Abstract
Pinus massoniana Lamb. is found in 17 Chinese provinces and is an important timber tree species in southern China. The current seasonal drought climate is becoming increasingly severe, threatening P. massoniana growth and limiting the development of the P. massoniana industry. Plant growth, development, and stress were all regulated by AP2/ERF. We identified 124 AP2/ERF transcription factor family members in this study and discovered that all the genes had their own conserved structural domains and that PmAP2/ERFs were divided into 12 subfamilies with high conservation and similarity in gene structure and evolutionary level. Nine PmAP2/ERF genes were constitutively expressed under drought treatment, and it was hypothesized that the PmAP2/ERF96 gene negatively regulated drought stress, PmAP2/ERF46 and PmAP2/ERF49 genes showed a positive or negative response to drought in different tissues, while the remaining six genes were positively regulated. The PmAP2/ERF genes responded to drought stress following treatment with the exogenous hormones SA, ABA, and MeJA, but the expression patterns differed, with each gene responding to at least one exogenous hormone to induce up-regulation of expression under drought stress, with PmAP2/ERF11, PmAP2/ERF44, PmAP2/ERF77, and PmAP2/ERF80 genes significantly induced by three hormones. The genes mentioned above may be involved in hormone signaling pathways in response to drought stress. The results indicate that the PmAP2/ERF genes may positively or negatively regulate the corresponding signaling pathways in P. massoniana to improve drought resistance.
1. Introduction
Drought, cold, salt, and other abiotic stressors have a significant impact on plant growth and development [1,2,3]. When plants are subjected to drought stress, they respond with a comprehensive series of physiological and molecular regulatory mechanisms [4,5]. To reduce plant damage, plants regulated osmoregulatory substances, antioxidant defense systems, and endogenous hormone levels to maintain cell morphology and scavenge excess oxygen radicals [4,6,7]. Drought induced the expression of plant-related genes, and the main regulatory genes that responded to drought were transcription factors. Such genes can respond quickly after the plant becomes stressed and form their own regulatory network by regulating downstream genes or collaborating with one another to resist drought stress [8,9].
AP2/ERF (APETALA2/ethylene -responsive factor) is one of the largest families of transcription factors in plants. AP2/ERF genes were firstly identified in Arabidopsis thaliana and were associated with flower development [10]. AP2/ERF gene families have been discovered in an increasing number of plant species, and AP2/ERF family genes are more numerous and functionally diverse, involved in physiological and biochemical processes such as growth and development, hormone signaling, and the response to biotic and abiotic stresses in plants [11,12]. The AP2 functional structural domain specific to the AP2/ERF gene consists of 60–70 conserved amino acid residues, which include the YRG and RAYD structural domains; the YRG structure is located at the N-terminus of the AP2 structural domain and consists of about 20 amino acid residues; the YRG structure’s role is to allow the AP2/ERF gene to contact DNA and recognize cis-acting elements [13]. A structural domain at the C-terminus with about 40 amino acid residues may participate in transcription factor interactions [13]. The AP2/ERF gene family is divided into four subfamilies based on sequence similarity and the number of AP2/ERF functional structural domains [14]. The AP2 subfamily contains two AP2/ERF structural domains that have been linked to plant flower development [15,16]; the RAV subfamily contains one AP2/ERF structural domain and one B3 structural domain; and the ERF and Soloist subfamilies have one AP2/ERF structural domain each.
Genes of the AP2/ERF family are considered to be plant-specific transcription factors. In A. thaliana [11] and Oryza sativa L. [17], a large number of AP2/ERF genes have been discovered; for instance, AP2/ERF genes can improve drought tolerance by specifically binding to downstream genes, for example, SpERF1 activated and regulated downstream genes Meanwhile, the function of AP2/ERF genes in model plants such as Arabidopsis and rice has been studied more frequently and intensively, and it has been shown that AP2/ERF genes play an important role in the molecular regulation mechanism of drought in transgenic plants to improve drought stress tolerance by binding to DRE/CRT elements in the promoters of drought-related genes HSP101, RD29A, P5CS, and others [18]. By regulating hormone signaling pathways such as abscisic acid (ABA) and jasmonic acid (JA), AP2/ERF genes play an important role in plant signaling and improve plant drought resistance [19,20]. Plant drought resistance was also improved by AP2/ERF genes, which regulate transpiration, photosynthesis, plant development, and endogenous hormone content [21,22]. In contrast, AP2/ERF genes have been relatively little studied in forest trees, due to the lack of genomic and related expression data, etc., in many tree species, and functional studies of AP2/ERF genes have also been carried out in recent years in forest trees, and the results showed that AP2/ERF genes are involved in the process of phellogen activity/phellem differentiation [23], in the early stage of leaf primordium development [24], in signal transduction such as ethylene [23] and gibberellin [25], in phosphorus stress and drought stress [7,26], etc.
Pinus massoniana Lamb. is distributed in 17 Chinese provinces and is an important timber species with significant economic value in southern China [27,28], as well as a pioneer tree species for afforestation [29]. Seasonal drought is common in southern China, which has a negative impact on P. massoniana growth and limited the development of the P. massoniana industry [6]. The mechanism of P. massoniana AP2/ERF genes in response to drought stress is not clear at the moment, and the mechanism of P. massoniana-related transcription factors involved in drought resistance from the molecular level study has rarely been investigated [5,30]. Since genome-wide data are not yet available for P. massoniana, the identification of the AP2/ERF gene family in P. massoniana is a feasible approach. In this study, we conducted gene family identification by identifying PmAP2/ERF genes in P. massoniana, explored related drought resistance genes, and explored the signaling pathways that the genes may be involved in, to provide a reference for revealing the function of PmAP2/ERF genes and drought response mechanism studies in P. massoniana.
2. Materials and Methods
2.1. Plant Materials and Treatments
Drought-tolerant line 19–220 seedlings and drought-sensitive line 19–214 seedlings were chosen as experimental materials, and seedlings with good development and consistency were chosen and put in pots (18 cm in diameter and 25 cm in height) with substrate (yellow clay soil:coconut coir = 3:1), one plant per pot, and the experiment was carried out after 1 month of normal cultivation in a greenhouse.
Five treatments were established based on the results of the previous pre-experiment: CK1 normal watering; CK2 drought stress; 50 mg/L salicylic acid (SA), SA + drought stress; 0.5 mmol/L methyl jasmonate (MeJA) + drought stress; and 25 mg/L ABA + drought stress, with three replications of each treatment and ten plants in each replicate. Following three days of continuous appropriate watering, the above concentrations of SA, MeJA, ABA, and distilled water (CK1 and CK2) were sprayed for four days, with 10 mL on the above-ground part (stems and needles) and 10 mL on the below-ground part (roots). Following that, all experimental seedlings were rehydrated and recorded as day 0 of drought stress for ongoing natural drought stress, with CK1 serving as the regular watering control group, which was watered once every two days. At soil drought levels [9] of mild drought (55%–70%), moderate drought (45%–55%), severe drought (30%–45%), and 48 h after rehydration, the needles (middle part of the area with needles), stems (3 cm long in the middle), and roots (2 cm in the apical part of the main and lateral roots) of P. massoniana were sampled and stored in a refrigerator at −80 °C.
2.2. Identification of PmAP2/ERFs Gene Family
The AP2/ERF genes in P. massoniana were identified based on the full-length transcriptome, insect resistance transcriptome [31], lateral branch differentiation transcriptome [25], and drought resistance transcriptome (unpublished) of the previous research group. Sequences from the three transcriptome databases were removed from redundant sequences and annotated by NR, SwissProt, and Pfam databases, and genes annotated as AP2/ERF were extracted, and their nucleic acid sequences and protein sequences were extracted using Perl language. Hidden Markov models of the AP2 structural domain were downloaded from the Pfam database [32] (https://pfam.xfam.org/, accessed on 7 June 2021) and the AP2/ERF genes were identified using HMMER software, while the protein sequences of the A. thaliana AP2/ERF genes were downloaded from the PlantTFBD database [33] (http://planttfdb.gao-lab.org/index_ext.php, accessed on 7 June 2021) to download the protein sequences of A. thaliana AP2/ERF genes, and the AP2/ERF protein sequences of A. thaliana and P. massoniana were compared using BLAST software, and the protein sequences obtained by the above two methods were taken as a concatenation. After removing the redundant sequences, NCBI CD Search [34] (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 8 June 2021), SMART [35] (http://smart.embl.de/, accessed on 8 June 2021), and Pfam were used to determine whether the candidate protein sequences contained AP2/ERF structural domains, and sequences lacking structural domains or containing incomplete structural domains were removed.
2.3. Analysis of Physicochemical Properties of PmAP2/ERFs Proteins
ExPASy [36] (https://web.expasy.org/, accessed on 14 December 2021) was used to predict the physicochemical properties of PmAP2/ERF molecular mass, isoelectric point, etc.; Plant-mPLoc [37] (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/, accessed on 14 December 2021) and WoLF PSORT [38] (https://psort.hgc.jp/, accessed on 14 December 2021) online sites for subcellular localization prediction analysis; TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/, accessed on 14 December 2021) for protein transmembrane structure analysis.
2.4. PmAP2/ERFs Protein Phylogenetic Analysis and Multiple Sequence Alignment
The A. thaliana AP2/ERF protein sequences were obtained from the TAIR database [39] and the PlantTFBD database, and the phylogenetic evolutionary trees of P. massoniana and A. thaliana were generated using MEGA7 software [40] with the following parameters: Using the Neighbor-Joining (NJ) approach, the P-distance model was chosen, and the evolutionary tree was decorated with iTOL [41].
2.5. PmAP2/ERFs Protein Conserved Motif Analysis
The conserved motifs of 124 P. massoniana PmAP2/ERFs proteins were evaluated online using the MEME online tool [42] (https://meme-suite.org/meme/, accessed on 26 July 2021), with the following parameters: The expected motif count was ten, and the motif length ranged from 6 to 60 AA.
2.6. Prediction of PmAP2/ERFs Protein Interactions
Using the STRING website [43] (https://string-db.org/cgi, accessed on 27 March 2022) and Cytoscape software [44], potential interaction networks and biological functions between PmAP2/ERFs proteins of P. massoniana were predicted based on the AP2/ERF protein analysis of A. thaliana.
2.7. RNA-seq Data Analysis of PmAP2/ERF Genes
The expression heat map of PmAP2/ERF genes under drought stress was drawn based on the pre-drought transcriptome data. The treatment groups were continuous natural drought stress (D) and the control group (C) was normal watering. The root systems (main and lateral root tips were 2 cm) of seedlings in the treatment and control groups corresponding to the three time points were taken at the 7th d (1), the 8th d before rehydration for 7 h (2), and the 8th d (3) of the drought stress treatment, respectively. Based on the obtained transcriptome data, the expression heat map of PmAP2/ERFs gene family under drought stress was drawn using TBtools.
2.8. Expression Analysis of PmAP2/ERFs Gene
RNA extraction was performed using the polyphenol polysaccharide plant RNA extraction kit from Tiangen (Beijing, China), cDNA synthesis was performed using M-MLV reverse transcriptase from Takara (Shanghai, China), and finally, the concentration of all cDNA samples was adjusted to 50 ng/μL. Fluorescent quantitative PCR was performed using the TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) kit (Shanghai, China) from Takara Bio, with the reaction system configured according to the instructions; the internal reference genes were PmUBI4 (tissue-specific internal reference gene) and PmCYP (drought stress-treated internal reference gene) [45], and primer information is provided in (Table S1); real-time fluorescence quantitative PCR was performed using a Bio-Rad CFX96 quantitative PCR instrument (San Diego, CA, USA), and each sample was technically repeated three times, and the relative expression of genes was calculated using the 2−ΔΔCt method [46], and the data were analyzed for significant differences using SPSS software (IBM, New York, NY, USA), and finally plotted using GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Identification and Naming of PmAP2/ERFs Gene Family
Non-redundant sequences were obtained based on the previous full-length transcriptome and annotated as AP2/ERF genes, and 453 sequences were identified by HMMER software and local Blast. After removing the redundant sequences, the candidate protein sequence structural domains were analyzed using NCBI CD Search, SMART, and Pfam, and 124 PmAP2/ERF genes of P. massoniana were finally identified and obtained (Table S2).
3.2. Analysis of Physicochemical Properties of PmAP2/ERFs Proteins
PmAP2/ERFs physicochemical properties and functional structure analysis showed that PmAP2/ERFs protein encoded 101 (PmAP2/ERF58) to 683 (PmAP2/ERF43) amino acids. The molecular weight of PmAP2/ERFs protein ranged from 11.34 (PmAP2/ERF58) to 76.18 (PmAP2/ERF43) kDa. The theoretical isoelectric points of PmAP2/ERFs proteins ranged from 4.48 (PmAP2/ERF27) to 11.65 (PmAP2/ERF89) with an average pI of 7.50; 61 PmAP2/ERFs proteins had isoelectric points < 7, which were acidic, and 63 PmAP2/ERFs proteins had isoelectric points >7, which were alkaline. A total of 9 PmAP2/ERFs proteins (PmAP2/ERF35\49\71\74\ 101\110\113\121\123) with instability coefficients less than 40 were stable proteins, while the remaining 115 PmAP2/ERFs proteins with instability coefficients greater than 40 were unstable proteins. The average hydrophobic value of 23 PmAP2/ERFs proteins was greater than −0.5, which were hydrophilic proteins, while the average hydrophobic value of the remaining 101 proteins was less than −0.5, which were hydrophobic. The subcellular localization showed that most of the proteins were localized in the nucleus, and some of them were also distributed in the cytoplasm. It was speculated that the PmAP2/ERF genes might play different regulatory roles in different organelles, and none of the PmAP2/ERFs had transmembrane structures (Table S2).
3.3. PmAP2/ERFs Protein Phylogeny and Multiple Sequence Alignment Analysis
The phylogenetic tree showed that the AP2/ERF gene family of P. massoniana can be divided into three subfamilies, AP2, RAV, and ERF, and did not contain the Soloist subfamily [11]. Including 8 members of the AP2 subfamily and 9 members of the RAV subfamily, 107 belong to the ERF subfamily. The ERF subfamily was further divided into two subfamilies, DREB and ERF, and in this study the DREB subfamily was divided into subgroups I, II, III, and IV, containing 6, 38, 14, and 4 genes, respectively, for a total of 62 genes; while the ERF subfamily was divided into subgroups V, VI, VII, VIII, IX, and X subfamilies, containing 1, 4, 7, 15, 13, and 5 member genes, respectively, for a total of 45 genes. The AP2 subfamily and the RAV subfamily clustered on one major branch and later on two different minor branches; the DREB and ERF subfamilies clustered on two different major branches, respectively (Figure 1).
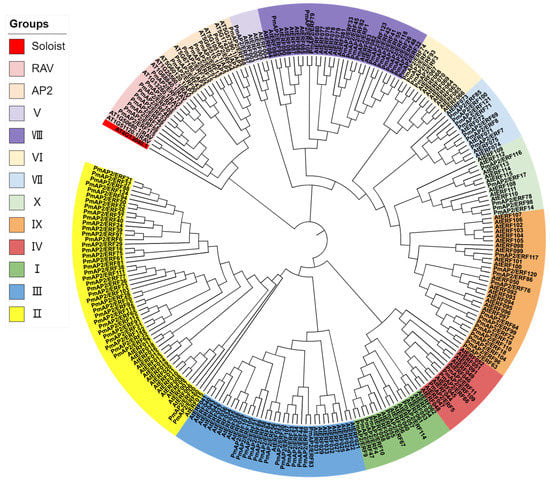
Figure 1.
Phylogenetic analysis of PmAP2/ERFs proteins in P. massoniana. A. thaliana AP2/ERF protein sequences were downloaded from TAIR database and PlantTFBD database, and the phylogenetic evolutionary trees of PmAP2/ERFs of P. massoniana and AtERFs of A. thaliana were constructed using MEGA7 software and iTOL, and different colors in the figure represent different groupings.
The AP2 subfamily genes included two AP2 structural domains (AP2-1 and AP2-2), both containing relatively conserved YRG and RAYD structural domains, with a C-terminal motif deletion in PmAP2/ERF102 in the second AP2 structural domain. The main differences between the ERF and DREB subfamilies were 14 and 19 amino acids of the ERF subfamily were alanine (A) and aspartic acid (D) and 14 and 19 amino acids of the DREB subfamily are valine (V) and glutamic acid (E) [14]. Both the ERF and DREB subfamily genes in the present study also contained the YRG and RAYD structural domains, and most genes were highly conserved in these two structural domains, while a few genes had amino acid residue variants or deletions at positions 14 and 19 (Figure 2).
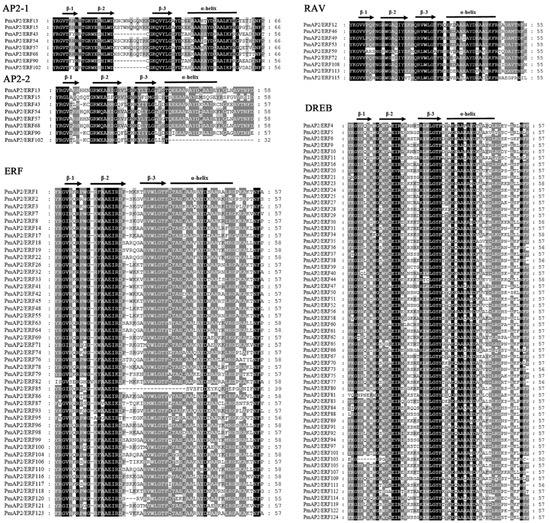
Figure 2.
Multiple sequence alignment of AP2 structural domain proteins from each subfamily of PmAP2/ERFs. The sequence comparison results of AP2 structural domain proteins of AP2, RAV, ERF, and DREB subfamilies are shown in the figure, where AP2 subfamily contains two AP2 structural domains, AP2-1 and AP2-2, respectively. Arrows represent β-sheets and horizontal lines represent α-helix.
3.4. Conserved Motif Analysis of PmAP2/ERFs Proteins
The conserved motif analysis of PmAP2/ERFs protein showed that 10 conserved Motifs were obtained, ranging from 29 to 58 amino acids in length. Among them, Motif 8, Motif 1, and Motif 10 formed the first AP2 structural domain of AP2 subfamily, and Motif 6 and Motif 1 formed the second AP2 structural domain. Motifs 6, 1, and 10 formed the AP2 structural domain of RAV subfamily, and Motif 5 and Motif 4 formed the B3 structural domain of RAV subfamily. Motif 2, Motif 1, and Motif 3 formed the AP2 domain of the ERF subfamily. The results showed that the genes of the same subfamily contained basically the same motifs, but there were a few differences, for example, members of the RAV subfamily contained Motif 6, Motif 1, Motif 10, Motif 5, and Motif 4, while PmAP2/ERF115 of the same RAV subfamily contained one less Motif 10 and one more Motif 4. This phenomenon also exists in other subfamilies, which may be due to mutations during protein evolution (Figure 3A,B).
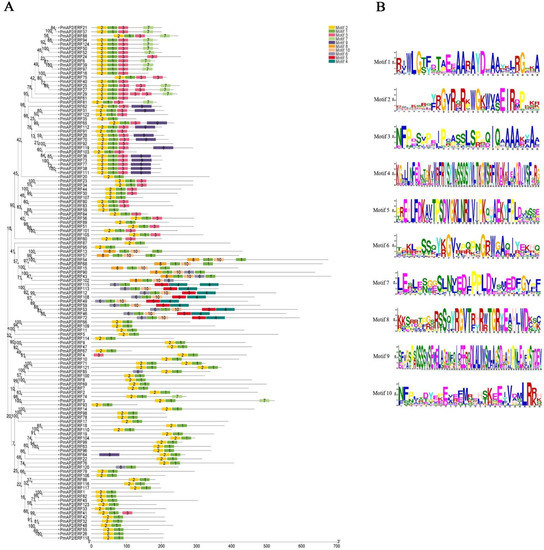
Figure 3.
Conserved motif analysis of PmAP2/ERFs proteins of P. massoniana and multiple sequence alignment of AP2 structural domain proteins: (A): Distribution of conserved motifs of PmAP2/ERFs proteins of P. massoniana. A total of 10 conserved Motifs were obtained, indicated by different numbers and colors, and arranged in order. (B): Conserved motifs of PmAP2/ERFs proteins of P. massoniana. Corresponds to Motif in A.
3.5. Protein Interaction Analysis of PmAP2/ERFs
The results of the protein interaction network map (Figure 4) showed that most of the PmAP2/ERFs could interact with more than one protein, among which there were interactions between PmAP2/ERF102 and PmAP2/ERF109. PmAP2/ERF102 (AT4G36920) and PmAP2/ERF109 (AT5G05410) played a vital role in the overall reciprocal network.
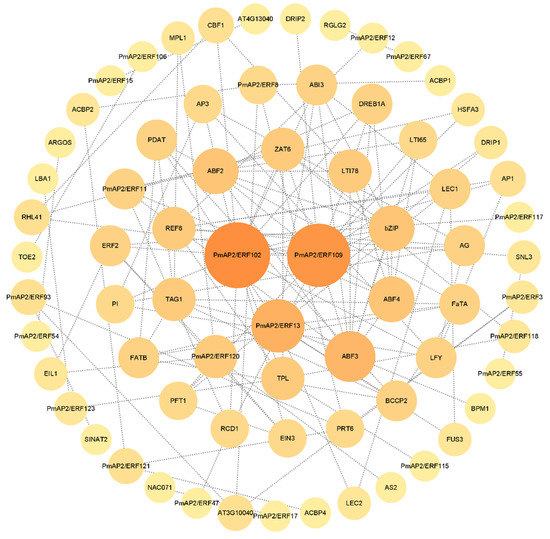
Figure 4.
Prediction of the protein interaction network between PmAP2/ERFs of P. massoniana and AP2/ERFs of A. thaliana. The protein interaction network map of PmAP2/ERFs was constructed by STRING and Cytoscape software, which was based on the AP2/ERFs proteins of A. thaliana for analysis and prediction. The circles of different colors and sizes represent the importance of different proteins in the whole interaction network, and the dashed lines represent the possible interactions between the proteins.
3.6. Expression Analysis of PmAP2/ERF Genes in RNA-seq
The expression heat map of PmAP2/ERF genes under drought based on transcriptome data (Figure 5) revealed that 118 genes expressed during drought stress, while 6 genes did not express. A total of 13 genes peaked at D1, 14 genes peaked at D2, 29 genes peaked at D3, and the rest of the genes peaked at CK1. Further, FDR ≤ 0.001 and |log2FC| ≥ 2 were used as the screening criteria for significantly different genes, and PmAP2/ERF11, PmAP2/ERF14, PmAP2/ERF44, PmAP2/ERF46, PmAP2/ERF49, PmAP2/ERF77, PmAP2/ERF80, PmAP2/ERF96, and PmAP2/ERF109 genes were studied for their expression patterns under hormonal and drought stresses.
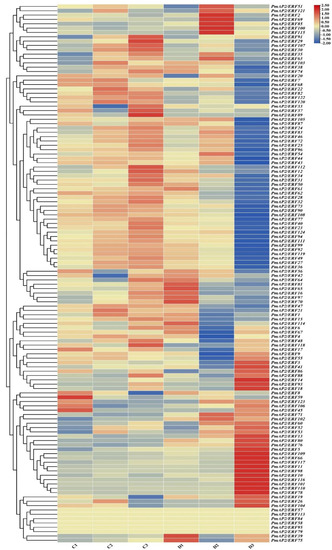
Figure 5.
Expression heat map of PmAP2/ERFs gene expression in RNA-seq data. Blue represents low expression levels and red represents high expression levels. C and D indicate control (normal growth) and treatment (drought stress) groups, respectively; 1, 2, and 3 indicate control and drought treatment groups on the 7th day, 8th day rehydration at 7 h, and 8th day, respectively. Gene expression heat map was plotted using TBtools.
3.7. Tissue-Specific Analysis of PmAP2/ERF Genes
The tissue-specific results showed that PmAP2/ERF genes were expressed in needles, stems, and roots, but the expression levels differed (Figure 6). The genes PmAP2/ERF14, PmAP2/ERF44, PmAP2/ERF49, and PmAP2/ERF109 were expressed in the roots of different drought-resistant materials. The highest expression was found in needle leaves. These genes may play an important role in regulating the growth and development of roots or needles (Figure 6A,B).
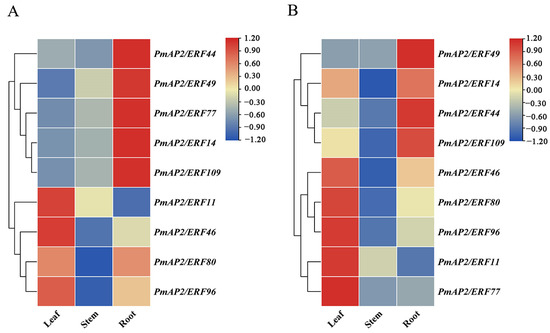
Figure 6.
Tissue-specific analysis of PmAP2/ERF genes in P. massoniana: (A): Heat map of PmAP2/ERFs gene expression in needle leaves, stems, and roots of drought-sensitive lines. (B): Heat map of PmAP2/ERFs gene expression in needles, stems, and roots of drought-resistant lines. The expression levels of PmAP2/ERF genes in needles, stems, and roots of P. massoniana were analyzed by real-time fluorescence quantitative PCR, and the expression of each gene in leaves was used as a control for quantification in stems and roots, respectively. Blue color represents low expression levels and red color represents high expression levels. Plotting was performed using TBtools software.
3.8. Expression Pattern Analysis of PmAP2/ERF Genes under Hormone Treatment and Drought Stress
Expression pattern studies showed that PmAP2/ERF genes expressed in all tissues as constitutive expression, but there were differences in expression patterns. In different drought-tolerant lines, drought stress (CK2) induced up-regulated expression of PmAP2/ERF11, PmAP2/ERF14, PmAP2/ERF44, PmAP2/ERF77, PmAP2/ERF80, and PmAP2/ERF109 genes, and down-regulated expression of PmAP2/ERF46, PmAP2/ERF49, and PmAP2/ERF96. Expression patterns of PmAP2/ERF genes induced by hormones differed in different tissues of different families (Figure 7 and Figure 8).
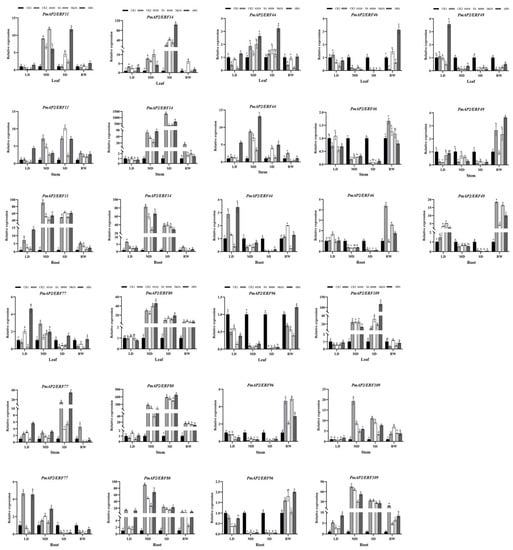
Figure 7.
Expression patterns of PmAP2/ERF genes in drought-sensitive lines during drought stress. LD indicates light drought, MD indicates moderate drought, SD indicates severe drought, and RW indicates rehydration. Different lowercase letters indicate differences in gene expression at the p < 0.05 level between treatments at each sampling site. The standard error of the mean for three biological replicates is represented by the error bars.
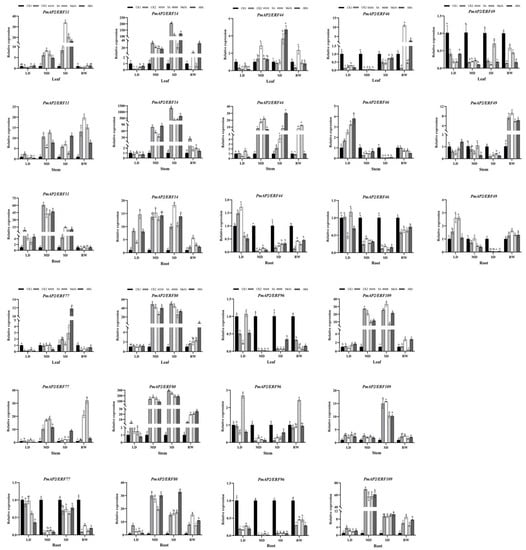
Figure 8.
Expression patterns of PmAP2/ERF genes in drought-resistant lines during drought stress. LD indicates mild drought, MD indicates moderate drought, SD indicates severe drought, and RW indicates rehydration. Different lowercase letters indicate differences in gene expression at the p < 0.05 level between treatments at each sampling site. The standard error of the mean for three biological replicates is represented by the error bars.
In needle leaves, 6 PmAP2/ERF genes were up-regulated in leaves of two lines, with PmAP2/ERF11 and PmAP2/ERF14 genes expressing more in drought-resistant lines than in drought-sensitive lines. The expression patterns of PmAP2/ERF genes induced by hormones under drought stress were similar and different in drought-sensitive and drought-resistant lines. Compared with CK2, PmAP2/ERF11 and PmAP2/ERF109 genes were significantly up-regulated when induced by SA, MeJA, and ABA in both lines, while PmAP2/ERF11 gene expression was higher in drought-resistant lines than in drought-sensitive lines. PmAP2/ERF14 and PmAP2/ERF80 genes were up-regulated by three hormones in drought-sensitive lines, while they were not significantly up-regulated by hormones in drought-resistant lines and both were smaller than CK2. PmAP2/ERF44 and PmAP2/ERF77 gene expression was induced by three hormones in drought-resistant lines, whereas PmAP2/ERF44 was induced by ABA at a higher expression level than CK2 in drought-sensitive lines; the PmAP2/ERF77 gene was significantly expressed when induced by SA and ABA during mild drought. The remaining three genes did not significantly express when induced by hormones. After rehydration, PmAP2/ERF genes were expressed at higher levels in drought-resistant lines (Figure 7 and Figure 8).
In the stems, there were similarities and also differences in the expression patterns of PmAP2/ERF genes induced by hormones in different lines. Compared with CK2, PmAP2/ERF11, PmAP2/ERF77, and PmAP2/ERF80 genes were significantly up-regulated when induced by SA, MeJA, and ABA in drought-sensitive lines, and PmAP2/ERF11, PmAP2/ERF44, PmAP2/ERF46, and PmAP2/ERF77 genes were significantly expressed when induced by three exogenous hormones in drought-resistant lines. The PmAP2/ERF14 gene was expressed when induced by SA and ABA in drought-sensitive lines, but only by ABA in drought-tolerant lines. The PmAP2/ERF49 gene was expressed when induced by ABA, and PmAP2/ERF96 gene was expressed when induced by MeJA. The expression of the PmAP2/ERF109 gene in drought-sensitive lines significantly increased by ABA treatment at mild drought and then decreased, which was always lower than CK2. In drought-resistant lines, the expression of the PmAP2/ERF109 gene induced by hormones was less than CK2 or not significantly different from CK2. After rehydration, the expression of PmAP2/ERF genes induced by different hormones was significantly higher than CK1 and CK2 after rehydration (Figure 7 and Figure 8).
In the roots, there were significant differences in expression levels of PmAP2/ERF genes in the two lines under hormone treatments. Compared with CK2, the PmAP2/ERF49 gene was significantly up-regulated by three hormones in drought-sensitive lines, whereas PmAP2/ERF49 genes were expressed when induced by SA and MeJA in the drought-resistant line. The PmAP2/ERF11, PmAP2/ERF14, and PmAP2/ERF80 genes were significantly expressed when induced by the three hormones in the drought-resistant lines, while the PmAP2/ERF11 gene was significantly induced by MeJA and ABA in the drought-sensitive lines, and the PmAP2/ERF14 and PmAP2/ERF80 genes were not significantly induced by the hormones. Both were basically smaller than CK2. In drought-sensitive lines, the PmAP2/ERF44 and PmAP2/ERF109 genes were expressed when induced by ABA, and the PmAP2/ERF46 gene was expressed when induced by SA; while in the drought-resistant lines, the PmAP2/ERF44 and PmAP2/ERF46 genes were induced by SA and MeJA, respectively, with the highest expression at mild drought, and PmAP2/ERF109 did not express. The PmAP2/ERF77 and PmAP2/ERF96 genes were less or not significantly different from CK2 in both lines, while the PmAP2/ERF44 and PmAP2/ERF46 genes were significantly expressed when induced by SA and MeJA, respectively, and the PmAP2/ERF109 gene was not significantly expressed in the drought-sensitive lines after rehydration (Figure 7 and Figure 8).
4. Discussion
AP2/ERF is a major transcription factor in plants that is involved in plant growth, development, biotic, and abiotic stresses [47,48,49]. Based on transcriptome data identification, 124 PmAP2/ERF genes were identified in this study, which was comparable to 122 in A. thaliana [11], less than Oryza sativa L. (163) [17], Zea mays L. (292) [3], and more than Taxus wallichiana var. chinensis (49) [20], implying that the number of AP2/ERF gene family may not be directly related to species and genome size. The current analysis was more detailed than previous investigations, which revealed 88 AP2/ERF genes in P. massoniana [50]. There were eight AP2 subfamily genes, nine RAV subfamily genes, and 107 ERF subfamily genes in PmAP2/ERFs. The ERF subfamily was divided into two subfamilies, DREB and ERF [11], which included 62 and 45 genes, respectively, and it has been shown that DREB genes bound to DRE elements in the promoter regions of downstream genes to regulate the expression of related genes, and ERF genes activated downstream gene expression by binding to GCC-box elements in the promoter regions of downstream target genes [51]. ERF subfamily genes were implicated in both plant hormone response and abiotic stress response [48,52]. The result showed that around half of the proteins were acidic, the majority were hydrophobic, and the protein structures were unstable. PmAP2/ERFs may perform various regulatory roles in different organelles based on their subcellular location in the nucleus or cytoplasm. Conservative motifs revealed that each cluster had its own distinct distribution pattern, and the motifs contained in each cluster branch and the same subfamily of genes were basically the same, implying that their roles may be similar, which was consistent with earlier research [51,53]. The current findings indicated that AP2/ERF genes were highly conserved and structurally and evolutionary comparable.
Protein interactions revealed that PmAP2/ERF102 (AT4G36920) and PmAP2/ERF109 (AT5G05410) played important roles in the overall interplay network. PmAP2/ERF10 may have a similar function to the AT4G36920 (APETALA 2) gene, which was discovered to be important in the development of the floral meristem, embryo, endosperm, and seed coat [54,55]. AT5G05410 (DREB2A) was associated with drought and high temperature [56,57], and A. thaliana plants with overexpressing DREB2A had significant drought tolerance [56], implying that PmAP2/ERF109 may have similar function with AT5G05410 (DREB2A), and the PmAP2/ERF109 gene was up-regulated under drought stress in our study, which indicated the result was accuracy.
Tissue-specific analysis of nine PmAP2/ERF genes revealed that PmAP2/ERF genes were expressed in all tissues, and there were differences in the expression levels of genes in different tissues of different lines, while the expression levels of some genes in the same tissues of different lines also differed significantly. For example, in drought-sensitive lines, PmAP2/ERF77 gene expression was highest in roots and lowest in needle leaves, whereas in drought-tolerant lines, PmAP2/ERF77 gene expression was the highest in needle leaves and the lowest in stems, and the tissue-specific expression of PmAP2/ERF77 gene was completely different in the two lines, which could be related to the difference in drought resistance.
The results of this study showed that nine PmAP2/ERF genes constitutively expressed under drought stress, PmAP2/ERF46, PmAP2/ERF49, and PmAP2/ERF96 genes were negatively regulated by drought stress, and the remaining six PmAP2/ERF genes were positively regulated to respond to drought stress. It was shown that the DREB subfamily of TtAP2/ERF genes, TtAP2/ERF-176, TtAP2/ERF-206, and TtAP2/ERF-227, were significantly up-regulated under drought stress [47]. Five DREB subfamily genes in this study (PmAP2/ERF11, PmAP2/ERF44, PmAP2/ERF77, PmAP2/ERF80, and PmAP2/ERF109) were up-regulated under drought stress and positively regulated drought stress. GmDREB1 regulated the expression of downstream stress-related genes by interacting with GmERF008 and GmERF106 to form a heterodimer, which significantly improved drought tolerance and increases yield in transgenic soybean [58]. The NtERF172 gene is directly bound to the promoter region of the downstream NtCAT gene and positively regulates NtCAT gene expression, resulting in higher catalase activity and less H2O2 accumulation in transgenic plants, indicating that the NtERF172 gene significantly improved the drought tolerance of the plants [59]. The PalERF2 gene directly regulates drought response genes PalRD20 and PalSAG113 to improve drought resistance in poplar. The above studies suggest that AP2/ERF genes can activate and regulate downstream gene expression in response to drought stress through intergenic interactions. Whether there are interactions between the nine PmAP2/ERF genes in this study and the regulatory mechanisms in response to drought needs to be further investigated.
Hormones played a crucial role in plant response to abiotic stresses such as drought. SA, ABA, and JA act as hormone signaling molecules in plant drought resistance [60,61,62]. AP2/ERF genes can improve plant resistance by participating in hormone signaling networks, e.g., the ZmEREB160 gene increased survival and proline accumulation in transgenic A. thaliana under drought stress. The expression levels of the ABA signaling pathway and drought-related genes such as ABI2, ABI5, and DREB2A were also found to be significantly up-regulated, indicating that the ZmEREB160 gene was involved in the ABA signaling pathway to improve drought resistance [63]. In this study, P. massoniana was pretreated with exogenous hormones SA, ABA, and MeJA, and the expression pattern of the PmAP2/ERF genes in P. massoniana was found to be different in different tissues of different lines induced by hormones under drought stress, in which the PmAP2/ERF96 gene significantly expressed when induced by MeJA only in the stem of the drought-resistant line at mild stress, and was not significantly affected by hormones in other tissues. PmAP2/ERF gene expression was induced by at least one exogenous hormone in response to drought stress in both lines and its expression was higher than that of CK2. It was hypothesized that PmAP2/ERF genes may be involved in the corresponding hormone signaling pathways.
The MdDREB2 gene directly bonded to the DRE motif in the promoters of MdNCED6 and MdNCED9 genes, activating the transcription of ABA biosynthesis genes to promote ABA synthesis, and the MdDREB2 gene interacted with the MdCoL gene to more effectively promote the expression of MdNCED6/9 for ABA synthesis [64]. A. thaliana overexpressing the sweet potato IbRAP2-12 gene showed up-regulated expression of genes related to ABA and JA signaling pathways under drought stress, while the IbRAP2-12 gene improved A. thaliana tolerance during abiotic stress [23]. PmAP2/ERF11, PmAP2/ERF44, PmAP2/ERF77, and PmAP2/ERF80 genes were significantly up-regulated when induced by three hormones, and it was hypothesized that these four PmAP2/ERF genes enhance drought tolerance in hormone signaling in P. massoniana, but the specific regulatory mechanisms need to be further investigated. The PmAP2/ERF46 and PmAP2/ERF49 genes were members of the RAV subfamily of the AP2/ERF gene family, and PmAP2/ERF11 and PmAP2/ERF109 were members of subfamily IV. In this study, we found similarities in expression patterns between the above two groups of genes under drought stress and hormone induction, further demonstrating that genes of the same subfamily may have similarities. The difference in expression of PmAP2/ERF genes induced by hormones in two different families was similar to the expression of CsPRX genes in two different Camellia sinensis varieties [65], which may be caused by the genetic background of two families with different drought tolerance levels. We also suggest that the high expression of some PmAP2/ERF genes in drought-resistant lineages increased the drought resistance of P. massoniana, and caused differences in drought resistance phenotypes.
5. Conclusions
In this study, we successfully identified 124 PmAP2/ERF genes and analyzed the physicochemical properties, phylogeny, and conserved motifs of the gene family members. The expression patterns of nine PmAP2/ERF genes were also analyzed under drought treatment, and it was found that all nine genes underwent expression changes in response to drought stress, but the expression patterns were different. The expression patterns showed that PmAP2/ERF11, PmAP2/ERF14, PmAP2/ERF44, PmAP2/ERF77, PmAP2/ERF80, and PmAP2/ERF109 genes were up-regulated in response to drought stress and were positively regulated; PmAP2/ERF96 gene was negatively regulated; PmAP2/ERF46 and PmAP2/ERF49 genes are up-regulated and down-regulated. The expression pattern of PmAP2/ERF genes induced by hormones differs in different tissues of different families, and the PmAP2/ERF genes responded to at least one hormone signal, suggesting that the PmAP2/ERF genes may positively or negatively regulate the response to hormones to improve drought tolerance in P. massoniana. Our study will provide a theoretical basis for the functional study of the AP2/ERF gene family and help to further investigate the molecular mechanism of PmAP2/ERF gene regulation in response to drought in P. massoniana. At the same time, this study also provides a reference for exploring the molecular mechanism of AP2/ERF genes in response to drought in other gymnosperms because of the close kinship between gymnosperms and the similarity in phylogeny and gene functions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f13091430/s1, Table S1: qRT-PCR Primer sequences of the genes; Table S2: Physical and chemical analysis of AP2/ERF in Pinus massoniana.
Author Contributions
S.S. and H.C. designed and conducted the experiments and wrote the manuscript; X.L. and H.C. contributed to manuscript writing and editing; L.H. executed the bioinformatics tools; and Z.Y. contributed to the experimental design and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The Natural Science Foundation of China (32060348, 32160382), The Guangxi Natural Science Foundation (2019GXNSFDA245033, 2019GXNSFBA245064), the Special Fund for Bagui Scholar (2019A26) and Bagui Young Scholar, and the Guangxi Science and Technology and Talents Special Project (AD19254004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials. It is also available from the correspondence author (yangzhangqi@163.com).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 3, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dubey, R. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing Rice seedlings. Plant Growth Regul. 2005, 3, 209–221. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, Y.; Wu, Y. Genome-Wide analysis of AP2/ERF transcription factor family in Zea mays. Curr. Bioinform. 2012, 7, 324–332. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Xu, H.; Gao, X.; Yu, C. Physiological and transcriptomic analysis of Pinus massoniana seedling response to osmotic stress. Biol. Plant. 2021, 65, 145–156. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhao, X.; Lu, Z.; Sun, X.; Ding, G. Role of Suillus placidus in improving the drought tolerance of Masson Pine (Pinus massoniana Lamb.) seedlings. Forests 2021, 12, 332. [Google Scholar] [CrossRef]
- Wang, X.; Han, H.; Yan, J.; Chen, F.; Wei, W. A New AP2/ERF transcription factor from the oil plant Jatropha curcas confers salt and drought tolerance to transgenic Tobacco. Appl. Biochem. Biotechnol. 2015, 176, 582–597. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, W.; Sun, Y.; Zhang, T.; Zhai, M. Two novel WRKY genes from Juglans regia, JrWRKY6 and JrWRKY53, are involved in abscisic acid-dependent stress responses. Biol. Plant. 2017, 61, 611–621. [Google Scholar] [CrossRef]
- Du, M.; Ding, G.; Cai, Q. The transcriptomic responses of Pinus massoniana to drought stress. Forests 2018, 9, 326. [Google Scholar] [CrossRef]
- Jofuku, K.D.; Boer, B.G.W.D.; Montagu, M.V.; Okamuro, J.K. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 1994, 9, 1211–1225. [Google Scholar]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide analysis of the ERF gene family in Arabidopsis and Rice. Plant Physiol. 2006, 2, 411–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lixia, Z.; Rajesh, Y. Genome-Wide identification and characterization of AP2/ERF transcription factor family genes in oil palm under abiotic stress conditions. Int. J. Mol. Sci. 2021, 22, 2821–2835. [Google Scholar]
- Okamuro, J.K.; Caster, B.; Villarroel, R.; Van Montagu, M.; Jofuku, K.D. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 13, 7076–7081. [Google Scholar] [CrossRef]
- Yoh, S.; Qiang, L.; Joseph, G.D.; Hiroshi, A.; Kazuo, S.; Kazuko, Y.S. DNA-Binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-Inducible gene expression. Biochem. Biophys. Res. Commun. 2002, 3, 998–1009. [Google Scholar]
- Kunst, L.; Klenz, J.E.; Haughn, M.Z.W. AP2 gene determines the identity of perianth organs in flowers of Arabidopsis thaliana. Plant Cell 1989, 1, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, S.; Chai, T.; Wang, T. OsAP2-1, an AP2-like gene from Oryza sativa, is required for flower development and male fertility. Sex. Plant Reprod. 2006, 19, 197–206. [Google Scholar] [CrossRef]
- Sharoni, A.M.; Nuruzzaman, M.; Satoh, K.; Shimizu, T.; Kondoh, H.; Sasaya, T.; Choi, I.R.; Omura, T.; Kikuchi, S. Gene structures, classification and expression models of the AP2/EREBP transcription factor family in Rice. Plant Cell Physiol. 2010, 2, 344–360. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, C.; Li, X.; Du, J.; Qian, M.; Sun, X.; Yang, Y. A novel Ap2/ERF transcription factor from Stipa purpurea leads to enhanced drought tolerance in Arabidopsis thaliana. Plant Cell Rep. 2016, 35, 2227–2239. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhang, Q.; Liu, Q.; Zhao, H.; Zhao, N.; He, S. An AP2/ERF gene, IbRAP2-12, from sweetpotato is involved in salt and drought tolerance in transgenic Arabidopsis. Plant Sci. 2019, 281, 19–30. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, M.; Li, L.; Xu, Z.; Chen, X.; Guo, J.; Ma, Y. Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J. Exp. Bot. 2009, 60, 3781–3796. [Google Scholar] [CrossRef]
- Abogadallah, G.M.; Nada, R.M.; Malinowski, R.; Quick, P. Overexpression of HARDY, an AP2/ERF gene from Arabidopsis, improves drought and salt tolerance by reducing transpiration and sodium uptake in transgenic Trifolium alexandrinum L. Planta 2011, 233, 1265–1276. [Google Scholar] [CrossRef]
- Mawlong, I.; Ali, K.; Srinivasan, R.; Rai, R.D.; Tyagi, A. Functional validation of a drought-responsive AP2/ERF family transcription factor-encoding gene from rice in Arabidopsis. Mol. Breed. 2015, 35, 163. [Google Scholar] [CrossRef]
- Lopes, S.T.; Sobral, D.; Costa, B.; Perdiguero, P.; Chaves, I.; Costa, A.; Miguel, C.M. Phellem versus xylem: Genome-wide transcriptomic analysis reveals novel regulators of cork formation in cork oak. Tree Physiol. 2020, 40, 129–141. [Google Scholar] [CrossRef]
- Zong, Y.; Hao, Z.; Tu, Z.; Shen, Y.; Zhang, C.; Wen, S.; Yang, L.; Ma, J.; Li, H. Genome-wide survey and identification of AP2/ERF genes involved in shoot and leaf development in Liriodendron Chinense. BMC Genom. 2021, 22, 807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jiang, L.; Wang, X.; Han, H.; Chen, D.; Qiu, D.; Yang, Y. Transcriptome-wide analysis of AP2/ERF transcription factors involved in regulating Taxol biosynthesis in Taxus × media. Ind. Crops Prod. 2021, 171, 113972. [Google Scholar] [CrossRef]
- Chen, N.; Qin, J.; Tong, S.; Wang, W.; Jiang, Y. One AP2/ERF transcription factor positively regulates Pi uptake and drought tolerance in Poplar. Int. J. Mol. Sci. 2022, 23, 5241. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Z.; Wei, Y.; Shen, D.; Feng, Z.; Hong, S. Genome-Wide identification of differentially expressed genes associated with the high yielding of oleoresin in secondary xylem of Masson pine (Pinus massoniana Lamb.) by transcriptomic analysis. PLoS ONE 2015, 7, e132624. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, Y.; Xu, L. Transcriptomic profiling reveals differentially expressed genes associated with pine wood nematode resistance in Masson pine (Pinus massoniana Lamb.). Sci. Rep. 2017, 1, 4693–4706. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tan, J.; Liang, X.; Tang, S.; Jia, J.; Yang, Z. Molecular mechanism of lateral bud differentiation of Pinus massoniana based on high-throughput sequencing. Sci. Rep. 2021, 11, 9033. [Google Scholar] [CrossRef]
- Fan, F.; Wang, Q.; Li, H.; Ding, G.; Wen, X. Transcriptome-wide identification and expression profiles of Masson Pine WRKY transcription factors in response to low phosphorus stress. Plant Mol. Biol. Report. 2021, 39, 1–9. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, H.; Jia, J.; Luo, Q.; Tang, S.; Li, K.; Wu, D.; Feng, Y. De novo assembly and discovery of metabolic pathways and genes that are involved in defense against pests in Songyun Pinus massoniana Lamb. Bangladesh J. Bot. 2016, 45, 855–863. [Google Scholar]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Yang, D.; Meng, Y.; Jin, J.; Gaom, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2020, 48, D1104–D1113. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Marc, G.; Hurwitz, D.I.; Marchler, G.H.; Song, J.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, 265–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Durinx, C. Expasy, the swiss bioinformatics resource portal, as designed by its users. Nucleic Acids Res. 2021, 49, 216–227. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H.B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, 585–587. [Google Scholar] [CrossRef]
- Swarbreck, D.; Wilks, C.; Lamesch, P.; Berardini, T.Z.; Garcia-Hernandez, M.; Foerster, H.; Li, D.; Meyer, T.; Muller, R.; Ploetz, L.; et al. The Arabidopsis Information Resource (TAIR): Gene structure and function annotation. Nucleic Acids Res. 2007, 36, 1009–1014. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Z.; Hu, Y.; Tan, J.; Jia, J.; Xu, H.; Chen, X. Reference genes selection for quantitative gene expression studies in Pinus massoniana L. Trees 2016, 30, 685–696. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Faraji, S.; Filiz, E.; Kazemitabar, S.K.; Vannozzi, A.; Palumbo, F.; Barcaccia, G.; Heidari, P. The AP2/ERF gene family in Triticum durum: Genome-wide identification and expression analysis under drought and salinity stresses. Genes 2020, 11, 1464. [Google Scholar] [CrossRef]
- Sharma, S.; Anuraj, C.; Heerendra, P.; Abhishek, W.; Raj, K.; Sneha, D. Identification, phylogeny and transcript profiling of ERF family genes during temperature stress treatment in Pea (Pisum sativum L.). J. Plant Biochem. Biotechnol. 2022, 31, 561–572. [Google Scholar] [CrossRef]
- Lv, K.; Li, J.; Zhao, K.; Chen, S.; Wei, H. Overexpression of an AP2/ERF family gene, BpERF13, in birch enhances cold tolerance through upregulating CBF genes and mitigating reactive oxygen species. Plant Sci. 2019, 292, 110375. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.; Zhang, J.; Wu, F.; Wang, X.; Pan, T.; Wei, Q.; Hao, Y.; Chen, X.; Jiang, C.; et al. Identification, classification, and characterization of AP2/ERF superfamily genes in Masson pine (Pinus massoniana Lamb.). Sci. Rep. 2021, 11, 5441. [Google Scholar] [CrossRef]
- Ohme-Takagi, M.; Shinshi, H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 1995, 2, 173–182. [Google Scholar]
- Han, D.; Han, J.; Xu, T.; Li, X.; Yao, C.; Li, T.; Sun, X.; Wang, X.; Yang, G. Overexpression of MbERF12, an ERF gene from Malus baccata (L.) Borkh, increases cold and salt tolerance in Arabidopsis thaliana associated with ROS scavenging through ethylene signal transduction. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 760–770. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, M.; Zhang, S.; Zhang, S.; Song, T.; Zhang, M.; Zhou, H.; Wang, Y.; Xiang, J.; Zhang, X. Transcriptomic identification of wheat AP2/ERF transcription factors and functional characterization of TaERF-6-3A in response to drought and salinity stresses. Int. J. Mol. Sci. 2022, 23, 3272. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, V.; Singh, S.K.; Thakur, S.; Siwach, P.; Sreenivasulu, Y.; Srinivasan, R.; Bhat, S.R. Promoter trapping and deletion analysis show Arabidopsis thaliana APETALA2 gene promoter is bidirectional and functions as a Pollen- and Ovule-specific promoter in the reverse orientation. Appl. Biochem. Biotechnol. 2017, 182, 1591–1604. [Google Scholar] [CrossRef]
- Ohto, M.; Floyd, S.K.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis. Sex. Plant Reprod. 2009, 22, 277–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, F.; Sakuma, Y.; Tran, L.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K.; et al. Arabidopsis DREB2A-Interacting proteins function as RING E3 ligases and negatively regulate plant drought Stress–responsive gene expression. Plant Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Mizoi, J.; Kanazawa, N.; Kidokoro, S.; Takahashi, F.; Qin, F.; Morimoto, K.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Heat-induced inhibition of phosphorylation of the stress-protective transcription factor DREB2A promotes thermotolerance of Arabidopsis thaliana. J. Biol. Chem. 2019, 294, 902–917. [Google Scholar] [CrossRef]
- Chen, K.; Tang, W.; Zhou, Y.; Chen, J.; Xu, Z.; Ma, R.; Dong, Y.; Ma, Y.; Chen, M. AP2/ERF transcription factor GmDREB1 confers drought tolerance in transgenic soybean by interacting with GmERFs. Plant Physiol. Biochem. 2022, 170, 287–295. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, R.S.; Liu, D.; Liu, X.; Wang, J.; Xiang, X.; Li, Y. The AP2 transcription factor NtERF172 confers drought resistance by modifying NtCAT. Plant Biotechnol. J. 2020, 18, 2444–2455. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Chen, L.; Huang, W.; Yu, D. Functional characterization of Arabidopsis thaliana WRKY39 in heat stress. Mol. Cells 2010, 5, 475–483. [Google Scholar] [CrossRef]
- Niu, F.; Cui, X.; Zhao, P.; Sun, M.; Jiang, Y. WRKY42 transcription factor positively regulates leaf senescence through modulating SA and ROS synthesis in Arabidopsis thaliana. Plant J. 2020, 104, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, F.; Fu, J.; Zhu, C.; Yan, J.; Li, X.; Meraj, T.A.; Shen, Q.; Hassan, B.; Wang, Q. Maize WRKY transcription factor ZmWRKY79 positively regulates drought tolerance through elevating ABA biosynthesis. Int. J. Mol. Sci. 2021, 22, 10080. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, B.; Chao, Q.; Wang, B.; Li, X. The Maize AP2/EREBP transcription factor ZmEREB160 enhances drought tolerance in Arabidopsis. Trop. Plant Biol. 2020, 13, 251–261. [Google Scholar] [CrossRef]
- Sun, X.; Wen, C.; Xu, J.; Wang, Y.; Zhu, J.; Zhang, Y. The apple columnar gene candidate MdCoL and the AP2/ERF factor MdDREB2 positively regulate ABA biosynthesis by activating the expression of MdNCED6/9. Tree Physiol. 2021, 41, 1065–1076. [Google Scholar] [CrossRef]
- Li, H.J.; Wang, H.B.; Chen, Y.; Ma, Q.P.; Chen, X. Isolation and expression profiles of class III PRX gene family under drought stress in Camellia sinensis. Biol. Plant. 2020, 64, 280–288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).