Abstract
Elms are threatened by Dutch elm disease, and conservation methods are needed to protect their genetic diversity. Cryopreservation of dormant buds allows large numbers of genotypes to be conserved with small space requirements and minimal upkeep. Cryopreservation through slow controlled cooling was tested for both elm species native to Finland, Ulmus glabra and Ulmus laevis. Regeneration of the thawed buds by micropropagation was studied on different basal media and using different growth regulators. Multiple surface sterilisation methods were tried out for bud explants. The multiplication of U. glabra was investigated with Driver and Kuniyuki walnut medium with either 0.5 mg/L meta-topolin or 0.5 mg/L 6-benzylaminopurine. Rooting with short indole-6-butyric acid induction in liquid medium and direct transplantation of the shoots to peat ex vitro after induction were tested. For initiation, either Murashige and Skoog or Driver and Kuniyuki walnut medium with 0.02 mg/L gibberellic acid 4 + 7 and 0.5 mg/L 6-benzylaminopurine were found to best promote shoot formation. Surface sterilisation remains the most challenging step. No significant differences were found between the multiplication media in either shoot production or rooting success. Rooting by direct transplanting was achieved in both species, but further development is required before application on a larger scale. With further improvements to sterilisation success especially in U. glabra, the method can be applied to the conservation of genetic resources of both U. laevis and U. glabra, and knowledge of regeneration success can be used to design the cryoconservation plan and optimise the sampling.
1. Introduction
Elms are deciduous trees valued in silviculture and urban landscaping due to their unique appearance and adaptability to city conditions [1]. Finland has two native elm species, European white elm (Ulmus laevis Pall.) and wych elm (Ulmus glabra Huds.), both at the northern edge of their distribution range [2,3]. The populations are mainly located in Southern Finland, separated from each other and too small to allow effective in situ conservation [4]. The genetic resources of the two species are therefore being conserved in outdoor ex situ collections bringing together elms from multiple populations to produce seed with higher genetic diversity than natural populations. In addition to habitat loss due to agriculture, logging and water regulation [3,4], elms are globally threatened by Dutch elm disease (DED), which is considered one of the most devastating known plant diseases [5]. The first DED pandemic occurred around 1910 when the fungal pathogen Ophiostoma ulmi Buisman spread throughout Europe, and later with infested timber imports into North America and parts of Central and Southwest Asia [6]. The second and still ongoing pandemic was recorded in the 1970s and is caused by the more aggressive Ophiostoma novo-ulmi Brasier [7]. DED disrupts xylem water transfer and results in vascular wilt syndrome, visible as crown defoliation followed by the eventual death of the tree [8,9]. No DED has yet been found in Finland [10], but the spread of DED to the genetic reserve collections could wipe out the valuable trees. The protecting factors are a less suitable cold climate for DED vectors, the genus Scolytus bark beetles [11], and the small size and fragmentation of Finnish elm populations. However, because of climate change, DED is expected to arrive in Finland, and is indeed already found, both in neighbouring Sweden and very close to the border with Russia [10]. As a way to use elms for urban landscaping, more DED-resistant elms have been bred using native material, or by crossing with more resistant elm species [5]. Asian elms are generally considered more resistant, but there is still high variation in how well the crossings perform [1]. Hybrids can also be susceptible to local pathogens [12], and the use of material of local origin is preferable because of the danger genetic pollution poses to natural populations [3]. In any case, elm genotypes adapted to northern conditions are needed for resistance breeding if such efforts are to take place in Finland. Because of global warming, elms may also become more attractive trees for landscaping in Finland. As part of the national conservation programme [13], cryopreservation and regeneration methods are being developed for U. laevis and U. glabra. The aim of genetic conservation is to ensure the survival of the elm species adapted to northern conditions. The advantages of cryopreservation are minimal labour requirements for collection upkeep and small space requirements [14]. The material is also protected from pathogens and pests. On the other hand, suitable facilities and staff with special skills are needed for plant regeneration. Cryopreservation provides a more genetically stable method of long-term ex situ conservation than continuous in vitro culture, which is prone to somaclonal variation [15]. Previous efforts to set up cryostorage gene banks for local elm species in Europe [16,17] provide a good starting point to develop the methods suitable for northern genotypes. In North America, efforts to store and propagate old Ulmus americana L. trees that have survived DED epidemics have taken place [18]. Cryopreservation of dormant winter buds is a space-effective and relatively easy way to conserve large amounts of genotypes. However, the methods must be tested and validated for the specific plant material used. The prerequisites for the cryopreservation of dormant buds by controlled rate cooling are cold-hardy plant material and access to a programmable freezer that can provide an adjustable steady decrease in temperature [19]. Controlled rate cooling aims to avoid lethal cryodamage caused by intracellular ice formation. As the sample is gradually cooled, extracellular ice forms first and draws water from the cells by osmotic pressure, thus preventing ice nucleation within the cells [20]. Precooled samples are then plunged into liquid nitrogen, in contact with which the cytoplasm of the cells vitrifies without ice crystal formation. The rewarming of samples is usually done rapidly to avoid crystal formation [21]. After thawing, a suitable micropropagation method is essential to regenerate the explants into ex vitro plants and for cryopreservation to be useful for genetic conservation. The aim of this study was to develop and test cryopreservation and micropropagation methods for U. glabra and U. laevis to function as a backup method for living genetic resource collections.
2. Materials and Methods
2.1. Plant Material
A total of 36 U. laevis and 13 U. glabra genotypes from three living genetic reserve collections and from the Punkaharju research forest were used in the experiments, as described in detail in the Supplementary Tables S1–S3. For the initiation of the media experiment, fresh U. laevis buds from five trees (E11630-E11634) from the Punkaharju research forest were collected at the beginning of September 2016. The branches from which fresh control buds were taken were kept in water in a cold room (+2 °C) until preparation. Cryopreserved buds were frozen in October 2016 and prepared starting in November 2016. For the first cryopreservation experiment, U. laevis buds from 20 trees from living gene reserve collections 205 and 232 (Paimio/Preitilä) were collected in January 2017, frozen in February 2017, and the experiment was initiated in February 2017. Reduced light intensity and Driver and Kuniyuki walnut (DKW) media were first tested with buds from the same five trees from Punkaharju (E11630-E11634) as previously. The branches were collected in November 2017 and cryopreserved in December 2017. Cultures from fresh buds were initiated in November, and from cryopreserved buds in December. Shoots from these cultures were used in later rooting experiments. Buds were collected from the following collections for the 2020 experiments: Preitilä 205 and 232 (U. laevis, ten trees), Preitilä 184 (U. glabra, five trees), and Solböle 192 (U. glabra, five trees). U. laevis buds were collected in in February 2019 (six trees, cryo in March), or January 2017 (four trees, cryo in February), and U. glabra buds were collected in March 2019 (cryo in March). For more details see Supplementary Table S2. Fresh controls of both species were collected in January 2020, and the experiment started in January 2020. Material from these experiments and from various surface sterilisation tests (not presented in this article) were also used in later cytokinin and rooting experiments. For the 2021 experiments, cryopreserved buds from five trees in U. laevis collections 205 and 232 and seven trees in U. glabra collection 184 were used, which were collected and cryopreserved at the end of January 2021. The buds were thawed, starting 13 days after cryopreservation. Twigs with dormant buds from both species were transported and stored, both with and without snow in the bags (for details on plant material see Supplementary Table S3). Shoots from these cultures were used for the 2021 rooting experiments.
2.2. Cryopreservation
For cryopreservation, elm branches or twigs with dormant buds were collected at varying times in different years between October and March (see Supplementary Tables) when the trees had been exposed to sub-zero temperatures. Twigs of 5 to 20 cm were placed in sealed plastic bags with snow or moistened tissue paper and transported with cold blocks in the container to sustain sub-zero temperature and dormancy. The branches collected from the nearby research forest in Punkaharju were up to 1 m long and placed in a bucket containing snow. All the branches were stored until preparation for cryopreservation in a cold room (–5 °C). The buds were cut with a small piece of the branch into 1.8 ml cryotubes (Sarstedt), and the tubes kept on ice overnight in a cold room (+2 °C). The buds were frozen the next day using a programmable liquid nitrogen freezer (Planer Kryo 10) 0.17 °C min−1 until they reached –38 °C [22], when they were submerged directly in liquid nitrogen. Thawing was carried out in a water bath (+38 °C, 2 min). Immediately after this, the tubes were placed on ice for at least 2 min.
2.3. Surface Sterilisation
In the first initiation and cryopreservation experiments, the buds were surface sterilised with 70% ethanol under agitation for 10 min. For the DKW experiment, the buds were kept in 10% H2O2 for 3 h before exposed to ethanol for 10 min. For the subsequent experiments, H2O2 was omitted, but disinfection time in ethanol was increased to 20 min. In 2021, sterilisation with H2O2 under agitation for 10 min was tested with four U. glabra and four U. laevis genotypes (for details on plant material see Supplementary Table S3) in the same way as for Norway spruce buds for explants [23]. The buds were kept on filter paper moistened with 100 mg/L polyvinylpyrrolidone after sterilisation until they were prepared for the initiation medium.
2.4. Initiation
Both fresh and thawed buds (Figure 1A) were prepared for culture under a stereo microscope by excising the outer bud scales and shaping the stem into a small wedge. The buds were placed into sterile De Wit polycarbonate tubes (130 × 27 mm, Duchefa Biochemie) containing 7 mL of initiation media. Modified Murashige and Skoog (MS) and modified woody plant medium (WPM) basal media (Appendix A), solidified with 6 g/L Plantagar S1000 (B&W, Parma, Italy) and with different hormone compositions were tested for U. laevis initiation: 0.1 mg/L gibberellic acid 4 + 7 (GA 4 + 7) (Ducheva Biochemie) and 0.5 mg/L 6-benzylaminopurine (BA) (Sigma Aldrich), 0.5 mg/L BA, 1 mg/L BA, or 0.02 mg/L thidiazuron (TDZ) (Sigma Aldrich) [24,25]. In later experiments, initiation media with DKW salts (5.2 g pouches for 1 L of medium, Sigma Aldrich, Appendix A) with 30 g/l sucrose and solidified with 6 g/L Plantagar S1000 and 0.1 mg/L GA 4 + 7 and 0.5 mg/L BA as growth regulators were used for both U. glabra and U. laevis [26,27,28]. All media were supplemented with varying concentrations (see Appendix A) of myo-inositol, glycine, nicotinic acid, pyridoxine-HCl, and thiamine-HCl. All media were adjusted to a pH of 5.8 prior to autoclaving for 20 min at 121 °C. GA 4 + 7, TDZ, and meta-topolin (Ducheva Biochemie) (mT) were added through filter sterilisation after autoclaving.

Figure 1.
(A) Comparison of U. laevis (left) and U. glabra (right) unprepared buds. The scale bar represents 0.1 cm. (B) Multiplication result of U. glabra genotype 0107 01 by cutting the apical part of the shoot and transferring the callus and basal part of the shoot onto new media. The scale bar represents 1 cm. (C) U. laevis genotype 0357 06 shoot grown from an in vitro bud excised for subculture. The scale bar represents 1 cm. (D) U. laevis and U. glabra shoots growing in peat 41 d after transplantation. The scale bar represents 5 cm.
After initiation, the buds were grown in the culture room at around +25 °C under Philips Master TL-D 36W/840 5G fluorescent lights (Poland) for a 16/8 light-dark photoperiod with light intensity of approximately 150 µmol m−2 s−1, with the first 2 to 3 days covered with veils to reduce initial light exposure. In the later experiments (from DKW experiments in 2017 and onwards), the veils were always kept on, keeping light intensity under 50 µmol m−2 s−1. The outcome of the initiations was classified as shoot grown, dead, or contaminated. If a shoot grew, but the culture was contaminated, it was not counted as a successful initiation.
2.5. Multiplication
After the shoots started to grow (1 to 2 weeks), they were moved into Magenta glass jars with approximately 25–30 ml of multiplication media, which was the same as initiation media, except without GA 4 + 7. Multiplication was carried out to produce enough shoots for rooting experiments. Subculture timing was determined based on the growth and visual appearance of the cultures, usually every three to four weeks. The subculture was mainly made by cutting off the apical part of the shoot and transferring both it and the basal part of the shoot with some callus onto new media (Figure 1B). With some genotypes, multiplication of nodal segments of varying length was also possible (Figure 1C). Until the 2020 experiments, the subculture was mainly made by cutting the shoot into two or more segments and transferring them without callus tissue onto fresh media. The effect of mT was tested as an alternative cytokinin at 0.5 mg/L. The tests were carried out with material initiated and multiplied with 0.5 mg/L BA as cytokinin, using four genotypes of U. glabra. For the cytokinin experiment, material with an equal number of shoots, nodal segments, or shoots with callus (Figure 2) was transferred into paired jars with either mT or BA as plant growth regulator (PGR). The shoot production was then assessed after 21 d by counting the number of shoots growing in each jar.
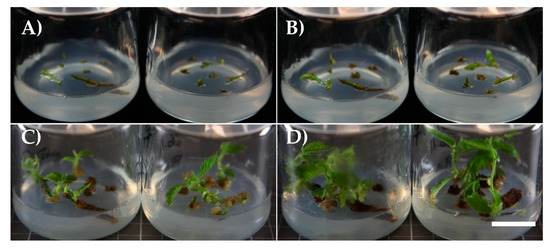
Figure 2.
An example of a jar pair in the cytokinin experiment: U. glabra genotype 0109 04 on 0.5 mg/L mT (jar on the left) and on 0.5 mg/L BA (jar on the right) (A) 1 d, (B) 7 d, (C) 14 d and (D) 21 d after subculture, with an equal number of shoots from previous media with 0.5 mg/L BA. The scale bar represents 2 cm.
2.6. Rooting
Rooting in vitro, in semi-solid media, was tried out with Punkaharju originated material grown on DKW media. Rooting was done in half-strength DKW media, either with 0.5 mg/L indole-3-butyric (IBA), no PGRs, or with 3 d induction with 3 mg/L IBA (Sigma Aldrich), after which the shoots were transferred onto hormone-free media. Liquid root induction was used for both U. glabra and U. laevis with 2020 initiated material according to Micheli et al. [29]. Induction was performed in a 5 mg/L IBA solution with 15 g/L sucrose and was tested for six genotypes of U. laevis and six genotypes of U. glabra. The shoots without callus tissue were cut and put into rooting liquid and kept in the dark for 2 to 3 days. After the induction period, the shoots were directly transplanted into growing containers (Pl 81f) filled with semi-coarse pre-fertilised light sphagnum peat in a greenhouse on 2 July in 2020 and 20 May in 2021. The large greenhouse had been sown with Norway spruce container seedlings, and the greenhouse conditions were maintained in conditions favourable for Norway spruce germination [30,31]. Relative humidity was kept high (above 80%), and the temperature was above +20 °C for three weeks, after which the humidity was gradually decreased by increasing ventilation. The survival of the shoots was evaluated 41 days (2020) (Figure 1D) or 21 days (2021) after transplantation. In the 2021 experiment, the largest leaves of the cuttings were trimmed to a smaller size to reduce evaporation. The survival of the shoots was evaluated on site in 2020 and from photographs in 2021. For the rooting of shoots from the multiplication experiment, the shoots were rooted and transplanted in the same way as for other experiments but were grown in the culture room at 25 °C in ventilated mini greenhouses and irrigated manually.
2.7. Statistical Analysis
Statistical analyses were conducted using SPSS Version 25 software. The differences in shoot production in the cytokinin experiment were investigated with independent samples using the Mann-Whitney U-test, because the data were not normally distributed. The differences in shoot formation and rooting success were analysed with a Chi-square test in all experiments. If any cells in the analysis had an expected value <5, Fischer’s exact test was used instead. A p-value < 0.05 was considered significant. The effect of location inside the containers was ruled out with logistic regression (not shown) with row and column covariates, which explained only 0.4% of correctly predicted cases after the species variable.
3. Results
3.1. Cryopreservation and Initiation
The different basal initiation media and hormone compositions were tried out for shoot culture initiation with five genotypes collected from Punkaharju (Figure 3). Both cryopreserved and fresh buds were cultured with GA 4 + 7; other media were tested only for fresh buds. No significant differences were found on MS with 0.1 mg/L GA 4 + 7 and 0.5 mg/L BA between cryopreserved and fresh buds, but on WPM with the same PGRs, the difference was significant (p < 0.001). When all the initiations were compared regardless of the PGRs, MS had a 45% success compared to 28% with WPM (χ2 (1) = 16.947; p < 0.001).
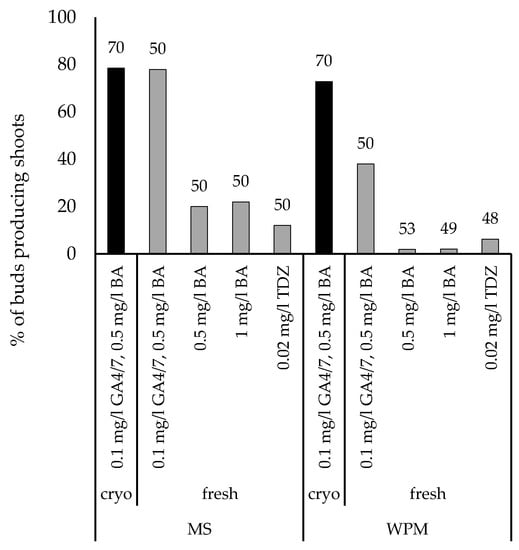
Figure 3.
The effect of initiation media base and PGR concentrations, and cryopreservation on the shoot regeneration of five genotypes of U. laevis collected from the Punkaharju research park. The total number of fresh (8–12 per genotype) or cryopreserved (14 per genotype) buds prepared for each media is shown on the top of the bar.
Based on the results, MS with 0.1 mg/L GA 4 + 7 and 0.5 mg/L BA was selected for further experiments, and further verified with 20 genotypes from Preitilä collections 205 and 232. A slightly higher proportion of prepared cryopreserved U. laevis buds produced a shoot (64%, n = 222, 10–12 per genotype) than fresh (57%, n = 200, 10 per genotype) U. laevis buds, but the difference was not significant (χ2 (1) = 2.138; p = 0.144). However, despite good initiation frequencies, the shoots tended to die after moving onto multiplication media. DKW-based initiation and multiplication media were therefore tried out as an alternative for buds collected from five trees from Punkaharju. With reduced light intensity (from 150 to less than 50 µmol m−2 s−1), this resulted in an improved shoot formation with 82% (n = 28) success for fresh and 94% (n = 51) for cryopreserved buds. Two hours of H2O2 treatment was added to the surface sterilisation protocol, because contaminations were a problem with earlier sets of buds (data not shown). No significant differences were found in survival between cryopreserved and fresh buds (Fischer’s exact test; p = 0.099). Cryopreservation experiments were conducted again in 2020 with a larger set of genotypes, and with both U. glabra and U. laevis (Figure 4A). Cryopreserved U. glabra buds showed poorer regeneration than fresh buds (χ2 (1) = 22.806; p < 0.001) or cryopreserved U. laevis buds (χ2 (1) = 51.598; p < 0.001). No significant differences were found between cryopreserved and fresh U. laevis buds. U. glabra buds had significantly more contaminations than U. laevis buds (χ2 (1) = 32.072; p < 0.001). Surface sterilisation was done for 20 min in 70% ethanol. Contaminations were more prevalent than in the first initiation and cryopreservation experiments. U. glabra appeared to suffer from cryopreservation more than U. laevis. Shoots were also lost after transfer to a multiplication medium. However, based on the results from initiated fresh buds, the same DKW medium also appears suitable for U. glabra, as nine out of ten genotypes produced shoots from fresh buds. Similar results were obtained for the 2021 experiment with only cryopreserved buds with U. glabra having a significantly lower initiation success rate (χ2 (1) = 27.842; p < 0.001) and higher contamination rate (χ2 (1) = 19.910; p < 0.001) than U. laevis (Figure 4B).
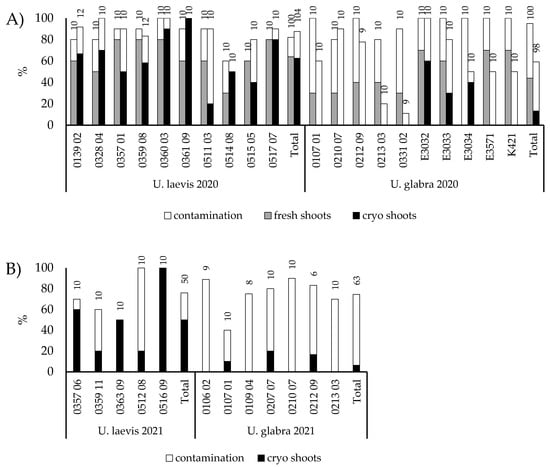
Figure 4.
Shoot production and contamination rates (i.e., both growing and non-growing explants showing contamination) of U. laevis and U. glabra buds of different genotypes in (A) 2020 and (B) in 2021. The remaining portion of the 100% represents failed but non-contaminated initiations. In 2020, both fresh and cryopreserved buds were used; in 2021, only cryopreserved ones. The number of buds prepared is shown at the top of the bar for each genotype and for the total number of genotypes in that treatment.
3.2. Surface Sterilisation
The sterilisation time in 70% ethanol was increased from 10 min to 20 min for buds prepared in 2020 and thereafter. Different sterilisation methods with PPM, antibiotics and sodium hypochlorite were tested in preliminary trials, but with not markedly better and sometimes worse results than the ethanol-sterilised controls (data not shown). The contamination rates from different experiments are not comparable, as different plant material was used. For the 2021 experiments, a surface sterilisation method that works for Norway spruce primordial shoot explants according to Varis et al. [23] with H2O2 was tested. This resulted in significantly higher contamination rates than with controls sterilised with ethanol for both U. laevis (49% vs 23%, χ2 (1) = 6.084; p = 0.014) and U. glabra (97% vs 82%, Fischer’s exact test; p = 0.049). No significant differences in contamination rate were found between dry (no snow) and wet storage (with snow or moistened tissue paper in the plastic bag) conditions after collection until preparation for cryopreservation.
3.3. Multiplication
As an alternative to BA, mT, was tested as a cytokinin for U. glabra (Figure 5). Slightly more shoots were obtained from mT-media (66 vs 50 shoots in total), but the difference was not statistically significant. The rooting of shoots was also tested with liquid IBA induction, and no differences were found between cytokinins used in the multiplication stage.
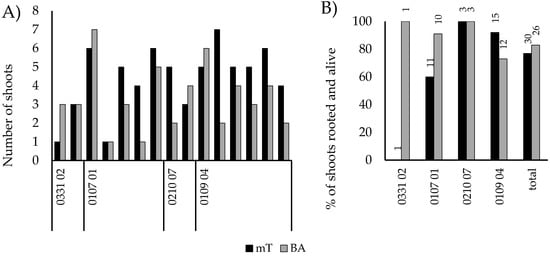
Figure 5.
The effect of cytokinin type on multiplication and rooting success of four genotypes of U. glabra. (A) Shoot production from each jar-pair with a comparison with similar starting material. (B) Rooting success of shoots from both media, with the number of shoots for rooting and transplanted shown on the top of each bar.
During the shoot multiplication phase, the growth habit and multiplication rate of different genotypes varied substantially. Some genotypes, especially in U. glabra, produced excessive amounts of callus with nodal segments, and the subculture with them was unsuccessful. Sometimes transferring a whole shoot, especially without a callus, resulted in withering of the shoots. However, this was not always the case. For some genotypes, subcultures were successful even with a very limited amount of starting material (Figure 2).
3.4. Rooting and Transplantation
From the first rooting experiments with U. laevis on solidified half-strength DKW medium, the best results were obtained from 3 mg/L induction treatment, with 67% (n = 30) of the shoots alive and with roots visible in the medium two weeks after induction. With constant 0.1 mg/L IBA rooted and alive were 45% (n = 31) and with hormone-free media 7% (n = 29). Rooting was tested for a larger set of shoots grown from buds prepared in 2020 and 2021, for both U. laevis and U. glabra. First, a small-scale test was done with both liquid and agar induction, with liquid media giving better results (data not shown). Therefore, liquid induction was tested in July 2020 on a larger scale, resulting in 18% of U. laevis shoots and 64% of U. glabra shoots being rooted and alive 41 days after transplantation (Figure 6A and Figure 1D). Rooting was significantly more successful for U. glabra than for U. laevis (χ2 (1) = 47.645; p < 0.001). Similar results were obtained U. laevis from the May 2021 experiment, with 20% of shoots surviving (Figure 6B). However, for U. glabra, the results were markedly worse than in 2020, with only 9% survival.
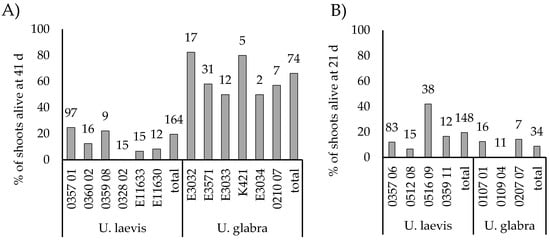
Figure 6.
(A) Rooting success of micro-propagated elm shoots following root induction in liquid 41 d from transplantation in July 2020. (B) Rooting success of micro-propagated shoots following root induction in liquid 21 d after transplantation in May 2021. The number at the top of the bar represents the total number of shoots transplanted from each genotype or species.
4. Discussion
The establishment of in vitro cultures and regeneration of explants into plants capable of surviving ex vitro from cryopreserved material is a multi-stage process with many steps requiring optimisation. However, many protocols for micropropagation and cryopreservation of plant material have been developed and function as an excellent starting point. Multiple protocols for elms can also be found [17,28,32]. However, the protocols can be improved upon and must always be tested for the specific plant materials in question. For cryopreservation to serve as a backup for the collections of living trees, the methods need to be validated and proven reliable. It was possible to regenerate U. laevis equally from both fresh and cryopreserved dormant buds. This is consistent with results from other deciduous trees, e.g., Betula pendula Roth [22] and hybrid aspen Populus tremula L.× Populus tremuloides Michx. [33]. The first results indicated slightly better regeneration with cryopreserved than with fresh buds, which is in agreement with the results of Harvengt et al. [17]. However, cryopreservation can decrease viability, for example, with Malus species [34,35]. This was the case with U. glabra, as in vitro cultures from cryopreserved buds were harder to establish than from fresh buds. According to Harvengt et al., micrografting can be used to successfully regenerate cryopreserved U. glabra buds but is unnecessary for U. laevis and Ulmus minor Mill. Compared to U. laevis, U. glabra buds were larger (see Figure 1A), which could lead to a more uneven temperature change within the tissues and subsequent tissue damage. With Prunus persica (L.), tissue cracking of the connecting tissue between the bud and the stem has been reported for cryopreserved material, resulting in their separation and bud falling off [36]. A similar phenomenon was occasionally observed with U. glabra. This could possibly be ameliorated by a slower cooling rate or desiccation to around 25% to 30% water content prior to freezing, as is often done with dormant bud cryopreservation [19,36]. Adding GA to initiation media improved the initial shoot formation of U. laevis. However, GA can also have negative effects on tissue cultures and was omitted after the first subculture [27]. Based on these results with Punkaharju U. laevis material, GA 4 + 7 was used for all the subsequent initiations. GA 3 has been used for U. americana proliferation [18], and GA 4 + 7 substantially improved U. laevis initiation frequencies. When the poor survival after successful shoot induction on multiplication medium in 2017 experiments was observed, several actions to improve the protocol were taken. First, the basal media was switched from MS to DKW following the recommendation of Fenning et al. [28]. Successful micropropagation of both U. laevis and U. glabra on MS has been reported [17,32]. However, DKW basal medium has been reported to suit U. americana [18] and Ulmus procera Salisb., which does not establish on MS [28]. Second, the light intensity used (more than 150 µmol m−2 s−1) was found to be excessive compared with published elm protocols and was reduced to less than 50 µmol m−2 s−1 found suitable for several elm species [17,28,37]. As both adjustments were made simultaneously, it is not certain if the better shoot survival in later experiments was due to one of the factors or their interaction. However, based on our results, DKW basal medium suits both U. glabra and U. laevis. High contamination rates were the biggest challenge, especially with U. glabra. Contamination reduces regeneration reliability from cryostorage and currently presents a major obstacle for the successful application of cryopreservation in U. glabra genetic resources conservation. Compared with U. laevis buds, U. glabra have larger buds and hairier bud scales. This could make it more difficult for sterilising agents to achieve the potential contamination sources. Multiple surface sterilisation methods and anti-microbial agents were tested for different sets of buds, but without better results than with controls sterilised with 70% ethanol (data not shown). The sterilisation method used initially was very light, with only a 10-min exposure to ethanol, and close to no contaminations were encountered in the first experiments. This was based on working sterilisation methods for aspen and birch [22,33], for which even lighter methods are used successfully. However, generally more extreme, and often chlorite-based methods are reported for elm species due to contaminations often presenting challenges [17,28,38]. Multiple methods were unsuccessfully tested on a small scale, with controls sometimes outperforming treatments. Antibiotics were also tested, with a decrease in bacterial but an increase in fungal contaminations (data not shown). U. laevis cultures were generally easier to multiply than U. glabra, although there were differences between genotypes. The subsequent rooting experiments therefore had highly varying numbers of shoots for testing from different genotypes, and almost twice the number of U. laevis shoots were obtained than from U. glabra. Alternative cytokinin, mT was tested for U. glabra cultures to improve shoot growth. MT has been shown to improve U. glabra shoot multiplication [37] and reduce physiological disorders like shoot tip necrosis [38]. No differences were found between the two cytokinins in this study. However, the culture time on mT media was only 21 d, and BA residues could be present in the plants. To obtain a more comprehensive view, the two cytokinins should be compared from the outset. MT is reported to cause less rooting inhibition than BA [39], but no differences were found between rooting from either culture. IBA or 1-naphthaleneacetic acid is used in elm rooting in most protocols [17,28], either as a continuous low concentration or as higher induction concentration, after which the shoots are transferred to hormone-free media. Auxin was needed for U. laevis rooting, as only 7% of shoots on hormone-free media survived rooting and produced roots, in contrast with 67% on the IBA induction media. However, some of the shoots with in vitro grown roots were lost after transplanting, resulting in greenhouse plant loss. Rooting in peat directly after liquid induction was more successful with U. glabra than with U. laevis in 2020, but not in 2021. Direct transplanting may be more sensitive to edaphic and ambient conditions and may require more advanced climate control than present in the large commercial greenhouse where the tests were conducted. However, the rooting of U. glabra for the multiplication experiment gave better results, suggesting advantages in using mini greenhouses. Rooting of the shoots directly after induction was done to eliminate damage caused to fragile in vitro roots when they were removed from agar. This also reduced labour because no solidified media was needed for rooting. Liquid induction still needs to be tested for U. laevis in mini greenhouses to see if this results in better survival. Alternatively, rooting induction should be further tested on semi-solid media. If either rooting success or multiplication rate are poor, they can be compensated with longer multiplication with more subcultures and with multiple rounds of rooting. Therefore, the initiation and establishment of vigorous and contamination-free in vitro cultures constitute the main limiting step for successful regeneration. Based on our results with the described method, approximately 40% of U. glabra and 80% of U. laevis genotypes could be regenerated. Although there are genotypical differences, few genotypes appear completely recalcitrant to regeneration. However, with the high contamination rates of U. glabra, this is difficult to estimate.
5. Conclusions
The aim of this study was to create reliable backup storage for living elm genetic reserve collections in the face of the potential DED threat in Finland. The micropropagation protocol works for both U. laevis and U. glabra, and the regeneration of most U. laevis genotypes from cryostorage is successful. Rooting without any agar media works for U. glabra but needs to be further tested for U. laevis. This can majorly reduce labour for micropropagation especially with potentially multiple rounds of rooting. However, contaminations in bud explants need to be reduced, especially in U. glabra, and the cryo-protocol then re-evaluated for U. glabra to improve the initiation frequency. After these improvements, the method can be applied to the conservation of elm genetic resources, and knowledge of regeneration success can be used to design the cryoconservation plan and optimise the sampling. Considering the approach of DED, this is an important complementary option for conservation of elm genotypes adapted to northern latitudes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/f12081121/s1, Table S1: Information on the plant material (bud explants per treatment) used in 2017 experiments. Table S2: Information on the plant material (bud explants per treatment) used in 2017 experiments. Table S3: Information on the plant material used in 2021 experiments.
Author Contributions
Conceptualisation, M.R. and T.A.; methodology, S.V., D.P., M.T., and T.A.; formal analysis, S.V.; investigation, S.V. and D.P.; resources, M.R. and T.A.; data curation, S.V.; writing—original draft preparation, S.V.; writing—review and editing, S.V., M.R., D.P., M.T., and T.A.; visualisation, S.V.; supervision, T.A.; project administration, M.R. and T.A.; funding acquisition, S.V., M.R., and T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Finnish Ministry of Agriculture and Forestry and the Finnish Cultural Foundation, South Savo Regional fund.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We wish to thank the technical staff at Luke Finland involved in bud collection, laboratory and greenhouse work, and Trevor Fenning for invaluable advice on the various steps of the micropropagation process.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Different basal media used in the experiments (mM). Both MS and WPM were modified media with stocks prepared in-house, whereas DKW was bought from Sigma Aldrich (Merck) as ready salt mix. Organic components were in line with instructions from Dr T. Fenning.
Table A1.
Different basal media used in the experiments (mM). Both MS and WPM were modified media with stocks prepared in-house, whereas DKW was bought from Sigma Aldrich (Merck) as ready salt mix. Organic components were in line with instructions from Dr T. Fenning.
| DKW | MS | WPM | |
|---|---|---|---|
| H4NO3 | 17.70 | 10.30 | 5.00 |
| KNO3 | 9.40 | ||
| KH2PO4 | 1.95 | 0.62 | 1.25 |
| MgSO4 | 3.00 | ||
| MgSO4·7H2O | 3.00 | 1.50 | |
| CaCl2 | 1.01 | ||
| CaCl2·2H2O | 1.50 | 0.65 | |
| Ca(NO3)2 | 8.33 | ||
| Ca(NO3)2·4H2O | 2.35 | ||
| K2SO4 | 8.95 | 5.68 | |
| MnSO4·H2O | 0.20 | 0.26 | 0.13 |
| ZnSO4·7H2O | 0.06 | 0.03 | |
| CuSO4 x 5H2O | 0.001 | 0.002 | 0.001 |
| KI | 0.005 | ||
| CoCl2·6H2O | 0.0001 | ||
| Zn(NO3)2 | 0.09 | ||
| NiSO4·6H2O | 0.00002 | ||
| H3BO3 | 0.08 | 0.10 | 0.10 |
| Na2MoO4·2H2O | 0.002 | 0.001 | 0.001 |
| Na2-EDTA | 0.12 | ||
| FeSO4·7H2O | 0.12 | ||
| NaFe-EDTA | 0.05 | 0.11 | |
| Myo-Inositol | 5.55 | 0.56 | 0.56 |
| Glycine | 0.027 | 0.027 | 0.027 |
| Nicotinic acid | 0.008 | 0.004 | 0.004 |
| Pyridoxine-HCl | 0.010 | 0.002 | 0.002 |
| Thiamine-HCl | 0.006 | 0.0003 | 0.003 |
References
- Buiteveld, J.; Van der Werf, B.; Hiemstra, J.A. Comparison of commercial elm cultivars and promising unreleased Dutch clones for resistance to Ophiostoma novo-ulmi. IForest 2015, 8, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Collin, E. EUFORGEN Technical Guidelines for Genetic Conservation and Use for European White elm (Ulmus laevis); International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 2003. [Google Scholar]
- Caudullo, G.; De Rigo, D. Ulmus-elms in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg City, Luxembourg, 2016; pp. 186–188. [Google Scholar]
- Vakkari, P.; Rusanen, M.; Kärkkäinen, K. High genetic differentiation in marginal populations of European white elm (Ulmus laevis). Silva Fenn. 2009, 43, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.A.; Sobrino-Plata, J.; Rodríguez-Calcerrada, J.; Collada, C.; Gil, L. Breeding and scientific advances in the fight against Dutch elm disease: Will they allow the use of elms in forest restoration? New For. 2019, 50, 183–215. [Google Scholar] [CrossRef] [Green Version]
- Brasier, C.M.; Buck, K.W. Rapid evolutionary changes in a globally invading fungal pathogen (Dutch elm disease). Biol Invasions 2001, 3, 223–233. [Google Scholar] [CrossRef]
- Brasier, C.M. Ophiostoma novo-ulmi sp. nov., causative agent of current Dutch elm disease pandemics. Mycopathologia 1991, 115, 151–161. [Google Scholar] [CrossRef]
- Newbanks, D.; Bosch, A.; Zimmermann, M.H. Evidence for xylem dysfunction by embolization in Dutch elm disease. Phytopathology 1983, 73, 1060–1063. [Google Scholar] [CrossRef] [Green Version]
- Whitten, R.R.; Single, R.U. The Dutch Elm Disease and Its Control; US Department of Agriculture: Washington, DC, USA, 1958.
- Hannunen, S.; Marinova-Todorova, M. Pest Risk Assessment for Dutch elm disease; Evira Research Reports 1/2016, Finnish Food Safety Authority Evira: Helsinki, Finland, 2016. [Google Scholar]
- Webber, J.F. Experimental studies on factors influencing the transmission of Dutch elm disease. For. Syst. 2004, 13, 197–205. [Google Scholar]
- Mittempergher, L.; Santini, A. The history of elm breeding. For. Syst. 2004, 13, 161–177. [Google Scholar]
- Pehu, T.; Kiviharju, E.; Rusanen, M.; Kantanen, J.; Heinimaa, P. Suomen maa-, metsä-ja kalatalouden kansallinen geenivaraohjelma (Finnish National Genetic Resources Programme for Agriculture, Forestry and Fishery); Ministry of Agriculture and Forestry: Helsinki, Finland, 2018; pp. 67–88.
- Kaviani, B. Conservation of plant genetic resources by cryopreservation. Aust. J. Crop. Sci. 2011, 5, 778–800. [Google Scholar]
- Häggman, H.; Rusanen, M.; Jokipii, S. Cryopreservation of in vitro tissues of deciduous forest trees. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 365–386. [Google Scholar]
- Collin, E.; Rondouin, M.; Joyeau, C.; Matz, S.; Raimbault, P.; Harvengt, L.; Bilger, I.; Guibert, M. Conservation and use of elm genetic resources in France: Results and perspectives. IForest 2020, 13, 41–47. [Google Scholar] [CrossRef]
- Harvengt, L.; Meier-Dinkel, A.; Dumas, E.; Collin, E. Establishment of a cryopreserved gene bank of European elms. Can. J. For. Res. 2004, 34, 43–55. [Google Scholar] [CrossRef]
- Shukla, M.R.; Jones, A.M.P.; Sullivan, J.A.; Liu, C.; Gosling, S.; Saxena, P.K. In vitro conservation of American elm (Ulmus americana): Potential role of auxin metabolism in sustained plant proliferation. Can. J. For. Res. 2012, 42, 686–697. [Google Scholar] [CrossRef]
- Towill, L.E.; Ellis, D.D. Cryopreservation of dormant buds In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 421–442. [Google Scholar]
- Benson, E.E. Cryopreservation theory. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 15–32. [Google Scholar]
- Reed, B.M.; Uchendu, E. Controlled rate cooling. In Plant Cryopreservation: A Practical Guide; Reed, B.M., Ed.; Springer: New York, NY, USA, 2008; pp. 77–92. [Google Scholar]
- Ryynänen, L. Survival and regeneration of dormant silver birch buds stored at super-low temperatures. Can. J. For. Res. 1996, 26, 617–623. [Google Scholar] [CrossRef]
- Varis, S.; Klimaszewska, K.; Aronen, T. Somatic embryogenesis and plant regeneration from primordial shoot explants of Picea abies (L.) H. Karst. somatic trees. Front. Plant. Sci. 2018, 9, 1551. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Intl. Plant Prop. Soc. Proc. 1980, 30, 421–427. [Google Scholar]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstock. HortScience 1984, 19, 507–509. [Google Scholar]
- Nickell, L.G.; Tulecke, W. Responses of plant tissue cultures to gibberellin. Bot. Gaz. 1959, 120, 245–250. [Google Scholar] [CrossRef]
- Fenning, T.M.; Gartland, K.; Brasier, C.M. Micropropagation and regeneration of English elm, Ulmus procera Salisbury. J. Exp. Bot. 1993, 44, 1211–1217. [Google Scholar] [CrossRef]
- Micheli, M.; Hafiz, I.A.; Standardi, A. Encapsulation of in vitro-derived explants of olive (Olea europaea L. cv. Moraiolo): II. Effects of storage on capsule and derived shoots performance. Sci. Hortic. 2007, 113, 286–292. [Google Scholar] [CrossRef]
- Rikala, R. Metsäpuiden Paakkutaimien Kasvatusopas (Container Seedling Growing Manual for Forest Trees); The Finnish Forest Research Institute: Suonenjoki, Finland, 2012; 247p. [Google Scholar]
- Landis, T.D.; Dumroese, R.K.; Haase, D. The Container Tree Nursery Manual. Vol 6. Seedling Propagation. Agricultural Handbook 674; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2010.
- Malá, J.; Cvikrová, M.; Chalupa, V. Micropropagation of mature trees of Ulmus glabra, Ulmus minor and Ulmus laevis. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Häggman, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 237–246. [Google Scholar]
- Aronen, T.; Ryynänen, L. Cryopreservation of dormant in vivo-buds of hybrid aspen: Timing as critical factor. Cryoletters 2014, 35, 385–394. [Google Scholar]
- Forsline, P.L.; Towill, L.E.; Waddell, J.W.; Stushnoff, C.; Lamboy, W.F.; McFerson, J.R. Recovery and longevity of cryopreserved dormant apple buds. J. Am. Soc. Hortic. Sci. 1998, 123, 365–370. [Google Scholar] [CrossRef]
- Höfer, M. Cryopreservation of winter-dormant apple buds: Establishment of a duplicate collection of Malus germplasm. Plant Cell Tissue Organ Cult. 2015, 121, 647–656. [Google Scholar] [CrossRef]
- Tanner, J.D.; Minas, I.S.; Chen, K.Y.; Jenderek, M.M.; Wallner, S.J. Antimicrobial forcing solution improves recovery of cryopreserved temperate fruit tree dormant buds. Cryobiology 2020, 92, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Malá, J.; Máchová, P.; Cvrčková, H.; Karady, M.; Novák, O.; Mikulík, J.; Dostál, J.; Strnad, M.; Doležal, K. The role of cytokinins during micropropagation of wych elm. Biol. Plant. 2013, 57, 174–178. [Google Scholar] [CrossRef]
- Mirabbasi, S.M.; Hosseinpour, B. Prevention of shoot tip necrosis, hyperhydricity and callus production associated with in vitro shoot culture of Ulmus glabra. J. Nov. Appl. Sci. 2014, 3, 683–689. [Google Scholar]
- Werbrouck, S.P.; Strnad, M.; Van Onckelen, H.A.; Debergh, P.C. Meta-topolin, an alternative to benzyladenine in tissue culture? Physiol. Plant. 1996, 98, 291–297. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).