Abstract
This study investigated the efficacy of Rotstop®, a native Latvian Phlebiopsis gigantea strain and 35% urea solution in combination with a stump cover treatment to control against natural spore infection by Heterobasidion spp. upon precommercial thinning of Norway spruce in three stands growing on former agricultural lands. The major findings were that (i) infection rates of Heterobasidion spp. on stumps treated with the native P. gigantea strain, Rotstop® or urea are similar when stumps are uncovered, and (ii) stump cover promotes stump colonization by the Latvian P. gigantea strain and Rotstop®, leading to a significantly smaller relative area colonized by Heterobasidion spp., as well greater efficiency against Heterobasidion in comparison with urea. Covering of stumps appears beneficial for controlling Heterobasidion stump colonization and may be valuable to forest owners if used in small-scale operations, but it is impractical in automatized thinnings, where managers should consider using regular Rotstop® without covering the stumps.
1. Introduction
Heterobasidion annosum sensu lato (Fr.) Bref. is a species complex of necrotrophic, root and white rot pathogens of conifers, comprising five species distributed in the Northern Hemisphere [1]. Three of the species are native to Europe: (i) Heterobasidion annosum sensu strictum (Fr.) Bref., primarily a pathogen of Scots pine (Pinus sylvestris L.) but also other pines and conifers; (ii) Heterobasidion parviporum Niemelä & Korhonen, a pathogen of Norway spruce (Picea abies (L.) Karst.); and (iii) Heterobasidion abietinum Niemelä & Korhonen, largely a pathogen of silver fir (Abies alba Mill.) and other Abies species. Heterobasidion irregulare (Underw.) Garbel. & Otorsina is native to North America; however, it was introduced into Europe in the 1940s and became invasive by spreading in Pinus pinea L. and Querqus spp. stands [2]. In intensively managed forests and plantations, Heterobasidion spp. is a major threat to timber production, owing to growth reduction and increased tree mortality, with financial losses estimated as more than 790 million euros per year in Europe alone. Moreover, these calculations do not include wind and storm damage in decay-affected stands, damage that may be or may become (due to climate change) extremely significant [1] and references therein. Disease development is largely dependent on forest management practices [3,4,5]. Primary infection of the fungus occurs by airborne spores infecting newly exposed wood surfaces [3,6]. Secondary infection from Heterobasidion spp. infected stumps and trees to healthy trees may occur belowground through interconnected root systems [3,6,7,8,9]. Stumps also serve as the main structure for developing fruiting bodies [10]. In spruce stands of Latvia, approximately 23% of cut trees are colonized by rot-causing fungi, most often H. parviporum [11].
While practically impossible to eradicate once established in a stand, the disease can be managed in healthy stands by certain preventative measures that limit primary infections. Stump and root removal of infected and neighboring tree material is an effective method, but it is expensive, requires specialized machinery and is hence rarely used in practice [12]. Harvesting and thinning during nonsporulation times greatly reduces the risk of new infections and should be done when possible. When logging during periods of sporulation, stump surfaces should be treated with a chemical or biological control agent (BCA) [13]. There are a few chemicals shown to be effective at reducing Heterobasidion colonization, the most important being urea. In efforts to reduce the use of chemicals in forestry, many countries in Europe have opted for the BCA Rotstop®. Rotstop® is a commercial formulation containing spores of the fungus Phlebiopsis gigantea (Fr.) Jülich, which is a naturally occurring saprotrophic fungus that effectively outcompetes Heterobasidion spp. for nutrients in the woody stumps. Different versions of Rotstop have been formulated based on local strains of P. gigantea, such as the PG suspension in the UK and PG IBL in Poland. BCA has been further developed to be compatible with the increased mechanization and use of harvesting machines and can now be applied directly to cut stumps at the time of felling through specialized sawblades [14,15,16,17,18,19,20]. BCA containing various strains of P. gigantea show higher efficacy in pine stumps in comparison to spruce stumps [21,22,23]. Urea is a chemical alternative to BCA [16,20,24,25] and registered for use in Finland, United Kingdom, Denmark, France, Ireland [2,16] and Latvia [19].
Urea and Rotstop® generally have various efficacy rates in spruce stumps [26,27,28]; however, BCA are considered to be more susceptible to biotic and abiotic factors, while urea is more stable [29]. The efficacy of Rotstop® and urea is dependent on stump coverage [28,30]. Oliva et al. [31] showed that urea is a reliable, long-term (at least 15 years) protection method against root and butt rot of Norway spruce. Only a few studies have directly compared the efficacy of BCA to urea in spruce stumps in the same experiment [20,28,30,32,33], and these yielded inconsistent results. The efficacy of stump treatment with urea solution and spore suspension of P. gigantea against infection by Heterobasidion spp. has been compared in field conditions in Abies cilicica wood, and urea showed higher efficacy than BCA [34]. Data obtained in Denmark showed that urea more effectively prevented the spread of Heterobasidion root rot to adjacent P. abies than Rotstop® or local strains of P. gigantea [33]. In Italy, the efficacy of urea at different concentration levels (10–30%) and Rotstop® has been compared [5], the data showing similar efficacy for a 30% urea (w/v) concentration and BCA. Contrastingly, Anselmi and Nicolotti [27] reported that the efficacy of P. gigantea was higher than that of 30% urea. In addition, the type of treated wood surface can have an impact; urea showed higher efficacy in logs, whereas Rotstop® and local strains of P. gigantea were more efficient in spruce stumps [26].
Heterobasidion spp. infection risk is particularly high in stands on former agricultural land [35,36]. Therefore, it is very important to analyze treatment agents against primary infection in spruce stands planted on former agricultural soil. In the literature available, there are only limited data where the efficacy of both BCA and urea against basidiospore infection in spruce stands on former agricultural lands has been compared.
Stumps are sometimes covered with wood discs, moss and soil to increase BCA efficacy [37,38,39]. However, stump cover could promote the development of other fungi, including Heterobasidion spp. [40,41]. Yet, the influence of stump cover with discs on the efficacy of urea against Heterobasidion spp. basidiospore infection is unknown. The aims of this study were to test the control efficiency of Rotstop®, a native Latvian Phlebiopsis gigantea strain and urea as control agents against natural spore infection of Heterobasidion spp. on pre-commercial thinning stumps of Norway spruce on former agricultural lands, and to analyze the effect of stump coverage on urea and BCA efficacy.
2. Materials and Methods
2.1. Plant and Fungal Material
The experiment was established in 2018 on three, first-rotation Norway spruce stands in Rezekne (Eastern Latvia). Site characteristics are detailed in Table 1. In Stands 1 and 3, precommercial thinning was conducted in 2016, prior to our experiment. To reduce the risk of secondary infections via root contacts from these thinned trees, a 3 m buffer zone between trees used in this experiment and old stumps was implemented. Commercial Rotstop® (Phlebiopsis gigantea strain VRA 1835) and Latvian P. gigantea strain 422 (in text P. gigantea 422), initially isolated from Norway spruce and previously characterized in vitro on malt agar for growth, asexual spore production and antagonism against H. annosum and H. parviporum [42,43], were used as BCA for stump treatments.
2.2. Experimental Description
At each of the three sites, 160 trees were cut using a chainsaw in July 2018 (in total 480 trees) to a stump height of 70 cm. None of the stumps showed signs of discoloration or decay and were presumed to be free of Heterobasidion infection at the time of cutting. Stumps were left at a 70 cm height for one week until they could be further treated. Outer bark was disinfected by treating them with 70% ethanol to reduce the unintended introduction of microbes to cut surfaces before treatment application [44]. For all sites, half of the stumps were cut to a height of 40 cm, while the other half were cut to 45 cm. The 45 cm high stumps then had a 5 cm thick disk cut from the top of the stump, which was kept and used for the subsequent stump cover treatment. After cutting, each stump was treated with one of four stump treatments: Rotstop® spore suspension, P. gigantea 422 spore suspension, 35% urea solution or distilled water. Rotstop® and P. gigantea 422 spore suspensions were prepared as described by Kenigsvalde et al. [45]. The amount applied varied according to the diameter of the stump surface so that the solution covered the surface with a thickness of about 1 mm [46].
After stump treatment, the 5 cm thick wood discs were replaced on top of their respective stumps, while the other stumps were left uncovered to create 8 unique treatment combinations per site (Rotstop® covered (n = 20), Rotstop® uncovered (n = 20), P. gigantea 422 covered (n = 20), P. gigantea 422 uncovered (n = 20), 35% urea covered (n = 20), 35% urea uncovered (n = 20), water covered (n = 20) and water uncovered (n = 20)). All stumps were subjected to natural Heterobasidion spp. infection. To avoid clustering of a certain treatment to one area of the site, treatments were assigned to stumps according to a randomized complete block design that was identical for all experimental sites. During the establishment of the experiments and the three subsequent weeks, the air temperature fluctuated between 8.9 and 30.5 °C, with a mean of 20.3 °C. Total precipitation in the three-week period following establishment was 51 mm.
2.3. Sampling, Heterobasidion spp. Infection Assessment and Identification of P. gigantea
The stumps were disinfected by treating them with 70% ethanol and sampled 14 weeks after cutting (Table 1). Identification tags from four stumps disappeared prior to sampling, so these trees were excluded, and samples were taken from the remaining 476 stumps. Two 3 cm thick discs were cut from each stump with a chainsaw. The top disc was discarded, and the second disc was taken to the laboratory and assessed for Heterobasidion spp. infection. Discs were examined for the presence of Heterobasidion spp. conidiophores [47], and the presence of P. gigantea was estimated by morphological inspection of the mycelia and presence of oidia (e.g., [17,18,30,48]). The area colonized by P. gigantea (either Rotstop®, P. gigantea 422 or naturally infected by airborne P. gigantea spores (in the text referred to as wild P. gigantea)) and Heterobasidion spp. was redrawn on a transparent sheet and measured using a planimeter (PLANIX 10S “Marble”, Tamaya, Japan). Re-isolations from 20 of the Rotstop® and P. gigantea 422 treated stumps were done to confirm successful colonization of the stumps. Somatic incompatibility assays for all isolates were performed. Isolates were paired on malt agar with the original strain used for inoculation to test for compatibility to confirm their identity [49].

Table 1.
Description of experimental sites and stump characteristics.
Table 1.
Description of experimental sites and stump characteristics.
| Site | Latitude, Longitude | Stand Age (Years) | Area (ha) | Forest Type | Number of Stumps | Mean Stump Diameter ±1 SD (cm) 5 | Stump Diameter, Min–Max, (cm) |
|---|---|---|---|---|---|---|---|
| 1 | 56.24088, 27.88769 | 15 1 | 5.83 | Oxalidosa2 | 160 | 11.5 ± 5.9 A | 8.4–16.0 |
| 2 | 56.22804, 27.97499 | 15 1 | 2.38 | Oxalidosa turf. Met.3 | 160 | 11.8 ± 5.9 A | 8.4–14.2 |
| 3 | 56.22430, 27.83745 | 15 1 | 8.44 | Hylocomisa4 | 160 | 7.8 ± 5.7 B | 4.1–14.5 |
1 No visual signs of heartwood; 2 Mesotrophic P. abies stands on mineral soil at the age of 100 years, tree height is 28–33 m [50]; 3 Highly productive mixed spruce and broad-leaved stands on eutrophic-rich drained peat soils [50]; 4 Mesotrophic P. abies on mineral soils at the age of 100 years, tree height is 30–33 m [50]; 5 Different letters represent significant differences in stump diameters as determined by the Kruskal–Wallis test at an α < 0.05 level.
2.4. Calculations and Statistical Analyses
The relative area colonized by P. gigantea (Rotstop® or Latvian strain) and H. annosum was calculated by dividing their occupied areas by the total area of the disc (Kenigsvalde et al., 2016). Control efficacy, expressed as the reduced proportion of stumps colonized by Heterobasidion spp. and the reduced proportion of wood colonized by this pathogen, for each treatment, was calculated according to the formula: where nt represents the proportion of colonized stumps or proportion of colonized wood for treated stumps, and nu represents the proportion of colonized stumps or proportion of colonized wood for control stumps [45]. Control efficacy was calculated within site, method and treatment.
Data were inspected for normality using the Shapiro–Wilk test and by manually evaluating Q–Q plots. Using these criteria, total area of discs, area of disc surface covered by P. gigantea and area of disc surface covered by Heterobasidion were considered to be not normally distributed (p = 0.00021, <2.2 × 10−16 and <2.2 × 10−16, respectively). The differences in diameter were determined using the Kruskal–Wallis test. The relationship between method (i.e., covered and uncovered stumps) and treatment effect (i.e., BCA, urea and untreated control) on the presence of Heterobasidion infection was determined using a generalized linear model (GLM) with a binomial distribution and logit as the link function. The relationship between method and treatment on relative infected areas for both Heterobasidion and P. gigantea was investigated with a GLM with a Poisson distribution and log as the link function. In order to determine differences between coverage methods and stump treatments on the frequency of Heterobasidion infection, and the relative areas occupied by Heterobasidion spp. and P. gigantea, pairwise comparisons of the model’s estimated marginal means (EMM) were carried out with a 95% confidence level, with p-value adjustment according to Tukey’s method. All statistical analyses were performed in the “R” environment [51].
3. Results
3.1. Effects of Treatments on Heterobasidion Incidence and Stump Colonization
Site did not have a significant influence on colonized area (p = 0.907) or infection frequency by Heterobasidion (p = 0.56). In the uncovered stumps, Heterobasidion infection frequency was significantly decreased compared to the untreated controls for the urea, Rotstop® and P. gigantea 422 treated stumps, but no statistical differences were found between the three treatments (Table 2). Heterobasidion infection frequency was significantly higher in the covered control stumps compared to the uncovered control stumps (p = 0.004). A similar trend was observed for urea-treated stumps, where coverage significantly increased Heterobasidion incidence (p = 0.026). Significantly fewer stumps were infected by Heterobasidion spp. when stumps were covered and treated with either Rotstop® or P. gigantea 422 compared to covered and uncovered urea and untreated control stumps. Stump coverage also decreased Heterobasidion infection in both Rotstop® and P. gigantea 422 treated stumps compared to the uncovered stumps.

Table 2.
Mean infection frequencies (%) of Heterobasidion spp. in Norway spruce stumps and percent of stump surface colonized by Heterobasidion spp. and P. gigantea treated with Rotstop®, native Latvian Phlebiopsis gigantea strain or urea (% ± standard deviation).
Significant differences in relative area occupied by Heterobasidion spp. between covered control stumps and other treatments were observed (p < 0.001; Table 2; Appendix A). Relative stump surface area occupied by Heterobasidion spp. was significantly less when Rotstop® or P. gigantea 422 (irrespective of coverage) were applied in comparison to covered and uncovered control stumps and covered urea-treated stumps (Table 2; Appendix A).
Mean relative area of P. gigantea was significantly greater (p < 0.001) than the area colonized by Heterobasidion spp. both in stumps treated with BCA and in control stumps (Table 2). Moreover, the surface area colonized by P. gigantea was significantly larger in uncovered control stumps than the area occupied by Heterobasidion spp. (p < 0.001). All re-isolations were vegetatively compatible with each respective inoculated strain.
A total of 47% of covered control stumps and 38% of uncovered control stumps were colonized by wild P. gigantea. Additionally, 24% of stumps (33% of covered and 15% of uncovered) had both Heterobasidion spp. and P. gigantea present. For these stumps, relative surface area colonized by Heterobasidion spp. varied from 3 to 49% (average 14%) and by P. gigantea from 1 to 69% (average 21%). The presence of naturally occurring P. gigantea had no influence on the natural infection rate of Heterobasidion spp. (p = 0.739). Eighteen percent of the uncovered urea-treated stumps were colonized by wild P. gigantea and 35% of the covered urea-treated stumps were infected by naturally occurring P. gigantea. However, the area occupied by wild P. gigantea was considerably smaller than that occupied by Rotstop® or P. gigantea 422 (for both p < 0.001; Table 2; Appendix B).
3.2. Control Efficacy
Control efficacy was calculated based on the proportion of infected stumps and area occupied by the pathogen. Based on infection frequency, Rotstop® and P. gigantea 422 showed the highest efficacy both in uncovered stumps (60.58% and 62.0%, respectively) and covered stumps (95.29% and 92.93%, respectively). For both covered and uncovered urea-treated stumps, the efficacy did not exceed 50% (47.71% and 45.78%, respectively).
The highest control efficacy was also found in BCA-treated and covered stumps in comparison to urea based on the relative surface area occupied by Heterobasidion spp., (99.39% for Rotstop®, 95.69% for P. gigantea 422 and 72.71% for urea). Also compared to uncovered stumps, the efficacy of both covered and Rotstop® and P. gigantea 422 treated stumps were higher (Figure 1).
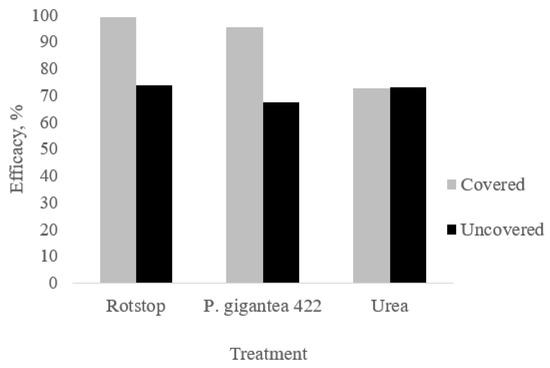
Figure 1.
Control efficacy (%) against Heterobasidion spp. based on the relative area of colonized wood.
4. Discussion
4.1. Effects of Treatments on Heterobasidion Incidence and Stump Colonization
The incidence of Heterobasidion infection did not differ between stumps treated by urea or P. gigantea suspensions. Treatment with either BCA significantly decreased the frequency of Heterobasidion spp. in comparison to control stumps. However, infection by Heterobasidion spp. was not completely prevented, as more than 13% of uncovered BCA-treated Norway spruce stumps were still infected. Such failure in preventing Heterobasidion infections is not uncommon for BCA such as Rotstop®. For example, Berglund and Rönnberg [30] regularly observed Heterobasidion infections (as high as 70% disease incidence at some sites) on Norway spruce stumps even when fully covered with Rotstop®. The efficacy of Rotstop® could be at least partially associated with the high natural infection rate of Heterobasidion spp. [30,45,52]. In Latvia, Rotstop® has proven to be an effective control agent against Heterobasidion spore infection [45], and P. gigantea 422 was equally effective. Several studies have reported that native isolates of P. gigantea are capable of achieving similar, if not higher, efficiency as Rotstop® [21,45,52]. Therefore, it seems possible to complement the conventionally used Rotstop® with a native strain also in Latvia (P. gigantea 422).
In this study, efficacy based on the proportion of infected stumps treated with urea did not exceed 50%; however, if efficacy was based on Heterobasidion infected area, then the efficacy of urea was almost the same as BCA. These results are in agreement with those obtained in other studies, where the efficacy of urea and P. gigantea were similar [5,20,29]; however, urea has been documented to have higher efficacy in comparison to P. gigantea in some studies [26,33,34]. Moreover, development of P. gigantea depends on (i) stump treatment coverage quality [28,30,53]; (ii) stump and root wood moisture content, which in turn depend on the humidity during the treatment period [53], weather conditions and seasonality [54,55,56]; (iii) growth characteristics of different P. gigantea isolates [43]; (iv) enzymatic activity of the fungi; (v) the characteristics of the wood; and (vi) the richness of the fungal biota [57]. Furthermore, Wang et al. [29] found that treatment of Larix x eurolepis stumps with urea resulted in more stable effects in control of Heterobasidion than using BCA. The average air temperature during experiment establishment was close to the optimal for P. gigantea development [57], and our data indicate that, although the total precipitation in the three-week period following establishment of the experiments was low, it was sufficient to ensure favorable conditions for fungal growth.
4.2. The Effect of Stump Cover on Heterobasidion spp. and P. gigantea Development
Although not used in practical forestry, stump cover treatments have been examined under experimental conditions, typically using plastic sheets or bags to protect stumps from environmental conditions and to the improve efficacy of P. gigantea [52,58,59,60] and other BCA, consisting of Hypholoma fasciculare (Huds.) P. Kumm., Phanerochaete velutina Karst., Vuilleminia comedens (Nees) Maire and Trichoderma harzianum [37].
As we analyzed covered and uncovered stumps, we had a possibility to compare results between these two groups. If the stump surface was uncovered, Rotstop® and P. gigantea 422 reached more than 60% efficacy based on the proportion of infected stumps and at least 65% efficacy based on the relative infected area. The results obtained about BCA efficacy based on incidence and colonized area are in agreement with previous research with Norway spruce in Finland, Sweden and Latvia [16,45,61,62,63]. Our results showed that the covered stumps had a greater relative surface colonized by P. gigantea. In two of the sites, treatment with BCA combined with stump cover completely excluded Heterobasidion infection (data not shown). Our data confirm that the development of both P. gigantea and Heterobasidion spp. increases with stump cover. This is in agreement with Redfern [41], who reported that covering stumps with freshly cut branches decreases variation in microclimate, thereby stimulating the development of various fungi, including Heterobasidion. Increased formation of Heterobasidion spp. fruiting bodies on covered Norway spruce stumps has also been reported by Paludan [40]. Redfern [55] found that Sitka spruce (Picea sitchensis (Bong.) Carr.) stumps covered with a polyethylene sheet 60 cm above their surface tended to be more infected by Heterobasidion spp. spores compared to uncovered stumps. Both Heterobasidion spp. and P. gigantea are primary colonizers of conifer stumps [64,65], so factors that positively affect P. gigantea likely favor Heterobasidion spp. as well. Despite this, our results indicate that covering of stumps with wooden discs significantly promotes Phlebiopsis gigantea growth over that of Heterobasidion spp. in treated stumps only. This was not the case in the control stumps. Our research demonstrates that stump cover can increase the efficacy of BCA by up to 90%. This may be of value for small-scale forestry, where cuttings are not mechanized, and manual placement of discs is feasible. Moreover, this study provides additional information about processes typically happening during commercial thinning and final felling, when stumps often become covered (with branches, leaves, logging residues, sawdust, moss and soil). However, in both large- and small-scale forestry, stump coverage increases efficacy of BCA only if stumps are treated correctly; otherwise, it may increase the risk of Heterobasidion colonization (clearly shown by high Heterobasidion infection frequency in covered control stumps; Table 2).
4.3. Treatment Effects on Wild P. gigantea
Wild P. gigantea was observed in 43% of the control stumps, which is higher than previous studies in Latvia, where wild P. gigantea inefficiently colonized spruce stumps at final felling [45,66]. Trees in this experiment were young and did not contain any heartwood yet. This likely benefited P. gigantea, as it prefers to colonize sapwood [17,59], unlike Heterobasidion spp., which is better adapted to heartwood in spruce stumps [67]. Moreover, it has been reported that Picea sitchensis (Bong.) Carr. heartwood remains susceptible to Heterobasidion basidiospores for longer than sapwood [68].
In uncovered control stumps, the mean relative surface area colonized by wild P. gigantea was three-fold larger than the area infected by Heterobasidion. Kenigsvalde et al. [45] showed that Heterobasidion spp. infection in untreated spruce stumps was low when wild P. gigantea covered more than 10% of the stump cross-section. However, our data indicate that stumps should be treated either with Rotstop® or P. gigantea 422 (equally effective) to protect stumps, as the area occupied by wild P. gigantea was at least sox-fold smaller than the area colonized by Rotstop® and P. gigantea 422. Moreover, colonization by wild P. gigantea did not show any significant effect on the occurrence of Heterobasidion infection.
Besides the treatment efficiency against Heterobasidion spore infection, the impact of different control agents on other stump-colonizing fungi and surrounding vegetation should be taken into account [64]. Previous studies have asserted that urea has a more negative effect on fungal biodiversity in treated stumps. In comparison to Rotstop®, short-term treatment with urea causes both radical changes in the fungal community structure and damage to bryothytes and vascular plants, while Rotstop®-treated stumps were mainly colonized by the same fungal species as untreated stumps, and no effect on ground-vegetation species was reported [69,70]. However, Varese et al. [37] concluded that the negative effects of urea treatment on fungal diversity are largely short term. We observed no difference in the colonization of wild P. gigantea in urea-treated stumps compared to the untreated controls, and hence the long-term effect from use of urea may be questioned and regarded as less important for fungal or biodiversity in general. When deciding between urea or Rotstop/P. gigantea as management options, managers should consider relevant factors that can affect treatment efficacy, fungal biodiversity and cost, including season, weather conditions, soil type and equipment availability. However, these issues were outside the scope of this study.
5. Conclusions
Overall, this study clearly shows that the efficacy of P. gigantea against Heterobasidion spp. in Norway spruce stumps is significantly increased by covering the stump surface with an autochthonous disk. Such a treatment is laborious and not practical for large-scale forestry. However, during manual cutting in private or urban forests stump cover should be considered. Commercial foresters should continue to protect against Heterobasidion infection by using urea or Rotstop® when appropriate. There is also a possibility to utilize native P. gigantea strains from Latvia rather than Rotstop® without compromising efficacy, which may lead to a higher acceptance by the public and contractors for using BCA.
Author Contributions
Conceptualization, T.G. and J.R.; methodology, T.G. and J.R.; software, A.Z. and U.S.; formal analysis, A.Z.; investigation, A.Z., L.B. and U.S.; data curation, A.Z. and U.S.; writing—original draft preparation, A.Z., U.S., T.G. and J.R.; writing—review and editing, A.Z., P.S., T.G. and J.R.; visualization, A.Z.; supervision, A.Z., T.G. and J.R.; project administration, T.G., A.Z. and J.R.; funding acquisition, T.G. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
In accordance with Contract No. 1.2.1.1/18/A/004 between the ‘Forest Sector Competence Centre of Latvia’ Ltd. and the Central Finance and Contracting Agency, the study ‘Development of chemical preparation for reducing root rot caused losses in Norway spruce stands on peat soils’ is conducted by LSFRI Silava with support from the European Regional Development Fund (ERDF) within the framework of the project ‘Forest Sector Competence Centre of Latvia’ Additional support was obtained by JSC “Latvian State Forests” and the Latvian Council of Sciences grant project No. lzp-2018/1-0431 “Investigations on the role of Phlebiopsis gigantea in restricting vegetative spread of Heterobasidion spp. in stumps of Norway spruce and Scots pine”. Further economic support was provided by Skogssällskapet and the Rattsjö foundation. Skogssällskapet provided field sites.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Liene Darta Lukstina, who provided help in field work.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Output of statistical tests reflecting relative Heterobasidion infected area, colonies per cm2. Relative infected area, Family = Poisson, Factors = Method + Treatment.
Table A1.
Output of statistical tests reflecting relative Heterobasidion infected area, colonies per cm2. Relative infected area, Family = Poisson, Factors = Method + Treatment.
| Treatment vs. Treatment | Estimate | SE | z | p-Value |
|---|---|---|---|---|
| Method: Covered | ||||
| Rotstop vs. Control | 5.114 | 0.5225 | 9.786 | p < 0.001 |
| Rotstop® vs. P. gigantea 422 | 1.969 | 0.5561 | 3.540 | p = 0.0095 |
| Rotstop® vs. Urea | −3.796 | 0.5267 | −7.207 | p < 0.001 |
| P. gigantea vs. Control | 3.145 | 0.1989 | 15.809 | p < 0.001 |
| P. gigantea vs. Urea | −1.828 | 0.2097 | −8.715 | p < 0.001 |
| Urea vs. Control | 1.317 | 0.0882 | 14.934 | p < 0.001 |
| Method: Not Covered | ||||
| Rotstop® vs. Control | 1.333 | 0.1556 | 8.570 | p < 0.001 |
| Rotstop® vs. P. gigantea 422 | 0.198 | 0.1860 | 1.604 | p = 0.9641 |
| Rotstop® vs. Urea | −0.035 | 0.1932 | −0.181 | p = 1.000 |
| P. gigantea 422 vs. Control | 1.136 | 0.1422 | 7.985 | p < 0.001 |
| P. gigantea vs. Urea | 0.163 | 0.1826 | 0.892 | p = 0.9868 |
| Urea vs. Control | 1.298 | 0.1514 | 8.574 | p < 0.001 |
Appendix B

Table A2.
Output of statistical tests reflecting relative P. gigantea colonized area, colonies per cm2. Relative colonized area, Family = Poisson, Factors = Method + Treatment.
Table A2.
Output of statistical tests reflecting relative P. gigantea colonized area, colonies per cm2. Relative colonized area, Family = Poisson, Factors = Method + Treatment.
| Treatment vs. Treatment | Estimate | SE | z | p-Value |
|---|---|---|---|---|
| Method: Covered | ||||
| Rotstop® vs. Control | −2.0176 | 0.0414 | 48.679 | p < 0.001 |
| Rotstop® vs. P. gigantea 422 | 0.0525 | 0.0195 | 2.687 | p = 0.126 |
| Rotstop® vs. Urea | 2.1386 | 0.0431 | 49.636 | p < 0.001 |
| P. gigantea vs. Control | −2.0700 | 0.0413 | 50.091 | p < 0.001 |
| P. gigantea vs. Urea | 2.1911 | 0.0430 | −50.991 | p < 0.001 |
| Urea vs. Control | 0.1211 | 0.0564 | 2.146 | p = 0.385 |
| Method: Not Covered | ||||
| Rotstop® vs. Control | −1.7333 | 0.0430 | 40.353 | p < 0.001 |
| Rotstop® vs. P. gigantea 422 | −0.0676 | 0.0241 | 2.806 | p = 0.0934 |
| Rotstop® vs. Urea | −2.6114 | 0.0636 | 41.091 | p < 0.001 |
| P.gigantea 422 vs. Control | −1.6658 | 0.0431 | 38.677 | p < 0.001 |
| P.gigantea vs. Urea | 2.5438 | 0.0636 | 39.979 | p < 0.001 |
| Urea vs. Control | 0.8781 | 0.0729 | 12.046 | p < 0.001 |
References
- Garbelotto, M.; Gonthier, P. Biology, epidemiology, and control of Heterobasidion species worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, P.; Lione, G.; Giordano, L.; Garbelotto, M.M. The American forest pathogen Heterobasidion irregulare colonizes. Ecol. Appl. 2012, 22, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Redfern, D.B.; Stenlid, J. Spore Dispersal and Infection. In Heterobasidion Annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 109–116. [Google Scholar]
- Rönnberg, J. Logging operation damage to roots of clear-felled Picea abies and subsequent spore infection by Heterobasidion annosum. Silva Fenn. 2000, 34, 29–36. [Google Scholar] [CrossRef]
- Nicolotti, G.; Gonthier, P. Stump treatment against Heterobasidion with Phlebiopsis gigantea and some chemicals in Picea abies stands in the western Alps. For. Pathol. 2005, 35, 365–374. [Google Scholar] [CrossRef]
- Rishbeth, J. Observations on the Biology of Fomes annosus, with Particular Reference to East Anglian Pine Plantations. III. Natural and Experimental Infection of Pines, and Some Factors affecting Severity of the Disease. Ann. Bot. 1951, 15, 221–246. [Google Scholar] [CrossRef]
- Piri, T. The spreading of the S type of Heterobasidion annosum from Norway spruce stumps to the subsequent tree stand. Eur. J. For. Pathol. 1996, 26, 193–204. [Google Scholar] [CrossRef]
- Piri, T.; Korhonen, K. Spatial distribution and persistence of Heterobasidion parviporum genets on a Norway spruce site. For. Pathol. 2007, 37, 1–8. [Google Scholar] [CrossRef]
- Möykkynen, T.; Pukkala, T. Optimizing the management of Norway spruce and Scots pine mixtures on a site infected by Heterobasidion coll. Scand. J. For. Res. 2010, 25, 127–137. [Google Scholar] [CrossRef]
- Vasaitis, R.; Stenlid, J.; Thomsen, I.M.; Barklund, P.; Dahlberg, A. Stump removal to control root rot in forest stands. Silva Fenn. 2008, 42, 457–483. [Google Scholar] [CrossRef]
- Arhipova, N.; Gatnieks, T.; Donis, J.; Stenlid, J.; Vasaitis, R. Butt rot incidence, causal fungi, and related yield loss in Picea abies stands of Latvia. Can. J. For. Res. 2011, 41, 2337–2345. [Google Scholar] [CrossRef]
- Gonthier, P.; Thor, M. Annosus Root and Butt Rots. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CAB International: Wallingford, UK, 2013; pp. 128–158. [Google Scholar]
- Asiegbu, F.O.; Adomas, A.; Stenlid, J. Conifer root and butt rot caused by Heterobasidion annosum (Fr.) Bref. s.l. Mol. Plant Pathol. 2005, 6, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Holdenrieder, O.; Greig, B.J.W. Biological methods of control. In Heterobasidion Annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 235–258. [Google Scholar]
- Pratt, J.E.; Niemi, M.; Sierota, Z.H. Comparison of Three Products Based on Phlebiopsis gigantea for the Control of Heterobasidion annosum in Europe. Biocontrol. Sci. Technol. 2000, 10, 467–477. [Google Scholar] [CrossRef]
- Thor, M. Optional stump treatment against Heterobasidion annosum in European forestry—Current situation. In Proceedings of the 10th IUFRO Conference on Root and Butt Rots of Forest Trees, Québec City, QC, Canada, 16–22 September 2003; Laflamme, G., Bérubé, J.A., Bussières, G., Eds.; Canadian Forest Service: Ottawa, AB, Canada, 2003; pp. 170–175. [Google Scholar]
- Oliva, J.; Zhao, A.; Zarei, S.; Sedlak, P.; Stenlid, J. Effect of temperature on the interaction between Phlebiopsis gigantea and the root-rot forest pathogen Heterobasidion spp. For. Ecol. Manag. 2015, 340, 22–30. [Google Scholar] [CrossRef]
- Oliva, J.; Messal, M.; Wendt, L.; Elfstrand, M. Quantitative interactions between the biocontrol fungus Phlebiopsis gigantea, the forest pathogen Heterobasidion annosum and the fungal community inhabiting Norway spruce stumps. For. Ecol. Manag. 2017, 402, 253–264. [Google Scholar] [CrossRef]
- Brauners, I.; Brūna, L.; Gaitnieks, T. Testing the ‘Rotstop’ biological preparation for controlling Heterobasidion root rot in Latvia. Res. Rural Dev. 2014, 2, 97–102. [Google Scholar]
- Blomquist, M.; Herrera, S.L.; Hofmann, J.; Beram, R.C.; Cleary, M.; Rönnberg, J. Size matters but is big always better? Effectiveness of urea and Phlebiopsis gigantea as treatment against Heterobasidion on Picea abies stumps of variable size. For. Ecol. Manag. 2020, 462, 1–8. [Google Scholar] [CrossRef]
- Korhonen, K. Simulated stump treatment experiments for monitoring the efficacy of Phlebiopsis gigantea against Heterobasidion. In Proceedings of the 10th IUFRO Conference on Root and Butt Rots of Forest Trees, Québec City, QC, Canada, 16–22 September 2003; Laflamme, G., Bérubé, J.A., Bussières, G., Eds.; Canadian Forest Service: Ottawa, AB, Canada, 2003; pp. 206–210. [Google Scholar]
- Webber, J.; Thorpe, K. Potential for biological control of Heterobasidion annosum in the UK using Rotstop. In Proceedings of the 10th IUFRO Conference on Root and Butt Rots of Forest Trees, Québec City, QC, Canada, 16–22 September 2003; Laflamme, G., Bérubé, J.A., Bussières, G., Eds.; Canadian Forest Service: Ottawa, AB, Canada, 2003; pp. 221–225. [Google Scholar]
- Drenkhan, T.; Hanso, S.; Hanso, M. Effect of the stump treatment with Phlebiopsis gigantea against Heterobasidion root rot in Estonia. Balt. For. 2008, 14, 16–25. [Google Scholar]
- Vollbrecht, G.; Jorgensen, B.B. Modelling the incidence of butt rot in plantations of Picea abies in Denmark. Can. J. For. Res. 1995, 25, 1887–1896. [Google Scholar] [CrossRef]
- Pratt, J.E.; Redfern, D.B. Infection of Sitka spruce stumps by spores of Heterobasidion annosum: Control by means of urea. Forestry 2001, 74, 73–78. [Google Scholar] [CrossRef]
- La Porta, N.; Grillo, R.; Ambrosi, P.; Korhonen, K. Stump treatment experiments against Heterobasidion in the Italian Alps. In Proceedings of the 10th IUFRO Conference on Root and Butt Rots of Forest Trees, Québec City, QC, Canada, 16–22 September 2003; Laflamme, G., Bérubé, J.A., Bussières, G., Eds.; Canadian Forest Service: Ottawa, AB, Canada, 2003; pp. 176–181. [Google Scholar]
- Anselmi, N.; Nicolotti, G. Biological control of Heterobasidion annosum in the forest by non-pathogenic wood-destroying fungi. In Proceedings of the 9th International Conference on root and butt rots, Carcans-Maubuisson, France, 1–7 September 1997; The University of Tuscia: Carcans-Maubuisson, France, 1997; pp. 421–428. [Google Scholar]
- Oliva, J.; Thor, M.; Stenlid, J. Long-term effects of mechanized stump treatment against Heterobasidion annosum root rot in Picea abies. Can. J. For. Res. 2010, 40, 1020–1033. [Google Scholar] [CrossRef]
- Wang, L.Y.; Pålsson, H.; Ek, E.; Rönnberg, J. The effect of Phlebiopsis gigantea and urea stump treatment against spore infection of Heterobasidion spp. on hybrid larch (Larix x eurolepis) in southern Sweden. For. Pathol. 2012, 42, 420–428. [Google Scholar] [CrossRef]
- Berglund, M.; Rönnberg, J. Effectiveness of treatment of Norway spruce stumps with Phlebiopsis gigantea at different rates of coverage for the control of Heterobasidion. For. Pathol. 2004, 34, 233–243. [Google Scholar] [CrossRef]
- Oliva, J.; Samils, N.; Johansson, U.; Bendz-Hellgren, M.; Stenlid, J. Urea treatment reduced Heterobasidion annosum s.l. root rot in Picea abies after 15 years. For. Ecol. Manag. 2008, 255, 2876–2882. [Google Scholar] [CrossRef]
- Lipponen, K. Stump infection by Heterobasidion annosum and its control in stands at the first thinning stage. Folia For. 1991, 770, 1–12. [Google Scholar]
- Thomsen, I.M. Effect of stump treatment on transfer of Heterobasidion annosum root rot in Norway spruce. In Proceedings of the 10th IUFRO Conference on Root and Butt Rots of Forest Trees, Québec City, QC, Canada, 16–22 September 2003; Laflamme, G., Bérubé, J.A., Bussières, G., Eds.; Canadian Forest Service: Ottawa, AB, Canada, 2003; pp. 217–218. [Google Scholar]
- Lehtijärvi, A.; Aday, G.; Lehtijärvi, T.D. Cedrus libani: The most susceptible Turkish conifer species to local Heterobasidion isolates in spring inoculations. For. Pathol. 2011, 41, 1–6. [Google Scholar] [CrossRef]
- Korhonen, K.; Delatour, C.; Greig, B.J.W.; Schönhar, S. Silvicultural control. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 283–313. [Google Scholar]
- Piri, T. Early development of root rot in young Norway spruce planted on sites infected by Heterobasidion in Southern Finland. Can. J. For. Res. 2003, 33, 604–611. [Google Scholar] [CrossRef]
- Varese, G.C.; Gonthier, P.; Nicolotti, G. Long-term effects on other fungi are studied in biological and chemical stump treatments in the fight against Heterobasidion annosum coll. Mycologia 2003, 95, 379–387. [Google Scholar] [CrossRef]
- Cech, T.L.; Steyrer, G.; Łakomy, P. Preliminary results of Norway spruce stump treatment with Hypholoma fasciculare and Phlebiopsis gigantea in an Austrian Alpine protection forest. In Proceedings of the 12th International Conference on root and Butt Rots of Forest Trees, Medford, OR, USA, 12–19 August 2007; Garbelotto, M., Gonthier, P., Eds.; The University of California Berkeley: Medford, OR, USA,, 2008; pp. 192–194. [Google Scholar]
- Volchenkova, G.A.; Zvyagintsev, V.B.; Savickij, A.V. Cкрининг штаммoв Phlebiopsis gigantea (Fr.) Jülich пoприживаемoсти на пнях сoсны пoсле рубoк ухoда (Screening of the strains of Phlebiopsis gigantea (Fr.) Jülich on ability to colonize pine stumps after thinning). Jul. Приживаемoсти Пнях Сoсны Пoсле Рубoк Ухoда 2013, 157, 219–222. [Google Scholar]
- Paludan, F. Infection and spread of Fomes annosus in young Norway spruce. Det Forstl. Forsøgsvæs. Dan. 1966, 30, 21–47. [Google Scholar]
- Redfern, D.B. Infection of Picea sitchensis and Pinus contorta stumps by basidiospores of Heterobasidion annosum. Eur. J. For. Pathol. 1982, 12, 11–25. [Google Scholar] [CrossRef]
- Sun, H.; Korhonen, K.; Hantula, J.; Kasanen, R. Variation in properties of Phlebiopsis gigantea related to biocontrol against infection by Heterobasidion spp. in Norway spruce stumps. For. Pathol. 2009, 39, 133–144. [Google Scholar] [CrossRef]
- Zaluma, A.; Bruna, L.; Klavina, D.; Burnevica, N.; Kenigsvalde, K.; Lazdins, A.; Gaitnieks, T. Growth of Phlebiopsis gigantea in wood of seven conifer species. For. Pathol. 2019, 49, e12555. [Google Scholar] [CrossRef]
- Gunulf-Åberg, A.; Witzell, J.; Rönnberg, J. Risk of False Positives during Sampling for Heterobasidion annosum s.l. Plant Dis. 2016, 100, 175–179. [Google Scholar] [CrossRef]
- Kenigsvalde, K.; Brauners, I.; Korhonen, K.; Zaluma, A. Evaluation of the biological control agent Rotstop in controlling the infection of spruce and pine stumps by Heterobasidion in Latvia. Scand. J. For. Res. 2016, 31, 254–261. [Google Scholar] [CrossRef]
- Gunulf, A.; Mc Carthy, R.; Rönnberg, J. Control efficacy of stump treatment and influence of stump height on natural spore infection by Heterobasidion spp. of precommercial thinning Stumps of Norway spruce and birch. Silva Fenn. 2012, 46, 655–665. [Google Scholar] [CrossRef]
- Gaitnieks, T.; Brauners, I.; Kenigsvalde, K.; Zaļuma, A.; Brūna, L.; Jansons, J.; Burņeviča, N.; Lazdiņš, A.; Vasaitis, R. Infection of pre-commercially cut stumps of Picea abies and Pinus sylvestris by Heterobasidion spp.—A comparative study. Silva Fenn. 2018, 52, 9911. [Google Scholar] [CrossRef]
- Gaitnieks, T.; Zaļuma, A.; Kenigsvalde, K.; Brūna, L.; Kļaviņa, D.; Burņeviča, N.; Stenlid, J.; Jankovský, L.; Vasaitis, R. Natural infection and colonization of pre-commercially cut stumps of Picea abies and Pinus sylvestris by Heterobasidion rot and its biocontrol fungus Phlebiopsis gigantea. Biol. Control 2020, 143, 104208. [Google Scholar] [CrossRef]
- Roy, G.; Cormier, M.; Hamelin, R.C.; Dessureault, M. Comparison of RAPD technique and somatic incompatibility tests for the identification of Phlebiopsis gigantea strains. Can. J. Bot. 1997, 75, 2097–2104. [Google Scholar] [CrossRef]
- Bušs, K. Forest ecosystems classification in Latvia. Proc. Latv. Acad. 1997, 51, 204–218. [Google Scholar]
- RR Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 10 October 2020).
- Berglund, M.; Rönnberg, J.; Holmer, L.; Stenlid, J. Comparison of five strains of Phlebiopsis gigantea and two Trichoderma formulations for treatment against natural Heterobasidion spore infections on Norway spruce stumps. Scand. J. For. Res. 2005, 20, 12–17. [Google Scholar] [CrossRef]
- Tubby, K.; Scott, D.; Webber, J. Relationship between stump treatment coverage using the biological control product PG Suspension, and control of Heterobasidion annosum on Corsican pine, Pinus nigra ssp. laricio. For. Pathol. 2008, 38, 37–46. [Google Scholar] [CrossRef]
- Kubiak, K.; Damszel, M.; Sikora, K.; Przemienieck, S.; Małecka, M.; Sierota, Z. Colonization of fungi and bacteria in stumps and roots of scots pine after thinning and treatment with Rotstop. J. Phytopathol. 2017, 165, 143–156. [Google Scholar] [CrossRef]
- Kallio, T.; Hallaksela, A.-M. Biological control of Heterobasidion annosum (Fr.). Bref. (Fomes annosus) in Finland. Eur. J. For. Pathol. 1979, 9, 298–308. [Google Scholar] [CrossRef]
- Vasiliauskas, R.; Larsson, E.; Larsson, K.H.; Stenlid, J. Persistence and long-term impact of Rotstop biological control agent on mycodiversity in Picea abies stumps. Biol. Control 2005, 32, 295–304. [Google Scholar] [CrossRef]
- Żółciak, A.; Sikora, K.; Wrzosek, M.; Damszel, M.; Sierota, Z. Why Does Phlebiopsis gigantea not Always Inhibit Root and Butt Rot in Conifers? Forests 2020, 11, 129. [Google Scholar] [CrossRef]
- Redfern, D.B. The effect of wood moisture on infection of Sitka spruce stumps by basidiospores of Heterobasidion annosum. For. Pathol. 1993, 23, 218–235. [Google Scholar] [CrossRef]
- Morrison, D.J.; Redfern, D.B. Long-term development of Heterobasidion annosum in basidiospore-infected Sitka spruce stumps. Plant Pathol. 1994, 43, 897–906. [Google Scholar] [CrossRef]
- Redfern, D.B.; Gregory, S.C.; Macaskill, G.A. Inoculum concentration and the colonization of Picea sitchensis stumps by basidiospores of Heterobasidion annosum. Scand. J. For. Res. 1997, 12, 41–49. [Google Scholar] [CrossRef]
- Kenigsvalde, K.; Brauners, I.; Zaļuma, A.; Jansons, J.; Gaitnieks, T. Biological protection of conifer against Heterobasidion infection -interaction between root-rot fungus and Phlebiopsis gigantea. Res. Rural Dev. 2017, 1, 69–75. [Google Scholar]
- Korhonen, K.; Lipponen, K.; Bendz, M.; Johansson, M.; Seiskari, P.; Niemi, M. Control of Heterobasidion annosum by stump treatment with ‘Rotstop’, a new commercial formulation of Phlebiopsis gigantea. In Proceedings of the Eighth International Conference on Root and Butt Rots, Wik, Sweden/Haikko, Finland, 9–16 August 1993; Johansson, M., Stenlid, J., Eds.; Swedish University of Agricultural Science: Uppsala, Sweden, 1994; pp. 675–683. [Google Scholar]
- Rönnberg, J.; Sidorov, E.; Petrylaitė, E. Efficacy of different concentrations of Rotstop and Rotstop S and imperfect coverage of Rotstop S against Heterobasidion spp. spore infections on Norway spruce stumps. For. Pathol. 2006, 36, 422–433. [Google Scholar] [CrossRef]
- Rishbeth, J. Dispersal of Fomes annosus Fr. and Peniophora gigantea (Fr.) massee. Trans. Brit. Mycol. Soc. 1959, 42, 243–260. [Google Scholar] [CrossRef]
- Meredith, D.S. Further Observations on Fungi Inhabiting Pine Stumps. Ann. Bot. 1960, 24, 63–78. [Google Scholar] [CrossRef]
- Gaitnieks, T.; Zaļuma, A.; Kenigsvalde, K.; Klavina, D.; Brauners, I.; Piri, T. Susceptibility of small-Diameter Norway Spruce Understory stumps to Heterobasidion spore infection. Forests 2019, 10, 521. [Google Scholar] [CrossRef]
- Oliva, J.; Bernat, M.; Stenlid, J. Heartwood stump colonisation by Heterobasidion parviporum and H. annosum s.s. in Norway spruce (Picea abies) stands. For. Ecol. Manag. 2013, 295, 1–10. [Google Scholar] [CrossRef]
- Woods, C.M.; Woodward, S.; Redfern, D.B. Receptivity of Picea sitchensis stumps to infection by Heterobasidion annosum basidiospores. Forestry 2000, 73, 457–465. [Google Scholar] [CrossRef]
- Westlund, A.; Nohrstedt, H.O. Effects of Stump-treatment Substances for Root-rot Control on Ground Vegetation and Soil Properties in a Picea abies forest in Sweden. Scand. J. For. Res. 2000, 15, 550–560. [Google Scholar] [CrossRef]
- Vasiliauskas, R.; Lygis, V.; Thor, M.; Stenlid, J. Impact of biological (Rotstop) and chemical (urea) treatments on fungal community structure in freshly cut Picea abies stumps. Biol. Control 2004, 31, 405–413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).