Abstract
The aim of this study was to enable searches for truffles (Tuber spp.), particularly the Burgundy truffle (T. aestivum Vittad.), to be carried out in forests based on a method that has been constantly developed since 2007 by the Forest Research Institute. The method is termed “Virtual Truffle Hunting” and it takes 12 parameters into account: bedrock, soil pH, Ca+ and CaCO3 content in soil, C/N ratio, soil structure, altitude of terrain, type of forest site, forest structure, the Burgundy truffle host trees, and the presence of particular species including orchids and insects. A simple “Virtual Truffle Hunting” software has also been developed, which makes the use of the method easy, fast, and effective. This method is to ascertain the truffle potential for all areas in which digital maps are not available. In 2015, the method was tested in 20 sites, representing forests in 5 Polish macroregions. Hunting for hypogeous fungi was conducted from June to October with the help of trained dogs. Thanks to this method, 14 new truffle sites were found. The knowledge of environmental conditions conducive to the Burgundy truffle growth enabled us to form an effective tool in order to identify new sites of truffle presence.
1. Introduction
Truffles are one of the most economically valued non-wood forest products owing to their taste and aroma. These subterranean mushrooms are especially appreciated in countries that have been associated with their cultivation for many decades, including France, Italy, and Spain. Prices of the highly esteemed truffle species, for example, white truffle (Tuber magnatum Picco) and black truffle (Tuber melanosporum Vittad.), can even reach 2000–3000 Euro per kilogram [1,2].
In Poland, the most common species is the Burgundy truffle (Tuber aestivum Vittad.) [3,4,5]. In 2012, the location of a population of the smooth black truffle (Tuber macrosporum Vittad.) was found [6], and the presence of winter (Tuber brumale Vittad.) and whitish (Tuber borchii Vittad.) truffles has also been confirmed [7,8,9,10]. Several other species of truffles that have no commercial value, including T. maculatum Vittad., T. excavatum Vittad., and T. rufum Picco, have been noted in various regions of the country [3,11]. In Poland, the distribution of sites suitable for individual species is poorly known. Despite the relatively rich tradition of using truffles in Polish cuisine, the fungi have disappeared from the table for many years, but today, they are slowly coming back into favor [4,5].
Truffles are the subterranean fruiting bodies of Tuber (Ascomycotina, Pezizales), a mycorrhizal genus of fungi that needs host plants and appropriate environmental conditions for development [12,13,14,15]. The process of fruiting body formation is affected by many biotic and abiotic factors, and research on this aspect has been conducted in many countries [16,17,18,19]. In Europe, Tuber spp. form a mycorrhizal symbiosis with many species of deciduous and coniferous trees and shrubs [20]. Truffles can also form mycorrhizas with some bushes, including representatives of the Mediterranean shrubs belonging to the family Cistaceae known as “rock roses”. Members of this family associated with several truffle species include Cistus L., commonly known as purge, as well as Helianthemum Mill. and Fumana procumbens (Dunal) Gren. et Godr. [21,22].
Previous studies have shown that orchids belonging to the genera Epipactis Zinn, Cephalanthera Rich, and Cypripedium L. also coexist with the Burgundy truffle [14,23]. Orchids have been reported to form mycorrhizal symbiosis with many species of truffles [24]. For example, mycelia of T. maculatum were isolated from the roots of Epipactis helleborine (L.) Crantz and Cephalanthera damasonium (Mill.) Druce, and mycelial T. excavatum was isolated from Epipactis microphylla (Ehrh.) Sw. root tissues [25]. Moreover, the orchid species and truffles mentioned here occupy a similar ecological niche. The species have similar soil preferences, and the key factors to their development are high calcium content in soil and high soil pH. For example, Cypripedium calceolus L. grows in soils with a pH range of 6.6–7.5 [26]. Thus, although the presence of orchid mycorrhiza does not guarantee fructification of truffles, several orchid species can be very helpful in indicating truffle sites.
Truffle species show different preferences for habitat, particularly for two environmental factors: soil pH and annual mean temperature [27]. Some species of truffles also have a high degree of specialization and selectivity towards plant partners [28]. On the other hand, the largest ecological generalists within the genus Tuber are considered whitish and Burgundy truffles, which form mycorrhizas with many species of angiosperms and gymnosperms, including plant species that are aliens in Europe, e.g., pecan nut (Carya illinoinensis (Wangenh.) K. Koch) [29]. Biotic and abiotic factors connected with presence of T. aestivum in Europe have been broadly investigated [14,15,17,18,19,30,31,32,33,34].
For many animals, including mammals (rodents, deer, wild boars), birds, and snails, truffles are a nutritious food source [35,36], but for some arthropods, most notably those from the orders of Coleoptera (beetles) and Diptera (flies), these fungi are the basis of their diet [16,37]. The fauna of mycophagic beetles associated with truffles are represented by Leiodes cinnamomea Panzer and L. oblonga Erichson (Coleoptera: superfamily Staphylinoidea) [38,39,40]. Adult females of round fungus beetles (Leiodes Latreille) are attracted by flavors emitted by the fruiting bodies of truffles at an early stage of their development, as even immature fungi can attract the beetles [41]. For the Leiodes beetles, some truffle species (T. melanosporum, T. aestivum, and T. excavatum) form a favorable environment for growth [38,42,43,44,45]. Flies belonging to the genus Suillia Robineau-Desvoidy [40] and the dor beetles (family Bolboceratidae Mulsant) (I.A. Bolbelasmus unicornis Schrank and Odonteus armiger Scopoli) can also feed and develop on truffles [46].
Factors affecting the life cycle of truffles can be divided into several groups, but it should be emphasized that they all interact with each other. The development of the fruiting bodies is shaped by the internal characteristics of the population (e.g., physiological factors) and environmental elements (biotic and abiotic) [47]. Soil and weather conditions as well as interactions with the plants are of the greatest importance among this group of factors. Büntgen et al. [47] also emphasize the role of other elements and processes, including terrain features (e.g., elevation, exposition), the way matter circulates in the ecosystem (particularly carbon, water, and nutrients), the presence of mycophagous fauna, and biotic disorders (e.g., defoliation of trees). Moreover, numerous biotic and abiotic factors mentioned here that drive truffle fructification are interrelated: soil chemistry depends on bedrock as well as land-use, while terrain elevation influences plant species composition, as well as weather conditions, and so on [47].
Truffles usually prefer well aerated, calcareous soils with an alkaline pH and an appropriate amount of organic matter (e.g., about 7.5 ± 3% for T. aestivum) [14,48]; however, moisture and temperature preferences vary depending on the truffle species. For example, T. melanosporum is a thermophilic species and prefers highly-drained soils, while T. macrosporum can grow in colder climates and in periodically flooded soils [49]. Truffle species also differ as a result of preferences in soil pH. The Burgundy truffle can occur in neutral soils, and the whitish truffle tolerates a pH of 5.0 [50].
Soil properties are shaped by many factors, including geological structure and altitude, climate, vegetation, soil organisms, and soil tillage [17]. The basic chemical and physical features of the soil, on the basis of which the soil is classified, stem from the geological history and the type of bedrock on which they were formed. They affect soil pH, content of macroelements such as exchangeable calcium and magnesium cations, soil structure, and other parameters important for the development of truffle fruiting bodies [14,15,32]. In the analysis of soils from natural sites and from truffle orchards, their granulometric composition is taken into account [33,34,51,52,53], because the share of individual fractions determines aeration and soil permeability.
Most truffle species occur in temperate regions and prefer a mild climate, free from extreme heat in summer on the one hand, and severe frosts on the other [54]. Büntgen et al. [47] list the five most important climate features shaping the life cycle of truffles: precipitation, temperature, snow cover length, number of sunny days per year, and distribution of negative temperatures throughout the year. The Burgundy truffle is an ecologically plastic species with high ecological amplitude, higher in terms of resistance to climatic anomalies, hydrological changes, and other abiotic stress than T. melanosporum [17,43,47,51,55].
The aim of this work was to create a comprehensive method of typing truffle sites based on knowledge obtained during ten years of research conducted by the authors and to check the effectiveness of the method in forest stands. An additional goal was to search for new truffle sites in Poland. This method could be successfully adopted in various areas of Europe to ascertain the truffle potential for the areas where there is a lack of digital maps based on the computer multilayer analysis [30,56,57]. Based on the results of the study conducted since 2007 and literature data, a comprehensive method of truffle site typing has been proposed. This method is named “Virtual Truffle Hunting” and is presented in the form of a practical table (Table 1) that can be easily applied with suitable free software.

Table 1.
“Virtual Truffle Hunting”—a method for typing truffle sites.
2. Materials and Methods
The method of “Virtual Truffle Hunting” is a scale for showing the possibility of the occurrence of the Burgundy truffle fruiting bodies based on chosen environmental factors and indicators. The concept of the method is similar to the assumptions of other commonly used natural scales, e.g., the Extended Biotic Index for water quality assessment (IBE) developed by Ghetti & Chierici [58].
The method included 12 biotic and abiotic parameters helpful in determining the Burgundy truffle sites (Table 1). Six parameters relate to soil conditions: bedrock, soil pH in H2O, calcium exchangeable cation content and calcium carbonate content in soil, soil carbon to nitrogen ratio, and soil granulometric structure, according to the soil texture triangle [34]. The information concerning bedrock (necessary for the method validation) was taken from the Central Geological Database [59] or the Forest Data Bank [60]. In order to determine other soil parameters, it was necessary to take soil samples from the sites and perform laboratory analyses in accordance with the methodology of Hilszczańska et al. [14]. Each parameter was assessed from 0 to 3 points (Table 1). Other important factors were altitude (m.a.s.l), forest habitat type and structure, and the presence and number of T. aestivum mycorrhizal partners (Table 2). Each of these traits is awarded a maximum of 3 points. The valorization was also based on the known bioindicators of truffles: orchids and mycophagous insects. Points are awarded (max. 3) for the presence of orchids at the site as well as for truffle-associated insect occurrence (3 points). To sum up, for each of 10 parameters, a four-step characteristic was proposed, according to which from 0 to 3 points are awarded. In the case of bioindicators (orchids and insects), the scale was modified and the criterion for awarding points is given in Table 1. The maximum number of points for one forest division is 36. The following classification thresholds were proposed: 31–36 pts.—high possibility of fruiting body occurrence, 25–30 pts.—medium possibility, 19–24 pts.—low possibility, and below 19 pts.—zero possibility. The calculation is facilitated by the software “Virtual Truffle Hunting” [61], which was created to speed up the calculation.

Table 2.
The most common trees and shrubs species forming mycorrhiza with the Burgundy truffle (Tuber aestivum Vittad.) in Poland [14,17,19,21,23].
In 2015, a comprehensive method for finding truffle sites using “Virtual Truffle Hunting” was tested in 20 selected forest divisions in five macro-regions of Poland (Figure 1). The choice of area was based on the Forest Data Bank database [60]. The basic characteristics of 20 selected test sites are given in Table 3. At the selected sites, truffle fruiting bodies and accompanying hypogeous fungi were searched for from June to the end of October 2015 with the help of a trained dog. Additionally, the following parameters were recorded at each site:
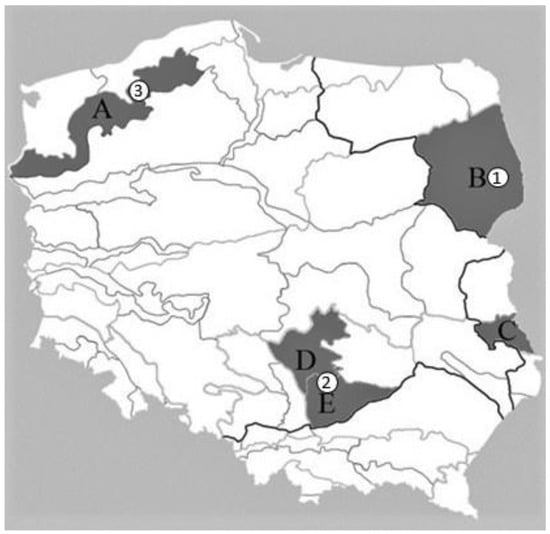
Figure 1.
Location of five Polish macroregions where the “Virtual Truffle Hunting” method was tested (A—West Pomeranian Lake District, B—North Podlasie Lowland, C—Volhynia Polesie, D—Przedbórz Upland, and E—Nida Basin) and three meteorological stations (1—Białystok, 2—Jędrzejów, and 3—Szczecinek).

Table 3.
Characteristics of 20 investigated forest sites (Fr. BF—fresh broadleaved forest, Fr. UBF—fresh upland broadleaved forest, Fr. MBF—fresh mixed broadleaved forest, Fr. UMBF—fresh upland mixed broadleaved forest).
- bedrock;
- chemical and granulometric composition analysis of soil samples (in the laboratory) [14];
- altitude;
- forest habitat type;
- the structure of the site and the undergrowth layer;
- description of trees and shrubs species that were associated with truffle mycorrhizae;
- information on the presence of orchids and indicator insects included in the method “Virtual Truffle Hunting”.
On the basis of information obtained in forest sites and the results of soil analyses, each of the 20 test areas was estimated for potential truffle presence in accordance with the proposed method for truffle typing site using “Virtual Truffle Hunting”.
3. Results
The results of sites’ valorization, as well as the scores for each parameter and the final assessment and the list of fruiting bodies of hypogeous fungi at each site, are presented in Table 4, Table 5, Table 6 and Table 7. Based on the method used, two sites were classified as unfavorable for the growth of truffles (zero probability of occurrence) and there was no fruiting of truffles or other species of hypogeous fungi. Five sites were classified as sites with a low probability of truffle occurrence. However, fruiting bodies were not confirmed on only one of the sites. At the other four sites, fruiting bodies of the genera Elaphomyces, Melanogaster and Genea and two species of truffles (Tuber ferrugineum and T. excavatum) were observed. Most of the tested forest districts (8) obtained a score indicating an average probability of truffle occurrence. In each of these forest districts, fruiting bodies of Tuber aestivum, T. excavatum, and T. rufum were found. According to the method used, five areas were characterized by a high probability of truffle fruiting bodies occurrence. On each of them, fruiting bodies of Burgundy truffle were found (from 3 to 38 fruiting bodies).

Table 4.
Characteristics of 20 investigated forest sites (Fr. BF—fresh broadleaved forest, Fr. UBF—fresh upland broadleaved forest, Fr. MBF—fresh mixed broadleaved forest, Fr. UMBF—fresh upland mixed broadleaved forest) (for sites 1–5).

Table 5.
Characteristics of 20 investigated forest sites (Fr. BF – fresh broadleaved forest, Fr. UBF—fresh upland broadleaved forest, Fr. MBF—fresh mixed broadleaved forest, Fr. UMBF—fresh upland mixed broadleaved forest), (for sites 6–10).

Table 6.
Characteristics of 20 investigated forest sites (Fr. BF—fresh broadleaved forest, Fr. UBF – fresh upland broadleaved forest, Fr. MBF—fresh mixed broadleaved forest, Fr. UMBF—fresh upland mixed broadleaved forest). (for sites 11–15).

Table 7.
Characteristics of 20 investigated forest sites (Fr. BF—fresh broadleaved forest, Fr. UBF—fresh upland broadleaved forest, Fr. MBF—fresh mixed broadleaved forest, Fr. UMBF—fresh upland mixed broadleaved forest). (for sites 16–20).
4. Discussion
The selection of truffle sites based on soil and vegetation analysis works especially well with Burgundy truffle (T. aestivum) [17,18,19,32,33]. Based on data in the literature and our own research [3,4,5,6,14,40], it was possible to create a new, methodical tool for typing sites conducive to locating truffles in Poland.
It should be noted that the proposed method is particularly adapted at a national level as it takes into account geological formations typical of Poland, as well as native species of flora and entomofauna. The comprehensive method for selecting truffle occurrences using “Virtual Truffle Hunting” is a pioneering tool and has no counterpart in other countries. Compared with the GIS (geographic information system)-based methods developed for the identification of potential naturally-occurring truffle areas [62,63], the advantage of this methodology is that it is much easier to apply. It does not require a high level of computer skills and the availability of thematic maps in ruster or vector format, and can thus also be applied in areas that have not been well studied in terms of georeferencing and by people who are not experts in numerical cartography.
The effectiveness and efficiency of “Virtual Truffle Hunting” are determined by both the number and the variety of biotic and abiotic parameters selected to create the method. The multiplicity of environmental factors affecting the development of truffle fruiting bodies means that the developed method focuses on key factors. For example, as previously mentioned, truffles can fruit in soils with a granulometric composition significantly different from the preferred soils, as long as the soil has an optimal calcium content. The “Virtual Truffle Hunting” method allows balancing the estimation of parameters while taking into account their variability.
It should be emphasized that the selection of precipitations conducive to the development of truffle fruiting bodies can take place regardless of the meteorological conditions prevailing in a given season; however, the search for fruiting bodies on selected sites should be carried out only in years favorable for truffle development. Optimal conditions for verification of field typing should be considered in those years in which the growing season is preceded by mild winters. Heavy rainfall in summer (July–August) seems to be conducive to fructification of truffles, hence the best search date is the period from mid-July to the first frost [64].
The results obtained in our work, as well as the development of new research methods, allow us to point out favorable conditions for the development of truffles in forest stands. Thanks to new information on the environmental preferences of truffles and by combining the various methods of searching, it is possible to accurately indicate those regions in which environmental conditions favor the occurrence of these fungi. Identification of their sites can be multifaceted—based on geological data, soil, and meteorological analyses, as well as vegetation features.
Given the precipitations (Figure 2) and temperatures (Figure 3) typical of European countries, the differences are not as high as could be expected. During the period June through September, precipitations in Poland are at the similar level as precipitations in France (Burgundy region) [33]. When we compare the period January through May in Huesca [65], precipitations are much higher than in Poland. Hence, taking into account weather and plasticity of T. aestivum, we are of the opinion that this method with some implementations could be used outside of Poland.
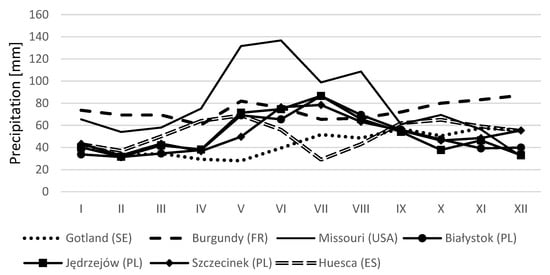
Figure 2.
Monthly mean precipitation (long—term average) for the chosen localization of five countries [33,34,65,66].
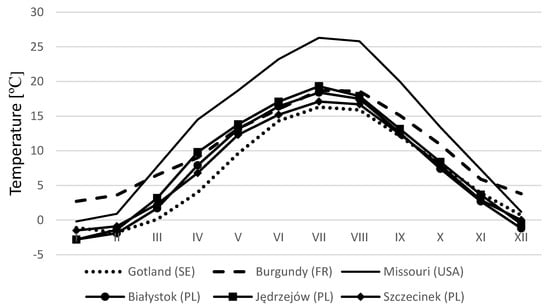
Figure 3.
Monthly mean temperature (long—term average) for the chosen localization of countries [33,34,66].
Soil conditions conducive to the development of truffles in Poland can be found in stands located mainly in the Uplands of Southern and South-Eastern Poland: Silesia-Cracow, Lesser Poland, and Lublin-Lviv. The macroregion of the Nida Basin is considered to be particularly conducive to truffles owing to soil and climate conditions, similar to those prevailing in some regions of France and Italy [15]. However, potentially favorable conditions for truffle development also exist in some locations in the north of Poland. The comprehensive “Virtual Truffle Hunting” truffle typing method can be particularly useful in determining potential truffle sites in these regions of the country. For example, in the Szczecin Landscape Park “Beech Woods”, although brown soils predominate, there are places, including sites in the vicinity of the Emerald Lake, with a soil rich in calcium carbonate, formed from chalk limestone. The geographical location and movement of atmospheric air masses from the Atlantic Ocean make this region, like the Nida Basin, one of the warmest in the country. There are some stands with photophilous xerothermic oak forest features and calciphilus plants [67], which are helpful in selecting truffle stands. It can thus be assumed that, despite many unique features, stands potentially conducive to the development of fruiting bodies of truffles are more numerous in Poland than was previously thought, and these valuable sites require discovery and examination.
5. Conclusions
Knowledge of the environmental requirements (especially soil) of truffles (Tuber spp.) has enabled the development of an effective tool for identifying new locations of these fungi in Poland. A comprehensive method of typing the truffle sites using “Virtual Truffle Hunting” has allowed us to indicate as many as 14 new forest divisions as the sites of four valuable truffle species (Tuber aestivum, T. excavatum, T. rufum, and T. ferrugineum). The “Virtual Truffle Hunting” method requires further estimation throughout Poland, which will probably allow us to widen the list of favorable sites in various parts of our country.
It seems that, owing to the similarities of weather conditions (rainfall and temperature) in France, Sweden, and Poland (Figure 2 and Figure 3), this method may be used in some applications in Central and Northern Europe. We are of the opinion that this method should be tested especially in neighboring countries (Germany and Czech Republic), and we hope that, with this article, we encourage studies conducting discussions and providing suggestions for the method’s development.
Author Contributions
Conceptualization, A.R.-G., D.H. and G.P.; methodology, A.R.-G. and D.H.; software, A.R.-G.; validation, A.R.-G., D.H. and G.P.; formal analysis, A.R.-G.; investigation, A.R.-G. and D.H.; data curation, A.R.-G.; writing—original draft preparation, A.R.-G.; writing—review and editing, D.H. and G.P.; visualization, A.R.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out as a part of research project No. 500446 entitled “Burgundy truffle management (Tuber aestivum Vitt.) in forests and plantations. Development of rules for the use and promotion of truffles as a special forest product” financed by the Directorate General of State Forests in Warsaw and in cooperation with the funds of the Ministry of Science and Higher Education under projects No. 240309 “Environmental factors affecting fruiting of truffle fungi (Tuber P. Micheli ex F.H. Wigg) in Polish forests” and 900129 “Hypogeous fungi as a component of the diet of selected species of forest fauna”. Cooperation with University of L’Aquila (Italy) was co-financed by a scholarship fund of the Forest Research Institute (IBL), Poland.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Tomasz Kłoszewski, who helped us in creating the “Virtual Truffle Hunting” software and transferring the method from paper to the virtual world.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Büntgen, U.; Egli, S.; Camarero, J.J.; Fischer, E.M.; Stobbe, U.; Kauserud, H.; Tegel, W.; Sproll, L.; Stenseth, N.C. Drought-induced decline in Mediterranean truffle harvest. Nat. Clim. Chang. 2012, 2, 827–829. [Google Scholar] [CrossRef]
- Pieroni, A. The changing ethnoecological cobweb of white truffle (Tuber magnatum Pico) gatherers in South Piedmont, NW Italy. J. Ethnobiol. Ethnomed. 2016, 12, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilszczańska, D.; Sierota, Z.; Palenzona, M. New Tuber species found in Poland. Mycorrhiza 2008, 18, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Gruszecka, A.; Hilszczańska, D.; Gil, W.; Kosel, B. Historia i perspektywy użytkowania i badań trufli w Polsce. Sylwan 2017, 16, 320–327. [Google Scholar] [CrossRef]
- Rosa-Gruszecka, A.; Hilszczańska, D.; Gil, W.; Kosel, B. Truffle renaissance in Poland–history, present and prospects. J. Ethnobiol. Ethnomed. 2017, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hilszczańska, D.; Rosa-Gruszecka, A.; Sikora, K.; Szmidla, H. First report of Tuber macrosporum occurrence in Poland. Sci. Res. Essays 2013, 8, 1096–1099. [Google Scholar] [CrossRef] [Green Version]
- Chmiel, M.A. Checklist of Polish larger Ascomycetes. In Biodiversity of Poland; Mirek, Z., Ed.; Szafer Institute of Botany: Cracow, Poland; Polish Academy of Sciences: Cracow, Poland, 2006; Volume 8, p. 152. [Google Scholar]
- Merenyi, Z.; Varga, T.; Geml, J.; Orczán, Á.K.; Chevalier, G.; Bratek, Z. Phylogenyand phylogeography of the Tuber brumale aggr. Mycorrhiza 2014, 24, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Mleczko, P.; Chachuła, P.; Kozak, M. Hypogeous fungi of Pieniny and Gorce, two mountain ranges of the Polish Carpathians. In Proceedings of the XVIIth Congress of European Mycologists, Madeira, Portugal, 21–25 September 2015. [Google Scholar]
- Chachuła, P. Macrofungi in the Dłubnia Landscape Park–preliminary results. Przegląd Przyr. 2018, 29, 58–71. [Google Scholar]
- Ławrynowicz, M. Four Tuber species accompanying T. mesentericum in natural sites in Poland. An. del Jardín Bot. Madr. 2009, 66, 145–149. [Google Scholar] [CrossRef]
- Delmas, J. Tuber spp. In The Biology and Cultivation of Edible Mushrooms; Chang, S.T., Hayes, W.A., Eds.; Academic Press: London, UK, 1978; pp. 645–681. [Google Scholar]
- Lulli, L.; Bragato, G.; Gardin, L. Occurrence of Tuber melanosporum in relation to soil surface layer properties and soil differentiation. Plant Soil 1999, 214, 85–92. [Google Scholar] [CrossRef]
- Hilszczańska, D.; Rosa-Gruszecka, A.; Gawryś, R.; Horak, J. The effect of soil properties and vegetation characteristic in determining the frequency of fruiting bodies of the Burgundy truffle in Southern Poland. Ecoscience 2019, 26, 113–122. [Google Scholar] [CrossRef]
- Hilszczańska, D.; Szmidla, H.; Sikora, K.; Rosa-Gruszecka, A. Soil properties conducive to Tuber aestivum Vitt. fruiting bodies formation in the Nida Basin stands. Pol. J. Environ. Stud. 2019, 28, 1713–1718. [Google Scholar] [CrossRef]
- Pacioni, G.; Ragnelli, A.M.; Miranda, M. Truffle development and interactions with the biotic environment. In Biotechnology of Ectomycorrhizae; Stocchi, V., Bonfante, P., Nuti, M., Eds.; Springer: Boston, MA, USA, 1995; pp. 213–227. [Google Scholar] [CrossRef]
- Stobbe, U.; Egli, S.; Tegel, W.; Peter, M.; Sproll, L.; Büntgen, U. Potential and limitations of Burgundy truffle cultivation. Appl. Microbiol. Biotechnol. 2013, 97, 5215–5224. [Google Scholar] [CrossRef] [PubMed]
- Gažo, J.; Miko, M.; Chevalier, G. First results of inventory research on economically important species of truffles (Tuber) in the Tribec Mountains. Acta Fytotech. Zootech. 2005, 8, 66–71. [Google Scholar]
- Moser, B.; Büntgen, U.; Molinier, V.; Peter, M.; Sproll, L.; Stobbe, U.; Tegel, W.; Egli, S. Ecological indicators of Tuber aestivum habitats in temperate European beech forests. Fungal Ecol. 2017, 29, 59–66. [Google Scholar] [CrossRef]
- Pacioni, G.; Comandini, O. Tuber. In Ectomycorrhizal Fungi: Key Genera in Profile; Cairney, J.W.G.S., Chambers, M., Eds.; Springer: Heidelberg, Germany, 1999; pp. 163–186. [Google Scholar]
- Granetti, B.; de Angelis, A.; Materozzi, G. Umbria Terra di Tartufi. Regione Umbria: Assessorato Regionale Agricoltura; Foreste, Caccia e Pesca: Perugia, Italy, 2005. [Google Scholar]
- García-Montero, L.G.; Casermeiro, M.A.; Manjón, J.L.; Hernando, I. Impact of active soil carbonate and burn size on the capacity of the rockrose Cistus laurifolius to produce Tuber melanosporum carpophores in truffle culture. Mycol. Res. 2007, 111, 734–739. [Google Scholar] [CrossRef]
- Hilszczańska, D.; Rosa-Gruszecka, A.; Szmidla, H. Characteristic of Tuber spp. localities in natural stands with emphasis on plant species composition. Acta Mycol. 2014, 49, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Selosse, M.A.; Faccio, A.; Scappaticci, G.; Bonfante, P. Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb. Ecol. 2004, 47, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Ouanphanivanh, N.; Merényi, Z.; Orczán, Á.K.; Bratek, Z.; Szigeti, Z.; Illyés, Z. Could orchids indicate truffle habitats? Mycorrhizal association between orchids and truffles. Acta Biol. Szeged. 2008, 52, 229–232. [Google Scholar]
- Ellenberg, H.E. Indicator values of plants in Central Europe. Scr. Geobot. 1974, 18, 1–248. [Google Scholar]
- Gryndler, M.; Šmilauer, P.; Šťovíček, V.; Nováková, K.; Hršelová, H.; Jansa, J. Truffle biogeography—A case study revealing ecological niche separation of different Tuber species. Ecol. Evol. 2017, 7, 4275–4288. [Google Scholar] [CrossRef]
- Molina, R.; Trappe, J.M. Patterns of ectomycorrhizal host specificity and potential among Pacific Northwest conifers and fungi. For. Sci. 1982, 28, 423–458. [Google Scholar]
- Benucci, G.M.; Bonito, G.; Falini, L.B.; Bencivenga, M. Mycorrhization of Pecan trees (Carya illinoinensis) with commercial truffle species: Tuber aestivum Vittad. and Tuber borchii Vittad. Mycorrhiza 2012, 22, 383–392. [Google Scholar] [CrossRef]
- Stobbe, U.; Büntgen, U.; Sproll, L.; Tegel, W.; Egli, S.; Fink, S. Spatial distribution and ecological variation of re-discovered German truffle habitats. Fungal Ecol. 2012, 5, 591–599. [Google Scholar] [CrossRef]
- Miko, M.; Gažo, J.; Priecel, J.; Vernarec, J.; Bratek, Z. Soils indicators of suitability for cultivation of burgundy truffle (Tuber aestivum Vitt.) in Slovak Republic. Sci. J. Phytotechny Zootech. 2009, 12, 70–73. [Google Scholar]
- Csorbainé, A.G. Studies on Cultivation Possibilities of Summer Truffle (Tuber aestivum Vittad.) and Smooth Black Truffle (Tuber macrosporum Vittad.) in Hungary. Ph.D. Thesis, Szent Istvan University, Gödöllő, Hungary, 2001. [Google Scholar]
- Wedén, C.; Chevalier, G.; Danell, E. Tuber aestivum (syn. T. uncinatum) biotopes and their history on Gotland, Sweden. Mycol. Res. 2004, 108, 304–310. [Google Scholar] [CrossRef]
- Bruhn, J.; Hall, M. Burgundy Black Truffle Cultivation in an Agroforestry Practice. In Action in Agroforestry; The Center for Agroforestry at the University of Missouri: Columbia, MO, USA, 2011. [Google Scholar]
- Johnson, C.N. Interactions between mammals and ectomycorrhizal fungi. Trends Ecol. Evol. 1996, 11, 503–507. [Google Scholar] [CrossRef]
- Vernes, K.; Cooper, T.; Green, S. Seasonal fungal diets of small mammals in an Australian temperate forest ecosystem. Fungal Ecol. 2015, 18, 107–114. [Google Scholar] [CrossRef]
- Santo, P.D. Interazioni Pianta–Micorriza–Insetto: Il Modello Quercus sp.–Tuber sp.–Leiodes cinnamomea (Panzer). Ph.D. Thesis, University of Molise, Campobasso, Italy, 2013. [Google Scholar]
- Arzone, A. Reperti ecologici ed etologici di Liodes cinnamomea Panzer vivente su Tuber melanosporum Vittadini (Coleoptera Staphylinoidea). Ann. Della Fac. Di Sci. Agrar. Della Univ. Degli Studi Di Torino 1970, 5, 317–357. [Google Scholar]
- Arzone, A. Nuovi reperti sulla biologia di Liodes cinnamomea Panzer in Tuber magnatum Pico (Coleoptera, Staphylinoidea). Allionia Boll. Dell’instituto ed Orto Bot. Dell’università Di Torino 1971, 17, 121–129. [Google Scholar]
- Rosa-Gruszecka, A.; Gange, A.C.; Harvey, D.J.; Jaworski, T.; Hilszczański, J.; Plewa, R.; Konwerski, S.; Hilszczańska, D. Insect-truffle interactions–potential threats to emerging industries? Fungal Ecol. 2017, 25, 59–63. [Google Scholar] [CrossRef]
- Hochberg, M.E.; Bertault, G.; Poitrineau, K.; Janssen, A. Olfactory orientation of the truffle beetle, Leiodes cinnamomea. Entomol. Exp. Appl. 2003, 109, 147–153. [Google Scholar] [CrossRef]
- Fogel, R.; Peck, S.B. Ecological Studies of Hypogeous Fungi, I. Coleoptera associated with sporocarps. Mycologia 1975, 67, 741–747. [Google Scholar] [CrossRef]
- Newton, A.F. Mycophagy in Staphylinoidea (Coleoptera). In Fungus–Insect Relationships: Perspectives in Ecology and Evolution; Wheeler, Q., Blackwell, M., Eds.; Columbia University Press: New York, NY, USA, 1984; pp. 302–353. [Google Scholar]
- Pacioni, G.; Bologna, M.A.; Laurenzi, M. Insect attraction by Tuber: A chemical explanation. Mycol. Res. 1991, 95, 1359–1363. [Google Scholar] [CrossRef]
- Bratek, Z.; Papp, L.; Merkl, O.; Takács, V. Föld alatti gombákon élő rovarok. Mikológiai Közlemények 1993, 31, 55–65. [Google Scholar]
- Byk, A.; Mokrzycki, T.; Rosa-Gruszecka, A.; Tylkowski, S.; Zamojski, M. Grzybolcowate (Bolboceratidae) i wygonakowate (Ochodaeidae) -aktywność, wymagania ekologiczne i metody obserwacji. SiM CEPL 2016, 49, 124–141. [Google Scholar]
- Büntgen, U.; Tegel, W.; Egli, S.; Stenseth, N.C. Truffles and climate change. Front. Ecol. Environ. 2011, 9, 150–151. [Google Scholar] [CrossRef]
- Ponce, R.A.; Ágreda, T.; Águeda, B.; Aldea, J.; Martínez-Peña, F.; Modrego, M.P. Soil physical properties influence “black truffle” fructification in plantations. Mycorrhiza 2014, 24, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Benucci, G.M.; Bonito, G.; Falini, L.B.; Bencivenga, M.; Donnini, D. Truffles, timber, food, and fuel: Sustainable approaches for multi-cropping truffles and economically important plants. In Edible Ectomycorrhizal Mushrooms; Zambonelli, A., Bonito, G.M., Eds.; Springer: Heidelberg, Germany, 2012; pp. 265–280. [Google Scholar] [CrossRef]
- Gardin, L. I tartufi minori della Toscana. In Gli Ambienti di Crescita Dei Tartufi Marzuolo e Scorzone; ARSIA: Florence, Italy, 2005; p. 55. [Google Scholar]
- Wedén, C.; Danell, E.; Tibell, L. Species recognition in the truffle genus Tuber–the synonyms Tuber aestivum and Tuber uncinatum. Environ. Microbiol. 2005, 7, 1535–1546. [Google Scholar] [CrossRef]
- Wedén, C.; Pettersson, L.; Danell, E. Truffle cultivation in Sweden: Results from Quercus robur and Corylus avellana field trials on the island of Gotland. Scand. J. For. Res. 2009, 24, 37–53. [Google Scholar] [CrossRef]
- Hilszczańska, D.; Szmidla, H.; Horak, J.; Rosa-Gruszecka, A. Ectomycorrhizal communities in a Tuber aestivum Vittad.orchard in Poland. Open Life Sci. 2016, 11, 348–357. [Google Scholar] [CrossRef]
- Trappe, J.M.; Molina, R.; Luoma, D.L.; Cázares, E.; Pilz, D.; Smith, J.E.; Castellano, M.A.; Miller, S.L.; Trappe, M.J. Diversity, Ecology, and Conservation of Truffle Fungi in Forests of the Pacific Northwest; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2009. [CrossRef] [Green Version]
- Streiblová, E.; Gryndlerová, H.; Gryndler, M. Truffle brûlé: An efficient fungal life strategy. FEMS Microbiol. Ecol. 2012, 80, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Notivoli, R.; Martín-Santafé, M.; Sánchez, S.; Barriuso, J.J. Cultivation potentiality of black truffle in Zaragoza province (Northeast Spain). J. Maps 2016, 12, 994–998. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Barreda, S.; Sánchez, S.; Marco, P.; Serrano-Notivoli, R. Agro-climatic zoning of Spanish forests naturally producing black truffle. Agric. For. Meteorol. 2019, 269, 231–238. [Google Scholar] [CrossRef]
- Ghetti, P.F.; Chierici, E. Indice Biotico Esteso (IBE): I Macroinvertebrati Nel Controllo Della Qualita Degli Ambienti di Acque Correnti: Manuale di Applicazione; Agenzia provinciale per la protezione dell’ambiente: Trento, Italy, 1997. [Google Scholar]
- Central Geological Database. Available online: https://geolog.pgi.gov.pl/ (accessed on 25 March 2021).
- Forest Data Bank. Available online: https://www.bdl.lasy.gov.pl (accessed on 25 March 2021).
- Virtual Truffle Hunting (Software). Available online: https://github.com/AleksandraRosa/Virtual-Truffle-Hunting/blob/main/VirtualTruffleHunting.exe (accessed on 27 April 2021).
- De Laurentis, G.; Spinelli, S. Carta Della Vocazionalità Tartuficola Della Regione Abruzzo Risultati Dell’indagine Conclusiva; ARSSA: Lanciano, Italy, 2009. [Google Scholar]
- Laruccia, N.; Marletto, V.; Leonardi, P.; Puliga, F.; Zambonelli, A. Map of suitability for the spontaneous growth of Tuber magnatum in Emilia-Romagna (Italy). Ital. J. Mycol. 2020, 49, 38–53. [Google Scholar] [CrossRef]
- Hilszczańska, D. Polskie Trufle. In Skarb Odzyskany. O hodowli i Kulinariach Podziemnego Przysmaku; CILP: Warsaw, Poland, 2016. [Google Scholar]
- Serrano-Notivoli, R.; Incausa-Ginés, A.; Barriuso-Vargas, J.; Sánchez-Durán, S.; Martín-Santafé, M. Modelización espacial del hábitat potencial de la trufa negra (Tuber melanosporum Vittad.) en la provincia de Huesca (España). ITEA-Inf. Técnica Económica Agrar. 2015, 111, 227–246. [Google Scholar] [CrossRef]
- Pogoda i klimat. Available online: https://meteomodel.pl/ (accessed on 29 April 2021).
- Zawadzka, D.; Kwiecień, E. Puszcze i lasy Polski. In Encyklopedia Ilustrowana; Oficyna Wydawnicza Multico: Warsaw, Poland, 2011. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).