Abstract
Enrichment planting is often suggested as a means of enhancing the productivity of logged rainforest. However, little is known about the long-term survival and growth of these trees. In this study, we used historical data from enrichment planting trials ranging from 15 to 32 years old to examine the survival and growth of 16 tree species across different sites in north Queensland, Australia. The results complement and extend current knowledge on the potential role of enrichment planting from a production perspective. A key finding was that the initial level of post-logging overwood did not appear to affect the immediate survival of enrichment plantings, but in the longer term (up to 30 years) survival decreased as post-logging overwood and regrowth increased. This suggests that removal of overwood should take place at the time of enrichment planting. A further key finding was that despite regular tending to remove vegetation adjacent to the plantings, competition from saplings and trees that were situated outside the tended area emerged as a major source of competition in some plots. By implication, the success of enrichment planting may depend on removal of competition from the entire logged area, not just adjacent to enrichment plantings. Results between individual species and trials varied widely. The best development of Flindersia brayleyana resulted in a mean tree diameter of 32.5 cm at age 22 for the 100 tallest trees per hectare. Although Eucalyptus grandis, Eucalyptus microcorys, and Eucalyptus pilularis all failed—as expected, because they do not normally grow in rainforest—Corymbia torelliana, Cardwellia sublimis, Araucaria bidwillii, Khaya senegalensis, Flindersia amboinensis, and Swietenia macrophylla also failed although they grow naturally in similar sites. In other trials, tree height and basal area growth were often poor. For example, Khaya ivorensis grew to a basal area of only 1.3 m2/ha and a mean height of 7.7 m at age 10, and Flindersia ifflaiana only grew to a basal area of 0.7 m2/ha and a height of 7.9 m, also at age 10. Overall, these results emphasise the necessity of site–species matching before enrichment planting begins and the necessity of post-planting monitoring and remedial tending.
1. Introduction
Tropical rainforests are important both as highly diverse ecosystems [1,2] and as an economic resource for rural communities in developing countries [3]. However, these forests are under intense pressure from the effects of uncontrolled harvesting and subsequent conversion of the land to agriculture. Between 1990 and 2015, the worldwide area of tropical forest declined by approximately 195 million ha [4]. Logging creates canopy gaps that may be invaded by vines and pioneer species. From a production perspective, the proportion of non-commercial tree species may be increased to the detriment of high-value species [5,6,7,8,9,10]. This provides an economic rationale for converting unproductive forest to agriculture.
In forest where there is a lack of natural regeneration, enrichment planting may improve the stocking of desired species without further disturbing the floristic structure of the forest [2,11,12,13]. Silvicultural techniques such as “tending”, that is, cutting down competing saplings or removing lianas, may maximise the growth and survival of enrichment plantings or natural regeneration in logged rainforest [14,15]. The ecological principle is to take advantage of increased light and soil moisture when canopies have been opened up—either naturally or by logging—to allow young trees to grow [16,17]. However, the success of enrichment planting depends on a detailed knowledge of how the new seedlings interact with the new environment in which they are planted [18,19].
The success of enrichment planting as a silvicultural technique can be measured by survival and growth in terms of tree and stand height and basal area. Hence, long-term information is needed to indicate whether the enrichment plantings are likely to provide a sufficient stocking of merchantable trees within an expected harvesting cycle, and whether the height and basal area development of these trees has not been suppressed by competition.
Previous studies that have assessed the efficacy of enrichment planting in tropical forests have mostly relied on short-term data, such as only 2 years after planting in Indonesia [20], 1 year in Mexico [21], 4 or 5 years in the Amazon [17,22], 4 years in Vietnam [11], 7 years in Argentina [19], and 7 years in Laos [23]. Hence, there have been few studies assessing the survival and growth of enrichment plantings in the longer term.
The discovery of historical records of enrichment planting trials (titled “experiments” or “Expts” in old records) in north Queensland, Australia, provided an opportunity to assess the efficacy of rainforest enrichment planting as a silvicultural technique over a long timeframe. The experiments were undertaken by the (then) Queensland Department of Forestry (QDF) to investigate the possibility of increasing the commercial viability of enrichment plantings in rainforest.
Early attempts at enrichment planting logged rainforest with Toona australis (F. Muell) Harms (red cedar) were carried out as early as 1903 [24]. Further enrichment planting experiments in north Queensland reached a peak between 1952 and 1970. After logging, undergrowth and small trees of non-commercial species were brushed or felled. Then, 12 commercial native timber species (most frequenly Flindersia brayleyana) were planted at across different sites in logged rainforest. In addition to 12 native species, the QDF included four exotic species (Khaya ivorensis, Khaya senegalensis, Swietenia macrophylla, and Flindersia amboinensis) as enrichment plantings. This reflects the attitude of the times, that is, that any species that could improve the productivity of the rainforest should not be excluded because it was not native to north Queensland [25]. The experiments were re-measured annually until 1987 when they were abandoned when much of the rainforest previously used for commercial forestry was handed over to the Wet Tropics Management Authority and Queensland Park and Wildlife Service for protection in conservation reserves.
The results of the QDF experiments have never been systematically evaluated except as an unpublished summary [25]. Fortunately, old plot measurement data were recently made available to the authors by the current custodians (the Queensland Department of Agriculture and Fisheries) as hard-copy and electronic records. This enabled us to evaluate the suitability of 16 rainforest species for use as enrichment plantings in logged rainforest. In particular, the data enabled us to assess the impact of competition from both existing overwood and regrowth that had never been removed in tending operations. As the first of three papers that use this data to assess the usefulness of enrichment planting as a silvicultural tool, in the following sections of this paper, we describe where the enrichment planting experiments were located and how they were established. We then present the results of the experiments in terms of enrichment planting survival, height, and basal area development. We also describe the silviculture that was used to manage the growth of the overwood and how this overwood competed with the enrichment plantings over time. Finally, we discuss the implications of our results for the usefulness of enrichment planting for rainforest management.
2. Materials and Methods
2.1. Background to the Establishment of the Experiments
The experiments described in this paper were located in a tropical rainforest within the Wet Tropics region of north Queensland, Australia, near the towns of Kuranda, Eacham, Atherton, Yungaburra, Ravenshoe, and Mission Beach. The mean annual rainfall of the sites ranges from 1250 mm at Atherton to 3500 mm at Mission Beach, and the elevation ranges from 6 m above sea level (ASL) at Mission Beach, to 1220 m asl at Atherton. The slopes of the sites range from flat to 15 degrees. The soil type is highly variable between sites and is derived from either rhyolite, basalt, granite, or metamorphic formations. Attributes of the study sites are presented in Appendix A Table A1.
The 29 experiments that were analysed in this paper were established between 1952 and 1970 following commercial logging (see Appendix A Table A2). After the completion of logging, the sites were “treated” by removing all non-commercial tree species with a DBH (stem diameter over bark at 1.3 m height) of greater than 5 cm, by ringbarking or herbicide application, and by felling all non-commercial smaller trees (<5 cm DBH). This treatment was used to reduce overwood basal area (Tree basal area is a measure of the over bark surface area of stumps at 1.3 m above ground level. The basal area of a stand is calculated as the sum of the basal area of all the trees, per hectare) to between 3 and 18 m2/ha. Following treatment, a range of commercially valuable tree species (mostly Flindersia brayleyana, Table 1) were planted, either randomly or in rows across the site. QDF practice was to plant seedlings in the wet season (variable but typically November to March), when the soil had become thoroughly wet.

Table 1.
Some characteristics of the tree species used in enrichment planting experiments in north Queensland.
At the time of planting, the stocking of the enrichment plantings ranged from 200 to 960 trees per hectare. Seedlings in most experiments were planted in rows. The distance between planted lines and among planted seedlings were different between experiments (Appendix A Table A1), but with the proviso that no seedlings were planted closer than 3 m to a retained tree and not closer than 0.5 m to a ringbarked tree.
After planting, all seedlings were tagged, mapped, and measured for height. When the seedlings became overtopped, all competing vegetation (i.e., lianas, vines, and non-commercial tree species) within 1.5 m around each planted seedling (1.5 m radial tending) and along planting lines to a width of 1.5 m (line tending) were removed. Overtopping woody regrowth and non-commercial trees were also removed by ringbarking or herbicide application to enhance light conditions for the enrichment planting. All enrichment plantings were measured for height until 5 years of age, and then for DBH and predominant height (Predominant height (PDH) is the mean height of the 50 tallest trees per hectare) after that age. The silvicultural treatments applied in each enrichment planting experiment are described in Appendix A Table A2.
2.2. Data Analysis
We calculated the survival and growth of the 16 enrichment planting species for the 29 experiments. In addition, eight plots from experiments 245, 246, and 322, in which the underplants had not died (e.g., from disease or grazing), were selected to follow the growth of the overwood (i.e., the original remnant overstory and regrowth saplings) throughout the duration of the experiment. The stocking and basal area development of the overwood in these eight plots were calculated. The original remnant trees in each plot were classified as cohort 1. At the time of each re-measurement, the recruitment of new saplings that had grown to a height of 6 m were tallied as a new cohort of overwood. By the time of the last measurement, this typically resulted in an original cohort of overwood plus 4–6 further cohorts of successively younger recruits.
We calculated an annualised mortality rate for the experiments for the different time periods between field measurements, using the formula provided by Sheil and May (1996) of mortality over any time period as: , where M is mortality rate of enrichment plantings, N and Nt are population counts at the beginning and end of the measurement interval, t [26].
To identify the effect of overwood growth on the survival of enrichment plantings, linear regression was used to identify the relationship between the dependent variables (i.e., survival of enrichment plantings) and independent variables (i.e., overwood basal area).
For experiment 373, a one-way analysis of variance (ANOVA) was used to compare significant differences in growth of Flindersia brayleyana between silvicultural treatments. Where significant differences were determined, a Tukey’s test was used to compare treatments. All data were tested for normality using the Shapiro–Wilk normality test and homogeneity of variance using Levene’s test.
3. Results
3.1. Survival
The long-term survival of enrichment plantings varied greatly between species and sites. (Figure 1; Figure 2). For example, the survival of F. brayleyana ranged between 32.2% and 84.5%, and the survival of Flindersia bourjotiana ranged between 14.3% and 77.3%. The survival of Araucaria cunninghamii ranged between 53.3% and 96.8%, except at experiment 350 where there was 100% mortality by the time of the last measurement (age 18). The survival of Flindersia ifflaiana was 43% and the survival of Cardwellia sublimis ranged between 13% and 39.6%. Both Khaya senegalensis and Swietenia macrophylla had an extremely low survival rate of 7.6% and 2%, respectively, at 10 years of age. Despite being replanted, all eucalypt species (i.e., Eucalyptus grandis, Eucalyptus microcorys, Eucalyptus pilularis, and Corymbia torelliana) completely failed (i.e., 100% mortality).
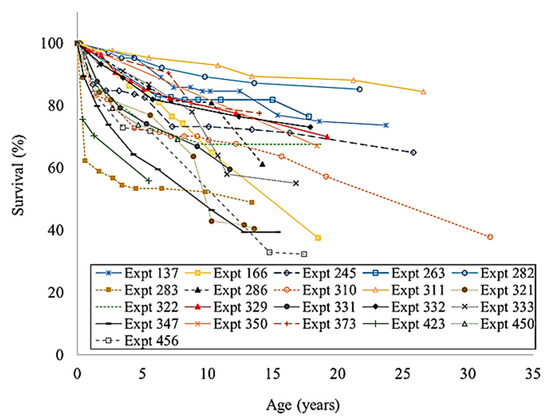
Figure 1.
The survival of Flindersia brayleyana in enrichment planting experiments at different sites in north Queensland.
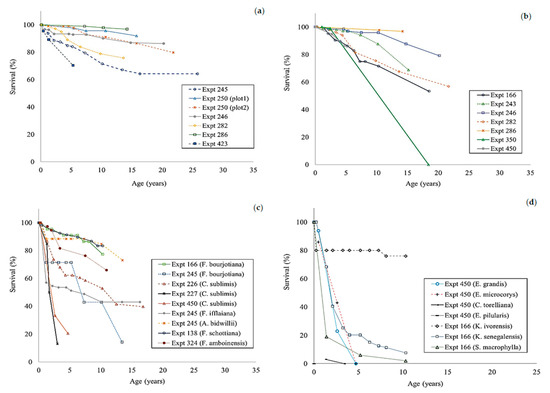
Figure 2.
The survival of Agathis robusta (a); Araucaria cunninghamii (b); Cardwellia sublimis, Araucaria bidwillii, Swietenia macrophylla, Flindersia bourjotiana, Flindersia schottiana, Flindersia ifflaiana, and Flindersia amboinensis (c); Khaya ivorensis, Khaya senegalensis, Eucalyptus grandis, Eucalyptus microcorys, Corymbia torelliana, and Eucalyptus pilularis (d) in enrichment planting experiments in north Queensland. Plot 1 of experiment 250 was planted with the southern provenance of Agathis robusta.
3.2. The Annualised Mortality Rate of Enrichment Plantings
The annualised mortality rate of 16 species over different periods between measurements (see Appendix A Table A3) showed high variation according to species and age. In the case of Flindersia brayleyana in experiment 282, annualised mortality fell to a very low level of 0.3% in the measurement period of 14–22 years. Other trends were less clear, for example, the high level of mortality of Cardwellia sublimis (approximately 10%) between 10 and 14 years of age. Some species showed a high level of continuing mortality, such as Khaya senegalensis in experiment 166, in which 15.4% of trees died 10 years after planting. Similarly, eucalyptus and corymbia species suffered 100% mortality soon after planting.
3.3. The Survival of Enrichment Plantings with Increasing Post-Logging Overwood
There is a significant relationship between the survival of enrichment plantings with overwood growth (computed as overwood basal area per hectare). The survival of enrichment plantings declined with increasing overwood basal area over time (Figure 3). However, the initial level of post-logging overwood (also computed as basal area per hectare) appeared to have little relationship with the initial survival of enrichment plantings (Table 2).
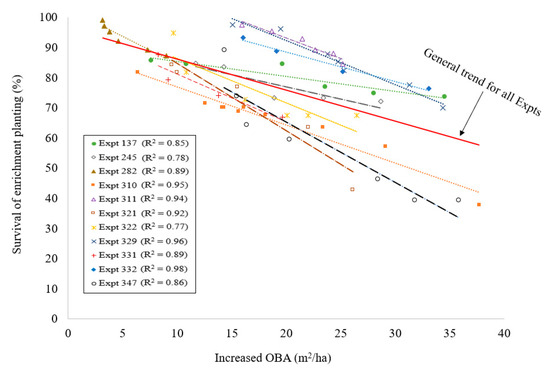
Figure 3.
The effect of growth of overwood basal area (OBA) over time on the survival of enrichment plantings (experiments 322 and 245 had a p-value < 0.05, all other experiments had a p-value < 0.01).

Table 2.
Initial overwood basal area and the survival of enrichment plantings at the age of their first measurement.
3.4. Height Development of the Enrichment Plantings
The height growth of enrichment plantings in all experiments was slow and showed substantial differences between species and sites (Figure 4 and Figure 5). Any comparison of the results was complicated by the varying ages at which measurements ceased and the limited dataset for some species. If the Flindersia brayleyana experiments are considered as a whole, the height growth trajectory of approximately 10 m at age 10 is clearly superior to all other species (Figure 4). By comparison, a lower height of approximately 6 m was recorded for Araucaria cunninghamii at the same age (Figure 5a). The southern provenance of Agathis robusta performed poorly in comparison to the northern provenance of this species (Figure 5b). Very poor height growth, that is, less than 3 m at 10 years of age, was recorded for Araucaria bidwillii, Flindersia amboinensis, Khaya senegalensis, and Swietenia macrophylla (Figure 5c,d). The early death of Eucalyptus grandis and Eucalyptus microcorys reduced the usefulness of the data (Figure 5d).
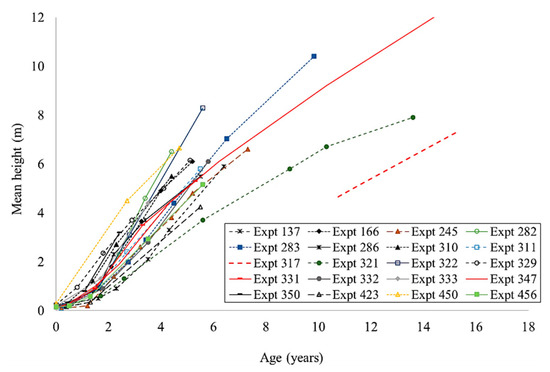
Figure 4.
The height growth of Flindersia brayleyana in enrichment planting experiments in north Queensland.
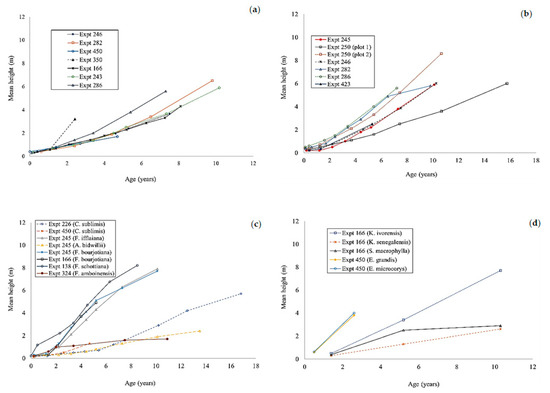
Figure 5.
The height growth of Araucaria cunninghamii (a); Agathis robusta (b); Cardwellia sublimis, Araucaria bidwillii, Swietenia macrophylla, Flindersia bourjotiana, Flindersia schottiana, Flindersia ifflaiana, and Flindersia amboinensis (c); Khaya ivorensis, Khaya senegalensis, Eucalyptus grandis, and Eucalyptus microcorys (5d) in enrichment planting experiments in north Queensland. Plot 1 of experiment 250 was planted with the southern provenance of Agathis robusta.
3.5. Basal Area Development of the Enrichment Plantings
Comparisons between the basal area growth of enrichment plantings (Figure 6) were complicated by the age at which specific measurements ceased for different species and sites. However, except for one experiment of Flindersia brayleyana, Flindersia schottiana, and Agathis cunninghamii, at experiments 282, 183, and 286, respectively, the basal area growth of all species at all sites was poor. The basal area growth of Flindersia brayleyana varied greatly between experiments (Figure 6a). In experiments 282, 311, 137, and 310, the basal area of Flindersia brayleyana was less than 4 m2 ha−1 at ages between 20 and 25. Individual results for Agathis robusta (Figure 6b) and Araucaria cunninghamii (Figure 6c) showed high variation between experiments and the very poor performance of A. robusta in particular. The limited data (one experiment each) available for Flindersia bourjotiana, Khaya ivorensis, and Flindersia ifflaiana showed basal areas of less than 2 m2 ha−1 at the last measurement at 10 years of age. The basal areas of Khaya senegalensis, Swietenia macrophylla, Flindersia amboinensis, Araucaria bidwillii, and Cardwellia sublimis were not calculated because the diameters of the stems were too small.
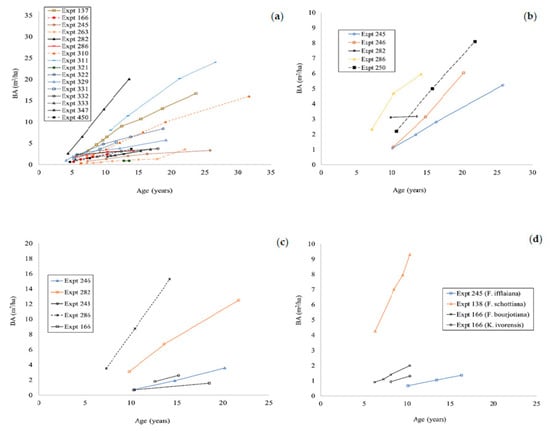
Figure 6.
The growth of basal area of Flindersia brayleyana (a); Agathis robusta (b); Araucaria cunninghamii (c); Flindersia ifflaiana, Flindersia schottiana, Flindersia bourjotiana, and Khaya ivorensis (d) in enrichment planting experiments in north Queensland.
3.6. The Height Growth of Three Commercially Desirable Species in the Enrichment Planting Experiments in Which They Grew Best
At 10 years of age, the PDH of three commercially desirable species in the experiments in which they grew best was highest (14 m) for Flindersia brayleyana, whereas the PDH of both Agathis robusta and Araucaria cunninghamii was less than 10 m. The greatest basal area was also achieved by Flindersia brayleyana at 22 years of age, followed by Araucaria cunninghamii (12.5 m2/ha) and Agathis robusta (6.0 m2/ha) at ages 22 and 20, respectively. The mean DBHs of the dominant trees (i.e., the tallest 100 trees/ha) for Flindersia brayleyana, Agathis robusta, and Araucaria cunninghamii were 32.5, 24.8, and 17.5 cm, respectively. The height and DBH growth of all these three species was less than those recorded by Bristow et al. (2005) at age 8 in plantations [27] in similar locations in north Queensland (Table 3).

Table 3.
A comparison of the early-age growth of three commercially desirable species in the enrichment planting experiments in which they grew best, with their expected growth rates in plantations.
3.7. The Growth Response of Flindersia brayleyana to Different Tending Regimes
The growth of Flindersia brayleyana under different tending regimes (as described in Table 4) in experiment 373 showed a clear response to increased light. The best growth was recorded for treatments 2 (no shade, complete herbicide application of overstory) and 4 (no shade, overstory ring-barked), with a DBH and basal area of 8.5 cm and 3.4 m2/ha, respectively, in treatment 2, and 8.1 cm and 3.2 m2/ha, respectively, in treatment 4. The lowest growth was found in treatment 1 (high shade), which resulted in a mean DBH of only 5.5 cm and a basal area of 1.8 m2/ha at age 14.

Table 4.
The diameter at breast height (DBH) (and standard errors) and the basal area of Flindersia brayleyana at age 14 under different tending regimes in experiment 373. The mean DBH of all four treatments were significantly different (p < 0.001).
Experiment “completion reports” after which the experiments were abandoned (see Appendix A Table A4) also provided qualitative information concerning the success (or otherwise) of individual experiments. Overall, the comments revealed a repeated message that the growth of enrichment plantings depended on an initial overwood basal area no greater than 15 m2/ha. In addition, in many experiments, competition from vines or overwood restricted growth of the enrichment plantings, particularly when the basal area of the overwood exceeded 30 m2/ha.
3.8. The Development of “Cohorts” of Overwood over Time
The development of ‘cohorts’ of overwood (see Appendix A Table A5) showed a general trend of strongly increasing overwood stocking and basal area in each experiment, but particularly in experiment 245. For example, in plot 2 of this experiment at age 13, the stocking of the original cohort of overwood was 150 trees per hectare, whereas recruited saplings numbered 2120 trees per hectare or 93% of the final overwood stocking. In other experiments, the number of recruited saplings was less. For example, in experiment 246 plot 2, the number of recruited saplings was only 60% of the final overwood stocking. The basal area of the recruits (not cohort 1) constituted 14.5 m2/ha or 51% of the total overwood basal area in experiment 245, plot 2, but only 2.4 m2/ha or 12% of the total overwood basal area in experiment 246, plot 2. Hence, in some experiments, recruitment of saplings to the overwood (in number and basal area) was high, whereas in other plots it was not. In each cohort, a constant feature of the cohorts of recruits over time was that stocking declined with increasing age. Both large DBH trees (cohort 1) and recruited saplings died or were ringbarked or herbicided in the intervals between measurements. For example, in experiment 245, plot 2, the stocking of cohort 2 declined by 52% (from 362 to 188 trees per hectare) between 1966 and 1969. However, cohorts 3, 4, and 5 grew to constitute 12.57 m2/ha or 44% of the total overwood before the plot was abandoned at age 13. Similarly, in experiment 245, plot 1, cohorts 3, 4, and 5 grew to constitute 55% or 11.3 m2/ha of final overwood at age 13. This was largely due to the emergence of a fast-growing secondary species (Acacia aulacocarpa A. Cunn. ex Benth) starting at 7 years of age, after the cessation of periodic tending.
3.9. Qualitative Evidence from Old QDF Office Memos and Summary Reports
Old QDF memos and summary reports indicate that, by 1976, enthusiasm for enrichment planting was waning (see Appendix A Table A4). In correspondence from the Atherton District office, research foresters commented that enrichment planting “just isn’t economically feasible” and that “efforts could be diverted to more fulfilling directions”. Similar memos indicated a growing realisation that weeds and competing undergrowth require regular tending, perhaps annually. At one experiment, “the long delay between establishment and first tending (6 months) resulted in severe damage”. Finally, in 1982, a report on the viability of enrichment planting noted that commercial timber yield would require “a ten-fold increase in timber value to become financially attractive” [25].
4. Discussion
4.1. Implications of These Results for Production Forestry
From a production perspective, the key message from this study is that collectively, the poor survival, height growth, and basal area of the enrichment plantings are indicative of trees under stress. Tree growth is affected by many factors, such as silvicultural treatment, competition from neighbouring trees, and microclimate [28]. Separating the effect of these factors can be difficult. The literature records many examples of enrichment plantings dying through insect attack [19] or drought [29,30], but the QDF records provided little information beyond isolated examples of thrips and coccids in Agathis robusta or browsing by herbivores. Hence, the major cause of mortality in these experiments appears to have come from competition from residual and regrowth overwood.
Data presented in Section 3.7 particularly demonstrate the influence of the overwood in suppressing the long-term growth of Flindersia brayleyana. This result cannot be directly applied to other species in this study, although the results of experiment 373 are corroborated by other research [16,17,31]. In office memos, QDF researchers also commented that, as a generality, the growth of enrichment plantings declines with overwood growth and a consequent lack of available light and water.
From a forest management perspective, despite silvicultural interventions being required to control excessively increased overwood growth, reducing this overwood by ringbarking or with chemicals causes further problems during harvesting, particularly after dead trees become rotten and are liable to fall at any time. Fortunately for Flindersia brayleyana at least, the lack of any clear relationship between the early-age survival of enrichment plantings and initial overwood basal area indicates that the initial level of overwood is not critical. Hence, an opportunity exists to reduce it to a low level at the time when enrichment planting is undertaken.
Except for the catastrophic effect of a lack of tending (e.g., as described in Section 3.1 for Araucaria cunninghamii in experiment 350), the very high variation in survival, basal area, and height across experiments and species provides little direction for the frequency and intensity of tending regimes. The historical records presented in Appendix A Table A2 show that almost all experiments were regularly tended. Part of the answer may lie in the recruitment of cohorts of fast-growing saplings, which can become a major component of the competing overwood. In experiment 245, for example, ceasing tending operations at age 7 allowed Acacia aulacocarpa to become a dominant part of the overstory. The general implication for enrichment planting is the need for continual monitoring and the funds to carry out remedial treatment. Extending tending beyond tending lines or circles could become very expensive.
4.2. Interpreting the Results of This Study for Individual Tree Species
For Flindersia brayleyana particularly, the continuing decrease in survival over time—across all experiments—indicates a fundamental inadequacy in growing conditions. This study showed that, compared to the other species, Flindersia brayleyana has adapted best to the enrichment planting environment. Although this species is described as a late secondary successional species [32], in rainforest, it can persist for many decades in the deep shade as suppressed seedlings [33]. Its growth is consequently slow until a suitable light gap occurs, after which it develops quickly [34]. The low annualised mortality (Appendix A Table A3) of the best performing experiment of this species (experiment 282) supports this finding. Where these conditions do not apply, mortality may become severe, even at later ages (e.g., Cardwellia sublimis in experiment 226).
The highly variable survival and growth of Araucaria cunninghamii was unexpected. This species is shade-tolerant [35], and able to tolerate a wide range of environmental conditions including drought [27]. Despite its failure as an enrichment planting, this species has proved very successful in plantations in southern and north Queensland. The complete failure of the Eucalypt and Corymbia species support the classifications of these species as shade-intolerant species [36]. A similar result was observed for Eucalyptus grandis when established under the canopy of Pinus elliottii in south-east Queensland [37]. The historical records do not provide any reason for failure of Khaya senegalensis and Swietenia macrophylla, despite the tending of these experiments being more intense than any of the other experiments. The extremely poor survival rate of Swietenia macrophylla was similar to that observed by previous studies [38,39,40]. At 5 years of age, for example, a study in Mexico reported a mortality of 95% for Swietenia macrophylla when planted under a forest canopy [40]. The cause of failure—as found by other researchers for both these species [16,17,30]—is likely to be a lack of light and water, which is dependent on the amount of retained overwood.
The stagnant growth combined with the relative high survival of Araucaria bidwillii suggests that this species can persist for a long time under limited available light without growing. In contrast, the poor survival of Flindersia bourjotiana and Cardwellia sublimis contrasts with their classifications as a shade-tolerant species [41,42]. However, the low survival of both these two species when grown under low light is consistent with the findings of other researchers [37,42]. Our results do not explain why Khaya ivorensis, as a light-demanding species [43], still survived much better than in similar studies [44].
4.3. Reflections on the Historical Records and Broader Impacts of the Research
Despite the substantial expenditure of resources to establish and maintain the many experiments, the key conclusion from QDF records is that enrichment planting is not an appropriate silvicultural technique to improve the economic value of logged rainforest in north Queensland. Even the best of the enrichment planting experiments has not been able to replicate the growth of plantation grown trees at a similar age and on similar sites. Furthermore, a repeated theme in the old QDF office memos—that enrichment planting growth slows once overstory basal area reaches 30–35 m2 ha−1—may be accurate as far as the enrichment plantings are concerned, but it neglects the timber volume and value of the overwood. The need for continued, intensive (and hence expensive) tending to remove competing overwood also neglects the biodiversity value of the overwood. These considerations provide an alternative perspective to the uncertain survival and growth of enrichment plantings.
4.4. The Implication of These Results for Enrichment Planting as Part of Assisted Natural Regeneration
Our results provide little encouragement for the potential success of assisted natural regeneration (ANR) as a forest restoration technique. On degraded sites, the basic principle of ANR is to protect and facilitate the growth of parent trees and regeneration [45]. In Australia, controlling non-native weeds and eliminating grazing has proved successful in regenerating highly disturbed rainforest [46]. Protecting roots and suckers also proved successful in rehabilitating slash-and-burn agricultural sites in the Democratic Republic of the Congo [47]. In southern Africa, it is reported that regeneration could be achieved by seed covered with soil, although planted seedlings were heavily predated by rodents [48]. In this study, although Flindersia brayleyana (in particular) showed that enrichment planting can be successful, the poor survival and growth of most of the other species tested in the experiments casts doubt on the efficacy of achieving ANR of rainforest through enrichment planting, without careful consideration of the species being planted.
5. Conclusions
This study has shown that enrichment planting can boost the productivity of tropical forests. However, it was also found that for the wide range of species investigated, neither the survival, height nor basal area growth of enrichment plantings is likely to be satisfactory unless extended tending regimes are employed that reduce competition from overwood. Although the initial level of post-logging overwood did not appear to affect the immediate survival of enrichment plantings, survival decreased in the longer term as post-logging overwood and regrowth increased. This suggests that overwood is best removed at the time of enrichment planting. We also found that saplings and trees that were situated outside tended areas may also emerge as a major source of competition. We conclude that unless competition from overwood is adequately managed, the best option would be for low-intensity selective harvesting followed by natural regeneration. In cases where management of overwood is not financially or practically feasible, timber requirements could also be supplemented by establishment of plantations on already cleared land.
Author Contributions
P.M.Q., J.B., J.H., and G.A. collected historical records and designed the study; P.M.Q. analysed data and drafted the manuscripts; P.M.Q., J.B., J.H., M.K., and G.A. contributed to the manuscript editing. All authors have read and agreed to the published version of the manuscript.
Funding
P.M.Q. was funded by the Vietnam Ministry of Agriculture and Rural Development and the University of the Sunshine Coast (USC) to undertake this study.
Acknowledgments
We would like to acknowledge the Queensland Department of Agriculture and Fisheries (DAF) for allowing us to access historical data files and commencement reports of enrichment planting experiments. We wish to recognise the substantial legacy of foresters from the Queensland Department of Forestry (QDF) who established and collected data from the many enrichment planting experiments that we have analysed. We would also like to thank Bruce Hogg (DAF) and David Lee (USC and DAF) for their help in providing access to the data files. The authors would like to acknowledge the work of Mr Don Nicholson who co-authored the original unpublished report [25].
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Some characteristics of the enrichment planting experiments in north Queensland.
Table A1.
Some characteristics of the enrichment planting experiments in north Queensland.
| Expt | Number of Plots | Study Site | Species | Date Expt Commenced (month/year) | Age (years) | Planted Spacing (m) | Soil Type | Mean Rainfall (mm/year) | Slope (degrees) | Elevation (m) | Aspect |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 137 | 1 | Atherton | F. brayleyana | 1–2/1954 | 23.7 | 2.7 × 2.4 | Granite | 1800 | Level | 1130 | Level |
| 138 | 1 | Atherton | F. schottiana | 2/1954 | 10.3 | Unknown | Gritty loam | 1778 | Unknown | 1158 | Unknown |
| 159 | 7 | Kuranda | F. brayleyana | 4/1954 | 9.3 | 2.4 × 3.7 | Basalt | 1400 | Unknown | 750 | Unknown |
| 166 | 5 | Kuranda | F. brayleyana | 5/1956 | 18.5 | 3.7 × 4.6 | Basalt | 1780–2030 | 5–10 | 790 | W |
| 166 | 1 | Ravenshoe | K. ivorensis | 5/1956 | 10.3 | 3.7 × 4.6 | Basalt | 1780–2030 | 5–10 | 790 | W |
| 166 | 2 | Ravenshoe | K. senegalensis | 5/1956 | 10.3 | 3.7 × 4.6 | Basalt | 1780–2030 | 5–10 | 790 | W |
| 166 | 1 | Ravenshoe | S. macrophylla | 5/1956 | 10.3 | 3.7 × 4.6 | Basalt | 1780–2030 | 5–10 | 790 | W |
| 166 | 2 | Ravenshoe | F. bourjotiana | 5/1956 | 10.3 | 3.7 × 4.6 | Basalt | 1780–2030 | 5–10 | 790 | W |
| 166 | 2 | Ravenshoe | A. cunninghamii | 5/1956 | 18.5 | 3.7 × 4.6 | Basalt | 1780–2030 | 5–10 | 790 | W |
| 226 | 1 | Eacham | C. sublimis | 3/1958 | 16.8 | 3.7 × 3 | Basalts | 1800 | 2–3 | 670 | N/N/E |
| 227 | 1 | Atherton | C. sublimis | 8/1958 | 3.0 | 3. 6 × 3 | Granite | 1778 | 10–15 | 1036 | S/W |
| 243 | 1 | Atherton | A. cunninghamii | 3/1959 | 15.2 | 2.4 × 3.7 | Granite | 1778 | 5–10 | 914 | W |
| 245 | 1 | Kuranda | F. brayleyana | 4/1959 | 25.8 | 4.6 × 3.7 | Metamorphic | 2040 | Level | 440 | Unknown |
| 245 | 1 | Kuranda | A. robusta | 4/1959 | 25.8 | 4.6 × 3.7 | Metamorphic | 2040 | Level | 440 | Unknown |
| 245 | 1 | Kuranda | F. ifflaiana | 4/1959 | 16.3 | 4.6 × 3.7 | Metamorphic | 2040 | Level | 440 | Unknown |
| 245 | 1 | Kuranda | A. bidwillii | 4/1959 | 13.5 | 4.6 x 3.7 | Metamorphic | 2040 | Level | 440 | Unknown |
| 245 | 1 | Kuranda | F. bourjotiana | 4/1959 | 13.4 | 4.6 x 3.7 | Metamorphic | 2040 | Level | 440 | Unknown |
| 246 | 2 | Kuranda | A. cunninghamii | 2–3/1959 | 20.2 | Unknown | Metamorphic | 1850 | 0–10 | 450 | W |
| 246 | 2 | Kuranda | A. robusta | 2–3/1959 | 20.2 | N/A | Metamorphic | 1850 | 0–10 | 450 | W |
| 250 | 2 | Kuranda | A. robusta (SP) | 1/1960 | 15.8 | N/A | Metamorphic | 2030 | <5 | 430 | E/S/E |
| A. robusta | 1/1960 | 21.8 | N/A | Metamorphic | 2030 | <5 | 430 | N | |||
| 263 | 1 | Kuranda | F. brayleyana | 5/1954 | 22.0 | 2.7 × 2.7 | Granite | 1800 | 5 | 1100 | N/W |
| 282 | 1 | Atherton | F. brayleyana | 12/1960 | 21.7 | 3.7 × 2.7 | Rhyolite | 2050 | 15 | 1220 | W |
| 282 | 1 | Atherton | A. robusta | 12/1960 | 13.6 | 3.7 × 2.7 | Rhyolite | 2050 | 15 | 1220 | W |
| 282 | 1 | Atherton | A. cunninghamii | 12/1960 | 21.7 | 3.7 × 2.7 | Rhyolite | 2050 | 15 | 1220 | W |
| 283 | 1 | Atherton | F. brayleyana | 12/1960 | 13.4 | Unknown | Granite | 2050 | Unknown | 1040 | Unknown |
| 286 | 1 | Atherton | F. brayleyana | 1/1961 | 14.2 | 3.7 × 2.4 | Metamorphic | 1400 | <5 | 610 | N |
| 286 | 1 | Atherton | A. cunninghamii | 1/1961 | 14.2 | 3.7 × 2.4 | Metamorphic | 1400 | <5 | 610 | N |
| 286 | 1 | Atherton | A. robusta | 1/1961 | 14.2 | 3.7 × 2.4 | Metamorphic | 1400 | <5 | 610 | N |
| 310 | 1 | Atherton | F. brayleyana | 6/1955–11/1956 | 31.7 | 3 × 3 | Basalt | 2090 | 5 | 670 | W |
| 311 | 1 | Atherton | F. brayleyana | 11/1960–1/1961 | 26.6 | 3 × 3 | Rhyolite | 1750 | 8–13 | 1040 | S/W |
| 317 | 1 | Atherton | F. brayleyana | 2/1952 | 23.7 | 3 × 2.7 | Metamorphic | 1400 | Level | 730 | Level |
| 321 | 1 | Atherton | F. brayleyana | 11/1960–1/1961 | 13.6 | 3 × 3 | Metamorphic | 1400 | 10 | 730 | N/E |
| 322 | 1 | Kuranda | F. brayleyana | 11/1960–1/1961 | 18.7 | 3 × 3 | Metamorphic | 2080 | 0–15 | 488 | W/N/W |
| 324 | 1 | Atherton | F. amboinensis | 2/1963 | 10.9 | 4.3 × 3 | Metamorphic | 2100 | 2 | 460 | E |
| 329 (R1) | 1 | Mission Beach | F. brayleyana | 2–8/1963 | 19.2 | 4.3 × 3 | Metamorphic | 3500 | 0 | 6 | N |
| 329 (R2) | 1 | Mission Beach | F. brayleyana | 2–8/1963 | 19.2 | 4.3 × 3 | Metamorphic | 3500 | 5–15 | 6 | S/W |
| 331 | 1 | Atherton | F. brayleyana | 11/1961–1/1962 | 11.7 | 3 × 3 | Metamorphic | 1250 | 7–15 | 730 | S/E |
| 333 | 1 | Yungaburra | F. brayleyana | 1961 | 16.8 | 3 × 3 | Metamorphic | 2090 | 5–10 | 670 | S/W |
| 347 | 1 | Eacham | F. brayleyana | 11/1958–6/1959 | 15.4 | Unknown | Basalt | 2000 | 2–5 | 670 | W/N/W |
| 350 | 1 | Atherton | F. brayleyana | 1963 | 18.4 | Unknown | Granite | 2150 | 0–5 | 580 | S/W |
| 350 | 2 | Atherton | A. cunninghamii | 1963 | 18.4 | Unknown | Granite | 2150 | 0–5 | 580 | S/W |
| 373 (R1) | 4 | Kuranda | F. brayleyana | 12/1966 | 14.0 | Unknown | Granite | 2032 | Unknown | 457 | Unknown |
| 373 (R2) | 4 | Kuranda | F. brayleyana | 12/1966 | 14.0 | Unknown | Metamorphic | 2032 | Unknown | 457 | Unknown |
| 423 | 1 | Atherton | F. brayleyana | 4/1968 | 5.5 | 4.6 × 6.1 | Basalt | 2800 | <10 | 825 | N/E |
| 423 | 1 | Atherton | A. robusta | 4/1968 | 5.5 | 4.6 × 6.1 | Basalt | 2800 | <10 | 825 | N/E |
| 450 | 1 | Eacham | F. brayleyana | 1–6/1970 | 13.9 | 6.1 × 4.6 | Basalt | 2000 | 0–5 | 730 | N/W |
| 450 | 1 | Eacham | F. brayleyana | 1–6/1970 | 13.9 | 6.1 × 4.6 | Basalt | 2000 | 0–5 | 730 | N/W |
| 450 | 1 | Eacham | A. cunninghamii | 1–6/1970 | 4.7 | 6.1 × 4.6 | Basalt | 2000 | 0–5 | 730 | N/W |
| 450 | 1 | Eacham | C. sublimis | 1–6/1970 | 4.7 | 6.1 × 4.6 | Basalt | 2000 | 0–5 | 730 | N/W |
| 450 | 1 | Eacham | E. grandis | 1–6/1970 | 3.4 | 6.1 × 4.6 | Basalt | 2000 | 0–5 | 730 | N/W |
| 450 | 1 | Eacham | E. microcorys | 1–6/1970 | 2.6 | 6.1 × 4.6 | Basalt | 2000 | 0–5 | 730 | N/W |
| 450 | 1 | Eacham | E. pilularis | 1–6/1970 | 1.3 | 6.1 × 4.6 | Basalt | 2000 | 0–5 | 730 | N/W |
| 450 | 1 | Eacham | C. torelliana | 1–6/1970 | 1.3 | 6.1 × 4.6 | Basalt | 2000 | 0–5 | 730 | N/W |
| 456 | 1 | Kuranda | F. brayleyana | 2/1955 | 17.4 | 3.7 × 2.4 | Granite | 1800 | n/a | 1160 | N/W |
(SP): A. robusta is a southern provenance. R1: replication 1, R2: replication 2, N/A: not applicable.

Table A2.
Post-planting silvicultural treatments carried out in the enrichment planting experiments in north Queensland.
Table A2.
Post-planting silvicultural treatments carried out in the enrichment planting experiments in north Queensland.
| Expt | Last Logged (year) | Date Expt Commenced (month/year) | Treatment after Enrichment Planting (month/year) | |||||
|---|---|---|---|---|---|---|---|---|
| Ringbarking and Herbicide Application | Brushing | Line Tending | 1.5m Radial Tending | Stinger Tending | Vine Tending | |||
| 137 | 1953 | 1–2/1954 | 1/1957, 8/1957 | 1/1956, 7/1957 | ||||
| 138 | Unknown | 2/1954 | 8/1958 | 10/1963 | ||||
| 159 * | 1954 | 4/1954 | ||||||
| 166 | 1942–1944 | 5/1956 | 10/1957, 9/1962, 1/1964 | 10/1957, 5/1958, 9/1962, 1/1964 | 10/1958, 8/1959, 6/1961 | 10/1957, 10/1958, 8/1959, 6/1961, 9/1962, 1/1964, 8/1964 | 10/1956 | 1/1964 |
| 226 | 1957 | 3/1958 | 10/1962; 11/1963 | 4/1974 | 6/1959; 10/1964 | |||
| 227 * | 1958 | 8/1958 | ||||||
| 243 | 1954–1956 | 3/1959 | 11/1961 | 7/1961 | 9/1960, 10/1964 | |||
| 245 | 1958–1959 | 4/1959 | 6/1959 | 11/1963; 4/1964;1/1965 | 7/1962; 11/1963, 4/1964 | |||
| 246 | Before 1958 | 2–3/1959 | 6/1974 | 12/1960; 1/1963; 12/1965 | ||||
| 250 | Unknown | 1/1960 | 12/1970 | 12/1970 | 7/1962 | 1/1965 | 11/1963 | |
| 263 | Unknown | 5/1954 | 1965 | |||||
| 282 | 1960 | 12/1960 | 10/1961 | 12/1962; 11/1963; 4/1964, 10/1964 | 5/1964 | |||
| 283 | 1957 | 12/1960 | 12/1961 | 12/1962, 4/1964 | 12/1961, 11/1963 | 10/1964 | ||
| 286 | 1930 | 1/1961 | 5/1975 | 4/1964 | 4/1968 | 2/1966 | ||
| 310 | Unknown | 6/1955–11/1956 | 2/1965 | 10/1964 | 7/1957; 3/1961 | 7/1957; 3/1961 | ||
| 311 | 1960 | 11/1960–1/1961 | 9/1962 | 10/1964 | 11/1963 | |||
| 317 | 1951 | 2/1952 | 12/1964 | 12/1963 | ||||
| 321 | 1945 | 11/1960–1/1961 | 12/1961 | 12/1961 | 1/1964; 2/1966 | |||
| 322 | 1956 | 11/1960–1/1961 | 9/1975 | 11/1963 | ||||
| 324 | 1948 | 2/1963 | 1/1974 | 1/1974 | 11/1965 | 7/1964, 1/1965 | 4/1964 | |
| 329 | 1960 | 2/–8/1963 | 12/1965 | 7/1964; 7/1965/7/1966 | 7/1965 | |||
| 331 | 1958 | 11/1961–1/1962 | 11/1963; 2/1966 | 11/1963 | ||||
| 333 | 1961 | 1961 | 1963 | |||||
| 350 | 350 | 1963 | 5/1964 | 1964, 1965 | ||||
| 347 | 1955 | 11/1958–6/1959 | 1961 | 9/1959; 10/1964 | 1958/1959 | 7/1961, 10/1964 | ||
| 373 * | 1964–1957 | 12/1966 | ||||||
| 423 | 1960 | 4/1968 | 10/1968, 10/1973 | 8/1974 | ||||
| 450 | 1970 | 1–6/1970 | 3/1973 | 3/1973 | 9/1972; 8/1974 | |||
| 456 | 1954 | 2/1955 | 8/1959, 4/1964 | 8/1955 | ||||
Ringbarking: non-commercial residual trees are girdled. Herbicide application: non-commercial residual trees were poisoned using 10% arsenic pentoxide and/or 1% to 5% 2,4,5T. Brushing (cutting): removing all shrubs and other competing ground vegetation near the planted seedlings. Line tending: clearing all vegetation along the planting line to a width from 1 to 1.5 m. 1.5 m radial tending: clearing all vegetation within 1.5 m around each planted seedling. Stinger tending: removing stinger trees (Dendrocide moroides) by spraying with a selective weedicide. Vine tending: cutting out vines. * Periodic tendings were not undertaken in the experiment.

Table A3.
Annualised mortality rate of enrichment plantings over different periods between measurements (years).
Table A3.
Annualised mortality rate of enrichment plantings over different periods between measurements (years).
| Expt/Species | Annualised Mortality Rate (%) for Time Period Intervals (years) | |||||
|---|---|---|---|---|---|---|
| Expt 310/Flindersia brayleyana | 0–4.4 years | 4.5–6.3 years | 6.4–10.1 years | 10.2–15.7 years | 15.8–19.1 years | 19.2–31.7 years |
| 7.0% | 0.9% | 1.0% | 1.4% | 3.1% | 3.2% | |
| Expt 311/Flindersia brayleyana | 0–2.7 years | 2.8–5.5 years | 5.6–10.8 years | 10. 9–13.4 years | 13.5–21.2 years | 21.3–26.6 years |
| 0.9% | 0.8% | 0.5% | 1.5% | 0.2% | 0.8% | |
| Expt 245/Flindersia brayleyana | 0–3.2 years | 3.3–7.3 years | 7.4–10.1 years | 10.2–13.4 years | 13.5–16.3 years | 16.3–25.8 years |
| 5.1% | 3.5% | 0% | 0.4% | 0.5% | 1% | |
| Expt 137/Flindersia brayleyana | 0–4.3 years | 4.4–7.4 years | 7.5–10.2 years | 10.3–15.4 years | 15.5–18.6 years | 18.7–23.7 years |
| 1% | 3.5% | 0.5% | 1.8% | 0.9% | 0.3% | |
| Expt 347/Flindersia brayleyana | 0–2.4 years | 2.5–4.3 years | 4.4–6.2 years | 6.3–10.3 years | 10.4–11.4 years | |
| 11.9% | 7.0% | 4.0% | 5.9% | 3.2% | ||
| Expt 322/Flindersia brayleyana | 0–2.8 years | 2.9–5.6 years | 5.7–9.7 years | 9.8–13.8 years | 13.9–18.7 years | |
| 6.9% | 4.2% | 1.8% | 0% | 0% | ||
| Expt 333/Flindersia brayleyana | 0–3.5 years | 3.6–5.5 years | 5.6–8.8 years | 8.9–10.8 years | 10.9–16.8 years | |
| 2.7% | 2.4% | 3.2% | 9.4% | 2.5% | ||
| Expt 321/Flindersia brayleyana | 0 - 2.6 years | 2.7 - 5.6 years | 5.7 - 8.9 years | 9 - 10.3 years | 10.4 - 13.6 years | |
| 3.2% | 2.1% | 5.6% | 24.6% | 1.8% | ||
| Expt 329/Flindersia brayleyana | 0 - 2.9 years | 3 - 4.1 years | 4.2 - 7.1 years | 7.2 - 12.2 years | 12.3 - 19.2 years | |
| 3.3% | 2.5% | 2.1% | 1.2% | 1.5% | ||
| Expt 263/Flindersia brayleyana | 0–6.2 years | 6.3 - 9.3 years | 9.4 - 11 years | 11.1 - 15 years | 15.1 - 17.8 years | |
| 3% | 0.4% | 0% | 0% | 2.4% | ||
| Expt 282/Flindersia brayleyana | 0-3.4 years | 3.5–6.5 years | 6.6–9.8 years | 9.9–13.6 years | 13.7–21.7 years | |
| 1.5% | 1.1% | 1.0% | 0.6% | 0.3% | ||
| Expt 332/Flindersia brayleyana | 0–3.5 years | 3.6–5.8 years | 5.9–12.4 years | 12.5–17.9 years | ||
| 3.3% | 3.4% | 1.1% | 0.8% | |||
| Expt 286/Flindersia brayleyana | 0–5.5 years | 5.6–7.2 years | 7.3–10.3 years | 10.4–14.2 years | ||
| 2.8% | 2.8% | 0.3% | 6.9% | |||
| Expt 166/Flindersia brayleyana | 0–3.3 years | 3.4–7.3 years | 7.4–10.3 years | 10.3–18.5 years | ||
| 3.3% | 3.9% | 5.3% | 6.5% | |||
| Expt 456/Flindersia brayleyana | 0–3.5 years | 3.6–5.6 years | 5.7–14.8 years | 14.9–17.4 years | ||
| 8.6% | 0.7% | 8.2% | 0.8% | |||
| Expt 283/Flindersia brayleyana | 0–3.4 years | 3.5–6.5 years | 6.6–9.8 years | 9.9–13.4 years | ||
| 16.4% | 0.7% | 0.6% | 1.8% | |||
| Expt 373/Flindersia brayleyana | 0–1.2 years | 1.3–7 years | 7–14 years | |||
| 2.2% | 1.3% | 2.2% | ||||
| Expt 450/Flindersia brayleyana | 0–4.7 years | 4.8–7.7 years | 7.8–13.9 years | |||
| 4.9% | 2.3% | 1.0% | ||||
| Expt 423/Flindersia brayleyana | 0–1.3 years | 1.4–5.5 years | ||||
| 23.8% | 5.3% | |||||
| Expt 245/Agathis robusta | 0–3.2 years | 3.3–7.3 years | 7.4–10.1 years | 10.2–13.4 years | 13.5–16.3 years | 16.4–25.8 years |
| 4.1% | 2.3% | 3.7% | 1.9% | 1.5% | 0% | |
| Expt 246/Agathis robusta | 0–5.3 year | 5.4–7.5 years | 7.6–10.3 years | 10.4–14.8 years | 14.9–20.2 years | |
| 1.4% | 0.0% | 1.1% | 0.8% | 0.1% | ||
| Expt 250/Agathis robusta | 0–3.7 years | 3.8–7.4 years | 7.5–10.7 years | 10.8–15.8 years | 15.9–21.8 years | |
| 0.3% | 1.7% | 0.6% | 1% | 1.3% | ||
| Expt 282/Agathis robusta | 0–3.4 years | 3.5–6.5 years | 6.6–9.8 years | 9.9–13.6 years | ||
| 1.5% | 3.9% | 1.9% | 1% | |||
| Expt 286/Agathis robusta | 0–7.2 years | 7.3–10.3 years | 10.3–14.2 years | |||
| 0.2% | 0.4% | 0.3% | ||||
| Expt 423/Agathis robusta | 0–1.3 years | 1.3–5.3 years | ||||
| 8.4% | 5.8% | |||||
| Expt 282/Araucaria cunninghamii | 0–3.4 years | 3.5–6.5 years | 6.6–9.8 years | 9.9–13.6 years | 13.7–21.7 years | |
| 1.2% | 5.2% | 2.2% | 2.9% | 2.1% | ||
| Expt 246/Araucaria cunninghamii | 0–4.6 years | 4.7–7.5 years | 7.6–10.3 years | 10.4–14.8 years | 14.9–20.2 years | |
| 0.3% | 0.9% | 0% | 2% | 1.9% | ||
| Expt 166/Araucaria cunninghamii | 0–3.3 years | 3.4–5.2 years | 5.3–7.3 years | 7.3–10.3 years | 10.4–18.5 years | |
| 3% | 2.5% | 6.5% | 1.5% | 3.5% | ||
| Expt 243/Araucaria cunninghamii | 0–3.4 years | 3.5–7.3 years | 7.4–10.2 years | 10.3–15.2 years | ||
| 0.6% | 1% | 2.5% | 4.7% | |||
| Expt 286/Araucaria cunninghamii | 0–10.4 years | 10.5–14.2 years | ||||
| 0.2% | 0.3% | |||||
| Expt 450/Araucaria cunninghamii | 0–2.7 years | 2.8–4.7 years | ||||
| 1.9% | 4.6% | |||||
| Expt 350/Araucaria cunninghamii | 0–2.4 years | 2.5–18.4 years | ||||
| 0.4% | 100% | |||||
| Expt 245/Flindersia bourjotiana | 0–3.2 years | 3.3–7.3 years | 7.4–10.1 years | 10.2–13.4 years | ||
| 10% | 11.7% | 0 | 28.3% | |||
| Expt 166/Flindersia bourjotiana | 0–4 years | 4.1–7.3 years | 7.4–10.3 years | |||
| 2.4% | 1.6% | 3.6% | ||||
| Expt 226/Cardwellia sublimis | 0–3.4 years | 3.5–6.6 years | 6.7–10.2 years | 10.3–12.5 years | ||
| 10.8% | 3.6% | 3.7% | 9.9% | |||
| Expt 227/Cardwellia sublimis | 0–1.7 years | 1.8–3 years | ||||
| 33.5% | 64.4% | |||||
| Expt 450/Cardwellia sublimis | 0–2.6 years | 2.7–4.7 years | ||||
| 34.5% | 20.7% | |||||
| Expt 245/Flindersia ifflaiana | 0–3.2 years | 3.3–7.3 years | 7.4–10.1 years | 10.2–13.4 years | 13.5–16.3 years | |
| 17.8% | 2.2% | 4.4% | 0.0% | 0.0% | ||
| Expt 245/Araucaria bidwillii | 0–3.2 years | 3.3–7.3 years | 7.4–10.1 years | 10.2–13 years | ||
| 3.8% | 0.0% | 1.6% | 4.9% | |||
| Expt 166/Khaya ivorensis | 0–3.3 years | 3.4–7.3 years | 7.4–10.3 years | |||
| 6.6% | 0.0% | 1.7% | ||||
| Expt 138/Flindersia schottiana | 0–3.4 years | 3.5–6.3 years | 6.4–8.5 years | 8.6–10.3 years | ||
| 2.3% | 1.1% | 1.5% | 2.2% | |||
| Expt 324/Flindersia amboinensis | 0–3.4 years | 3.5–7.5 years | 7.6–10.9 years | |||
| 5.81% | 1.63% | 4.23% | ||||
| Expt 166/Swietenia macrophylla | 0–1.4 years | 1.5–5.2 years | 5.3–10.3 years | |||
| 69.5% | 26.2% | 19.4% | ||||
| Expt 166/Khaya senegalensis | 0–3.3 years | 3.4–7.3 years | 7.4–10.3 years | |||
| 34.5% | 15.9% | 15.4% | ||||
| Expt 450/Eucalyptus grandis | 0–2.6 years | 2.7–4.7 years | ||||
| 43.2% | 100% | |||||
| Expt 450/Eucalyptus microcorys | 0–2.6 years | 2.7–4.7 years | ||||
| 27.7% | 100% | |||||
| Expt 450/Corymbia torelliana | 0–1.3 years | 1.4–3.4 years | ||||
| 93.3% | 100% | |||||
| Expt 450/Eucalyptus pilularis | 0–0.1 years | |||||
| 100% | ||||||

Table A4.
Summarized qualitative comments extracted from historical records of enrichment planting experiments in north Queensland.
Table A4.
Summarized qualitative comments extracted from historical records of enrichment planting experiments in north Queensland.
| Expt | Enrichment Plantings | Purpose of the Expt | Age of Enrichment Plantings (year) | Comment/Conclusion |
|---|---|---|---|---|
| 137 | Flindersia brayleyana | To observe the growth of enrichment plantings in treated rainforest. | 23.8 | Maple grown successfully, possibly because overwood basal area was below 15 m2/ha. |
| 138 | Flindersia schottiana | To observe the growth of planted Flindersia schottiana in treated rainforest. | 10.3 | Wallabies and borer attack caused significant mortality. |
| 159 | Flindersia brayleyana | To test the suitability of planted Flindersia brayleyana in treated eucalypt forest. | 0.3 | Maple was severely damaged by wallabies. A total of 60% of under-plantings died 3 months after planting. |
| 1.1 | Very poor survival: deaths caused by consistent browsing by wallabies (almost complete death). | |||
| 226 | Cardwellia sublimis | To test the suitability of planted Cardwellia sublimis in treated rainforest. | 16.8 | Overwood has not been reduced since 1963. |
| 12.5 | Overwood suppressed the enrichment plantings. | |||
| 227 | Cardwellia sublimis | To test the suitability of planted Cardwellia sublimis in treated rainforest. | 3 | Poor survival was caused by wallaby browsing. |
| 243 | Araucaria cunninghamii | To test the suitability of planted Araucaria cunninghamii in treated rainforest. | 5.2 | Poor growth caused by heavy growth of overwood. |
| 245 | Flindersia brayleyana, Agathis robusta, Flindersia ifflaiana, Flindersia bourjotiana, Araucaria bidwillii | To study the growth of enrichment plantings on a wet site in treated rainforest. | 16.3 | Growth of all species is falling because overwood basal area is now 27 m2/ha. Bunya pine had high survival rate, but its growth was very poor. |
| 246 | Araucaria cunninghamii, Agathis robusta | To test the suitability of enrichment plantings in treated rainforest. | 7.3 | The form of both kauri pine and hoop pine was good but vigour was fair. |
| 250 | Agathis robusta | To compare relative growth rate of the northern and southern provenances of Agathis robusta planted in treated rainforest. | 21.8 | At age 16, DBH for the best 100 tree/ha was 7 and 18 cm for plot 1 and plot 2, respectively. |
| 15.8 | Northern kauri pine in this experiment was better than on Expt 245 due to lower basal area. | |||
| 263 | Flindersia brayleyana | To test the suitability of planted Flindersia brayleyana in treated eucalypt forest. | 17.8 | Enrichment plantings were suppressed by eucalyptus species. |
| 263 | Flindersia brayleyana | To test the suitability of planted Flindersia brayleyana in treated eucalypt forest. | 17.8 | Enrichment plantings were suppressed by eucalyptus species. |
| 283 | Flindersia brayleyana | To test the suitability of enrichment planting Flindersia brayleyana in treated rainforest. | 13.4 | Initial mortality (reason not known) was high, but growth since then has been satisfactory. The trees had good form. |
| 286 | Flindersia brayleyana, Araucaria cunninghamii, Agathis robusta. | To observe growth of rainforest species when planted under ringbarked wattle. | 14.2 | Hoop pine performed well, whereas maple and kauri pine grew poorly due to compacted subsoil. |
| 310 | Flindersia brayleyana | To follow the development of Flindersia brayleyana planted in treated rainforest. | 16.1 | Growth satisfactory. DBH growth peaked at age 8 to 9 when stand overwood was 15 m2/ha. However, when overwood increased to 29 m2/ha, growth declined. |
| 311 | Flindersia brayleyana | To observe the growth of planted Flindersia brayleyana in treated rainforest. | 13.4 | Maple grew very well despite a high initial overwood basal area of 14.7 m2/ha, which increased to 34.1 m2/ha at age 13. DBH increment still 0.8 cm/year. |
| 13.4 | Form and vigor were satisfactory. Branching was heavy but shedding is being stimulated by rainforest growth. | |||
| 317 | Flindersia brayleyana | To observe the growth of planted Flindersia brayleyana in treated rainforest. | 18.7 | Poor growth because overwood basal area ranged from 10 to 15 m2/ha when planted but increased. Overwood was reduced at age 10 and growth of the maple improved. |
| 321 | Flindersia brayleyana | To observe the development of planted Flindersia brayleyana in treated rainforest. | 13.6 | Competition from regrowth and vine resulted in poor growth. |
| 322 | Flindersia brayleyana | To observe the development of maple planted in treated rainforest. | 13.8 | Good growth with the best trees having free access to overhead light and planted away from retained trees. |
| 324 | Flindersia brayleyana | To observe growth of Flindersia amboinensis in treated rainforest. | 10.9 | Very slow growth due to overwood basal area of 32 m2/ha. |
| 329 | Flindersia brayleyana | To observe and record the growth of Flindersia brayleyana in treated rainforest. | 19.2 | Growth in both plots is slowing dramatically because of high overwood measured as 33 m2/ha in 1975. |
| 331 | Flindersia brayleyana | To collect increased data for GBH, basal area, and volume to determine the yield potential of Flindersia brayleyana planted in silviculturally treated rainforest. | 9.2 | A total of 30% of underplants were suppressed by overwood. Growth was poor. |
| 11.7 | Too much overwood remained in plot, resulted in very poor growth of underplants. | |||
| 332 | Flindersia brayleyana | To observe the growth of Flindersia brayleyana planted in treated rainforest. | 12.4 | Slow growth: caused by the initial basal area of overwood (15 m2/ha), which rose to 33 m2/ha at age 12. |
| 333 | Flindersia brayleyana | To follow the growth of planted Flindersia brayleyana in treated rainforest. | 16.8 | Large retained rainforest trees restricted the growth of enrichment plantings. |
| 11.3 | Most enrichment plantings had free access to overhead light and side light. Form and log lengths were consequently good. | |||
| 347 | Flindersia brayleyana | To assess the potential yield of planted Flindersia brayleyana in silviculturally treated rainforest. | 15.4 | Poor growth due to suppressed by overwood. DBH increments were falling steadily at age 12 to 15 due to overwood basal area being 30 m2/ha. |
| 350 | Flindersia brayleyana, Araucaria cunninghamii. | To observe the growth rate of enrichment plantings in logged rainforest. | 18.4 | Hoop pine failed due to lack of tending. Maple showed that it can grow successfully with minimum follow-up treatment. |
| 373 | Flindersia brayleyana | To observe growth under different tending regimes in treated rainforest. | 8.8 | The plots that had received no tending grew poorly. |
| 423 | Flindersia brayleyana, A. robusta | To test the suitability of enrichment plantings in treated rainforest. | 5.5 | Growth of enrichment plantings was good, but the survival was poor from the start due to heavy vine growth. The long delay between establishment and first tending resulted in severe damage. |
| 450 | Flindersia brayleyana, Araucaria cunninghamii, Cardwellia bidwillii, Eucalyptus grandis, Eucalyptus microcorys, Corymbia torelliana, Eucalyptus pilularis | To calculate the cost of enrichment planting and to assess growth and development of the enrichment plantings. | 7.7 | The eucalyptus species planted failed, despite replanting. This is the best maple plot in north Queensland. |
| 7.7 | The form of maple was reasonable and improving; some maples were damaged from rung overwood; some suffered a setback to form and vigour due to rampant vines growth. | |||
| 4.7 | Form of the hoop pine and bull oak was below average due to vine damage and suppression by weeds. Eucalyptus species failed completely. |

Table A5.
The development of cohorts of overwood over time in enrichment planting experiments in north Queensland.
Table A5.
The development of cohorts of overwood over time in enrichment planting experiments in north Queensland.
| Expt/Plot | Planted Species | Surveyed Date (month/year) | Age of Enrichment Plantings (year) | Overwood Basal Area (m2/ha) and Stocking (stems/ha) of Each Cohort | Stocking of Enrichment Plantings (stems/ha) | BA of Enrichment Plantings (m2/ha) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Cohort 5 | Cohort 6 | Total BA and Stocking | ||||||
| 322/1 | Flindersia brayleyana | 3/1961 | 0.3 | 11.5 (198) | 11.5 (198) | 721 | ||||||
| 7/1963 | 2.8 | 12.5 (198) | 0.2 (69) | 12.7 (267) | 623 | |||||||
| 5/1966 | 5.6 | 14.4 (188) | 0.3 (69) | 1.5 (475) | 16.2 (732) | 553 | 2.2 | |||||
| 7/1970 | 9.7 | 16.8 (178) | 0.4 (59) | 3.4 (475) | 1.5 (554) | 22.1 (126) | 514 | 4.8 | ||||
| 7/1974 | 13.8 | 18.9 (178) | 0.4 (50) | 4.5 (436) | 2.6 (535) | 0.17 (119) | 26.5 (1318) | 514 | 6.5 | |||
| 6/1979 | 18.7 | 15.3 (89) | 0.4 (40) | 2.2 (248) | 2.0 (406) | 0.21 (99) | 0.01 (10) | 20.1 (892) | 514 | 8.4 | ||
| 245/2 | Flindersia brayleyana | 8/1959 | 0.2 | 11.2 (179) | 11.2 (179) | 385 | ||||||
| 6/1961 | 2.2 | 11.7 (179) | 11.7 (179) | 326 | ||||||||
| 7/1963 | 4.4 | 12.0 (169) | 2.3 (362) | 14.3 (531) | 322 | |||||||
| 7/1966 | 7.3 | 13.3 (169) | 3.0 (362) | 2.7 (556) | 18.9 (1087) | 282 | 0.8 | |||||
| 4/1969 | 10.1 | 13.8 (164) | 2.4 (188) | 4.8 (556) | 2.5 (874) | 23.4 (1738) | 282 | 1.3 | ||||
| 7/1972 | 13.4 | 14.2 (150) | 1.9 (130) | 6.8 (478) | 4.1 (784) | 1.67 (628) | 28.7 (2170) | 278 | 2.0 | |||
| 245/1 | Agathis robusta | 8/1959 | 0.2 | 6.1 (58) | 6.1 (58) | 495 | ||||||
| 6/1961 | 2.2 | 6.3 (58) | 0.01 (4) | 6.3 (62) | 438 | |||||||
| 7/1963 | 4.4 | 6.8 (58) | 0.01 (4) | 1.5 (261) | 8.3 (323) | 420 | ||||||
| 7/1966 | 7.3 | 7.7 (53) | 0.0 (0) | 2.5 (261) | 3.0 (606) | 13.2 (920) | 393 | |||||
| 4/1969 | 10.1 | 7.9 (53) | 0.0 (0) | 2.1 (164) | 5.3(602) | 1.28 (518) | 16.5 (1337) | 354 | 1.1 | |||
| 7/1972 | 13.4 | 8.5 (53) | 0.0 (0) | 1.3 (102) | 8.0 (540) | 1.99 (522) | 0.65 (270) | 20.5 (1487) | 332 | 2.0 | ||
| 246/2 | Agathis robusta | 5/1959 | 0.4 | 15.0 (233) | 15.0 (233) | 424 | ||||||
| 3/1961 | 2.2 | 15.6 (233) | 15.6 (233) | 401 | ||||||||
| 7/1963 | 4.6 | 16.6 (233) | 16.6 (233) | 401 | ||||||||
| 7/1966 | 7.5 | 18.2 (233) | 2.9 (555) | 21.0 (788) | 401 | |||||||
| 4/1969 | 10.3 | 15.8 (214) | 3.76 (517) | 1.45 (476) | 21.0 (1207) | 382 | 0.32 | |||||
| 1/1974 | 14.8 | 17.1 (210) | 5.13 (471) | 2.11 (448) | 0.16 (5) | 24.5 (1134) | 364 | 1.67 | ||||
| 4/1979 | 20.2 | 17.6 (177) | 1.6 (131) | 0.7 (117) | 0.0 (0) | 0.1 (14) | 20.0 (439) | 359 | 3.97 | |||
| 246/4 | Agathis robusta | 5/1959 | 0.4 | 23.3 (158) | 23.3 (158) | 485 | ||||||
| 3/1961 | 2.2 | 23.9 (158) | 23.9 (158) | 480 | ||||||||
| 7/1963 | 4.6 | 23.8 (153) | 23.8 (153) | 480 | ||||||||
| 7/1966 | 7.5 | 25.2 (153) | 1.5 (403) | 26.6 (556) | 475 | |||||||
| 4/1969 | 10.3 | 26.1 (148) | 2.06 (357) | 0.31 (153) | 28.4 (658) | 470 | 2 | |||||
| 1/1974 | 14.8 | 28.1 (148) | 2.94 (332) | 0.52 (153) | 31.6 (633) | 459 | 4.5 | |||||
| 4/1979 | 20.2 | 26.3 (128) | 1.8 (153) | 0.2 (51) | 0.1 (15) | 28.4 (347) | 459 | 8.1 | ||||
| 246/1 | Araucaria cunninghamii | 5/1959 | 0.4 | 11.0 (213) | 11.0 (213) | 383 | ||||||
| 3/1961 | 2.2 | 11.5 (213) | 11.5 (213) | 376 | ||||||||
| 7/1963 | 4.6 | 12.5 (213) | 12.5 (213) | 376 | ||||||||
| 7/1966 | 7.5 | 16.7 (213) | 2.7 (616) | 19.4 (829) | 356 | |||||||
| 4/1969 | 10.3 | 14.1 (201) | 3.9 (585) | 1.1 (457) | 19.2 (1243) | 356 | 0.4 | |||||
| 1/1974 | 14.8 | 14.8 (190) | 5.8 (542) | 1.7 (426) | 22.1 (1158) | 321 | 1.7 | |||||
| 4/1979 | 20.2 | 14.9 (151) | 2.7 (217) | 1.4 (159) | 19.0 (527) | 321 | 4.1 | |||||
| 246/3 | Araucaria cunninghamii | 5/1959 | 0.4 | 17.2 (178) | 17.2 (178) | 403 | ||||||
| 3/1961 | 2.2 | 17.5 (178) | 17.5 (178) | 403 | ||||||||
| 7/1963 | 4.6 | 18.0 (178) | 18.0 (178) | 403 | ||||||||
| 7/1966 | 7.5 | 18.7 (173) | 1.7 (503) | 20.4 (676) | 403 | |||||||
| 4/1969 | 10.3 | 19.1 (160) | 2.4 (472) | 1.0 (455) | 22.6 (1087) | 403 | 1.0 | |||||
| 1/1974 | 14.8 | 20.6 (156) | 3.5 (468) | 1.8 (442) | 26.2 (1066) | 373 | 2.1 | |||||
| 4/1979 | 20.2 | 20.8 (134) | 1.5 (147) | 0.7 (91) | 0.1 (13) | 23.1 (385) | 303 | 3.8 | ||||
| 245/3 | Flindersia ifflaiana | 8/1959 | 0.2 | 11.4 (118) | 11.4 (118) | 535 | ||||||
| 6/1961 | 2.2 | 11.8 (118) | 11.8 (118) | 292 | ||||||||
| 7/1963 | 4.4 | 10.9 (106) | 4.1 (516) | 15.0 (621) | 286 | |||||||
| 7/1966 | 7.3 | 11.8 (99) | 5.9 (516) | 3.4 (752) | 21.1 (1366) | 261 | ||||||
| 4/1969 | 10.1 | 12.2 (99) | 5.5 (373) | 5.2 (752) | 2.4 (839) | 25.3 (2062) | 230 | |||||
| 7/1972 | 13.4 | 12.6 (99) | 5.3 (311) | 7.4 (689) | 3.8 (832) | 1.5 (634) | 30.5 (2565) | 230 | ||||
References
- Montagnini, F.; Jordan, C.F. Tropical Forest Ecology: The Basis for Conservation and Management; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Lawal, A.; Adekunle, V.J. A silvicultural approach to volume yield, biodiversity and soil fertility restoration of degraded natural forest in South-West Nigeria. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2013, 9, 201–214. [Google Scholar] [CrossRef][Green Version]
- Keenan, R.; Hambleton, A.; Robson, K.; Webb, M. Growth response of rainforest cabinet timber species to fertiliser application in North Queensland plantations. In Soils of Tropical Forest Ecosystems; Springer Science and Business Media LLC: Berlin, Germany, 1998; pp. 107–114. [Google Scholar]
- Singh, K.D. Global Forest Resources Assessments. Sustain. Land Use Mount. Regions Southeast Asia 2013, 3, 203–211. [Google Scholar]
- Putz, F.E. The Natural History of Lianas on Barro Colorado Island, Panama. Ecology 1984, 65, 1713–1724. [Google Scholar] [CrossRef]
- Putz, F.E.; Chai, P. Ecological Studies of Lianas in Lambir National Park, Sarawak, Malaysia. J. Ecol. 1987, 75, 523. [Google Scholar] [CrossRef]
- Mills, D.J.; Bohlman, S.A.; Putz, F.E.; Andreu, M. Liberation of future crop trees from lianas in Belize: Completeness, costs, and timber-yield benefits. For. Ecol. Manag. 2019, 439, 97–104. [Google Scholar] [CrossRef]
- Marshall, A.R.; Coates, M.A.; Kivambe, E.; Mnendendo, H.; Mtoka, S.; Mwakisoma, R.; Figueiredo, R.J.R.L.; Njilima, F.M.; Archer, J. Liana cutting for restoring tropical forests: A rare palaeotropical trial. Afr. J. Ecol. 2016, 55, 282–297. [Google Scholar] [CrossRef]
- Sabogal, C.; Nasi, R. Restoring Overlogged Tropical Forests. In Forest Restoration in Landscapes; Springer: New York, NY, USA, 2006; pp. 361–369. [Google Scholar]
- Nabe-Nielsen, J.; Severiche, W.; Fredericksen, T.; Nabe-Nielsen, L.I. Timber tree regeneration along abandoned logging roads in a tropical Bolivian forest. New For. 2007, 34, 31–40. [Google Scholar] [CrossRef]
- Millet, J.; Tran, N.; Ngoc, N.V.; Thi, T.T.; Prat, D. Enrichment planting of native species for biodiversity conservation in a logged tree plantation in Vietnam. New For. 2012, 44, 369–383. [Google Scholar] [CrossRef]
- Yeong, K.L.; Reynolds, G.; Hill, J.K.; Loong, Y.K. Enrichment planting to improve habitat quality and conservation value of tropical rainforest fragments. Biodivers. Conserv. 2016, 25, 957–973. [Google Scholar] [CrossRef]
- Ruslandi, R.; Romero, C.; Putz, F. Financial viability and carbon payment potential of large-scale silvicultural intensification in logged dipterocarp forests in Indonesia. For. Policy Econ. 2017, 85, 95–102. [Google Scholar] [CrossRef]
- Pariona, W.; Fredericksen, T.S.; Licona, J. Natural regeneration and liberation of timber species in logging gaps in two Bolivian tropical forests. For. Ecol. Manag. 2003, 181, 313–322. [Google Scholar] [CrossRef]
- Schwartz, G.; Lopes, J.C.; Mohren, F.; Peña-Claros, M. Post-harvesting silvicultural treatments in logging gaps: A comparison between enrichment planting and tending of natural regeneration. For. Ecol. Manag. 2013, 293, 57–64. [Google Scholar] [CrossRef]
- Hartshorn, G.S. Application of Gap Theory to Tropical Forest Management: Natural Regeneration on Strip Clear-cuts in the Peruvian Amazon. Ecology 1989, 70, 567–576. [Google Scholar] [CrossRef]
- Peña-Claros, M.; Boot, R.G.; Dorado-Lora, J.; Zonta, A. Enrichment planting of Bertholletia excelsa in secondary forest in the Bolivian Amazon: Effect of cutting line width on survival, growth and crown traits. For. Ecol. Manag. 2002, 161, 159–168. [Google Scholar] [CrossRef]
- Forbes, A.S.; Norton, D.A.; Carswell, F.E. Underplanting degraded exotic Pinus with indigenous conifers assists forest restoration. Ecol. Manag. Restor. 2015, 16, 41–49. [Google Scholar] [CrossRef]
- Montagnini, F.; Eibl, B.; Grance, L.; Maiocco, D.; Nozzi, D. Enrichment planting in overexploited subtropical forests of the Paranaense region of Misiones, Argentina. For. Ecol. Manag. 1997, 99, 237–246. [Google Scholar] [CrossRef]
- Ådjers, G.; Hadengganan, S.; Kuusipalo, J.; Nuryanto, K.; Vesa, L. Enrichment planting of dipterocarps in logged-over secondary forests: Effect of width, direction and maintenance method of planting line on selected Shorea species. For. Ecol. Manag. 1995, 73, 259–270. [Google Scholar] [CrossRef]
- Alvarez-Aquino, C.; Williams-Linera, G.; Newton, A. Experimental Native Tree Seedling Establishment for the Restoration of a Mexican Cloud Forest. Restor. Ecol. 2004, 12, 412–418. [Google Scholar] [CrossRef]
- D’Oliveira, M.V. Artificial regeneration in gaps and skidding trails after mechanised forest exploitation in Acre, Brazil. For. Ecol. Manag. 2000, 127, 67–76. [Google Scholar] [CrossRef]
- Phongoudome, C.; Sawathvong, S.; Woo, S.-Y.; Ho, W.M.; Park, Y.-D. Effects of Enrichment Planting with Five Native Species and Different Plantation Treatments on Seedling Growth Characteristics at Logged-over Forest in Lao PDR. Korean J. Agric. For. Meteorol. 2012, 14, 45–52. [Google Scholar] [CrossRef][Green Version]
- Vanclay, J. Lessons from the Queensland Rainforests. J. Sustain. For. 1996, 3, 1–27. [Google Scholar] [CrossRef]
- Keys, M.; Nicholson, D. Underplanting of silviculturally treated rainforest in north Queensland. Qld Dep. For. 1982, 7, 23. [Google Scholar]
- Sheil, D.; May, R.M. Mortality and recruitment rate evaluation in heterogeneous ropical forests. J. Ecol. 1996, 81, 91–100. [Google Scholar] [CrossRef]
- Bristow, M.; Erskine, P.D.; McNamara, S.; Annandale, M. Species performance and site relationships for rainforest timber species in plantations in the humid tropics of Queensland. In Reforestation in the Tropics and Subtropics of Australia: Using Rainforest Tree Species; Erskine, P.D., Lamb, D., Bristow, M., Eds.; Rainforest CRC: Cairns, Australia, 2005; pp. 84–100. [Google Scholar]
- Girona, M.M.; Rossi, S.; Lussier, J.-M.; Walsh, D.; Morin, H. Understanding tree growth responses after partial cuttings: A new approach. PLoS ONE 2017, 12, e0172653. [Google Scholar]
- Gerhardt, K. Leaf defoliation of tropical dry forest tree seedlings–implications for survival and growth. Trees 1998, 13, 88–95. [Google Scholar] [CrossRef]
- Engelbrecht, B.M.J.; Kursar, T.A.; Tyree, M.T. Drought effects on seedling survival in a tropical moist forest. Trees 2005, 19, 312–321. [Google Scholar] [CrossRef]
- Agyeman, V.K.; Swaine, M.D.; Thompson, J. Responses of tropical forest tree seedlings to irradiance and the derivation of a light response index. J. Ecol. 1999, 87, 815–827. [Google Scholar] [CrossRef]
- Osunkoya, O.; Ash, J.E.; Hopkins, M.S.; Graham, A.W. Factors affecting survival of tree seedlings in North Queensland rainforests. Oecologia 1992, 91, 569–578. [Google Scholar] [CrossRef]
- Thompson, W.; Stocker, G.; Kriedemann, P. Growth and Photosynthetic Response to Light and Nutrients of Flindersia brayleyana F. Muell., A Rainforest Tree With Broad Tolerance to Sun and Shade. Funct. Plant Boil. 1988, 15, 299–315. [Google Scholar] [CrossRef]
- Goosem, S.P.; Tucker, N.I. Repairing the Rainforest; Wet Tropics Management Authority: Cairns City QlD 4870, Australia, 2013.
- Enright, N.J. The ecology of Araucaria species in New Guinea. II. Pattern in the distribution of young and mature individuals and light requirements of seedlings. Austr. Ecol. 1982, 7, 39–48. [Google Scholar] [CrossRef]
- Florence, R. Ecology and Silviculture of Eucalypt Forests; CSIRO Publishing: Clayton, VIC, Australia, 2004. [Google Scholar]
- Simpson, J.; Osborne, D. Performance of seven hardwood species underplanted to Pinus elliottii in south-east Queensland. For. Ecol. Manag. 2006, 233, 303–308. [Google Scholar] [CrossRef]
- Ramos, J.; Del Amo, S. Enrichment planting in a tropical secondary forest in Veracruz, Mexico. For. Ecol. Manag. 1992, 54, 289–304. [Google Scholar] [CrossRef]
- Negreros-Castillo, P.; Mize, C. Enrichment planting and the sustainable harvest of mahogany (Swietenia macrophylla King) in Quintana Roo, Mexico. In Big-leaf Mahogany: Genetics, Ecology Management; Lugo, A.E., Alayón, M., Eds.; Springer: New York, NY, USA, 2003; pp. 278–287. [Google Scholar]
- Snook, L.K.; Negreros-Castillo, P. Regenerating mahogany (Swietenia macrophylla King) on clearings in Mexico’s Maya forest: The effects of clearing method and cleaning on seedling survival and growth. For. Ecol. Manag. 2004, 189, 143–160. [Google Scholar] [CrossRef]
- Applegate, G.; Robson, K. Establishment of mixed rainforest species on degraded land-a case study from the coastal lowlands of northeastern Australia. J. Trop. For. Sci. 1994, 7, 8–17. [Google Scholar]
- Bloor, J.M.G.; Grubb, P.J. Growth and mortality in high and low light: Trends among 15 shade-tolerant tropical rain forest tree species. J. Ecol. 2003, 91, 77–85. [Google Scholar] [CrossRef]
- Hawthorne, W. Forest Regeneration after Logging: Findings of a Study in the Bia South Game Production Reserve, Ghana; Overseas Development Administration (ODA): London, UK, 1993. [Google Scholar]
- Opuni-Frimpong, E.; Karnosky, D.; Storer, A.; Cobbinah, J. Silvicultural systems for plantation mahogany in Africa: Influences of canopy shade on tree growth and pest damage. For. Ecol. Manag. 2008, 255, 328–333. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Yang, Z.; Xu, C.; Xie, J.; Chen, G.; Lin, C.; Guo, J.; Liu, X.; Xiong, D.; et al. Large Ecosystem Service Benefits of Assisted Natural Regeneration. J. Geophys. Res. Biogeosci. 2018, 123, 676–687. [Google Scholar] [CrossRef]
- Uebel, K.; A Wilson, K.; Shoo, L.P. Assisted natural regeneration accelerates recovery of highly disturbed rainforest. Ecol. Manag. Restor. 2017, 18, 231–238. [Google Scholar] [CrossRef]
- Peltier, R.; Diowo, S.; Gigaud, M.; Marien, J.-N.; Marquant, B.; Vermeulen, C.; Dubiez, E.; Peroches, A.; Proces, P. Assisted Natural Regeneration in slash-and-burn agriculture: Results in the Democratic Republic of the Congo. Bois Forets Des Trop. 2014, 321, 67. [Google Scholar] [CrossRef]
- De Cauwer, V.; Chaka, M.; Chimwamurombe, P.M.; George, D.; Ham, H.; Heita, H.; Makoi, T.; Mashungwa, G.; Reinhold-Hurek, B.; Tshwenyane, S. Artificial and assisted natural regeneration of socio-economically important southern African tree species. Biodivers. Ecol. 2018, 6, 324–331. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).