Abstract
The aim of this study was to compare the foraging activity of bats in coniferous, deciduous, and mixed forests and to test whether this activity was subject to seasonal variation. Sample points were selected in stands of similar spatial structure in coniferous (Pinus sylvestris L.), in mixed (Pinus sylvestris and Quercus petraea (Matt.) Liebl.), and in deciduous (Quercus petraea) managed forests in western Poland. Bat calls were recorded using automated ultrasound recording devices (Batcorder 3.0, ecoObs, Nürnberg, Germany) during five consecutive nights from May to September in each of the six stands. A total of 4250 bat passes were recorded. Overall, 63.1% of bat passes were identified to species, 31.6% were identified to genus or sonotype group, and 5.3% remained unidentified. In total, eight species of bats and seven sonotype groups were recorded. The dominant species in all types of forests were Pipistrellus pygmaeus (44.5% of recorded bat passes), followed by Nyctalus noctula (10.3%) and Pipistrellus nathusii (5.7%). There were no significant differences in the total activity of bats between the three types of forests; however, high seasonal fluctuations in bat foraging activity were found. This study demonstrates that when coniferous, deciduous, and mixed stands with similar spatial structure are compared, forest type does not affect the foraging activity of bats.
1. Introduction
Forests are a key habitat for bats throughout the world [1,2,3], and almost half of known bat species worldwide use trees as roosts for at least part of the year [4]. Forests provide both foraging and roosting resources for bats, and moreover, bats provide significant ecosystem services to forests [5], including control of phytophagous insects [6,7]. Thus, bat conservation in forests is crucial not only for maintaining biodiversity, but also for sustainable forest management.
Many studies have examined the foraging activity of bats in various types of forests [8,9,10,11]. Several studies have shown that bat assemblages respond to forest structure, and in particular, to forest type, age, and the amount of structural clutter [10,12,13,14,15]. Most often, studies of habitat use have compared deciduous and coniferous forests. Some of them have shown bat activity to be lower in coniferous forests than in deciduous or mixed forests [16,17,18,19,20,21]. By contrast, some showed no difference between these forest types [22,23,24], and some showed that bat activity was higher in coniferous forests [25,26,27]. The question is: why is there such ambiguity in the results? Additional factors related to the type of forest may be the answer.
Studies of habitat use in mosaic landscapes have sometimes compared bat activity in intensively managed coniferous plantations with that in deciduous woodlands [12,28,29,30]. These two types of habitats differ in structural heterogeneity and amount of clutter, which may significantly affect their usage by bats [10,31,32,33]. The presence of a high number of internal service roads on plantations may also be an important factor, which allow bats to access and use sites that are otherwise too cluttered [30].
The age of stands may be another factor explaining the differences between forest types. Coniferous forests—especially plantations‚ may be younger due to their generally shorter rotations, whereas bats tend to prefer older forests [11,34,35], which may be due to the greater availability of roosts in older trees [13,36,37]. Furthermore, when comparing forest types, their spatial structure is also important. For example, coniferous forests may have less structural clutter, as in mature pine stands with reduced midstory vegetation, or they may have more clutter, as in middle-aged densely branched spruce stands.
Coniferous and deciduous forests can also contain different numbers of tree species. Coniferous stands are often single-species monocultures, while deciduous and mixed stands usually have a more diverse species composition. In addition, deciduous forests often grow on more fertile habitats, which may result in higher vegetation diversity and richer arthropod fauna [38,39]. Furthermore, insect abundance in coniferous forests often fluctuates due to the higher risk of insect outbreaks in more simplified systems [40]. This is likely to affect comparisons between forest types if bat activity is recorded over a short period of time [21,31,41].
Higher bat activity has been observed in deciduous or coniferous forest types depending on the dominant forest composition in the surrounding landscape. Bat activity was higher in stands that were rarer at the landscape level and potentially provided non-substitutional resources to bats for both foraging and roosting [31].
Considering how many additional factors have influenced comparisons of bat activity in different forest types, it becomes important to determine whether deciduous or coniferous forests provide better foraging conditions for bats. To test this, we carried out a study in selected single-species pine and oak stands and mixed oak–pine stands. We tried to select sample stands in which the only differentiating factor was forest type, with very limited influence of other factors. To reduce the influence of fluctuations in insect abundance, bat recording was repeated over several months (different phenological seasons).
The aim of this study was to compare the foraging activity of bats in coniferous, deciduous, and mixed forests and to test whether this activity is subject to seasonal variation. We hypothesize that: (1) foraging activity of bats is lower in coniferous forests than in deciduous and mixed forests; and that (2) bat activity is greater in late summer and autumn, when juveniles are foraging along with adults.
2. Materials and Methods
2.1. Study Area
The study area is located in western Poland (Figure 1) in the Włoszakowice Forest District. In total, 28.4% of the area is covered by mixed, deciduous, and coniferous forests (Figure 2), managed by the State Forests National Forest Holding according to the forest management plan. The forests are composed of native tree species, the most abundant being Scots pine Pinus sylvestris L. (83%), sessile oak Quercus petraea (Matt.) Liebl. (10%), and silver birch Betula pendula Roth (4%), which consist of several age and size cohorts. These forests are managed for multiple ecosystem services (e.g., wood and non-wood products, climate regulation, soil protection, water supply, recreation, biodiversity conservation, etc.). Due to its commercial use, the forest area is crossed by a network of small roads, which facilitate the maintenance of equipment on site. Standard management practices are applied in these forests, including tree planting and natural regeneration, a rotation period of 100–140 years, and thinning once per decade.
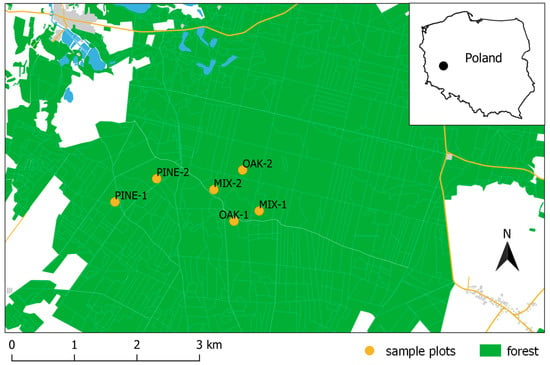
Figure 1.
Distribution of sampled stands (see Table 1 for stand description; source of spatial data: OpenStreetMap.org and https://www.bdl.lasy.gov.pl, accessed on 12 November 2021).

Figure 2.
Three types of stands sampled: coniferous (Pinus sylvestris), mixed (Pinus sylvestris and Quercus petraea), and deciduous (Quercus petraea).
The forests are located in a temperate climate zone. This is a low-elevation area, 110–130 m above sea level. The most common soil type is Brunic Arenosols (74%). The average annual rainfall is 530 mm, the average temperature is 8.2 °C, and the length of the growing season is 220 days [42].
2.2. Bat Acoustic Surveys
We selected six sample points in stands of similar spatial structure: two in coniferous (Pinus sylvestris), two in mixed (Pinus sylvestris and Quercus petraea) and two in deciduous (Quercus petraea) stands (Table 1, Figure 2). The process of choosing sample stands was carried out in two stages. First, stands meeting certain criteria (min. area of 2 hectares, only 1 tree layer, located min. 500 m from the forest edge and average DBH of 75–85 cm) were selected in the forest database of the study area (N: 51°53′40″–51°53′00″, E: 16°16′40″–16°19′10″). Then, 2 stands for each forest type were randomly selected (using a random number generator to choose the stand ID). Each sampling plot retained structural homogeneity in terms of management and understory cover. The stands were composed of only two layers: the canopy layer and the understory.

Table 1.
Main characteristics of coniferous (PINE-1, PINE-2), mixed (MIX-1, MIX-2), and deciduous (OAK-1, OAK-2) sampled stands.
We sampled bats in each of the six stands in 2014 on five consecutive nights of each main phenological season (characteristic for most temperate bats): gestation period (12–16 May), births period (18–22 June), lactation period (18–22 July), mating/dispersion period (16–20 August and 13–17 September). All sample points were recorded simultaneously. For each season, we chose a period in which the forecast did not predict rain. Consequently, rain did not occur on any survey night. Due to the simultaneous recording, it can be assumed that all sites had similar weather conditions (wind, temperature, moonlight, etc.). In addition, we collected selected parameters (average daily temperature and average daily wind speed) from the nearest weather station and the length of night to analyze their influence on bat activity.
To record bat calls, we used six Batcorder 3.0 automated ultrasound recording devices (ecoObs, Nürnberg, Germany, http://www.ecoobs.de). Each detector was placed on a tree located in the interior of the sampled stand (the minimum distance from the edge/nearest road was 50 m). We selected a tree with a single branch at a height of 4–6 m above ground, on which the detector was attached. The microphone was angled 30–60 degrees above horizontal.
Batcorders digitally record ultrasonic signals in real time (500 kHz, 16 bit) and use online analysis to distinguish between bat calls and ultrasound signals from other origins (e.g., bush crickets). Further strengths of this system are the comparability of results between different devices (calibrated sensitivity) and the omnidirectionality of the microphone. This system was originally designed for studies on microhabitat use by bats in forests. The devices were calibrated and configured by ecoObs, and no further adjustments were made to the settings (400 ms post-trigger, −27 dB threshold level). The timers of the batcorders were set to record throughout the night (from 20:30 to 06:00). It should be noted that there is probably a pseudo-replication of individuals traversing across the sites, and therefore, one individual may be recorded more than once within and across the surveyed sites. Thus, bat activity is simply the total number of calls, but does not necessarily indicate the abundance of individuals.
The data (full-spectrum samples) were then fed into bcAdmin 2.0 and Batident 1.5 (ecoObs, Nürnberg, Germany) software for the automatic identification of bat echolocation calls up to species or sonotype group level. Some of the identifications given by the program were manually verified (about 30%), especially findings of rare or unusual species for the habitat. Manual verification was carried out using bcAnalyze 2.0 (ecoObs, Nürnberg, Germany) software. The basic parameters that were taken into account in the identification were: call shape, frequency of maximum energy, call duration, and interval between calls. For manual identification, we used published guides [43,44,45,46] and our own library of bat sounds recorded mainly in western and central Poland (the individuals were recorded by hand release method after being captured end properly identified).
2.3. Statistical Analysis
One-way ANOVA on ranks was used to find the differences between the activity of bats in individual months. After rejection of the null hypothesis in a Kruskal–Wallis non-parametric test, a multiple comparisons (post hoc) test using Fisher’s Least Significant Difference Criterion and the Bonferroni correction was performed at a significance level of 0.05. Data were analyzed using the open-source software R for statistical computing (Version 4.0.4). Visualization of data was performed with the R package ‘ggplot2’.
These preliminary results were extended by a comprehensive analysis taking into account weather parameters and the structure of the studied stands. Average daily temperature and average daily wind speed as well as DBH (continuous) were included in analysis as predictors. Night length was significantly correlated with temperature (Pearson’s correlation, r = −0.450, p < 0.001), while tree age, stand density, tree height, basal area, and stand volume were significantly correlated with DBH (r = 0.87, p < 0.001; r = −0.75, p < 0.001, r = −0.55, p < 0.001, r = −049, p < 0.001, r = −0.41, p < 0.001) and were excluded from the analysis to avoid collinearity. Poisson generalized linear mixed models (‘glmer’ function in package lme4 in R) fitted by maximum likelihood using Laplace approximation have been used to investigate the forest type fix effect. Day was considered in the model as random-effect slope, and month as well as plot as random-effect intercepts. Analysis of deviance with Type II Wald chi-square tests was performed with the use of ‘car’ package. Fixed effects and related confidence intervals were extracted using the ‘tidy’ function (package ‘broom.mixed’ in R). If categorical predictors were significant (criterion p < 0.05), estimated marginal means (EMMs) were computed (‘emmeans’ (emmeans)) and Tukey post hoc test conducted (‘cld’ (multcomp)).
3. Results
Across all types of forests, a total of 4250 bat passes were recorded. Overall, 63.1% of bat passes were identified to species, 31.6% were identified to genus or sonotype group, and 5.3% remained unidentified. In total, eight species of bats and seven sonotype groups were recorded. The dominant species in all types of forests was soprano pipistrelle Pipistrellus pygmaeus (Leach, 1825) (44.5% of recorded bat passes), followed by common noctule Nyctalus noctula (Schreber, 1774) (10.3%) and Nathusius’ pipistrelle Pipistrellus nathusii (Keyserling & Blasius, 1839) (5.7%). The remaining five species, which made up 2.6% of total passes, were common pipistrelle Pipistrellus pipistrellus (Schreber, 1774), western barbastelle Barbastella barbastellus (Schreber, 1774), serotine bat Eptesicus serotinus (Schreber, 1774), Daubenton’s bat Myotis daubentonii (Kuhl, 1817), and greater mouse-eared bat Myotis myotis (Borkhausen, 1797) (Table 2).

Table 2.
Species of bats recorded and identified from coniferous (PINE), mixed (MIX), and deciduous (OAK) forests.
There were no significant differences in the total activity of bats between the three types of forests (coniferous, mixed, and deciduous) in any of the analyzed seasons or for all seasons combined (Figure 3). However, significant differences in bat activity were found between months (Table 3). The highest number of bat passes per night for coniferous and deciduous forests were recorded in August, and for mixed forests in July and August. The lowest bat activity for all types of forest was recorded in May and June (Figure 3, Table 3).
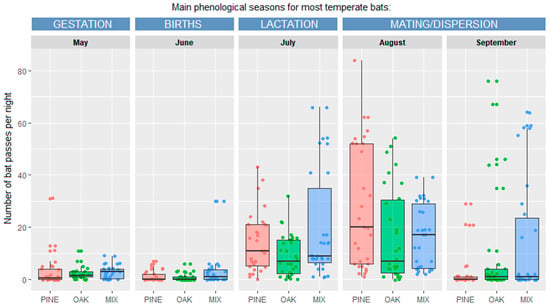
Figure 3.
Bat foraging activity in coniferous (PINE), mixed (MIX), and deciduous (OAK) forests for main phenological seasons characteristic for most temperate bats (from May to September).

Table 3.
Mean number of bat passes recorded in different months (phenological seasons) for coniferous, mixed, and deciduous forests. Different letters indicate significant differences in bat activity between months (p < 0.05).
The most abundant bat genera, Pipistrellus, Nyctalus, and Myotis, were analyzed separately. For no genus was there any significant differences in bat activity between coniferous, mixed, and deciduous forest types (Figure 4).
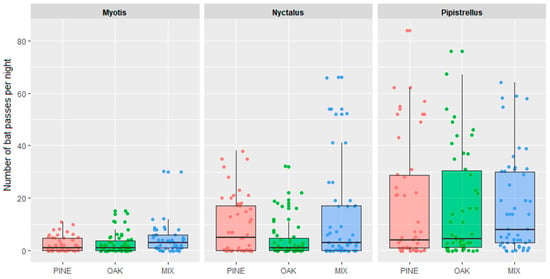
Figure 4.
Bat foraging activity in coniferous (PINE), mixed (MIX), and deciduous (OAK) forests for three genera of bats (Myotis, Nyctalus, and Pipistrellus).
The results of generalized linear mixed models (GLMM) for the effect of the forest type on foraging activity of all bats (Table 4) were consistent with those of the correlational analyses. The GLMM analyses showed no effect of forest type (coniferous, mixed and deciduous) on total activity of bats. In contrast, the GLMM analyses for three genera of bats (Myotis, Pipistrellus, and Nyctalus) separately yielded different results. GLMM revealed a significant effect (p < 0.0001) of forest type on bat activity for all genera of bats (Table 4 and Table 5).

Table 4.
Outcome of analyses of deviance after computation of generalized linear mix models (GLMM) of bat passes for all bat species together and three genera of bats (Myotis, Pipistrellus, and Nyctalus) separately.

Table 5.
Estimated marginal means (EMMs) and asymptotic confidence intervals (in parentheses) of bat passes in coniferous (PINE), mixed (MIX), and deciduous (OAK) forests for all bat species together and three genera of bats (Myotis, Pipistrellus, and Nyctalus) separately. Results are given on the log (not the response) scale. Different letters indicate statistically significant (α = 0.05) differences between EMM computed for factor levels, as a result of post hoc Tukey test.
Furthermore, the GLMM results showed a significant effect of wind speed on the activity of all bats (p = 0.0039) and on Myotis (p < 0.0001), as well as an effect of temperature on Myotis (p = 0.0075) and on Nyctalus (p = 0.0013), and an effect of tree size (DBH) on Pipistrellus (p < 0.0001).
4. Discussion
In our study, we compared coniferous, deciduous, and mixed even-aged stands of similar age and very similar spatial structure. The compared forests were composed of only two layers: the canopy layer and the understory. Below the well-developed canopy they contained open space with little clutter. Coniferous and deciduous stands were formed by only one tree species, and mixed stands by two species (oak and pine). We found no significant differences in bat activity between these sampled stands (differences were only for bat species/genus). This could indicate that there is no universal pattern of bat preferences for forest type (coniferous or deciduous). Thus, our findings suggest that the spatial structure of the stand rather than the forest type determines its attractiveness as a foraging site for bats. Contrary to expectations, there were no differences in the overall preference of bats for coniferous, deciduous, and mixed stands. The results did not support hypothesis 1.
We have found very few studies comparing bat activity in coniferous and deciduous forests of similar age, spatial structure, and clutter conditions (the amount of vegetation in the forest canopy and understory). This is because these types of forests usually differ not only in tree species, but also in many other characteristics, such as habitat fertility, vegetation diversity, layer arrangement, rotation period, and harvesting system. In these situations, it is difficult to distinguish whether bat activity is affected by forest type or by some other factor [47,48].
Suitable conditions for determining bat preferences for forest type can be found in the boreal zone, where pure stands of simple spatial structure are more common. Kalcounis et al. [16] sampled bat activity in mature stands in Canada to determine differences in activity among forest types. They found that there was significantly more bat passes per night in mixed wood than in aspen or pine forest. Wermundsen and Siivonen [25] determined the foraging habitats of bats in southern Finland. Among the 20 habitat types classified, those most used by bats were coniferous woodland (29%) and mixed woodland (11%). Vasko et al. [2] studied the presence of certain boreal bats in different types of forest in Finland. They found that Eptesicus nilssonii shifts between a preference from coniferous forests to deciduous forests in August and September, but observed no such trend for Myotis species. In many other studies that have shown differences in bat activity between forest types, other factors may have influenced the results [10,12,13]. As in our case, some studies have shown no differences in bat activity between coniferous and deciduous forests [22,23,24].
We also performed a comparison of bat activity in different forest types separately for the most abundant bat genera, Pipistrellus, Nyctalus, and Myotis. The results were inconclusive, with preliminary analysis of variance on ranks showing no effect and GLMM indicating a significant effect of forest type on the foraging activity of tested bat groups. This may mean that despite the lack of effect of forest type on all bats analyzed together, forest type or even specific tree species may be important for some bat species or groups.
Some other studies also have shown differences in pipistrelle bat activity between different forests [49]. For example, Pipistrellus pipistrellus foraged more actively in the presence of deciduous trees than conifers [21], but the foraging activity of Pipistrellus pygmaeus was higher in a broadleaved–conifer mix than in broadleaved-only woodlands [50]. In addition, Nyctalus spp. were found to prefer deciduous or mixed stands [21,31,51]. In turn, Patriquin and Barclay [27] indicated that Myotis spp. were more active in conifer forests than in other forest types, but Russ and Montgomery [17] found that Myotis bats more often selected deciduous stands.
Another aspect of our study was the seasonal variability of bat activity. In all forest types, the foraging activity of bats was significantly influenced by month (phenological season). Bats had low activity in May (gestation) and June (births), followed by high activity in July (lactation) and August (mating/dispersion), and again, low activity in September (continuation of mating/dispersion). Our results partially supported hypothesis 2. Bats exhibited greater activity in late summer, but not in autumn. The reason for these differences may be seasonal changes in foraging strategies associated with reproductive phenology [52,53]. During lactation, females may forage more often in sub-optimal habitats because of the energy costs of long-distance flights [21,54]. Increased bat activity in late summer may be due to juveniles foraging along with adults [41]. Conversely, low activity in autumn may be related to the fact that bats aggregate elsewhere to feed before hibernation [54]. Other reasons proposed for seasonal variations in bat activity in different forest types include changes in arthropod abundance [55,56], availability of adequate roosts within particular stands [51,57], and the fact that roost microclimates differ between seasons [56,58].
Charbonnier et al. [21] sampled bat communities in different periods of the summer season in pine plantation forests of southwestern France. They reported that bat activity was significantly lower in June than in the other sampling periods (May and August). Pereira et al. [54] evaluated bat species’ richness and activity during the three phenological seasons in managed pine forests in central Portugal. Bat species’ richness and activity varied with the season and was higher in September, when mating, swarming, and dispersion from nurseries to hibernacula took place; it was lower during the lactation period (July). Deeley et al. [59] sampled activity of Eptesicus fuscus and Lasiurus borealis in the Mid-Atlantic region of the United States. They observed lower levels of acoustic call activity during late summer than in spring. They determined that the highest levels of acoustic activity within the maternity season were most associated with the lactation period, rather than the period of peak activity of juvenile bats, as is often assumed. Randall et al. [41] measured Myotis lucifugus bat activity from June to August in the boreal forest of the southwestern area of Yukon, Canada. They found that bat activity did not vary significantly with season. Such varied results indicate the need for further studies of the variability of bat activity in different forest types, combining multiple spatial and temporal scales across the entire summer season.
5. Conclusions
To date, numerous acoustic studies of the habitat preferences of bats in different forest types have yielded very inconclusive results, whereby bats were sometimes most active in coniferous stands and sometimes in deciduous or mixed stands. Such variation in results is probably a consequence of the fact that coniferous and deciduous forests usually differ not only in tree species, but also in many other features, especially spatial structure. This means that the results obtained may have been misinterpreted, since it was not known which factor could have influenced bat activity.
Our study demonstrates that when coniferous, deciduous, and mixed stands with similar spatial structure are compared, forest type does not affect the total activity of bats. However, some bat species may have individual preferences for forest type or for the presence of selected tree species. This finding may have implications for forest management. Mature coniferous forests should receive more attention in Europe, as they represent valuable habitats for bats.
In addition, our study showed high seasonal fluctuations in bat foraging activity. Therefore, when examining the intensity of bat use of different forest types, it is necessary to take seasonal variability into account.
Author Contributions
Conceptualization, A.W. and W.G.; methodology, A.W., W.G., A.Ł. and R.J.; software, A.Ł.; formal analysis, R.J. and A.Ł.; investigation, A.W., W.G., R.J. and J.W.; writing—original draft preparation, A.W.; writing—review and editing, A.W. and W.G.; visualization, A.W. and A.Ł. All authors have read and agreed to the published version of the manuscript.
Funding
The publication is co-financed within the framework of the Ministry of Science and Higher Education program “Regional Initiative Excellence” in the years 2019–2022, project number 005/RID/2018/19.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to its large size.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rodríguez-San Pedro, A.; Simonetti, J.A. The relative influence of forest loss and fragmentation on insectivorous bats: Does the type of matrix matter? Landsc. Ecol. 2015, 30, 1561–1572. [Google Scholar] [CrossRef]
- Vasko, V.; Blomberg, A.S.; Vesterinen, E.J.; Suominen, K.M.; Ruokolainen, L.; Brommer, J.E.; Norrdahl, K.; Niemelä, P.; Laine, V.N.; Selonen, V.; et al. Within-season changes in habitat use of forest-dwelling boreal bats. Ecol. Evol. 2020, 10, 4164–4174. [Google Scholar] [CrossRef] [PubMed]
- Langridge, J.; Pisanu, B.; Laguet, S.; Archaux, F.; Tillon, L. The role of complex vegetation structures in determining hawking bat activity in temperate forests. For. Ecol. Manag. 2019, 448, 559–571. [Google Scholar] [CrossRef]
- Brigham, R.M. Bats in forests: What we know and what we need to learn. In Bats in Forests: Conservation and Management; Lacki, M.J., Hayes, J.P., Kurta, A., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 1–15. [Google Scholar]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.M.; Wells, K.; Kalko, E.K.V. Top-down control of herbivory by birds and bats in the canopy of temperate broad-leaved oaks (Quercus robur). PLoS ONE 2011, 6, e17857. [Google Scholar] [CrossRef] [PubMed]
- Lacki, M.J.; Johnson, J.S.; Dodd, L.E.; Baker, M.D. Prey consumption of insectivorous bats in coniferous forests of north-central Idaho. Northwest Sci. 2007, 81, 199–205. [Google Scholar] [CrossRef]
- Krusic, R.A.; Yamasaki, M.; Neefus, C.; Pekins, P. Bat habitat use in White Mountain National Forest. J. Wildl. Manag. 1996, 60, 625–631. [Google Scholar] [CrossRef]
- Grindal, S.D.; Brigham, R.M. Impacts of forest harvesting on habitat use by foraging insectivorous bats at different spatial scales. Ecoscience 1999, 6, 25–34. [Google Scholar] [CrossRef]
- Jung, T.S.; Thompson, I.D.; Titman, R.D.; Applejohn, A.P. Habitat selection by forest bats in relation to mixed-wood stand types and structure in central Ontario. J. Wildl. Manag. 1999, 63, 1306–1319. [Google Scholar] [CrossRef]
- Loeb, S.C.; O’Keefe, J.M. Habitat use by forest bats in South Carolina in relation to local, stand, and landscape characteristics. J. Wildl. Manag. 2006, 70, 1210–1218. [Google Scholar] [CrossRef]
- Russo, D.; Jones, G. Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: Conservation implications. Ecography 2003, 26, 197–209. [Google Scholar] [CrossRef]
- Erickson, J.L.; West, S.D. Associations of bats with local structure and landscape features of forested stands in western Oregon and Washington. Biol. Conserv. 2003, 109, 95–102. [Google Scholar] [CrossRef]
- Crampton, L.H.; Barclay, R.M.R. Selection of roosting and foraging habitat by bats in different-aged aspen mixedwood stands. Conserv. Biol. 1998, 12, 1347–1358. [Google Scholar] [CrossRef]
- Bender, M.J.; Perea, S.; Castleberry, S.B.; Miller, D.A.; Wigley, T.B. Influence of insect abundance and vegetation structure on site-occupancy of bats in managed pine forests. For. Ecol. Manag. 2021, 482, 118839. [Google Scholar] [CrossRef]
- Kalcounis, M.C.; Hobson, K.A.; Brigham, R.M.; Hecker, K.R. Bat activity in the boreal forest: Importance of stand type and vertical strata. J. Mammal. 1999, 80, 673–682. [Google Scholar] [CrossRef]
- Russ, J.M.; Montgomery, W.I. Habitat associations of bats in Northern Ireland: Implications for conservation. Biol. Conserv. 2002, 108, 49–58. [Google Scholar] [CrossRef]
- Tibbels, A.E.; Kurta, A. Bat activity is low in thinned and unthinned stands of red pine. Can. J. For. Res. 2003, 33, 2436–2442. [Google Scholar] [CrossRef]
- Rudolph, B.-U.; Liegl, A.; Helversen, O.V. Habitat selection and activity patterns in the greater mouse-eared bat Myotis myotis. Acta Chiropterologica 2009, 11, 351–361. [Google Scholar] [CrossRef]
- Luszcz, T.M.J.; Barclay, R.M.R. Influence of forest composition and age on habitat use by bats in southwestern British Columbia. Can. J. Zool. 2016, 94, 145–153. [Google Scholar] [CrossRef]
- Charbonnier, Y.; Gaüzère, P.; van Halder, I.; Nezan, J.; Barnagaud, J.-Y.; Jactel, H.; Barbaro, L. Deciduous trees increase bat diversity at stand and landscape scales in mosaic pine plantations. Landsc. Ecol. 2016, 31, 291–300. [Google Scholar] [CrossRef]
- Ciechanowski, M. Habitat preferences of bats in anthropogenically altered, mosaic landscapes of northern Poland. Eur. J. Wildl. Res. 2015, 61, 415–428. [Google Scholar] [CrossRef]
- Bontadina, F.; Schmied, S.F.; Beck, A.; Arlettaz, R. Changes in prey abundance unlikely to explain the demography of a critically endangered Central European bat. J. Appl. Ecol. 2008, 45, 641–648. [Google Scholar] [CrossRef]
- Burns, L.K.L.; Loeb, S.C.; Bridges, W.C. Effects of fire and its severity on occupancy of bats in mixed pine-oak forests. For. Ecol. Manag. 2019, 446, 151–163. [Google Scholar] [CrossRef]
- Wermundsen, T.; Siivonen, Y. Foraging habitats of bats in southern Finland. Acta Theriol. 2008, 53, 229–240. [Google Scholar] [CrossRef]
- Lacki, M.J.; Cox, D.R.; Dodd, L.E.; Dickinson, M.B. Response of northern bats (Myotis septentrionalis) to prescribed fires in eastern Kentucky forests. J. Mammal. 2009, 90, 1165–1175. [Google Scholar] [CrossRef]
- Patriquin, K.J.; Barclay, R.M.R. Foraging by bats in cleared, thinned and unharvested boreal forest. J. Appl. Ecol. 2003, 40, 646–657. [Google Scholar] [CrossRef]
- Smith, P.G.; Racey, P.A. Natterer’s bats prefer foraging in broad-leaved woodlands and river corridors. J. Zool. 2008, 275, 314–322. [Google Scholar] [CrossRef]
- Walsh, A.L.; Harris, S. Foraging habitat preferences of vespertilionid bats in Britain. J. Appl. Ecol. 1996, 33, 508–518. [Google Scholar] [CrossRef]
- Rodríguez-San Pedro, A.; Simonetti, J.A. Foraging activity by bats in a fragmented landscape dominated by exotic pine plantations in central Chile. Acta Chiropterologica 2013, 15, 393–398. [Google Scholar] [CrossRef]
- Froidevaux, J.S.P.; Barbaro, L.; Vinet, O.; Larrieu, L.; Bas, Y.; Molina, J.; Calatayud, F.; Brin, A. Bat responses to changes in forest composition and prey abundance depend on landscape matrix and stand structure. Sci. Rep. 2021, 11, 10586. [Google Scholar] [CrossRef]
- O’Keefe, J.M.; Loeb, S.C.; Hill, H.S., Jr.; Lanham, J.D. Quantifying clutter: A comparison of four methods and their relationship to bat detection. For. Ecol. Manag. 2014, 322, 1–9. [Google Scholar] [CrossRef]
- Patriquin, K.J.; Hogberg, L.K.; Chruszcz, B.J.; Barclay, R.M.R. The influence of habitat structure on the ability to detect ultrasound using bat detectors. Wildl. Soc. Bull. 2003, 31, 475–481. [Google Scholar]
- Thomas, D.W. The distribution of bats in different ages of Douglas-fir forest. J. Wildl. Manag. 1988, 52, 619–626. [Google Scholar] [CrossRef]
- Węgiel, A.; Grzywiński, W.; Ciechanowski, M.; Jaros, R.; Kalcounis-Rüppell, M.; Kmiecik, A.; Kmiecik, P.; Węgiel, J. The foraging activity of bats in managed pine forests of different ages. Eur. J. For. Res. 2019, 138, 383–396. [Google Scholar] [CrossRef]
- Campbell, L.A.; Hallett, J.G.; O’Connell, M.A. Conservation of bats in managed forests: Use of roosts by Lasionycteris noctivagans. J. Mammal. 1996, 77, 976–984. [Google Scholar] [CrossRef]
- Kalcounis-Rüppell, M.C.; Psyllakis, J.M.; Brigham, R.M. Tree roost selection by bats: An empirical synthesis using meta-analysis. Wildl. Soc. Bull. 2005, 33, 1123–1132. [Google Scholar] [CrossRef]
- Müller, J.; Mehr, M.; Bässler, C.; Fenton, M.B.; Hothorn, T.; Pretzsch, H.; Klemmt, H.-J.; Brandl, R. Aggregative response in bats: Prey abundance versus habitat. Oecologia 2012, 169, 673–684. [Google Scholar] [CrossRef]
- Hagar, J.C. Wildlife species associated with non-coniferous vegetation in Pacific Northwest conifer forests: A review. For. Ecol. Manag. 2007, 246, 108–122. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Björkman, C. Mixed forests to mitigate risk of insect outbreaks. Scand. J. For. Res. 2018, 33, 772–780. [Google Scholar] [CrossRef]
- Randall, L.A.; Barclay, R.M.R.; Reid, M.L.; Jung, T.S. Recent infestation of forest stands by spruce beetles does not predict habitat use by little brown bats (Myotis lucifugus) in southwestern Yukon, Canada. For. Ecol. Manag. 2011, 261, 1950–1956. [Google Scholar] [CrossRef]
- Woś, A. Climate of Poland [Klimat Polski]; PWN Scientific Publishers: Warszawa, Poland, 1999; p. 301. [Google Scholar]
- Russ, J. British Bat Calls: A Guide to Species Identification; Pelagic Publishing Ltd.: London, UK, 2012. [Google Scholar]
- Runkel, V.; Gerding, G.; Marckmann, U. The Handbook of Acoustic Bat Detection; Pelagic Publishing: Exeter, UK, 2021. [Google Scholar]
- Ahlén, I. Identification of Bats in Flight; Swedish Society for Conservation of Nature & Swedish Youth Association for Environmental Studies and Conservation: Stockholm, Sweden, 1990; p. 50. [Google Scholar]
- Barataud, M. Acoustic ecology of European bats. In Species Identification and Studies of Their Habitats and Foraging Behaviour; Biotope Editions, Mèze; National Museum of Natural History: Paris, France, 2015; p. 352. [Google Scholar]
- Menzel, J.M.; Menzel, M.A.; Kilgo, J.C.; Ford, W.M.; Edwards, J.W.; McCracken, G.F. Effect of habitat and foraging height on bat activity in the Coastal Plain of South Carolina. J. Wildl. Manag. 2005, 69, 235–245. [Google Scholar] [CrossRef]
- Ford, W.M.; Menzel, J.M.; Menzel, M.A.; Edwards, J.W.; Kilgo, J.C. Presence and absence of bats across habitat scales in the Upper Coastal Plain of South Carolina. J. Wildl. Manag. 2006, 70, 1200–1209. [Google Scholar] [CrossRef]
- Kirkpatrick, L.; Maher, S.J.; Lopez, Z.; Lintott, P.R.; Bailey, S.A.; Dent, D.; Park, K.J. Bat use of commercial coniferous plantations at multiple spatial scales: Management and conservation implications. Biol. Conserv. 2017, 206, 1–10. [Google Scholar] [CrossRef]
- Fuentes-Montemayor, E.; Goulson, D.; Cavin, L.; Wallace, J.M.; Park, K.J. Fragmented woodlands in agricultural landscapes: The influence of woodland character and landscape context on bats and their insect prey. Agric. Ecosyst. Environ. 2013, 172, 6–15. [Google Scholar] [CrossRef]
- Ruczyński, I.; Nicholls, B.; MacLeod, C.D.; Racey, P.A. Selection of roosting habitats by Nyctalus noctula and Nyctalus leisleri in Bialowieza Forest-Adaptive response to forest management? For. Ecol. Manag. 2010, 259, 1633–1641. [Google Scholar] [CrossRef]
- Cisneros, L.M.; Fagan, M.E.; Willig, M.R. Effects of human-modified landscapes on taxonomic, functional and phylogenetic dimensions of bat biodiversity. Divers. Distrib. 2015, 21, 523–533. [Google Scholar] [CrossRef]
- Klingbeil, B.T.; Willig, M.R. Seasonal differences in population-, ensemble- and community-level responses of bats to landscape structure in Amazonia. Oikos 2010, 119, 1654–1664. [Google Scholar] [CrossRef]
- Pereira, M.J.R.; Peste, F.; Paula, A.; Pereira, P.; Bernardino, J.; Vieira, J.; Bastos, C.; Mascarenhas, M.; Costa, H.; Fonseca, C. Managing coniferous production forests towards bat conservation. Wildl. Res. 2016, 43, 80–92. [Google Scholar] [CrossRef]
- Akasaka, T.; Nakano, D.; Nakamura, F. Influence of prey variables, food supply, and river restoration on the foraging activity of Daubenton’s bat (Myotis daubentonii) in the Shibetsu River, a large lowland river in Japan. Biol. Conserv. 2009, 142, 1302–1310. [Google Scholar] [CrossRef]
- Loeb, S.C. Qualitative synthesis of temperate bat responses to silvicultural treatments—Where do we go from here? J. Mammal. 2020, 101, 1513–1525. [Google Scholar] [CrossRef]
- Ciechanowski, M. Utilization of artificial shelters by bats (Chiroptera) in three different types of forest. Folia Zool. 2005, 54, 31–37. [Google Scholar]
- Rueegger, N. Variation in summer and winter microclimate in multi-chambered bat boxes in Eastern Australia: Potential eco-physiological implications for bats. Environments 2019, 6, 13. [Google Scholar] [CrossRef]
- Deeley, S.; Ford, W.M.; Kalen, N.J.; Freeze, S.R.; St. Germain, M.; Muthersbaugh, M.; Barr, E.; Kniowski, A.; Silvis, A.; De La Cruz, J. Mid-Atlantic Big Brown and Eastern Red Bats: Relationships between Acoustic Activity and Reproductive Phenology. Diversity 2022, 14, 319. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).