Abstract
Biomass carbon foams are extensively utilized across various fields due to their favorable properties and cost-effectiveness. In this study, triethylene glycol (TEG), nylon 66 (PA66), and 3-glycidyl-oxypropyl-trimethoxy-silane (KH560) were incorporated into pine wood liquefaction resin to successfully prepare three novel modified carbon foams (MCFs), and their characteristics were investigated. The results indicate that the compressive strength and specific surface area of the three MCFs were significantly enhanced. Specifically, the compressive strength increased by 37%, 46%, and 89% following modification with TEG, PA66, and KH560, respectively, while the specific surface areas ranged from 383.4 to 499.3 m2/g. Additionally, the cell structures of the three MCFs exhibited greater uniformity, with larger average pore sizes, thinner ligament thicknesses, and increased opening porosities. Notably, the opening porosity of KH560-modified carbon foam (KH560-PLP-PF-CF) reached its maximum value at 87.95%. XPS analysis confirmed the successful introduction of Si-containing molecular bonds, including Si-OH-Si, Si-OH, and Si-CH, into KH560-PLP-PF-CF. Furthermore, FT-IR analysis revealed characteristic Si-O vibration peaks, PA66 amide peaks, and TEG ether bond absorption peaks in the three MCFs. The incorporation of flexible functional groups effectively enhanced their compressive properties. The findings of this study expand the potential for utilizing biomass waste to partially replace phenol in the development of novel carbon foams.
1. Introduction
Biomass, a naturally carbon-rich and renewable raw material derived from agricultural, forestry, and urban residues, is the only renewable resource capable of generating gas, liquid, and solid fuels, as well as chemical feedstocks [1]. However, conventional disposal techniques not only negatively impact the environment—such as open burning that produces smog and air pollution [2]—but also lead to a significant waste of resources. Therefore, the resource utilization of biomass is crucial for conserving energy, protecting the environment, and enhancing production. Biomass can serve as a feedstock for the preparation of various carbon materials, value-added chemicals, and biofuels. As an abundant and renewable resource, biomass presents an ideal alternative carbon source to fossil fuels [3], allowing for partial replacement in the creation of new carbon materials. This approach can alleviate the depletion of fossil resources, reduce global carbon emissions, and improve the ecological environment while also promoting the high-value applications of biomass waste in the field of carbon materials.
Carbon foam, a lightweight and porous carbon material, possesses a three-dimensional reticular architecture. Because of its low density, high temperature tolerance, ablation resistance, mechanical strength, electrical conductivity, and large surface area [4], it is extensively employed in the production of electrode materials [5,6], heat storage materials [7], adsorbent materials [8,9], wave-absorbing materials [10], catalyst carriers [11,12], and thermal insulation materials [13]. It has exhibited promising prospects for application in the fields of aerospace, wastewater degradation, electronics, and electrical. Currently, carbon foams are predominantly synthesized from precursors, such as phenol formaldehyde resin [14], biomass [9], petroleum pitch [15], polyurethane [16], and coal [17]. Among these, biomass-based carbon foams are particularly advantageous due to their cost-effectiveness, abundance, accessibility, and sustainability compared to fossil-derived resources like graphite, pitch, and organic polymers [18], leading to their widespread application across various fields. Previous research has elucidated methods for the preparation of biomass-based carbon foams. For instance, Sun et al. synthesized carbon foam from V. natans waste biomass by integrating the mole method with ZnCl2 thermal dilation, followed by carbonization at 600 °C for 90 min [19]. Qu et al. obtained carbon foam from lignin using a process that required neither additives nor additional pressure [20]. Additionally, in a separate study, Qu and his co-workers successfully developed a novel lignin-based carbon foam using pure industrial corncob lignin as the feedstock, employing pre-firing and carbonization processes [21].
Nevertheless, the structural brittleness of carbon foams post-carbonization poses a significant barrier to their widespread application. To mitigate this limitation, researchers have increasingly adopted strategies such as the incorporation of various modifiers and the optimization of processing parameters to enhance the structural integrity and properties of carbon foams [22,23,24]. Recent investigations have concentrated on augmenting the mechanical properties of carbon foam through the integration of graphene, nanoparticles, or carbon nanotubes [25,26,27,28]. The fabrication of carbon foam epoxy composites incorporating graphene oxide (GO) has been successfully executed via the vacuum-assisted resin transfer molding (VARTM) technique, resulting in substantial improvements in mechanical performance, with flexural and Mode I fracture properties of the composites enhanced by 25% and 14%, respectively [26]. Li et al. [27] reported the development of graphene-modified carbon foam composites, achieving a 53.41% increase in compressive strength compared to unmodified carbon foam. Similarly, Wang et al. [25] achieved enhancements in the structural and mechanical properties of carbon foam through the addition of multi-walled carbon nanotubes (MWCNTs) and graphene oxide (GO) into the carbon foam matrix. The compressive strength was 19.3 MPa with 2 wt.% MWCNTs-GO additive loadings.
Tang et al. [29] employed triethylene glycol (TEG) as a modifier in the synthesis of TEG-modified phenolic foams (T-Pre-PFs), which demonstrated remarkable toughness. The flexural strength of T-Pre-PF was measured at 0.263 MPa, while its compressive strength reached 0.160 MPa. In a separate study, Zhang et al. [30] developed silicone foam composites (FG-M/SF) by incorporating FG-M as fillers in the presence of triethoxyvinylsilane. The inclusion of FG-M was shown to significantly enhance the electrical, thermal, and mechanical properties of the composites. Despite the prevalent use of triethylene glycol (TEG), nylon 66 (PA66), or 3-glycidyl-oxypropyl-trimethoxy-silane (KH560) as modifiers in the synthesis of modified resins for foam composites, aimed at improving material properties [29,30], there have been no documented instances of subsequent carbonization of these materials to produce carbon foam.
In this study, carbon foam was synthesized utilizing a pine wood liquefaction product resin (PLP resin) as a precursor. For the first time, TEG, KH560, and PA66 were incorporated into the PLP resin as modifiers. The resulting materials, namely pine resin carbon foam (PLP-PF-CF), TEG-modified pine resin carbon foam (TEG-PLP-PF-CF), KH560-modified pine resin carbon foam (KH560-PLP-PF-CF), and PA66-modified pine resin carbon foam (PA66-PLP-PF-CF), were successfully fabricated through foaming and carbonization processes. Comprehensive characterization and analysis of the microstructure, elemental composition, phase composition, compressive strength, and apparent density of the PLP-PF-CF and three MCFs were conducted using FT-IR, SEM, XRD, and XPS. These analyses facilitated the evaluation of the enhancement effects of the three modifiers on the properties of the biomass-based carbon foam, providing valuable insights for the advancement and application of carbon foam materials.
2. Materials and Methods
2.1. Materials
Pine sawdust for this study was obtained from Taida Woodware Factory (Kunming, China), which was dried at 102 °C, pulverized, and sieved through 80 mesh. Phenol, formaldehyde, KH560, and PA66 were purchased from Chengdu Chron Chemicals Co., Ltd. (Chengdu, China). Hydrochloric acid, sulfuric acid, sodium hydroxide, and Tween-80 were provided by Yunnan Yanglin Industrial Development Zone Shandian Drug Co., Ltd. (Kunming, China). TEG was purchased from Shanghai Aladdin Chemical Technology Co., Ltd. (Shanghai, China) The reagents listed above are analytical grade. n-Hexane (analytical grade) and activated carbon (industrial grade) were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China).
2.2. Preparation of Carbon Foams
2.2.1. Liquefaction and Resinification of Pine Powder
Pine wood flour was utilized as a raw material and combined with phenol in a weight ratio of 1:5 within a three-necked flask equipped with a condenser and a stirrer. The mixture was then heated and stirred in an oil bath maintained at 150 °C. Concentrated sulfuric acid, constituting 5% of the phenol’s mass, was subsequently added dropwise to the mixture as a catalyst, allowing the reaction to proceed for 160 min. Upon cooling to room temperature, the liquefaction products were obtained.
The liquefaction products were then placed in a water bath and combined with formaldehyde (37% aqueous solution) at a phenol-to-formaldehyde molar ratio of 1:1.8, alongside a 5 wt% sodium hydroxide solution. The reaction mixture was allowed to react at 60 °C for 30 min, followed by an increase in temperature to 80 °C, which was maintained for 120 min. Subsequently, a 1:1 (v/v) dilution of hydrochloric acid was employed to adjust the pH of the reaction mixture to a range of 7.0–8.0. The resulting product was divided into four equal portions. One portion was directly subjected to rotary evaporation to obtain pine resin (PLP resin), with its viscosity maintained within the range of 500–700 mPa·s. The remaining three portions underwent modification through continuous heating and stirring. Specifically, the first portion was treated with KH560 at 4% of the resin mass, the second with TEG at 15% of the resin mass, and the third with PA66 at 6% of the resin mass. Following thorough mixing, each modified resin was rotary evaporated to separately yield KH560-modified pine resin (KH560-PLP resin), TEG-modified pine resin (TEG-PLP resin), and PA66-modified pine resin (PA66-PLP resin), with all of the resins maintaining a viscosity within the range of 500–700 mPa·s.
2.2.2. Preparation of PLP-PF-CF and MCFs
Samples weighing 20 g each of pine resin and modified pine resin were accurately weighed and placed in plastic cups. Tween-80 (4% of the resin mass), a foaming agent (6% of the resin mass), and hydrochloric acid (5% of the resin mass) were subsequently added. The mixture was homogenized using a high-speed stirrer until a silver-gray consistency was achieved, after which it was poured into a foaming mold, weighed, and then foamed in an oven at 80 °C for 30 min. Following the cooling process, the samples were removed from the oven and demolded, resulting in the production of pine resin foam (PLP-PF), KH560-modified pine resin foam (KH560-PLP-PF), TEG-modified pine resin foam (TEG-PLP-PF), and PA66-modified pine resin foam (PA66-PLP-PF).
The obtained PLP-PF and the resin foams modified with KH560, TEG, and PA66 were processed and shaped into cubic samples. These samples were subsequently placed in a vacuum tube furnace under nitrogen protection and subjected to a programmed heating carbonization treatment. The heating program was as follows: the temperature was increased from room temperature to 500 °C at a rate of 5 °C/min, followed by an increase from 500 °C to 700 °C at a rate of 2 °C/min. This temperature was maintained for 2 h before allowing the samples to cool naturally to room temperature. Consequently, PLP-PF-CF, TEG-PLP-PF-CF, KH560-PLP-PF-CF, and PA66-PLP-PF-CF were prepared, respectively.
2.3. Properties Characterization
Fourier transform infrared spectroscopy (FT-IR, TENSOR27, BRUKER, Karlsruhe, Germany) was employed to investigate the functional groups in carbon foams both before and after modification. The morphology of the carbon foams was investigated by a scanning electron microscopy–energy dispersive spectrometer (SEM-EDS, VEGA3SBH, Tesken Trading Co., Ltd., Shanghai, China), operating at a 10 kV acceleration voltage following gold sputtering of the samples. The surface elements of the carbon foams were characterized by X-ray photoelectron spectroscopy (XPS, K-Alpha+, Thermo Fisher Scientific, Waltham, MA, USA). The structural properties of the carbon foams were analyzed using X-ray diffraction (XRD, TTRIII, Beijing Physics and Chemistry Saisi Technology Co., Ltd., Beijing, China), employing Cu Kα1 radiation with a wavelength (λ) of 1.5406 Å, a tube voltage of 40 kV, and a tube current of 30 mA. The scanning range was set from 10 to 90° with a step size of 0.01° and a scanning rate of 5°/min. Additionally, the surface area and porosimetry instrument (ASAP-2000, Micromeritics, GA, USA) was used for N2 adsorption–desorption analysis. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method, while the t-plot model was employed to determine the micropore volume and average pore diameter.
The opening porosity was determined using a fully automated true density analyzer (3H-2000 TD1, Beishide Instrument Technology (Beijing) Co., Ltd., Beijing, China).
The compressive strength of the carbon foams was assessed using an electronic universal testing machine (FR100-C, Shenzhen Sansi Zongheng Technology Co., Ltd., Shenzhen, China). The samples were cut into cubes with a height of 20 mm. Compression Platens were employed at a displacement rate of 2 mm/min. Three specimens from each sample were tested, and the results were averaged. The compressive strength (σ) was calculated using the following Equation (1):
where σ represents the compressive strength (MPa), P denotes the maximum failure load (N), and A is the cross-sectional area (mm2).
The volume shrinkage rate (S) was calculated using the following Formula (2):
where S is the volume shrinkage rate (%), V0 is the volume of the foam before combustion (cm3), and V1 is the volume of the foam after combustion (cm3).
3. Results and Discussion
3.1. Carbonization Temperature Effects on Carbon Foam Volume Stability
In this study, carbon foam is produced from lignocellulose-based organic polymers, which must first be foamed and then carbonized at high temperatures while being protected by inert gas. The temperature at which the resin is fully carbonized significantly influences the morphology, microstructure, and mechanical properties of the carbon foam [31]. Thus, it is crucial to investigate this aspect. After 2 h at 700 °C, the PLP-PF-CF, TEG-PLP-PF-CF, KH560-PLP-PF-CF, and PA66-PLP-PF-CF did not exhibit any deformation or collapse, as shown in Figure 1 and Figure 2. The axial and radial shrinkage rates were nearly identical, demonstrating a consistent trend as the carbonization temperature increased, which suggests stable foam synthesis and structural integrity. As illustrated in Figure 1a, at a carbonization temperature of 400 °C, the axial, radial, and volumetric shrinkage rates of PLP-PF-CF were 8.1%, 7.9%, and 24.7%, respectively. At 700 °C, these rates increased to 20.0%, 20.1%, and 47.1%, representing increases of 2.46, 2.5, and 1.9 times, respectively. When comparing the other two MCFs (TEG-PLP-PF-CF and PA66-PLP-PF-CF), KH560-PLP-PF-CF exhibited slightly higher axial, radial, and volumetric shrinkage rates (see Figure 1b,c). However, in general, the MCFs displayed higher shrinkage rates than PLP-PF-CF, indicating that the addition of modifiers facilitates the foaming of the resin. When the carbonization temperature was raised to 800 °C, the axial, radial, and volumetric shrinkage rates of the four carbon foams only showed a slight increase compared to those at 700 °C. Therefore, it can be inferred that when the carbonization temperature exceeds 700 °C, the volumes of the carbon foams remain relatively constant.
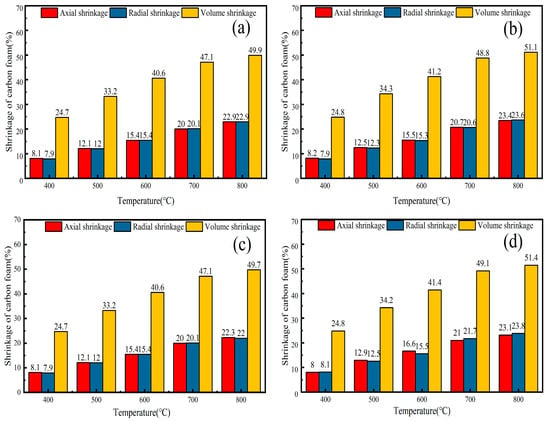
Figure 1.
The shrinkage rates of PLP-PF-CF (a), TEG-PLP-PF-CF (b), PA66-PLP-PF-CF (c), and KH560-PLP-PF-CF (d) at different carbonization temperatures.
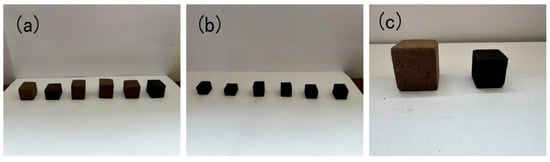
Figure 2.
PFs and corresponding carbon foams before (a) and after (b) carbonization at 700 °C and close comparison before and after carbonization (c) at 700 °C.
3.2. Structural Characterization and Physicochemical Properties of Carbon Foams
To investigate the effect of different modifiers on the microstructure of carbon foam, we analyzed the microstructures of carbon foams derived from pine resin foam, TEG-modified pine resin foam, KH560-modified pine resin foam, and PA66-modified pine resin foam after carbonization at 700 °C for 2 h. SEM images of the four carbon foams are shown in Figure 3. As illustrated, all four carbon foams exhibited honeycomb-like architectures with cells approximating spherical shapes, indicating that the foam structures can be achieved regardless of whether modifiers are added or not. However, the cell size of the resulting carbon foams grew to varied degrees when the modifiers were added. Notably, KH560-PLP-PF-CF demonstrated the most significant increase, with an average pore size approximately twice that of PLP-PF-CF, reaching 82 µm (Table 1). Furthermore, the ligament thickness between the pores became noticeably thinner, and the cell structure more uniform, suggesting that KH560 facilitated the foaming process of the resin. As shown in Table 1, the larger the pore size, the thinner the cell wall thickness. Combined with the data on opening porosity in Table 1, it was observed that the opening porosity of PLP-PF-CF was 70.66%, which was lower than that of the three MCFs. The opening porosity of the three MCFs increased, which can be attributed to the enlargement of the average pore size and the thinning of ligaments [32]. Among the three MCFs, KH560-PLP-PF-CF exhibited the highest open porosity at 87.95%, a 17% increase compared to PLP-PF-CF. Additionally, the pHPZC and the water contact angles of the four carbon foams were approximately the same. The pHPZC was around 7.5, and the water contact angles were approximately 120°, all exceeding 90°, indicating that the carbon foams were hydrophobic materials.
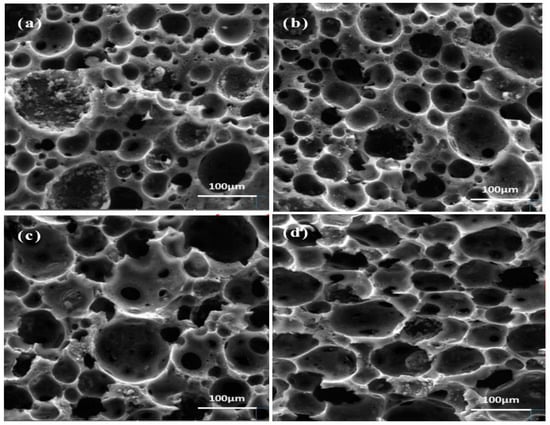
Figure 3.
SEM images of PLP-PF-CF (a), TEG-PLP-PF-CF (b), PA66-PLP-PF-CF (c), and KH560-PLP-PF-CF (d).

Table 1.
Physical properties of different carbon foams.
3.3. Analysis of Specific Surface Area, Pore Volume, and Micropore Size of Carbon Foams
Figure 4 illustrates the N2 adsorption–desorption isotherms (a) and pore size distribution curves (b) for PLP-PF-CF, TEG-PLP-PF-CF, KH560-PLP-PF-CF, and PA66-PLP-PF-CF. As depicted in Figure 4a, the adsorption–desorption isotherms of the four carbon foams are classified as type IV according to the IUPAC standard for physical adsorption isotherms, indicating a strong interaction between the carbon foams and nitrogen, with a hysteresis loop of H4 type. Additionally, Figure 4b reveals that the pores of the carbon foam consist of both micropores and mesopores. Table 2 presents the specific surface area (SBET), average pore diameter (Dpore), total pore volume (Vtotal), and micropore volume (Vmicro), all calculated using the BET method and the t-plot model. The results demonstrate that the specific surface areas of PA66-PLP-PF-CF and KH560-PLP-PF-CF are significantly larger than that of PLP-PF-CF (354.0 m2/g), reaching 471.0 m2/g and 499.3 m2/g, respectively. This corresponds to an improvement of 33% and 44%, with both the micropore volume and total pore volume also showing some increase. From this analysis, several observations can be made: (1) Regardless of whether the PLP-PF resin is modified, a significant number of micropores can form in the resulting carbon foam, produced during the foaming and pyrolysis processes of the resin. (2) The three modifiers, namely TEG, PA66, and KH560, enhance the formation of micropores on the surface of carbon foam cell walls. (3) Microstructural characteristics such as pore size and cell wall thickness significantly influence the specific surface area, total pore volume, and micropore volume of the carbon foam. Appropriate pore sizes and cell wall thicknesses facilitate the formation of additional micropores, whereas excessively large pore sizes and overly thin cell walls impede micropore development.
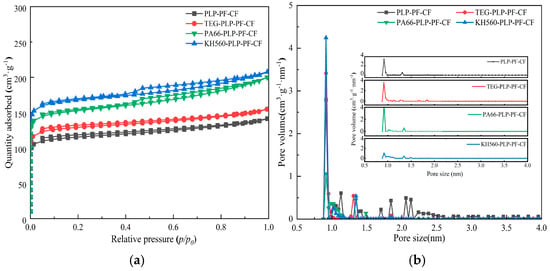
Figure 4.
N2 adsorption–desorption isotherms (a) and pore size distribution curves (b) of carbon foams.

Table 2.
The specific surface area, pore capacity, and average pore size of different carbon foams.
3.4. Mechanical Properties of Carbon Foams
The mechanical characteristics of carbon foam are strongly linked to its apparent density; the lower the density, the more porous the structure of the carbon foam, which results in lower compressive strength. Conversely, higher density results in greater compressive strength in the carbon foam. The compressive strength and volume shrinkage of the carbon foam before and after carbonization were analyzed by measuring the foam’s volume before and after combustion, and the maximum compressive stress was determined. As shown in Table 3, the carbon foams experienced significant volume shrinkage after carbonization, almost 50%. This was attributed to the loss of water, residual phenolic compounds from resin synthesis, and the decomposition of small molecules as the combustion temperature increased during carbonization. However, after modification with TEG, PA66, and KH560, the compressive strength of the carbon foams notably improved, while the effect of apparent density became less significant. Specifically, the modified carbon foams exhibited a 37%, 46%, and 89% increase in compressive strength, respectively, compared to PLP-PF-CF, with KH560-PLP-PF-CF showing the maximum value of 3.40 MPa. The apparent density of PLP-PF-CF exhibited the largest change before and after carbonization, almost halving, while the MCFs showed much smaller changes, with a maximum reduction of only 18%. This indicates that the rate of mass reduction of the MCFs was lower than that of PLP-PF-CF.

Table 3.
Volume shrinkage and compressive strength of different carbon foams.
Carbon foams are classified as elastic brittle materials, characterized by a tendency for stress to increase rapidly with strain, followed by a fluctuating plateau, which is a typical feature of such materials [33,34]. Figure 5 illustrates the stress–strain curves of carbon foams. Due to their inherent structure, which includes cell walls, ligaments, and joint structures, all four types of carbon foam displayed a sharp increase in stress with a minimal strain when subjected to external loading. Consequently, the stress–strain curves exhibited a rapid increase. As the load intensified, local cell walls, ligaments, or joints within the carbon foam began to fracture and fail, leading to a decrease in stress with increasing strain. Subsequently, as the indenter load penetrated further, damage progressively propagated from the contact surface into the internal layers of the carbon foam. This behavior resulted in the stress–strain curves of carbon foams displaying an extended sawtooth pattern. Overall, the stress levels of the MCFs were higher than those of PLP-PF-CF, as shown in Figure 5. This difference can be attributed to the mechanical enhancements provided by the modifiers. Specifically, PA66, known for its excellent mechanical strength and toughness, reinforced the resin foam matrix. Additionally, the hydrolysis of KH560 facilitated the formation of flexible, long-chain silanol groups bonded to the resin, which increased the strain capacity of the carbon foam. The flexible ether linkages of TEG and the resin contribute to this chemical modification. Therefore, the stress levels of the MCFs were higher than those of PLP-PF-CF.
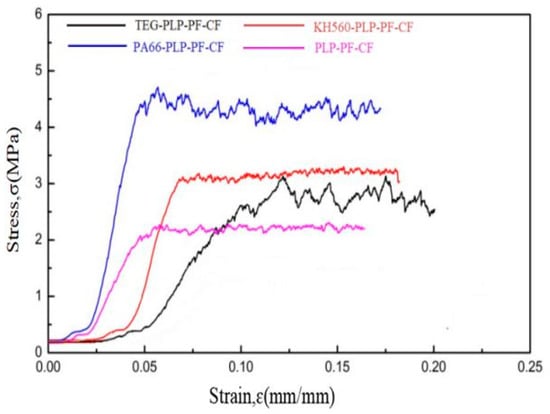
Figure 5.
Stress–strain curves of carbon foams.
To further analyze the mechanical properties of the four carbon foams, the results of this study were compared with those from other relevant research. Table 4 presents the modifiers utilized in the various modified bio-based carbon foams, listed in the following order from top to bottom: calcium oxide, carbon nanotubes, activated carbon, kaolinite clay, and graphite powder. According to the data in Table 4, with the exception of graphite powder and activated carbon modified carbon foams, the compressive strength of the other three modified carbon foams is significantly lower than that of the three modified carbon foams developed in this study (2.46–3.40 MPa). Notably, the compressive strength of the activated carbon-modified carbon foam aligns with that of KH560-PLP-PF-CF from this study. This finding indicates that the three modified carbon foams produced in this study exhibit a distinct advantage in compressive performance. The incorporation of these modifiers substantially enhances the mechanical properties of carbon foam, underscoring the potential of these materials for practical applications.

Table 4.
Compressive strength of other studies.
3.5. XPS Analysis
The XPS test provides detailed insights into the elemental composition of solid material surfaces. The wide scan results (Figure 6a–d) indicate the presence of elements such as carbon, sodium, sulfur, and oxygen in all carbon foams. As shown in Figure 6a, the PLP-PF-CF obtained after carbonization at 700 °C is primarily composed of 85.69% C, 11.02% O, 1.6% Na, 0.71% S, and 0.41% Cl. Additionally, silicon (Si) was detected in KH560-PLP-PF-CF, with a relative atomic content of 3.47%, confirming the successful modification of KH560 on the surface of the resin. The high-resolution XPS spectra for the C 1s and O 1s regions are presented in panels a1–a4 and b1–b4 of Figure 7, respectively. The C 1s spectra of the PLP-PF-CF (Figure 7(a1)) reveal three distinct peaks, attributed to aromatic carbon C-C (284.8 eV) [40,41], phenolic C-O (285.5 eV) [42], and carboxylated O-C=O (289.8 eV) [43]. Notably, C-C exhibits the highest relative content at 52.5%. The next is the C-O bond, with a relative content of 37%, while the O=C-O bond has the lowest relative content, at 10.6%. These results suggest that even at a carbonization temperature of 700 °C, a small quantity of oxygen-containing functional groups, such as phenolic C-O and carboxyl O-C=O groups, persist on the surface of the carbon foams. The O 1s spectra of the PLP-PF-CF (Figure 7(b1)) indicate four distinct peaks, with O-C-OC-OH representing the highest proportion at 46.4%, while O-H accounts for the lowest at 5.3%.
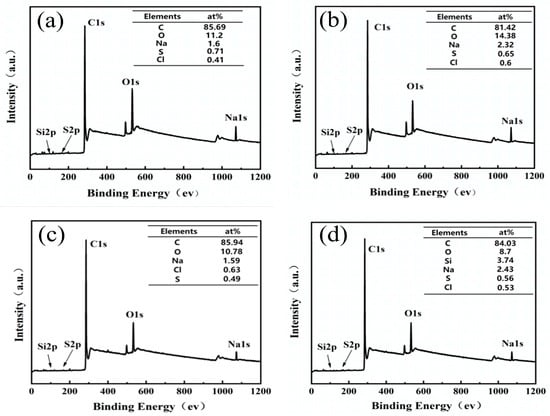
Figure 6.
XPS full spectra relative atomic content of elements of PLP-PF-CF (a), TEG-PLP-PF-CF (b), PA66-PLP-PF-CF (c), and KH560-PLP-PF-CF (d).
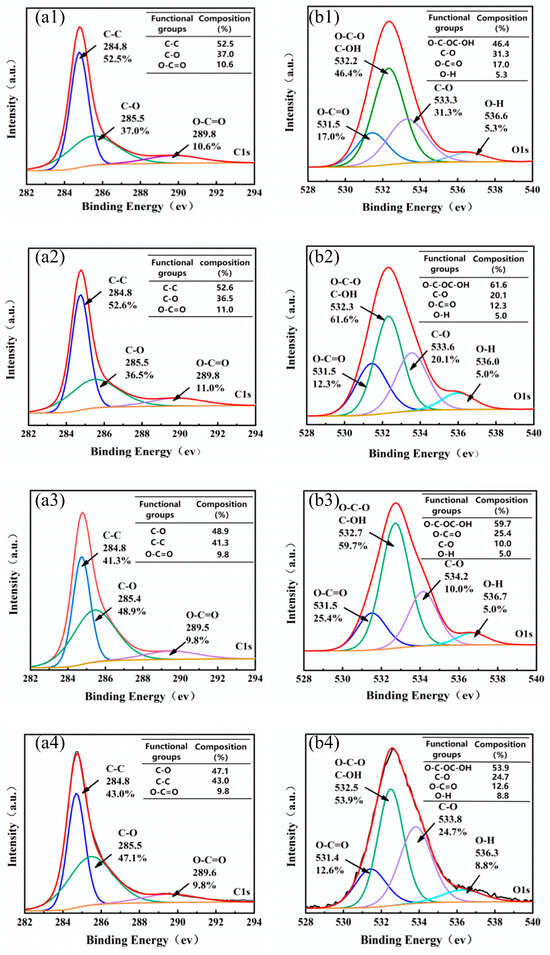
Figure 7.
C 1s spectrum and relative content of different carbon bonds of PLP-PF-CF (a1), TEG-PLP-PF-CF (a2), PA66-PLP-PF-CF (a3), and KH560-PLP-PF-CF (a4). O 1s spectrum and relative content of different carbon bonds of PLP-PF-CF (b1), TEG-PLP-PF-CF (b2), PA66-PLP-PF-CF (b3), and KH560-PLP-PF-CF (b4).
When comparing the C 1s spectra of PLP-PF-CF to those of the three MCFs, the MCFs exhibited 52.6%, 41.3%, and 43% of aromatic carbon C-C, alongside 36.5%, 48.9%, and 47.1% of phenolic C-O, respectively. The C-C content in the MCFs decreased by approximately 4%–5%, while the C-O content increased by a similar margin. In the O 1s spectra, the proportion of O-C-OC-OH in the MCFs rose to 61.6%, 59.7%, and 53.9%, respectively, indicating that the original bonds were broken and that the modifiers bonded more strongly to this functional group. Conversely, the C-O content decreased to 20.1%, 10%, and 24.7%, respectively, suggesting that further reactions occurred on the C-O after modification. Furthermore, the Si element appeared following modification (Figure 8), confirming that KH560 successfully introduced Si atoms into the PLP resin. The Si 2p spectra (Figure 8) reveal three specific peaks corresponding to the silicon atoms from the Si-O-Si (103.2 eV), Si-OH (102 eV), and Si-CH3 (104.1 eV) groups. These results indicate that new Si bonds were successfully formed during the reaction between KH560 and PLP resin, contributing to the improved toughness and compressive strength of the carbon foam.
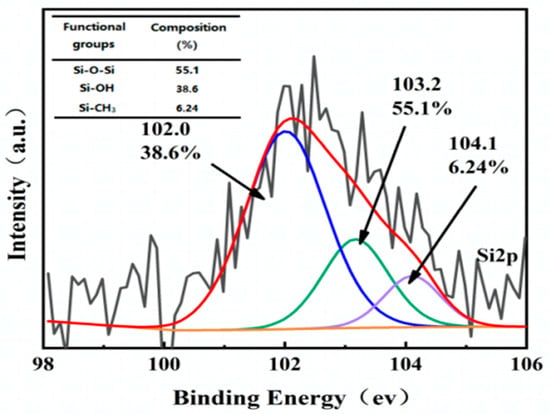
Figure 8.
Si 2p spectrum and relative content of different of KH560-PLP-PF-CF.
3.6. FT-IR Analysis
Figure 9 illustrates the changes in the functional groups of PLP-PF-CF, TEG-PLP-PF-CF, KH560-PLP-PF-CF, and PA66-PLP-PF-CF. The FT-IR curves of these four carbon foams exhibit significant similarities, indicating that the resin foams formed after the foaming and curing processes of the three modified resins retained functional group compositions consistent with those of the unmodified resin. This observation suggests that the principal characteristics of the liquefaction product resin were preserved. In the infrared spectrum of both the MCFs and PLP-PF-CF, the first peak, observed between 3200 and 3600 cm−1, is attributed to the stretching vibration of O-H groups in alcohol and phenol hydroxyls [44,45]. The second peak, characterized by medium-intensity vibration between 1510 and 1600 cm−1, corresponds to non-conjugated β-carbonyl groups in lignin, carbonyl in polysaccharides, non-conjugated C=O in ester groups, and C=C ring skeleton vibration [46]. The peak at 1384 cm−1 is associated with the in-plane bending vibration of C-H. The fourth peak, which appears between 800 and 1209 cm−1, primarily consists of the stretching vibration peaks of C-C [47], C-O [48], and C-O-C [49]. Additionally, the peak at 740 cm−1 is linked to the out-of-plane bending vibration of the C-H group on the benzene ring [50,51]. Furthermore, compared to PLP-PF-CF, the characteristic peaks of KH560-PLP-PF-CF at 3400 cm−1 were significantly enhanced, with symmetric and asymmetric stretching vibration peaks of the Si-O group appearing at 796 and 1051 cm−1, respectively [51]. The adsorption peak of Si-O-C at 1051 cm−1 in the KH560-PLP resin indicates that the resin successfully reacted with KH560, effectively incorporating silicon into the resin. These findings are consistent with the results of the XPS analysis. For TEG-PLP-PF-CF, distinct peaks were observed around 3000 cm−1, primarily due to the presence of characteristic ether bonds following modification. For PA66-PLP-PF-CF, an amide characteristic peak was observed at 1653 cm−1, while nylon characteristic peaks were noted at 3297 and 1540 cm−1, indicating the formation of chemical bonds between PLP-PF and PA66.
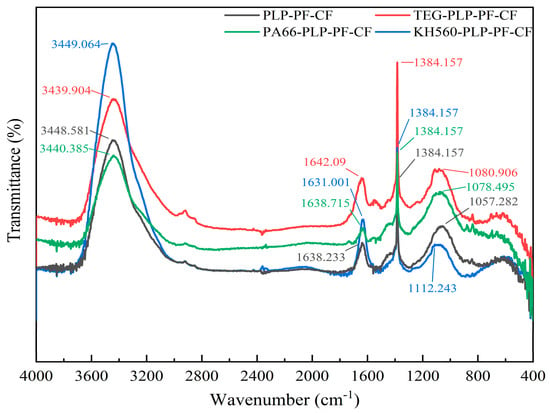
Figure 9.
FT-IR curves of different carbon foams.
3.7. XRD Analysis of Carbon Foams
XRD is a critical tool for analyzing the structure of solid materials. To investigate the effects of the three modifiers on PLP-PF-CF, XRD characterization was employed to assess the crystallinity of the carbon foams. Figure 10 presents the XRD patterns of the four carbon foams. The presence of diffuse diffraction peaks in the XRD pattern indicates that the carbon foams contain a significant amount of amorphous carbon. Additionally, sharp characteristic peaks were observed at 38°, 43.5°, and 63° in the XRD patterns of the three MCFs, which correspond to the characteristic peaks of NaCl. This finding suggests the presence of NaCl crystals in the MCFs, which can be attributed to the use of NaOH and HCl during the preparation of PLP resin in the pre-experimental stage, where these reagents reacted to produce NaCl crystals [52]. Compared to the XRD pattern of PLP-PF-CF, the intensity of the peak at 38° was significantly higher in the MCFs, indicating an increased content of the modified crystalline phase. Among the three MCFs, KH560-PLP-PF-CF exhibited the highest intensity at this peak, suggesting that KH560 most effectively enhanced the content of the crystalline phase in the carbon foam.
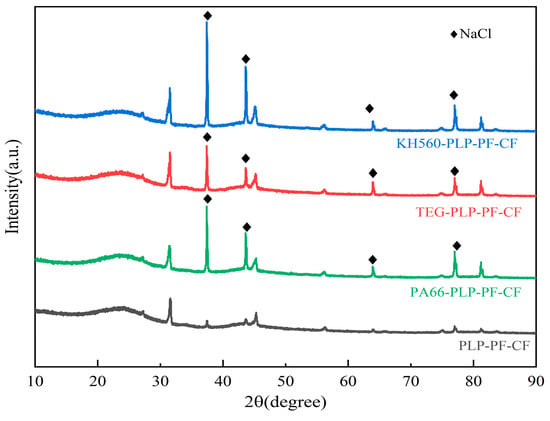
Figure 10.
XRD of different carbon foams.
4. Conclusions
This study successfully developed novel carbon foam materials characterized by high compressive strength and a large specific surface area, utilizing unseparated and unpurified liquefied pine whole products. The modifiers employed were TEG, PA66, and KH560. Compared to PLP-PF-CF, the compressive strength and specific surface area of the three MCFs were noticeably enhanced, with compressive strength values ranging from 2.46 to 3.4 MPa. Notably, KH560-PLP-PF-CF demonstrated the highest specific surface area, measuring 499.3 m2/g. SEM analysis indicated that KH560-PLP-PF-CF possessed a more uniform cell structure and the highest opening porosity at 87.95%, suggesting that KH560 was the most effective modifier. Furthermore, KH560-PLP-PF-CF successfully formed silicon-containing molecular bonds, which contributed to improved toughness and compressive strength. Additionally, the presence of the Si-O characteristic peak specific to KH560, the PA66 amide characteristic peak, and the TEG ether bond characteristic peak were observed, which effectively enhanced the flexibility of the carbon foam. Meanwhile, the crystalline phase content of all three MCFs increased significantly. Therefore, we can optimize the mechanical properties of carbon foam by effectively adjusting its structure through the addition of TEG, PA66, and KH560. Moreover, it provides a new idea for the high-value application and disposal of biomass waste.
Author Contributions
Conceptualization, S.L. (Shiyu Lu), J.L. (Jianwei Ling) and J.L. (Jianxiang Liu); methodology, S.L. (Shiyu Lu), J.L. (Jianwei Ling), S.L. (Shouqing Liu) and X.L.; data curation, S.L. (Shiyu Lu), J.L. (Jianwei Ling) and X.L.; writing—original draft preparation, S.L. (Shiyu Lu); writing—review and editing, S.L. (Shiyu Lu) and J.L. (Jianxiang Liu); funding acquisition, J.L. (Jianxiang Liu). All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (32460370).
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Ge, L.; Zhao, C.; Zuo, M.; Tang, J.; Ye, W.; Wang, X.; Zhang, Y.; Xu, C. Review on the preparation of high value-added carbon materials from biomass. J. Anal. Appl. Pyrolysis 2022, 168, 105747. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, Y.; Balasubramanian, R. Synthesis of biowaste-derived carbon foam for CO2 capture. Resour. Conserv. Recycl. 2022, 185, 106453. [Google Scholar] [CrossRef]
- Gou, G.; Huang, F.; Jiang, M.; Li, J.; Zhou, Z. Hierarchical porous carbon electrode materials for supercapacitor developed from wheat straw cellulosic foam. Renew. Energy 2020, 149, 208–216. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S.; Tian, N.; Yan, F.; You, C.; Yang, Y. Carbon foams: 3D porous carbon materials holding immense potential. J. Mater. Chem. A 2020, 8, 23699–23723. [Google Scholar] [CrossRef]
- Cao, M.; Feng, Y.; Tian, R.; Chen, Q.; Chen, J.; Jia, M.; Yao, J. Free-standing porous carbon foam as the ultralight and flexible supercapacitor electrode. Carbon 2020, 161, 224–230. [Google Scholar] [CrossRef]
- Yang, N.; Ji, L.; Fu, H.; Shen, Y.; Wang, M.; Liu, J.; Chang, L.; Lv, Y. Hierarchical porous carbon derived from coal-based carbon foam for high-performance supercapacitors. Chin. Chem. Lett. 2022, 33, 3961–3967. [Google Scholar] [CrossRef]
- Ola, O.; Chen, Y.; Zhu, Y. Three-dimensional carbon foam nanocomposites for thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 191, 297–305. [Google Scholar] [CrossRef]
- Qi, J.; Wei, G.; Sun, X.; Wang, L.; Li, J. Enhanced removal for H2S by Cu-ordered mesoporous carbon foam. J. Hazard. Mater. 2020, 396, 122710. [Google Scholar] [CrossRef] [PubMed]
- Licona-Aguilar, A.I.; Torres-Huerta, A.M.; Dominguez-Crespo, M.A.; Palma-Ramirez, D.; Conde-Barajas, E.; Negrete-Rodriguez, M.X.L.; Rodriguez-Salazar, A.E.; Garcia-Zaleta, D.S. Reutilization of waste biomass from sugarcane bagasse and orange peel to obtain carbon foams: Applications in the metal ions removal. Sci. Total Environ. 2022, 831, 154883. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Zheng, S.; Wang, F.; Liu, Y.; Liu, J. High-performance microwave absorption of MOF-derived Co3O4@N-doped carbon anchored on carbon foam. J. Colloid Interface Sci. 2021, 602, 197–206. [Google Scholar] [CrossRef]
- Varila, T.; Mäkelä, E.; Kupila, R.; Romar, H.; Hu, T.; Karinen, R.; Puurunen, R.L.; Lassi, U. Conversion of furfural to 2-methylfuran over CuNi catalysts supported on biobased carbon foams. Catal. Today 2021, 367, 16–27. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, C.; Song, L.; Du, P.; Yang, Y.; Xiong, J. A facile controllable preparation of highly porous carbon foam and its application in photocatalysis. Mater. Res. Bull. 2020, 122, 110697. [Google Scholar] [CrossRef]
- Alifanov, O.M.; Budnik, S.A.; Nenarokomov, A.V.; Salosina, M.O. Design of thermal protection based on open cell carbon foam structure optimization. Appl. Therm. Eng. 2020, 173, 115252. [Google Scholar] [CrossRef]
- Wang, C.; Peng, L.; Shi, Z.-h.; Li, B.-l.; Li, K.-z. Preparation, thermal stability and deflection of a density gradient thermally-conductive carbon foam material derived from phenolic resin. Results Phys. 2019, 14, 102448. [Google Scholar]
- Yu, M.; Ao, X.; Chen, Q. Fabrication of ultralight reticulated carbon foams for thermal insulation from raffinate pitch of low-temperature coal tar. J. Anal. Appl. Pyrolysis 2022, 163, 105494. [Google Scholar] [CrossRef]
- Ge, C.; Song, J.; Qin, Z.; Wang, J.; Fan, W. Polyurethane Foam-Based Ultramicroporous Carbons for CO2 Capture. ACS Appl. Mater. Interfaces 2016, 8, 18849–18859. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Diez, M.A.; Antuña-Nieto, C.; López-Antón, M.A.; García, R.; Martínez-Tarazona, M.R. An insight into the role of biomass, biocompounds and synthetic polymers as additives to coal for the synthesis of carbon foams. J. Anal. Appl. Pyrolysis 2021, 160, 105359. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Tan, J.; Ma, C.; Luo, S.; Li, W.; Liu, S. Hierarchical porous graphene oxide/carbon foam nanocomposites derived from larch for enhanced CO2 capture and energy storage performance. J. CO2 Util. 2021, 52, 101666. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Yuan, D.; Yu, Z. Adsorption of nitroimidazole antibiotics from aqueous solutions on self-shaping porous biomass carbon foam pellets derived from Vallisneria natans waste as a new adsorbent. Sci. Total Environ. 2019, 664, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Zhao, Z.; Liang, C.; Hu, P.; Ma, Z. Simple, additive-free, extra pressure-free process to direct convert lignin into carbon foams. Int. J. Biol. Macromol. 2022, 209, 692–702. [Google Scholar] [CrossRef]
- Liang, C.; Xia, H.; Yin, L.; Du, C.; Wu, X.; Wang, J.; Li, S.; Xu, J.; Zhang, X.; Wang, Y.; et al. Carbon foam directly synthesized from industrial lignin powder as featured material for high efficiency solar evaporation. Chem. Eng. J. 2024, 481, 148375. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, X.; Liu, X.; Xu, Y.; Sun, Z.; Zhang, L.; Huang, Z.; Mi, R.; Min, X. Polyethylene glycol/modified carbon foam composites for efficient light-thermal conversion and storage. Polymer 2021, 228, 123894. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Zhang, K.; Liu, H.; Luo, B.; Lin, Q. Preparation of near net-shape carbon foams from allyl COPNA-modified bismaleimide resin: Structures and properties. J. Anal. Appl. Pyrolysis 2016, 117, 125–131. [Google Scholar] [CrossRef]
- Song, W.; Cui, S.; Zhang, J.; Fan, S.; Chen, L.; Zhang, H.M.; Zhang, Y.; Meng, X. Three-Dimensional Carbon Foam Modified with Mg3N2 for Ultralong Cyclability of a Dendrite-Free Li Metal Anode. ACS Appl. Mater. Interfaces 2023, 15, 9421–9430. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Khan, M.; Hussain, A.; Khan, I.; Nawaz, A.; Ragab, A.H.; Sayqal, A.; Lei, T.; Zada, A. Carbon foam composites containing carbon nanotubes and graphene oxide as additives for enhanced mechanical, thermal, electrical and catalytic properties. J. Mater. Res. Technol. 2023, 24, 608–622. [Google Scholar] [CrossRef]
- Bhanuprakash, L.; Parasuram, S.; Varghese, S. Experimental investigation on graphene oxides coated carbon fibre/epoxy hybrid composites: Mechanical and electrical properties. Compos. Sci. Technol. 2019, 179, 134–144. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Deng, W.; Kong, S. Preparation and Adsorption Properties of Graphene-Modified, Pitch-Based Carbon Foam Composites. Polymers 2022, 14, 4455. [Google Scholar] [CrossRef]
- Xie, Y.; Xiao, S.; Chen, W.; Hu, X.; Liu, Y.; Jiang, L.; Luo, L.; Luo, W.; Ma, Y.; Jiang, X.; et al. Shape-stabilized nanosilver-modified grapefruit peel-based porous carbon composite phase change material with high thermal conductivity, photothermal conversion performance and thermal management capability. J. Energy Storage 2024, 83, 110819. [Google Scholar] [CrossRef]
- Tang, K.; Tang, X.; Liu, X.; Zhang, A.; Ge, T.; Li, Y. Phenolic Foams Toughened with Triethylene Glycol by In Situ Polymerization and Prepolymerization Processes. ACS Appl. Polym. Mater. 2022, 4, 8303–8314. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zang, C.G.; Jiao, Q.J. Electrical, thermal, and mechanical properties of silicone foam composites filled with carbon-based nanofillers. J. Appl. Polym. Sci. 2020, 137, 49191. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Yang, L.; Xu, T.; Wu, H.; Zhang, J.; He, L. Effect of pre-heat treatment and carbonization temperature on comprehensive properties of melamine-derived carbon foam. Ceram. Int. 2024, 50, 31548–31558. [Google Scholar] [CrossRef]
- Banerjee, C.; Chandaliya, V.K.; Dash, P.S.; Meikap, B.C. Effect of different parameters on porosity and compressive strength of coal tar pitch derived carbon foam. Diamond Relat. Mater. 2019, 95, 83–90. [Google Scholar] [CrossRef]
- Nagel, B.; Pusz, S.; Trzebicka, B. Tailoring the properties of macroporous carbon foams. J. Mater. Sci. 2014, 49, 1–17. [Google Scholar] [CrossRef]
- Seo, J.; Park, H.; Shin, K.; Baeck, S.H.; Rhym, Y.; Shim, S.E. Lignin-derived macroporous carbon foams prepared by using poly (methyl methacrylate) particles as the template. Carbon 2014, 76, 357–367. [Google Scholar] [CrossRef]
- Wu, H.; Pu, X.; Li, X.; Li, T.; Jiang, S.; Liu, S. Self-foaming calcium modified carbon foam derived from a liquefied product resin of tobacco stalks for the removal of Pb2+ in water. Ind. Crop. Prod. 2024, 216, 118743. [Google Scholar] [CrossRef]
- Farhan, S.; Wang, R.; Jiang, H.; Li, K.; Wang, C. A novel combination of simple foaming and freeze-drying processes for making carbon foam containing multiwalled carbon nanotubes. Ceram. Int. 2016, 42, 8980–8989. [Google Scholar] [CrossRef]
- Narasimman, R.; Prabhakaran, K. Preparation of carbon foams by thermo-foaming of activated carbon powder dispersions in an aqueous sucrose resin. Carbon 2012, 50, 5583–5593. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Wu, X.; Huang, J.; Chen, K.; Fang, M.; Liu, Y. Preparation, microstructure, and compressive strength of carbon foams derived from sucrose and kaolinite. J. Mater. Res. 2014, 29, 1018–1025. [Google Scholar] [CrossRef]
- Jana, P.; Fierro, V.; Celzard, A. Sucrose-based carbon foams with enhanced thermal conductivity. Ind. Crop. Prod. 2016, 89, 498–506. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, Z.; Guo, T.; Huang, X.; Zhu, T.; Tang, X.-Z. Three-dimensional carbon foam modified with starlike-ZnO@ reduced graphene oxide for microwave absorption with low filler content. J. Alloys Compd. 2022, 897, 163200. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Liu, D.; Gai, L.; Wang, Y.; Wang, P.; Han, X.; Du, Y. A Self-foaming Strategy to Construct Small Mo2C Nanoparticles Decorated 3D Carbon Foams as Superior Electromagnetic Wave Absorbing Materials with Strong Corrosion Resistance. Small Methods 2024, 9, 2400734. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Li, S.; Wang, D.; Zheng, Z. Rational design of freestanding and high-performance thick electrode from carbon foam modified with polypyrrole/polydopamine for supercapacitors. Chem. Eng. J. 2022, 447, 137562. [Google Scholar] [CrossRef]
- Zhang, R.; Gu, X.; Liu, Y.; Hua, D.; Shao, M.; Gu, Z.; Wu, J.; Zheng, B.; Zhang, W.; Li, S.; et al. Hydrophilic nano-porous carbon derived from egg whites for highly efficient capacitive deionization. Appl. Surf. Sci. 2020, 512, 145740. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, R.; Zhou, Y.; Lin, Q.; Fang, C. A novel surface-oxidized rigid carbon foam with hierarchical macro-nanoporous structure for efficient removal of malachite green and lead ion. J. Mater. Sci. Technol. 2022, 103, 15–28. [Google Scholar] [CrossRef]
- Laksaci, H.; Khelifi, A.; Trari, M.; Addoun, A. Synthesis and characterization of microporous activated carbon from coffee grounds using potassium hydroxides. J. Clean. Prod. 2017, 147, 254–262. [Google Scholar] [CrossRef]
- Song, M.-X.; Xie, L.-J.; Cheng, J.-Y.; Yi, Z.-L.; Song, G.; Jia, X.-Y.; Chen, J.-P.; Guo, Q.-G.; Chen, C.-M. Insights into the thermochemical evolution of maleic anhydride-initiated esterified starch to construct hard carbon microspheres for lithium-ion batteries. J. Energy Chem 2022, 66, 448–458. [Google Scholar] [CrossRef]
- Li, C.-P.; Wu, Y.-Q.; An, J.-J.; Gao, L.-X.; Zhang, D.-Q.; Li, J.; An, Z.-X. Preparation of carbon foam from depolymerization-reforming lignin for capacitive deionization. Desalination 2023, 559, 116656. [Google Scholar] [CrossRef]
- Song, G.; Fan, W.; Zhang, J.; Xue, T.; Shi, Y.; Sun, Y.; Ding, G. Adsorption of anionic dyes from aqueous solutions by a novel CTAB/MXene/carbon nanotube composite: Characterization, experiments, and theoretical analysis. Appl. Surf. Sci. 2024, 661, 160036. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Li, Z.; Yang, D.; Xu, J.; Liu, X. MgO-laden biochar enhances the immobilization of Cd/Pb in aqueous solution and contaminated soil. Biochar 2021, 3, 175–188. [Google Scholar] [CrossRef]
- Jana, P.; Fierro, V.; Pizzi, A.; Celzard, A. Biomass-derived, thermally conducting, carbon foams for seasonal thermal storage. Biomass Bioenergy 2014, 67, 312–318. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhan, L. Carbon foams prepared from coal tar pitch for building thermal insulation material with low cost. Chin. J. Chem. Eng. 2018, 26, 415–420. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Hyeon, T. Direct synthesis of uniform mesoporous carbons from the carbonization of as-synthesized silica/triblock copolymer nanocomposites. Carbon 2004, 42, 2711–2719. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).