Abstract
There are few studies on the vertical distribution and seasonal activity of arthropods in open habitats (in glades) in temperate forests due to methodological problems associated with the arrangement of certain structures for trapping. However, glades in forests are places of significant biodiversity of native animals, which are attracted to such areas by the possibilities of nutrition, reproduction, and wintering. The research was carried out on the territory of the Republic of Mordovia (the center of the European part of Russia). Fermental traps (bait—fermenting beer with sugar) were used to collect Coleoptera. They were installed on a special structure at heights of 2, 4, 6, 8, and 10 m. The collections were carried out from May to October 2020 in a glade with an area of 0.93 hectares in a deciduous forest. At the end of the research, 745 specimens of 80 species were registered from 30 Coleoptera families. The greatest species diversity was recorded in Nitidulidae (11 species), Cerambycidae (10 species), Scarabaeidae (7 species), Elateridae, Coccinellidae, and Curculionidae (5 species each). The greatest species diversity (53 species) and numerical abundance were obtained at a height of 2 m, and the smallest one (16 species) at a height of 10 m. The largest differences in the Jaccard similarity index were obtained between samples from a height of 2 and 10 m. The maximum values of the Shannon index and the minimum values of the Simpson index were recorded at the height of 2 m. The most significant relative number of saproxylic species was obtained at a height of 4 m. The relative number of anthophilic species was minimal at a height of 10 m. The seasonal dynamics of Coleoptera abundance were the same at different heights and the highest abundance was observed in late May and early June. However, the seasonal dynamics were different for some Coleoptera species in the glade located and inside the forest. Our data confirm the relevance of sampling in open biotopes at different heights in the study of arthropod biodiversity.
1. Introduction
Coleoptera is one of the most species-rich and widespread insect orders worldwide [1]. They significantly contribute to the biodiversity of forest ecosystems and play an important role in the functioning and dynamics of ecosystems. Among them, there are numerous predators, phytophages, as well as other groups [2,3,4,5]. The significant species diversity of beetles allowed them to occupy a variety of biotopes in all ecosystems of the globe [6,7,8,9]. Among beetles, some species prefer forests of various types and can be found in these closed biotopes [10,11,12]. However, there are also species that prefer open biotopes [13,14,15].
According to Allison et al. [16], who conducted studies using semiochemical-baited traps, some species of Monochamus (Cerambycidae) are more abundant in glades near the forest than in the forest itself. The overall abundance and diversity of some families of wood-dwelling beetles (Buprestidae, Cerambycidae, Brentidae, and others) were higher in the center of open biotopes located in the forest than inside the forest [17]. Different species of Cerambycidae were caught in open biotopes at different distances from the forest, and traps with attractants collected more individuals. However, some species were not attracted to open biotopes, even to traps with bait, despite their abundance in the forest [18]. In the glades that appeared after logging, the species diversity of Coleoptera increased from the first to the third year after logging [19]. On the other hand, the abundance and species richness of Scolytidae and Cerambycidae were lower in glades in pine plantations than in closed biotopes and on the edges. However, the largest number of unique species were caught at the edge and glade [20]. The combination of open and closed biotopes played a positive role in increasing the number and species diversity of dung beetles [21].
Many studies have shown that in the forests, different groups of insects are distributed along different horizontal and vertical environmental gradients [22,23,24,25,26,27,28,29]. In natural continuous woodlands, there are separate open biotopes (meadows, glades), which are habitats for species that do not belong to forest inhabitants. Such a mosaic structure of the landscape has a very different impact on the settlement, behavior, life cycles of insects, and influences the dynamics of populations and the conservation of species on a broader scale [30,31,32,33]. However, if vertical stratification in temperate forests is under study and is becoming clearer, then the vertical distribution of insects is little known in open areas due to methodological problems associated with the construction of certain structures. Our research aims to determine the vertical stratification and seasonal dynamics of Coleoptera in a glade in a forest area using a simple structure. We used fermental traps, which are easy to make and equipped with baits.
2. Materials and Methods
2.1. Study Area and Sampling Procedures
All studies were conducted in the center of the European part of Russia (the Republic of Mordovia, Temnikov region, the territory of the Mordovia State Nature Reserve). The Mordovia State Nature Reserve is located on the right bank of the Moksha River and covers an area of 321.62 km2. This natural large forest area is located in the zone of coniferous-deciduous forests on the border with the forest steppe. Forest communities occupy 89.3% of the entire protected area, representing the largest refugium for threatened invertebrate species. However, there are some small areas of open biotopes in this forest. These are meadows and glades of different configurations, areas, and microclimatic characteristics. Usually, such areas are surrounded by forests [34,35,36].
The research installation is a hollow tube with a diameter of 72 mm, 10 m high from the soil surface (54°43′39″ N, 43°09′08″ E, Figure 1). There are transverse crossbars at every 2 m on this pipe. There are hanging traps on them. There were no obstacles to the movement of species between the hanging traps. Insects could freely change their height.

Figure 1.
A special installation for conducting research on the territory of the Mordovia State Nature Reserve.
The installation was located in a glade with an area of 0.93 hectares bounded by a lake on the south side. On the north, west, and east there is a border of mixed forest with Pinus sylvestris Linn., Quercus robur L., Populus tremula L., Tilia cordata Mill., Picea abies (L.) Karst., Betula pendula Ehrh., Betula alba L., and Alnus glutinosa L. Euonymus verrucose L., Lonicera xylosteum L., and Frangula alnus P.Mill. grow in the undergrowth. Prunus domestica L. and Pyrus communis L. grow on the border of the glade and the forest near small wooden buildings. Carex praecox Schre., Calamagrostis epigeios (L.) Roth, Bromopsis inermis (Leyss.) Holub, Elytrigia repens L., and Dactylis glomerate L. dominate in the grassy layer in the glade. In addition, there are Acinos arvensis (Lam.), Veronica prostrata L., Galium verum L., Galium mollugo L., Achillea millefolium L., Fragaria viridis Duch., Erigeron annuus L., and Conysa canadensis L. Verbascum Thapsus L., Rumex confertus Willd., Poa bulbosa L., and Alchemilla sp., and other herbaceous plants are single specimens [37].
Coleoptera was collected from May to October 2020, when insect activity was very high. All collections were carried out using traps of our own design. A five-leather plastic container with a window cut out on one side at a distance of 10 cm from the bottom was used as a trap [38]. Beer was used as bait. Sugar was added for fermentation. The collected samples were delivered in plastic bags containing 70% alcohol from the forest to the laboratory, then sorted and stored in alcohol. A total of 745 species have been studied.
2.2. Identification and Taxonomic Position of Samples
The classification of the family-group taxa used in this checklist follows Bouchard et al. [38] predominantly, with subsequent additions [39]. The changes are taken into account from the Catalog of Coleoptera of the Palearctic [40,41,42,43,44,45,46], as well as data on Cucujoidea from the research of Robertson et al. [47] on Curculionoidea from the research of Alonso-Zarazaga et al. [48]. The cited works were used to clarify the nomenclature, as well as the Catalog of Coleoptera of the Palearctic [49,50]. The years of the description of some species are specified according to Bousquet [51]. The identification of the species was carried out by L.V. Egorov.
2.3. Data Analyses
When analyzing the results of the studies, data were used only on the quantitative parameter (number) of all Coleoptera individuals in traps for exposure time. Saproxious species were determined using the approaches adopted by authors [52,53,54]. The anthophilic species were classified according to their own long-term observations. Jaccard similarity index was used to compare species similarity between study plots. We did not take into account insects that were not identified as species. Based on the collected data on the total number of Coleoptera for the season, we calculated the Shannon index and the Simpson index [55,56].
3. Results
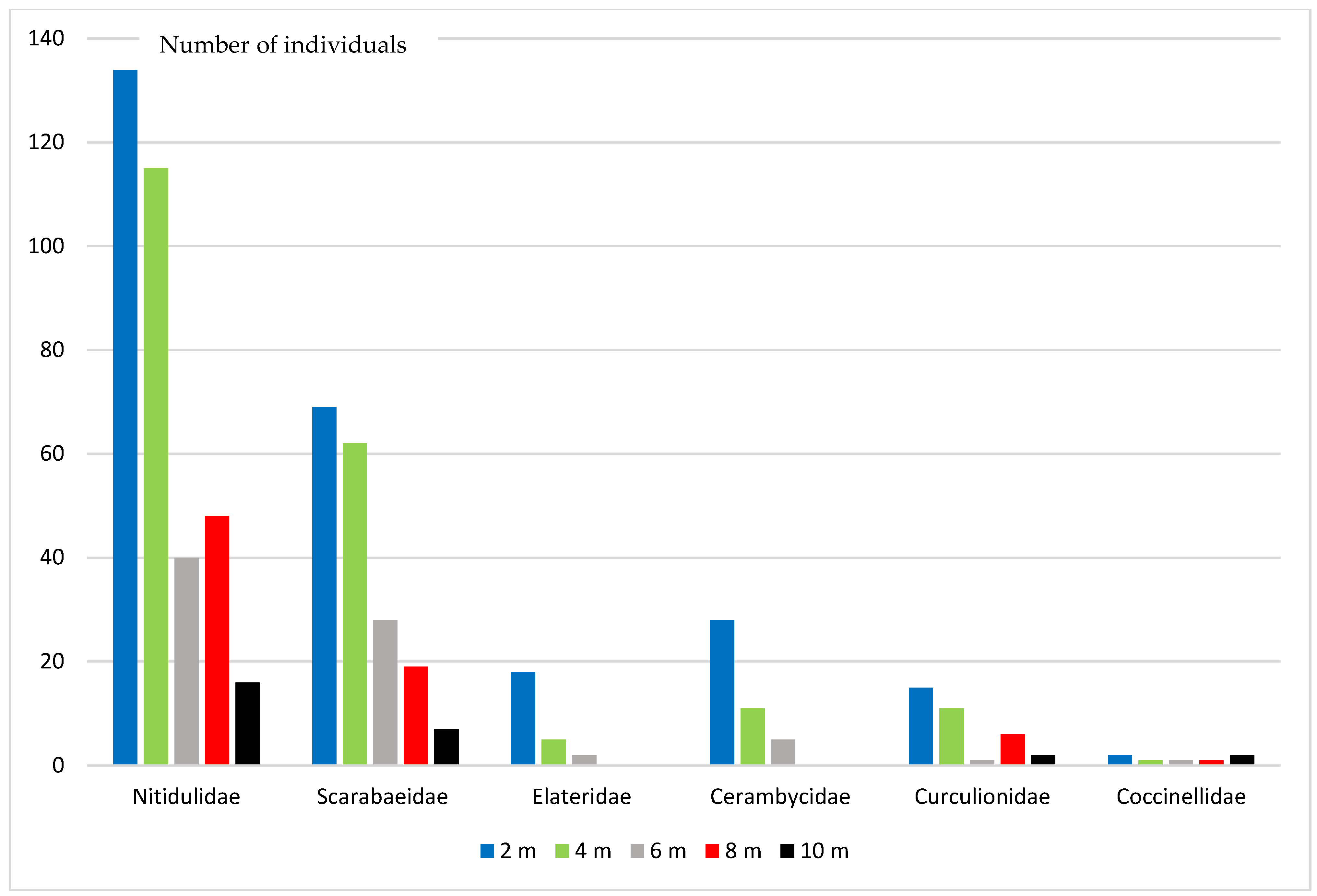
During the research, 80 species from 30 Coleoptera families were registered (Table 1, Figure 2). Some specimens from several families could not be identified as species. The greatest species diversity was registered in Nitidulidae (11 species), Cerambycidae (10 species), Scarabaeidae (7 species), Elateridae, Coccinellidae, Curculionidae (5 species each). Representatives of these families in total accounted for 87.1% of all studied specimens.

Table 1.
Species diversity and abundance of Coleoptera were collected using fermental traps at different heights on the territory of the Mordovia State Nature Reserve.
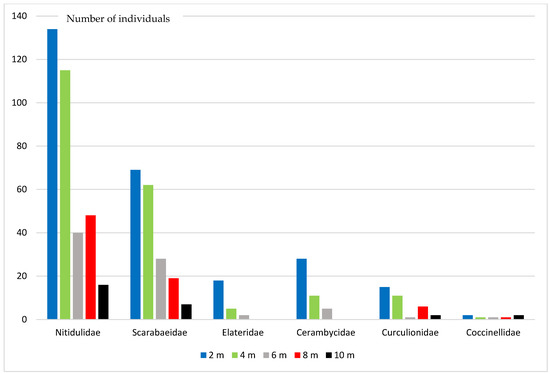
Figure 2.
Numerical abundance of some Coleoptera families at different heights.
Cryptarcha strigata (Fabricius, 1787) (total 228 specimens) and Protaetia marmorata (Fabricus, 1792) (113 specimens) had the largest numbers in beer traps. At all heights, only five species were observed in traps: P. marmorata, Malachius bipustulatus (Linnaeus, 1758), C. strigata, Cryptarcha undata (G.-A. Olivier, 1790), and Soronia grisea (Linnaeus, 1758).
The largest abundance of P. marmorata was registered at heights from 2 to 6 m, with a maximum abundance at a height of 4 m. The abundance of C. strigata was greatest at a height of 4 m and slightly smaller at a height of 2 m. The highest abundance of S. grisea was registered at a height of 2 m. The data has not been obtained on the abundance of M. bipustulatus and C. undata at different heights.
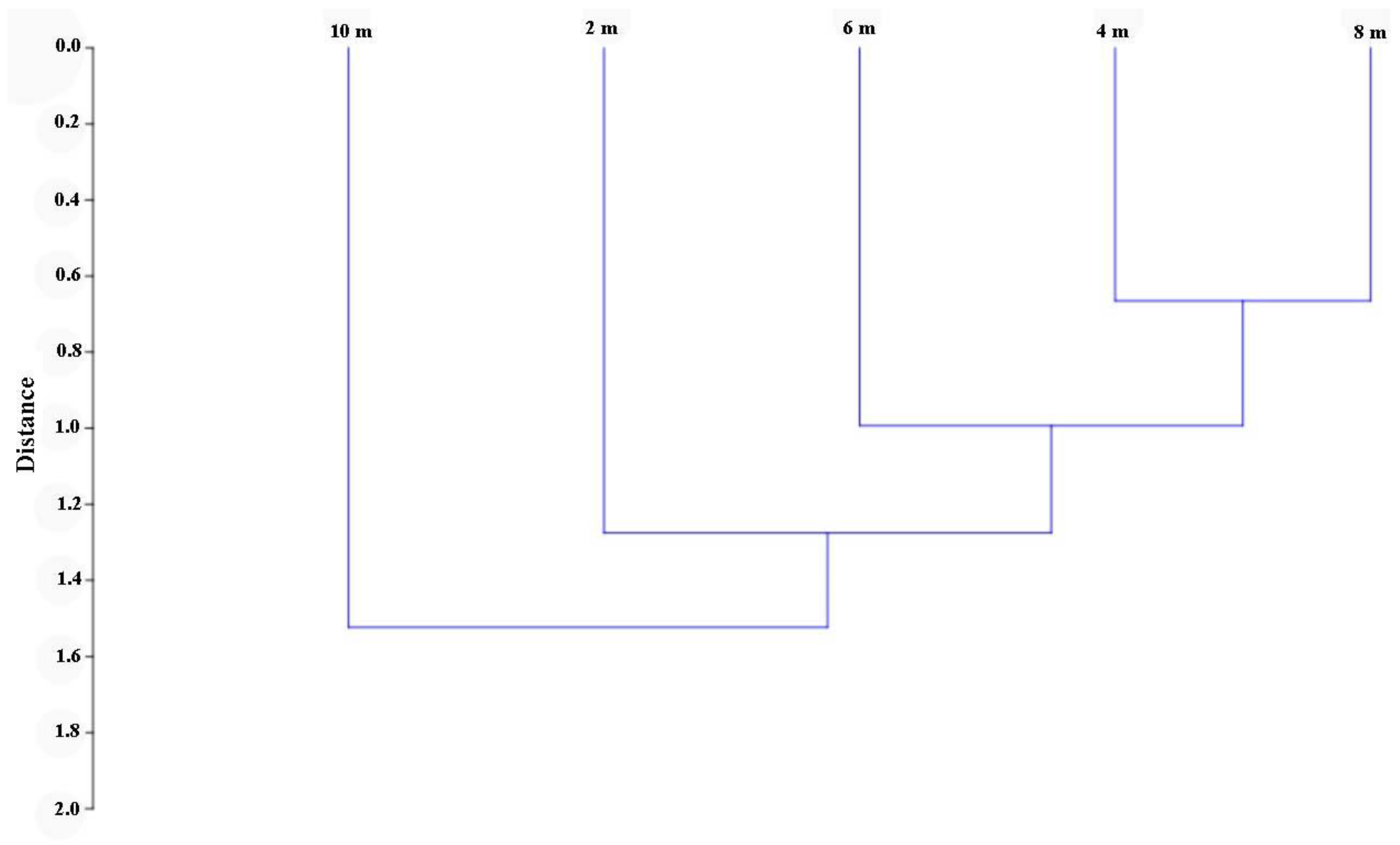
The calculation of the Jaccard similarity index demonstrated certain differences between the Coleoptera species caught at different heights (Figure 3). The greatest differences were obtained between samples from a height of 2 and 10 m. The differences were minimal between the heights of 4 and 8 m.
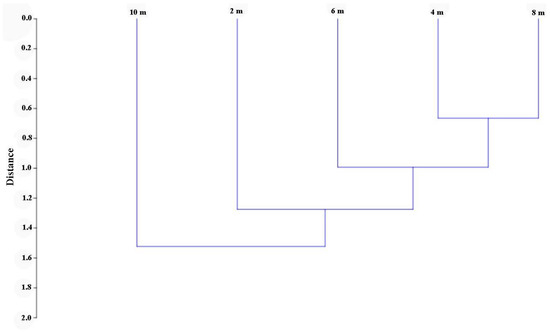
Figure 3.
The insect species composition similarity for four studied heights is based on the Jaccard similarity index by using Ward’s method and Euclidean distances as a measurement of Linkage Distance (cophenetic correlation coefficient = 0.690).
The greatest species diversity (53 species) was obtained at a height of 2 m and the smallest one (16 species)—at a height of 10 m (Table 1). According to the average number of specimens, the greatest one was observed at heights of 2 and 4 m (302 and 229 specimens, respectively). The minimum number of specimens was caught at a height of 10 m.
In total, we identified 48 saproxylic and 38 anthophilic beetle species. The most significant relative number of saproxylic beetle species was obtained at a height of 4 m. At other heights, the number of saproxylic species was almost the same. The relative number of anthophilic species was minimal at a height of 10 m. However, at other heights, it increased very much (Table 1).
The calculated Shannon and Simpson indices showed the following results. We obtained the maximum values of the Shannon index and the minimum values of the Simpson index at the heights of 2 m. Conversely, the minimum values of the Shannon index and the maximum values of the Simpson index are calculated for heights of 4 and 8 m. At other heights, the average values are obtained between these indicators (Table 1).
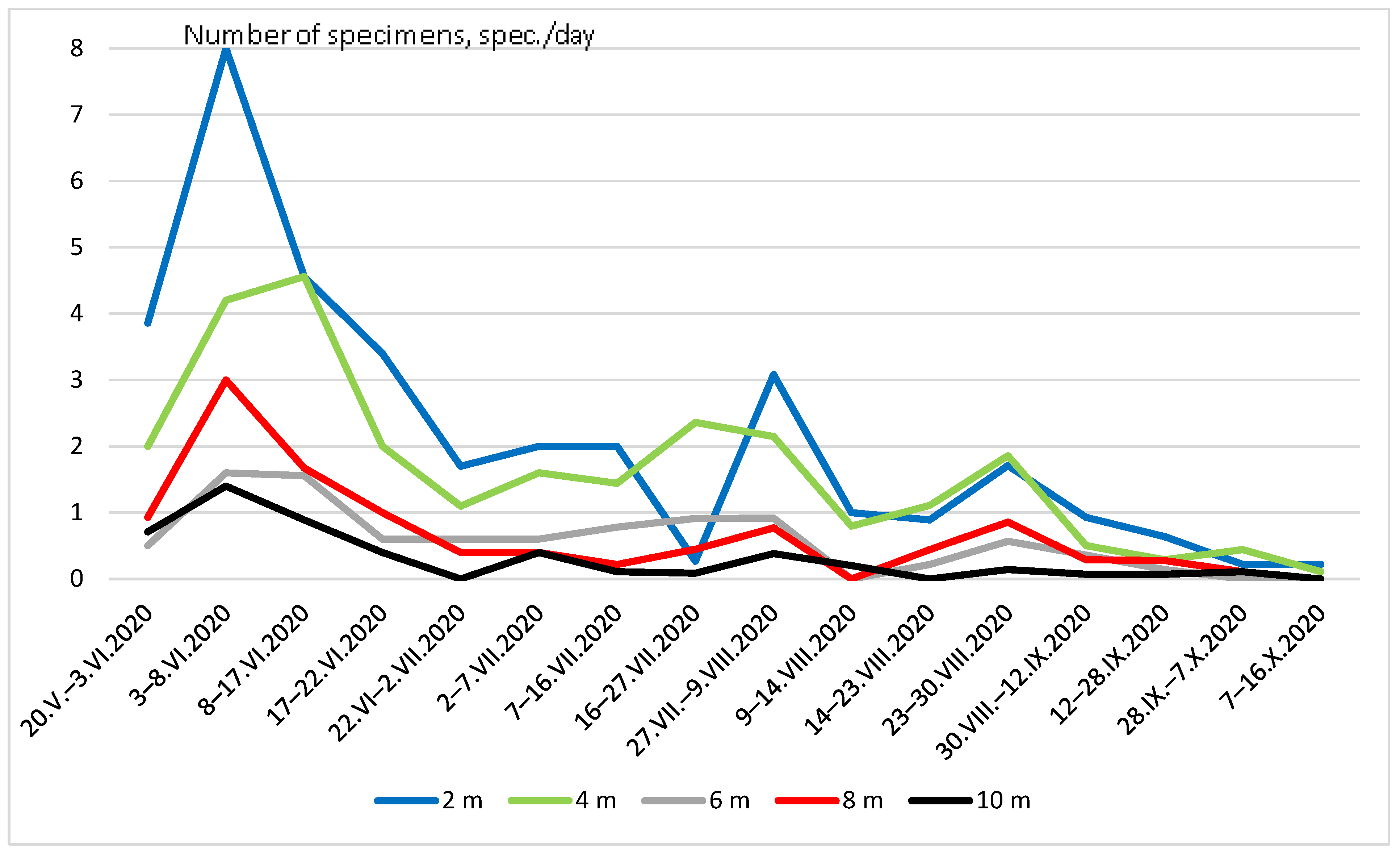
Seasonal dynamics of Coleoptera abundance were the same at different heights.
(Figure 4). The greatest peak in numbers was recorded in late May and early June. A smaller peak in numbers occurred from the end of July to the beginning of August.
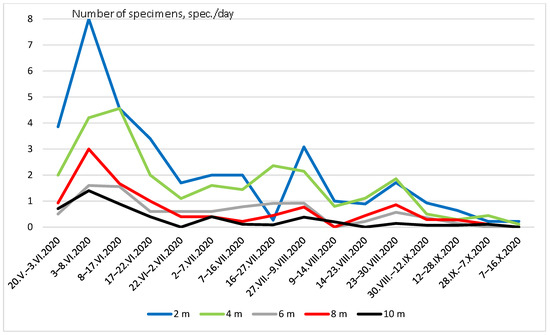
Figure 4.
Seasonal dynamics of Coleoptera abundance at different heights.
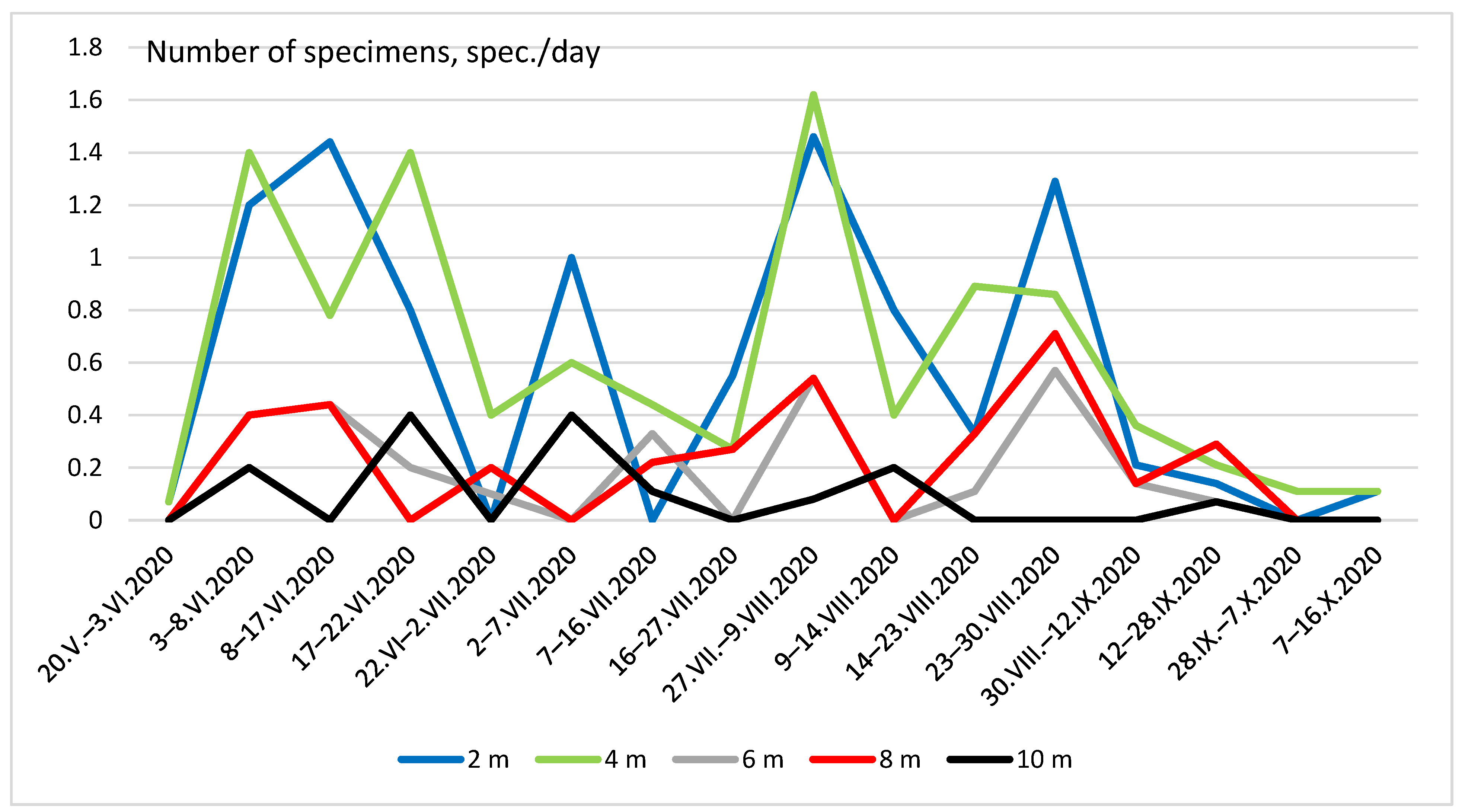
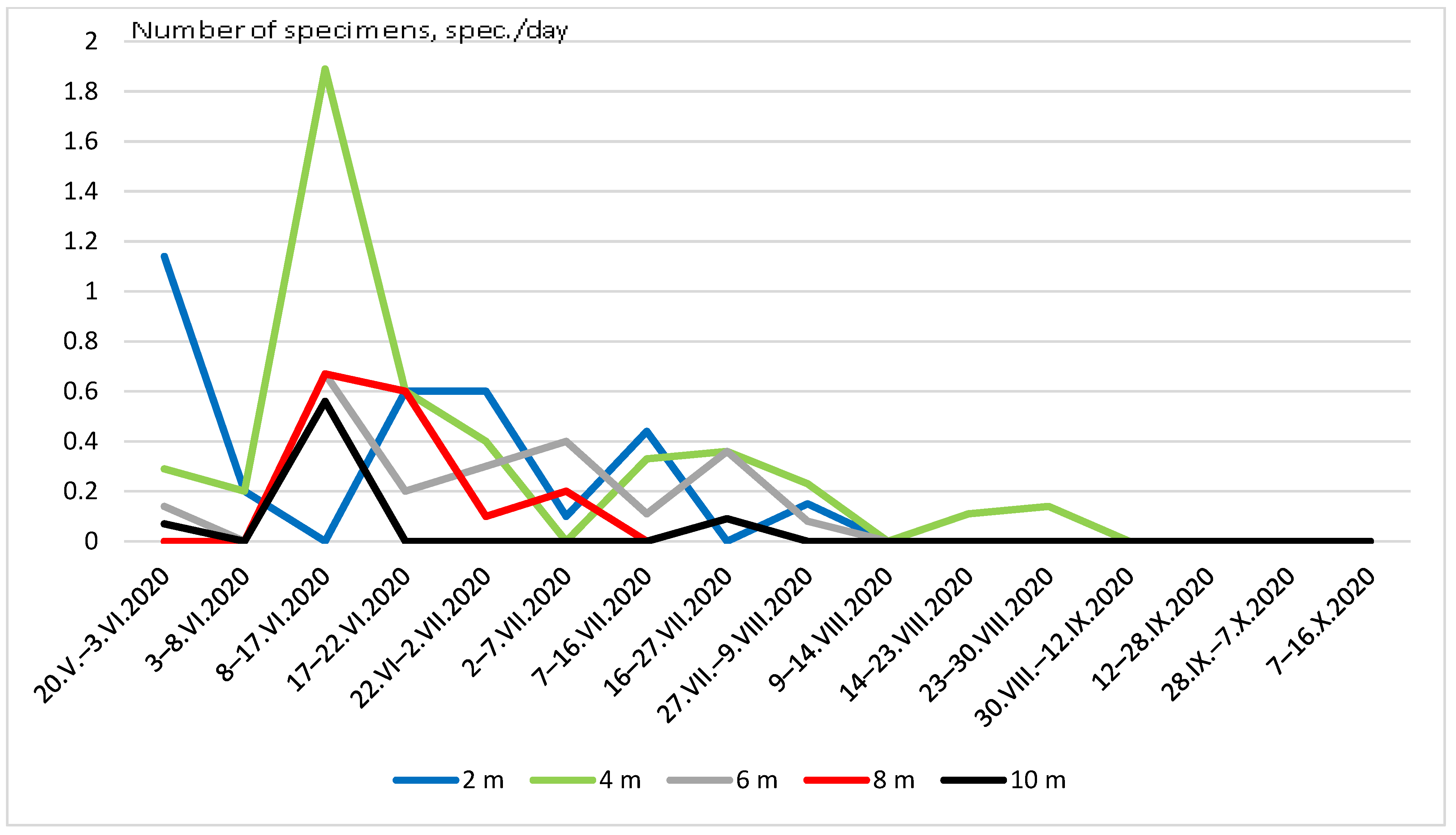
Figure 5 and Figure 6 show the seasonal dynamics of the abundance of the two most widespread species (C. strigata and P. marmorata) at different heights. In the first species, the population dynamics did not show a clear pattern and there were several maxima and minima practically at all heights (Figure 5). The graph of the total seasonal abundance of C. strigata at all heights (Figure 7) shows the same. Nevertheless, the largest number of the species was observed mainly in early summer (June) and August–early September.
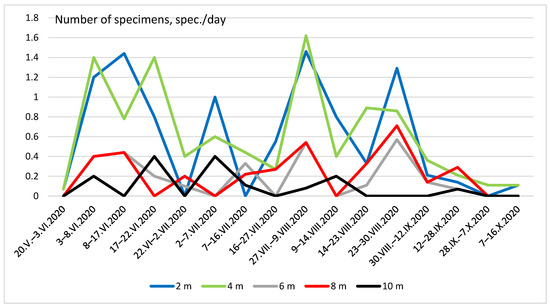
Figure 5.
Seasonal dynamics of Cryptarcha strigata abundance at different heights.
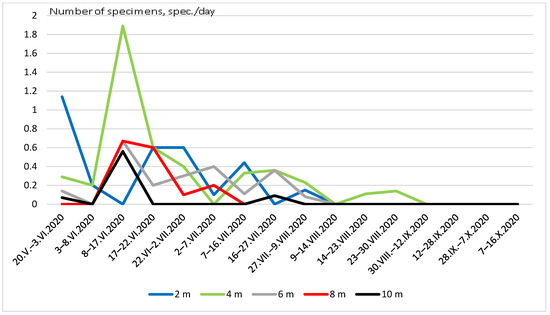
Figure 6.
Seasonal dynamics of Protaetia marmorata abundance at different heights.
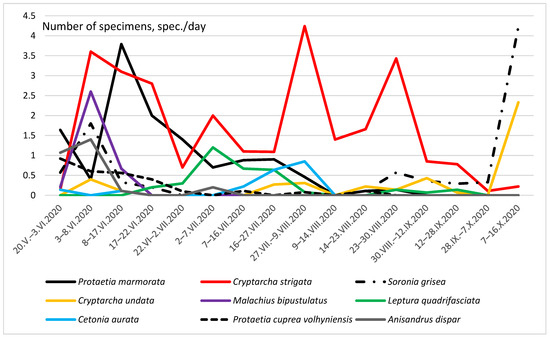
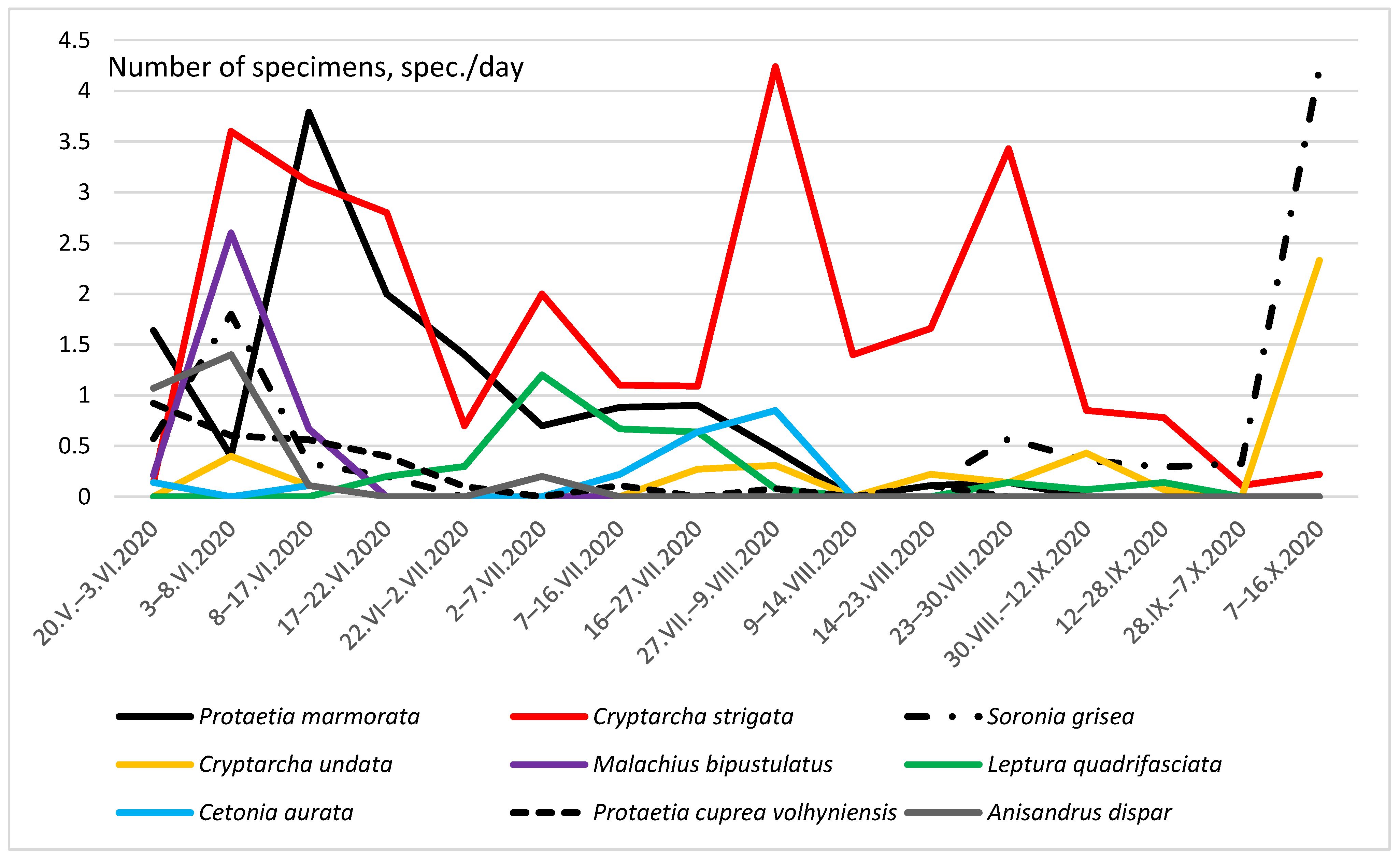
Figure 7.
The total seasonal dynamics of the number of nine Coleoptera taxon in a glade in the forest.
The seasonal dynamics of P. marmorata abundance were more regular (Figure 6 and Figure 7). The maximum number was recorded in mid-June, and then it decreased. In August, isolated individuals were observed in traps; since September, this species has not been observed.
Figure 7 shows the seasonal total dynamics of nine species, whose number exceeded 20 specimens in traps for the entire season. The first individuals of Cetonia aurata (Linnaeus, 1758) appeared in traps already in May. However, this species reached its greatest number in traps only in the second half of July. After 9 August, this species was no longer found in traps. The maximum abundance of Protaetia cuprea volhyniensis (Gory & Percheron, 1833) was recorded in May. Then there was a gradual decrease in the number of the species and from 23 August, it stopped falling into traps. Seasonal dynamics of the number of C. undata was three-vertex in beer traps. The maximum numbers were observed in early June, late July–August, and in the first half of October.
Seasonal dynamics of S. grisea abundance in the glade was two-vertex. The first smaller peak in numbers was detected in the first half of June; the second and more significant one occurred in the first half of October. M. bipustulatus was caught in beer traps from 20 May to 17 June, with a peak in the first decade of June. In the subsequent time, this species was not observed in traps. The first individuals of Leptura quadrifasciata Linnaeus, 1758 appeared in traps in mid-June and in the first half of July, the peak of the number of this species was already noted. Single specimens were also trapped in August and September. The peak number of Anisandrus dispar (Fabricius, 1792) occurred in the first half of June.
4. Discussion
Our study shows the idea of the diversity, height preferences, and seasonal dynamics of Coleoptera in open biotopes (in glades) in the forest ecosystems of European Russia. Different species of Coleoptera fall into beer traps set at different heights, but most of them are species that fly to the wandering bait of beer and sugar. Some authors [57,58,59] indicate that for traps similar to ours, alcoholic fermentation is a key process for attracting beetles, which in the wild are attracted by fermented tree sap. In recent years, a lot of information has accumulated about the height preference of Coleoptera in various forest layers in the temperate zone, from the undergrowth to tree crowns [60,61,62,63,64,65,66].
In our research, vertical stratification was studied in an open biotope in a glade in the forest. It was found that the number and species diversity of Coleoptera is higher when the trap is installed at a height of 2 m. This is consistent with our previous studies conducted in nearby deciduous forests [67]. The Shannon index was higher near the ground than at high heights. Similar results were obtained in the studies of Ulyshen and Hanula [68]. Thus, at high heights in the open biotope, there is a small species diversity of Coleoptera and one or several species dominate.
In deciduous forests, C. strigata imagos were mainly found at medium heights (3.5–7.5 m) with relatively high numbers [67]. In our studies, the abundance of C. strigata was greatest in the glade at a height of 4 m and lower at a height of 2 m. Imagos are usually found on the trunks of oaks and aspens near the flowing brooding sap. In biotopes with the predominance of these tree species, the abundance of C. strigata is greatest [69,70]. Thus, both inside forest biotopes and in open biotopes, C. strigata prefers to stick to small heights and does not move too high or low.
In our studies in a glade in the forest, the highest number of S. grisea was obtained at a height of 2 m and, partly, at a height of 4 m. Earlier S. grisea was reported to be more common inside deciduous forests at a height of 3.5 m [70]. Thus, this species prefers the lower layers (undergrowth level) in closed and open biotopes.
Imago P. marmorata had clear preferences in relation to the highest layer of the deciduous forest (tree crowns) [70]. However, in the glade, the largest number of P. marmorata was obtained at heights from 2 to 6 m, with a maximum number at a height of 4 m. The larvae of this species develop in the hollows of dead deciduous trees [71,72]. Imagos are anthophiles and are often found on flowering plants and shrubs [73,74]. Apparently, the anthophilicity of this species is due to the high-height preference in open habitats. Most of the flowers that beetles visit are located at a height of no more than 1.5–2 m and partially up to 3 m on some shrubs (mountain ash, bird cherry).
The largest sum of saproxylic and anthophilic species was obtained at a height of 2 m, and the smallest number of these species was obtained at a height of 10 m. However, the calculation with respect to the sum of species at a certain height showed that the number of saproxylic species was almost the same at heights of 2, 6, 8, and 10 m. Thus, saproxylic species prefer a height of 4 m to a greater extent and are equally found at other heights in open biotopes.
Seasonal cycles of Coleoptera activity have been studied for a long time and in various ways. There are many publications in which the authors describe in detail the seasonal activity of many species and families [10,75,76,77,78]. One publication estimates the seasonal dynamics of Cerambycidae abundance in the Cerrado of Distrito Federal (Brazil) when caught on fermented sugar cane juice with the addition of various fruits [79]. In fact, this fermenting liquid is similar to our bait. It turned out that the largest number of individuals and species is observed shortly after the first rains, especially in November. The authors also noted that this type of bait proved to be effective for collecting insects, comparable in effectiveness to synthetic baits that are commonly used in such cases [79]. Similar studies can also be successfully carried out in temperate forests [70].
The peak number of P. marmorata was observed in mid-June, and later the number decreased. Only at some heights were there isolated individuals. However, since September, this species has not been noted in traps. It was previously shown that in forest biotopes, the maximum number is observed in the second half of May and June, and after it decreases. At the same time, P. marmorata individuals have not been observed in all forest biotopes since the end of July [70]. Apparently, the first imagos appear inside forest biotopes and subsequently migrate to open biotopes for feeding. They are anthophiles, which is why they prefer open glades with a variety of herbaceous plants. This fact explains greater activity in open stations (until the end of August) than in closed forest biotopes, where they have not been found since the end of July.
Interesting results were obtained on the dynamics of the abundance of C. aurata. The species lives in different biotopes, more often in open ones (meadows, glades, roadsides, etc.). It is quite common on the flowers of plants from the families Umbelliferae, Rosacea, and Asteraceae, where it feeds on pollen and nectar [80,81]. According to our observations, it appears on flowering plants in mid-May and occurs until September [82]; unpublished information]. It often falls into beer traps [83]. Thus, the first appearance of individuals of this species (20 May) in traps is quite logical. However, then its number in traps dropped sharply to zero, and it began to be caught again only from 7 July, peaking in the second half of July. After 9 August, this species was no longer found in traps. At the same time, the number of flowering plants in the glade near the traps was quite high until August. Probably, C. aurata, in general, attracts worse in crown traps because it is mainly anthophilic.
In forest biotopes, the peak abundance of C. strigata was observed in early June, and solitary imagos were found throughout the season in different biotopes [70]. However, in the open biotope, the number of these species varied very significantly, with sharp rises and falls in the number of individuals in traps. The largest number of the species is observed mainly in early summer (June) and August–early September. It is possible that this species gives more than one generation per season, which is reflected in the graph of its abundance.
The main peak of S. grisea abundance within forest biotopes was observed in late May–early June. At the same time, a small number of beetles were active in late August and September [70]. However, the seasonal dynamics were different in the glade. If the spring and early summer population peaks were obvious, then in the first half of October, we observed a more significant increase in the number. It can be explained by the fact that the species migrates to wintering grounds. We assume that at this time, at relatively high air temperatures, it is the open biotopes that are warming up better and this species is activated in such places. To a certain extent, a similar regularity in seasonal dynamics was also found in C. undata. The greatest number of these species also occurred in the first half of October.
Adults of M. bipustulatus are predators and feed on small insects living on flowers; their larvae feed on the nymphs of some xylophagous insects [84]. Apparently, its entry into beer traps is accidental and is associated with predation on small insects lured by fermented liquid.
The number of anthophilic longicorns L. quadrifasciata was not very high in traps. It had a single peak with a maximum in mid-June. This species lives in a wide range of biotopes. Larvae develop in dead or rotting wood, especially in the lower parts of standing trees, stumps, fallen trunks, and branches of various trees. Usually, the imago of this species prefers flowering plants growing in meadows, glades, and forest edges [83,85]. The activity of the bark beetle A. dispar was recorded in spring-early summer. Since mid-June, this species has not been observed in traps. Similar activity was recorded in forest biotopes [70].
This study showed that glades in the natural undisturbed forests of the temperate zone are a place of clusters of many species of Coleoptera. At the same time, the maximum species diversity and abundance fall on the surface layer (at a height of 2 m). It is believed that isolated glades in forests do not have significant species diversity and numerical abundance of Coleoptera, unlike glades that are connected to the edges of forests by certain corridors. This is due to the lack of corridors for migrations of flying Coleoptera species [19]. We point out that our study took place simultaneously with the study of the vertical stratification of Coleoptera in the forests of the temperate zone of the European part of Russia and the sites for this were located in the same forest ecosystem [67]. However, the data indicate that the species diversity of Coleoptera is also very significant in isolated glades in undisturbed forests and the range of species is different from that in the inner parts of the forest (closed biotopes). In addition, this study showed that some Coleoptera species could change their height preference and seasonal population dynamics can change.
5. Conclusions
As a result of studies in a glade in a mixed forest of the temperate zone of the European part of Russia, 80 species from 30 Coleoptera families were identified at different heights. The greatest species diversity was recorded in Nitidulidae (11 species), Cerambycidae (10 species), Scarabaeidae (7 species), Elateridae, Coccinellidae, Curculionidae (5 species each). The greatest species diversity (53 species) and numerical abundance were obtained at a height of 2 m, the smallest one (16 species)—at a height of 10 m. The values of the Shannon index and the Simpson index confirm that. The most significant relative number of species of saproxylic species was obtained at a height of 4 m. The relative number of anthophilic species was significantly lower at a height of 10 m. The seasonal dynamics of Coleoptera abundance were the same at different heights, and the greatest abundance was observed in late May and early June. However, the seasonal dynamics in the glade in the forest and inside the forest were different for some Coleoptera species.
Author Contributions
Conceptualization, A.B.R. and L.V.E.; methodology, A.B.R.; software, A.B.R.; validation, A.B.R. and L.V.E.; formal analysis, A.B.R. and L.V.E.; investigation, A.B.R.; resources, A.B.R.; data curation, L.V.E.; writing—original draft preparation, A.B.R.; writing—review and editing, L.V.E.; visualization, L.V.E.; supervision, A.B.R.; project administration, A.B.R.; funding acquisition, A.B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This article was funded by the Russian Science Foundation, grant number 22-14-00026.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to A.A. Khapugin (Saransk, Tyumen) and E.V. Ershkova (Saransk) for their help in conducting a statistical analysis of the results obtained and in describing the vegetation in the studied glade.
Conflicts of Interest
Not applicable.
References
- Bouchard, P.; Bousquet, Y.; Davies, A.E.; Alonso-Zarazaga, M.A.; Lawrence, J.F.; Lyal, C.H.C.; Newton, A.F.; Ried, C.A.M.; Schmitt, M.; Ślipiński, S.A.; et al. Family-group names in Coleoptera (Insecta). ZooKeys 2011, 88, 1–972. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Johansson, T.; Jonsson, B.G.; Hjältén, J.; Edman, M.; Ericson, L. Landscape and substrate properties affect species richness and community composition of saproxylic beetles. For. Ecol. Manag. 2012, 286, 108–120. [Google Scholar] [CrossRef]
- Dedyukhin, S.V. Phytophagous beetles (Coleoptera: Chrysomelidae and Curculionoidea), protected and recommended for protection in the regions of the Middle Volga and the Urals. Nat. Conserv. Res. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Glotov, S.V.; Hushtan, K.V. Rove beetles of the subfamily Aleocharinae (Coleoptera: Staphylinidae) from the Hutsulshchyna National Nature Park. Biosyst. Divers. 2020, 28, 364–369. [Google Scholar] [CrossRef]
- Sundukov, Y.N.; Makarov, K.V. The ground beetles of the tribus Trechini (Carabidae) on the Southern Kuril Islands. Nat. Conserv. Res. 2021, 6, 15–51. [Google Scholar] [CrossRef]
- Prokin, A.A.; Sazhnev, A.S.; Philippov, D.A. Water beetles (Insecta: Coleoptera) of some peatlands in the North Caucasus. Nat. Conserv. Res. 2019, 4, 57–66. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Zamotajlov, A.S.; Belyi, A.I.; Khomitskiy, E.E. Fauna and ecological characteristics of ground beetles (Coleoptera, Carabidae) of the Nature Sanctuaries «Prichernomorskiy» and «Tuapsinskiy» (Russia). Nat. Conserv. Res. 2020, 5, 66–85. [Google Scholar] [CrossRef]
- Khalimov, F. The ground beetles (Coleoptera, Carabidae) of the Karatepa and Chakilkalyan mountains (west part of Zarafshan Mountains Range, Uzbekistan). Biosyst. Divers. 2020, 28, 265–271. [Google Scholar] [CrossRef]
- Avtaeva, T.A.; Sukhodolskaya, R.A.; Brygadyrenko, V.V. Modeling the bioclimatic range of Pterostichus melanarius (Coleoptera, Carabidae) in conditions of global climate change. Biosyst. Divers. 2021, 29, 140–150. [Google Scholar] [CrossRef]
- Zamotajlov, A.S.; Serdyuk, V.Y.; Khomitskiy, E.E.; Belyi, A.I. New data on distribution and biology of some rare ground beetles (Coleoptera, Carabidae) in South Russia. Nat. Conserv. Res. 2019, 4, 81–90. [Google Scholar] [CrossRef]
- Kabak, I.I.; Liang, H.-B. An annotated list of the genus Chlaenius Bonelli, 1810 (Coleoptera: Carabidae) of Xinjiang Uygur Autonomous Region of China. Far East. Entomol. 2021, 429, 12–28. [Google Scholar] [CrossRef]
- Tomaszewska, W.; Egorov, L.V.; Ruchin, A.B.; Vlasov, D.V. First record of Clemmus troglodytes (Coleoptera: Coccinelloidea, Anamorphidae) for the fauna of Russia. Nat. Conserv. Res. 2018, 3, 103–105. [Google Scholar] [CrossRef]
- Gobbi, M.; Fontaneto, D. Biodiversity of ground beetles (Coleoptera: Carabidae) in different habitats of the Italian Po lowland. Agric. Ecosyst. Environ. 2008, 127, 273–276. [Google Scholar] [CrossRef]
- Sergeev, M.E. Species composition and biotopic distribution of leaf beetles (Coleoptera: Megalopodidae, Chrysomelidae) in the Sikhote-Alin State Nature Reserve (Russia). Nat. Conserv. Res. 2020, 5, 80–88. [Google Scholar] [CrossRef]
- Asbeck, T.; Großmann, J.; Paillet, Y.; Winiger, N.; Bauhus, J. The use of tree-related microhabitats as forest biodiversity indicators and to guide integrated forest management. Curr. For. Rep. 2021, 7, 59–68. [Google Scholar] [CrossRef]
- Allison, J.; Strom, B.; Sweeney, J.; Mayo, P. Trap deployment along linear transects perpendicular to forest edges: Impact on capture of longhorned beetles (Coleoptera: Cerambycidae). J. Pest Sci. 2019, 92, 299–308. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Hanula, J.L.; Horn, S.; Kilgo, J.C.; Moorman, C.E. Spatial and temporal patterns of beetles associated with coarse woody debris in managed bottomland hardwood forests. For. Ecol. Manag. 2004, 199, 259–272. [Google Scholar] [CrossRef]
- Dunn, E.; Hough-Goldstein, J.; Hanks, L.M.; Millar, J.G.; D’Amico, V. Range of attraction of pheromone lures and dispersal behavior of cerambycid beetles. Ann. Entomol. Soc. Am. 2016, 109, 872–880. [Google Scholar] [CrossRef]
- Kozel, P.; Sebek, P.; Platek, M.; Benes, J.; Zapletal, M.; Dvorsky, M.; Lanta, V.; Dolezal, J.; Bace, R.; Zbuzek, B.; et al. Connectivity and succession of open structures as a key to sustaining light-demanding biodiversity in deciduous forests. J. Appl. Ecol. 2021, 58, 2951–2961. [Google Scholar] [CrossRef]
- Dodds, K. Effects of habitat type and trap placement on captures of bark (Coleoptera: Scolytidae) and longhorned (Coleoptera: Cerambycidae) beetles in semiochemical-baited traps. J. Econ. Entomol. 2011, 104, 879–888. [Google Scholar] [CrossRef]
- Estrada, A.; Coates-Estrada, R. Dung beetles in continuous forest, forest fragments and in an agricultural mosaic habitat island at Los Tuxtlas, Mexico. Biodivers. Conserv. 2002, 11, 1903–1918. [Google Scholar] [CrossRef]
- Schroeder, B.; Buddle, C.; Saint-Germain, M. Activity of flying beetles (Coleoptera) at two heights in canopy gaps and intact forests in a hardwood forest in Quebec. Can. Entomol. 2009, 141, 515–520. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Arthropod vertical stratification in temperate deciduous forests: Implications for conservation-oriented management. For. Ecol. Manag. 2011, 261, 1479–1489. [Google Scholar] [CrossRef]
- Kirstová, M.; Pyszko, P.; Šipoš, J.; Drozd, P.; Kočárek, P. Vertical distribution of earwigs (Dermaptera: Forficulidae) in a temperate lowland forest, based on sampling with a mobile aerial lift platform. Entomol. Sci. 2017, 20, 57–64. [Google Scholar] [CrossRef]
- Sheehan, T.N.; Ulyshen, M.D.; Horn, S.; Hoebeke, E.R. Vertical and horizontal distribution of bark and woodboring beetles by feeding guild: Is there an optimal trap location for detection? J. Pest Sci. 2019, 92, 327–341. [Google Scholar] [CrossRef]
- Weiss, M.; Didham, R.K.; Procházka, J.; Schlaghamerský, J.; Basset, Y.; Odegaard, F.; Tichechkin, A.; Schmidl, J.; Floren, A.; Curletti, G.; et al. Saproxylic beetles in tropical and temperate forests—A standardized comparison of vertical stratification patterns. For. Ecol. Manag. 2019, 444, 50–58. [Google Scholar] [CrossRef]
- Makarkin, V.N.; Ruchin, A.B. Materials on the Neuroptera and Raphidioptera fauna in Mordovia and adjacent regions of European Russia. Proc. Mordovia State Nat. Reserve 2020, 24, 161–181. [Google Scholar]
- Perry, K.I.; Sivakoff, F.S.; Wallin, K.F.; Wenzel, J.W.; Herms, D.A. Forest disturbance and arthropods: Small-scale canopy and understory disturbances alter movement of mobile arthropods. Ecosphere 2021, 12, e03771. [Google Scholar] [CrossRef]
- Urban-Mead, K.R.; Muñiz, P.; Gillung, J.; Espinoza, A.; Fordyce, R.; van Dyke, M.; McArt, S.H.; Danforth, B.N. Bees in the trees: Diverse spring fauna in temperate forest edge canopies. For. Ecol. Manag. 2021, 482, 118903. [Google Scholar] [CrossRef]
- Hanski, I. Habitat fragmentation and species richness. J. Biogeogr. 2015, 42, 989–993. [Google Scholar] [CrossRef]
- Ekström, A.L.; Bergmark, P.; Hekkala, A.M. Can multifunctional forest landscapes sustain a high diversity of saproxylic beetles? For. Ecol. Manag. 2021, 490, 119107. [Google Scholar] [CrossRef]
- Kärvemo, S.; Jönsson, M.; Hekkala, A.M.; Sjögren, J.; Strengbom, J. Multi-taxon conservation in northern forest hot-spots: The role of forest characteristics and spatial scales. Landsc. Ecol. 2021, 36, 989–1002. [Google Scholar] [CrossRef]
- Cicort-Lucaciu, A.Ș. Road-killed ground beetles prove the presence of Carabus hungaricus (Coleoptera: Carabidae) in North-Western Romania. Nat. Conserv. Res. 2020, 5, 134–138. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Makarkin, N.V. Neuroptera and Raphidioptera in the Mordovia State Nature Reserve. Nat. Conserv. Res. 2017, 2, 38–46. [Google Scholar] [CrossRef][Green Version]
- Ruchin, A.B.; Khapugin, A.A. Red Data Book Invertebrates in a Protected Area of European Russia. Acta Zool. Acad. Sci. Hung. 2019, 65, 349–370. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Alekseev, S.K.; Khapugin, A.A. Post-fire fauna of carabid beetles (Coleoptera, Carabidae) in forests of the Mordovia State Nature Reserve (Russia). Nat. Conserv. Res. 2019, 4 (Suppl. S1), 11–20. [Google Scholar] [CrossRef]
- Chugunov, G.G.; Vargot, E.V.; Khapugin, A.A. Materials for the flora of the Mordovia State Nature Reserve named after P.G. Smidovich (Report 3). Proc. Mordovia State Nat. Reserve 2017, 19, 220–228. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A.; Vikhrev, N.E.; Esin, M.N. The use of simple crown traps for the insects collection. Nat. Conserv. Res. 2020, 5, 87–108. [Google Scholar] [CrossRef]
- Bouchard, P.; Bousquet, Y. Additions and corrections to “Family-group names in Coleoptera (Insecta)”. ZooKeys 2020, 922, 65–139. [Google Scholar] [CrossRef]
- Löbl, I.; Smetana, A. (Eds.) Curculionoidea I. In Catalogue of Palaearctic Coleoptera; Apollo Books: Stenstrup, Denmark, 2011; Volume 7, p. 373. [Google Scholar]
- Löbl, I.; Smetana, A. (Eds.) Curculionoidea II. In Catalogue of Palaearctic Coleoptera; Apollo Books: Stenstrup, Denmark, 2013; Volume 8, p. 707. [Google Scholar]
- Löbl, I.; Löbl, D. (Eds.) Revised and Updated Version. Hy-Drophiloidea–Staphylinoidea. In Catalogue of Palaearctic Coleoptera; Brill: Leiden, The Netherlands; Boston, MA, USA, 2015; Volume 2/1, p. 1702. [Google Scholar]
- Löbl, I.; Löbl, D. (Eds.) Revised and Updated Version. Scara-Baeoidea–Scirtoidea–Dascilloidea–Buprestoidea–Byrrhoidea. In Catalogue of Palaearctic Coleoptera; Brill: Leiden, The Netherlands; Boston, MA, USA, 2016; Volume 3, p. 983. [Google Scholar]
- Löbl, I.; Löbl, D. (Eds.) Revised and Updated Version. Archostemata–Adephaga–Myxophaga. In Catalogue of Palaearctic Coleoptera; Brill: Leiden, The Netherlands; Boston, MA, USA, 2017; Volume 1, p. 1443. [Google Scholar]
- Iwan, D.; Löbl, I. (Eds.) Revised and Updated Second Edition. Tenebrionoidea. In Catalogue of Palaearctic Coleoptera; Brill: Leiden, The Netherlands; Boston, MA, USA, 2020; Volume 5, p. 945. [Google Scholar]
- Danilevsky, M. (Ed.) Updated and Revised Second Edition. Chrysomeloidea I (Vesperidae, Disteniidae, Cerambycidae). In Catalogue of Palaearctic Coleoptera; Brill: Leiden, The Netherlands; Boston, MA, USA, 2020; Volume 6/1, p. 712. [Google Scholar]
- Robertson, J.; Ślipiński, A.; Moulton, M.; Shockley, F.W.; Giorgi, A.; Lord, N.P.; McKenna, D.D.; Tomaszewska, W.; Forrester, J.; Miller, K.B.; et al. Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Syst. Entomol. 2015, 40, 745–778. [Google Scholar] [CrossRef]
- Alonso-Zarazaga, M.A.; Barrios, H.; Borovec, R.; Bouchard, P.; Caldara, R.; Colonnelli, E.; Gültekin, L.; Hlaváč, P.; Korotyaev, B.; Lyal, C.H.C.; et al. Cooperative Catalogue of Palaearctic Coleoptera Curculionoidea. Monogr. Electrón. SEA 2017, 8, 1–729. [Google Scholar]
- Löbl, I.; Smetana, A. (Eds.) Elateroidea–Derodontoidea–Bostrichoidea–Lymexyloidea–Cleroidea–Cucujoidea. In Catalogue of Palaearctic Coleoptera; Apollo Books: Stenstrup, Denmark, 2007; Volume 4, p. 935. [Google Scholar]
- Löbl, I.; Smetana, A. (Eds.) Chrysomeloidae. In Catalogue of Palaearctic Coleoptera; Apollo Books: Stenstrup, Denmark, 2010; Volume 6, p. 924. [Google Scholar]
- Bousquet, Y. Litteratura Coleopterologica (1758–1900): A guide to selected books related to the taxonomy of Coleoptera with publication dates and notes. ZooKeys 2016, 583, 1–776. [Google Scholar] [CrossRef]
- Lachat, T.; Wermelinger, B.; Gossner, M.M.; Bussler, H.; Isacsson, G.; Müller, J. Saproxylic beetles as indicator species for dead-wood amount and temperature in European beech forests. Ecol. Indic. 2012, 23, 323–331. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Baviera, C.; Biscaccianti, A.B.; Brandmayr, P.; Mazzei, A.; Mason, F.; Battistoni, A.; Teofili, C.; Rondinini, C.; Fattorini, S.; et al. A red list of Italian saproxylic beetles: Taxonomic overview, ecological features and conservation issues (Coleoptera). Fragm. Entomol. 2015, 47, 53–126. [Google Scholar] [CrossRef]
- Gutowski, J.M.; Sućko, K.; Borowski, J.; Kubisz, D.; Mazur, M.A.; Melke, A.; Mokrzycki, T.; Plewa, R.; Żmihorski, M. Post-fire beetle succession in a biodiversity hotspot: Białowieża Primeval Forest. Forest Ecol. Manag. 2020, 461, 117893. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423; 623–659. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Chapman & Hall: London, UK, 1996; 179p. [Google Scholar]
- Allemand, R.; Aberlenc, H.P. Une methode efficace d’echantillonnage de l’entomofaune des frondaisons: Le piege attractif aerien. Bull. De La Soc. Entomol. Suisse 1991, 64, 293–305. [Google Scholar]
- Brustel, H. Coleopteres Saproxyliques et Valeur Biologique des Forets Françaises. Perspectives Pour la Conservation du Patrimoine Naturel; Les Dossiers Forestiers 13, Office National des Forets: Paris, France, 2004; 297p. [Google Scholar]
- Li, Y.; Meng, Q.; Silk, P.; Gao, W.; Mayo, P.; Sweeney, J. Effect of semiochemicals and trap height on catch of Neocerambyx raddei in Jilin province, China. Entomol. Exp. Appl. 2017, 164, 94–101. [Google Scholar] [CrossRef]
- Vance, C.C.; Kirby, K.R.; Malcolm, J.R.; Smith, S.M. Community composition of longhorned beetles (Coleoptera: Cerambycidae) in the canopy and understorey of sugar maple and white pine stands in south-central Ontario. Environ. Entomol. 2003, 32, 1066–1074. [Google Scholar] [CrossRef]
- Leksono, A.S.; Takada, K.; Koji, S.; Nakagoshi, N.; Anggraeni, T.; Nakamura, K. Vertical and seasonal distribution of flying beetles in a suburban temperate deciduous forest collected by water pan trap. Insect Sci. 2005, 12, 199–206. [Google Scholar] [CrossRef]
- Hirao, T.; Murakami, M.; Kashizaki, A. Importance of the understory stratum to entomofaunal diversity in a temperate deciduous forest. Ecol. Res. 2009, 24, 263–272. [Google Scholar] [CrossRef]
- Bouget, C.; Brin, A.; Brustel, H. Exploring the “last biotic frontier”: Are temperate forest canopies special for saproxylic beetles? For. Ecol. Manag. 2011, 261, 211–220. [Google Scholar] [CrossRef]
- Graham, E.E.; Poland, T.M.; McCullough, D.G.; Millar, J.G. A comparison of trap type and height for capturing cerambycid beetles (Coleoptera). J. Econ. Entomol. 2012, 105, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Vodka, Š.; Cizek, L. The effects of edge-interior and understorey-canopy gradients on the distribution of saproxylic beetles in a temperate lowland forest. For. Ecol. Manag. 2013, 304, 33–41. [Google Scholar] [CrossRef]
- Procházka, J.; Cizek, L.; Schlaghamerský, J. Vertical stratification of scolytine beetles in temperate forests. Insect Conserv. Divers. 2018, 11, 534–544. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V. Vertical stratification of beetles in deciduous forest communities in the Centre of European Russia. Diversity 2021, 13, 508. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Hanula, J.L. A comparison of the beetle (Coleoptera) fauna captured at two heights above the ground in a North American temperate deciduous forest. Am. Midl. Nat. 2007, 158, 260–278. [Google Scholar] [CrossRef]
- Kurochkin, A.S. Fauna and bionomy of sap beetles (Coleoptera, Nitidulidae) and kateretid beetles (Coleoptera, Kateretidae) of Krasnosamarskoe forestry farm (Samara Region, Russia). Vestn. Samara Univ. Nat. Sci. Ser. 2007, 8, 120–128. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Seasonal activity of Coleoptera attracted by fermental crown traps in forest ecosystems of Central Russia. Ecol. Quest. 2021, 32, 37–53. [Google Scholar] [CrossRef]
- Oleksa, A.; Chybicki, I.J.; Gawronski, R.; Svensson, G.P.; Burczyk, J. Isolation by distance in saproxylic beetles may increase with niche specialization. J. Insects Conserv. 2013, 17, 219–233. [Google Scholar] [CrossRef]
- Urban, P.; Schulze, W. Ein aktueller Nachweis des Marmorierten Rosenkäfers Pro-taetia marmorata (Fabricius, 1792) in der Senne (Nordrhein-Westfalen) (Mitteilungen zur Insektenfauna Westfalens XXII). Mitt. Der Arb. Westfälischer Entomol. 2017, 33, 15–19. [Google Scholar]
- Berglund, H.L.; Milberg, P. Sampling of flower-visiting insects: Poor correspondence between the catches of colour pan-trap and sweep netting. Europ. J. Entomol. 2019, 116, 425–431. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvořák, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef]
- Nasir, S.; Akram, W.; Ahmed, F. The population dynamics, ecological and seasonal activity of Paederus fuscipes Curtis (Staphylinidae; Coleoptera) in the Punjab, Pakistan. APCBEE Procedia 2012, 4, 36–41. [Google Scholar] [CrossRef][Green Version]
- Ruchin, A.B.; Egorov, L.V.; Semishin, G.B. Fauna of click beetles (Coleoptera: Elateridae) in the interfluve of Rivers Moksha and Sura, Republic of Mordovia, Russia. Biodiversitas 2018, 19, 1352–1365. [Google Scholar] [CrossRef]
- Batschynskaja, J.A.; Komisova, T.E.; Lykova, I.O. Peculiarities of the development and seasonal dynamics of the activity of epigean beetles of the Polyphaga suborder (Coleoptera) in fields of winter wheat in the conditions of Southern Ukraine. Biosyst. Divers. 2020, 28, 243–249. [Google Scholar] [CrossRef]
- Zemoglyadchuk, A.V.; Ruchin, A.B.; Egorov, L.V. An annotated checklist of the tumbling flower beetles (Coleoptera, Mordellidae) of the Republic of Mordovia, with a short review of the family in European Russia. Entomol. Rev. 2020, 100, 771–787. [Google Scholar] [CrossRef]
- Evangelista, J.; Rocha, M.V.C.; Monné, M.L.; Monné, M.A.; Frizzas, M.R. Diversity of Cerambycidae (Insecta: Coleoptera) in the Cerrado of Central Brazil using a new type of bait. Biota Neotrop. 2021, 21, e20201103. [Google Scholar] [CrossRef]
- Karolyi, F.; Gorb, S.N.; Krenn, H.W. Trapping pollen by the moist mouth: Structure and function of the mouthparts in the flower visiting Cetonia aurata (Scarabeidae, Coleoptera). Arthropod Plant Interact. 2009, 3, 1–8. [Google Scholar] [CrossRef]
- Fremlin, M. The rose chafer Cetonia aurata L. (Coleoptera: Scarabaeidae: Cetoniinae) in Essex: Distribution and some aspects of its ecology. Essex Nat. 2018, 35, 167–178. [Google Scholar]
- Egorov, L.V.; Ruchin, A.B.; Semishin, G.B. Some data concerning the Coleoptera fauna of the Mordovia State Nature Reserve. Information 9. Proc. Mordovia State Nat. Reserve 2020, 24, 61–150. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V.; Khapugin, A.A. Usage of fermental traps for studying the species diversity of Coleoptera. Insects 2021, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Maican, S.; Munteanu, C. The diversity of urban coleopterans. In Species Monitoring in the Central Parks of Bucharest/Coord.: Marilena Onete; Ars Docendi: Bucureti, Romania, 2009; pp. 106–112. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V. Fauna of longicorn beetles (Coleoptera: Cerambycidae) of Mordovia. Rus. Entomol. J. 2018, 27, 161–177. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).