Abstract
Eutypella parasitica R.W. Davidson and R.C. Lorenz is the causative agent of Eutypella canker of maple, a destructive disease of maples in Europe and North America. The fungus E. parasitica infects the trunk through a branch stub or bark wound. Because the fungal community may have an impact on infection and colonization by E. parasitica, the composition of fungi colonizing wood of dead branches of sycamore maple (Acer pseudoplatanus L.) was investigated in five sampling sites in Slovenia. Forty samples from each sampling site were collected between the November 2017 and March 2018 period. Isolations were made from the wood in the outer part of dead branches and from discoloured wood in the trunk that originated from a dead branch. Pure cultures were divided into morphotypes, and one representative culture per morphotype was selected for further molecular identification. From a total of 2700 cultured subsamples, 1744 fungal cultures were obtained, which were grouped into 212 morphotypes. The investigated samples were colonized by a broad spectrum of fungi. The most frequently isolated species were Eutypa maura (Fr.) Sacc., Eutypa sp. Tul. and C. Tul., Fusarium avenaceum (Fr.) Sacc., Neocucurbitaria acerina Wanas., Camporesi, E.B.G. Jones and K.D. Hyde and E. parasitica. In this study, we distinguished species diversity and the fungal community. There were no significant differences in the diversity of fungal species between the five sampling sites, and branch thickness did not prove to be a statistically significant factor in fungal species diversity. Nevertheless, relatively low Jaccard similarity index values suggested possible differences in the fungal communities from different sampling sites. This was confirmed by an analysis of similarities, which showed that the isolated fungal community distinctly differed between the five sampling sites and between the different isolation sources. Eutypella parasitica was isolated from all five investigated sampling sites, although Eutypella cankers were observed in only three sampling sites, indicating the possibility of asymptomatic infection.
1. Introduction
Sycamore maple (Acer pseudoplatanus L.) is the most common maple and also one of the most valuable tree species in Europe [1]. It is a temperate climate tree originating from the mountainous areas of Central Europe. The current distribution of A. pseudoplatanus extends from Turkey and Spain to Ireland and Sweden [2], and it is adapted to a wide range of site conditions [3]. It is characterized by rapid growth and potentially high timber prices [4]. It is light demanding and grows best on highly productive sites [5,6,7]. In 2018, A. pseudoplatanus represented 3.14% of the wood stock of Slovenian forests [8].
Nectria cinnabarina (Tode) Fr., Cryptostroma corticale (Ellis and Everh.) P.H. Greg. and S. Waller, Rhytisma acerinum (Pers.) Fr., Verticillium dahliae Kleb., Cristulariella depraedans (Cooke) Höhn., Sawadaea Miyabe, Diplodina acerina (Pass.) B. Sutton, Acericecis vitrina Kieffer, Zeuzera pyrina L. and Eriophyes Nalepa are the most typical harmful organisms for A. pseudoplatanus worldwide [9]. The fungus Eutypella parasitica R.W. Davidson and R.C. Lorenz, the causative agent of Eutypella canker of maple, has also a high potential to damage sycamore maple. The disease was reported for the first time in Europe from Slovenia [10], and later from Austria, Croatia, Germany, Hungary, the Czech Republic, Poland and Italy [11,12,13,14,15,16,17]. It is a serious disease that affects the aesthetic and economic value of infected maple trees [10]. It is believed to originate from North America [18] and represents a considerable risk for an extensive area of naturally distributed maples in Europe [17,19]. Eutypella parasitica is believed to enter the trunk through branch stubs or bark wounds [20] and consequently creates a characteristic canker mostly on the lower portions of the trunk [18,21]. Fruiting bodies (i.e., perithecia) develop in the central part of six to eight-year-old cankers. Their black necks protrude slightly above the surface [18] and release ascospores during wet periods at moderate temperatures [20,22]. The high number of discharged ascospores is an important factor of successful disease spread [21]. Spores are dispersed by wind, over long distances by trade of plants for planting or wood [23]. The optimal temperature for fungus growth is 24–28 °C [22,24].
Fungal communities in the dead branches of A. pseudoplatanus and other maples are still not well known. There have been very few studies of the fungal endophytes and saprotrophs present on sycamore branches, and none of these species have been studied in connection with E. parasitica. Most fungi on the dead twigs of A. pseudoplatanus belong to Ascomycota and Deuteromycota [25]. Fungal communities of Aceraceae are usually dominated by a few species that belong to the Diaporthales [26]. Different authors [25,27,28,29,30,31] have studied the fungal communities of the wood and bark of living or dead branches of A. pseudoplatanus (Table 1). These studies provide a context for this study and a reference point for comparison with our results. There are also some research papers on studies of fungal communities in Acer saccharum Marshall [32], A. ginnala Maxim. [33], A. truncatum Bunge [34] and A. rubrum L. [35], but extensive research of fungi in wood of dead branches of A. pseudoplatanus in connection with E. parasitica is lacking.

Table 1.
Prevailing fungal taxa 6 isolated from the branches of Acer pseudoplatanus in other studies.
The aim of our study was to determine the species composition of fungi colonizing wood of dead branches of young A. pseudoplatanus in connection with E. parasitica in the central part of Slovenia.
2. Materials and Methods
2.1. Definitions of Repeatedly Used Terms
We use a number of terms repeatedly throughout the text. Short explanations of these terms are given here for easier reading and understanding:
- Sampling site—a site or an area in the forest stand where samples were collected
- Sample—a dead A. pseudoplatanus branch with a section of the trunk where it was attached (Figure 1)
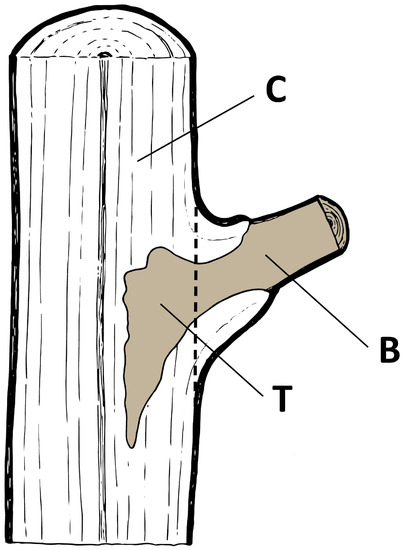 Figure 1. Sources of fungal isolations in a sample: B, branch; T, trunk; C, control (illustration by S. Zidar, Slovenian Forestry Institute).
Figure 1. Sources of fungal isolations in a sample: B, branch; T, trunk; C, control (illustration by S. Zidar, Slovenian Forestry Institute). - Isolation source—a location in a sample from which subsamples were cut (B—branch; T—trunk; C—control) (Figure 1)
- Subsample—a small piece of approximately 1 × 2 × 2 mm cut from the wood and representing three isolation sources (if possible) in each sample
- Culture—an outgrown mycelium from a subsample
2.2. Inventory of Eutypella Canker of Maple
One-hectare plots (100 × 100 m) were established in five sampling sites (Table 2) to assess the presence of the Eutypella canker of maple. Every sycamore maple with a diameter at breast height of at least ten centimetres was carefully checked for typical symptoms of E. parasitica—depressed or flattened areas covered by firmly attached bark; broad, slightly raised concentric rings of callus tissue; white to buff mycelial fans under the bark at the margins of the canker; and the presence of black perithecia in the centres of older cankers [18].

Table 2.
Characteristics of sampling sites.
2.3. Sampling
Field sampling was performed between the November 2017 and March 2018 period. In each sampling site, twenty individuals of A. pseudoplatanus with a diameter at breast height of less than 6.5 cm were randomly chosen. From each tree, one to three samples were randomly collected (Figure 1) and altogether 40 samples were collected from each sampling site. A total of 200 samples were analysed (Table 3).

Table 3.
Mean and standard deviation (in parenthesis) of the height, length and DBH (diameter at breast height) of sampled trees and branches in sampling sites.
2.4. Isolation of Fungi
Collected samples were labelled, placed in paper bags and transported to the laboratory. Samples were stored at 4 °C and processed within two days. After rinsing and scrubbing under running tap water, samples were surface sterilized by 70% (v/v) ethanol (1 min), followed by sodium hypochlorite with 1% available chlorine (30 sec) and again by 70% (v/v) ethanol (1 min). Finally, samples were rinsed under distilled water. After surface sterilization, samples were dried, halved and cut into smaller subsamples with sterilized equipment. Fungal isolations were made from wood representing three sources (if possible) in each sample: wood in the outer part of the dead branch (eight subsamples; labelled “B”; branch), discoloured wood in the trunk that originated from the dead branch (eight subsamples; labelled “T”; trunk) and visually healthy, non-discoloured wood in the trunk (four subsamples; labelled “C”; control) (Figure 1). Subsamples were evenly plated on 2% (w/v) malt extract agar (MEA; Becton Dickinson, Sparks, MD, USA), four subsamples per plate (70 mm in diameter). Petri dishes were sealed, incubated at 19.6 °C ± 1.0 °C and examined periodically. Outgrown mycelium from the wood subsamples were immediately transferred to new Petri dishes with 2% (w/v) MEA.
Obtained fungal cultures were grouped into morphotypes according to the morphological characteristics of the mycelium cultures. One representative culture from those morphotypes, with more than five cultures, was selected for further molecular identification. Representative cultures were deposited in the culture collection of the Laboratory of Forest Protection at the Slovenian Forestry Institute.
2.5. DNA Extraction, Amplification and Sequencing
Genomic DNA was extracted from the mycelium scraped from the MEA plates using a NucleoSpin® Plant II (Macherey Nagel, Düren, Germany) following the manufacturer’s instructions, after homogenizing the fungal material with a Lysing Matrix A tube (MP Biomedicals, Solon, OH, USA) using a Precellys Evolution device (Bertin Technologies, Montigny-le-Bretonneux, France). The ITS rDNA region was amplified using primer pairs ITS1 and ITS4 [38]. The 50 µL PCR mixture consisted of PCR® Master Mix (2x) (Thermo Fisher Scientific, Waltham, MA, USA), 1 µL each of 10 µM primers (Sigma-Aldrich, St. Louis, MO, USA), 3 µL of DNA (approx. conc. 25 µg/mL) and 20 µL of molecular grade water (Sigma-Aldrich, St. Louis, MO, USA). The reaction conditions were as follows: 3 min initial denaturation at 95 °C, followed by 39 cycles of 30 s denaturation at 95 °C, 45 s primer annealing at 55 °C and 90 s extension at 72 °C, and a final extension at 72 °C for 10 min.
For determination of Fusarium spp., nucleotide sequences of elongation factor (EF-1α) were amplified using primers EF1 and EF2 [39]. The reaction conditions were as follows: 5 min initial denaturation at 95 °C, followed by 45 cycles of 30 s denaturation at 95 °C, 30 s primer annealing at 51.5 °C and 60 s extension at 72 °C, and a final extension at 72 °C for 6 min.
The obtained PCR products were cleaned using a Wizard SV Gel and PCR Clean-Up System (Promega, Fitchburg, WI, USA) kit according to the manufacturer’s protocol and sequenced at the DNA sequencing facility of Eurofins Genomics (Ebersberg, Germany) in both directions. Sequences were visualised and manually edited using Geneious Prime® version 2019.0.4 (Biomatters Ltd., Auckland, New Zealand). Each consensus sequence, representing one morphotype, was used to perform individual searches with the BLASTn algorithm against nr/nt database from the NCBI website on different dates from 23 January to 2 August 2019. Sequences were deposited in GenBank.
2.6. Data Analysis
The colonization rate was calculated as the total number of infected subsamples (subsamples with outgrown mycelium) divided by the total number of incubated subsamples [40]. The relative colonization frequency (F) of an individual taxon was expressed as the number of cultures of a certain species divided by the total number of cultures. The density index (DI) was defined as the number of cultures produced by one species divided by the number of samples in which the species was present [28].
The Shannon diversity index (H’) of fungal taxa was calculated from the equation:
where pi is the proportion of individuals found in the ith species [41], to measure the diversity of populations in different sampling sites and isolation sources. To obtain more information from the Shannon diversity index, we calculated the corresponding effective number of species [42] for each sampling site and isolation source:
H’ = − ∑ pi ln(pi)
ENS = exp (H’)
The species evenness (J’) was estimated according to Pielou’s formula:
where H’ represents the Shannon diversity index, and Hmax = ln(S) (S is the species richness, defined as the number of species of a given taxon) [41]. The Jaccard similarity index (CJ) [41] was used to compare fungal communities from different sampling sites:
where a is the total number of species present in both sampling sites, b is the number of species present only in site 1 and c is the number of species present only in site 2.
J’ = H’/Hmax
CJ = a/(a + b + c)
To study frequencies and diversity of sample colonization by the most common fungal taxa in relation to branch base diameter, we designed three branch thickness classes: 0.2–0.7 cm, 0.8–1.2 cm and 1.3–3.2 cm. They covered approximately the same number of branches in individual classes and had more or less equal diameter.
Calculations of colonization rate, relative colonization frequency, density index and the Jaccard similarity index were performed in Microsoft Excel version 1902, while the Shannon diversity index, effective number of species, species evenness, t-tests and analysis of similarities were performed in the R software environment for statistical computing [43] with the “vegan” library [44]. The Mann–Whitney U test was used to compare the diversity index between samples with and without E. parasitica. Similarly, the Kruskal–Wallis test was used to compare the diversity index between different sampling sites, between different isolation sources and between different branch base diameter classes. Differences in fungal community structure between sampling sites, isolation sources, branch base diameter classes and samples with or without E. parasitica were tested with an analysis of similarities (ANOSIM) based on Jaccard dissimilarities. A non-parametric test, ANOSIM uses ranked dissimilarities instead of raw data and is similar to an ANOVA hypothesis test. ANOSIM is used to determine if the differences between two or more groups are significant [45].
3. Results
3.1. Eutypella Parasitica
The Eutypella canker of maple was observed in three sampling sites—Rožnik, Mala voda and Smrekovec (Table 4). The highest number of symptomatic sycamore maples were observed at Rožnik and Smrekovec (1.39% and 1.29% of surveyed maples). In contrast, only one of 216 surveyed maples from Mala voda exhibited a typical canker, and the field survey did not detect the Eutypella canker of maple in Mokrc and Samotorica.

Table 4.
Eutypella canker of maple: number of visually healthy and symptomatic A. pseudoplatanus in sampling sites.
Eutypella parasitica was isolated in all sampling sites. It was most frequently found in Rožnik (61.3%), but in other sampling sites, the relative colonization frequency was much lower (3.2–14.5%) (Table 5). The fungus represented 8.5% of all isolations from Rožnik, 2.6% from Mokrc, 1.4% from Samotorica, 0.9% from Smrekovec and 0.5% from Mala voda. The species yielded 62 colonies from 19 different samples. With a density index of 3.26, it was one of the most densely occurring species (Table 6). Eutypella parasitica was 1.5 times more frequent in the discoloured wood of trunks (T) than in the outer parts of dead branches (B).

Table 5.
Relative colonization frequencies (F) of subsamples by E. parasitica.

Table 6.
List of taxa identified in samples from wood of dead branches of A. pseudoplatanus based on BLASTn queries in the NCBI, their relative colonization frequency (F), density index (DI), GenBank and the Laboratory of Forest Protection at the Slovenian Forestry Institute (ZLVG) accession numbers.
The most frequent fungal species isolated from samples with E. parasitica were Eutypa sp. 2 and Neonectria sp. Among the ten most frequently isolated species, only Peniophora incarnata was not isolated from A. pseudoplatanus samples where E. parasitica was also present. No fungal species was strictly associated with the occurrence of E. parasitica—all co-isolated species were also present in samples without E. parasitica. No significant difference was found with the Mann–Whitney U test for the Shannon diversity of fungal species between samples with and without E. parasitica (p = 0.081). Similarly, the isolated fungal community did not differ between samples with and without E. parasitica (p = 0.297), based on the results of the ANOSIM.
3.2. Colonization Rate, Relative Colonization Frequency and Density Index
Cultures were obtained from 98.5% of the investigated samples from wood of sycamore dead branches, and out of 200 samples, only three did not yield any mycelium growth on agar plates. From a total of 2700 cultured subsamples, 1744 fungal cultures were grouped into 212 morphotypes. Ninety-one morphotypes were represented by more than five cultures. Out of the 91 morphotypes, a total of 58 fungal taxa were identified. Seven out of 800 control subsamples yielded cultures, which were identified as Eutypa sp. 3, Eutypa maura, Daldinia sp. and Dendryphion europaeum.
The relative colonization frequency and density index is given in Table 6. The number of fungal taxa in each sampling site ranged from 35 to 42 (Table 7). The overall colonization rate of fungi in different sampling sites and in different isolation sources in the sample is presented in Table 7.

Table 7.
Total and average number of fungal taxa, colonization rates, Shannon diversity index and species evenness for different sampling sites and isolation sources.
A high-density index was observed for Cadophora sp., Eutypa lata, E. parasitica, Eutypa sp. 2, Fusarium lateritium and Neonectria sp. (values above 3.00). Furthermore, Fusarium avenaceum, Neocucurbitaria acerina, Coprinellus sp. and Nigrograna obliqua had a high relative colonization frequency and low-density index (Table 6).
We compared the results about the number of identified fungal taxa from A. pseudoplatanus branches in foreign studies with results of our study (Table 1). In our study, 58 different fungal taxa were identified. Kowalski and Kehr [28] isolated 41 endophytic taxa in the basal part of the living branches of A. pseudoplatanus in Poland. In contrast, dead twigs were colonized by only 23 species in two independent studies from Poland and Germany [27,30]. The number of identified fungal taxa in our study is two times higher than the 27 taxa identified on the basis of dead branches still attached to the main stem of A. pseudoplatanus trees recorded by Butin and Kowalski [25]. However, we identified a higher number of taxa in comparison to other studies (Table 1). Our results on the average number of fungal taxa isolated per sample is consistent with the results of Kowalski and Kehr [28], who found that 56% of A. pseudoplatanus branches were colonized by three or four fungal species (Table 7).
3.3. Community Composition
Forty-three per cent of taxa were identified to the species level, and 55% to the genus level. One sequenced morphotype (1%) was identified only to the family level, and one (1%) to the order level. The level of taxon identification was conditioned with relevant BLASTn matches. Of the 58 taxa recovered from A. pseudoplatanus, four taxa belonged to Basidiomycota and all others to Ascomycota. Identified species were grouped into five classes, Agaricomycetes, Dothideomycetes, Eurotiomycetes, Leotiomycetes and Sordariomycetes, all belonging to Pezizomycotina and Agaricomycotina. The most frequently isolated species on A. pseudoplatanus samples were Eu. Maura, Eutypa sp. 2, F. avenaceum, N. acerina and E. parasitica (Figure 2).
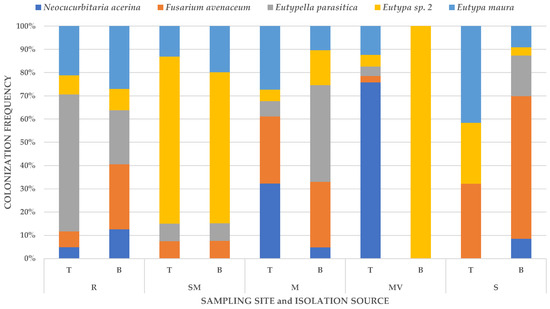
Figure 2.
Colonization frequency of the five most frequently isolated fungi of wood of dead branches of young A. pseudoplatanus from different sampling sites and isolation sources. Sampling site: R, Rožnik; SM, Smrekovec; M, Mokrc; MV, Mala voda; S, Samotorica; Isolation source: T, discoloured wood in the trunk linked with discolouration in wood of dead branches; B, wood in outer part of a dead branch.
These taxa were isolated from an average of 6.03% of subsamples. Neonectria sp., Petrakia irregularis, Ph. Pustulata, P. incarnata and Coprinellus sp. were recorded in an average of 3.22% of the examined subsamples. Other taxa occurred in frequencies of less than 2.70% (Table 6). The ten most frequently isolated fungal taxa were found in almost all sampling sites, with the exception of N. acerina, Neonectria sp. (both not found in Smrekovec) and P. incarnata (not found in Rožnik). Prosthecium sp. and D. europaeum occurred only in Smrekovec, while Clonostachys sp. was found only in Rožnik. The isolated fungal community differed distinctly between the five sampling sites (p = 0.001).
The number of species isolated from the wood in the outer part of the dead branch (B) and discoloured wood in the trunk (T) was almost the same (52 and 55, respectively), but there were significant differences in isolated fungal species composition (p = 0.001). Petrakia sp., Aureobasidium pullulans and Prosthecium sp. were isolated only from isolation source B, while Cadophora sp., Cytospora sp. and Penicillium brevicompactum were specific for isolation source T. Species of Eu. Maura, Eutypa sp. 2, N. acerina, E. parasitica, Neonectria sp., Ph. Pustulata and Coprinellus sp. were on average two times more frequently isolated from isolation source T (63.60%) than from B (35.06%). Petrakia irregularis was isolated almost exclusively from B (94.64%). The frequency of F. avenaceum and P. incarnata was higher in isolation source B (average 69.13%) than in T (average 30.87%).
3.4. Species Diversity
The Shannon diversity index (H’) values varied between 3.01 in Smrekovec and 3.30 in Rožnik (Table 7). No significant differences were found for the diversity of fungal species between the five sampling sites (p = 0.076). The average species evenness (J’) at different sampling sites was 0.87 (Table 7). H’ was higher in B than in T, but the difference was not significant (p = 0.212). Similarly, J’ was higher in B than in T (Table 8). The effective number of species (ENS) ranged from 20 in Smrekovec to 27 in Rožnik. In different isolation sources, the difference in ENS was smaller (T: 33 and B: 36). To describe the beta diversity of pairs of sampling sites, we calculated the Jaccard similarity index, which uses presence–absence data and does not entail any information on the abundance of species [41]. The highest overlap (CJ = 0.65) was observed for the fungal communities in the Samotorica–Rožnik pair (Table 9). The lowest similarity between communities of fungi was observed for the Samotorica–Smrekovec pair (CJ = 0.51). Jaccard similarity index (CJ) for fungal communities between sampling sites was relatively low with an average of 0.59 (Table 9).

Table 8.
Shannon diversity index (H’) and species evenness (J’) in different isolation sources.

Table 9.
Jaccard similarity index (CJ) between sampling sites.
3.5. Branch Diameter and Species Diversity
The number of fungal species colonizing different branch base diameter classes ranged from 46 to 57. Differences in the average number of fungal species between different branch base diameter classes were not significant (p = 0.810). On average, 3.71 (±1.78) species of fungi were found per one sample. Branches were most frequently colonized by three (19%) or four species (22.5%). Approximately 10% of samples were colonized by a single fungal species and 17% by two species. Branches colonized by five or more fungal species represented 29.5% of investigated samples. Fusarium avenaceum, N. acerina, Phomopsis pustulata and Peniophora incarnata were the most frequent in thin branches (0.2–0.7 cm), while the other most frequently isolated species (Coprinellus sp., Eu. Maura, Eutypa sp. 2, E. parasitica, Neonectria sp. and Petrakia irregularis) were isolated mostly from branches with a 0.8–1.2 cm base diameter (Table 10). Branches in the first (0.2–0.7 cm) diameter class were most frequently colonized by F. avenaceum, while branches in the second (0.8–1.2 cm) and third (1.3–3.2 cm) diameter classes were usually colonized by Eu. Maura (Table 10).

Table 10.
The most common fungal taxa in relation to branch base diameter.
Shannon diversity index (H’) values varied between 3.56 for thin branches (0.2–0.7 cm) and 3.71 for branches with a 0.8–1.2 cm base diameter (Table 10). No significant differences were found for the diversity of fungal species between the three branch base diameter classes (p = 0.822). Average species evenness (J’) in different branch base diameter classes was 0.93 (Table 10). The effective number of species (ENS) ranged from 35 in the first (0.2–0.7 cm) branch diameter class to 40 in the second (0.8–1.2 cm) and third (1.3–3.2 cm) diameter classes. The Jaccard similarity index (CJ) for fungal communities between three branch base diameter classes was relatively high with an average of 0.80. The isolated fungal community differed distinctly between the three branch base diameter classes (p = 0.003), based on the ANOSIM results.
4. Discussion
Isolations from the wood of dead branches of young A. pseudoplatanus yielded a wide selection of fungal species. The fungus Eutypella parasitica, which is believed to be a non-native pathogen in Europe, was detected in all five studied sampling sites, although Eutypella cankers were observed only in three sites. The fungal communities were affected by sampling site and isolation source, but not by the presence of E. parasitica.
The USDA database currently lists 691 fungal species occurring on A. pseudoplatanus [46]. In the literature search, we found only four holistic studies of fungal species from A. pseudoplatanus branches [25,27,28,30], which served as a reference point for comparison with our results. The number of identified taxa in our study would have been even higher if we had also identified less frequently isolated species. It should also be noted that we identified only the species with the fastest growth and ability to grow under the employed conditions of isolation and incubation, and we can expect that the total number of fungal species present is much higher (e.g., Wu et al. [47]).
In our study, the average number of fungal species colonizing the wood of dead branches increased with increasing branch base diameter, which could be one of the indicators of branch age, thus suggesting that older branches are colonized by higher numbers of different fungal species. The most promising explanation for the non-significant differences in the average number of fungal species between different branch base diameter classes is that all examined branches were relatively uniformly thin and taken from young A. pseudoplatanus.
Danti et al. [48] posit that geographic origin and twig age and size, as well as different methods of surface sterilization, contribute to the differences in the number of reported fungal taxa between studies. We believe that differences may also arise from different sources of isolation. Tedersoo et al. [49] stated that global fungal richness can be best predicted by climatic factors. A favourable climate in Slovenia is one of the possible explanations for the greater number of isolated fungal taxa compared to other studies performed in more northern latitudes such as Germany, Poland and the Czech Republic (Table 1). The detection of 58 taxa out of 91 sequenced morphotypes in five sampling sites located relatively close together (up to 26 km apart) suggests a high diversity of fungal species. Among the fungi isolated, Eutypa maura, Eutypa sp. 2, Fusarium avenaceum, Neocucurbitaria acerina and E. parasitica colonized more than 17% of the plated subsamples. These five species accounted for about 30% of all cultures and appear to play a dominant role in the colonization of dead samples of A. pseudoplatanus in our study. In comparison with other studies (Table 1), only Eu. maura was commonly detected, while other species were specific to our study.
The investigated samples in our study were densely colonized by a broad spectrum of fungi. In our case, almost all samples were colonized by fungi. A similar result was obtained by Kowalski and Kehr [28] in the basal parts of living branches. Fungi that colonized dead branches almost exclusively belong to Ascomycota, and to Basidiomycota in only a few cases, as already described for the living and dead basal parts of sycamore branches [25,28]. Only two taxa (Eu. maura and F. avenaceum) were present in more than 20% of samples. This is consistent with the results of Kowalski and Kehr [28].
Eutypa sp. 3, Eu. maura, Daldinia sp. and Dendryphion europaeum were also isolated from seven control subsamples in our study. We assume that the reason for this are endophytic life strategies of those species. Interestingly, D. europaeum belongs to Torulaceae, as does Torula sp., which was one of the most frequent taxa isolated from living branch bases in Kowalski and Kehr [28].
The species isolated and identified in our study are mainly saprotrophs on hardwood species. Identification of known pathogens of Eutypella and Eutypa is very interesting, since our study focuses on E. parasitica, and co-isolated species with hyperparasitic impact have the potential to be used as biological control agents. In this context, Bloxamia sp., Cosmospora sp. and Pseudocosmospora rogersonii are of great interest. Pseudocosmospora rogersonii is characterised by its mycoparasitism on Eutypella spp. in the USA [50]. A similar strategy has been reported for Cosmospora spp. Fungi in this genus parasitize other fungi, particularly species in the Xylariales [51]. Glawe [52] reported B. truncata on the partially decayed stromata of Eutypella spp. He suggested further studies to determine whether B. truncata is capable of mycoparasitic activity.
Eutypella parasitica was among the five most frequently isolated species in this study. It was isolated from samples acquired from all sampling sites, although the Eutypella canker of maple was observed only in Rožnik (3), Smrekovec (2) and Mala voda (1). On inventory plots in Mokrc and Samotorica, there were no cankers discovered, but those two sampling sites lie in close proximity to other sampling sites with the Eutypella canker of maple (Samotorica–Mala voda: 1680m and Mokrc–Smrekovec: 850m). We assume that the reason for the isolation of E. parasitica in samples from sampling sites without the Eutypella canker of maple is the wind dispersal of actively discharged ascospores from cankered trees during wet periods and possible asymptomatic infections.
In Rožnik, 45% of sampled trees were infected with E. parasitica. This is much higher than the usual disease occurrence, which has been estimated to be 3–5% in a stand, but similar to incidences of up to 30% recorded in a stand in the eastern part of Slovenia [21]. On average, 19% of all sampled trees in our study were infected by E. parasitica, which is higher than the usual incidence of 5% reported by Gross [53]. No significant difference was found for diversity (p = 0.081) and the fungal community (p = 0.297) between samples with and without E. parasitica, which might indicate that other fungi do not suppress or promote its growth. Furthermore, E. parasitica was more frequently isolated from discoloured wood in the trunks (T) than in the wood of the outer parts of dead branches (B). It is likely that E. parasitica in the wood of the outer parts of dead branches (B) is overgrown or replaced by other fungi, and because of strong competition, the fungus quickly progresses into the wood of the trunk. Based on the results of isolations and maple inventory, it is likely that E. parasitica has an even wider distribution than previously thought. We assume that the disease was simply overlooked previously, since there was no systematic monitoring in those sampling sites and young infections are very inconspicuous [14], or E. parasitica is capable of causing asymptomatic infections.
In addition to E. parasitica, different species of Eutypa spp. were also identified. Eutypa spp. were among the most frequently isolated taxa in our study. They were identified in 23% of plated subsamples. Fungi from the genus Eutypa were represented by Eu. maura and Eu. lata, two well-known species from woody tissues of trees, and five other species not identified to the species level. Unterseher and Tal [54] reported Eu. maura as a dominant component of dead twigs and branches. In their study the stromata of Eu. maura covered most of the branch surface and probably made it impossible for saprophytic secondary invaders to successfully colonize the substrate. This could also be a possible explanation for our results. Eutypa lata is a worldwide pathogen of many woody plants [55,56,57]. It is a well-known cause of one of the most destructive diseases of Vitis vinifera L.—Eutypa dieback or dead-arm disease of grapevine [55,58]. Rappaz [59] found a host specific variety of Eu. lata on the wood and bark of A. campestre L. in France and A. pseudoplatanus in Switzerland. The old name (Eu. lata var. aceri Rappaz) has now been changed to Eu. lata, after Index Fungorum [60].
Our results suggest high fungal species diversity in wood of dead branches of A. pseudoplatanus. The Shannon diversity index (H’) for fungal species from different sampling sites ranged from 3.01 to 3.30 (p = 0.076), from 3.51 to 3.59 in different isolation sources (p = 0.212) and from 3.56 to 3.71 in different branch base diameter classes (p = 0.822). These values are consistent with Magurran [41], who stated that typical values of H’ lie between 1.5 and 3.5, and only rarely exceed four. Gennaro et al. [61] reported Shannon diversity indices in the range between 0.21 and 0.80 for endophytic fungi from different tissues of healthy and declining Quercus robur L. and Q. cerris L. in Italy. Hanácková et al. [62] found significantly higher diversity in the winter shoots of Fraxinus excelsior. The Shannon diversity index of endophytic fungi from Ulmus macrocarpa Hance, Q. liaotungensis Koidz. and Betula platyphylla Sukaczev ranged from 1.28 to 2.11 [63]. Therefore, this comparison suggests a relatively high diversity of fungal taxa in our study. The above authors also detected differences in diversity between different tissue types. In general, the similarity between communities of different sampling sites was relatively low, with an average of 0.59. Kowalski et al. [64], for example, found higher values of similarity between fungal communities on the living and dead stems and twigs of F. excelsior (ranging from 0.65 to 0.92).
The isolated fungal community differed distinctly between the five sampling sites (p = 0.001), between the different isolation sources (p = 0.001), and between the different branch base diameter classes (p = 0.003). The fungal community structure of A. pseudoplatanus-dead branches in our study could have been affected by the decay rate of the samples, age of trees and branches, season of sampling and overall tree health status, as already reported by Gennaro, Gonthier and Nicolotti [61] and Hanácková, Havrdová, Černý, Zahradník and Koukol [62]. The distribution and diversity of fungal species is also dependent on environmental factors, such as temperature, rainfall, tree composition, water availability and soil characteristics [30,54,64]. The degree of colonization may also be dependent on the plant community and branch diameter [28]. Danti, Sieber and Sanguineti [48] and Kowalski, Kraj and Bednarz [64] suggested the dependency of observed species composition and frequency on the method of isolation. The authors pointed out the possibility that the method of isolation used does not yield a complete picture of the real number and frequency of species. This could be also the case in our study. The observed fungal community could have also been a consequence of the generalized incubation conditions. However, we are aware that only the fastest growing and the most frequently isolated culturable fungal species were identified, and that there are many other species left to be identified. Species classified as “sp.” and identified only to genus or an even higher level would need further morphological and molecular analyses.
5. Conclusions
Isolations from the wood of dead branches of young A. pseudoplatanus yielded 1744 fungal cultures, which were grouped into 212 morphotypes. Fifty-eight fungal taxa were identified from morphotypes represented by more than five cultures. The most frequently isolated species were Eutypa maura, Eutypa sp. 2, Fusarium avenaceum, Neocucurbitaria acerina and Eutypella parasitica. Since there was no significant difference in the fungal community of samples with or without E. parasitica, we assume that E. parasitica did not have a strong impact on the success of tissue colonization with other isolated species. On the other hand, the overall fungal communities of samples were affected by the sampling site, isolation source and branch base diameter. In contrast, branch thickness did not prove to be a significant factor in the fungal species diversity of the dead branches of A. pseudoplatanus in our study because they were relatively thin and young. The most interesting finding of our research is the isolation of E. parasitica from all five investigated sampling sites, although Eutypella cankers were observed only in three, indicating the possibility of asymptomatic infection and the long-distance wind dispersal of its ascospores.
Author Contributions
Conceptualization, A.B., B.P. and N.O.; methodology, A.B., B.P. and N.O.; validation, A.B., B.P. and N.O.; formal analysis, A.B., B.P. and N.O.; investigation, A.B., B.P. and N.O.; resources, A.B., B.P. and N.O.; data curation, A.B., B.P. and N.O.; writing—original draft preparation, A.B.; writing—review and editing, A.B., B.P. and N.O.; visualization, A.B.; supervision, A.B., B.P. and N.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency (Young Research Scheme for A.B.; Research Program P4-0107 Forest Biology, Ecology and Technology) and by the Ministry of Agriculture, Forestry and Food (Public Forestry Service).
Acknowledgments
Many thanks go to our colleagues from the Slovenia Forest Service who helped with providing appropriate sampling sites–Rok Havliček, Marija Kolšek, Vinko Maček, Milan Podlogar and Barbara Slabanja. The authors acknowledge enormous help in the field from Robert Krajnc, Peter Smolnikar and Saša Vöchl (Slovenian Forestry Institute) and laboratory assistance from Zina Devetak and Špela Jagodic (Slovenian Forestry Institute). We thank Maarten de Groot from Slovenian Forestry Institute for providing initial help in analysis of similarities. We are grateful to Jan Nagel for providing language help. We are especially grateful to two reviewers for their constructive comments which improved the manuscript greatly.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Spiecker, H.; Hein, S.; Makkonen-Spiecker, K.; Thies, M. Valuable Broadleaved Forests in Europe; Brill: Leiden, The Netherlands; Boston, MA, USA, 2009; p. 256. [Google Scholar]
- Rusanen, M.; Myking, T. Euforgen Technical Guidelines for Genetic Conservation and Use for Sycamore (Acer pseudoplatanus); International Plant Genetic Resources Institute: Rome, Italy, 2003; p. 6. [Google Scholar]
- Hein, S. Distribution of valuable broadleaved forests in Europe, Appendix B. In Valuable Broadleaved Forests in Europe; Spiecker, H., Hein, S., Makkonen-Spiecker, K., Thies, M., Eds.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2009; pp. 251–256. [Google Scholar]
- Hein, S.; Collet, C.; Ammer, C.; Le Goff, N.; Skovsgaard, J.P.; Savill, P. A review of growth and stand dynamics of Acer pseudoplatanus L. in Europe: Implications for silviculture. Forestry 2008, 82, 361–385. [Google Scholar] [CrossRef]
- Brus, R. Dendrology for Foresters, 2nd ed.; University of Ljubljana, Biotechnical Faculty, Department of Forestry and Renewable Forest Resources: Ljubljana, Slovenia, 2008; p. 408. [Google Scholar]
- Savill, P. Future Prospects for the Production of Timber from Valuable Broadleaves. In Valuable Broadleaved Forests in Europe; Spiecker, H., Hein, S., Makkonen-Spiecker, K., Thies, M., Eds.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2009; pp. 11–26. [Google Scholar]
- Spiecker, H. Increasing Interest in Valuable Broadleaved Tree Species. In Valuable Broadleaved Forests in Europe; Spiecker, H., Hein, S., Makkonen-Spiecker, K., Thies, M., Eds.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2009; pp. 3–6. [Google Scholar]
- ZGS. Forest Funds Database, 2018; Slovenia Forest Service. 2019. Available online: https://www.stat.si/StatWeb/en/News/Index/8384 (accessed on 27 September 2019).
- Wulf, A.; Kehr, R. Diseases, Disorders and Pests of Selected Valuable Broadleaved Tree Species. In Valuable Broadleaved Forests in Europe; Spiecker, H., Hein, S., Makkonen-Spiecker, K., Thies, M., Eds.; Brill: Leiden, The Netherlands; Boston, MA, USA, 2009; pp. 61–84. [Google Scholar]
- Jurc, D.; Ogris, N.; Slippers, B.; Stenlid, J. First report of Eutypella canker of Acer pseudoplatanus in Europe. Plant Pathol. 2006, 55, 577. [Google Scholar] [CrossRef]
- Cech, T.L. Erstnachweis von Eutypella parasitica in Österreich. Forstsch. Aktuell 2007, 40, 10–13. [Google Scholar]
- Ogris, N.; Diminić, D.; Piškur, B.; Kraigher, H. First report of Eutypella parasitica causing cankers on field maple (Acer campestre) in Croatia. Plant Pathol. 2008, 57, 785. [Google Scholar] [CrossRef]
- Cech, T.L.; Schwanda, K.; Klosterhuber, M.; Straßer, L.; Kirisits, T. Eutypella canker of maple: First report from Germany and situation in Austria. For. Pathol. 2016, 46, 336–340. [Google Scholar] [CrossRef]
- Jurc, D.; Ogris, N.; Piškur, B.; Csóka, G. First report of Eutypella canker of maple (Eutypella parasitica) in Hungary. Plant Dis. 2016, 100, 1241. [Google Scholar] [CrossRef]
- Černý, K.; Hrabětová, M.; Svobodová, I.; Mrázková, M.; Kowalski, T. Eutypella parasitica naturalised in Bohemian and Polish Silesia. For. Pathol. 2017, 47, e12347. [Google Scholar] [CrossRef]
- Bregant, C. Eutypella parasitica: Primo Rinvenimento e Monitoraggio in Fruili Venezia Giulia. MSc Thesis, Università degli Studi di Padova, Legnaro, Italy, 2018. [Google Scholar]
- Jurc, D.; Piškur, B.; Ogris, N.; Brglez, A.; Linaldeddu, B.T.; Bregant, C.; Montecchio, L. First Report of Eutypella Canker Caused by Eutypella parasitica on Acer campestre in Italy. Plant Dis. 2020, 104. [Google Scholar] [CrossRef]
- Davidson, R.W.; Lorenz, R.C. Species of Eutypella and Schizoxylon associated with cankers of maple. Phytopathology 1938, 28, 733–745. [Google Scholar]
- Ogris, N.; Jurc, D.; Jurc, M. Spread risk of Eutypella canker of maple in Europe. EPPO Bull. 2006, 36, 475–485. [Google Scholar] [CrossRef]
- French, W.J. Eutypella Canker on Species of Acer in New York State. Ph.D. Thesis, State University College of Forestry at Syracuse University, Syracuse, New York, NY, USA, 1967. [Google Scholar]
- Ogris, N.; Piškur, B.; Jurc, D. Some morphological aspects of Eutypella canker of maple (Eutypella parasitica). In Proceedings of the IUFRO Working Party 7.02.02, Eğirdir, Turkey, 11–16 May 2009; pp. 150–161. [Google Scholar]
- Lachance, D. Discharge and germination of Eutypella parasitica ascospores. Can. J. Bot. 1971, 49, 1111–1118. [Google Scholar] [CrossRef]
- EPPO. Mini Data Sheet on Eutypella parasitica, 2008/028 ed.; European and Mediterranean Plant Protection Organization: Paris, France, 2008; p. 2. [Google Scholar]
- Johnson, D.W. Eutypella Canker of Maple: Ascospore Discharge and Dissemination. Phytopathology 1979, 69, 130. [Google Scholar] [CrossRef]
- Butin, H.; Kowalski, T. Die natürliche Astreinigung und ihre biologischen Voraussetzungen, III. Die Pilzflora von Ahorn, Erle, Birke, Hainbuche und Esche. For. Pathol. 1986, 16, 129–138. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Boil. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Chlebicki, A. Some ascomycetous fungi or their anamorphs occurring on trees in Poland. I. Acta Mycol. 2014, 24, 77–92. [Google Scholar] [CrossRef][Green Version]
- Kowalski, T.; Kehr, R.D. Endophytic fungal colonization of branch bases in several forest tree species. Sydowia 1992, 44, 137–168. [Google Scholar]
- Ellis, M.B.; Ellis, J.P. Microfungi on Land Plants: An Identification Handbook; The Richmond Publishing Co. Ltd.: Slough, UK, 1997; p. 868. [Google Scholar]
- Unterseher, M.; Otto, P.; Morawetz, W. Species richness and substrate specificity of lignicolous fungi in the canopy of a temperate, mixed deciduous forest. Mycol. Prog. 2005, 4, 117–132. [Google Scholar] [CrossRef]
- Johnová, M. Diversity and ecology of selected lignicolous Ascomycetes in the Bohemian Switzerland National Park (Czech Republic). Czech Mycol. 2009, 61, 81–97. [Google Scholar] [CrossRef]
- Yang, D.-Q. Isolation of wood-inhabiting fungi from Canadian hardwood logs. Can. J. Microbiol. 2005, 51, 1–6. [Google Scholar] [CrossRef]
- Qi, F.-H.; Jing, T.-Z.; Wang, Z.-X.; Zhan, Y.-G. Fungal endophytes from Acer ginnala Maxim: Isolation, identification and their yield of gallic acid. Lett. Appl. Microbiol. 2009, 49, 98–104. [Google Scholar] [CrossRef]
- Sun, X.; Guo, L.-D.; Hyde, K.D. Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Divers. 2011, 47, 85–95. [Google Scholar] [CrossRef]
- Green, D.J.; Shortle, W.C.; Shigo, A.L. Compartmentalization of Discolored and Decayed Wood in Red Maple Branch Stubs. Forest Sci. 1981, 27, 519–522. [Google Scholar]
- ARSO. Monthly and Yearly Average Temperature in Slovenia for the Period from 1981 to 2010: 12 Monthly and One Yearly Map in Raster Format with 1 km Resolution; Slovenian Environment Agency, Meteorology Office: Ljubljana, Slovene, 2012. [Google Scholar]
- ARSO. Monthly and Yearly Average Sum of Corrected Precipitations in Slovenia for the Period from 1981 to 2010: 12 Monthly and One Yearly Map in Raster Format with 1 km Resolution; Slovenian Environment Agency, Meteorology Office: Ljubljana, Slovene, 2012. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Academic Press, Inc.: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Petrini, O.; Stone, J.; Carroll, F.E. Endophytic fungi in evergreen shrubs in western Oregon: A preliminary study. Can. J. Bot. 1982, 60, 789–796. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing Company: Hoboken, NJ, USA, 2004; p. 256. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 3.6.1; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solmyos, P.; et al. Vegan: Community Ecology Package. R package version 2.5–5. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 28 January 2020).
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal Databases. Available online: https://nt.ars-grin.gov/fungaldatabases/index.cfm (accessed on 18 February 2020).
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef]
- Danti, R.; Sieber, T.N.; Sanguineti, G. Endophytic mycobiota in bark of European beech (Fagus sylvatica) in the Apennines. Mycol. Res. 2002, 106, 1343–1348. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef]
- Herrera, C.S.; Rossman, A.Y.; Samuels, G.J.; Chaverri, P. Pseudocosmospora, a new genus to accommodate Cosmospora vilior and related species. Mycologia 2013, 105, 1287–1305. [Google Scholar] [CrossRef]
- Herrera, C.S. Systematics of the Genus Cosmospora (Nectriaceae, Hypocreales), and Cospeciation of Cosmospora Species. Ph.D. Thesis, Faculty of the Graduate School of the University of Maryland, College Park, MD, USA, 2014. [Google Scholar]
- Glawe, D.A. Bloxamia truncata in Artificial Culture. Mycologia 1984, 76, 741–745. [Google Scholar] [CrossRef]
- Gross, H.L. Impact of Eutypella Canker on the Maple Resource of the Owen Sound and Wingham Forest Districts. For. Chron. 1984, 60, 18–21. [Google Scholar] [CrossRef]
- Unterseher, M.; Tal, O. Influence of small scale conditions on the diversity of wood decay fungi in a temperate, mixed deciduous forest canopy. Mycol. Res. 2006, 110, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, F.P.; Gubler, W.D. Host Range, Biological Variation, and Phylogenetic Diversity of Eutypa lata in California. Phytopathology 2010, 100, 1048–1056. [Google Scholar] [CrossRef]
- Wenneker, M.; Van Raak, M.M.J.P.; Van Brouwershaven, I.R.; Martin, W.; Kox, L.F.F. Eutypa lata, the causal agent of dieback in red currant (Ribes rubrum) and gooseberry (R. uva-crispa) in the Netherlands. Eur. J. Plant Pathol. 2011, 131, 441–449. [Google Scholar] [CrossRef]
- Travadon, R.; Baumgartner, K.; Rolshausen, P.E.; Gubler, W.D.; Sosnowski, M.R.; LeComte, P.; Halleen, F.; Péros, J.-P. Genetic structure of the fungal grapevine pathogen Eutypa lata from four continents. Plant Pathol. 2011, 61, 85–95. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Greve, L.C.; Labavitch, J.M.; Mahoney, N.E.; Molyneux, R.J.; Gubler, W.D. Pathogenesis of Eutypa lata in Grapevine: Identification of Virulence Factors and Biochemical Characterization of Cordon Dieback. Phytopathology 2008, 98, 222–229. [Google Scholar] [CrossRef]
- Rappaz, F. Taxonomie et nomenclature des diatrypacées à aseas octospores. Mycol. Helvetica 1987, 2, 285–648. [Google Scholar]
- Index Fungorum. Available online: http://www.indexfungorum.org (accessed on 2 September 2019).
- Gennaro, M.; Gonthier, P.; Nicolotti, G. Fungal Endophytic Communities in Healthy and Declining Quercus robur L. and Q. cerris L. Trees in Northern Italy. J. Phytopathol. 2003, 151, 529–534. [Google Scholar] [CrossRef]
- Hanácková, Z.; Havrdová, L.; Černý, L.; Zahradník, D.; Koukol, O. Fungal Endophytes in Ash Shoots-Diversity and Inhibition of Hymenoscyphus fraxineus. Balt. For. 2017, 23, 89–106. [Google Scholar]
- Sun, X.; Ding, Q.; Hyde, K.D.; Guo, L.-D. Community structure and preference of endophytic fungi of three woody plants in a mixed forest. Fungal Ecol. 2012, 5, 624–632. [Google Scholar] [CrossRef]
- Kowalski, T.; Kraj, W.; Bednarz, B. Fungi on stems and twigs in initial and advanced stages of dieback of European ash (Fraxinus excelsior) in Poland. Eur. J. For. Res. 2016, 135, 565–579. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).