Heterogeneity in Decomposition Rates and Nutrient Release in Fine-Root Architecture of Pinus massoniana in the Three Gorges Reservoir Area
Abstract
1. Introduction
2. Materials and Methods
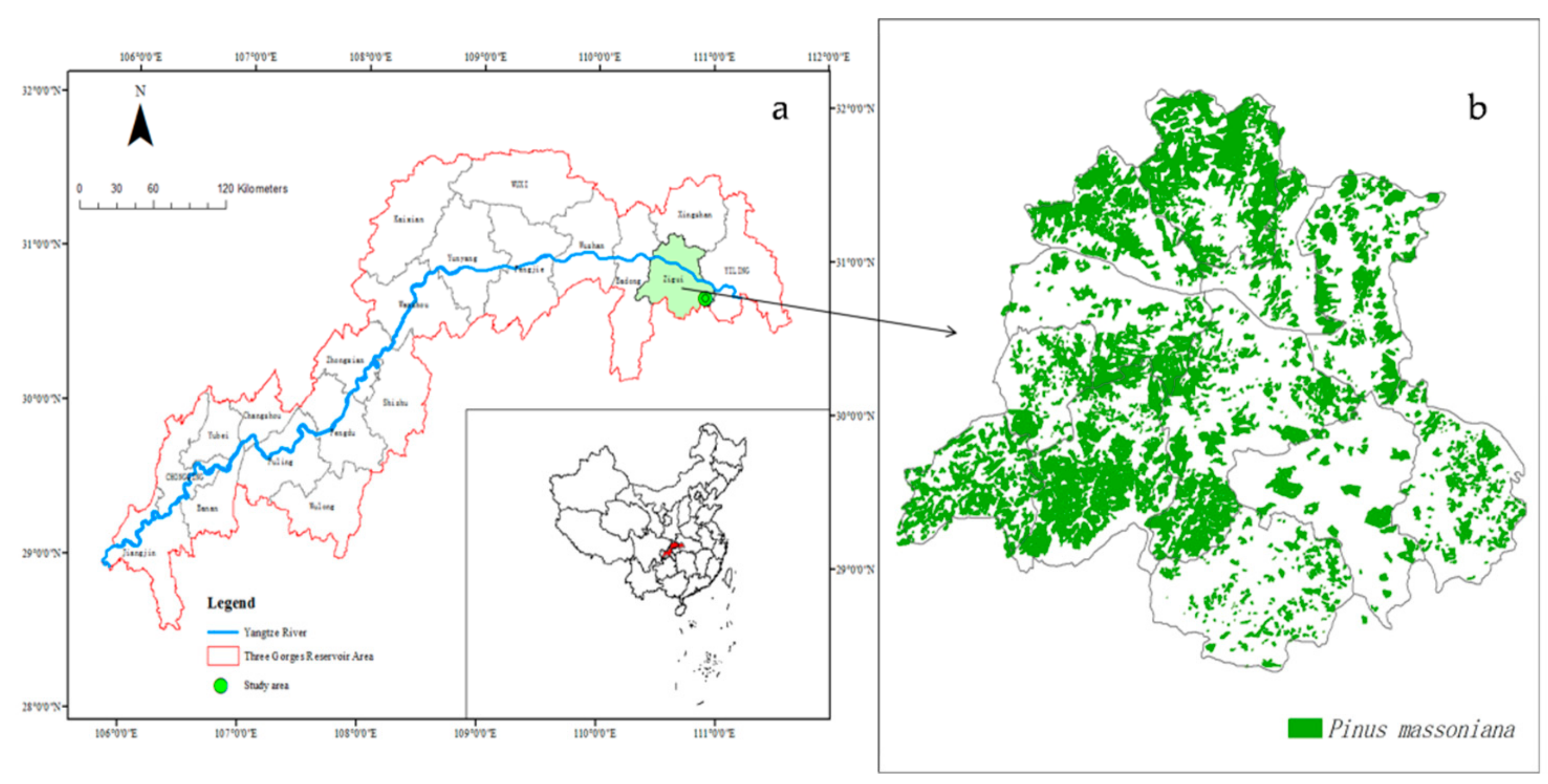
2.1. Site Description
2.2. Decomposition Experiment
2.3. Chemical Analyses
2.4. Statistical Analysis
3. Results
3.1. Initial Fine Roots Chemistry
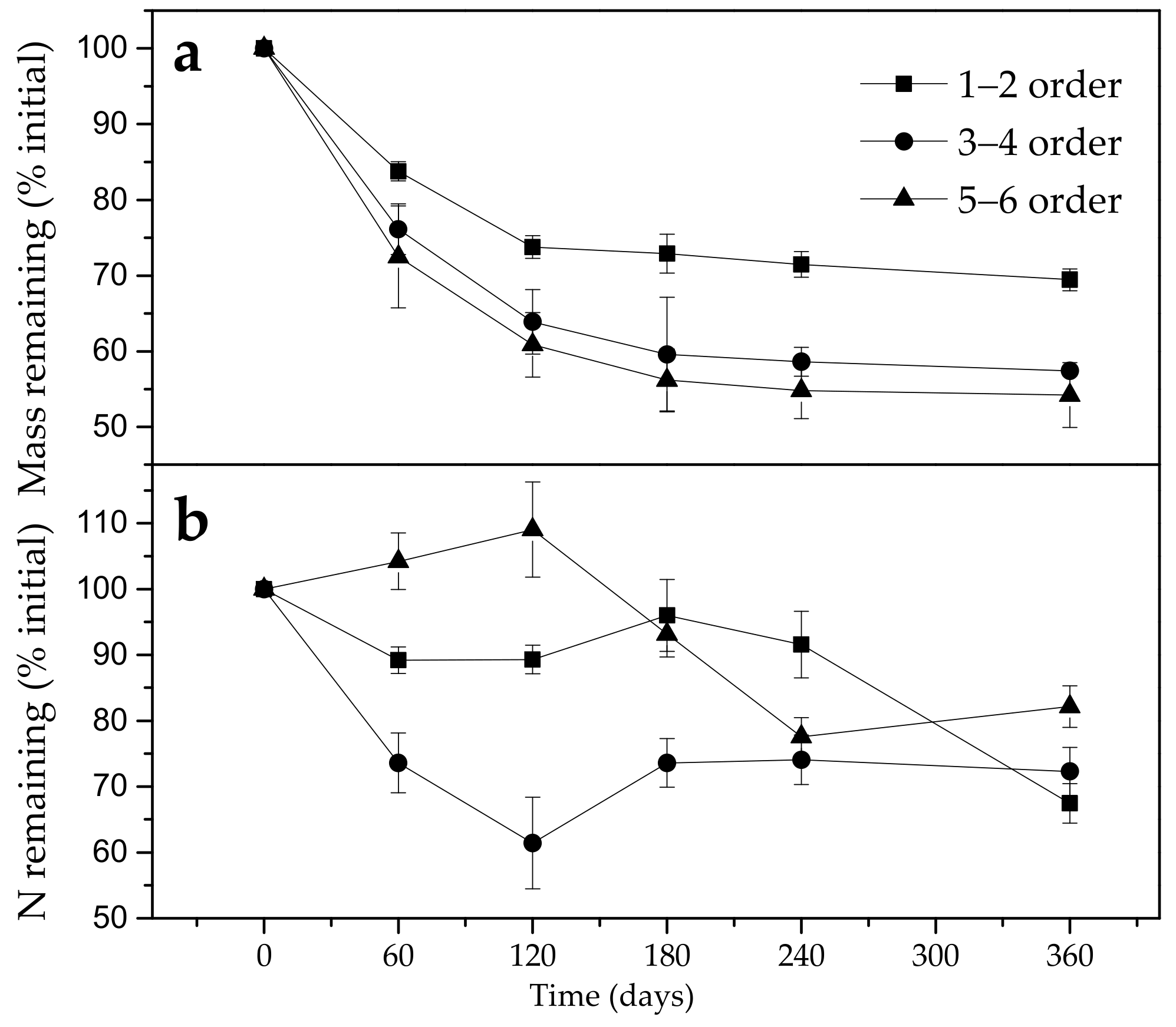
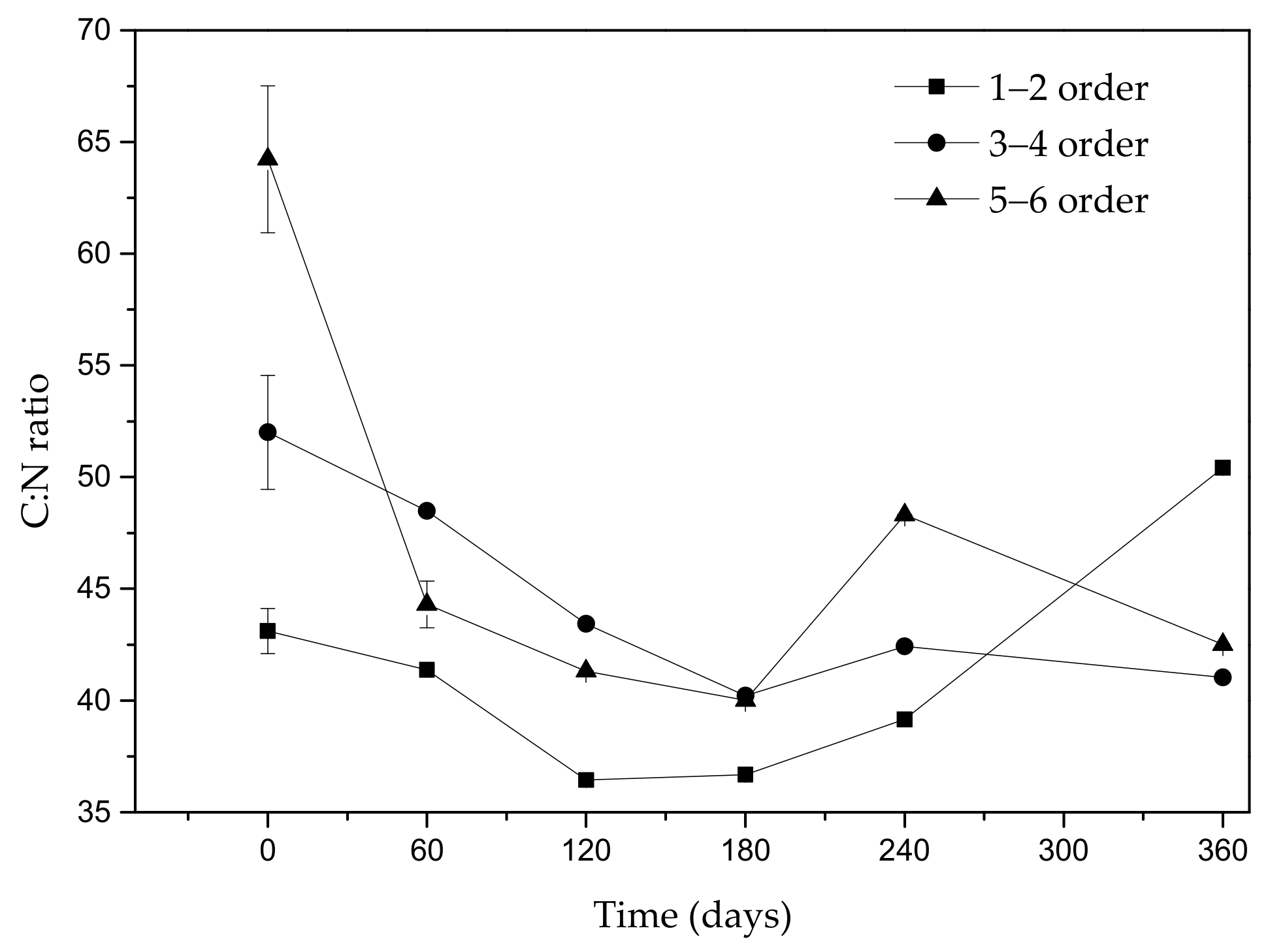
3.2. Effect of Fine Roots Order Class on Decomposition and N Release Pattern
3.3. Linear Relationships between the k-Value and Initial Concentrations of N and AUR
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Mambelli, S.; Bird, J.A.; Gleixner, G.; Dawson, T.E.; Torn, M.S. Relative contribution of foliar and fine root pine litter to the molecular composition of soil organic matter after in situ degradation. Org. Geochem. 2007, 42, 1099–1108. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornwell, W.K.; Wardle, D.A.; Elumeeva, T.G.; Liu, W.; Jackson, B.G.; Onipchenko, V.G.; Soudzilovskaia, N.A.; Tao, J.; Cornelissen, J.H.C. Linking litter decomposition of above and belowground organs to plant-soil feedbacks worldwide. J. Ecol. 2013, 101, 943–952. [Google Scholar] [CrossRef]
- Smith, A.R.; Lukac, M.; Bambrick, M.; Miglietta, F.; Godbold, D.L. Tree species diversity interacts with elevated CO2 to induce a greater root system response. Glob. Chang. Biol. 2012, 19, 217–228. [Google Scholar] [CrossRef]
- Beidler, K.V.; Pritchard, S.G. Maintaining connectivity: Understanding the role of root order and mycelial networks in fine root decomposition of woody plants. Plant Soil 2017, 420, 19–36. [Google Scholar] [CrossRef]
- Burton, A.J.; Jarvey, J.C.; Jarvi, M.P.; Zak, D.R.; Pregitzer, K.S. Chronic N deposition alters root respiration–tissue N relationship in northern hardwood forests. Glob. Chang. Biol. 2012, 18, 258–266. [Google Scholar] [CrossRef]
- Joslin, J.D.; Gaudinski, J.B.; Torn, M.S.; Riley, W.J.; Hanson, P.J. Fine-root turnover patterns and their relationship to root diameter and soil depth in a 14C-labeled hardwood forest. New Phytol. 2006, 172, 523–535. [Google Scholar] [CrossRef]
- Ouimette, A.; Guo, D.; Hobbie, E.; Gu, J. Insights into root growth, function, and mycorrhizal abundance from chemical and isotopic data across root orders. Plant Soil 2013, 367, 313–326. [Google Scholar] [CrossRef]
- Park, B.B.; Yanai, R.D.; Fahey, T.J.; Bailey, S.W.; Siccama, T.G.; Shanley, J.B.; Cleavitt, N.L. Fine root dynamics and forest production across a calcium gradient in northern hardwood and conifer ecosystems. Ecosystems 2008, 11, 325–341. [Google Scholar] [CrossRef]
- Pregitzer, K.S.; Laskowski, M.J.; Burton, A.J.; Lessard, V.C.; Zak, D.R. Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol. 1998, 18, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S.; Deforest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine north American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Rewald, B.; Rechenmacher, A.; Godbold, D.L. It’s complicated-intra-root system variability of respiration and morphological traits in four deciduous tree species. Plant Physiol. 2014, 166, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Mao, Z. Functional relationships between morphology and respiration of fine roots in two Chinese temperate tree species. Plant Soil 2011, 346, 375–384. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Wells, C.E.; Yanai, R.D.; Whitbeck, J.L. Building roots in a changing environment: Implications for root longevity. New Phytol. 2000, 147, 33–42. [Google Scholar] [CrossRef]
- Guo, D.L.; Mitchell, R.J.; Hendricks, J.J. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 2004, 140, 450–457. [Google Scholar] [CrossRef]
- Guo, D.L.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Mccormack, M.L.; Adams, T.S.; Smithwick, E.A.H.; Eissenstat, D.M. Predicting fine root lifespan from plant functional traits in temperate trees. New Phytol. 2012, 195, 823–831. [Google Scholar] [CrossRef]
- Valenzuela-Estrada, L.R.; Vera-Caraballo, V.; Ruth, L.E.; Eissenstat, D.M. Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae). Am. J. Bot. 2008, 95, 1506–1514. [Google Scholar] [CrossRef]
- Valenzuela-Estrada, L.R.; Richards, J.H.; Andres, D.; Eissensat, D.M. Patterns of nocturnal rehydration in root tissues of Vaccinium corymbosum L. under severe drought conditions. J. Exp. Bot. 2009, 60, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.E.; Eissenstat, D.M. Marked differences in survivorship among apple roots of different diameters. Ecology 2001, 82, 882–893. [Google Scholar] [CrossRef]
- Du, X.; Wei, X. Definition of fine roots on the basis of the root anatomy, diameter and branch orders of one-year old Fraxinus mandshurica seedlings. J. For. Res. 2018, 29, 1321–1327. [Google Scholar] [CrossRef]
- Jia, S.; McLaughlin, N.B.; Gu, J.; Li, X.; Wang, Z. Relationships between root respiration rate and root morphology, chemistry and anatomy in Larix gmelinii and Fraxinus mandshurica. Tree Physiol. 2013, 33, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Guo, D.; Pregitzer, K.S. Ephemeral root modules in fraxinus mandshurica. New Phytol. 2010, 188, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.S.; McCormack, M.L.; Eissenstat, D.M. Foraging strategies in trees of different root morphology: The role of root lifespan. Tree Physiol. 2013, 33, 940–948. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Lin, G.; Zeng, D.H. Heterogeneity in decomposition rates and annual litter inputs within fine-root architecture of tree species: Implications for forest soil carbon accumulation. For. Ecol. Manag. 2017, 389, 386–394. [Google Scholar] [CrossRef]
- Fan, P.; Guo, D. Slow decomposition of lower order roots: A key mechanism of root carbon and nutrient retention in the soil. Oecologia 2010, 163, 509–515. [Google Scholar] [CrossRef]
- Goebel, M.; Hobbie, S.E.; Bulaj, B.; Zadworny, M.; Archibald, D.D.; Oleksyn, J.; Reich, P.B.; Eissenstat, D.M. Decomposition of the finest root branching orders: Linking belowground dynamics to fine-root function and structure. Ecol. Monogr. 2011, 81, 89–102. [Google Scholar] [CrossRef]
- Sun, T.; Dong, L.L.; Zhang, L.L.; Wu, Z.J.; Wang, Q.K.; Li, Y.Y.; Zhang, H.G.; Wang, Z.W. Early stage fine-root decomposition and its relationship with root order and soil depth in a Larix gmelinii plantation. Forests 2016, 7, 234. [Google Scholar] [CrossRef]
- Xiong, Y.; Fan, P.; Fu, S.; Zeng, H.; Guo, D.L. Slow decomposition and limited nitrogen release by lower order roots in eight Chinese temperate and subtropical trees. Plant Soil 2013, 363, 19–31. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Y.; Fu, X.; Xu, M.; Dai, X.; Wang, H.M. Thinning effect on understory community and photosynthetic characteristics in a subtropical Pinus massoniana plantation. Can. J. For. Res. 2017, 47, 1104–1115. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.G.; Rossi, S.; Ma, Q.; Yu, B.; Zhai, L.; Luo, D.; Guo, X.; Fu, S.; Zhang, W. Intra-annual dynamics of xylem growth in Pinus massoniana submitted to an experimental nitrogen addition in Central China. Tree Physiol. 2017, 37, 1546–1553. [Google Scholar] [CrossRef]
- Piao, S.L.; Fang, J.Y.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef]
- Lin, C.; Yang, Y.; Guo, J.; Chen, G.; Xie, J. Fine root decomposition of evergreen broadleaved and coniferous tree species in mid-subtropical china: Dynamics of dry mass, nutrient and organic fractions. Plant Soil 2011, 338, 311–327. [Google Scholar] [CrossRef]
- Shen, Y.F.; Wang, N.; Cheng, R.M.; Xiao, W.; Yang, S.; Guo, Y. Characteristics of fine roots of Pinus massoniana in the Three Gorges Reservoir Area, China. Forests 2017, 8, 183. [Google Scholar] [CrossRef]
- Berg, B.; Mcclaugherty, C. Plant Litter-Decomposition, Humus Formation, Carbon Sequestration; Springer: Berlin, Germany, 2003. [Google Scholar] [CrossRef]
- Talbot, J.M.; Treseder, K.K. Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 2012, 93, 345–354. [Google Scholar] [CrossRef]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The global stoichiometry of litter nitrogen mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef]
- Gong, Z.T. Chinese Soil Taxonomy; China Science Press: Beijing, China, 2003. [Google Scholar] [CrossRef]
- Wang, R.; Cheng, R.; Xiao, W.; Feng, X.; Liu, Z.; Wang, X. Influencing factors of fine root production and turnover in forest ecosystem. World For. Res. 2012, 25, 19–24, (In Chinese with English Abstract). [Google Scholar]
- Mcclaugherty, C.A.; Aber, J.D.; Melillo, J.M. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 1982, 63, 1481–1490. [Google Scholar] [CrossRef]
- Ryan, M.G.; Melillo, J.M.; Ricca, A. A comparison of methods for determining proximate carbon fractions of forest litter. Can. J. For. Res. 1990, 20, 166–171. [Google Scholar] [CrossRef]
- Hendricks, J.J.; Aber, J.D.; Nadelhoffer, K.J.; Hallett, R.D. Nitrogen controls on fine root substrate quality in temperate forest ecosystems. Ecosystems 2000, 3, 57–69. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Wieder, R.K.; Lang, G.E. A critique of the analytical method used in examining decomposition data obtained from litter bags. Ecology 1982, 63, 1636–1642. [Google Scholar] [CrossRef]
- Fan, P.; Jiang, Y. Nitrogen dynamics differed among the first six root branch orders of Fraxinus mandshurica and Larix gmelinii during short-term decomposition. J. Plant Res. 2010, 123, 433–438. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.L. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Copley, J. Ecology goes underground. Nature 2000, 406, 452–454. [Google Scholar] [CrossRef]
- Morgan, J.A. Looking beneath the Surface. Science 2002, 298, 1903–1904. [Google Scholar] [CrossRef]
- Yang, Y.S.; Chen, G.S.; Guo, J.F.; Lin, P. Decomposition dynamic of fine roots in a mixed forest of Cunninghamia lanceolata and Tsoongiodendron odorum in mid-subtropics. Ann. For. Sci. 2004, 61, 65–72. [Google Scholar] [CrossRef]
- Sun, T.; Mao, Z.; Han, Y. Slow decomposition of very fine roots and some factors controlling the process: A 4-year experiment in four temperate tree species. Plant Soil 2013, 372, 445–458. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Murray, P.; Johnson, S.; Murray, P.J. Root herbivory in grassland ecosystems. Papers Presented at the Workshop: Integrative Approaches for the Investigation of Root Herbivory in Agricultural and Natural Systems, Berkshire, UK, 17 September 2008. [Google Scholar] [CrossRef]
- Silver, W.L. Global Patterns in Root Decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef] [PubMed]

| Order Class | Initial Root Nutrients (mg Element/g Root) | Ratios | Initial C Fraction (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | C:N | AUR | AHR | Extractives | |
| 1–2 | 10.22 ± 0.26 a | 0.52 ± 0.01 a | 2.25 ± 0.07 a | 5.95 ± 0.05 a | 1.43 ± 0.08 a | 43.11 ± 1.01 a | 45.27 ± 2.71 a | 22.43 ± 1.51 a | 32.30 ± 3.01 a |
| 3–4 | 8.55 ± 0.41 b | 0.43 ± 0.03 b | 1.84 ± 0.05 b | 5.23 ± 0.34 b | 1.21 ± 0.06 b | 52.00 ± 2.55 b | 38.13 ± 1.25 ab | 29.17 ± 1.33 ab | 32.70 ± 2.35 a |
| 5–6 | 7.08 ± 0.34 c | 0.35 ± 0.01 c | 1.38 ± 0.03 c | 3.97 ± 0.36 c | 1.05 ± 0.06 c | 64.22 ± 3.29 c | 34.63 ± 1.90 b | 32.30 ± 2.41 b | 33.07 ± 3.73 a |
| Root Class | k (Year−1) | R2 | p |
|---|---|---|---|
| Order 1–2 | 0.37 a | 0.80 | <0.05 |
| Order 3–4 | 0.56 b | 0.77 | <0.05 |
| Order 5–6 | 0.62 b | 0.75 | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Cheng, R.; Xiao, W.; Shen, Y.; Wang, L.; Guo, Y.; Sun, P. Heterogeneity in Decomposition Rates and Nutrient Release in Fine-Root Architecture of Pinus massoniana in the Three Gorges Reservoir Area. Forests 2020, 11, 14. https://doi.org/10.3390/f11010014
Yang S, Cheng R, Xiao W, Shen Y, Wang L, Guo Y, Sun P. Heterogeneity in Decomposition Rates and Nutrient Release in Fine-Root Architecture of Pinus massoniana in the Three Gorges Reservoir Area. Forests. 2020; 11(1):14. https://doi.org/10.3390/f11010014
Chicago/Turabian StyleYang, Shao, Ruimei Cheng, Wenfa Xiao, Yafei Shen, Lijun Wang, Yan Guo, and Pengfei Sun. 2020. "Heterogeneity in Decomposition Rates and Nutrient Release in Fine-Root Architecture of Pinus massoniana in the Three Gorges Reservoir Area" Forests 11, no. 1: 14. https://doi.org/10.3390/f11010014
APA StyleYang, S., Cheng, R., Xiao, W., Shen, Y., Wang, L., Guo, Y., & Sun, P. (2020). Heterogeneity in Decomposition Rates and Nutrient Release in Fine-Root Architecture of Pinus massoniana in the Three Gorges Reservoir Area. Forests, 11(1), 14. https://doi.org/10.3390/f11010014




