Abstract
Ginkgo biloba L. is a valuable medicinal plant known for its high content of flavonoids and terpenoids in the leaves of young trees. Pruning can increase leaf yield in ginkgo plantations; however, it is unclear how the intensity of pruning affects leaf yield and quality. In addition, G. biloba exhibits low cutting rooting rates, which limits its efficiency in asexual propagation. In our study, we compared consecutive pruning with varying levels of intensity, including top pruning, light pruning, and heavy pruning, to evaluate the effects of pruning on leaf yield and cutting rooting. The results showed that these three pruning methods all contributed to an increase in the number of new branches, the leaf weight, and the flavonoid content in five-year-old trees. Among them, the effect of light pruning was the best, with a 150% increase in branch number, a 130% increase in leaf weight, and a 40.6% increase in flavonoid content. The secondary pruning further increased leaf area by 22.3%, indicating that secondary pruning further enhanced the rejuvenation of plants and increased leaf yield. At the transcriptional level, pruning can significantly change the expression of genes related to bud sprouting, resulting in a particularly significant increase in SHR expression in the buds. Pruning also promoted the expression of important genes related to flavonoid synthesis, including chalcone synthase (CHS), flavonoid 3′-hydroxylase (F3′H), flavonol synthase (FLS), and dihydroflavonol reductase (DFR). Furthermore, we demonstrated a significant increase in the rooting rate of these second-pruned branch cuttings and screened the optimal hormone ratio for rooting, which is 1.5 μM MeJA + 400 mg/L NAA + 100 mg/L Uniconazole-P. These results suggest that secondary pruning can effectively rejuvenate plants to promote cutting rooting in G. biloba. This method can not only be used to improve the yield and quality of ginkgo leaves, but also for cutting propagation.
1. Introduction
Ginkgo biloba L. is an economically important tree species, with a very wide planting area in China of more than 400,000 hectares, and the total output value of the ginkgo industry reaching more than CNY 18 billion in 2021 [1,2,3]. Ginkgo leaves contain a large amount of active medicinal ingredients, such as flavonoids and terpenoids, and G. biloba leaf extract (GbE) is widely used as a raw material for the treatment of diseases such as hypertension, cardiovascular diseases, and cerebrovascular diseases [4,5,6,7]. It is worth noting that only the leaves of young ginkgo trees contain high levels of biologically active compounds such as flavonoids (e.g., kaempferol, quercetin, isorhamnetin) and terpenoids (e.g., ginkgolides, bilobalides) [3,8]. Therefore, the leaves of ginkgo saplings, aged 1 to 5 years, are used for GbE extraction [9,10]. Cutting propagation is a simple and economical asexual reproduction technique that exhibits advantages such as maintaining the excellent characteristics of parent plants and promoting reproduction to obtain more abundant saplings. However, propagating ginkgo saplings through cuttings may be challenging because of their slow rooting process and low survival rate (only about 40%) [11,12]. Therefore, developing cuttings suitable for asexual propagation and establishing a rapid propagation technique for ginkgo cuttings is of great significance to the development of the ginkgo leaf industry.
Age is an important factor affecting the rate of rooting and the survival of cuttings. With the increase in mother plant age, the cutting rooting rate significantly decreases [13,14]. Therefore, maintaining the juvenile state of cuttings is vital for successful propagation. Pruning refers to the cutting, shaping, and other processing of plant organs to adjust the structure of the plant [15,16,17]. Pruning can stimulate the growth of new branches in mature or aging trees, thereby achieving the purpose of tree rejuvenation or revitalization [18,19]. Generally, pruning can be categorized into different intensities, such as top pruning [20], light pruning, and heavy pruning [21]. Top pruning can break the dominance of the plant’s top and promote an increase in the number of short branches. Light pruning can increase the budding rate of plants, resulting in more and faster thickening of mother plant branches, while heavy pruning can revive weak trees and weak branches. For example, different pruning methods applied to cherimoya (Annona squamosa L.), sour cherry (Prunus cerasus L.), and tea trees (Camellia sinensis L.) resulted in the production of more branches and leaves, both of which showed a state of rejuvenation [22,23,24]. In addition, after consecutive pruning of apple trees, the number of branches and leaves above the ground significantly increases, and the stomatal conductance, photosynthetic rate, and photosynthetic characteristics of the leaves also increase [25] These studies all indicate that pruning and consecutive pruning can overcome age effects, promote the production of branches and leaves, and rejuvenate plants.
Several previous studies have found that hormones play a crucial role in promoting shoot formation and the rejuvenation of plants after pruning. In yellowhorn trees (Xanthoceras sorbifolium B.), pruning increases the levels of development-related hormones (such as gibberellins and auxins), while reducing the levels of growth-inhibiting hormones (such as abscisic acid), thereby promoting the growth of shoots and leaves and resulting in a juvenile state [21]. In olive trees (Olea europaea L.), pruning can also induce changes in the levels of cytokinins, gibberellins, and auxins in the stem, indicating significant alterations in hormone synthesis, transport, and distribution in plants, thereby promoting bud sprouting and shoot formation [26]. On the other hand, pruning-induced shoot formation and the rejuvenation of plants can be attributed to various factors, including increased root-to-shoot ratio, enhanced leaf photosynthetic capacity, and increased tree resource storage [27]. For example, pruning oil tea (Camellia oleifera L.) trees enhances the efficiency of light energy utilization, increases the leaf photosynthetic rate, and promotes the formation of sprout shoots and the rejuvenation of plants [28].
In our previous research, we found that pruning trees can produce a large number of buds and stimulate the development of new branches on the trunk of G. biloba. Compared to unpruned trees, pruned plants exhibit larger and thicker leaves, as well as an increase in the number and depth of the leaf lobes. All of these indicate that pruning can promote the recovery and vigorous growth of ginkgo trees [8,29]. Here, we further compare the effects of different pruning methods on five-year-old G. biloba trees and increase the pruning frequency to determine if it is possible to further enhance leaf yield and medicinal ingredient content. Additionally, we identify key genes involved in bud germination and the flavonoid synthesis pathways and determine their expression in lateral branch and leaf development. Furthermore, we compare the rooting rate of branches after different pruning frequencies and screen the optimal hormone ratio for rooting cuttings. Our research results contribute to optimizing leaf yield and cutting rooting in G. biloba.
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Pruning
The ginkgo experimental field was located at Yangzhou University (latitude 32°38′, longitude 119°42′) in Yangzhou, Jiangsu Province, China. Five-year-old ginkgo trees were used in our experiments. The first pruning (referred to as primary pruning) treatment was conducted on 5 February 2022 (winter dry season, −1 °C to 10 °C). A total of 50 plants were selected as experimental materials, of which 10 were pruned at the top (removing apical buds of branches), 10 were lightly pruned (removing one-third of the branches), and 10 were heavily pruned (removing two-thirds to three-fourths of the branches), while the others (20 plants) were not pruned, representing control conditions. On 5 May 2022 (spring drought season, 15 °C to 25 °C), we chose the best pruning method from the primary pruning results for the second pruning (referred to as secondary pruning). A total of 5 out of 10 pruned trees were pruned at the secondary pruning (as the secondary pruning group), while allowing the remaining 5 to continue growing. At the same time, we selected 5 out of the 20 unpruned trees and performed the primary pruning using the light pruning method (as the primary pruning group), leaving the rest as the control group. On 1 May 2022 and 5 June 2022, after the trees had sprouted and grown, we examined the shoot length (cm), number of lateral branches (pcs), stem diameter (mm), lobing number (pcs), lobing depth (cm), thickness (mm), area (cm2), fresh weight (g), dry weight (g), and water content (%) of these pruned trees.
2.2. Morphological Observation
For different treatments, 10 leaves were measured for each replication (each plant), with three replications (three plants) for each treatment to determine the lobing number, lobing depth, thickness, and area. The leaves were photographed using a digital camera, and ImageJ software (V1.8.0.112, National Institutes of Health, Bethesda, MD, USA) was utilized to analyze their area. We measured the thickness of the center of the leaves from the main lobe using a vernier caliper (Figure 1). For deeply lobed leaves, we measured the thickness at the center of the leaf lobe. For leaves with shallow lobes, we measured the thickness at the center of the leaf. Specifically, we selected ten leaves and measured their thickness at these specific locations. From these measurements, we calculated the average thickness. We conducted repeated measurements, starting from 5 May 2022, by pruning the trees and recording their growth every seven days.

Figure 1.
Leaf thickness measurement.
2.3. Physiological Measurement
We randomly selected 10 leaves (from the middle part of the middle branch of each plant) to determine their fresh weight, dry weight, and water content. Fresh weight (FW) was measured using an analytical balance, and then the leaves were dried at 75 °C until a constant dry weight (DW) was reached. Water content (%) was calculated using the formula (FW − DW)/FW × 100.
2.4. Determination of Flavonoid Content
The leaves were collected from samples taken on 1 May 2022 and 5 June 2022. The total flavonoid content of 0.02 g of leaves was determined according to the methods of the plant flavonoid detection kit (China Limited, Suzhou, China). After fixing the samples at 120 °C for 30 min, the sample was dried to a constant weight at 75 °C. The leaves were then pressed through an 80-mesh sieve, 0.02 g of leaf powder were weighed out, and 2 mL of 60% ethanol was added. The resulting mixture was shaken in a 60 °C water bath for 2 h and centrifuged at 10,000 rpm for 10 min at 25 °C. The supernatant was retained and each reagent was then added to it. The extract was then allowed to stand for 6, 5, and 15 min, the absorbance at 510 nm was measured, and the total flavonoid content was calculated.
2.5. Total RNA Extraction and qRT-PCR Experiment
The total RNA was isolated from the leaves and buds using the plant RNA prep Pure Plant Plus Kit (TianGen Biotechnology Co., Ltd., Beijing, China). The total RNA was then reverse transcribed into cDNA using ChamQ SYBR qPCR Master Mix (Vazyme Co., Ltd., Nanjing, China). Finally, the cDNA was used as a template for the qRT-PCR (CFX96TM, Bio-Rad, Hercules, CA, USA) experiments. Based on previous studies, we selected several structural genes related to bud formation and flavonoid biosynthesis, such as WIND, SHR, SPL, PIN, CHS, FLS, DFR, and F3′H. We performed qRT-PCR analysis on these genes [8,13]. GADPH expression was used as an internal control [29,30], and qRT-PCR was performed, as described previously [31]. The relative expression levels of the genes were calculated using the 2−ΔΔCt method. All reactions were repeated as three biological replicates.
2.6. Rooting of Stem Cutting
On 5 June 2022 (which marked the end of spring and the beginning of summer, 20 °C to 30 °C, clear weather), the unpruned, once-pruned, and twice-pruned branches were collected and trimmed to approximately 5 cm long, leaving one node, with the top pruned flat (to minimize the exposed surface area and reduce water loss through evaporation) and the bottom cut into a single oblique shape (to maximize the contact area between the scion and the substrate, enhancing the potential for successful rooting). Then, the branches were planted in pots that were placed outside the greenhouse in an experimental propagation bed (latitude 32.391, longitude 119.418, Wenhui Road campus of Yangzhou University). These pots were filled with a 1:1 mixture of vermiculite and perlite, treated with 400 mg/L of naphthalene acetic acid (NAA). The rooting rate of the cuttings after the secondary pruning was observed and compared to that of the unpruned cuttings used as the control. In total, we evaluated 450 cuttings from all treatments (150 cuttings for each treatment). Each treatment contained three replicates, with 50 cuttings in each replicate.
On 25 July 2022 (during the summer season, with temperatures ranging from 28 °C to 35 °C and occasional thunderstorms), the cuttings after the secondary pruning were treated with different concentrations of methyl jasmonate (MeJA) at 1 μM and 1.5 μM, 400 mg/L of NAA, and a combination of Uniconazole-P at 10 mg/L and 100 mg/L, in addition to hormone treatment. The specific operation method is to first soak the cuttings in MeJA for 30 min, quickly dip them in NAA for 6–10 s before inserted them into the substrate, and then spraying them with Uniconazole-P on top. After 7 days, another treatment of Uniconazole-P was applied. A total of 750 cuttings were selected after the secondary pruning. The cutting experiment comprises four treatment groups and one control group. Each treatment group consists of three replicates, with 50 cuttings per replicate (50 × 3 × 5 = 750). A total of 400 mg/L NAA was used individually as the control in order to screen the optimal hormone formula that promotes the rooting of cuttings. The cutting pots were placed in the propagation bed, automatic sprinklers were set up (with periodic misting for 10 min per hour during the day for humidity control), and the pots were regularly sprayed with 50 mg/L of 40% carbendazim (FENGDE, Lanfeng Bio-chemical Co., Ltd., Xuzhou, China) every 7 days. Next, we sampled three cuttings per replicate (nine cuttings per treatment) to approximate the time for both rooting and callus formation, and the time of first rooting and callus formation was recorded. A total of 1.5 months after the start of the experiment, we analyzed and counted the number of roots, the root length, and the rooting rate. We also calculated the rooting rate using the following formula: rooting rate = number of rooted cuttings/total number of cuttings.
2.7. Statistical Analysis
The experimental data from both the control group and the treatment groups in this experiment are presented as the mean ± standard deviation (SD) of at least three independent experiments. Analysis was performed using a one-way analysis of variance (one-way ANOVA), followed by Tukey’s post hoc testing for significance analysis. Differences between lowercase letters indicate significance at the p < 0.05 level.
3. Results
3.1. Effects of Various Pruning Methods on Plant Growth and Leaf Development
The 5-year-old G. biloba trees were pruned using top pruning, light pruning, and heavy pruning methods (Figure 2A–H). Compared with the control, the new shoot growth of the plant increased by 28.3% (68 ± 1.2 cm) and 33.9% (71 ± 1.1 cm) after the treatment of heavy and light pruning, respectively, while the number of branches also increased by 150% in both treatments (Figure 2I,J). After top pruning, the number of branches increased by 75%. Interestingly, compared with the control, the stem diameter of the branches decreased by 14.8%, to 0.58 ± 0.08 cm, after heavy pruning, while it increased by 19.1%, to 0.81 ± 0.04 cm, after light pruning. The stem diameter of the branches after top pruning was 0.72 ± 0.05, with no significant change (Figure 2K).
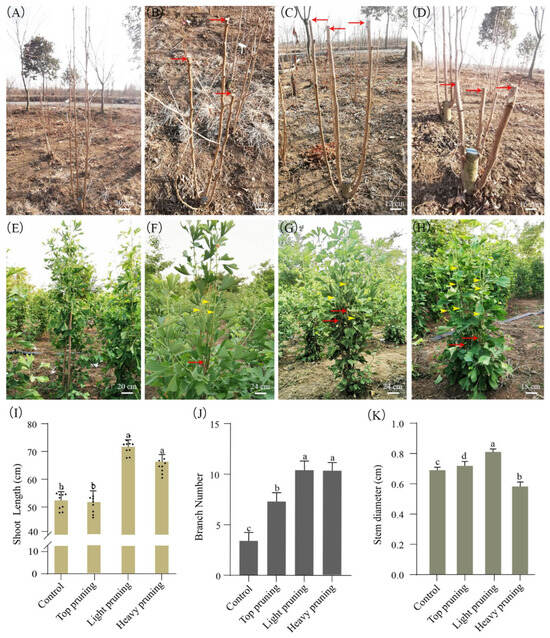
Figure 2.
The growth of branches after different pruning methods. (A) Control plant; (B) top pruning plant; (C) light pruning plant; (D) heavy pruning plant. (E) The growth of unpruned control plant; (F) the growth of top pruning plant; (G) the growth of light pruning plant; (H) the growth of heavy pruning plant. (I) The shoot length; (J) the number of lateral branches; (K) the stem diameter. The red arrow represents the pruned cutting area, and the yellow arrow represents the newly sprouted lateral branches after pruning. Each black dot represents a repetition. Means ± SD, n = 10. Letters indicate significant differences based on one-way ANOVA (p < 0.05).
The shape and size of G. biloba leaves changed significantly as a result of different pruning methods (Figure 3A–D). Compared with the control, the size and lobe number of the leaves after light pruning, heavy pruning, and top pruning increased, with the best effect observed in the light pruning groups (Figure 3E–H). The fresh weight of leaves after the heavy pruning treatment was 11.46 ± 0.16 g, an increase of 1.7 times. Similarly, the fresh weight of leaves after light pruning was 12.36 ± 0.12 g, an increase of 1.8 times, and the fresh weight of leaves after top pruning was 8.24 ± 0.11 g, an increase of 1.3 times (Figure 3I). Following heavy pruning, the dry weight of the leaves was 2.71 ± 0.09 g, an increase of 1.5 times, while the water content of the leaves increased by 6%. After light pruning, the dry weight of the leaves was 2.59 ± 0.08 g, an increase of nearly 1 time, and the water content of the leaves increased by 8%. Top pruning led to a more significant increase in dry leaf weight, which increased by 60% to 2.08 ± 0.06 g, accompanied by a 5% increase in water content (Figure 3J,K). Considering that flavonoids are important bioactive constituents in G. biloba leaves, we compared the total flavonoid content in leaves from different pruning treatments and found that, compared with the control, the content of flavonoids increased by 46% and 45% after light and heavy pruning, respectively, and by 25% after top pruning (Figure 3L).

Figure 3.
Changes in morphology, water content, and flavonoids in leaves after primary pruning. (A) Control plant leaves; (B) top pruning plant leaves; (C) light pruning plant leaves; (D) heavy pruning plant leaves. (E–L) The leaf area, leaf lobing number, leaf lobing depth, leaf thickness, fresh weight, dry weight, water content, and flavonoid content were measured after pruning. In (E–H), light green represents the control group and dark green represents the treatment group. Means ± SD, n = 3. Letters indicate significant differences based on one-way ANOVA (p < 0.05).
3.2. Effects of Pruning Times on Plant Growth and Leaf Development
Among the three pruning methods, light pruning can significantly promote branch number, leaf biomass, and flavonoid content. Therefore, we chose light pruning for the secondary pruning study (Figure 4D). Compared to the primary pruning, the number of branches increased by 3–4 after the secondary pruning, but the new shoot growth and the stem diameter of the branches slightly decreased (Figure 4F–H). The plants generally exhibit dwarfism, increased branch density, and an expanded canopy after two light prunings.

Figure 4.
Branch growth after different pruning times. (A) Multiple pruning model; (B) primary pruning; (C) plant growth after the primary pruning; (D) secondary pruning; (E) plant growth after the secondary pruning; (F) shoot length after pruning; (G) number of new branches after pruning; (H) stem diameter after pruning. The red arrow represents the pruned cutting area, and the yellow arrow represents the newly sprouted lateral branches after pruning. Means ± SD, n = 5. Letters indicate significant differences based on one-way ANOVA (p < 0.05).
At the same time, the size and shape of the leaves have undergone significant changes after being pruned twice. Compared to the control, the leaves were larger, with more and deeper leaf lobing after the primary pruning, indicating that pruning can rejuvenate G. biloba (Figure 5A–C). Moreover, compared with the primary pruning, after the secondary pruning, the leaf area increased by 22.6%, the depth of leaf lobing increased by 61.1%, and the leaf thickness increased by 19.5%, indicating that secondary pruning can further enhance the rejuvenation of G. biloba (Figure 5D–G). Furthermore, compared with the control, after the secondary pruning, the fresh weight of the leaves was 8.34 ± 0.17 g, an increase of 189% (Figure 5I), and the dry weight was 1.82 ± 0.18 g, an increase of 104% (Figure 5H,J). However, compared with the primary pruning, the content of flavonoids in the leaves slightly increased after the secondary pruning (Figure 5K).
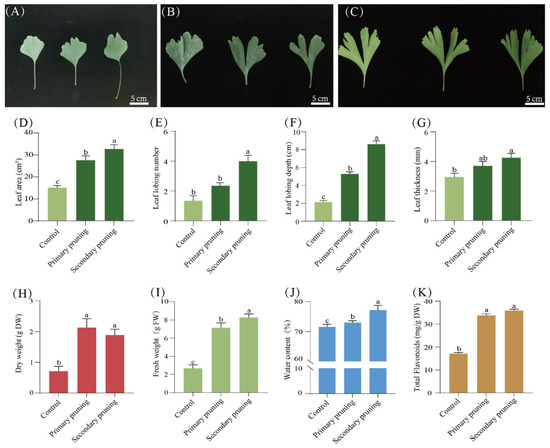
Figure 5.
Changes in leaf morphology, water content, and flavonoid accumulation after secondary pruning. (A) Control leaves; (B) leaves of primary pruning; (C) leaves of secondary pruning. (D–K) The area, lobing number, lobing depth, thickness, fresh weight, dry weight, water content, and flavonoid content of the leaves after pruning. In (D–G), light green represents the control group and dark green represents the treatment group. Means ± SD, n = 3. Letters indicate significant differences based on one-way ANOVA (p < 0.05).
3.3. Expression Changes of Genes Related to Bud Sprouting and Flavonoid Biosynthesis in Pruned Seedlings
Due to the significant promotion of bud sprouting achieved by pruning, we used qRT-PCR to detect the expression of genes related to sprouting in the buds. After pruning, the expression of growth-inhibiting factors GbSHR (17251) and GbSHR (30494) was significantly upregulated (Figure 6A,B), while the expression of genes responding to auxin, GbPIN (02144), and GbSPL14 (00228) was significantly downregulated (Figure 6C,D). Because pruning caused damage to the plants, and previous research found that genes involved in the response to wounding also participate in bud development, we also analyzed genes that respond to damage. We found that GbWIND1 (08541) and genes responding to cytokinin, GbWUS (28733), showed an upward trend of expression (Figure 6E,F).

Figure 6.
Expression of genes related to bud formation after pruning. QRT-PCR expression profiling the analysis of six candidate genes involved in bud formation (A–F). The data represent mean ± SD of the relative expression levels (n = 3). Lowercase letters indicate significant differences compared to the control, p < 0.05, as determined using a Student’s t-test.
Considering that the flavonoid content significantly increases after pruning, we analyzed the dynamic expression changes of five key enzyme-coding genes related to flavonoid synthesis. We found that after pruning, the expression of the flavonoid biosynthesis-related genes GbFLS (06949), GbF3′H (24534), and GbDFR (26256) increased significantly compared with the control (Figure 7A–C), while the expression of GbFLS (14030) and GbCHS (19002) increased slightly (Figure 7D,E).

Figure 7.
Expression of genes related to flavonoid synthesis after multiple prunings. QRT-PCR expression profiling analysis of five candidate genes involved in flavonoid synthesis (A–E). The data represent mean ± SD of the relative expression levels (n = 3). Letters indicate significant differences based on one-way ANOVA (p < 0.05).
3.4. Repetitive Pruning Promotes the Rooting of Cuttings
Next, we conducted a cutting experiment using the cuttings obtained after each pruning (Figure 8A–C). Compared with the control, the formation of callus tissue and the development of roots occurred early after pruning. Specifically, the occurrence of rooted cuttings was accelerated by 6 days following the secondary pruning (Figure 8D,E). In addition, the number and length of roots increased by 46.9% and 17.9%, respectively, in the cuttings after the primary pruning and by 53.1% and 18.2%, respectively, in the cuttings after the secondary pruning (Figure 8F,G). It is worth noting that the rooting rate of the cuttings increased by 12.5% after primary pruning and by 16.6% after the secondary pruning (Figure 8H).

Figure 8.
The rooting of cuttings after pruning: (A) control cuttings; (B) cuttings after the primary pruning; (C) cuttings after the secondary pruning; (D) the time of cutting callus generation; (E) the time of cutting root formation; (F) the number of cutting roots; (G) the length of cutting roots; (H) rooting rate of cuttings. The right end of the red arrow is a close-up of the left end. Means ± SD, n = 3. Letters indicate significant differences based on one-way ANOVA (p < 0.05).
Due to the high rooting rate in the cuttings after consecutive pruning, we specifically selected the cuttings after the secondary pruning to further screen for the most optimal hormone combination for root development (Figure 9A–E). Compared with the control, the combined treatment of three hormones significantly increased the number of roots, the root length, and the rooting rate of the branches. Among them, the combination of 1.5 μM MeJA + 400 mg/L NAA + 100 mg/L Uniconazole-P had the most favorable effect, promoting callus and root formation 14 days and 17 days earlier, respectively (Figure 9F,G). This particular combination also resulted in a 93.9% increase in the number of roots, a 116.0% increase in root length, and a 21.9% increase in the rooting rate (Figure 9H–J).
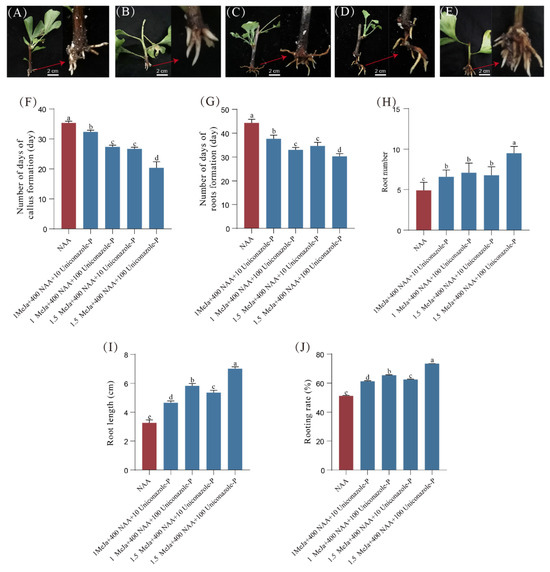
Figure 9.
The root development of cuttings after NAA, MeJA, and Uniconazole-P treatments. (A–E) Root development of cuttings after different hormone treatments; (F) time of cutting callus formation; (G) time of cutting root formation; (H) number of cutting roots; (I) length of cutting roots; (J) rooting rate. MeJA unit is μM; NAA unit is mg/L; Uniconazole-P unit is mg/L. The right end of the red arrow is a close-up of the left end. Means ± SD, n = 3. Letters indicate significant differences based on one-way ANOVA (p < 0.05).
4. Discussion
Pruning can effectively promote the sprouting of adventitious buds or axillary buds, thereby achieving the goal of the rejuvenation and revitalization of plants [32]. The number of branches and leaves in eucommia (Eucommia ulmoides O.) significantly increases after pruning in the third year [33]. In this study, we initially applied various pruning techniques to five-year-old G. biloba trees and observed that light pruning had the most pronounced effect on lateral branch growth and plant rejuvenation. Subsequently, a second light pruning on this basis further increased the number of lateral branches, with an increase of up to 3–4 times. Pruning experiments on tea trees (Camellia sinensis L.) have shown that different degrees of pruning can significantly increase branch weight and fresh weight, while also promoting rejuvenation and vitality. There are significant differences between light pruning and mild to heavy pruning. Compared to light pruning, mild pruning increased branch and leaf biomass by 41.2%, while heavy pruning increased it by 61.2% [24]. Increasing the frequency of pruning in raspberry (Rubus corchorifolius L.) led to a significant increase in both branch growth and leaf yield [34]. These studies showed that pruning can increase branch and leaf biomass by stimulating the growth of buds, and consecutive pruning can further stimulate bud sprouting and promote the development of lateral branches and rejuvenation.
The G. biloba leaves, due to their high content of active medicinal ingredients, such as flavonoids and terpenoids, have significant economic value [3]. Therefore, increasing the yield of ginkgo leaves and their active medicinal ingredients is of great importance. After pruning the ginkgo trees, there is a significant increase in leaf size, leaf quantity, leaf water content, and flavonoid content, indicating a clear rejuvenation state [8]. Previous studies have found that ginkgo leaf yield significantly increases after pruning, and the leaves exhibit more desirable characteristics, such as deeper leaf lobes, larger leaf size, and higher water content. Additionally, the content of compounds such as flavonoids and terpenoids in the leaves also increases significantly [29]. In this study, we conducted consecutive pruning on 5-year-old ginkgo trees and found that compared to the control group, the flavonoid content in the leaves significantly increased after both rounds of pruning. Furthermore, on the basis of the primary pruning, the leaf yield further increased after the secondary pruning, and the flavonoid content in the leaves increased by an additional 10.3%. Our results indicate that secondary pruning can increase the leaf yield and accumulation of flavonoid compounds in ginkgo trees by continuously stimulating the plant’s rejuvenation.
Multiple genes have been found to be involved in bud sprouting. The WOX family belongs to the homologous domain (HD) superfamily and plays an important role in plant lateral branch development [35]. WUS (WUSCHEL) is the first WOX gene to be discovered. Overexpression of WUS in birch (Betula platyphylla Suk.) trees can enhance lateral branch formation [36]. Additionally, SPL and SHR have been identified to participate in the regulation of branch development in Populus. Overexpression of SPL13 leads to slow plant growth, while silencing SPL13 increases the number of lateral branches [37]. SHR is primarily expressed in axillary buds and is significantly upregulated during bud maturation and activation [38,39]. Furthermore, other crucial genes involved in regulating lateral branch formation have been identified, such as CUC (CUP-SHAPED COTYLEDON) and BRC1 (BRANCHED1). Ectopic overexpression of CUC1/CUC2 in Arabidopsis thaliana promotes bud formation, contributes to axillary meristem development, and aids in lateral branch formation [40], while mutants of BRC1 and BRC2 exhibit excessive branching [41]. To further explore the gene expression changes related to bud sprouting in G. biloba following pruning, we conducted qRT-PCR analysis on GbSHR, GbPIN, GbSPL, GbWUS, and GbWIND1. Our results revealed that GbSHR showed significant upregulation after pruning, while GbPIN and GbSPL14 were significantly downregulated. Based on these findings, it is hypothesized that GbSHR auxin transport-related genes may play a role in the regulation of bud sprouting in G. biloba.
The biosynthesis of flavonoids is directly controlled by many key enzyme coding genes [42]. In G. biloba, several important genes related to the flavonoid synthesis pathway have been identified, including GbPAL, GbC4H, Gb4CL, GbCHS, GbFLS, GbDFR, GbANS, GbANR, GbCHI, GbF3H, GbF3′H, and GbLAR [13,43,44,45]. Among them, CHS, FLS, F3′H, and DFR have been found to participate in the biosynthesis of flavonoids [8]. Previous research has indicated that pruning can significantly increase the expression level of genes associated with flavonoid biosynthesis in sandalwood [46]. To explore whether multiple prunings can continuously affect the expression of flavonoid biosynthesis-related genes [8,29], we chose the key genes of flavonoid biosynthesis, including GbCHS, GbFLS, GbDFR, and GbF3′H, for qRT-PCR detection. We found that the expression of the GbFLS, GbF3′H, and GbDFR genes increased significantly after pruning, especially the expression of GbF3′H in the leaves, which increased by 7.3 times, confirming that pruning could enhance flavonoid biosynthesis. Due to a significant increase in the total flavonoid content in G. biloba leaves, we hypothesize that GbF3′H might serve as the key enzyme-coding gene responsible for regulating flavonoid biosynthesis in G. biloba.
Many studies have shown that the quality of cuttings and exogenous hormone treatments have a significant impact on the rooting of cuttings [47,48,49]. The young apple tree branch has a 24.02% higher rooting rate than the mature branch [50]; the highest rooting rate of African blackwood (Dalbergia melanoxylon Guill. & Perr.) cuttings is 71.11%, while the rooting rate of mature cuttings is only 24.42% [51]. This is because the young branches have strong cell division ability and a reduced rate of substances that inhibit rooting in the plant, making rooting easier [52]. In this study, we compared the rooting ability of branches after each pruning and found that the cutting rooting rate of branches after secondary pruning was higher than that of branches after primary pruning and that of the control group.
Previous research utilizing NAA treatment on ginkgo cuttings reported an average rooting rate of 51.04% for various types of cuttings [53]. Additionally, the application of both NAA and IBA led to rooting approximately 60 to 70 days post-treatment, with a rooting rate of approximately 50% [54]. In the present study, we added MeJA and Uniconazole-P to the NAA treatment and screened a hormone formula that promotes cutting rooting. We found that the best effect was obtained with 1.5 μM MeJA + 400 mg/L NAA + 100 mg/L Uniconazole-P, with rooting taking about 20 days and a rooting rate reaching 71.2%. Compared to those of previous studies, our cutting technique significantly reduces rooting time by over 30 days and increases the rooting rate by about 20%. This is likely because Uniconazole-P inhibits the synthesis of GA3 and ABA, promoting the induction of root primordia into adventitious roots, while MeJA responds to wound signals to promote auxin accumulation and improve the ability to regenerate adventitious roots [55,56]. Our results suggest that pruning can promote the rejuvenation of plants and improve their rooting ability, while MeJA and NAA can serve as supplemental hormones for rooting agents, applied for the rapid rooting of ginkgo cuttings.
5. Conclusions
Pruning treatments resulted in a significant increase in branch number, leaf lobe depth, leaf area, leaf weight, and leaf flavonoids. Among the treatments, light pruning had the most positive effect on increasing branch number, leaf area, and leaf flavonoids. Secondary light pruning also led to an increase in branch number. Additionally, cuttings taken from secondary pruning showed a higher rate of rooting. Further research is needed to understand the molecular mechanisms behind these improved economic traits.
Author Contributions
L.Z. and L.W. designed the experiment and drafted the article; L.Z. performed the experiment and conducted the data analysis; L.W. and B.J. reviewed and revised the manuscript; S.X. (Shiyuan Xu) and W.Z. conducted material and data collection; S.X. (Shuwen Xu) performed additional data analysis; Z.L. assisted in modifying the review comments. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 32171838, 31971686), the Forestry Sci-Tech Innovation and Promotion Project of Jiangsu Province (LYKJ [2021]35), and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (SJCX22_1787).
Data Availability Statement
The data are included in the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Zhang, H.F.; Huang, L.B.; Zhong, Y.B.; Zhou, Q.H.; Wang, H.L.; Zheng, G.Q.; Lin, Y. An overview of systematic reviews of Ginkgo biloba extracts for mild cognitive impairment and dementia. Front. Aging Neurosci. 2016, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Baharara, H.; Amiri, M.S.; Moghadam, A.T.; Sahebkar, A.; Emami, S.A. Ginkgo biloba: An update review on pharmacological, ethnobotanical, and phytochemical studies. Pharmacol. Res.-Mod. Chin. Med. 2023, 9, 100331. [Google Scholar] [CrossRef]
- Mao, D.Y.; Zhong, L.; Zhao, X.Y.; Wang, L. Function, biosynthesis, and regulation mechanisms of flavonoids in Ginkgo biloba. Fruit Res. 2023, 3, 0018. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, X.; Wang, T.L.; Wang, G.B.; Cao, F.L. Regulation of flavonoid metabolism in ginkgo leaves in response to different day-night temperature combinations. Plant. Physiol. Bioch. 2020, 147, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Rethinking Ginkgo biloba L.: Medicinal uses and conservation. Pharmacogn. Rev. 2015, 9, 140–148. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.F.; Gan, Q.; Liu, S.A.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Wang, Q.J.; Jiang, Y.; Mao, X.Y.; Yu, W.W.; Lu, J.K.; Wang, L. Integration of morphological, physiological, cytological, metabolome and transcriptome analyses reveal age inhibited accumulation of flavonoid biosynthesis in Ginkgo biloba leaves. Ind. Crop. Prod. 2022, 187, 115405. [Google Scholar] [CrossRef]
- Lu, Z.G.; Zhu, L.K.; Lu, J.K.; Shen, N.; Wang, L.; Liu, S.A.; Wang, Q.J.; Yu, W.W.; Noguchi, H.K.; Li, W.X.; et al. Rejuvenation increases leaf biomass and flavonoid accumulation in Ginkgo biloba. Hortic Res. 2022, 9, uhab018. [Google Scholar] [CrossRef]
- Vellas, B.; Coley, N.; Ousset, P.J.; Berrut, G.; Dartigues, J.F.; Dubois, B.; Grandjean, H.; Pasquier, F.; Piette, F.; Robert, P.; et al. Long-term use of stand-ardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012, 11, 851–859. [Google Scholar] [CrossRef]
- Herrschaft, H.; Nacu, A.; Likhachev, S.; Sholomov, I.; Hoerr, R.; Schlaefke, S. Ginkgo biloba extract EGb 761(R) in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. Psychiatr. Res. 2012, 46, 716–723. [Google Scholar] [CrossRef]
- Bitencourt, J.; Zuffellato-Ribas, K.C.; Koehler, H.S. Ginkgo biloba L. cutting using three substrates. Rev. Bras. Plantas Med. 2010, 12, 135–140. [Google Scholar] [CrossRef]
- Kosenko, I.S.; Tsybrovska, N.V.; Balabak, O.A.; Hrabovyi, V.M.; Muzyka, H.I.; Shvets, T.A.; Oksantiuk, V.M. Introduction of Ginkgo biloba and its cultivars by vegetative propagation. Ukr. J. Ecol. 2021, 11, 65–76. [Google Scholar]
- Wang, L.; Lu, Z.; Li, W.; Xu, J.; Luo, K.; Lu, W.; Zhang, L.; Jin, B. Global comparative analysis of expressed genes in ovules and leaves of Ginkgo biloba L. Tree Genet. Genomes. 2016, 12, 29. [Google Scholar] [CrossRef]
- Zhang, G.F.; Zhao, F.; Chen, L.; Pan, Y.; Sun, L.; Bao, N.; Zhang, T.; Cui, C.X.; Qiu, Z.; Zhang, Y.; et al. Jasmonate-mediated wound signalling promotes plant regeneration. Nat. Plants. 2019, 5, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; Matheny, N. The research foundation to tree pruning: A review of the literature. Arboric. Urban For. 2010, 36, 110. [Google Scholar] [CrossRef]
- Long, H.; James, S. Sensing and automation in pruning of apple trees: A Review. Agronomy 2018, 8, 211. [Google Scholar] [CrossRef]
- Forrester, D.I. Growth responses to thinning, pruning and fertiliser application in eucalyptus plantations: A review of their production ecology and interactions. Forest Ecol. Manag. 2013, 310, 336–347. [Google Scholar] [CrossRef]
- De la Paz, A.V.C.; Espinosa, B.G.G.; Brizuela, E.I.L.; Bravo, D.E.L.; Cueto, D.O.G. Fructification pruning in guava Crop (Psidium guajava L.) and its influence on yield. Rev. Cienc. Técnicas Agropecu. 2019, 28, 81–88. [Google Scholar]
- Wendling, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry—Part I: Concepts, regulation and consequences of phase change. New For. 2014, 45, 449–471. [Google Scholar] [CrossRef]
- Pereira, A.C.; Scaloppi Junior, E.J.; Costa, E.; Maitins, G.L.M.; Souza, N.C. Efeito da poda apical nos atributos morfofisiológicos do porta-enxerto clonal de seringueira GT 1. Ciência Florest. 2019, 29, 900–912. [Google Scholar] [CrossRef]
- Su, M.L.; Wu, S.; Ma, L.Y.; Duan, J.; Rong, G.C.; Su, S.C.; Ao, Y. Effects of three pruning methods on endogenous hormone and shoot growth of Xanthoceras sorbifolia Bunge buds. J. Northwest A F Univ. 2017, 45, 101–108. [Google Scholar]
- Duan, Y.J.; Meng, F.X.; Yang, S.Y.; Sun, M.Y.; Yang, Y.J.; Guo, S.P.; Liu, H.G.; Fang, H.D. Effects of different pruning methods on the growth of new shoots of Annona atemoya. Yunnan Acad. Agric. Sci. 2021, 7, 63–65. [Google Scholar]
- Csihon, Á.; Dremák, P.; Gonda, I. Partial and total rejuvenation pruning of sour cherry trees. Int. J. Hortic. Sci. 2015, 21, 11–15. [Google Scholar] [CrossRef]
- Zhu, L.G.; Sun, J.; Zhang, W.J.; Chen, Z.Z.; Wu, Z.D.; Jiang, F.Y. Water retention and biomass of litter from varied pruning practices on tea bushes. Fujian J. Agric. Sci. 2016, 31, 1210–1215. [Google Scholar]
- Zhang, C.; Liu, D.H.; Yang, T.F.; Zhang, Q.; Li, M.J.; Zhang, J.K. Different winter pruning amounts affect the growth and fruiting of ‘Fuji’ apples. Acta Agric. Boreali-Occident. Sin. 2016, 25, 1650–1655. [Google Scholar] [CrossRef]
- Atmaca, S.; Ulger, S. The effects of different planting densities and pruning methods on changes of endogenous hormone levels in shoot tips and flowering in ‘Gemlik’ Olive cultivar. Erwerbs-Obstbau 2021, 63, 201–207. [Google Scholar] [CrossRef]
- Chen, M.; Cao, M.; Lin, L.X. Research advances in regeneration of woody plants by sprouting. J. Plant Ecol. 2007, 26, 1114–1118. [Google Scholar]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Cao, M.; Gan, Q.; Xu, Y.; Lu, J.K.; Zhong, L.; Wang, M.X.; Liu, S.A.; Wang, L. Pruning improves seedling development and bioactive secondary metabolite accumulation in the leaves of Ginkgo biloba. Trees 2022, 36, 953–966. [Google Scholar] [CrossRef]
- Wang, Q.J.; Xu, S.Y.; Zhong, L.; Zhao, X.Y.; Wang, L. Effects of Zinc Oxide Nanoparticles on Growth, Development, and Flavonoid Synthesis in Ginkgo biloba. Int. J. Mol. Sci. 2023, 24, 15775. [Google Scholar] [CrossRef]
- Zhang, R.; Ding, J.; Liu, C.; Cai, C.; Zhou, B.; Zhang, T.; Guo, W. Molecular evolution and phylogenetic analysis of eight COL superfamily genes in group I related to photoperiodic regulation of fowering time in wild and domesticated cotton (Gossypium) species. PLoS ONE 2015, 10, e0118669. [Google Scholar]
- Xu, Y.; Lu, J.K.; Liu, S.A.; Wang, L. Research progress on the mechanism of resprouting in trees. J. Plant Physiol. 2023, 59, 803–816. [Google Scholar]
- Yang, B.; Hu, L.; Shen, H.; Sun, X.; Zhang, P. Effect of mounding and top-pruning on survival and growth of manchurian ash seedlings planted under the secondary forest of the species. Sci. Silvae Sin. 2018, 51, 104–113. [Google Scholar]
- Guo, C.J. Impact of pruning frequency and retention of branches on raspberry yield and benefits. North. Fruits 2016, 3, 15–17. [Google Scholar]
- Yu, Y.J.; Zhang, D.B.; Yuan, Z. The updated functional study of WOX protein family in regulating stem cell development. Chin. Bull. Bot. 2016, 51, 565–574. [Google Scholar]
- Lou, H.; Huang, Y.T.; Wang, W.Z.; Cai, Z.Y.; Cai, H.Y.; Liu, Z.Q.; Sun, L.; Xu, Q.J. Overexpression of the AtWUSCHEL gene promotes somatic embryogenesis and lateral branch formation in birch (Betula platyphylla Suk.). Plant Cell Tissue Organ Cult. (PCTOC) 2022, 150, 371–383. [Google Scholar] [CrossRef]
- Gao, R.; Gruber, M.Y.; Amyot, L.; Hannoufa, A. SPL13 regulates shoot branching and flowering time in Medicago sativa. Plant. Mol. Biol. 2018, 96, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.L.; Yang, H.Y.; Yang, S.H.; Wang, J.H. Overexpression of short-root2 transcription factor enhances the outgrowth of mature axillary buds in poplar trees. J. Exp. Bot. 2022, 73, 2469–2486. [Google Scholar] [CrossRef]
- Tian, Y.T.; Wang, J.N.; Guo, H.P.; Qu, K.; Xu, D.; Hou, L.L.; Li, J.H. Transcriptome analysis of active axillary buds from narrow-crown and broad-crown poplars provides insight into the phytohormone regulatory network for branching angle. Plant Mol. Biol. Rep. 2021, 39, 595–606. [Google Scholar] [CrossRef]
- Kareem, A.; Durgaprasad, K.; Sugimoto, K.; Du, Y.J.; Pulianmackal, A.J.; Trivedi, Z.B.; Abhayadev, P.V.; Pinon, V.; Meyerowitz, E.M.; Scheres, B.P. PLETHORA genes control regeneration by a two-step mechanism. Curr. Biol. 2015, 25, 1017–1030. [Google Scholar] [CrossRef]
- Seale, M.; Bennett, T.; Leyser, O. BRC1 expression regulates bud activation potential but is not necessary or sufficient for bud growth inhibition in Arabidopsis. Development 2017, 144, 1661–1673. [Google Scholar] [PubMed]
- Zou, K.; Liu, X.; Zhang, D.; Yang, Q.; Fu, S.; Meng, D.; Chang, W.; Li, R.; Yin, H.; Liang, Y. Flavonoid biosynthesis is likely more susceptible to elevation and tree age than other branch pathways involved in phenylpropanoid biosynthesis in Ginkgo leaves. Front. Plant. Sci. 2019, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.A.; Meng, Z.L.; Zhang, H.Y.; Chu, Y.X.; Qiu, Y.Y.; Jin, B.; Wang, L. Identification and characterization of thirteen gene families involved in flavonoid biosynthesis in Ginkgo biloba. Ind. Crop. Prod. 2022, 188, 115576. [Google Scholar] [CrossRef]
- Zhao, B.B.; Wang, L.; Pang, S.Y.; Jia, Z.C.; Wang, L.; Li, W.X.; Jin, B. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind. Crop. Prod. 2020, 151, 112483. [Google Scholar] [CrossRef]
- Jia, Z.; Zhao, B.; Liu, S.; Lu, Z.; Chang, B.; Jiang, H.; Cui, H.; He, Q.; Li, W.; Jin, B.; et al. Embryo transcriptome and miRNA analyses reveal the regulatory network of seed dormancy in Ginkgo biloba. Tree Physiol. 2020, 41, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, M.; Kang, S.C.; Yang, C.M.; Meng, H.; Yang, Y.; Zhao, X.S.; Gao, Z.H.; Xu, Y.H.; Jin, Y.; et al. Molecular mechanism underlying mechanical wounding-induced flavonoid accumulation in Dalbergia odorifera T. Chen, an endangered tree that produces Chinese rosewood. Genes 2020, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, Y.; Li, Y. Plant rejuvenation: From phenotypes to mechanisms. Plant Cell Rep. 2020, 39, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Hechmi, M.; Khaled, M.; Abed, S.; EL-Hassen, A.; Faiez, R.; M’hamed, A. Performance of olive cuttings (Olea europaea L.) of different cultivars growing in the agro-climatic conditions of Al-Jouf (Saudi Arabia). Am. J. Plant Physiol. 2013, 8, 41–49. [Google Scholar] [CrossRef][Green Version]
- Fan, H.Y.; Wang, Y.; Tang, F.; Lu, C. Determination of the mimic epitope of the M-like protein adhesin in swine Streptococcus equi subsp zooepidemicus. BMC Microbiol. 2008, 8, 170. [Google Scholar] [CrossRef]
- Xiao, Z.F.; Ji, N.; Zhang, X.Z.; Zhang, Y.Z.; Wang, Y.; Wu, T.; Xu, X.F.; Han, Z.H. The lose of juvenility elicits adventitious rooting recalcitrance in apple rootstocks. Plant Cell 2014, 119, 51–63. [Google Scholar] [CrossRef]
- Amri, E.; Lyaruu, H.V.M.; Nyomora, A.S.; Kanyeka, Z.L. Vegetative propagation of African Blackwood (Dalbergia melanoxylon Guill. & Perr.): Effects of age of donor plant, IBA treatment and cutting position on rooting ability of stem cuttings. New For. 2010, 39, 183–194. [Google Scholar] [CrossRef]
- Hong, H.H. The effect of cutting age on the rooting process, biochemical characteristics, and seedling growth of white poplar. Beijing For. Univ. 2018, 2, 274–281. [Google Scholar]
- Tong, P.X.; Ma, K.P.; Hu, Y.; Wang, L. Relationship between ginkgo cutting types and rooting rate. Mod. Hortic. 2019, 11, 10–11. [Google Scholar]
- Wang, R.M.; Zhu, L.G.; Chen, Y.; Yao, X.W.; Bai, J.W.; Chen, X.; Zhang, Q.Q.; Cao, F.L. Study on the rooting process and mechanism of hard branch cuttings of Ginkgo biloba. J. Cent. South Univ. For. Technol. 2020, 40, 28–37. [Google Scholar]
- He, Q.Q.; Zhang, Q.; Mu, X.P.; Zhang, J.C.; Du, J.J.; Wang, P.F. Effects of foliar spraying plant growth regulator on rooting and physiological changes of Cerasus humilis softwood cuttings. J. Plant Physiol. 2024, 60, 108–116. [Google Scholar] [CrossRef]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A jasmonate signaling network activates root stem cells and promotes regeneration. Cell 2019, 177, 942–956.e14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).