Abstract
In the Mediterranean region, some areas of the Vesuvius National Park (southern Italy) are subjected to a severe anthropogenic impact, especially during spring and summer seasons. The continuous trampling of tourists and buses leads to the formation of “dust-clouds”, exposing plants, especially along the paths, to a great deposition of powder particles on leaves. The aim of this study was to analyze if the dust deposition induces changes in leaf morpho-anatomical and eco-physiological traits of the alien, invasive, species Robinia pseudoacacia L., with particular attention to the photosystem II (PSII) efficiency. We selected plants located near the paths with a high deposition of dust (HD) and plants far away from the paths (low deposition, LD), and tested them over three dates along summer. We analyzed PSII photochemistry, photosynthetic pigments content, and leaf functional (e.g., relative water content and leaf dry matter content) and morpho-anatomical traits (e.g., parenchyma thickness, mesophyll density). HD plants presented a more efficient PSII activity, indicated by the higher quantum yield of PSII electron transport (FPSII) (9%) and electron transport rate (ETR) (38%) in the end of July. Dust deposition also reversibly altered photosynthetic pigments concentration and some lamina traits, adjustable in the short-term (e.g., intercellular spaces and phenolics distribution). We hypothesize that HD leaves were shielded by dusts which would protect their photosynthetic apparatus from the excess of light.
1. Introduction
In recent times, many native species in urban and forest areas have been replaced by the invasion of alien species that cope better with environmental disturbances causing severe damage to natural ecosystems and leading to a significant loss of biodiversity [1,2]. Robinia pseudoacacia L. (black locust) is an invasive, alien, deciduous tree that has been introduced throughout Europe in the last two centuries, and in Italy in 1662 [1]. In Central Europe, black locust has mainly invaded areas with subcontinental or sub-Mediterranean climates, especially dry forests and shrublands [2]. For this light-demanding pioneer species, precipitation is not a limiting factor, so it can spread rapidly and colonize dry habitats. It has been predicted that both climate change and large-scale disturbance may increase the range of habitats colonized by black locust in the future [2,3,4]. Indeed, this species spreads well in open dry forests, shrublands, urban forests, and disturbed sites [5], strongly competing and possibly causing the extinction of many endangered light-demanding native species.
In the Mediterranean area, the loss of biodiversity due to alien species invasion and climate change is also worsened by the anthropogenic impact, responsible for soil and air pollution.
Current issues, much studied by the scientific community, are those related to the release of heavy metals from road traffic and industries, which harm human and ecosystem health [6,7]. Despite heavy metals contamination limits plant growth and biomass accumulation by altering many different metabolic pathways [8], many studies focus on the beneficial roles of tree species in counteracting environment pollution and contamination. Among the alien species, black locust is recognized to be one of the most tolerant to soil and air pollution and, therefore, it is widely utilized in reclamation of degraded sites [9,10,11] and in urban forestation and landscaping [12,13].
However, even in non-polluted areas, dust suspended in air, especially roadside, is deposited on leaves causing specific plant responses. Indeed, the deposition of particulate dust can determine changes in plant morphology, anatomy, physiology, and biochemistry [14,15], also affecting light use efficiency of plants.
The term light use efficiency refers to the ratio of biomass accumulation (primary production) with respect to absorbed PAR (Photosynthetically Active Radiation) [16] and is strictly related to many photoprotective strategies involved in plant photoprotection under excessive radiation. It has been demonstrated that the endorsement of photoprotective strategies is the main reason for the adaptative success of several species of Mediterranean forest and shrublands, such as Phyllirea angustifolia L., Cistus spp. and Quercus ilex L. [17,18,19,20] in presence of environmental constraints.
The excess of light that cannot be used for the photosynthesis can be dissipated through the not-radiative processes, regulated or not by the xanthophyll cycle [21]. Generally, sun leaves, thanks to specific biochemical and morphoanatomical traits, are more tolerant to high radiation, compared to shade leaves, which are, conversely, more prone to photoinhibition [22]. More specifically, the shade leaves are characterized by a more expanded but thinner leaf lamina and by an increased content of chlorophylls compared to sun leaves [23]. These are all morpho-physiological adjustments that shade leaves undergo in order to capture more light to maintain a high net photosynthetic rate [24].
Among species growing on the Vesuvius protected areas, Robinia pseudoacacia L. has a very peculiar strategy to avoid photoinhibition in addition to the usual anatomical features. More specifically, it presents paraheliotropic movements which enable leaves to modify their orientation in the space, allowing leaf lamina to intercept the light in optimal way even during the hottest and sunny hours of the day compared to the other species [25]. The invasion of black locust in the Vesuvius forest areas is interesting not only in terms of changes in primary production, but also for the impact on soil ecological and biological properties, because the presence of black locust trees increases carbon and nitrogen stocks in the upper soil organic layers improving soil fertility [26].
The present study was conducted on R. pseudoacacia plants growing on the slope of the Vesuvius volcano in southern Italy. In recent years, the number of tourists who visit the Vesuvius National Park annually far exceeds 600,000 visitors, just considering access to the Grand Cone [27]. In this regard, in the different areas of the park and especially along the paths that lead to the cone, the continuous trampling of tourists and tour buses leads to the formation of “dust-clouds”, exposing plants, especially those growing along the roads, to a heavy deposition of particulate matter on their leaves. In a previous study, the effect of dust deposition was analyzed on Centranthus ruber [27], a very common, herbaceous species of the Vesuvius Grand Cone [28]. C. ruber showed plastic response to the dust deposition, raising the hypothesis that species-specific plant responses can result in different plant competitiveness, thus altering vegetation dynamics under the limiting conditions of Mediterranean environments.
The aim of this study was to assess if the dust deposition may alter the morpho-anatomical development of leaves also in a tree species, thus affecting their physiological and biochemical behavior. To investigate this aim, we selected R. pseudoacacia plants located roadside and subjected to a high amount of dust deposition (HD) and plants located far from the paths, subjected to a low amount of dust deposition (LD). R. pseudoacacia represents an interesting study model because its invasion in Vesuvius national Park continued despite the tourist pressure, suggesting a high plasticity of this species to also overcome the anthropic disturbance.
Taking into account that roadside plants play a fundamental role in the mitigation of air pollution in urban and peri-urban environments, it is, therefore, crucial to investigate the performance of invasive species at the expense of native species, especially on disturbed sites, considering the extent of anthropogenic pressure. This could help implement appropriate and targeted management strategies for such sites and maintain the ecological value of urban forestry.
2. Materials and Methods
2.1. Study Site and Species Selection
This study was conducted on the south-east side of the Vesuvius Grand Cone along the Matrone road (40°49′17.3″ N 14°26′06.8″ E, 1036 m a.s.l.) on a slope of about 30°–45° within the Vesuvius National Park. The area is subjected to a severe anthropogenic impact, particularly during spring and summer months, when frequent buses and pedestrians cause the phenomenon known as “dust-clouds” formation. Indeed, the raising of a great deal of dusts from the soil causes the deposition of a thick layer of dust on leaf lamina of plants growing close to the paths [27,29,30]. The measurements were performed in June and July when the touristic pressure on the Vesuvius National Park reaches the highest level [29,30]. The climate in the study area is Mediterranean with an arid period which usually lasts from May to July, and mild and wet winters [31]. More specifically the monthly average maximum, mean, and minimum temperatures were, respectively, 28.6 ± 1.8 °C, 22.9 ± 1.6, and 17.6 ± 1.3 °C in June, while they were, respectively, 31.3 ± 0.6 °C, 25.1 ± 0.6, and 19.8 ± 0.4 °C in July (data as mean values and standard deviation for the years 2018–2022, from the nearby Boscotrecase, NA, meteorological station—www.agricoltura.regione.campania.it/meteo/agrometeo.htm, accessed on 17 January 2023). Cumulative monthly precipitation ranged between 0 and 60 mm in June and between 0.4 and 34 mm in July over the same period.
The area of investigation included two different plots of 5 × 3 m (about 15 m2) located at different distances from the paths. The first plot was located along the path and was characterized by a high amount of dust deposition; thus, it is defined in the text as HD (High Dust); the second plot was located about 8–10 m away from the path, thus being characterized by a low amount of dust deposition (defined as LD, Low Dust). Both sites had the same environmental characteristics and species composition, only differing in dust deposition amount.
The study was conducted on Robinia pseudoacacia L., a native tree species from North America, which has a pioneer characteristic that makes it a threat to native woody species [32]. Its leaves are characterized by a paraheliotropic movement which can act as a photo-protective strategy [25]. R. pseudoacacia is also known to be well-adapted to live on polluted soil [33]. However, the study area was characterized by slight contamination of Cd, Cu and Si, according to Memoli [29].
The black locust trees selected for our experiment were about 5 years old. To carry out a standardized sampling for chlorophyll fluorescence measurements, we chose fully expanded, well-exposed mature leaves collected from the outer part of the tree crown at 2 m height from the ground. After measuring in vivo, without cleaning the leaf lamina, the same leaves were detached from trees, stored in ice bags for leaf functional trait, and photosynthetic pigment content determination, and in F.A.A. (38% formaldehyde, glacial acetic acid, 50% ethanol solution, 5/5/90 by volume) for leaf anatomy.
2.2. Dust Deposition Quantification
At both HD and LD sites, the quantification of dust was performed on three different dates between June and July: 1st June (DOY 152), 24th June (DOY 175), and 24th July (DOY 205). On each date, 5 g of leaves were collected from about 20 to 25 twigs and promptly put into iced plastic bags for transferring to the laboratory. The dust quantification was performed following the approach described by De Micco [27] and previously described by Younis [34]. More specifically, after collection, each leaf was weighted using a fine scale; soon after, the dust was carefully removed from the lamina using a dry cloth, making attention not to crash or spoil the leaf lamina. Then, each leaf was re-weighted. The amount of dust was quantified as the difference between the two weights (before and after cleaning) and referred to the leaf area, expressed in µg cm−2.
2.3. Leaf functional Traits and Leaf Anatomical Traits
At both HD and LD sites, on each date, 10 fully expanded leaves were collected from 5 plants per site, cleaned as reported in the previous paragraph and used for the quantification of leaf functional traits following Cornelissen [35]. More specifically, Leaf area (LA) was expressed in cm2 and calculated by using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). The specific leaf area (SLA) was calculated as the ratio between leaf area and leaf dry weight (cm2 g−1). Leaf dry matter content (LDMC) was obtained as the ratio of leaf dry weight and saturated weight and expressed as g g−1. Leaf dry weight was obtained oven dried the leaves at 70 °C for 48 h, while saturated weight was determined maintaining leaves for 24 h in the dark at 4 °C with the petiole submerged in distilled water. The leaf relative water content (RWC) was calculated as percentage of (fresh weight − dry weight)/(saturation weight − dry weight): [(FW − DW)/(TW − DW)] × 100.
Concerning anatomical analyses of leaf lamina, 9 leaves from 3 plants were collected at DOY 205 and promptly stored in the F.A.A. Each leaf was then dissected to obtain subsamples of about 5 × 5 mm which were subjected to: (i) dehydration, (ii) embedding, (iii) sectioning, (iv) staining, (v) observing under microscopes, (vi) photographing, and (vii) digital image analysis. The dehydration procedure was performed in ethanol series (up to 95%) where each sub-sample remained for about an hour. The sub-samples were then embedded in the acrylic resin JB4® (Polysciences, Eppelheim, Germany) and cross-sectioned (5 μm thickness) using a rotatory microtome. For the staining phase, the Toluidine Blue in water was used following Feder and O’Brien [36]. The sections were mounted in mineral oil for epi-fluorescence microscopy, observed under a light microscope (BX61, Olympus, Tokyo, Japan) and photographed using a camera (XC50, Olympus, Tokyo, Japan), to calculate the following parameters: (i) Palisade parenchyma thickness (PPT), spongy parenchyma thickness (SPT) and total parenchyma thickness (TPT), measured in six positions along the lamina, avoiding veins; and (ii) Intercellular Spaces (IS), measured as the percentage of tissue occupied by intercellular spaces over a given surface, in four regions along the leaf lamina. Stomata frequency was determined on the lower epidermis by quantifying the number of stomata per mm of epidermis in three transects along the lamina.
Furthermore, unstained sections were observed under an epi-fluorescence microscope (BX60, Olympus) to detect the presence of simple phenolics in the mesophyll, which are auto-fluorescent when observed with a mercury lamp, band-pass filter of 330–385 nm, dichromatic mirror of 400 nm and above, and a barrier filter of 420 nm and above [37,38]. The incidence of phenolic compounds was quantified, previously standardizing the background [39] as the percentage of tissue occupied by phenolic compounds over a given surface in four regions along the leaf lamina (PC%). Moreover, the relative intensity of the phenolic compounds (PCi) was measured as the mean gray value as previously reported by De Micco (2014, 2020) [27,40].
2.4. Chlorophyll a Fluorescence Analysis and Photosynthetic Pigment Content
Chlorophyll fluorescence analysis was performed in vivo on 5 leaves per 3 plants per both HD and LD plots, by using a portable pulse amplitude-modulated fluorometer (FluorPen FP 100max), equipped with a light sensor (Photon System Instruments, Brno, Czech Republic) following the procedure reported in Arena (2020) [41]. The basal fluorescence (F0) was induced on 30 min dark-adapted leaves by an internal blue light pulse of about 1–2 μmol photons m−2 s−1. The maximal fluorescence level in the dark (Fm), was obtained by a 1s saturating light pulse of 3000 μmol photons m−2 s−1. The maximal fluorescence level in the dark, Fm, was induced by a 1s saturating light pulse of 3000 μmol photons m−2 s−1. The maximum quantum efficiency of PSII photochemistry, Fv/Fm, was calculated as (Fm–F0)/Fm, according to Kitajima and Butler [42]. The measurements in the light were conducted at midday under natural sunlight of 1800 ± 200 μmol photons m−2 s−1). The maximal fluorescence signal in the light (Fm’) was obtained by a pulse of 1 sec at 3000 μmol photons m−2 s−1. The quantum yield of PSII electron transport (ΦPSII) was determined following Genty [43].
The electron transport rate (ETR) was calculated following the equation of Krall and Edwards (1992) [44], while the non-photochemical quenching (NPQ) was determined as (Fm–Fm’)/‘Fm’ according to Bilger and Björkman [45].
The photosynthetic pigment content was determined on the same leaves used for measurements of photochemistry. Briefly, chlorophyll a and b and total carotenoids were extracted on area basis from a leaf disk of 0.283 cm2 in ice-cold 100% acetone, by using a mortar and a pestle. The concentration of chlorophylls and carotenoids was determined spectrophotometrically (UV-VIS Cary 100; Agilent Technologies, Santa Clara, CA, USA) at wavelengths of 662, 645 and 470 nm, according to Lichtenthaler [46] and expressed in μg cm−2.
2.5. Statistical Analyses
For statistical analyses, the SPSS 10.0 for Windows statistical package (SPSS Inc., Chicago, IL, USA) was used. All parameters were analyzed through one-way analysis of variance (ANOVA) to evaluate the differences between HD and LD leaves, within each sampling date. Shapiro–Wilk and Kolmogorov–Smirnov tests were performed to check for normality.
3. Results
3.1. Dust Accumulation on Leaf Lamina
There was a significant difference in dust deposition over the leaf lamina between HD and LD plants in all analyzed dates, with HD plants always having the highest deposition of dust on their lamina (Figure 1). Moreover, values in HD plants, although being always bigger than LD plants, showed a decreasing trend along the time, whereas, in LD plants, dust deposition was highest at the second date.
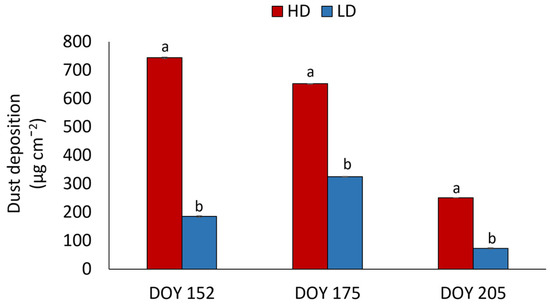
Figure 1.
Dust deposited on leaf lamina of R. pseudoacacia HD and LD plants at DOY 152, 175 and 205. Mean values and standard errors are shown. Different letters correspond to statistically significant differences (p < 0.05) within the date of sampling.
3.2. Leaf Functional Traits and Leaf Anatomical Traits
Leaf functional traits are reported in Table 1. HD plants maintained a significantly higher leaf area (LA) only on the first observation date (DOY 152), compared to LD plants. Differently, the specific leaf area (SLA) was higher in HD compared to LD plants at DOY 175 and 205, but not on the first observation date (DOY 152) where no differences were detected. No statistically significant differences were detected for leaf dry matter content (LDMC) on the first two dates, whereas, at DOY 205, a significant increment in LDMC in HD plants was found. Similarly, the relative water content (RWC) showed a different behavior on the three observation dates. More specifically, no differences were found at DOY 152, but at DOY 175 and 205, higher values were detected in HD plants than LDs.

Table 1.
Leaf area (LA), specific leaf area (SLA), leaf dry matter content (LDMC), and relative water content (RWC) of R. pseudoacacia HD and LD plants at DOY 152, 175, and 205. Mean values and standard errors are shown. Different letters correspond to statistically significant differences (p < 0.05) within each date of sampling.
The quantification of leaf anatomical traits is reported in Table 2, while a selection of images representative of HD and LD leaf laminas is reported in Figure 2 showing that the usual tissue organization is maintained irrespective of the dust amount deposited. Although a tendency to the increase in the thickness of both palisade and spongy parenchymas was observed in response to high dust deposition, data reported in Table 2 show that the differences in the thickness of the palisade and spongy parenchymas (PPT and SPT) between HD and LD leaves were not significant. However, the total parenchyma thickness (TPT) resulted significantly higher in HD than LD plants. HD plants also developed a significantly lower percentage of intercellular spaces (IS) (reduced by 30%) compared to LD plants. Moreover, a significant decrease not only in the percentage of tissue occupied by phenolic compounds (PC), but also in the intensity of phenolics (PCi) was detected in HD compared to LD plants (reduction of 28% and 45% respectively). No significant differences between HD and LD plants were found in stomatal frequency (SF).

Table 2.
Total parenchyma thickness (TPT), palisade parenchyma thickness (PPT), spongy parenchyma thickness (SPT), intercellular spaces (IS), percentage of tissue occupied by phenolic compounds (PC), phenolics content intensity (PCi), and stomata frequency (SF) in R. pseudoacacia HD and LD plants. Mean values and standard errors are shown. Different letters correspond to statistically significant differences (p < 0.05) within each date of sampling.
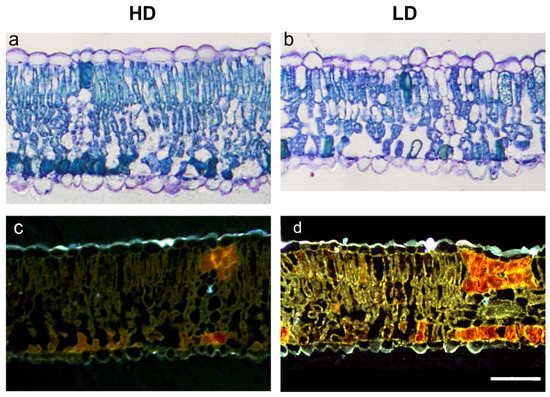
Figure 2.
Light (a,b) and epi-fluorescence (c,d) microscopy views of leaf lamina cross-section of R. pseudoacacia HD (a,c) and LD (b,d) plants. Images are at the same magnification; scale bar = 50 µm.
3.3. Chlorophyll a Fluorescence Analysis and Photosynthetic Pigment Content
Chlorophyll a fluorescence of R. pseudoacacia leaves at DOY 152, 175, and 205 is shown in Figure 3. On all dates, the PSII maximum photochemical efficiency (Fv/Fm) showed no significant difference between HD and LD plants. In contrast, statistically significant differences between HD and LD leaves were found in quantum yield of PSII electron transport (ΦPSII), electron transport rate (ETR), and non-photochemical quenching (NPQ).
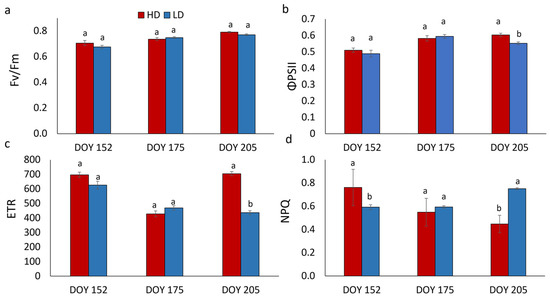
Figure 3.
Maximum PSII photochemical efficiency (Fv/Fm) (a), quantum yield of PSII electron transport (ΦPSII) (b), linear electron transport rate (ETR) (c), and non-photochemical quenching (NPQ) (d) in R. pseudoacacia HD and LD plants at DOY 152, 175, and 205. Mean values and standard errors are shown. Different letters correspond to statistically significant differences (p < 0.05) within each date of sampling.
More specifically, ΦPSII and ETR measured at DOY 205 showed higher values in HD compared to LD plants. The NPQ at DOY 152 was significantly higher in HD plants, it did not change between HD and LD at DOY 175, while it was significantly lower in HD plants at DOY 205.
Total chlorophyll (chl a + b) and carotenoid (x + c) contents were higher in HD compared to LD plants at DOY 175 (Figure 4). At DOY 152 and 205, no significant difference in chlorophyll content was found between HD and LD plants, whilst LD leaves exhibited a higher carotenoid concentration than HDs.
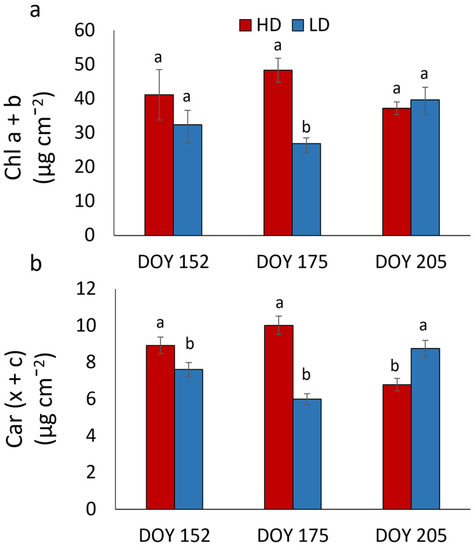
Figure 4.
Concentration of total chlorophyll (a) and total carotenoids (b) in R. pseudoacacia HD and LD plants at DOY 152, 175, and 205. Mean values and standard errors are shown. Different letters correspond to statistically significant differences (p < 0.05) within the date of sampling.
4. Discussion
This study evaluates the changes in morpho-anatomical and eco-physiological traits caused by different dust deposition on R. pseudoacacia, an alien invasive species in the Mediterranean area.
Under environmental unfavorable conditions, plants rely on several strategies to cope with stress, by modulating their anatomical and physiological features [20,47]. The synergy of such structural alterations and physiological acclimation mechanisms allow the photosynthetic apparatus to maintain carbon gain, thus allowing plants to survive [24], given that plant structure establishes the limits for physiological regulation [48,49,50]. At the leaf level, the main adaptations regard mesophyll, stomata, and within cells, the chloroplast traits and ultrastructure [51]. This latter primary affects plant photosynthesis.
Invasive species, such as R. pseudoacacia are particularly prone to modulate their functional traits in response to environmental changes and to use resources more efficiently under stressful conditions [52]. Black locust has typically higher CO2 assimilation rates [53,54], and higher photosynthetic pigments content and carbohydrates [55] compared to the autochthonous species of the Mediterranean area, which represents an advantage in replacing the natives.
In this study, the high amount of dust found on HD leaf lamina during the whole period of observation (June–July), caused by the great touristic pressure in these months, seems to elicit a positive outcome in R. pseudoacacia plants in terms of photoprotection. Although the higher amount of dust deposited on the lamina may induce problems to gas exchanges, including limitation in stomatal conductance, it may better act as a shield against the excess of solar radiation in the hottest and sunny hours of the day, when irradiance of 2000–2500 PPFD (photosynthetic photon flux density) are reached on the leaves. This shielding effect due to the dust deposition on the leaf lamina likely reduces the amount of light on the photosynthetic apparatus that, also thanks to intrinsic strategies (i.e., paraheliotropism), is able to optimize the light use efficiency at photosystems, minimizing the risks of photodamages.
It is noteworthy that during summertime, a prolonged combination of high temperatures and irradiances are reached in the Mediterranean Basin, especially Southern Italy ecosystems [18], where these multiple stressors enhance the risks of photoinhibition [56] making Mediterranean species vulnerable to photodamages. To counteract this situation, changes in photochemical and non-photochemical strategies, and gas-exchange regulation, as well as biochemical adjustments, such as the modulation of chlorophyll and carotenoid content, have often been found in Mediterranean plant species under field conditions during summer [57]. One of the main targets of the light stress is the photosystem II (PSII) [58]. Our data demonstrated that in black locust plants, the maximum PSII photochemical efficiency, as suggested by Fv/Fm ratio, did not show any significant differences between HD and LD leaves, indicating that the dust deposition did not affect photosynthetic apparatus of R. pseudoacacia. Furthermore, the values of Fv/Fm between 0.79–0.84 also confirmed a healthy status for plants with no signal of stress [41,59]. However, a more fine analysis revealed that some differences occur between HD and LD leaves as regards other photochemical indexes, namely ΦPSII, ETR, and NPQ. Even if the potential intrinsic photochemical efficiency (Fv/Fm) in both leaf type is the same, the HD and LD leaves exhibited a different light regulation strategy to photosystems. More specifically, on the last date (DOY 205), when the plants are exposed to prolonged disturbance in terms of elevated temperature and light intensity, accompanied by low water availability, HD plants increased the ΦPSII and ETR and contextually decreased the NPQ as if the high dust deposition would counteract the stressors. This behavior may be interpreted as a compensatory strategy by photosynthetic apparatus: in the dust-shielded leaves, the light energy which reaches the photosystems is utilized mainly in photochemistry (ΦPSII and ETR rise) and the need for thermal dissipation is reduced (decline of NPQ). The increased photochemical efficiency in HD leaves may also be favored by the increased lamina thickness, reduced percentage of intercellular spaces (i.e., denser mesophyll) based on the general rule that higher photosynthesis can be obtained by increasing tissue density which is directly related to increased Rubisco activity, mesophyll conductance and water use efficiency in terms of increased enhanced CO2 uptake for the same amount of transpired water [46,60,61,62]. In LD plants, we observed an opposite trend with a reduced photochemistry and an enhanced NPQ on the last date. The diverse photochemical regulation can be attributed to the difference in dust deposition on the leaf lamina. The lower NPQ in HD plants at DOY 205 may be the result of a reduced light energy pressure to photosystems due to high amount of dust deposited on the leaf which acts as a shield against solar radiation, thus reducing the risk for photoinhibition. This result is in agreement with previous studies conducted by De Micco [27] on another species (C. ruber) in the same location during summertime.
Consistent with our hypothesis, the chlorophyll and carotenoid contents were often higher in HD plants shielded by dust. The hypothesis arises that the presence of dust on HD leaves acts as a stimulus for the production of photosynthetic pigments to increase light interception. The HD leaves should be similar to “shade leaves” exhibiting some usual traits: shade leaves are generally characterized by a higher pigment content to enhance light harvesting and are more susceptible to photoinhibition compared to sun leaves, which are suited for the high irradiance [63,64]. Moreover, shade leaves are usually thinner and more expanded than sun leaves. In our study, a higher leaf area in HD than LD leaves was found at DOY 152 as a way to harvest more light. Since SLA is comparable between HD and LD plants, it may be hypothesized that they maintain a comparable dry mass production despite the different dust deposition which may have influence on gas exchanges. However, the dust deposition did not prime any changes in stomata differentiation to compensate for possible obstacle to gas exchanges, as confirmed by unchanged stomata frequency between HD and LD leaves. In our study, HD leaves can be considered as “standard” sun leaves in their structure that behave instead as shade leaves. In fact, leaf anatomical traits usually show acclimation when stressors act during cell differentiation. Considering leaf phenology, leaf unfolding in R. pseudoacacia occurs when the anthropogenic-induced dust-clouds formation is still low; therefore, organogenesis determines the formation of sun leaves, likely not different in the general structure from leaves formed in LD plants. This hypothesis is confirmed by the fact that parameters such as parenchyma thickness and stomatal frequency are not influenced by the dust level. The occurrence of a slightly thicker lamina in HD plants, reduced intercellular spaces together with the higher RWC would likely indicate some limitations of water losses in the presence of high dust amount. During summer, the leaves of R. pseudoacacia have proved to promptly respond to the environmental perturbations, by modulating the other functional traits (such as photosynthesis or production of pigments) that are prone to short-term acclimation [23,65]. Among promptly-modulable traits, apart from eco-physiological traits and pigments content, there is also the synthesis and distribution of phenolic compounds along the leaf tissues. Fluorescence microscopy analysis revealed that HD plants present a lower percentage of tissue occupied by phenolic compounds despite the occurrence of a denser mesophyll (e.g., lower percentage of intercellular spaces) and a lower intensity of phenolics (autofluorescent compounds). This result is in line with the hypothesis that the screen of dust on leaf lamina, reducing the photo-inhibitory damage risks, also decreases the need for the synthesis of phenolic compounds active in cell protection against the excess of light during the summer season [66] as also found before in C. ruber [27].
The regulation of photosynthesis and photoprotective strategies in plant of Mediterranean environments as summer progresses act together with modification in plant structure and anatomy [25,67,68]. The paraheliotropic movements of black locust leaves, by which leaves progressively close avoiding the direct sunlight beats leaf lamina in the hottest hours of the day, also significantly affects the leaf functional traits. The presence of paraheliotropism could explain the higher relative water content (RWC) and leaf dry matter content (LDMC) found in HD compared to LD leaves, meaning that HD plants minimize the loss of water and enhance the investments in photosynthate in terms of structural support. Besides differences in light-use efficiency, differences in water-use efficiency between HD and LD plants were also detected. Indeed, LD leaves have a greater quantity of intracellular spaces, and this variation in leaf tissue density can determine a different water use efficiency in the two leaf types, with plants characterized by more intercellular spaces posing a higher resistance to water-use [69,70].
5. Conclusions
R. pseudoacacia plants seem to straightforwardly acclimate to the presence of dust likely benefiting from a shield against the high solar radiation levels of Mediterranean summer. The acclimation regards the regulation of some reversible traits at the leaf lamina level, adjustable in the short-term (e.g., intercellular spaces amount and phenolics distribution), as well as in the pigment content and eco-physiological control. The coordination of structural and eco-physiological traits, together with the paraheliotropism in R. pseudoacacia, confirm the high capacity of this alien species to cope with environmental stressors suggesting that the anthropogenic dust-induced “disturbe” may even be a favorable stimulus for this species allowing avoidance of any photo inhibitory damages. Such a response should be taken into account in the management of disturbed natural areas to evaluate the vegetation dynamics of invasive species at the expense of native species.
Author Contributions
Conceptualization, V.D.M. and C.A. (Carmen Arena), methodology, V.D.M., C.A. (Chiara Amitrano), and C.A. (Carmen Arena); formal analysis, all the authors; investigation, V.D.M., C.A. (Chiara Amitrano), L.G.I., A.B., E.V., and C.A. (Carmen Arena); data curation, V.D.M., C.A. (Chiara Amitrano), C.C., A.B., and C.A. (Carmen Arena); writing—original draft preparation, V.D.M., C.A. (Chiara Amitrano), A.B., and C.A. (Carmen Arena); writing—review and editing, all the Authors; supervision, V.D.M., C.A.(Carmen Arena), and C.C.; project administration, V.D.M.; funding acquisition, V.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Vesuvius National Park (Ente Parco Nazionale del Vesuvio), within the project “Monitoraggio della biodiversità vegetale e valutazione di indicatori dell’impatto delle polveri sulla crescita delle piante nel territorio del Parco Nazionale del Vesuvio (BIO-IND-2)” through azione di Sistema “Impatto antropico da pressione turistica nelle aree protette: interferenze su territorio e biodiversità”—Direttiva Conservazione della Biodiversità n. 15956 del 27 July 2016 del Ministero dell’Ambiente e della Tutela del Territorio e del Mare.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Acknowledgments
We wish to thank Enrica Zalloni and Adriano Stinca for their support in the field. We also thank Rossella Barile and Paola Conti of the Vesuvius National Park for their technical and administrative support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gentili, R.; Ferrè, C.; Cardarelli, E.; Montagnani, C.; Bogliani, G.; Citterio, S.; Comolli, R. Comparing negative impacts of Prunus serotina, Quercus rubra and Robinia pseudoacacia on native forest ecosystems. Forests 2019, 10, 842. [Google Scholar] [CrossRef]
- Sukopp, H.; Wurzel, A. The effects of climate change on the vegetation of central European cities. Urban Habitats 2003, 1, 66–86. [Google Scholar]
- Chmielewski, F.M.; Müller, A.; Küchler, W. Possible impacts of climate change on natural vegetation in Saxony (Germany). Int. J. Biometeorol. 2005, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Kleinbauer, I.; Dullinger, S.; Peterseil, J.; Essl, F. Climate change might drive the invasive tree Robinia pseudacacia into nature reserves and endangered habitats. Biol. Conserv. 2010, 143, 382–390. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, M.; Capozzi, F.; Amitrano, C.; Giordano, S.; Arena, C.; Spagnuolo, V. Performance of three cardoon cultivars in an industrial heavy metal-contaminated soil: Effects on morphology, cytology and photosynthesis. J. Hazard. Mater. 2018, 351, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Fatema, K.; Shoily, S.S.; Ahsan, T.; Haidar, Z.; Sumit, A.F.; Sajib, A.A. Effects of arsenic and heavy metals on metabolic pathways in cells of human origin: Similarities and differences. Toxicol. Rep. 2021, 8, 1109–1120. [Google Scholar] [CrossRef]
- Woś, B.; Pająk, M.; Krzaklewski, W.; Pietrzykowski, M. Verifying the utility of black locust (Robinia pseudoacacia L.) in the reclamation of a lignite combustion waste disposal site in Central European conditions. Forests 2020, 11, 877. [Google Scholar] [CrossRef]
- Środek, D.; Rahmonov, O. The properties of Black Locust Robinia pseudoacacia L. to selectively accumulate chemical elements from soils of ecologically transformed areas. Forests 2021, 13, 7. [Google Scholar] [CrossRef]
- Nicolescu, V.-N.; Rédei, K.; Mason, W.L.; Vor, T.; Pöetzelsberger, E.; Bastien, J.-C.; Brus, R.; Benčať, T.; Đodan, M.; Cvjetkovic, B. Ecology, growth and management of black locust (Robinia pseudoacacia L.), a non-native species integrated into European forests. J. For. Res. 2020, 31, 1081–1101. [Google Scholar] [CrossRef]
- Moser, A.; Rötzer, T.; Pauleit, S.; Pretzsch, H. Structure and ecosystem services of small-leaved lime (Tilia cordata Mill.) and black locust (Robinia pseudoacacia L.) in urban environments. Urban For. Urban Green. 2015, 14, 1110–1121. [Google Scholar] [CrossRef]
- Esfandiary, M.; Sodaiezadeh, H.; Hakimzadeh, M.A. Assessment some of heavy metals in black locust (Robinia pseudoascacia) in the Yazd highway green belt. J. Ornam. Plants 2019, 9, 193–203. [Google Scholar]
- Rai, P.K. Biodiversity of roadside plants and their response to air pollution in an Indo-Burma hotspot region: Implications for urban ecosystem restoration. J. Asia- Pac. Biodivers. 2016, 9, 47–55. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Rathore, D. Dust pollution: Its removal and effect on foliage physiology of urban trees. Sustain. Cities Soc. 2019, 51, 101696. [Google Scholar] [CrossRef]
- Medlyn, B.E. Physiological basis of the light use efficiency model. Tree Physiol. 1998, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Arena, C.; Vitale, L.; Aronne, G.; Virzo De Santo, A. Anatomy and photochemical behaviour of Mediterranean Cistus incanus winter leaves under natural outdoor and warmer indoor conditions. Botany 2011, 89, 677–688. [Google Scholar] [CrossRef]
- Vitale, L.; Arena, C.; De Santo, A.V. Seasonal changes in photosynthetic activity and photochemical efficiency of the Mediterranean shrub Phillyrea angustifolia L. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2012, 146, 443–450. [Google Scholar]
- Arena, C.; Mistretta, C.; Di Natale, E.; Mennella, M.R.F.; De Santo, A.V.; De Maio, A. Characterization and role of poly (ADP-ribosyl) ation in the Mediterranean species Cistus incanus L. under different temperature conditions. Plant Physiol. Biochem. 2011, 49, 435–440. [Google Scholar] [CrossRef]
- Vitale, L.; Magliulo, V.; Arena, C. Morphological and physiological modifications of Cistus salvifolius L. winter leaves in response to the rise in winter temperatures. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 148, 1093–1101. [Google Scholar]
- Gonsamo, A.; Chen, J.M. 3.11—Vegetation Primary Productivity. In Comprehensive Remote Sensing; Liang, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 163–189. [Google Scholar]
- Osmond, B.; Förster, B. Photoinhibition: Then and now. In Photoprotection, photoinhibition, gene regulation, and environment; Springer: Berlin/Heidelberg, Germany, 2008; pp. 11–22. [Google Scholar]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf Anatomy and Function. In The Leaf: A Platform for Performing Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 97–139. [Google Scholar]
- Calzavara, A.; Rocha, J.; Lourenço, G.; Sanada, K.; Medri, C.; Bianchini, E.; Pimenta, J.; Stolf-Moreira, R.; Oliveira, H. Acclimation responses to high light by Guazuma ulmifolia Lam.(Malvaceae) leaves at different stages of development. Plant Biol. 2017, 19, 720–727. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, L.; De Santo, A.V. Paraheliotropism in Robinia pseudoacacia L.: An efficient strategy to optimise photosynthetic performance under natural environmental conditions. Plant Biol. 2008, 10, 194–201. [Google Scholar] [CrossRef] [PubMed]
- De Marco, A.; Arena, C.; Giordano, M.; Virzo De Santo, A. Impact of the invasive tree black locust on soil properties of Mediterranean stone pine-holm oak forests. Plant. Soil 2013, 372, 473–486. [Google Scholar] [CrossRef]
- De Micco, V.; Amitrano, C.; Stinca, A.; Izzo, L.; Zalloni, E.; Balzano, A.; Barile, R.; Conti, P.; Arena, C. Dust accumulation due to anthropogenic impact induces anatomical and photochemical changes in leaves of Centranthus ruber growing on the slope of the Vesuvius volcano. Plant Biol. 2020, 22, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Stinca, A.; Ricciardi, M. Flora e Vegetazione. Laboratorio per il monitoraggio della biodiversità e cartografia del Parco Nazionale del Vesuvio. Napoli. Vesuvio; Carpino, F., Sammichelu, F., Eds.; Ente Parco Nazionale del Vesuvio: Napoli, Italy, 2009; pp. 17–64. [Google Scholar]
- Memoli, V.; Eymar, E.; García-Delgado, C.; Esposito, F.; Santorufo, L.; De Marco, A.; Barile, R.; Maisto, G. Total and fraction content of elements in volcanic soil: Natural or anthropogenic derivation. Sci. Total Environ. 2018, 625, 16–26. [Google Scholar] [CrossRef]
- Memoli, V.; Eymar, E.; García-Delgado, C.; Esposito, F.; Panico, S.C.; De Marco, A.; Barile, R.; Maisto, G. Soil element fractions affect phytotoxicity, microbial biomass and activity in volcanic areas. Sci. Total Environ. 2018, 636, 1099–1108. [Google Scholar] [CrossRef]
- Balzano, A.; Čufar, K.; Battipaglia, G.; Merela, M.; Prislan, P.; Aronne, G.; De Micco, V. Xylogenesis reveals the genesis and ecological signal of IADFs in Pinus pinea L. and Arbutus unedo L. Ann. Bot. 2018, 121, 1231–1242. [Google Scholar] [CrossRef]
- Montecchiari, S. Syntaxonomical and Ecological Characterization of Two Main Alien Forest Communities: Robinia Pseudoacacia and Ailanthus Altissima at Their Southern Limit in Europe. Ph.D. Thesis, Polytechnic University of Marche, Ancona, Italy, 2021. [Google Scholar]
- Capozzi, F.; Di Palma, A.; Sorrentino, M.C.; Adamo, P.; Giordano, S.; Spagnuolo, V. Morphological Traits Influence the Uptake Ability of Priority Pollutant Elements by Hypnum cupressiforme and Robinia pseudoacacia Leaves. Atmosphere 2020, 11, 148. [Google Scholar] [CrossRef]
- Younis, U.; Bokhari, T.Z.; Malik, S.A.; Ahmad, S.; Raja, R. Variations in leaf dust accumulation, foliage and pigment attributes in fruiting plant species exposed to particulate pollution from Multan. Int. J. Agric. Sci. Res. 2013, 3, 1–12. [Google Scholar]
- Cornelissen, J.; Cerabolini, B.; Castro-Díez, P.; Villar-Salvador, P.; Montserrat-Martí, G.; Puyravaud, J.; Maestro, M.; Werger, M.; Aerts, R. Functional traits of woody plants: Correspondence of species rankings between field adults and laboratory-grown seedlings? J. Veg. Sci. 2003, 14, 311–322. [Google Scholar] [CrossRef]
- Feder, N.; O’brien, T. Plant microtechnique: Some principles and new methods. Am. J. Bot. 1968, 55, 123–142. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Fujii, T. Peroxide dependent and independent lipid peroxidation: Site-specific mechanisms of initiation by chelated iron and inhibition by α-tocopherol. Lipids 1992, 27, 227. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; Volume 198. [Google Scholar]
- Kolb, C.A.; Kaser, M.A.; Kopecký, J.; Zotz, G.; Riederer, M.; Pfundel, E.E. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef] [PubMed]
- De Micco, V.; Arena, C.; Aronne, G. Anatomical alterations of Phaseolus vulgaris L. mature leaves irradiated with X-rays. Plant Biol. 2014, 16, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Conti, S.; Francesca, S.; Melchionna, G.; Hájek, J.; Barták, M.; Barone, A.; Rigano, M.M. Eco-physiological screening of different tomato genotypes in response to high temperatures: A combined field-to-laboratory approach. Plants 2020, 9, 508. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Butler, W. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta (BBA)-Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Krall, J.P.; Edwards, G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- De Micco, V.; Aronne, G. Morpho-Anatomical Traits for Plant Adaptation to Drought. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 37–61. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.; Carins Murphy, M.R. Xylem and stomata, coordinated through time and space. Plant Cell Environ. 2017, 40, 872–880. [Google Scholar] [CrossRef]
- Amitrano, C.; Arena, C.; Rouphael, Y.; De Pascale, S.; De Micco, V. Vapour pressure deficit: The hidden driver behind plant morphofunctional traits in controlled environments. Ann. Appl. Biol. 2019, 175, 313–325. [Google Scholar] [CrossRef]
- Amitrano, C.; Junker, A.; D’Agostino, N.; De Pascale, S.; De Micco, V. Integration of high-throughput phenotyping with anatomical traits of leaves to help understanding lettuce acclimation to a changing environment. Planta 2022, 256, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.; Crescente, M.F.; Varone, L. Effect of Water Stress on Physiological and Morphological Leaf Traits: A Comparison among the Three Widely-Spread Invasive Alien Species Ailanthus altissima, Phytolacca americana, and Robinia pseudoacacia. Plants 2022, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Leishman, M.R.; Haslehurst, T.; Ares, A.; Baruch, Z. Leaf trait relationships of native and invasive plants: Community-and global-scale comparisons. New Phytol. 2007, 176, 635–643. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Jorgensen, A.; Sorrell, B.K.; Eller, F. Carbon assimilation through a vertical light gradient in the canopy of invasive herbs grown under different temperature regimes is determined by leaf and whole-plant architecture. AoB Plants 2020, 12, plaa031. [Google Scholar] [CrossRef]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, X.; Wang, H.; Chen, Y.; Sun, Y. Heat-induced down-regulation of photosystem II protects photosystem I in honeysuckle (Lonicera japonica). J. Plant Res. 2021, 134, 1311–1321. [Google Scholar] [CrossRef]
- Zhang, R.; Sharkey, T.D. Photosynthetic electron transport and proton flux under moderate heat stress. Photosynth. Res. 2009, 100, 29–43. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Lehmeier, C.; Pajor, R.; Lundgren, M.R.; Mathers, A.; Sloan, J.; Bauch, M.; Mitchell, A.; Bellasio, C.; Green, A.; Bouyer, D. Cell density and airspace patterning in the leaf can be manipulated to increase leaf photosynthetic capacity. Plant J. 2017, 92, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Way, D.A.; Pearcy, R.W. Sunflecks in trees and forests: From photosynthetic physiology to global change biology. Tree Physiol. 2012, 32, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.C. The physiological significance of variation in leaf structure. Sci. Prog. 1972, 60, 25–51. [Google Scholar]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic efficiency in sun and shade plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Du, Q.; Liu, T.; Jiao, X.; Song, X.; Zhang, J.; Li, J. Leaf anatomical adaptations have central roles in photosynthetic acclimation to humidity. J. Exp. Bot. 2019, 70, 4949–4962. [Google Scholar] [CrossRef]
- Cruces, E.; Flores-Molina, M.R.; Díaz, M.J.; Huovinen, P.; Gómez, I. Phenolics as photoprotective mechanism against combined action of UV radiation and temperature in the red alga Gracilaria chilensis? J. Appl. Phycol. 2018, 30, 1247–1257. [Google Scholar] [CrossRef]
- Valladares, F.; Pearcy, R. Drought can be more critical in the shade than in the sun: A field study of carbon gain and photo-inhibition in a Californian shrub during a dry El Niño year. Plant Cell Environ. 2002, 25, 749–759. [Google Scholar] [CrossRef]
- Hall, F.G.; Hilker, T.; Coops, N.C.; Lyapustin, A.; Huemmrich, K.F.; Middleton, E.; Margolis, H.; Drolet, G.; Black, T.A. Multi-angle remote sensing of forest light use efficiency by observing PRI variation with canopy shadow fraction. Remote Sens. Environ. 2008, 112, 3201–3211. [Google Scholar] [CrossRef]
- Xiong, D.; Douthe, C.; Flexas, J. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant Cell Environ. 2018, 41, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Amitrano, C.; Arena, C.; Cirillo, V.; De Pascale, S.; De Micco, V. Leaf morpho-anatomical traits in Vigna radiata L. affect plant photosynthetic acclimation to changing vapor pressure deficit. Environ. Exp. Bot. 2021, 186, 104453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).