Abstract
(1) Background: The invasive ambrosia beetle Xylosandrus compactus (Coleoptera, Curculionidae, Scolytinae) poses an increasing risk of being introduced into new environments, mostly unintentionally, via wood products, live plants, and wood packaging materials. It is already spreading across Europe and causing growing concern due to its destructive behavior and by infesting a wide range of woody plants. The aim of this research is to identify X. compactus in Croatia and compile a list of recorded host plants. (2) Methods: Fieldwork was conducted through the assessment of beetle presence and infestation levels on host plants showing visible symptoms of attack, as well as through sampling of the beetles. Adult specimens of bark beetles were examined under a microscope, and molecular analysis was done. DNA was extracted from three randomly chosen individuals. The sequences were compared using the BLAST tool in the NCBI GenBank database. (3) Results and Conclusions: X. compactus was confirmed as a new record in Croatia, infesting laurel (Laurus nobilis), holm oak (Quercus ilex), laurestine (Viburnum tinus), olive (Olea sp.), myrtle (Myrtus communis), strawberry tree (Arbutus unedo), and pittosporum (Pittosporum tobira).
1. Introduction
Bark and ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) are frequently introduced into new regions unintentionally through wood products, live plants, and wood packaging materials [1]. These beetles pose a significant threat to ecosystems [2] and have demonstrated remarkable adaptability in Europe [3], which can be attributed to their broad range of host plants—both in their native environments and new habitats—their symbiotic relationships with fungi, and their ability to thrive in various climates [4]. These beetles are small, usually less than 2 mm in length, making them difficult to detect during the early stages of spread and infestation. The host preference of newly introduced invasive bark beetle species is very important in terms of already recorded hosts to fulfill the list, as they could potentially thrive due to the species spreading in the new region. So, attention to the inspection and identification of all host species is crucial.
Global trade has been identified as a key driver of their spread, particularly through the movement of wood products, live plants, and wood packaging materials [5]. Beetles of the genus Xylosandrus are attracted to stressed or dead wood, and infested wood packaging can carry larvae or adults over long distances undetected. This facilitates the transport of infested wood between continents, significantly increasing the risk of introduction into new environments. Species such as Xylosandrus germanus Blandford, X. crassiusculus Motschulsky, and X. compactus Eichhoff are globally invasive ambrosia beetles native to Asia. Their introduction and spread in Europe have caused growing concern due to their destructive behavior and the threats they pose to ecosystems, forestry, and horticulture [2].
Xylosandrus beetles belong to the Scolytinae subfamily and are classified as ambrosia beetles due to their symbiotic relationship with fungi. These beetles bore into trees, introducing specific fungal species that serve as a food source for both the beetles and their larvae. While the fungi benefit the beetles, they often cause significant damage to host plants, leading to symptoms such as wilting, dieback, and, in severe cases, the death of the host tree [6]. Beetles in this genus are known to infest a wide range of woody plants, including both native and ornamental species, and are frequently introduced unintentionally by human activity. Although they primarily target weakened or stressed trees, they can also infest seemingly healthy trees.
The life cycle of Xylosandrus beetles revolves around their symbiotic fungi. After females bore into the wood, they lay eggs in galleries they construct. The larvae then feed on the fungi introduced by the beetles. Unlike traditional wood-boring beetles, Xylosandrus species do not consume the wood itself. Instead, they rely on fungal cultivation, earning them the term “fungal farmers”. This fungal activity can disrupt vascular flow within the tree, leading to wilting and dieback [7].
One of the main challenges in managing these invasive beetles is their cryptic behavior. They tend to attack the inner bark, making early detection difficult until significant damage has occurred. Furthermore, their high reproductive rates and ability to infest a wide range of hosts complicate management efforts. Effective management of these invasive pests requires a coordinated approach, including early detection and strict regulation of high-risk commodity movements on both global and local scales. Quarantine regulations, particularly concerning wood, live plants, and packaging materials, are essential to prevent further spread. Monitoring systems and traps have been established in high-risk areas, such as ports and container terminals, to detect infestations as early as possible. Chemical treatments, along with sanitation measures, have shown limited effectiveness due to stringent insecticide regulations and the beetles’ cryptic biology.
Xylosandrus compactus (synonyms: Xyleborus compactus Eichhoff; Xyleborus morstatti Hagedorn; Xylosandrus morstatti Hagedorn) originates from East Asia but is also widely distributed across tropical and subtropical regions of Africa and South America. The species has been introduced to North America, several Pacific islands, and Europe [8]. In Europe, X. compactus was first detected in Italy in 2011 [9], followed by reports in France in 2015 [10], Greece [11], and Spain in 2019 [12]. More recently, infestations were documented in Malta in 2021 [13] and Turkey in 2022 [14], with detections in Slovenia [15] and on the Russian Black Sea coast in Sochi in 2023 [16]. A recent publication [16] mentions that the first four X. compactus specimens were collected in Alsace, France, as early as 1999. However, these specimens were kept in private collections and not published until recently. Numerous infestations have been documented across Europe, particularly in Italy and France, affecting 54 genera of forest, agricultural, and ornamental plants [17]. This species represents a significant threat to several Mediterranean plant species [18].
In 2023, wilting and shoot dieback were observed in various trees and shrubs in the Lokrum Island Reserve area, just 0.35 nautical miles southeast of Dubrovnik’s old city walls, Croatia (Figure 1). The phenomenon was first noticed on bay laurel (Laurus nobilis L.) specimens. Upon closer inspection, the same symptoms were found in several other species. The aim of the subsequent research was to identify the ambrosia beetle species responsible and compile a list of recorded host plants, assessing the levels of infestation per host where possible.
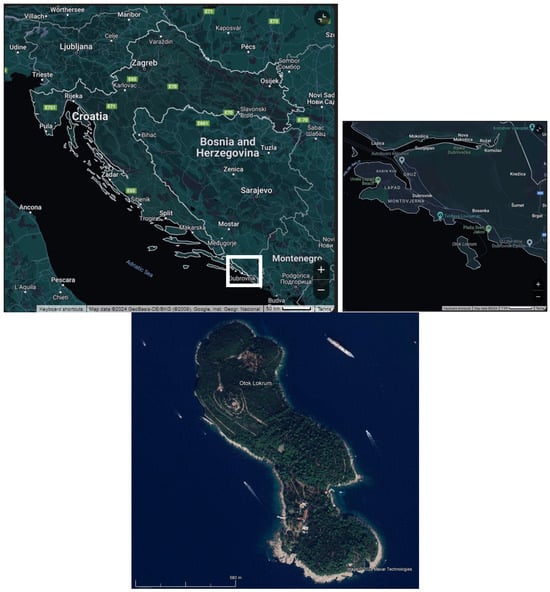
Figure 1.
Study area (island Lokrum) where the invasive Xylosandrus compactus was found for the first time in Croatia. (Google, Inst. Geogr. National).
2. Materials and Methods
Fieldwork was conducted in two steps: (i) field assessment of the beetle’s presence and level of infestation on host plants with visible symptoms of infestation, and (ii) sampling beetles.
The presence and level of infestation were recorded through visual examination of twigs on plants showing possible infestation symptoms. The visual survey was conducted by walking along paved paths and documenting the plants. Plants without symptoms were also counted to determine the overall level of infestation. The infestation was confirmed by visually inspecting twigs for characteristic ambrosia beetle entry holes. These holes were further investigated by dissecting the twigs to confirm the presence of larvae and adults. For each host plant with confirmed infestation, the number of infested host plants was divided by the total number of that host species present in the area to establish the relative level of infestation. The infestation level was calculated as a percentage of infested versus available host plants on the island.
Sample collection involved cutting twigs containing live broods of ambrosia beetles and storing them in Ziplock® bags, with separate bags for each host plant species. The samples were then transferred to the entomology laboratory, where further morphological analyses and molecular sampling of larvae were conducted. During the first sample collection (26 July 2023), one infected twig was sampled per tree species, with five adults (imago) collected from each sample. In total, 45 adults were examined under the microscope. Beetles collected from the infested hosts were examined using a standard Olympus SZX7 stereomicroscope equipped with two objective lenses, providing magnifications of up to 112x. Identification keys were consulted, including those relevant to Palearctic bark and ambrosia beetles [19], newer keys covering species that have recently spread globally [6,20], and online pictorial datasets [21].
Out of the microscopically examined adults, 15 were sent for molecular analysis. Samples for DNA barcoding were collected from Quercus ilex L., Arbutus unedo L., and Ceratonia siliqua L. and were immediately put into vials with ethanol (>70%). DNA was extracted from three randomly chosen individuals using the PureLink® Genomic DNA kit (Invitrogen, Waltham, MA, USA) and following the manufacturer’s protocol, whereas the initial grinding of samples was performed with sterile pistils. Polymerase chain reaction (PCR) was run in 25 μL volumes, with HCO/LCO primers [22] that amplify a 678-bp fragment of mtDNA’s Cytochrome Oxidase I gene (COI). PCR conditions included an initial denaturation stage at 94 °C (1 min), followed by 6 cycles of 45 s at 94 °C (denaturation), 30 s at 47 °C (annealing), and 45 s at 72 °C (extension), and then by 40 cycles of 45 s at 94 °C (denaturation), 30 s at 51 °C (annealing), and 45 s at 72 °C (extension). The final extension was carried out at 72 °C over the course of 5 min. Purification of PCR products was carried out with ExoSAP-IT™ PCR Product Cleanup Reagent (ThermoFischer Scientific, Waltham, MA, USA). Finally, Double-stranded DNA sequencing was conducted in the automated sequencer ABI3730XL of CeMIA Company (Larisa, Greece) using the Big-Dye Terminator v.3.1 Cycle Sequencing kit®, following the manufacturer’s protocol and using the same primers as in PCR. Finally, sequences were initially visualized with Chromas Lite software version 2.01 to cut off the rear and back nucleotides that were blurred and then blasted in the NCBI GenBank database.
3. Results
3.1. Morphological Identification
Especially useful in using ID keys were two recent publications that included compiled dichotomous keys comparing two or three morphologically similar species recorded in the European Mediterranean in recent years: Xylosandrus germanus, X. crassiusculus, and X. compactus [14,16]. The identification of sampled beetles confirmed the presence of X. compactus on the island of Lokrum, marking it as the first part of Croatian territory colonized by this ambrosia beetle, to our knowledge.
Xylosandrus compactus was found on several host plants, including laurel (Laurus nobilis), holm oak (Quercus ilex), laurestine (Viburnum tinus L.), olive (Olea sp.), myrtle (Myrtus communis L.), strawberry tree (Arbutus unedo), pittosporum (Pittosporum tobira (Thunb.) W.T. Aiton), carob tree (Ceratonia siliqua), and mock privet (Phillyrea sp.) (Table 1). In the branches of Italian Cypress (Cupressus sempervirens L.), exit holes and galleries were found that match the characteristics of X. compactus, and they were first classified as infested during fieldwork. Because there has been no confirmed record of this host species until now, adults or larvae were sought in the autumn of 2024 but could not be found. As a result, it cannot be stated with certainty that C. sempervirens is a host, and the trees will need to be sampled again next year.

Table 1.
List of hosts and intensity (%) of infestation over the three instances of field research.
For nearly all species (except holm oak and pittosporum), a pattern was observed: after the initial attack, there was a sudden increase in the number of infected trees during the second observation, followed by stagnation in the third and final observation. Thus far, no tree mortality has been recorded; however, severe infestations of shoots have been observed around the edges of the canopy.
3.2. Molecular Identification
After removing the back and rear nucleotides of the three sequences, a 635-bp locus was available for all three individuals screened. Blasting these sequences in the NCBI GenBank (https://www.ncbi.nlm.nih.gov/ (accessed on 20 October 2024) revealed that they resembled by 100% the sequence accession ID: MW961427, which stands for Xylosandrus compactus, verifying unambiguously the identity of the samples.
4. Discussion
The introduction of Xylosandrus beetles in Europe has become an escalating ecological and economic issue, posing risks to forestry, horticulture, and agriculture. Broadleaf species, some conifers, and various Mediterranean plants are particularly vulnerable to infestations. The introduction of fungal symbionts exacerbates the problem, causing dieback and tree mortality that can impact timber production and forest biodiversity. Additionally, these beetles pose a threat to ornamental trees and shrubs, jeopardizing nurseries and urban landscapes. Infestation of fruit trees, such as olives and figs, has the potential to significantly affect agricultural productivity. The discovery of X. compactus in Croatia, therefore, represents a potential problem that requires urgent intervention and the development of protective measures.
It took about 12 years since the first published record of X. compactus presence in Europe (Italy) until the first discovery of this invasive beetle in the summer of 2023, on the island of Lokrum, at the very south of Croatian part of the Adriatic Sea. Classical entomological field research was done on the island regarding monitoring of Orthotomicus erosus Wollastone, which poses a potential problem on Aleppo pine [23]. Pheromone traps were set in 2019. The catches in the pheromone traps were collected mainly from 7 to 14 days [24], depending on the weather conditions. The catches were continuously monitored every year and examined under a microscope. Xyleborus species as incidental catches have never been found in them until now, which is not surprising, considering that the pheromone traps were not designed for these species. The fact that the recorded level of infestation on this small island (72 ha) on some woody plants was pretty high could indicate that it must have been invaded a few years ago and/or that the typical Mediterranean climate on the island might have had enabled more than just two generations per year, as has been reported earlier [14,18,25]. Given the favorable climatic conditions [26], X. compactus is expected to spread further in the region. To date, no tree death caused by X. compactus has been observed in Croatia.
Interestingly enough, the other two invasive beetles from this genus, X. germanus and X. crassiusculus have either not yet been detected in Croatia (X. crassiusculus) or have not been confirmed in the coastal part of the country. Xylosandrus germanus is now a common member of the ambrosia beetle assemblages throughout continental Croatia [27] but has not yet been recorded along the Adriatic coast in spite of the several recent (2018–2021) bark beetle monitoring efforts stretching from the island of Brijuni in Northern Adriatic to the Southern Dalmatia, near Makarska and Biokovo mountains (Hrašovec, unpublished data).
Climatologically, the island Lokrum resides in the typical Mediterranean climate belt with preserved vegetation that thrives on the island. It was designated as a special reserve of forest vegetation in 1948. Rich in native growing trees, shrubs, and non-woody vegetation, it also has a solid number of exotic plants cultivated in the small botanical garden (https://www.enciklopedija.hr/clanak/lokrum) (accessed on 20 October 2024). All this contributes to the abundance of host choices for X. compactus, and it may be expected that more hosts will be recorded in the future on this island.
The host preference of newly introduced invasive bark beetle species is very important in terms of already recorded hosts to fulfill the list, as they could potentially thrive due to the species spreading in the new region. That is why we pay attention to the inspection and identification of all tree species on the island. Xylosandrus compactus, by all accounts, is very opportunistic in terms of host preference. The EPPO database [26] lists 159 hosts, either at the species or genus level, each linked to the source publications. Many publications deal with host species from very different regions, describing from a few specific to over 200 host species [27,28,29,30,31,32]. The list of recorded hosts will likely grow as the species spreads in the region. In our fieldwork, among the already known and common hosts, one new host has raised a puzzling question: Cupressus sempervirens. In the branches of C. sempervirens, exit holes and galleries were found that match the characteristics of X. compactus, and they were classified as infested during fieldwork. Upon later review of the literature, it became evident that there has been no confirmed record of this host species until now. Therefore, adults or larvae were sought in the autumn of 2024 but could not be found for further analysis. As a result, it cannot be stated with certainty that C. sempervirens is a host, and the trees will need to be sampled again next year. More than a decade after X. compactus started appearing in Mediterranean countries, knowing that in many of these instances C. sempervirens is widely present and a common part of the woody assemblage, it remains unclear how it was not confirmed in earlier instances as a X. compactus host. In Karpun et al. [16], where the authors published the first records of two non-native bark beetles from Russia, the survey of the vegetation was conducted in a similar way to this research. X. compactus has been discovered on Laurus nobilis, Magnolia grandiflora L., and Prunus laurocerasus L. but not on C. sempervirens, which turned out to be infested by Phloeosinus armatus Reitter, a new bark beetle species for Russia. Clearly, they must have conducted a thorough examination of infested tree parts and would have found the presence of X. compactus on C. sempervirens had that been the case. On the other hand, the successful entry and development of X. compactus in C. sempervirens might not be a big surprise. According to Guigliuzzo et al. [18], the use of essential oil extracted from C. sempervirens, among other substances, applied on known Laurus nobilis host plants revealed neither attractiveness nor repellency in laboratory choice bioassay tests. They conclude in their work that “X. compactus females did not exhibit any significant preference for stem sections treated with EO nanoemulsions of C. sempervirens compared with untreated ones, confirming results of previous studies about the lack of an attractive effect of this substance for X. compactus”. The fact that no repelling effect was recorded might explain why C. sempervirens should, in fact, be regarded as a new host, but it remains to be seen if this will become just a minor host for X. compactus.
Over the past decade, new non-native species have been reported in Croatia for the first time, like neighboring Italy, which has one of the highest numbers of intercepted and established non-native bark and ambrosia beetles in the EU [33]. Further research should be performed to investigate the presence and spread of this species throughout the region.
Author Contributions
Conceptualization, M.P., B.H. and N.L.; methodology, M.P., N.L. and D.A.; validation, M.P., D.A. and N.L.; formal analysis, M.P. and D.A.; investigation, M.P., B.H., N.L., O.D. and D.A.; writing—original draft preparation, M.P. and B.H.; writing—review and editing, M.P., B.H., N.L., O.D. and D.A.; visualization, M.P.; supervision, B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Public institution Lokrum, contract number 2.4/23 and receiving order 17/10/24.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We express our gratitude to Vedrana Križanić and Josip Kovač (Arbofield Ltd., Jastrebarsko) and Mirko Fabijanić (Jastrebarsko) for their elaborate contribution to field investigations, sampling, and laboratory preparations.
Conflicts of Interest
Nikola Lacković is employed by the company Arbofield Ltd., Jastrebarsko. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article has been republished with a minor correction to the correspondence contact information. This change does not affect the scientific content of the article.
References
- Lantschner, M.V.; Corley, J.C.; Liebhold, A.M. Drivers of global Scolytinae invasion patterns. Ecol. Appl. 2020, 30, e02103. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Auger-Rozenberg, M.A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Kirkendall, L.R.; Faccoli, M. Bark beetles and pinhole borers (Curculionidae, Scolytinae, Platypodinae) alien to Europe. ZooKeys 2010, 56, 227–251. [Google Scholar] [CrossRef]
- Grousset, F.; Grégoire, J.-C.; Jactel, H.; Battisti, A.; Benko Beloglavec, A.; Hrašovec, B.; Hulcr, J.; Inward, D.; Orlinski, A.; Petter, F. The Risk of Bark and Ambrosia Beetles Associated with Imported Non-Coniferous Wood and Potential Horizontal Phytosanitary Measures. Forests 2020, 11, 342. [Google Scholar] [CrossRef]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Dole, S.A.; Cognato, A.I. Phylogenetic Revision of Xylosandrus Reitter (Coleoptera: Curculionidae: Scolytinae: Xyleborina). Proc. Calif. Acad. Sci. USA 2010, 61, 451–545. [Google Scholar]
- Peris, D.; Delclos, X.; Jordal, B. Origin and evolution of fungus farming in wood-boring Coleoptera—A palaeontological perspective. Biol. Rev. 2021, 96, 2476–2488. [Google Scholar] [CrossRef]
- EPPO. Pest information sheet on Xylosandrus compactus. In EPPO Study on the Risk of Bark and Ambrosia Beetles Associated with Imported Non-Coniferous Wood; EPPO Technical Document no. 1081; EPPO: Paris, France, 2020; pp. 161–168. Available online: https://pra.eppo.int/getfile/572ee5a3-64e1-49cd-92ac-69e3b1e6b8a9 (accessed on 27 November 2024).
- Garonna, A.P.; Dole, S.A.; Saracino, A.; Mazzoleni, S.; Cristinzio, G. First record of the black twig borer Xylosandrus compactus (Eichhoff) (Coleoptera: Curculionidae, Scolytinae) from Europe. Zootaxa 2012, 3251, 64–68. [Google Scholar] [CrossRef]
- Barnouin, T.; Soldati, F.; Roques, A.; Faccoli, M.; Kirkendall, L.R.; Mouttet, R.; Daubree, J.-B.; Noblecourt, T. Bark beetles and pinhole borers recently or newly introduced to France (Coleoptera: Curculionidae, Scolytinae and Platypodinae). Zootaxa 2020, 4877, 51–74. [Google Scholar] [CrossRef]
- Spanou, K.; Marathianou, M.; Gouma, M.; Dimou, D.; Nikoletos, L.; Milonas, P.G.; Papachristos, D.P. First record of black twig borer Xylosandrus compactus (Coleoptera: Curculionidae) in Greece. In Proceedings of the 18th Panhellenic Entomological Congress, Komotini, Greece, 15–18 October 2019; p. 77. (In Greek). [Google Scholar]
- Leza, M.; Núñez, L.; Riba-Flinch, J.M.; Comparini, C.; Roca, A.; Gallego, D. First record of the black twig borer, Xylosandrus compactus (Coleoptera, Curculionidae, Scolytinae), in Spain. Zootaxa 2020, 4767, 345–350. [Google Scholar] [CrossRef] [PubMed]
- EPPO. Xylosandrus compactus (XYLSCO). 2024. Available online: https://gd.eppo.int/taxon/XYLSCO (accessed on 22 November 2024).
- Hýzal, E.; Acer, S.; Altunýþýk, S. First record of the invasive alien species Xylosandrus compactus (Eichhoff) (Coleoptera: Curculionidae: Scolytinae) in Turkey. BioInvasions Rec. 2023, 12, 93–102. [Google Scholar] [CrossRef]
- Hauptman, T.; Pavlin, R.; Grošelj, P.; Jurc, M. Distribution and abundance of the alien Xylosandrus germanus and other ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in different forest stands in central Slovenia. iForest 2019, 12, 451–458. [Google Scholar] [CrossRef]
- Karpun, N.N.; Petrov, A.V.; Zhuravleva, E.N.; Shoshina, E.I.; Kirichenko, N.I.; Mandelshtam, M.Y.; Musolin, D.L. Two invasive bark beetles Phloeosinus armatus Reitter and Xylosandrus compactus (Eichhoff) (Coleoptera, Curculionidae: Scolytinae) newly recorded in Russia. EPPO Bull. 2024, 54, 166–181. [Google Scholar] [CrossRef]
- Riba-Flinch, J.M.; Leza, M.; Gallego, D. First records of Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) in the Iberian Peninsula: An expanding alien species? Zootaxa 2021, 4970, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Gugliuzzo, A.; Criscione, G.; Biondi, A.; Aiello, D.; Vitale, A.; Polizzi, G. Seasonal changes in population structure of the ambrosia beetle Xylosandrus compactus and its associated fungi in a southern Mediterranean environment. PLoS ONE 2020, 15, e0239011. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, A. Zentral- und westpaläarktische Borken- und Kernkäfer (Coleoptera: Scolytidae, Platypodidae). In Pro entomologia c/o; Naturhistorisches Museum: Basel, Switzerland, 1995; p. 310. [Google Scholar]
- Smith, S.M.; Beaver, R.A.; Cognato, A.I. A monograph of the Xyleborini (Coleoptera, Curculionidae, Scolytinae) of the Indochinese Peninsula (except Malaysia) and China. ZooKeys 2020, 983, 1–442. [Google Scholar] [CrossRef]
- Atkinson, T.H. Bark and Ambrosia Beetles of the Americas. 2024. Available online: https://barkbeetles.info (accessed on 19 October 2024).
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Pernek, M.; Lacković, N.; Lukić, I.; Zorić, N.; Matošević, D. Outbreak of Orthotomicus erosus (Coleoptera, Curculionidae) on Aleppo Pine in the Mediterranean Region in Croatia. SEEFOR-South-East Eur. For. 2019, 10, 19–27. [Google Scholar] [CrossRef]
- Pernek, M.; Milas, T.; Kovac, M.; Lackovi´c, N.; Koren, M.; Hrašovec, B. Effective Reduction in Natural Enemy Catches in Pheromone Traps Intended for Monitoring Orthotomicus erosus (Coleoptera, Curculionidae). Forests 2024, 15, 1298. [Google Scholar] [CrossRef]
- Faccoli, M. Scolitidi d’Europa: Tipi, Caratteristiche e Riconoscimento dei Sistemi Riproduttivi. In WBA Handbooks 2015; WBA Handbooks: Verona, Italy, 2015; pp. 1–160. [Google Scholar]
- Urvois, T.; Auger-Rozenberg, M.A.; Roques, A.; Rossi, J.P.; Kerdelhue, C. Climate change impact on the potential geographical distribution of two invading Xylosandrus ambrosia beetles. Sci. Rep. 2021, 11, 1339. [Google Scholar] [CrossRef]
- Chong, J.-H.; Reid, L.; Williamson, M. Distribution, Host Plants, and Damage of the Black Twig Borer, Xylosandrus compactus (Eichhoff), in South Carolina. J. Agric. Urban Entomol. 2009, 26, 199–208. [Google Scholar] [CrossRef]
- Dixon, W.N.; Woodruff, R.E.; Foltz, J.L. Black Twig Borer, Xylosandrus compactus (Eichhoff)(Insecta: Coleoptera: Curculionidae: Scolytinae). 2003. Available online: https://edis.ifas.ufl.edu/in577 (accessed on 1 December 2024).
- Egonyu, J.P.; Baguma, J.; Ogari, I.; Ahumuza, G.; Ddumba, G. Host preference by the twig borer Xylosandrus compactus (Coleoptera: Scolytidae) and simulated infuence of shade trees on its populations. Int. J. Trop. Insect Sci. 2017, 37, 183–188. [Google Scholar] [CrossRef]
- Greco, E.B.; Wright, M.G. Ecology, Biology, and Management of Xylosandrus compactus (Coleoptera: Curculionidae: Scolytinae) with Emphasis on Coffee in Hawaii. J. Integr. Pest Manag. 2015, 6, 7. [Google Scholar] [CrossRef]
- Ngoan, N.D.; Wilkinson, R.C.; Short, D.E.; Moses, C.S.; Mangold, J.R. Biology of an Introduced Ambrosia Beetle, Xylosandrus compactus, in Florida. Ann. Entomol. Soc. Am. 1976, 69, 872–876. [Google Scholar] [CrossRef]
- Franjević, M.; Šikić, Z.; Hrašovec, B. First occurrence of Xylosandrus germanus (Blandford, 1894)—Black steam borer in pheromone baited panel traps and population build up in Croatian oak stands. Sumar. List. 2019, 143, 215–219. [Google Scholar] [CrossRef]
- Ruzzier, E.; Morin, L.; Zugno, M.; Tapparo, A.; Bani, L.; Di Giulio, A. New records of non-native Coleoptera in Italy. Biodivers. Data J. 2023, 11, e111487. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).