Abstract
In the twentieth century, a substantial decline in Pinus thunbergii populations in Japan occurred due to the outbreak of pine wood nematode (PWN), Burshaphelencus xylophilus. A PWN-P. thunbergii resistant trees-breeding project was developed in the 1980s to provide reforestation materials to minimalize the pest damage within the population. Since climate change can also contribute to PWN outbreaks, an intensive reforestation plan instated without much consideration can impact on the genetic diversity of P. thunbergii populations. The usage and deployment of PWN-P. thunbergii resistant trees to a given site without genetic management can lead to a genetic disturbance. The Iki-no-Matsubara population was used as a model to design an approach for the deployment management. This research aimed to preserve local genetic diversity, genetic structure, and relatedness by developing a method for deploying Kyushu PWN-P. thunbergii resistant trees as reforestation-material plants into Iki-no-Matsubara. The local genotypes of the Iki-no-Matsubara population and the Kyushu PWN-P. thunbergii resistant trees were analyzed using six microsatellite markers. Genotype origins, relatedness, diversity, and structure of both were investigated and compared with the genetic results previously obtained for old populations of P. thunbergii throughout Japan. A sufficient number of Kyushu PWN-P. thunbergii resistant trees, as mother trees, within seed orchards and sufficient status number of the seedlings to deploy are needed when deploying the Kyushu PWN-P. thunbergii resistant trees as reforestation material planting into Iki-no-Matsubara population. This approach not only be used to preserve Iki-no-Matsubara population (genetic diversity, genetic structure, relatedness, and resilience of the forests) but can also be applied to minimize PWN damage. These results provide a baseline for further seed sourcing as well as develop genetic management strategies within P. thunbergii populations, including Kyushu PWN-P. thunbergii resistant trees.
1. Introduction
In general, forests can be categorized based on their purpose as conservation forests, protected forests, production forests, and forests with specific functions such as mitigation or tourism [1]. Different management strategies are required to protect forests with multiple functions [2], such as in Indonesia [3], China and Germany [4], and recently genetic approach methods have been proposed for long-term management [5,6]. Forests today face numerous threats, including diseases and pests [7], human interference [8], loss of unique or rare species and genetic resources [9,10], and loss of genetic diversity, which provides forest ecosystem resilience [11,12]. In Japan, climate change has led to changes in environmental conditions in, such as increased annual sunshine, temperature, and rainfall (precipitation); rapid sea-level rise at a rate of 3.2 mm/year from 1993–2010; and higher intensity and more frequent storm surges (27 tropical cyclones slightly above normal) [13,14]. Climate change is an unpredictable factor and one of the most serious threats to forest ecosystems [15].
By area, Japanese forests comprise almost 50% conifers; however, Pinus thunbergii accounts for only 1% of the conifer composition [16]. In the Kyushu area, the species has been planted in coastal areas since more than 400 years ago [17]. A characteristic of P. thunbergii is tolerance to extreme conditions such as high salinity, high temperature, and low precipitation. Moreover, as a pine forest, it provides protection to coastal areas, by reducing wind damage, inhibiting sand movement, and decreasing tsunami wave energy [18,19].
Severe outbreaks of Burshaphelencus xylophilus (pine wood nematode; PWN) depleted P. thunbergii populations in Japan between the 1900s and 2000s. The spread of PWN in Japan is the most significant occurrences of pest-disease damage than another country. The individuals damaged by PWN reached its peak in 1979, exceeded 2.43 million m3; as of 2016, the damage was one-fifth of the peak volume [16]. Air temperatures significantly influence the growth of PWN [20,21]. From a forest pest/disease perspective, climate change can directly or indirectly affect forest dynamics, changing the way that host trees and pathogens interact [22]. The warming climate may provide conditions for further PWN outbreaks and damage in the future [23].
In 1978, a breeding project to develop a PWN-P. thunbergii resistant trees as a countermeasure against outbreaks [24] was established at Breeding Region Institutions in Japan. This breeding project was initiated to select surviving pine trees from heavily damaged forests in Southwestern Japan. In the case of P. thunbergii, 14,620 trees were selected as candidate, and after the artificial inoculation tests, 16 clones were certified as resistant trees [24,25]. Three rounds of breeding program, based on individual performance selection-trial, have been performed throughout Japan until 2018 with gradual changes in the methodology [26]. In each prefecture of Japan, seed orchards were designed based on these resistant trees breeding program and the seedlings were used as reforestation-material plant. To date, 211 PWN-P. thunbergii resistant trees from 71 forests had been developed [27]. The purpose of the P. thunbergii breeding project was to create PWN-resistant trees for use as reforestation materials to enhance old populations of P. thunbergii in Japan for mitigation functions. Before the existence of breeding project, artificial planting with natural seedling recruitment, as reforestation-material plants, had repeatedly performed to maintain the forest. Unfortunately, the mitigation functions have been given priority with little consideration of the seed sources or genetic impacts of artificial planting.
From a forest protection perspective, the deployment of PWN-P. thunbergii resistant trees at a given site would indeed protect forests against PWN infection, minimizing damage. However, deployment without proper genetic management could lead to a genetic disturbance within the population, such as genetic diversity loss and modification of the genetic structure, reduced adaptability to local environments, “gene swamping,” and increased homogeneity; thus, negatively impacting the population as a gene resource [7,10,28,29,30]. Therefore, genetic diversity management must be considered when implementing tree improvement-products such as PWN-P. thunbergii resistant trees [31]. Genetic management and silviculture are fundamental components of forest management systems that have the potential to affect one another [2]. Both strategies are important for preserving local genetic diversity and maintaining forest resilience against environmental changes [32] even to the ecosystem [33], especially in extreme environments such as coastal areas.
In Japan, genetic diversity as well as genetic management of P. thunbergii populations and PWN-P. thunbergii resistant trees topics have not been discussed. In this study, we developed a genetic management based on the current genetic informations (genetic diversity, genetic structure, and relatedness) of a local pine forest, Iki-no-Matsubara, that has been repeatedly planted for mitigation functions under the situation of PWN damage is not yet under control. The origin of seedlings in this site were inferred based on their genetic relationships with neighboring P. thunbergii populations in Kyushu area and throughout Japan. In addition, we investigated the genetic diversity, genetic structure, and relatedness of Kyushu PWN-P. thunbergii resistant trees with P. thunbergii populations in Kyushu area. In this way, this study aimed to preserve the Iki-no-Matsubara P. thunbergii population (current genetic diversity, genetic structure, and relatedness) as genetic resources throughs the use of Kyushu PWN-P. thunbergii resistant trees with the possibility of genetic disturbance when deploying it into the site. The genetic knowledges obtained from this case study are expected to provide a baseline for further seed sourcing as well as develop genetic management strategies within P. thunbergii populations, including Kyushu PWN-P. thunbergii resistant trees.
2. Materials and Methods
2.1. Study Field
Total individual and diversity of P. thunbergii within the populations in Japan has been declined. Most of current P. thunbergii populations in Japan, including Iki-no-Matsubara, are an uneven-aged forest, because it has been replanted repeatedly to preserve the forest. There was no historical record of the origin of material-planted and genetic information.
The research area of Iki-no-Matsubara (33°34′52.8″ N 130°17′59.7″ E) was 12.56 hectares. A folktale claims that the forest was established in tribute to Empress Jingu for Silla conquest around 200 or 300 AD (Yamato periods) [34]. Iki-no-Matsubara is one of Japan’s top 100 beautiful green pine forests [35]. It is not only an education forest that belongs to Kyushu University since 1922 but also as an urban forest and conservation forest with mitigation functions since Edo Era (1603–1868) or earlier [36]. Iki-no-Matsubara locates within Genkai Quasi-national Park, which under Nature Conservation Law based on Natural Park Act, designated by prefectural government as a conservation forest for mitigation functions [34]. Field survey, tree census, and measurement of the trees’ diameter at Iki-no-Matsubara was conducted from January 2017 until June 2019. From tree census data, the diameter was classified into three classes: 1–30 cm DBH (Diameter at breast height) range, 31–60 cm DBH range, and 61–90 cm DBH range (Table 1). Then, measured the stumps to estimate the age based on the DBH class ranges [37]. Based on cross-dating dendrochronology observation of annual ring of the stump in different location within Iki-no-Matsubara [38], the oldest tree was estimated to be around 200 years old (Table 1). P. thunbergii was highly regarded by Japan’s religion and culture and became Japan’s cultural identity. Hence, it possible that domesticated and artificial regeneration has been conducted repeatedly by local people since 1500 BP [39].

Table 1.
DBH class range based on stump wood and number of samples within the Iki-no-Matsubara population.
2.2. DNA Analysis
A total of 269 mature leaves were collected from selected trees at Iki-no-Matsubara experimental research field representing each DBH ranges class (Table 1). Selected trees were chosen randomly which represented each DBH classification and research field. Genomic DNA was extracted from 50 mg of tissue per individual by using the cetyltrimethyl ammonium bromide (CTAB) method [40] with slightly modifications and a DNeasy Plant Kit (Qiagen Inc., Valencia, CA, USA) following the manufacturer’s protocol. Simple Sequence Repeat (SSR) analysis was carried out by six markers, bcpt1075, bcpt1671, bcpt834, bcpt1823, bcpt2532, and bcpt1549 [18]. A total of 12 µL for PCR analysis was carried out by using 2 µL DNA elution, 1 µL of primer mix, DNase/RNase-free water, and 2× multiplex PCR kit by Qiagen (Qiagen Inc., USA). PCR reaction was carried out by Touchdown PCR [41]. PCR protocol began with denaturing 95 °C for 15 min, two step annealing: (1) 10 cycles of denaturation 94 °C (30 s), annealing 60 °C (90 s), annealing temperature was decreased by 0.5 °C per cycle until 55 °C, and extension 72 °C (1 min); (2), 20 cycles of 94 °C (30 s), 55 °C (90 s), and 72 °C (1 min), and final extension 60 °C for 30 min. Then, 10 µL of DNA amplicon mixed with Genescan 500 Liz Size Standard and Hi-Di Formamide (Applied Biosystems Inc., Bedford, MA, USA) was electrophoresed by ABI PRISM 3730 Genetic Analyzer (Applied Biosystems Inc., USA). Genotype data was analyzed using Genemapper 4.0 software (Applied Biosystems Inc., USA).
2.3. Statistical Analysis
Genotype data of 42 old populations of P. thunbergii from Iwaizumi et al. (2018) and PWN-P. thunbergii resistant trees (Watanabe, unpublished data, see Appendix A Table A2), which have been selected based on three breeding programs, was analyzed with data from Iki-no-Matsubara. Old populations are remaining populations of P. thunbergii that had decline due to overbreak of PWN. PWN-P. thunbergii resistant trees are tree improvement products that have high PWN resistance, which managed by Japan Tree Breeding Institution office in each region (Tohoku, Kansai, Kanto, and Kyushu) except Hokkaido. GeneAlex version 6.503 [42] was used to measure genetic diversity, Hardy-Weinberg Equilibrium, private alleles, genetic differentiation pattern through by principal coordinates analysis (PCoA) among populations and investigated gene flow (Nm) for examining the relationship between genetic differentiation and number of migrants variable per generation at each locus. Allelic richness (AR) and FIS (inbreeding coefficient) at each locus was calculated by Fstat version 2.9.3.2 software [43]. Structure 2.3.4 [44] was used to determine individual-based genetic structure assessment by Bayesian method with a simulation run 15 times replicated, K-set 1–6 for 30,000 iterations burn-in period, and 30,000 iterations LOCPRIOR model under admixture ancestral model. The optimum value of each cluster K and the ΔK value within the genetic structure was determined by Evanno method [45] then upload the results to structure harvester [46].
3. Results
3.1. Inference of Origin and Genetic Structure in Iki-no-Matsubara Based on DBH
Table 2 shows the genetic diversity in Iki-no-Matsubara. Na values was ranged from 14 (bcpt1549) to 29 (bcpt2532), Ne value from 3.18 (bcpt1549) to 7.83 (bcpt2532), AR value from 5.75 (bcpt1549) to 11.49 (bcpt2532), HO and HE from 0.57 (bcpt2532) to 0.85 (bcpt1075), and 0.69 (bcpt1549) to 0.87 (bcpt2532), respectively. Lowest value on FIS was −0.03 (bcpt1075) and highest was 0.35 (bcpt2532). Three markers, bcpt834, bcpt1823, and bcpt2532 showed deviation from Hardy-Weinberg equilibrium (p < 0.05, p < 0.001, and p < 0.001, respectively).

Table 2.
Genetic diversity of the Iki-no-Matsubara P. thunbergii population using six primer markers.
The Na, AR, Ho, and FIS values for Iki-no-Matsubara were higher than those reported by Iwaizumi et al. (2018). Iki-no-Matsubara had more private alleles than another population within the Kyushu region and the presence private alleles in the same loci were none to be found in nearby populations in Kyushu area. Among 269 trees, 92 carried a total of 18 private alleles at four out of six loci (Table 3). Four trees were in the 61–90 cm DBH range, six trees were in the 31–60 cm DBH range, and the remaining were in the 1–30 cm DBH range (Appendix A Table A1).

Table 3.
Private alleles in Iki-no-Matsubara with 42 old populations of P. thunbergii throughout Japan using six primers.
The genetic structure of Iki-no-Matsubara showed two color patterns (Figure 1A), even on K2, K3, or K4 structure result (number 8 in Appendix A Figure A1). Further analysis of the spatial distribution of genetic structure at Iki-no-Matsubara showed that the blue pattern (blue color ≥ 55%) was dominantly observed on the east side of the research field (Appendix A Figure A2). In the 1–30 cm DBH range, 20 out of 109 trees showed the blue pattern, while in the 31–60 cm and 61–90 cm DBH range 12 out of 108 trees and no trees, respectively, showed the blue pattern
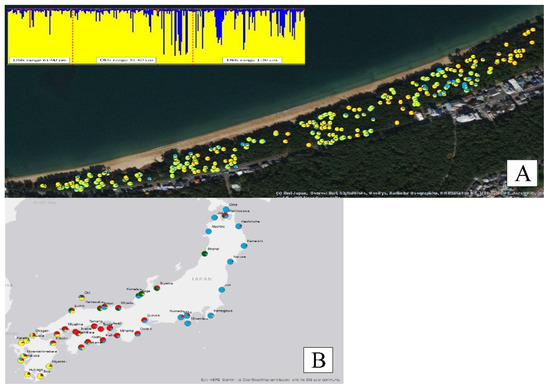
Figure 1.
Spatial distribution of genetic structure at Iki-no-Matsubara (A); Iki-no-Matsubara with 42 old populations of P. thunbergii throughout Japan (B).
Figure 1B shows the genetic structure among P. thunbergii populations throughout Japan. Iki-no-Matsubara was dominated by the yellow pattern, same with the other populations from the Kyushu area. Principle Coordinate Analysis (PCoA) showed that Iki-no-Matsubara was more similar to the Minami-Shimabara population than the Karatsu population, which is geographically closer to Iki-no-Matsubara. The Minami-Shimabara and Amakusa populations had the highest probability of taking part in the gene flow (Nm) into Iki-no-Matsubara, at 47.99% and 35.35%, respectively (Figure 2). Based on DBH class range (Figure 3) specifically, the similarity between the Minami-Shimabara and Iki-no-Mastubara 61–90 cm DBH, 31–60 cm DBH, and 1–30 cm DBH ranges were 14.18%, 36.93%, and 15.99%, respectively. More importantly, the relationship between the DBH ranges indicated that the 61–90 cm DBH range shared 53.44% of genetic similarity with the 31–60 cm range, and 6.51% with the 1–30 cm DBH range. This finding suggests that the origin of the young trees, 1–30 cm DBH range class, were not from the Iki-no-Matsubara population but another area.
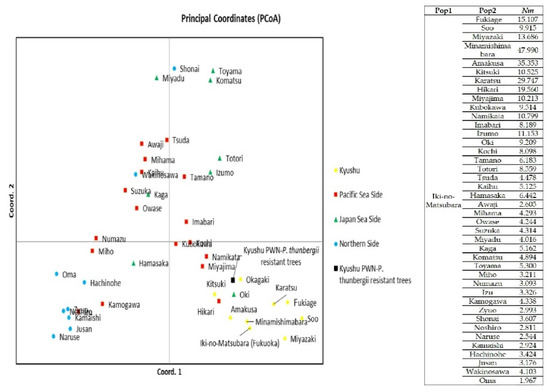
Figure 2.
PCoA of Iki-no-Matsubara with other populations of P. thunbergii and the Kyushu PWN-P. thunbergii resistant trees with gene flow (Nm) between Iki-no-Matsubara and the other populations.
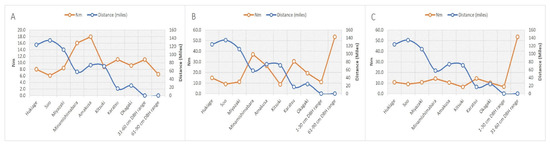
Figure 3.
Relationship between gene flow (Nm) and distance with other populations in the Kyushu area on the following basis: (A) 1–30 cm DBH class range; (B) 31–60 cm DBH class range; (C) 61–90 cm DBH class range.
3.2. Genetic Diversity and Genetic Structure of PWN-P. thunbergii Resistant Trees
Since the 1990s, PWN-P. thunbergii resistant trees have been planted to enhance the old populations of P. thunbergii. Therefore, analyzing the local genotype of the Iki-no-Matsubara population (genetic diversity, genetic structure, and relatedness with other populations in Kyushu area) provides a baseline when deploying Kyushu PWN-P. thunbergii resistant trees. In general, the genetic structure of PWN-P. thunbergii resistant trees within each region (Figure 4) showed Kyushu (yellow color) and Kanto (green) PWN-P. thunbergii resistant trees had the most distinct genetic structure (dominated by region’s structure pattern). In contrast, Tohoku and Kansai PWN-P. thunbergii resistant trees exhibited mixed patterns. The PCoA results show that the Kyushu PWN-P. thunbergii resistant trees are similar to the Okagaki populations (Figure 2). Some North Kyushu populations likely had a higher possibility of contributing to the gene flow than populations on the other side of Kyushu (see Appendix A Table A3). The genetic diversity of Kyushu PWN-P. thunbergii resistant trees was low compared with the mean genetic diversity of the P. thunbergii populations in entire Kyushu area (Table 4).
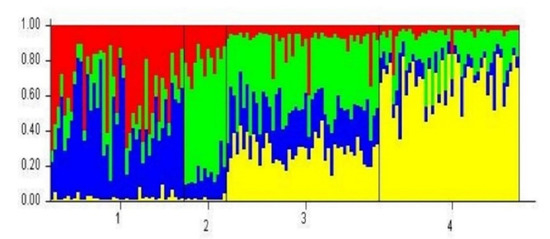
Figure 4.
Genetic structure of PWN-P. thunbergii resistant trees on K4 (1) Tohoku region, (2) Kanto region, (3) Kansai region, and (4) Kyushu region.

Table 4.
Genetic diversity of the Kyushu PWN-P. thunbergii resistant trees using six primer markers compared with the overall genetic diversity of populations in the Kyushu area.
4. Discussion
4.1. Inference of Origin and Genetic Structure in Iki-no-Matsubara based on DBH
Most P. thunbergii forests are located in coastal regions, including the Iki-no-Matsubara population. They have been expected for conservation area, especially to preserve mitigation functions such as reducing wind damage, inhibiting sand movement, and decreasing tsunami wave energy [19]. Before the existence of breeding project, artificial planting with natural seedling recruitment had repeatedly performed to maintain the forest.
In wind-pollinated conifers, the genetic diversity within the population has a tendency to be higher than that among populations. However, the genetic diversity within Iki-no-Matsubara was low in this study. Many P. thunbergii in Japan were damaged by the strong impact of PWN. After the 1980s, individuals with pest damage in the Iki-no-Matsubara population were removed and replanting has been continuously performed; however, the origin of seedlings were unknown. The number of private alleles was highest in Iki-no-Matsubara, and the presence of private alleles in the same loci were none to be found in the nearby populations in the Kyushu area. The lack of private alleles in a particular population within the Kyushu area is likely due to the small sample size compared to Iki-no-Matsubara [47]. The presence of private alleles in the Iki-no-Matsubara (Appendix A Table A1), interestingly, showed 31–60 cm DBH class range and 61–90 cm DBH class range shared on the same loci, while 1–30 cm DBH class range on different loci. Based on the structure analysis and PCoA results (Figure 2), we postulate that the Iki-no-Matsubara could be derived from the Kyushu area, especially the Minami-Shimabara or Amakusa population, which was farther from Iki-no-Matsubara than the Karatsu or Okagaki populations. In detail, 1–30 cm DBH class range was highly associated with Minami-shimabara and Amakusa. Meanwhile the 31–60 cm and 61–90 cm DBH class ranges displayed strong associated each other and the closest neighbour, Karatsu population (Figure 3). Such results, showed the recently planted the 1–30 cm DBH class range indicate that they were planted without considering genetic origin.
The genetic structure within the population was clearly divided into two patterns, and younger individual corresponding to DBH was remarkable. Furthermore, the genetic structure deviated to the area in the field. In more detail, some individuals exhibited the same pattern, yet different diameter class range (Figure 1A). The yellow patterns observed in Iki-no-Matsubara were common among populations in the Kyushu area (Figure 1B), while the blue pattern was not recognized in Karatsu nor Okagaki. There two possible explanations for this finding: (1) the materials planted in Iki-no-Matsubara were introduced from a different origin area, especially at DBH range 1–30 cm, which show dominantly blue color patterns; (2) Iki-no-Matsubara had more than two patterns of genetic structure in the past, including the patterns observed in Karatsu and Okagaki, but the population was reduced as a result of a bottleneck [18]. The exact cause is still uncertain due to the lack of historical records regarding the artificially-planted materials and the P. thunbergii genetic structure of Iki-no-Matsubara in the past. It would be reasonable to assume that the origin of the seedling was not considered when new planting was performed after removing individuals damaged by pine wilt disease.
4.2. Genetic Management of P. thunbergii in Iki-no-Matsubara with Kyushu PWN-P. thunbergii Resistant Trees
From a forest protection viewpoint, artificially planting Kyushu PWN-P. thunbergii resistant trees to enhance Iki-no-Matsubara population and counter PWN infection still has its merits; however, the genetic aspects such as genetic diversity (avoid homogeneity), genetic structure, resilience of the forest, and relatedness with another populations must also be properly considered. Thus, there are two crucial points to consider: (1) how well the PWN-P. thunbergii resistant trees as seed-sourcing strategy and (2) genetic management within the population, including the PWN-P. thunbergii resistant trees.
In addition, there two aspects should be considered for the genetic management of PWN-P. thunbergii resistant trees as seed-source strategy: (1) How well the mother trees represent the genetic diversity and relatedness in the selected area? (2) A sufficient number of resistant trees should be sourced as mother trees? [48,49,50,51]. The mother trees will represent the genetic diversity, structure, and gene flow pattern of the population where it was taken [52,53]. The extent of gene flow among populations shows how alleles are shared (similarities) and play an important role in genetic differentiation among populations [54,55].
From the perspective of genetic structure, Kyushu PWN-P. thunbergii resistant trees were noticeably displayed a yellow pattern (Kyushu region’s structure pattern) (Figure 4). However, from relatedness viewpoint, Kyushu PWN-P. thunbergii resistant trees were located in the middle between the Kyushu area and Pacific seaside area and shared similarity with the Okagaki populations (Figure 2). This may have occurred because the selected trees for Kyushu PWN-P. thunbergii resistant trees were not sufficiently balanced to represent all Kyushu area populations. In fact, among 43 Kyushu PWN-P. thunbergii resistant trees, ten were from Okagaki, and none were from Iki-no-Matsubara (Appendix A Table A2).
The sufficient number of mother trees, act as effective population size in seed orchard, must be examined first to manage the diversity and relatedness within Iki-no-Matsubara with other populations in Kyushu area [56]. The effective population size is a concept used to predict the ideal size of the population, considering that the genes transmitted to seeds will still possess the same level of genetic diversity after many generations [57]. However, this study case only provide the Iki-no-Matsubara, not of the entire P. thunbergii in Kyushu area. Thus, in the future, the breeding project of Kyushu PWN-P. thunbergii resistant trees need to develop a perspective based on genetic management according to the genetic characteristics in each local pine forest in the Kyushu area.
4.3. Kyushu PWN-P. thunbergii Resistant Trees Deployment Management as Part of Genetic Management
To maintain P. thunbergii population in Iki-no-Matsubara, both PWN resistance and genetic diversity must be considered as part of genetic management, which is PWN-P. thunbergii resistant trees deployment management. Only using clones (vegetative) or seeds of specific Kyushu PWN-P. thunbergii resistant trees as reforestation-material plants on a large scale repeatedly for long-terms would cause a genetic disturbance such as increased homogeneity, inbreeding depression, reduced genetic diversity and adaptability to local environments [30,31,58]; thus, negatively impacting the population as a gene resource. Therefore, it is necessary to determine the status numbers of Kyushu PWN-P. thunbergii resistant trees [59] using information from genetic analysis within the population by practice selective seed-cone harvesting to balance genetic gain and diversity [48,59] for the necessary reforestation. When considering genetic diversity in the next generation and the status number of Kyushu PWN-P. thunbergii resistant trees, we can first refer to the local seed pool for reference, where at least 24 seedlings (generative) from each of the 30 mother trees will be needed to provide complete coverage for genetic diversity in the Iki-no-Matsubara population in the next generation [60]. Genetic diversity is defined as the genetic variation carried by individuals within a population as a part of their evolutionary path, providing a basis to form responses to environmental changes, as resilience of the forest [61].
Seedlings from a local seed pool or a neighbour population, such as Karatsu (geographically near of Iki-no-Matsubara), should be given priority. A seedling’s adaptive potential from the local seed pool will have the optimal genotype because it has undergone many life cycles within the local environment over several generations. Proper seedling selection for planting is necessary to avoid maladaptation and improve the survival rate [62,63]. Furthermore, determining the origin of seedlings according to the Japan Forest Seeds and Seedlings Law 1939 [64] so that, at least, the structure pattern among the four areas shown in Figure 1B could be maintained. Subsequently, PWN-P. thunbergii resistant trees should be managed separately in each Japan Tree Breeding Institution office region. Using a non-local seed pool or non-local genetic pattern could lead to uncertain results in terms of adaptation and genetic differentiation among populations [30,65].
5. Conclusions
Declining P. thunbergii populations as a result of PWN outbreaks triggered to the consideration of genetic diversity management of the current populations for necessary genetic resources [18]. A forest with high genetic diversity provides a foundation for individuals to survive and adapt through evolution, especially when the forest has undergone human intervention [9,47,66,67]. Nevertheless, understanding the current genetic informations of Iki-no-Matsubara (genetic diversity, genetic structure, and relatedness) are essential for deploy Kyushu PWN-P. thunbergii resistant trees into the site, as part of genetic management. Genetic diversity (HO) in Iki-no-Matsubara was 0.71 and dominated by yellow pattern from structure viewpoint. However, information based on DBH class range showed high relatedness with Minami-Shimabara and Amakusa, and there was a possibility that the origin of the materials that had been planted were not from the local seed pool was proposed, which was especially likely for the 1–30 cm DBH class range.
Additionally, the genetic structure of Kyushu PWN-resistant trees revealed a clear yellow genetic pattern. The Kyushu PWN-P. thunbergii resistant trees genetic diversity was lower than that of the overall population in the Kyushu area. An insufficient number of Kyushu PWN-P. thunbergii resistant trees unbalanced the gene flow, thus genetically to be found similar to the Okagaki population. A sufficient number of Kyushu PWN-P. thunbergii resistant trees, as mother trees, within seed orchards and sufficient status number of the seedlings need to be considered to safely deploy Kyushu PWN-P. thunbergii resistant trees as reforestation-material plants into Iki-no-Matsubara population. This approach can be used not only to preserve Iki-no-Matsubara population (genetic diversity, genetic structure, relatedness, and resilience of the forests) but can also be applied to minimize PWN damage. These results provide a baseline for further seed sourcing as well as develop genetic management strategies within P. thunbergii populations, including the PWN-P. thunbergii resistant trees.
Author Contributions
Methodology, Investigation, Conceptualisation, Formal Analysis, Writing—original draft and editing, Validation, A.A.M.; Methodology, Investigation, Conceptualisation, Resources, M.T.; Investigation, Resources, Supervision, Writing—review, K.M., T.I., M.G.I.; Methodology, Investigation, Conceptualisation, Supervision, Resources, Validation, Writing—review and editing, A.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Japan Society for the Promotion of Science, KAKENHI, grant number 17K07853.
Institutional Review Board Statement
This study did not require ethical approval. This study did not involve humans or animals.
Informed Consent Statement
This study did not involve humans or animals.
Data Availability Statement
Data available on request due to restrictions eg privacy or ethical. The data presented in this study are available upon request to the authors. The data are not publicly available due to the data are managed by Instituional authority in Japan.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Number of private alleles within Iki-no-Matsubara.
Table A1.
Number of private alleles within Iki-no-Matsubara.
| No | DBH Class Range | Number of Trees | Loci |
|---|---|---|---|
| 1 | 1–30 cm | 82 | bcpt2532 |
| 1 | bcpt1823 | ||
| 2 | 31–60 cm | 5 | bcpt1671 |
| 1 | bcpt834 | ||
| 3 | 61–90 cm | 1 | bcpt1671 |
| 4 | bcpt834 |

Table A2.
List of PWN-P. thunbergii resistant trees based on the region [68].
Table A2.
List of PWN-P. thunbergii resistant trees based on the region [68].
| No | PWN-P. thunbergii Resistant Trees | |||
|---|---|---|---|---|
| Tohoku | Kansai | Kanto | Kyushu | |
| 1 | Naruse39 | Tanabe54 | Odaka37 | Shima64 |
| 2 | Naruse72 | Bizen143 | Odaka203 | Tsuyazaki50 |
| 3 | Naruse6 | Mitoyo103 | Iwaki27 | Karatsu1 |
| 4 | Watari5 | Namikata37 | Osuga5 | Karatsu4 |
| 5 | Yamamoto82 | Namikata73 | Osuga6 | Karatsu7 |
| 6 | Yamamoto84 | Misaki90 | Osuga12 | Karatsu9 |
| 7 | Yamamoto90 | Yoshida2 | Osuga15 | Karatsu11 |
| 8 | Yuza27 | Yasu37 | Osuga23 | Karatsu16 |
| 9 | Yuza72 | Tosashimizu63 | Utchihara5 | Karatsu17 |
| 10 | Yuza33 | Kumihama10 | Tomiura7 | Obama30 |
| 11 | Yuza54 | Kumihama21 | Okazaki25 | Oseto12 |
| 12 | Yuza56 | Kumihama109 | Okazaki34 | Kawaura8 |
| 13 | Yuza58 | Amino31 | Okazaki35 | Kawaura13 |
| 14 | Yuza60 | Amino43 | Amakusa20 | |
| 15 | Yuza57 | Tango47 | Oita8 | |
| 16 | Yuza59 | Tango50 | Sadowara8 | |
| 17 | Yuza77 | Tango51 | Sadowara14 | |
| 18 | Murakami2 | Tango58 | Sadowara15 | |
| 19 | Murakami5 | Tango60 | Miyazaki20 | |
| 20 | Murakami11 | Tango65 | Sendai20 | |
| 21 | Murakami16 | Tango69 | Ei425 | |
| 22 | Murakami44 | Tango71 | Hiyoshi1 | |
| 23 | Murakami9 | Totori7 | Hiyoshi5 | |
| 24 | Murakami15 | Totori13 | Hukiage25 | |
| 25 | Nigata8 | Iwami63 | Okagaki1 | |
| 26 | Nigata40 | Nisinosima142 | Okagaki5 | |
| 27 | Nigata3 | Komatsu99 | Okagaki6 | |
| 28 | Aikawa27 | Ota39 | Okagaki8 | |
| 29 | Nagaoka15 | Hamada6 | Okagaki25 | |
| 30 | Nagaoka8 | Hamada12 | Okagaki29 | |
| 31 | Ozika151 | Hamada24 | Okagaki31 | |
| 32 | Sendai35 | Hamada28 | Okagaki32 | |
| 33 | Ishimaki251 | Gotsu29 | Okagaki35 | |
| 34 | Ishimaki260 | Yunotsu52 | Okagaki20 | |
| 35 | Ishimaki259 | Hukube51 | Munakata2 | |
| 36 | Atsumi43 | Hukube54 | Munakata4 | |
| 37 | Tsuruoka38 | Hukube60 | Munakata12 | |
| 38 | Tsuruoka44 | Hukube61 | Munakata19 | |
| 39 | Tsuruoka46 | Hukube71 | Shingu2 | |
| 40 | Zyoetsu1 | Koryo60 | Shingu5 | |
| 41 | Zyoetsu10 | Koryo77 | Shingu11 | |
| 42 | Kaga387 | Shingu14 | ||
| 43 | Kaga388 | Shingu17 | ||
| 44 | Kaga295 | |||
| 45 | Shiga396 | |||
| 46 | Tsuruga14 | |||
| 47 | Tsuruga15 | |||

Table A3.
Gene flow (Nm) of Kyushu PWN-P. thunbergii resistant trees with the populations within Kyushu area.
Table A3.
Gene flow (Nm) of Kyushu PWN-P. thunbergii resistant trees with the populations within Kyushu area.
| Pop1 | Pop2 | FST | Nm |
|---|---|---|---|
| Hukiage | Kyushu PWN-P. thunbergii resistant trees | 0.020 | 12.308 |
| Soo | Kyushu PWN-P. thunbergii resistant trees | 0.033 | 7.349 |
| Miyazaki | Kyushu PWN-P. thunbergii resistant trees | 0.027 | 8.956 |
| Minamishimabara | Kyushu PWN-P. thunbergii resistant trees | 0.007 | 34.354 |
| Amakusa | Kyushu PWN-P. thunbergii resistant trees | 0.008 | 32.530 |
| Kitsuki | Kyushu PWN-P. thunbergii resistant trees | 0.016 | 15.698 |
| Karatsu | Kyushu PWN-P. thunbergii resistant trees | 0.008 | 32.144 |
| Iki-no-Matsubara (Fukuoka) | Kyushu PWN-P. thunbergii resistant trees | 0.010 | 25.961 |
| Okagaki | Kyushu PWN-P. thunbergii resistant trees | 0.010 | 24.077 |
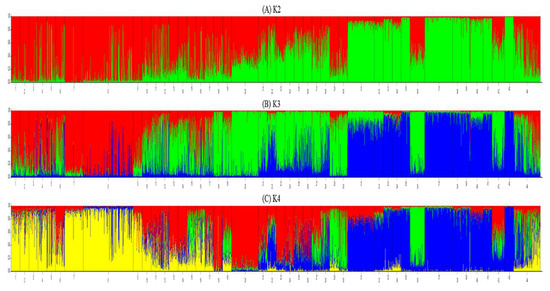
Figure A1.
Genetic structure of Iki-no-Matsubara (Fukuoka) with 42 old populations of P.thunbergii on K2, K3, and K4 (From South-West (Left) to North-East (Right)).
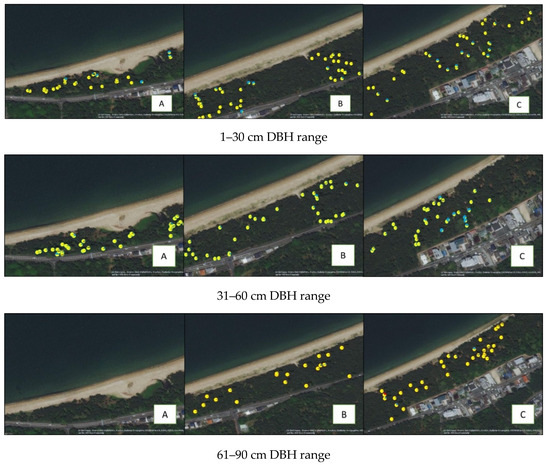
Figure A2.
Spatial distribution of Iki-no-Matsubara genetic structure per DBH range (A) West side, (B) Central side, and (C) East side.
References
- Fuhrer, E. Forest function, ecosystem stability and management. For. Ecol. Manag. 2000, 132, 29–38. [Google Scholar] [CrossRef]
- Krott, M. Forest Policy Analysis; European Forest Institute; Springer: Amsterdam, The Netherlands, 2005; ISBN 978-1-4020-3485-5. [Google Scholar]
- Santika, T.; Meijaard, E.; Wilson, K.A. Designing multifunctional landscapes for forest conservation. Environ. Res. Lett. 2015, 10. [Google Scholar] [CrossRef]
- Benz, J.P.; Chen, S.; Dang, S.; Dieter, M.; Labelle, E.R.; Liu, G.; Hou, L.; Mosandl, R.M.; Pretzsch, H.; Pukall, K.; et al. Multifunctionality of forests: A white paper on challenges and opportunities in China and Germany. Forests 2020, 11, 266. [Google Scholar] [CrossRef]
- Aravanopoulos, F. Do silviculture and forest management affect the genetic diversity and structure of long-impacted forest tree populations? Forests 2018, 9, 355. [Google Scholar] [CrossRef]
- Ratnam, W.; Rajora, O.P.; Finkeldey, R.; Aravanopoulos, F.; Bouvet, J.M.; Vaillancourtf, R.E.; Kanashiro, M.; Fady, B.; Tomita, M.; Vinson, C. Genetic effects of forest management practices: Global synthesis and perspectives. For. Ecol. Manag. 2014, 333, 52–65. [Google Scholar] [CrossRef]
- Tubby, K.V.; Webber, J.F. Pests and diseases threatening urban trees under a changing climate. For. Int. J. For. Res. 2010, 83, 451–459. [Google Scholar] [CrossRef]
- Milner-Gulland, E.J. Interactions between human behaviour and ecological systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 270–278. [Google Scholar] [CrossRef]
- Aravanopoulos, F.A. Conservation and monitoring of tree genetic resources in temperate forests. Curr. For. Rep. 2016, 2, 119–129. [Google Scholar] [CrossRef]
- Ledig, F.T. The conservation of diversity in forest trees: Why and how should genes be conserved? BioScience 1988, 38, 471–479. [Google Scholar] [CrossRef]
- Rajora, O.P.; Pluhar, S.A. Genetic diversity impacts of forest fires, forest harvesting, and alternative reforestation practices in black spruce (Picea mariana). Theor. Appl. Genet. 2003, 106, 1203–1212. [Google Scholar] [CrossRef]
- White, T.L.; Adams, W.T.; Neale, D.B. Forest Genetics; Cabi: Cambridge, MA, USA, 2007; ISBN 978-0-85199-083-5. [Google Scholar]
- Japan Meteorological Agency. Climate Change Monitoring Report 2017; Japan Meteorological Agency: Tokyo, Japan, 2018; pp. 31–55.
- Ministry of the Environment; Ministry of the Education Culture, Sports, Science, and Technology; Ministry of the Agriculture, Forestry, and Fisheries; Ministry of the Land, Infastructure, Transport, and Tourism; Japan Meteorological Agency. Synthesis Report on Observations, Projections, and Impact Assesments of Climate Change, 2018: Climate Change in Japan and It’s Impacts; Ministry of the Environment: Tokyo, Japan, 2018; pp. 1–7.
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Forestry Agency. State of Japan’s Forest and Forest Management: 3rd Country Report of Japan to the Montreal Process; Forest Agency: Tokyo, Japan, 2019.
- Ministry of Agriculture, Forestry, and Fisheries. Niji-no-Matsubara (Pine Grove) Recreation Forest. Available online: https://www.rinya.maff.go.jp/e/national_forest/recreation_forest/niji.html (accessed on 20 April 2020).
- Iwaizumi, M.G.; Miyata, S.; Hirao, T.; Tamura, M.; Watanabe, A. Historical seed use and transfer affect geographic specificity in genetic diversity and structure of old planted Pinus thunbergii population. For. Ecol. Manag. 2018, 408, 211–219. [Google Scholar] [CrossRef]
- Suwa, R. Evaluation of the wave attenuation function of a coastal black pine Pinus thunbergii forest using the individual-based dynamic vegetation model SEIB-DGVM. J. For. Res. 2013, 18, 238–245. [Google Scholar] [CrossRef]
- Ichihara, Y.; Fukuda, K.; Suzuki, K. Early symptom development and histological change associated with migration of Bursaphelenchus xylophilus in seedling tissues of Pinus thunbergii. Plant Dis. 2000, 84, 675–680. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Matsunaga, K.; Watanabe, A. Influence of temperature on pine wilt disease progression in Pinus thunbergii seedlings. Eur. J. Plant Pathol. 2019, 156, 1–10. [Google Scholar] [CrossRef]
- Linnakoski, R.; Kasanen, R.; Dounavi, A.; Forbes, K.M. Forest health under climate change: Effects on tree resilience, and pest and pathogen dynamics. In Frontiers in Plant Science; Frontiers Media SA: Lausanne, Switzerland, 2019; pp. 5–7. [Google Scholar] [CrossRef]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Toda, T.; Nishimura, K.; Yamate, H.; Fuyuno, S. Breeding project on resistance to pine-wood nematode-an outline of the research and the achievement of the project for ten years. Bull. For. Tree Breed. Inst. 1989, 3, 1–84. [Google Scholar]
- Kurinobu, S. Current status of resistance breeding of Japanese pine species to pine wilt disease. For. Sci. Technol. 2008, 4, 51–57. [Google Scholar] [CrossRef]
- Matsunaga, K.; Watanabe, A. characterization of pine wood nematode and development of more improved second generation resistant trees [In Japanese]. For. Genet. Tree Breed. 2018, 7, 115–119. [Google Scholar] [CrossRef]
- FFPRI. Forest tree breeding center and forest bio-research center brochure. In Forest Research and Management Organization National Research and Development Agency; FFPRI: Tokyo, Japan, 2018. [Google Scholar]
- Aitken, S.N.; Whitlock, M.C. Assisted gene flow to facilitate local adaptation to climate change. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 367–388. [Google Scholar] [CrossRef]
- Içgen, Y.; Kaya, Z.; Çengel, B.; Velioǧlu, E.; Öztürk, H.; Önde, S. Potential impact of forest management and tree improvement on genetic diversity of Turkish red pine (Pinus brutia Ten.) plantations in Turkey. For. Ecol. Manag. 2006, 225, 328–336. [Google Scholar] [CrossRef]
- Konnert, M.; Fady, B.; Gomory, D.; A’Hara, S.; Wolter, F.; Ducci, F.; Koskela, J.; Bozzano, M.; MaKowalczyk, J. Use and transfer of forest reproductive material in europe in the context of climate change. In European Forest Genetic Resources Programme (EUFORGEN), Bioversity International; Euforgen: Rome, Italy, 2015; ISBN 978-92-9255-031-8. [Google Scholar]
- Ingvarsson, P.K.; Dahlberg, H. The effect of clonal forestry on genetic diversity in wild and domesticated stands of forest trees. Scandinavian J. For. Res. 2019, 34, 370–379. [Google Scholar] [CrossRef]
- Kavaliauskas, D.; Fussi, B.; Westergren, M.; Aravanopoulos, F.; Finzgar, D.; Baier, R.; Alizoti, P.; Bozic, G.; Avramidou, E.; Konnert, M.; et al. The interplay between forest management practices, genetic monitoring, and other long-term monitoring systems. Forests 2018, 9, 133. [Google Scholar] [CrossRef]
- Bailey, J.K.; Schweitzer, J.A.; Ubeda, F.; Koricheva, J.; LeRoy, C.J.; Madritch, M.D.; Rehill, B.J.; Bangert, R.K.; Fischer, D.G.; Allan, G.J.; et al. From genes to ecosystems: A synthesis of the effects of plant genetic factors across levels of organization. Philos. Trans. Royal Soc. B Biol. Sci. 2009, 364, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka City. The Iki-no-Matsubara pine forest and genko borui. Hakata Cult. 2016, 133. Available online: http://www.fukuoka-now.com (accessed on 23 December 2019).
- Awano, T. An evaluation of the intrinsic value of the takada pine forest (Takakada-no-Matsubara) as a scenic beauty spot in japan. Urban Reg. Plan. Rev. 2015, 2, 18–30. [Google Scholar] [CrossRef]
- Park, K.W.; Sokh, H.; Inoue, S. Local inhabitants consciousness of using and managing urban forest Aaea-a case study of iki no matsubara in fukuoka city. J. Fac. Agric. Kyushu Univ. 2003, 47, 267–275. [Google Scholar]
- Studhalter, R.A.; Glock, W.S.; Agerter, S.R. Tree gowth: Some historical chapters in the study of diameter growth. Bot. Rev. 1963, 29, 245–365. [Google Scholar] [CrossRef]
- Pilcher, J.R. Sample preparation, cross-dating, and measurement. In Method of Dendrochronology: Application in the Environmental Sciences; Editor Cook, E., Kairiukstis, L., Eds.; Springer Science & Business Media Dordrecht: Berlin, Germany, 1992; pp. 40–50. ISBN 978-94-015-7879-0. [Google Scholar]
- Omura, H. Tree, forests, and religion in japan. Mt. Res. Dev. 2004, 24, 179–182. [Google Scholar] [CrossRef]
- Fukatsu, E.; Isoda, K.; Hirao, T.; Watanabe, A. Development and characterization of simple sequence repeat dna markers for Zelkova serrata. Mol. Ecol. Notes 2005, 5, 378–380. [Google Scholar] [CrossRef]
- Korbie, D.J.; Mattick, J.S. Protocol: Touchdown pcr for increased specifiy and sensitivity in pcr amplification. Nat. Protoc. 2008, 3, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GENALEX 6.5: Genetic analysis in excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. FSTAT, a Program to Estimate and Test Gene Diversities and Fixation Indices (Version 2.9.3.2). 2001. Available online: http://www2.unil.ch/popgen/softwares/fstat.html (accessed on 20 December 2019).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; von Holdt, B.M. Stcuture harvester: A website and program for visualizing structure output and implementing the evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Welt, R.S.; Litt, A.; Franks, S.J. Analysis of population genetic structure and gene fow in an annual plant before and after a rapid evolutionary response to drought. AoB Plants 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Funda, T.; lstibůrek, M.; Lachout, P.; Klápště, J.; El-Kassaby, Y.A. Optimization of combine genetic gain and diversity for collection and deployment of seed orchard crops. Tree Genet. Genomes 2009, 5, 583–593. [Google Scholar] [CrossRef]
- Kang, K.S.; Lindgren, D.; Mullin, T.J. Prediction of genetic gain and genetic diversity in seed orchard crops under alternative management strategies. Theor. Appl. Genet. 2001, 103, 1099–1107. [Google Scholar] [CrossRef]
- David, A.; Pike, C.; Stine, R. Comparison of selection methods for optimizing genetic gain and gene diversity in a red pine (Pinus resinosa Ait.). Theor. Appl. Genet. 2003, 107, 843–849. [Google Scholar] [CrossRef]
- Funda, T. Population Genetics of Conifer Seed Orchard. Ph.D. Thesis, The Faculty of Graduate Studies (Forestry), The University of British Columbia, Vancouver, BC, Canada, 2012. [Google Scholar]
- Kitzmiller, J.H. Managing genetic diversity in a tree improvement program. For. Ecol. Manag. 1990, 35, 131–149. [Google Scholar] [CrossRef]
- Wheeler, N.C.; Jech, K.S. The use of electrophoretic markers in seed orchard research. New For. 1992, 6, 311–328. [Google Scholar] [CrossRef]
- Slatkin, M. Gene flow and the geographic structure of natural population. Science 1987, 236, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Stefenon, V.M.; Gailing, O.; Finkeldey, R. The role of gene flow in shaping genetic structures of the subtropical conifer species Araucaria angustifolia. Plant Biol. 2008, 10, 356–364. [Google Scholar] [CrossRef]
- Lindgren, D.; Mullin, T.J. Relatedness and status number in seed orchard crops. Can. J. For. Res. 1998, 28, 276–283. [Google Scholar] [CrossRef]
- Crow, J.F.; Kimura, M. An Introduction to Population Genetic Theory; Blackburn Press: Caldwell, NJ, USA, 2009; pp. 345–364. ISBN 978-1-932846-12-6. [Google Scholar]
- Wu, H.X. Benefits and risks of using clones in forestry-a review. Scandinavian J. For. Res. 2018, 34, 352–359. [Google Scholar] [CrossRef]
- Lindgren, D.; El-Kassaby, Y. Genetic consequences of combining selective cone harvesting and genetic thinning in clonal seed orchards. Silvae Genet. 1989, 38, 65–70. [Google Scholar]
- Iwaizumi, M.; Kawai, Y.; Miyamoto, N.; Nasu, J.; Kubota, M.; Mukasyaf, A.A.; Tamura, M.; Watanabe, A. Evaluation of Genetic Diversity of Mother Trees and Seed Pool for Gene Conservation of A Pinus thunbergii Population. Jpn. For. Soc. Congr. 2020, 131. (In Japanese) [Google Scholar] [CrossRef]
- Jost, L.; Archer, F.; Flanagan, S.; Gaggiotti, O.; Hoban, S.; Latch, E. Differentiation measures for conservation genetics. Evol. Appl. 2018, 11, 1139–1148. [Google Scholar] [CrossRef]
- Johnson, G.R.; Sorensen, F.C.; St Clair, J.B.; Cronn, R.C. Pacific northwest forest tree seed zones-a template for native plants? Nativ. Plants J. 2004, 5, 131–140. [Google Scholar] [CrossRef]
- O’Brien, E.K.; Krauss, S.L. Testing the home-site advantage in forest trees on disturbed and undisturbed sites. Restor. Ecol. 2010, 18, 359–372. [Google Scholar] [CrossRef]
- Nagamitsu, T.; Shimada, K.; Kanazashi, A. A reciprocal transplant trial suggests a disadvantage of northward seed transfer in survival and growth of japanese red pine (Pinus densiflora) trees. Tree Genet. Genomes 2015, 11, 1–10. [Google Scholar] [CrossRef]
- Adams, W.T.; Burczyk, J. Magnitude and implications of gene flow in gene conservation reserves. In Forest Conservation Genetics: Principles and Practice; CABI: Wallingford, UK, 2000; pp. 215–224. ISBN 0-643-06260-2. [Google Scholar]
- Jump, A.S.; Marchant, R.; Peñuelas, J. Environmental change and the option value of genetic diversity. Trends Plant Sci. 2009, 14, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.J.; Underwood, N.; Vellen, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef] [PubMed]
- FFPRI. Variety of Pine Wood Nematode Resistant Tree Brochure; Forest Tree Breeding Center and Forest Bio-Research Center; Forest Research and Management Organization National Research and Development Agency: Tokyo, Japan, 2019. Available online: https://www.ffpri.affrc.go.jp/ftbc/business/sinhijnnsyu/teikousei.html (accessed on 21 April 2020). (In Japanese)
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).