagemeijer, M.C.; Takvorian, P.M.; Pau, C.; Cali, A.; Brantner, C.A.; Stempinski, E.S.; Connelly, P.S.; Ma, H.-C.; et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 2015, 160, 619–630. [Google Scholar] [CrossRef] [Green Version]
- Altan-Bonnet, N.; Perales, C.; Domingo, E. Extracellular vesicles: Vehicles of en bloc viral transmission. Virus Res. 2019, 265, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Münz, C. The Autophagic Machinery in Viral Exocytosis. Front. Microbiol. 2017, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Li, Y.; McKnight, K.L.; Hensley, L.; Lanford, R.E.; Walker, C.M.; Lemon, S.M. Human pDCs preferentially sense enveloped hepatitis A virions. J. Clin. Investig. 2015, 125, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E.K. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef] [Green Version]
- Böker, K.O.; Lemus-Diaz, N.; Ferreira, R.R.; Schiller, L.; Schneider, S.; Gruber, J. The Impact of the CD9 Tetraspanin on Lentivirus Infectivity and Exosome Secretion. Mol. Ther. 2018, 26, 634–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundquist, W.I.; Krausslich, H.G. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2012, 2, a006924. [Google Scholar] [CrossRef] [PubMed]
- Goila-Gaur, R.; Strebel, K. HIV-1 Vif, APOBEC, and Intrinsic Immunity. Retrovirology 2008, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotič, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitaš, A.; Peterlin, B. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Ariën, K.K.; Verhasselt, B. HIV Nef: Role in pathogenesis and viral fitness. Curr. HIV Res. 2008, 6, 200–208. [Google Scholar] [CrossRef]
- Raab-Traub, N.; Dittmer, D.P. Viral effects on the content and function of extracellular vesicles. Nat. Rev. Microbiol. 2017, 15, 559–572. [Google Scholar] [CrossRef]
- Palomino, R.A.Ñ.; Vanpouille, C.; Laghi, L.; Parolin, C.; Melikov, K.; Backlund, P.; Vitali, B.; Margolis, L. Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV-1 infection of human tissues. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Grandi, N.; Tramontano, E. Human Endogenous Retroviruses Are Ancient Acquired Elements Still Shaping Innate Immune Responses. Front. Immunol. 2018, 9, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiller, L.T.; Lemus-Diaz, N.; Ferreira, R.R.; Böker, K.O.; Gruber, J. Enhanced Production of Exosome-Associated AAV by Overexpression of the Tetraspanin CD9. Mol. Ther. Methods Clin. Dev. 2018, 9, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Heider, S.; Metzner, C. Quantitative real-time single particle analysis of virions. Virology 2014, 462–463, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Burnie, J.; Tang, V.A.; Welsh, J.A.; Persaud, A.T.; Thaya, L.; Jones, J.C.; Guzzo, C. Flow Virometry Quantification of Host Proteins on the Surface of HIV-1 Pseudovirus Particles. Viruses 2020, 12, 1296. [Google Scholar] [CrossRef]
- Heider, S.; Muzard, J.; Zaruba, M.; Metzner, C. Integrated Method for Purification and Single-Particle Characterization of Lentiviral Vector Systems by Size Exclusion Chromatography and Tunable Resistive Pulse Sensing. Mol. Biotechnol. 2017, 59, 251–259. [Google Scholar] [CrossRef]
- Ghose, J.; Dona, A.; Murtadha, M.; Gunes, E.G.; Caserta, E.; Yoo, J.Y.; Russell, L.; Jaime-Ramirez, A.C.; Barwick, B.G.; Gupta, V.A.; et al. Oncolytic herpes simplex virus infects myeloma cells in vitro and in vivo. Mol. Ther. Oncolytics 2021, 20, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Heider, S.; Dangerfield, J.A.; Metzner, C. Biomedical applications of glycosylphosphatidylinositol-anchored proteins. J. Lipid Res. 2016, 57, 1778–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heider, S.; Kleinberger, S.; Kochan, F.; Dangerfield, J.A.; Metzner, C. Immune Protection of Retroviral Vectors upon Molecular Painting with the Complement Regulatory Protein CD59. Mol. Biotechnol. 2016, 58, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Metzner, C.; Kochan, F.; Dangerfield, J.A. PostexitSurface Engineering of Retroviral/Lentiviral Vectors. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Metzner, C.; Kochan, F.; Dangerfield, J.A. Fluorescence molecular painting of enveloped viruses. Mol. Biotechnol. 2013, 53, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Metzner, C.; Mostegl, M.M.; Günzburg, W.H.; Salmons, B.; Dangerfield, J.A. Association of glycosylphosphatidylinositol-anchored protein with retroviral particles. FASEB J. 2008, 22, 2734–2739. [Google Scholar] [CrossRef]
- Metzner, C.; Salmons, B.; Günzburg, W.H.; Dangerfield, J.A.; Information, P.E.K.F.C. Comment on Patel et al; “Protein transfer-mediated surface engineering to adjuvantate virus-like nanoparticles for enhanced anti-viral immune responses” Nanomedicine, 2015. 11(5): P. 1097-107. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 665–666. [Google Scholar] [CrossRef]
- Metzner, C.; Salmons, B.; Günzburg, W.H.; Dangerfield, J.A. Rafts, anchors and viruses—A role for glycosylphosphatidylinositol anchored proteins in the modification of enveloped viruses and viral vectors. Virology 2008, 382, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Hadac, E.M. Fluorescein and radiolabeled Function-Spacer-Lipid constructs allow for simple in vitro and in vivo bioimaging of enveloped virions. J. Virol. Methods 2011, 176, 78–84. [Google Scholar] [CrossRef]
- Thery, C. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Witwer, K.W. Updating the MISEV minimal requirements for extracellular vesicle studies: Building bridges to reproducibility. J. Extracell. Vesicles 2017, 6, 1396823. [Google Scholar] [CrossRef] [Green Version]
- Minh, A.D.; Star, A.; Stupak, J.; Fulton, K.; Haqqani, A.; Gélinas, J.-F.; Li, J.; Twine, S.; Kamen, A. Characterization of Extracellular Vesicles Secreted in Lentiviral Producing HEK293SF Cell Cultures. Viruses 2021, 13, 797. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).