Abstract
Swietenia macrophylla King, commonly known as Brazilian mahogany, is a high-value neotropical tree species currently threatened due to intensive logging in previous decades. Technologies aimed at clonal production are essential for this species’ conservation and sustainable use at times of climate change and increasing demand for ecological restoration. The aim of the present study is to develop a low-cost protocol for mahogany clonal propagation through mini-cutting by assessing clonal mini-hedge nutrition, vegetative propagule type and indole-3-butyric acid (IBA) application effects on rooting and early clone growth. The experiment was conducted in nursery under controlled conditions based on using basal and apical mini-cuttings rooted in a low-cost mini-greenhouse subjected to three nutrient solution concentrations (50%, 100%, and 200%) and five IBA doses (0–8000 ppm). The mini-cutting technique proved efficient and led to over 90% survival after the hardening phase. The 200% nutrient solution concentration allowed balanced performance between cutting types and optimized clonal yield. IBA concentration at 4000 ppm accounted for higher root percentages at the bottom of the tube and the trend towards higher dry biomass production at 160 days. The results highlighted mini-cutting’s potential as a viable mahogany conservation and sustainable production technique. It also supported tropical forestry sector adaptation to challenges posed by climate change.
1. Introduction
Swietenia macrophylla King, commonly known as Brazilian mahogany, big-leaf mahogany, aguano, araputanga and caoba, is a high-value endangered neotropical tree species acknowledged for its wood’s excellent aesthetic, physical and mechanical properties []. Mahogany is included in the list of threatened species due to intensive logging, mainly in the 1970s and 80s [].
Therefore, Brazil is now committed to restore 12 million hectares of forests by 2030 as part of its contribution to the Paris Agreement []; this must be performed by promoting public policies aimed at large-scale ecological restoration. This progress has increased the demand for Amazonian forest seedlings, whose production mostly relies on sexual propagation [].
According to data from the Forest Restoration Observatory, of the 153,000 hectares currently under restoration in Brazil, only about 15,000 are located in the Amazon biome []. This indicates a limited concentration of initiatives in the region and signals an imminent pressure on the seedling production sector, considering that the national target of restoring 12 million hectares must be achieved by 2030.
Consequently, the development of alternative propagation methods has become a strategic priority for the conservation and sustainable use of Amazonian species, particularly given that mahogany produces winged seeds that must be harvested from fruits still attached to the tree, prior to full dehiscence []. This requirement demands specialized labor, specific equipment, and deliberate anthropogenic intervention in seed trees, further emphasizing the need for efficient clonal propagation alternatives.
Mini-cutting has been standing out for its efficiency among the several forms of vegetative propagation. Although it enhances rooting, especially in species that are difficult to propagate, its application requires costly technological structures, such as greenhouses with temperature, humidity, and ventilation control, which limits access for small-scale producers []. Therefore, in addition to understanding the physiological and nutritional factors that influence rooting, it is essential to develop low-cost and easily implemented infrastructure alternatives capable of democratizing clonal production and expanding its use in resource-limited contexts.
Propagule type choices and adequate mini-stump nutrition are decisive factors for mini-cutting success, since they influence shoot production and rooting—although this element changes depending on branches’ juvenility gradient [,]. Furthermore, the use of growth regulators such as indole-3-butyric acid (IBA) is widely adopted to promote effective rooting through mini-cuttings due to the role it plays in root induction. However, outcomes change depending on species, propagule type, applied concentration and on cultivation environmental and seasonal conditions [].
Although recent studies have reported promising results in the clonal propagation of neotropical forest species using mini-cuttings [,,], there remains a need for a more robust theoretical and technical foundation to support the development of efficient protocols for Amazonian species. Overcoming the inherent recalcitrance of many forest species, including Swietenia macrophylla, continues to be a major challenge, as these species often exhibit low responsiveness to vegetative propagation due to limited regeneration and transformation capacities [].
In this context, recent studies have demonstrated the feasibility of vegetative propagation of Swietenia macrophylla through mini-cutting techniques [,]. However, survival rates below 70% [], combined with preliminary assessments using non-destructive methods [,], indicate the need for further investigation into the quality of clonal seedlings of this species. Moreover, studies specifically focused on the productivity of S. macrophylla clonal mini-hedges remain scarce, particularly those evaluating the inputs used in nutritional supplementation and the rooting capacity of different types of vegetative material.
Accordingly, Experiment 1 of this study was based on the hypothesis that increasing fertigation in the mini-hedge system positively influences the production of vegetative propagules. Experiment 2 was designed under the hypothesis that the interaction between nutritional enhancement of mini-stumps and the type of mini-cutting results in the production of more vigorous clones, especially from hardwood mini-cuttings, which are traditionally considered difficult to root. Finally, Experiment 3 was conducted under the hypothesis that the application of exogenous auxins enhances rooting even in juvenile propagules, which naturally exhibit high internal auxin concentrations.
The aim of the current study was to develop a low-cost protocol for mahogany clonal propagation by using mini-cuttings based on assessing clonal mini-hedge nutrition, vegetative propagule type and synthetic auxin indole-3-butyric acid (IBA) application effects on rooting and early clone growth.
2. Materials and Methods
2.1. Experimental Area
This study was conducted in a nursery at the Zoobotanical Park of Federal University of Acre, Rio Branco municipality, Acre State, Southwestern Amazon, Brazil. The rooting of vegetative propagules was carried out between October and March, during the rainy season (Figure 1). According to the Köppen climate classification, the region has Am climate (tropical monsoon) [].
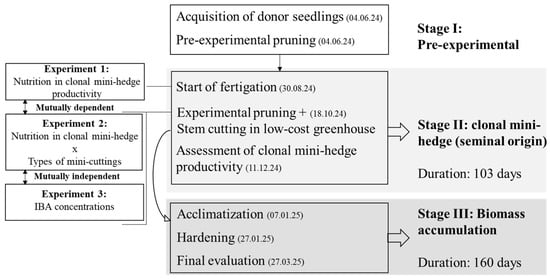
Figure 1.
Flowchart illustrating stages of clonal mini-hedge management, mini-cutting rooting, and acclimatization of clonal seedlings of Swietenia macrophylla King.
2.2. Clonal Mini-Hedge
The experiment was carried out with Swietenia macrophylla King (mahogany) seedlings at the transplant stage, with a mean height of 15 cm, in order to ensure appropriate mini-stump establishment at the target height of 10 cm. Genetic representativeness was ensured by sampling the largest number possible of donor trees []. The clonal mini-hedge was established in protected environment by using 5 L pots filled with commercial forest substrate mix (Pilar Produtos Agroecológicos®, Lages, Brazil) and washed sand, at a 3:1 (v/v) ratio, which totaled 81 mini-stumps (Experiment 1). Seedlings were pruned to 10 cm above base to induce sprouting after a 40-day acclimation period. Fertilization was applied through manual fertigation with 150 mL nutrient solution applied every three days. Nutrient solutions were formulated based on previous studies [], and they comprised the following soluble salts (in mg L−1): monoammonium phosphate (40), magnesium sulfate (133), potassium nitrate (219), ammonium sulfate (123), calcium nitrate (200), boric acid (5.88), manganese sulfate (5.33), sodium molybdate (0.05), copper sulfate (0.4), zinc sulfate (5) and iron sulfate (33).
Factor A, which corresponded to nutrient solutions, consisted of three base-solution concentration levels: 50%, 100% and 200%. Experimental design in the clonal mini-hedge followed a randomized block design (RBD) with three replications, nine plants per plot and three treatment levels. The following Equation (1) was used to quantify the mini-cutting production by taking into consideration the full use of sprouts:
wherein the following applies:
- Y5cm = the number of mini-cuttings with the minimum length required for rooting;
- Hi = the length of the i-th sprout;
- N = the total number of sprouts, and 5 = minimum adopted cutting length (in cm);
- U.E. = experimental unit (number of plants per plot).
2.3. Mini-Cutting Rooting
Mahogany mini-cuttings were obtained from mini-stump sprouts 60 days after the beginning of nutrient solution application (Factor A). Cuttings were classified as basal when they were collected from the sprout’s base to its intermediate region and apical, when they were collected from its intermediate region to its tip (Factor B) [].
Rooting was carried out for 80 days in a closed-system mini-greenhouse under high-temperature (≈30 °C) and -humidity (>80%) conditions. This experiment (Experiment 2) followed a randomized block design (RBD) at 3 × 2 factorial scheme, with three replications and eight mini-cuttings per plot (totaling 144 plants). The mini-greenhouse was designed to be easy to assemble, transport and maintain, using low-cost materials available in local markets. The structure was primarily built with PVC pipes. The total cost of the system, including an independent irrigation setup added with a low-power pump (0.3 hp), remained under USD 100, and this highlighted its affordability. Cuttings were acclimatized for 20 days in a shade house and hardened for 60 days under a full sun.
Five indole-3-butyric acid (IBA) concentrations (0, 1000, 2000, 4000, and 8000 ppm)—in liquid form, dissolved in sodium hydroxide (NaOH) at 1 mol/L and diluted with distilled water—were tested in a complementary experiment (Experiment 3) on intermediate stem mini-cuttings from mini-stumps fertilized with 100% base nutrient solution. This trial also followed a randomized block design (RBD) with three replications and seven mini-cuttings per plot (totaling 105 plants).
The analyzed variables included visible root formation at the bottom of the tubes 60 days after planting; clones’ survival in greenhouse at 80 days; and morphological quality at 160 days.
Shoot height (H) was measured with a ruler placed from substrate level to shoot apex; collar diameter (CD) was measured 1 cm above the node near the surface with the aid of a digital caliper.
The shoot and root systems were separated, placed in Kraft paper bags and oven-dried at 65 °C under forced air circulation until constant weight for destructive analysis purpose. Dry masses were determined on a semi-analytical scale at 0.01 g precision. Total dry mass (TDM) was calculated as the sum of shoot dry mass (SDM) and root dry mass (RDM).
Morphological indices were also calculated, namely the SDM:RDM ratio (shoot/root dry mass ratio) and the Dickson Quality Index (DQI), according to Equation (2) []:
wherein the following applies:
- TDM = total dry mass (g);
- H:CD = collar height/diameter ratio;
- SDM:RDM = shoot/root dry mass ratio (g).
2.4. Statistical Analysis
The data of the treatments were subjected to tests for variance height (Bartlett, p < 0.05) and error normality (Shapiro–Wilk, p < 0.05). An analysis of variance (ANOVA, p < 0.05) was performed based on a randomized block design (RBD). A two-way ANOVA was applied in Experiment 2 to evaluate the interaction between factors, while a one-way ANOVA was used in Experiments 1 and 3 to assess the effect of a single factor. When assumptions were met, Tukey’s test (p < 0.05) was adopted for mean comparison. All statistical analyses were performed in R Studio version 2024.09.1+394.
3. Results
Although no statistically significant differences were detected among treatments in mini-cutting production (p > 0.05), there was a trend that indicated that higher nutrient solution concentrations can enhance yield (Table 1). Mini-stumps treated with 200% nutrient solution produced more shoots per plant, and this finding suggested that higher nutrient availability can have a positive influence on mahogany clones’ vegetative vigor.

Table 1.
Nutrient solution concentration effect on mahogany mini-stump yield 60 days after pruning.
Accordingly, the mini-cutting technique led to excellent results and a high survival rate regardless of the mini-cutting type, nutrient solution concentration or tested IBA dose. Survival remained consistently high across all treatments, 90%, on average, and this pointed towards this propagation method’s robustness and effectiveness for mahogany. This finding also suggested that the developed low-cost mini-greenhouse system was highly efficient for this species’ clonal production (Figure 2).
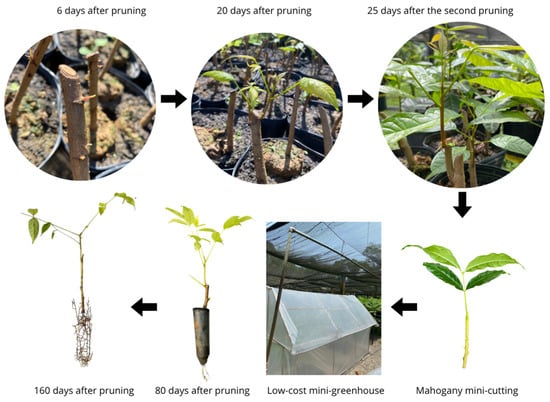
Figure 2.
Swietenia macrophylla King (mahogany) clonal production process through mini-cutting conducted in low-cost mini-greenhouse installed in Southwestern Amazon, Acre State, Brazil.
According to the nutrient solution concentration/mini-cutting type interaction in biomass production, the performed basal cuttings performed just as good as, or even better than, apical cuttings, even under the standard nutrient concentration (100%) (Table 2). This outcome highlighted that basal propagules were highly efficient in biomass accumulation, and it reinforced their potential for large-scale mahogany clonal propagation.

Table 2.
Nutrient solution concentration/mini-cutting type interaction effect on mahogany clones’ biomass production 160 days after cutting (80 days in low-cost greenhouse and 80 days in nursery).
Notably, applying the nutrient solution at 200% increases production costs. However, the significant gains observed in dry mass accumulation offset this increase, making this practice technically feasible, especially due to the excellent utilization of inputs under the controlled conditions of the clonal mini-hedge environment.
Only the variable ‘root at the bottom of the tube (RBT)’ showed a statistically significant difference among treatments (p < 0.05) when different IBA doses were assessed for early S. macrophylla clones’ development. The other analyzed variables, height (H), collar diameter (CD), the shoot/root dry mass ratio (SDM/RDM) and the Dickson Quality Index (DQI), did not significantly differ, as shown in Table 3.

Table 3.
The effect of different indole-3-butyric acid (IBA) concentrations on root formation at the bottom of the tube (RBT) 60 days after cutting and on the morphological and quality variables of Swietenia macrophylla King clones at 160 days.
Although no statistically significant differences were observed for the other variables, Swietenia macrophylla clones reached mean heights ranging from 11.40 to 13.55 cm; the highest values were recorded at an IBA concentration of 4000 ppm. It is worth noting that increasing the auxin concentration to this level does not significantly raise production costs, especially considering that a single prepared solution can be used to treat a large number of cuttings simultaneously.
Mahogany clones in the present study were produced in tubes, and the SDM:RDM ratio ranged from 1.96 to 3.41. Lower ratios were observed at the highest indole-3-butyric acid (IBA) doses, and this indicated a trend towards a higher balance between root growth and shoot development under stronger hormonal stimulus.
The Dickson Quality Index (DQI) ranged from 0.31 to 0.64; its highest value was recorded at an IBA concentration of 4000 ppm, which also led to the highest rate of visible roots at the bottom of the tube at 60 days. Although the effects were not statistically significant, this concentration consistently indicated an improvement in plant physiological quality, and this suggested that intermediate regulator doses can enable morphophysiological balance at the rooting phase.
The biomass accumulation analysis applied to mahogany clones showed a consistent response trend to indole-3-butyric acid (IBA), even in the absence of statistically significant differences (p > 0.05) in shoot dry mass (SDM), root dry mass (RDM) and total dry mass (TDM). A quadratic biological response pattern was observed when the variable increased up to the concentration of 4000 ppm. This was followed by reductions at higher doses, although this behavior suggested a promotive IBA effect at intermediate concentrations and likely negative physiological interference at higher levels, as shown in Figure 3.
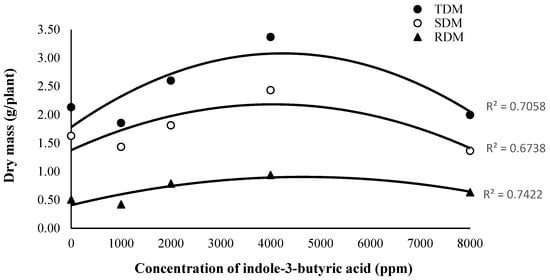
Figure 3.
Total dry mass (TDM), shoot dry mass (SDM) and root dry mass (RDM) of Swietenia macrophylla clones produced through mini-cuttings as function of different indole-3-butyric acid (IBA) concentrations 160 days after cutting.
The 4000 ppm IBA concentration led to more favorable hormonal environment for cell division and differentiation, which resulted in higher biomass accumulation. In addition, the visual comparison between clones under IBA treatment ranging from 0 and 4000 ppm highlighted auxins’ positive effect on root development. Clones treated at 4000 ppm recorded higher root density and volume, as well as a more vigorous shoot system (Figure 4).

Figure 4.
Swietenia macrophylla King clones derived from mini-cuttings treated with different indole-3-butyric acid (IBA) concentrations of (A) 0 ppm and (B) 4000 ppm.
4. Discussion
Yield in commercial clonal mini-hedges is directly influenced by mini-stump density and harvest frequency []. This suggests that investments in genetic enhancement programs and in refining silvicultural practices tailored to each species’ ecological and physiological features are essential to make the clonal propagation of Amazonian species commercially viable.
The ratio of mini-cuttings per mini-stump recorded in the current study was similar to findings reported in the scientific literature for Khaya anthotheca (1.8 mini-cuttings every 60 days) [] and Handroanthus heptaphyllus (1.76 mini-cuttings every 90 days) []. These results are in compliance with previous studies that analyzed experimental clonal mini-hedges of Brazilian woody species and found values often ranging from one to four mini-cuttings per mini-stump, per month [].
Although promising, this yield remains below the levels observed for commercial Eucalyptus hybrids, which often range from 6 to 20 mini-cuttings per mini-stump per month [,,]. Proper silvicultural management plays a key role in enabling the use of Amazonian species in large-scale production systems. Accordingly, the achieved yield of mahogany clonal mini-hedge highlighted the importance of artificial selection and domestication to enhance the clonal silviculture.
When it comes to survival at 160 days, similar results have been reported for forest species’ mini-cuttings known for having more established silvicultural research such as Eucalyptus hybrids and African mahogany (Khaya anthotheca), which commonly achieve survival rates higher than 90% [,,,]. On the other hand, experimental neotropical forest species’ clonal mini-hedges often show inconsistent survival rates, below 50%, on average [,], and this finding highlights the effectiveness of the herein adopted production method.
The observed survival rate (≈90%) was also higher and showed less variation when compared specifically to previous studies on Swietenia macrophylla clones propagated via mini-cutting. These studies reported average survival rates ranging from 43% to 86% [], 55% to 69% [], and 73% to 97% [], highlighting the consistency and effectiveness of the adopted protocol.
Cuttings collected from the apical portions of shoots tend to present less mature tissues and lower lignification levels []. Higher lignification is often associated with higher resistance to dehydration, mainly under water and heat stress conditions, like those observed at the final experimental stage under a full sun.
This behavior can be related to plants’ physiological strategy under stress, when resources are used for survival mechanisms rather than for growth []. This hypothesis is supported by the apical mini-cuttings’ performance at 200% nutrient concentration, whose results did not statistically differ from those of basal mini-cuttings. This finding suggests that higher nutrient availability may have increased plant vigor and resistance under field-like conditions.
Although seedlings exhibit greater growth plasticity compared to mature trees, which allows them to respond more efficiently to moderate biomass loss, this advantage is constrained by their low reserves of non-structural carbohydrates (sugars and starch). Consequently, apical mini-cuttings are more sensitive to prolonged stress, as they rely heavily on recently assimilated carbon. As a result, sustained or severe reductions in photosynthetic rate directly compromise the survival of young tissues, whose lower maturity makes them more vulnerable to immediate fluctuations in carbon gain [].
The superior basal mini-cuttings’ performance in mahogany cloning has also been reported by other authors [] and observed in other Amazonian forest species, even without the application of exogenous auxin, such as Handroanthus heptaphyllus [] and Apuleia leiocarpa []. However, in some cases, apical mini-cuttings can result in better rooting, as reported for Cedrela odorata [] and Anadenanthera macrocarpa []. Upper sprout portions tend to present higher natural concentrations of auxins, which, in some cases, can offset tissue maturity and environmental stress effects on mini-cutting development [].
The findings in the current study have shown that intermediate IBA concentrations, mainly 4000 ppm (28.57%), promote rooting in mahogany mini-cuttings and reinforce the regulator’s positive effect on rhizogenesis. These results are consistent with those reported previously in the scientific literature, which observed improved survival and root formation in mahogany cuttings treated with 3.000 and 4.000 ppm IBA [,].
These concentrations also appeared to work well for Schizolobium parahyba var. amazonicum, as concentrations ranging from 2000 to 3000 ppm led to the best rooting rates, root number, and mean root length, in addition to reducing mortality []. However, in Handroanthus heptaphyllus mini-cuttings, although IBA is not essential for root emission, it increases both the number and length of roots [].
The presence of a functional root system developed at the nursery phase is critical to achieve successful plant establishment, growth and survival after planting due to the role it plays in both water uptake and new root formation []. The exogenous application of growth regulators such as auxins plays a key role in inducing adventitious rooting in forest species’ vegetative propagules. These substances are naturally produced in actively dividing meristematic regions such as buds and young leaves; yet, they are transported to propagules’ base where they trigger root formation, provide hormonal balance with gibberellins and cytokinins, and maintain the cofactors [].
In many cases, endogenous auxin levels are not enough to fully start rhizogenesis. Exogenous application at carefully adjusted doses is necessary to optimize the results []. Indole-3-butyric acid (IBA) stands out among synthetic auxins for its superior chemical stability and lower susceptibility to biological degradation, which favor the formation of more uniform and vigorous root systems [,,].
Mean heights observed for Swietenia macrophylla clones were comparable to those reported in previous studies for Cedrela fissilis propagated by mini-cuttings, which recorded mean heights ranging from 10.6 to 15.2 cm at 120 days []. The mean collar diameter was 5.13 mm, and it fell in the optimal range of 5 to 10 mm for quality forest plants [].
On the other hand, the subsequent reduction in morphological variables at higher IBA concentrations suggested that auxin excess can impair key physiological processes, such as the activity of enzymes involved in cell division and hormonal balance, and have negative effect on plant development []. Previous studies suggest a phytotoxic effect when 8000 ppm of IBA is applied to Eucalyptus urophylla mini-cuttings, inhibiting root formation []. Recent studies have also identified phytotoxic effects associated with the application of exogenous auxins at high concentrations, as observed in Pongamia pinnata, where doses above 800 ppm inhibited rooting []. Likewise, in Vaccinium myrtillus, the application of IBA at concentrations above 4000 ppm significantly increased cutting mortality and reduced leaf retention [].
Similar patterns were observed in Paratecoma peroba, with higher IBA concentrations leading to decreased rooting and to lower root numbers []. In Peltophorum dubium, higher IBA concentrations led to the reduced survival of both apical and basal mini-cuttings, along with visible toxicity symptoms such as chlorosis, necrosis, and propagule death []. These findings indicate that excessive auxin levels can suppress the rooting process.
Shoot (SDM), root (RDM) and total dry mass (TDM) linear reduction at higher doses can also be linked to the degree of juvenility of donor plants, to the physiological condition of the propagules, and to the presence of endogenous auxins and rhizogenesis-inducing enzymes like starch phosphorylase, amylases and kinases []. It is worth noticing that juvenile cuttings often present endogenous indole-3-acetic acid (IAA) levels good enough to trigger the rooting process without the need for exogenous auxin application [].
Mini-stumps in the present study were obtained from juvenile seed-derived plants that, in theory, ensured IAA physiological reserves and cofactors essential for an adventitious root formation []. Therefore, supplementation with high IBA doses may have exceeded the species’ physiological threshold and damaged the mini-cuttings rather than promoting growth.
Biomass values and ratios were similar to those previously reported for African mahogany (Khaya grandifoliola) clones propagated by mini-cuttings in an experiment focused on mini-stump nutrition []. This finding indicated a compatible growth pattern among Meliaceae species under similar clonal cultivation conditions, and it is indicative of adequate morphophysiological balance.
The SDM:RDM ratio reflects the functional balance between the transpiring area and plants’ water absorption capacity []. This is a key post-planting physiological performance indicator. This balance is quite important for container production systems, where a ratio below 2:1 is recommended in comparison to the 3:1 ratio for bare-root plants.
The herein recorded Dickson Quality Index (DQI) values exceeded the minimum threshold of 0.20 proposed in the literature as a reference for forest plants with satisfactory performance potential []. The superior morphophysiological quality achieved in the present experiment is evident, especially when compared to previous findings, which reported a mean DQI of 0.056 for Plathymenia reticulata clones produced in tubes []. The DQI is one of the most understandable and reliable parameters to assess plant quality, as it integrates multiple morphological variables into a single index []. Furthermore, higher DQI values are closely associated with higher vigor, post-planting performance and adaptability to adverse field conditions [].
The root development of mahogany seedlings at 90 days grown in forest nurseries presents results similar to those observed in the current study [,]. However, corrective phosphorus fertilization seems to have a significant impact on early seedling development [,]. No fertilization was applied to the rooting substrate in this experiment, since its primary goal was to isolate the effects of fertilization applied to the mini-stump substrate.
Mahogany clones previously produced via mini-cutting and grown in commercial substrate without plant growth regulators showed a root dry mass of 0.020 g, 32 days after cutting []. If one bears in mind the 0.41 g, on average, at 160 days, observed in the present study, this number corresponds to a mean root biomass accumulation rate close to 0.00305 g a day for the assessed species. However, specific studies are needed to more precisely quantify this variable.
The study results show that mini-cutting in combination with appropriate nutritional management and exogenous auxin application is a viable strategy for mahogany clonal propagation. These findings and low-cost production systems’ technical feasibility support the advancement of clonal forestry for Amazonian species and contribute to sustainable forest production and to the development of genetic conservation strategies.
5. Conclusions
- The low-cost mini-cutting technique showed high potential for the clonal propagation of Swietenia macrophylla King (Brazilian mahogany), with a mean survival rate higher than 90% across all treatments, which confirmed the effectiveness of the developed low-cost mini-greenhouse system.
- High nutrient solution (200%) concentrations led to higher shoot production and highlighted the positive influence of increased nutrient availability on mini-stump yield.
- Clone dry biomass showed increasing trend up to the intermediate IBA dose at 4000 ppm. This was followed by a decline at higher concentrations, which suggested its likely phytotoxicity at excessive doses.
- Basal cuttings recorded equal or superior morphophysiological performance in comparison to apical cuttings, mainly under higher nutrient levels. This finding reinforced their preferential use in clonal propagation systems set for this species.
- The Dickson Quality Index ranged from 0.31 to 0.64, exceeded the minimum reference value (0.20) and confirmed the high quality of the produced clones, which recorded performance superior to that reported for other Amazonian species under similar conditions.
Overall, the current study contributed to advancing clonal forestry for native Amazonian species, with emphasis on the relevance of proper management practices, careful propagule selection, the strategic use of biostimulants and balanced nutrition for the production of high-quality plants. This study also demonstrated that vegetative propagation can be successfully carried out in the absence of highly technological permanent structures, which makes the technique feasible and replicable for small-scale forest nurseries.
Author Contributions
Conceptualization, R.B.D.L., A.S.d.C. and P.A.T.; methodology, R.B.D.L., A.S.d.C. and P.A.T.; software, R.B.D.L., A.S.d.C. and P.A.T.; validation, R.B.D.L., A.S.d.C., P.A.T., L.G.S., B.V.d.O. and F.C.d.S.; formal analysis, R.B.D.L., A.S.d.C., P.A.T., L.G.S., B.V.d.O. and F.C.d.S.; investigation, R.B.D.L., A.S.d.C., P.A.T., L.G.S., B.V.d.O. and F.C.d.S.; resources, P.A.T.; data curation, R.B.D.L., A.S.d.C. and P.A.T.; writing—original draft preparation, R.B.D.L., A.S.d.C. and P.A.T.; writing—review and editing, R.B.D.L., A.S.d.C. and P.A.T.; visualization, R.B.D.L., A.S.d.C. and P.A.T.; supervision, P.A.T.; project administration, R.B.D.L., A.S.d.C. and P.A.T.; funding acquisition, P.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq), Grant No. 408601/2023-0.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available as they form part of ongoing research. Access to the data may be granted upon reasonable request, subject to the completion of the associated studies.
Acknowledgments
We thank the National Council for Scientific and Technological Development for granting financial support for the research project. We also express our gratitude to Viveiro da Floresta (the Acre State Department of the Environment, Rio Branco, Brazil) for supplying materials and donating forest seedlings.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IBA | Indole-3-butyric acid |
| TDM | Total Dry Mass |
| SDM | Shoot Dry Mass |
| RDM | Root Dry Mass |
| RBT | Root at the Bottom of the Propagation Tube |
| H | Height |
| CD | Collar Diameter |
| DQI | Dickson Quality Index |
References
- Carvalho, P.E.R. Espécies Arbóreas Brasileiras; Coleção Espécies Arbóreas Brasileiras, v. 2; Embrapa Informação Tecnológica: Brasília, Brazil; Embrapa Florestas: Colombo, PR, Brazil, 2006; 627p. [Google Scholar]
- CITES. Apéndices I, II and III of CITES; Secretariat of the Convention on International Trade in Endangered Species of Wild Fauna and Flora: Geneva, Switzerland, 2019. [Google Scholar]
- Ministério do Meio Ambiente. Plano Nacional de Recuperação da Vegetação Nativa—Planaveg; MMA: Brasília, Brazil, 2018; 76p.
- Da Silva, A.P.M.; Schweizer, D.; Marques, H.R.; Teixeira, A.M.C.; Santos, T.V.M.N.d.; Sambuichi, R.H.R.; Badari, C.G.; Gaudare, U.; Brancalion, P.H.S. Can current native-tree seedling production and infrastructure meet an increasing forest-restoration demand in Brazil? Restor. Ecol. 2016, 25, 509–515. [Google Scholar] [CrossRef]
- ORR—Observatório da Restauração e Reflorestamento. Dashboard de Monitoramento da Restauração Florestal no Brasil. 2025. Available online: https://observatoriodarestauracao.org.br/dashboard (accessed on 15 July 2025).
- Xavier, A.; Wendling, I.; Silva, R.L. Silvicultura Clonal: Princípios e Técnicas, 3rd ed.; Editora UFV: Viçosa, Brazil, 2021; p. 407. [Google Scholar]
- Xu, X.; Li, X.; Hu, X.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. High miR156 expression is required for auxin-induced adventitious root formation via MxSPL26 independent of PINs and ARFs in Malus xiaojinensis. Front. Plant Sci. 2017, 8, 1059. [Google Scholar] [CrossRef]
- Borelli, K.; Rocha, J.H.T.; Silva, M.R.; Scaloppi, E.J., Jr.; Gonçalves, A.N.; Tecchio, M.A. Rubber tree mini clonal garden: Electric conductivity of the nutritional solution in the production of propagules. Rev. Árvore. 2024, 48, e4811. [Google Scholar] [CrossRef]
- Freire, J.M.; Varíssimo, L.N.; Pereira, B.R.; Rouws, J.R.C.; Arthur, J.C.A., Jr. Vegetative propagation of Hymenaea courbaril L. and Apuleia leiocarpa (Vogel) J. F. Macbr. by mini-cutting. Rev. Árvore 2020, 44, e4405. [Google Scholar] [CrossRef]
- Sant’Ana, B.T.; Berude, M.C.; Feletti, T.A.; Caldeira, M.V.W.; Gonçalves, E.O. Produtividade de minicepas e enraizamento de miniestacas de sapucaia (Lecythis lanceolata). Pesq. Florest. Bras. 2023, 43, e4304. [Google Scholar]
- Villalobos, J.M.; Melara, M.V.; Díaz, E.G.; Umaña, J.M.Z.; Arias, A.G. Review of biotechnological advances in mahogany (Swietenia macrophylla King): In vitro culture and genetic transformation. Plant Cell Tissue Organ Cult. 2025, 160, 78. [Google Scholar] [CrossRef]
- Torres, V.G.; Gaona, N.; Ordoñez, L.; García, P.; Mendoza, W.; Saavedra, J.; Macedo, W.; Reátegui, K.; Baselly, J.R.; Marín, C. Cutting propagation technique of mahogany (Swietenia macrophylla) in microtunnels from the Peruvian Amazon. Bosque 2024, 45, 485–495. [Google Scholar] [CrossRef]
- Azad, S.; Matin, A. Effect of indole-3-butyric acid on clonal propagation of Swietenia macrophylla through branch cutting. J. Bot. 2015, 2015, 249308. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.C.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Vencovsky, R. Tamanho efetivo populacional na coleta e preservação de germoplasmas de espécies alógamas. Indo-Pac. Econ. Framew. 1987, 35, 79–84. [Google Scholar]
- Filho, J.B.; Di Carvalho, M.A.; de Oliveira, L.S.; Konzen, E.R.; Brondani, G.E. Mini-cutting technique for Khaya anthotheca: Selection of suitable IBA concentration and nutrient solution for its vegetative propagation. J. For. Res. 2017, 29, 73–84. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant Propagation: Principles and Practices, 9th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2017; p. 1024. [Google Scholar]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white-spruce and white-pine seedling stock in nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Alfenas, A.C.; Zauza, E.A.V.; Mafia, R.G.; Assis, T.F. Clonagem e Doenças do Eucalipto, 2nd ed.; UFV: Viçosa, Brazil, 2009; pp. 1–500. [Google Scholar]
- Oliveira, T.P.d.F.d.; Barroso, D.G.; Lamônica, K.R.; Carneiro, J.G.d.A.; de Oliveira, M.A. Productivity of polyclonal minigarden and rooting of Handroanthus heptaphyllus Mattos minicuttings. Rev. Bras. Ciênc. Agrar. 2015, 10, 553–558. [Google Scholar] [CrossRef]
- Dias, P.C.; de Oliveira, L.S.; Xavier, A.; Wendling, I. Estaquia e miniestaquia de espécies florestais lenhosas do Brasil. Pesq. Florest. Bras. 2012, 32, 453–465. [Google Scholar] [CrossRef]
- Vilasboa, J.; Da Costa, C.T.; Fett-Neto, A.G. Environmental modulation of mini-clonal gardens for cutting production and propagation of hard- and easy-to-root Eucalyptus spp. Plants 2022, 11, 3281. [Google Scholar] [CrossRef]
- Rocha, F.M.; Maravilha, L.F.; Titon, M.; Fernandes, S.J.O.; Machado, E.L.M.; Martins, N.S. Productivity of mini-cuttings of a hybrid clone of Eucalyptus urophylla × Eucalyptus pellita as a function of exposure time of mini-stumps to mini-tunnel. Bosque 2023, 44, 595–603. [Google Scholar] [CrossRef]
- Canguçu, V.S.; Titon, M.; Silva, L.F.M.; Pena, C.A.A.; Assis, S.L.A., Jr.; Santos, P.H.R.; Oliveira, M.L.R. Mini-tunnel models influence the productivity of eucalyptus mini-stumps? Bosque 2022, 43, 211–219. [Google Scholar] [CrossRef]
- Brondani, G.E.; Wendling, I.; Brondani, A.E.; Araujo, M.A.; da Silva, A.L.L.; Gonçalves, A.N. Dynamics of adventitious rooting in mini-cuttings of Eucalyptus benthamii × Eucalyptus dunnii. Bosque 2012, 33, 129–137. [Google Scholar] [CrossRef]
- Wendling, I.; Warburton, P.M.; Trueman, S.J. Maturation in Corymbia torelliana × C. citriodora stock plants: Effects of pruning height on shoot production, adventitious rooting capacity, stem anatomy, and auxin and abscisic acid concentrations. Forests 2015, 24, e032. [Google Scholar] [CrossRef]
- Costella, C.; Araujo, M.M.; Aimi, S.C.; Barghetti, A.L.P.; Griebeler, A.M.; Lima, M.S.; Gasparin, E.; Santos, O.P.; Valente, B.M.R.T. Mini-tunnel and season influence in clonal garden on the production of clonal seedlings for two subtropical clones: Eucalyptus saligna and Corymbia torelliana × Corymbia citriodora. iForest 2025, 18, 154–162. [Google Scholar] [CrossRef]
- Hossain, M.A.; Islam, M.A.; Hossain, M.M. Rooting ability of cuttings of Swietenia macrophylla King and Chukrasia velutina Wight et Arn as influenced by exogenous hormone. Int. J. Agric. Biol. 2004, 6, 560–564. [Google Scholar]
- Broetto, F.; Gomes, E.R.; Joca, T.A.C. O Estresse das Plantas: Teoria & Prática; Cultura Acadêmica: São Paulo, Brazil, 2017; p. 194. [Google Scholar]
- Ninemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Cordeiro, I.M.C.C.; Lameira, O.A. Propagação de mogno (Swietenia macrophylla King) por miniestaquia. In Proceedings of the Congresso Brasileiro de Recursos Genéticos, Belém, PA, Brazil, 24–28 September 2012; Sociedade Brasileira de Recursos Genéticos: Brasília, Brazil, 2012. [Google Scholar]
- Oliveira, T.P.F.; Barroso, D.G.; Lamônica, K.R.; Carvalho, G.C.M.W. Aplicação de AIB e tipo de miniestacas na produção de mudas de Handroanthus heptaphyllus Mattos. Ciênc. Florest. 2016, 26, 313–320. [Google Scholar]
- Maldonado, S.S.; Casas, M.J.; Upton, J.L.; Upton, J.L.; Monsalvo, V.S.; Mata, J.J.; Martínez, A.E.; Martínez, C.R.C. Enraizado de miniestacas de Cedrela odorata L. Agrociencia 2016, 50, 919–929. [Google Scholar]
- Dias, P.C.; Xavier, A.; Oliveira, L.S.; Paiva, H.N.; Correia, A.C.G. Propagação vegetativa de progênies de meios-irmãos de angico-vermelho (Anadenanthera macrocarpa (Benth) Brenan) por miniestaquia. Rev. Árvore 2012, 36, 485–494. [Google Scholar] [CrossRef]
- Lima, C.C.; Ohashi, S.T.; Silveira, A.S. Efeito de diferentes concentrações de aib e procedências geográficas no enraizamento de estacas de paricá. Ciênc. Florest. 2018, 28, 1282–1292. [Google Scholar] [CrossRef]
- Haase, D. Seedling Root Targets. In National Proceedings of the Forest and Conservation Nursery Associations—2010; Riley, L.E., Haase, D.L., Pinto, J.R., Eds.; United States Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 80–82. [Google Scholar]
- Silva, C.P.; Pistori, M.F.; Blini, R.C.B.; Santana, A.P.L. Reguladores vegetais no crescimento in vitro. In Agricultura 4.0, 1st ed.; Zuffo, A.M., Aguilera, J.G., Eds.; Pantanal Editora: Nova Xavantina, Brazil, 2020; pp. 46–57. [Google Scholar]
- Hussain, I.; Assis, A.M.; Yamanoto, L.Y.; Koyama, R.; Roberto, S.R. Indole butyric acid and substrates influence on multiplication of blackberry ‘Xavante’. Ciênc. Rural 2014, 44, 1761–1765. [Google Scholar] [CrossRef]
- Bastos, F.E.A.; Grimaldi, F.; Kretzschmar, A.; Rufato, L. Propagation of native plants with ornamental potential from Serra do Oratório, Santa Catarina State, Brazil. Ornam. Hortic. 2020, 26, 298–305. [Google Scholar] [CrossRef]
- Xavier, A.; Santos, G.A.; Wendling, I.; Oliveira, M.L. Propagação vegetativa de cedro-rosa por miniestaquia. Rev. Árvore 2003, 27, 139–143. [Google Scholar] [CrossRef]
- Gonçalves, J.L.M.; Santarelli, E.G.; Moraes Neto, S.P.; Manara, M.P. Produção de mudas de espécies nativas: Substrato, nutrição, sombreamento e fertilização. In Nutrição e Fertilização Florestal, 1st ed.; IPEF: Piracicaba, Brazil, 2000; pp. 427–450. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer: Sunderland, MA, USA, 2014; p. 700. [Google Scholar]
- Lana, R.M.Q.; Garcia, S.L.R.; Sampaio, R.L.; Leite, I.T. Doses do ácido indolbutírico no enraizamento e crescimento de estacas de eucalipto (Eucalyptus urophylla). Biosci. J. 2008, 24, 13–18. [Google Scholar][Green Version]
- Sahoo, G.; Swamy, S.L.; Singh, A.K.; Mishra, A. Propagation of Pongamia pinnata (L.) Pierre: Effect of auxins, age, season and C/N ratio on rooting of stem cuttings. Trees For. People 2021, 5, 100091. [Google Scholar] [CrossRef]
- Biasi, L.A.; Pereira, J.R.; Cosmo, A.C.; Ayub, R.A. Minicutting Is an Efficient Method for Blueberry Propagation. Int. J. Plant Biol. 2024, 15, 855–864. [Google Scholar] [CrossRef]
- Araújo, E.F.; Gonçalves, E.O.; Santos, A.R.; Gibson, E.L.; Caldeira, M.V.W.; Pezzopane, J.E.M. Controlled release fertilizer in the rooting and performance of clones of Paratecoma peroba. CERNE 2020, 26, 202–211. [Google Scholar] [CrossRef]
- Mantovani, N.; Roveda, M.; Tres, L.; Fortes, F.O.; Grando, M.F. Cultivo de canafístula (Peltophorum dubium) em minijardim clonal e propagação por miniestacas. Ciênc. Florest. 2017, 27, 225–236. [Google Scholar] [CrossRef]
- Quan, J.; Meng, S.; Guo, E.; Zhang, S.; Zhao, Z.; Yang, X. De novo sequencing and comparative transcriptome analysis of adventitious root development induced by indole-3-butyric acid in cuttings of tetraploid black locust. BMC Genom. 2017, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Wendling, I.; Brooks, P.R.; Trueman, S.J. Topophysis in Corymbia torelliana × C. citriodora seedlings: Adventitious rooting capacity, stem anatomy, and auxin and abscisic acid concentrations. New For. 2015, 46, 107–120. [Google Scholar] [CrossRef]
- Azevedo, M.L.; Titon, M.; Machado, E.L.M.; Assis Júnior, S.L.; Freitas, E.C.S. Influência do ácido indolbutírico no enraizamento de miniestacas caulinar e foliar de mogno-africano (Khaya grandifoliola C. DC.). Ciênc. Florest. 2021, 31, 898–919. [Google Scholar] [CrossRef]
- Hunt, G.A. Effect of Styroblock design and copper treatment on morphology of conifer seedlings. In Proceedings of the Western Forest Nursery Association, 13–17 August 1990, Roseburg, OR, USA; Rose, R., Campbell, S.J., Landis, T.D., Eds.; Rocky Mountain Forest and Range Experiment Station, Forest Service, United States Department of Agriculture: Fort Collins, CO, USA, 1990; pp. 218–222. [Google Scholar]
- Carvalho, G.C.M.W.; Pessanha, D.S.; Silva, R.D.; Silva, M.K.F.; Barroso, D.G. Mini-cutting of Plathymenia reticulata Benth. with mini-stumps conducted in suspended seedbed and tubes. CERNE 2021, 27, e102584. [Google Scholar] [CrossRef]
- Gomes, J.M.; Paiva, H.N. Viveiros Florestais; UFV: Viçosa, Brazil, 2011; 116p. [Google Scholar]
- Neimog, W.; Gomes, E.P.; Carvalho, M.B.F.; Mendonça, A. Produção de mudas de mogno (Swietenia macrophylla King) em substrato com resíduos de agroindústria. Sci. For. 2022, 50, e3906. [Google Scholar] [CrossRef]
- Liévano, A.D.; Rodríguez, M.A.; Zaragoza, S.E.; Aldrete, A.; Villarreal, A.W.; Pérez de la O., N.B. Substrates and fertilization to produce Swietenia macrophylla King and Tabebuia donnell-smithii Rose plants in trays. Rev. Mex. Cienc. For. 2023, 14, 56–75. [Google Scholar]
- Ahmed, D.A.E.A.; El-Din, A.S.; EL-Dayem, A.A.; Motawee, M.; EL-Atreby, M. The impact of fertilizing Swietenia mahagoni (L.) Jacq. seedlings under water stress with various nitrogen and phosphorous sources. J. Ecol. Environ. 2025, 49, 94–109. [Google Scholar] [CrossRef]
- Cardoso, A.A.S.; Santos, J.Z.L.; Oka, J.M.; Ferreira, M.S.; Barbosa, T.M.B.; Tucci, C.A.F. Ammonium supply enhances growth and phosphorus uptake of mahogany (Swietenia macrophylla) seedlings compared to nitrate. J. Plant Nutr. 2021, 44, 1349–1364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).