Abstract
This study investigates how Iyengar yoga postures influence autonomic nervous system (ANS) activity by analyzing multimodal physiological signals collected via wearable sensors. The goal was to explore whether subtle postural variations elicit measurable autonomic responses and to identify which sensor features most effectively capture these changes. Participants performed a sequence of yoga poses while wearing synchronized sensors measuring electrodermal activity (EDA), heart rate variability, skin temperature, and motion. Interpretable machine learning models, including linear classifiers, were trained to distinguish physiological states and rank feature relevance. The results revealed that even minor postural adjustments led to significant shifts in ANS markers, with phasic EDA and RR interval features showing heightened sensitivity. Surprisingly, micro-movements captured via accelerometry and transient electrodermal reactivity, specifically EDA peak-to-RMS ratios, emerged as dominant contributors to classification performance. These findings suggest that small-scale kinematic and autonomic shifts, which are often overlooked, play a central role in the physiological effects of yoga. The study demonstrates that wearable sensor analytics can decode a more nuanced and granular physiological profile of mind–body practices than traditionally appreciated, offering a foundation for precision-tailored biofeedback systems and advancing objective approaches to yoga-based interventions.
1. Introduction
The autonomic nervous system (ANS) governs involuntary physiological processes through its sympathetic and parasympathetic branches, which respectively mediate arousal (“fight or flight”) and recovery (“rest and digest”) responses to maintain homeostatic balance []. Increasing parasympathetic autonomic activity is associated with numerous health benefits, including improved cardiovascular health [] and anti-inflammatory effects [], which are particularly valuable for managing chronic conditions. Chronic stress, cardiovascular risks, hypertension, and other ill health conditions are associated with sympathetic activity dominance or dysregulation [,]. Among the various yoga styles, Iyengar yoga [] is uniquely suited for improving parasympathetic activity of the autonomic nervous system (ANS) due to its focus on precise body alignment while performing the posture (asana), targeted engagement of muscle groups, and sustained postural engagement. In Iyengar yoga, props like blocks, blankets, and straps are used to assist with alignment, deepen poses, allowing participants to spend more time in the asana, which often leads to the yoga only being performed under the guidance of certified instructors. This limits the extensive use of such therapeutic yoga for individuals on their own, which, in turn, decreases the possibility of gathering data in such settings. This study is one such setting where data was collected with the agreement of both the yoga practitioner and participants. The analysis of physiological mechanisms of Iyengar yoga, a relatively data-scarce area, was carried out in this study using robust and interpretable machine learning models. To that end, the study was guided by the following research questions:
- Can machine learning models distinguish between different physiological states (relaxed vs. parasympathetic-inducing/sympathetic poses) based on features derived from physiological signals captured by the wearable device?
- Can the models differentiate between sympathetic and parasympathetic autonomic responses based on pose-induced physiological changes?
- What features contribute strongly to this distinction? Are there any physiological patterns specific to each autonomic response?
2. Related Work
Yoga therapy is known to improve cardiac autonomic tone and vagal modulation [], and the practice of yoga therapy and breath work (pranayama) influences autonomic nervous system (ANS) function by enhancing parasympathetic activity and reducing sympathetic activation []. Preliminary studies, such as [], have reported improvements in ambulation, mental health, and pain management, while others, such as [,], have highlighted its potential in addressing clinical depression and stress-related symptoms. Iyengar yoga’s practice of holding poses for extended durations (5–10 min), as recommended by [], facilitates the release of the myotatic reflex, increasing muscular range of motion and potentially eliciting distinct physiological responses.
However, these studies are limited to specific populations or conditions, and there is a need for more rigorous longitudinal research to better understand the physiological mechanisms underlying these benefits. To address this gap, a multi-case longitudinal study design is proposed through this study to investigate the physiological effects of Iyengar yoga at the pose level. Building on prior research, such as [], which demonstrated improvements in acute physiological measures like arterial blood pressure and heart rate during 90 min yoga sessions, these measures were analyzed in this study at the pose-level. While the previous studies employed survey-based [,] and Holter ECG monitoring-based [] data collection, EmbracePlus, a wearable smart watch, was employed in this study to record physiological data and ANS activity was measured using skin temperature, electrodermal activity (EDA), heart-rate variability, and other vitals, to assess sympathetic and parasympathetic responses. Prior studies [] used statistical analyses and report-based methods, where as, machine learning models were implemented in this study to advance the understanding of differences in physiological responses at pose-level, lasting an average of 5–10 min, as opposed to entire 90 min sessions in previous studies [].
3. Materials and Methods
3.1. Experimental Design
The EmbracePlus smartwatch (Empatica, Oakland, CA, USA), a wearable device equipped with multisensor capabilities, was used to continuously capture high-frequency physiological data from participants before and during the yoga sessions. The EmbracePlus device was configured to record raw data from a range of biosensors, including body-movement signals, blood volume pulse (BVP), electrodermal activity (EDA), skin temperature, and heart rate variability signals. Data were recorded under two primary conditions: a baseline phase, during which participants remained at their normal and restful state for approximately 5 minutes prior to the yoga session, and a postural phase, in which participants engaged in 1–2 specific Iyengar yoga poses altogether for an average duration of 11.75 min. The choice of yoga poses for each participant was made at the discretion of a certified yoga practitioner, Dr. Anuradha Oak, based on the participant’s physical condition and needs, with the goal of eliciting either sympathetic or parasympathetic autonomic responses. Poses aimed at eliciting sympathetic activation, as shown in Figure 1a, involved greater muscular engagement or sustained effort, such as holding challenging positions for extended periods, thus promoting alertness and physiological arousal. In contrast, poses designed to elicit parasympathetic activation were generally passive and restorative, as shown in Figure 1b, requiring minimal muscular effort and allowing the participant to settle into the posture over time. Example poses/asanas are as in Table 1. Each pose was annotated with its intended autonomic effect (see Table 2) according to the expert assessment of the certified practitioner, rather than being grounded in direct physiological measurement. While this approach carries inherent subjectivity, it is consistent with other biomedical studies, such as [], where expert interpretation serves as a reference standard in the absence of direct ground-truth measures. In addition to that, a subjective score of the perceived difficulty level by the participant, on a scale out of 5 was recorded using a post yoga session survey, with 50% of the participants reporting a difficulty level of 4 and 5. A chi-square test of independence was conducted to examine the association between autonomic response (sympathetic vs. parasympathetic) and participant’s perceived difficulty level (rated 1 to 5) during postural engagement. The association was statistically significant, , (N = 202, accounting for the postural samples only), indicating that the difficulty level varied depending on the physiological state. The effect size, measured by Cramér’s V, was 0.475, suggesting a moderate to strong association between autonomic state and perceived difficulty, implying that participants’ autonomic responses are meaningfully related to their reported difficulty during yoga postures.

Figure 1.
Example poses for eliciting (a) sympathetic and (b) parasympathetic autonomic responses.

Table 1.
Asanas (poses) selected by the certified practitioner for the subjects.

Table 2.
Definitions of baseline, parasympathetic, and sympathetic conditions used in the study.
Overall, eleven wearable sensor signals were collected using the sensors of EmbracePlus, as shown in Table 3, to analyze physiological responses. Sessions were tagged in EmbracePlus at respective intervals for further analysis, i.e, the start and end of baseline and postural phases, respectively. The tagged activities were also cross-verified using the manual entry of timestamps recorded during the sessions. Each session lasted for approximately 20 min combining the baseline and postural phases. The baseline phase ranged from 2 to 10 min with an average of 3 min, while postural phase ranged from 10 to 20 min with an average of 11.75 min. An imbalance in the distribution of the sampled data was observed due to the unequal durations of the baseline and postural phases and our one-minute sampling interval, as seen in Figure 2, to predict the physiological states. An analysis of these physiological states based on differences in physiological features between the baseline and postural phases was performed using machine learning models such as logistic regression, Gaussian Naive Bayes, Decision Tree, Random Forest, and Support Vector Machines.

Table 3.
Summary of sensor types and the physiological metrics recorded.
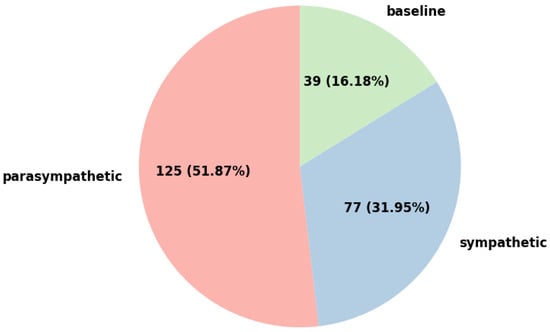
Figure 2.
Distribution of subjects across baseline, sympathetic, and parasympathetic groups.
Using a convenience sampling approach, based on the availability, different subjects were recruited for the study. The risk of complications included subjects with a low risk (general fitness), moderate risk (musculoskeletal disorders), and high risk (chronic cardio-endocrine-musculoskeletal disorders). A total of 16 subjects were studied, with 10 female and 6 male subjects, aged between 30 and 65 years. The majority, 75%, reported having 0–1 years of experience with yoga, with a notable proportion (62.5%) falling within the 50–60-year age group. Targeted yoga poses were recommended to subjects based on their profiles. For example, subjects who lacked strength and tone of muscles to even hold the poses were recommended poses that elicit sympathetic response to build and improve the tone of muscles. An assessment of their engagement and postural alignment in a particular yoga pose was conducted using the judgment of the certified yoga practitioner. In total, 93.75% of subjects were observed to be engaged as required during the yoga poses, as per the therapist’s assessment.
Data were collected using EmbracePlus at a frequency of 64 Hz, leaving us with sixty-four data points per second. Especially in a therapeutic yoga setting like Iyengar yoga, unlike strenuous activities such as running or any vinyasa practice, the physiological response does not vary drastically in a short period of time; therefore, the data were downsampled to one data point per minute. For example, the continuous data from the 5 min baseline phase, with data being recorded 3840 times a minute, was reduced to 1 time per minute, thus giving five data points for the baseline phase. This was carried out by averaging the 64 data points per sec to 1 data point per sec and 60 such data points (or less for few samples) per minute were averaged down to 1 data point per minute. With this approach, for the baseline and postural phases of 16 participants, a total of 247 min of data were obtained, i.e., 247 data points. Given this experimental design, wherein each participant contributed data from only two distinct sessions (baseline and postural) across 16 individuals, yielding 32 sessions in total, there was no complex nested repeated measures structure in the dataset. Therefore, traditional ANOVA and MANOVA approaches, with appropriate corrections such as Tukey’s Honest Significant Difference (HSD) test [] for multiple comparisons, were considered valid for the current scope of the study.
3.2. Feature Extraction
To transform the raw sensor signals listed in Table 3 to a more suitable data representation format, feature extraction was applied on the raw signals. Within the current Iyengar style of yoga therapy, the poses focus mainly on the alignment of the musculoskeletal system in a particular pose and holding that pose for a certain period. Consequently, the steps count and three-axis gyroscope signals were not considered for further analysis in this study. From the three-axis accelerometer signals, the accelerometer magnitude feature was extracted and the RR interval was extracted from systolic peaks. For the five signals, (extracted accelerometer magnitude, blood volume pulse, electrodermal activity, skin temperature, extracted RR interval), eight features for each of the signals were extracted. Table 4 provides a detailed description of all the features. The feature extraction yielded up to a total of 40 (5 × 8) features. These extracted features represented the properties of the original raw signals while reducing the volume of data. To reduce the redundant information, all features that correlated positively or negatively with other features at a level of at least 0.8 were eliminated, and 26 features were left in the feature set.

Table 4.
Summary of computed features and their mathematical descriptions.
Embedded feature selection methods that perform feature selection in the process of training, such as L1-Regularized Logistic Regression (LASSO), Random Forest (RF) feature importance, Decision Tree (DT) feature importance, Gaussian Naive Bayes individual feature probabilities, Support Vector Machine (SVM) coefficient magnitudes, were used. LASSO applies an L1 penalty, which enforces sparsity by shrinking some coefficients to zero, effectively eliminating less relevant features. RF feature importance was computed based on the mean decrease in impurity (Gini index), aggregating information across multiple decision trees to provide a stable ranking of feature relevance. Additionally, DT feature importance, derived from a single decision tree, measures the contribution of each feature to reducing node impurity. While DT feature importance may be more sensitive to data variations compared to RF, it provides an interpretable, tree-structured assessment of feature relevance. Gaussian Naive Bayes (GNB) evaluates feature importance based on the probability distributions of each feature given the class label. Since GNB assumes conditional independence among features, it assigns importance by analyzing how strongly individual features contribute to class separation based on their likelihood estimates. This method is particularly useful for identifying features with distinct distributions across different classes, although it may not fully capture complex feature interactions. Linear Support Vector Machine (SVM) models assess feature relevance based on the absolute magnitude of learned weights, with larger weight magnitudes indicating greater influence on the classification decision boundary.
To systematically identify the most informative features while mitigating redundancy and overfitting, all embedded feature selection methods were evaluated under a stratified 10-fold cross-validation framework. This approach preserves class balance within each fold and provides a population-level assessment of physiological state separability, highlighting differences across the baseline, sympathetic, and parasympathetic phases irrespective of individual variation. To complement this holistic view, Leave-One-Subject-Out Cross-Validation (LOGO-CV) [] was additionally employed, which offers a stricter estimate of subject-independent generalization by ensuring that no individual’s data appear simultaneously in training and testing. In this approach, data from a single participant were held out as the test set, while the remaining participants’ data were used to train the model. This process was repeated iteratively until every subject had served once as the test set, ensuring that no data from the same individual appeared simultaneously in training and testing, i.e, 16-fold CV. Unlike the stratified 10-fold cross-validation that was implemented, LOGO-CV provides a stricter and more realistic estimate of performance in scenarios where models must generalize to entirely new individuals. Performance metrics were computed for each held-out subject, and both the area under the receiver operating characteristic curve (AUC) and the F1-score were reported to capture the overall discriminative ability and class-balanced predictive accuracy, respectively. Final results are presented as mean ± standard deviation across all subjects, and per-subject performance distributions were analyzed to highlight inter-individual variability.
4. Results and Discussion
A multivariate analysis of variance (MANOVA) was conducted to evaluate the overall effect of the physiological state on a set of 40 physiological features. The multivariate test revealed a highly significant effect of the state on the combined physiological profile (Wilks’ Lambda = 0.111, F(80, 398) = 9.98, 0.001), with a large effect size ( = 0.889), indicating that approximately 89% of the variance in physiological responses is attributable to the underlying state. To further investigate which specific features contributed to this effect, follow-up univariate analyses were conducted on each feature. Thirty-two features were found to be significantly different across states at a stringent threshold of 0.01 and power > 0.8. For each of these features, the group means and standard deviations for baseline, parasympathetic, and sympathetic states are reported in Table 5. To identify which physiological states differed significantly on each feature, post hoc comparisons using Tukey’s Honest Significant Difference (HSD) test were performed, which was also leveraged in other studies [].

Table 5.
Univariate ANOVA results with effect sizes and descriptive statistics across physiological states.
These comparisons revealed consistent patterns of autonomic modulation across sensor modalities. Notably, sympathetic states were associated with significantly elevated skin conductance variability (e.g., EDA std dev, EDA peak to peak), increased cardiovascular reactivity (e.g., BVP std dev, RR interval peak to peak), and greater temperature fluctuations (temperature std dev, peak to peak), relative to both baseline and parasympathetic conditions. In contrast, parasympathetic states exhibited increased mean RR intervals and lower accelerometer-derived motion features, consistent with reduced physiological arousal and physical movement. Baseline states typically showed intermediate values but were statistically distinct from the other two states for several features. These univariate results, supported by robust post hoc evidence, provide strong validation that specific physiological features reliably differentiate between autonomic states. The consistent group-wise differences across modalities affirm the potential of multimodal sensor fusion for state classification and underscore the biological plausibility of the derived features in capturing autonomic state dynamics. Based on this, subsequent analyses focused on feature-level importance using supervised machine learning models, aiming to identify core physiological features that are predictive of each physiological state.
In this study, the feasibility and performance of various machine learning models were explored for distinguishing three physiological states: baseline, parasympathetic-dominant, and sympathetic-dominant poses from wearable sensor data in the context of Iyengar yoga. Given that this is the first known study exploring physiological signal classification within the specific practice of Iyengar yoga using machine learning techniques, we prioritized generalizability, interpretability, and robustness in our model selection process. Simpler and computationally efficient models were deliberately chosen to reduce the risk of overfitting in a low-data regime, a common concern when initiating modeling in novel application domains with no prior machine learning baselines. All models were evaluated using 10-fold stratified cross-validation to ensure performance estimates were robust and class proportions preserved across folds. This approach enhanced the reliability of our findings despite limited data availability. Table 6 with comparative model performances reveals that ensemble learning and margin-based approaches are more suited to distinguish physiological states than probabilistic or linear models. The Random Forest model achieved the highest performance across all evaluation metrics, with an accuracy, precision, recall, and F1-score of 0.94 and an AUC of 0.99, which can be attributed to its ability to capture complex, nonlinear relationships and reduce variance through ensembling. In physiological signals, such nonlinearities are common due to individual variability, sensor noise, and the interaction of autonomic responses (e.g., heart rate, respiration, and electrodermal activity often covary under stress).

Table 6.
Stratified 10-fold cross-validation model performances for physiological state prediction.
The SVM model also performed well, with an F1-score of 0.86 and AUC of 0.95, demonstrating strong performance particularly with the use of a linear kernel indicating a strong discrimination capability and balanced precision–recall tradeoff. The Decision Tree, outperforming logistic regression (F1-score = 0.70) and Naive Bayes (F1-score = 0.65), provided decent results (F1-score = 0.80) but was still susceptible to overfitting. These differences highlight the varying capacity of models to handle nonlinear patterns and interdependent physiological features. In contrast, logistic regression’s performance indicated that linear models lack the flexibility to capture interdependencies in physiological signals, despite L1 regularization aiding in sparsity and interpretability. Naive Bayes exhibited the weakest performance, reflecting the impracticality of its core assumption of feature independence. Given the biological correlations among physiological channels (e.g., respiration affecting both BVP and temperature), this assumption is violated, leading to degraded model fidelity. Despite their reduced flexibility, both models showed reasonable performance. Failing to detect a sympathetic state (false negative) may result in missed intervention opportunities, whereas false positives can lead to unnecessary feedback or false alarms. Hence, maximizing both recall and precision is essential, making F1-score a central metric for model selection. These findings suggest that, for real-world deployment in biofeedback systems, models like Random Forest and SVM offer a favorable balance of accuracy, reliability, and interpretability.
4.1. Leave-One-Group-Out (LOGO) Cross-Validation
Leave-One-Subject-Out Cross-Validation (LOGO-CV) revealed substantial heterogeneity in classification performance across participants and models as seen in Table 7. On average, SVM achieved the highest discriminative power with an AUC of 0.89 ± 0.13, closely followed by logistic regression (LR, 0.88 ± 0.15) and Random Forest (RF, 0.82 ± 0.19), while Decision Tree (DT)’s performance was markedly less stable (0.66 ± 0.24). In terms of F1-scores, which better reflect balanced precision and recall, LR (0.62 ± 0.38) and RF (0.60 ± 0.37) provided the strongest results, with SVM (0.57 ± 0.36), DT (0.51 ± 0.37), and Naive Bayes (NB, 0.50 ± 0.36) performing less consistently. Importantly, the large standard deviations across all models highlight pronounced subject-level variability: while some participants (e.g., Subject 4, Subject 6, Subject 9) as in Figure 3, were classified with near-perfect accuracy across models (AUC and F1 ≈ 1.0), others (e.g., Subject 1, Subject 13, Subject 15) consistently approached chance-level outcomes (AUC ≈ 0.5, F1 ≤ 0.1). In several cases, models achieved high AUC but low F1 (e.g., Subject 3, Subject 8, Subject 14), suggesting that class probabilities were ranked correctly but hard decision thresholds failed, likely due to class imbalance or weak physiological separability. These patterns reinforce that model success is not uniform—for some individuals, physiological signals aligned well with the intended sympathetic/parasympathetic labels, while for others, either signal noise, atypical autonomic responses, or limited generalizability. Overall, while population-level metrics suggested strong average discriminative ability, the LOGO-CV analysis underscores the importance of inter-individual variability and points toward the need for personalized calibration or subgroup-specific modeling approaches in future work.

Table 7.
Per-subject model performance: AUC and F1-score.
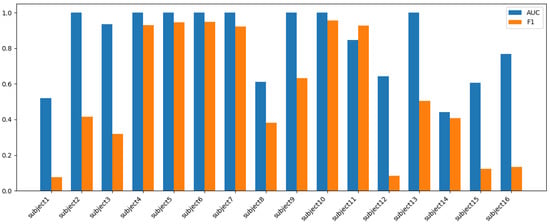
Figure 3.
Random Forest (RF) model’s per-subject AUC vs. F1.
4.2. Building Sparse Models
Given the variability in model performance and inherent differences in learning paradigms, model-specific feature selection and interpretation were performed. Importantly, for building sparse models, we analyzed the feature importances, coefficients, and probabilities from the 10-fold cross-validation metrics and chose top-k, where k was the minimal number of features per model, as shown in Figure 4; thus, when used on a hold-out test set, it gave the best performance when compared with the base models using full feature set on a single train-test split setting, shown in Table 8. This individual top-k features per model approach avoids the risk of overinterpreting features from underperforming models and provides a more grounded understanding of physiological drivers for each classifier. Furthermore, any convergent features across models were noted as candidates for robust domain-level significance, for future investigation.
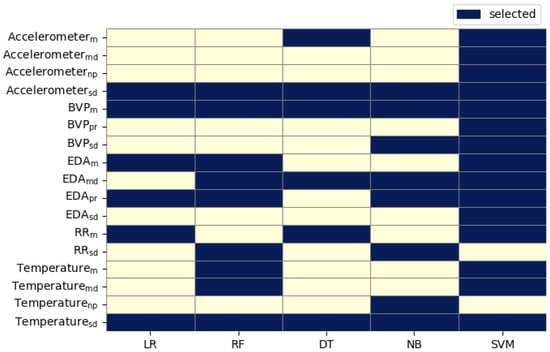
Figure 4.
Top K optimal number of features per model based on the best performance. Feature abbreviations: = Signal Standard Deviation; = Signal Mean; = Signal Mean Derivative; = Signal Number of Peaks; = Signal Peak to RMS.

Table 8.
Comparison of full vs. sparse (top-k) feature models across classifiers.
Several features appeared repeatedly in the top-k sets across models, indicating their robust relevance across different models and that they likely encode the most physiologically salient information for differentiating between relaxed, sympathetic-inducing, and parasympathetic-inducing physiological states. For instance, accelerometer-related features may reflect postural transitions, while BVP-related features relate to cardiovascular activity, which can vary under sympathetic arousal. EDA-related features capture phasic electrodermal responses and temperature-related features may reflect thermoregulatory shifts associated with relaxation or physical effort.
Random Forest emphasized both EDA dynamics (peak_rms, mean_derivative) and cardiorespiratory indicators like rr_std_dev, with its top features balancing well across modalities: EDA, BVP, temperature, RR interval, and accelerometry, indicating an ensemble’s ability to capture inter-modality patterns. Logistic regression’s top weights were assigned to features like eda_mean, peak_rms, and bvp_mean, aligning with known markers of autonomic state. Its reliance on a smaller but informative set of linearly separable features helped construct a compact and effective model. Naive Bayes highlighted more basic statistical descriptors (means, std devs), avoiding derivatives or entropy measures, making sense given NB’s independence assumptions, and confirming it can serve as a lightweight, reasonably performant baseline.
Consistent top features across the five models include acc_std_dev: a strong proxy for physical stillness vs. movement; bvp_mean: reflecting average blood flow changes tied to autonomic tone; eda_peak_rms: capturing phasic EDA response directly correlating sympathetic arousal; temp_std_dev: fluctuating skin temperature demonstrating transient autonomic reactions across conditions; and rr_std_dev: indicating variability in heart rate (HRV), a widely used marker of autonomic balance. Movement (accelerometer) and Cardiac Signals (RR interval, BVP) were consistent top contributors, reflecting the interplay between posture, movement, and cardiac autonomic regulation during Iyengar yoga. Electrodermal and Temperature Signals underline the importance of arousal and peripheral circulation as non-invasive autonomic markers. Derivative and standard deviation signals were frequently selected over simple means, reinforcing the strength of dynamic features over static values in distinguishing subtle physiological states. A small number of features (especially those related to EDA, BVP, and HRV) consistently drove the classifiers’ performance. These overlaps point to robust candidate features for autonomic state differentiation, offering stable physiological markers across multiple algorithmic viewpoints, despite their inherent modeling differences.
To assess the potential confounding impact of movement-related (accelerometer) features, we conducted an ablation experiment excluding all accelerometer-derived features and re-trained each classifier. As summarized in Table 9, the stratified cross-validation performance remained high across models (e.g., Random Forest: accuracy 0.94, F1 0.94, AUC 0.99 without the accelerometer), closely matching the results obtained with the full feature set that included movement metrics. These observations confirm that the models are not exclusively leveraging pose-related movement differences, but also capture meaningful autonomic signal modulation reflected in the heart rate variability, EDA, temperature, and BVP.

Table 9.
Model performances with all features vs. without accelerometer features (stratified 10-fold CV).
4.3. Feature Explanation
There is notable convergence between the insights gained from the SHapely Additive exPlanations (SHAP) analysis [] of the top-performing Random Forest model and feature weights from classical models (logistic regression, SVM, Naive Bayes). This intersection highlights a set of physiological features that robustly predict autonomic states—sympathetic, parasympathetic, and baseline (relaxed)—within the Iyengar yoga dataset. These features are not model-specific artifacts; they encode stable, physiologically meaningful information evidenced across fundamentally different analytical approaches. From the best performing Random Forest model, the highly discriminative features were rr_std_dev (0.098), acc_std_dev (0.097), bvp_mean (0.094), eda_peak_rms (0.074), and temp_mean (0.066). SHAP analysis on this best performing RF model formed the basis for further analysis of how each of these features contributed to each physiological states. Feature importances from other classical models such as LR, GNB and SVM were used in complement to support the physiological robustness.
4.3.1. Heart Rate Variability (RR Interval Mean and Standard Deviation)
RR Interval Mean and standard deviation are markers of heart rate variability and cardiac autonomic balance. Long RR intervals (i.e., low heart rate) and high variability (std_dev) consistently increased parasympathetic predictions. The SHAP values in Figure 5 show strong positive effects; LR (+1.11; OR: 3.04) and SVM (+0.49; OR: 1.64) agree. This aligns well with the established physiology where parasympathetic dominance increases HRV and is strongly indicative of vagal dominance. Shorter RR intervals (lower HRV) drove sympathetic predictions in SHAP Figure 5 and LR (−0.88; OR: 0.42), matching known associations between sympathetic arousal and cardiac acceleration. RR interval values near the mean were associated with relaxed state in SHAP Figure 5, suggesting moderate HRV distinct from the extremes defining parasympathetic or sympathetic states. This reflects the relaxed state’s position as a physiological middle ground. Parasympathetic activity promotes HRV and slower HR; sympathetic arousal suppresses HRV and raises heart rate.
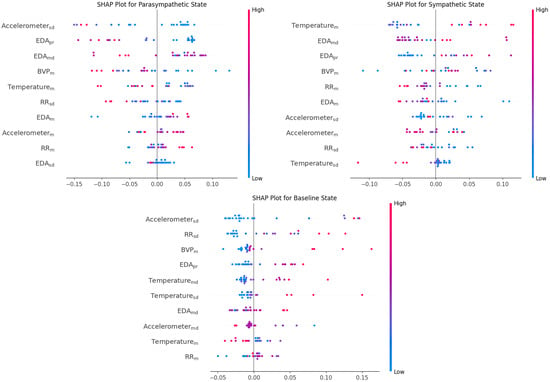
Figure 5.
SHAP values illustrating the impact of each feature on the model’s output across the three physiological states: parasympathetic, sympathetic, and baseline. (feature notation is same as in Figure 4).
4.3.2. Movement Variability (Accelerometer Standard Deviation)
Accelerometer standard deviation reflects movement variability/postural change. Unsurprisingly, low accelerometer standard deviation was strongly predictive of parasympathetic state, reflecting restorative poses. The SHAP values in Figure 5 were sharply negative for higher values, pushing the model away from parasympathetic classification. This was corroborated by LR (−1.99; OR: 0.14) and SVM (−1.27; OR: 0.28). Stillness aligns with the restorative, restful parasympathetic state. While high accelerometer variability increased sympathetic prediction in SHAP values in Figure 5 and SVM (coef: +1.00; OR: 2.72), suggesting movement during challenging or arousing postures. Interestingly, high accelerometer variability also contributed to relaxed class predictions in the SHAP values in Figure 5 and LR (coef: +2.06; OR: 7.82). This may reflect postural transitions or free movement within a calm, baseline context. Parasympathetic activation is associated with stillness, while sympathetic tone increases during exertion or challenging postures manifesting as more movement.
4.3.3. Blood Volume Pulse Amplitude (BVP Mean)
Blood volume pulse, a proxy for peripheral circulation, is a measure of cardiac and vascular activity often modulated by sympathetic tone. Low BVP amplitude was associated with parasympathetic predictions in SHAP Figure 5, suggesting reduced cardiac output and vasoconstriction typical of a resting state. High BVP amplitude pushed predictions toward a sympathetic state. This aligns with elevated cardiac activity and pulse wave amplitude under stress or arousal. Intermediate BVP levels were associated with relaxed predictions in SHAP Figure 5, again emphasizing the mid-range nature of this class.
4.3.4. Phasic Electrodermal Activity (EDA Peak to RMS)
EDA Peak to RMS represents the phasic, rapid component of skin conductance changes. Low peak-to-RMS values strongly promoted parasympathetic predictions in the SHAP values in Figure 5 and LR (−2.82; OR: 0.06), reflecting the dampening of sympathetic arousal typical of restful states. High values robustly indicated sympathetic activation, the SHAP values in Figure 5 showed a large positive push; LR (+0.77; OR: 2.17) and SVM (+0.14; OR: 1.15) supported this, reflecting a gold-standard biomarker for SNS activity. Mid-range values were weakly associated with relaxed state predictions, with the SHAP values contributions in Figure 5 being notably smaller, suggesting that neither extreme phasic arousal nor complete flatness defines this class. Electrodermal activity is governed by the sympathetic branch; phasic EDA increases during stress or effort, not during rest-and-digest periods.
4.3.5. Skin Temperature (Temperature Mean)
The temperature mean reflects the peripheral skin temperature responding to vascular changes and showed class-distinct but partially inconsistent behavior across models. The SHAP values in Figure 5 indicated slightly positive association, with higher skin temperature increasing parasympathetic prediction. However, LR (−0.67; OR: 0.51) and SVM (−0.18; OR: 0.83) suggested the opposite. While parasympathetic activity is classically linked to increased peripheral temperature due to vasodilation, because the parasympathetic data here is labeled in accordance with passive poses (e.g., lying down, resting), the body core temperature might drop due to less muscular activity. The data were collected through the wrist, as this region is very sensitive to peripheral vasoconstriction/vasodilation; thus, there may have been delayed or inverse changes compared to the core parasympathetic activity. The SHAP values in Figure 5 showed a positive influence, higher temperatures pushed predictions toward the sympathetic class, and LR (+0.68; OR: 1.97) agreed. However, SVM showed the reverse (−1.12; OR: 0.33). These mixed results suggest skin temperature may reflect non-autonomic processes. The SHAP values in Figure 5 associated mid-to-high temperatures with a relaxed state prediction. This aligns with a comfort zone interpretation of neither cold-stressed nor vasodilated extremes.
Among these best predictors, features such as temp_mean, eda_mean, eda_std_dev, eda_peak_rms, eda_mean_deriv, rr_mean, rr_std_dev, acc_mean, acc_std_dev, and bvp_mean appear to be the key discriminators for parasympathetic and sympathetic autonomic response-eliciting poses. This feature-centric analysis reveals that EDA peak to RMS, HRV, movement variability, and, to a lesser extent, skin temperature and BVP amplitude are consistent and interpretable predictors of autonomic state classification. Parasympathetic states are defined by stillness, high HRV, and low phasic arousal. Sympathetic states arise from dynamic movement, short RR intervals, and phasic EDA surges. Relaxed states are distinguished by moderate movement and balanced physiological values across modalities.
For instance, an increase in the magnitude of the RR interval from baseline to the postural phase is indicative of parasympathetic activity, aligning with previous findings that associate elevated RR intervals with higher vagal tone [], can be observed in Figure 6. Skin temperature tends to rise under parasympathetic dominance due to a reduction in sympathetic vasoconstriction, reflecting increased peripheral blood flow. In contrast, EDA typically decreases as parasympathetic activity prevails. However, it is important to note that during the initial phase of posture execution, a brief surge in EDA may occur triggered by transient sympathetic arousal in response to physical effort before it subsides as the parasympathetic state establishes itself. BVP, reflecting peripheral vascular tone, also increases under parasympathetic influence, signifying vasodilation.
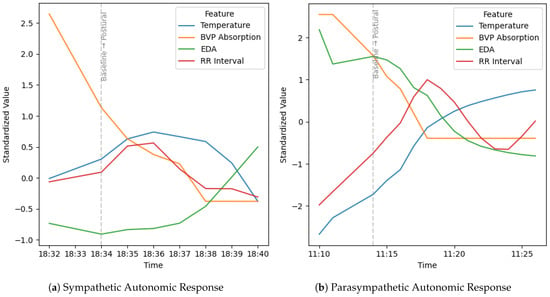
Figure 6.
Comparison of physiological signals over time from baseline to postural phases for (a) sympathetic and (b) parasympathetic autonomic responses.
The parasympathetic response differs markedly from the sympathetic response across these modalities. While parasympathetic activation is associated with relaxation and recovery manifesting as increased RR interval (slower heart rate), elevated skin temperature (due to vasodilation), decreased EDA, and increased BVP absorption, the sympathetic response produces the opposite pattern. During sympathetic activation, such as in stress or challenge, the RR interval decreases (faster heart rate), skin temperature typically drops (stemming from vasoconstriction), EDA rises sharply (from sweat gland activation), and BVP absorption decreases (indicating vasoconstriction). These physiology-driven contrasts underscore the fundamental opposition between calming (parasympathetic) and arousing (sympathetic) branches of the ANS, and highlight the nuanced, sometimes overlapping, shifts detected by multimodal physiological monitoring.
5. Limitations
- In this study, the autonomic states were labeled based on a certified yoga practitioner’s expertise. This practitioner served as a clinical expert to validate the physiological annotations. This approach aligns with established practices in physiological and clinical research where clinician-driven labeling is widely accepted as a valid and robust method, especially when purely data-driven ground truths are unavailable or limited []. Nonetheless, we recognize this as a limitation and plan to incorporate objective physiological validation (e.g., validating data against a rule-based algorithm with known biology of ANS) to strengthen construct validity in future work.
- Our work provides valuable information and insights into the physiological effects of yoga as a therapeutic tool, focusing on elderly Indian adults with limited to moderate yoga experience. While these findings are most applicable to the population studied, caution should be exercised when extrapolating to other groups. This contribution serves as a foundational resource and may inform future studies seeking to broaden the evidence base for Iyengar yoga’s effects across diverse demographic settings.
- Our evaluation employed two complementary cross-validation strategies—stratified 10-fold CV to capture population-level separability of physiological states and Leave-One-Subject-Out CV (LOGO-CV) to assess subject-independent generalization. While stratified CV provides a holistic view of state differences across the dataset, it may overestimate performance due to potential subject-specific artifacts leaking across folds. We, therefore, emphasize LOGO-CV as the more realistic estimate of model generalizability to new individuals. Nevertheless, the relatively small sample size (N = 16) means that LOGO-CV estimates can still be unstable, and results should be interpreted as exploratory until validated in larger, more diverse cohorts.
6. Conclusions
This study establishes a robust methodological framework using wearable sensor data and machine learning models to discriminate different autonomic physiological states (baseline/relaxed, parasympathetic/restorative poses, and sympathetic/stimulating poses) during Iyengar yoga practice. Differentiating between phases (baseline and postural) and ANS response (parasympathetic and sympathetic) helps in providing the user with feedback for their engagement which is an essential step towards automated and personalized practice of such therapeutic yoga, which is currently limited to being performed under the guidance of certified instructors only. While the feedback about phases (relaxed or in-pose) provides information about getting into the pose, feedback about the type of autonomic response provides information on whether the required physiological response is elicited or not after holding the poses for sometime. As poses elicit a physiological response, this provides a chance to course-correct along the way, which makes it more feasible, reliable, and personalized. This work emphasizes that not only intense bodily movements but even slow, static yoga postures can induce rapid, multidimensional changes detectable via wearable sensors. While many studies documented ANS modulation using biomarkers such as HRV, EDA, or respiratory markers, the mechanisms are often attributed to breathing, meditation, or relaxation aspects rather than the pose mechanics themselves, which is the key aspect of this study, especially in Iyengar yoga. By mitigating overfitting and improving generalization, the developed sparse models create a strong foundation for future wearable-based health monitoring systems. Interpretable feature selection and model interpretation consistently highlighted acceleration and cardiac variability features, phasic EDA measures, and vascular/thermal dynamics as core predictors of the autonomic state. These results elucidate the complex interplay among movement, cardiovascular activity, electrodermal fluctuations, and thermoregulation during yoga, providing a physiologically sound and computationally validated pathway for future real-time, adaptive biofeedback and posture-aware health interventions. The findings from this study herein offer a scalable solution for objective autonomic assessment, enabling precision yoga therapy, self-monitoring, and broader applications in digital health and behavior medicine.
Author Contributions
Conceptualization, C.V.A. and T.B.; methodology, W.L.R., C.V.A. and T.B.; literature review, C.V.A.; validation, W.L.R. and C.V.A.; formal analysis, W.L.R. and C.V.A.; investigation, T.B. and C.V.A.; resources, W.L.R. and T.B.; data curation, T.B. and C.V.A.; writing-original draft preparation, C.V.A., T.B. and W.L.R.; writing-review and editing, W.L.R., C.V.A. and T.B.; visualization, C.V.A.; supervision, T.B. and W.L.R.; project administration, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Wright State University Institutional Review Board (FWA00002427; Study ID: IRB-2024-651) on 4 December 2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study. Please reach out to the authors with questions regarding data access.
Acknowledgments
The authors wish to thank Anuradha Oak for her expert guidance in designing individualized Iyengar yoga sessions for each participant at the Atmaja Wellness Awareness Organization. Based on her assessment of each participant’s condition, Oak selected and adapted the yoga poses, determined their duration, and provided in-person instruction to ensure correct form and technique throughout the sessions.
Conflicts of Interest
The authors report no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANS | Autonomic Nervous System |
| EDA | Electrodermal Activity |
| BVP | Blood Volume Pulse |
| RF | Random Forest |
| LR | Logistic Regression |
| DT | Decision Tree |
| GNB | Gaussian Naive Bayes |
| NB | Naive Bayes |
| SVM | Support Vector Machine |
| AUC | Area Under the Curve |
| SHAP | SHapely Additive exPlanations |
| OR | Odds Ratio |
| RMS | Root Mean Square |
| HRV | Heart Rate Variability |
| HSD | Tukey’s Honest Significant Difference |
References
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology, 13th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kay, M.W.; Jain, V.; Panjrath, G.; Mendelowitz, D. Targeting Parasympathetic Activity to Improve Autonomic Tone and Clinical Outcomes. Physiology 2022, 37, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lujan, H.L.; DiCarlo, S.E. Physical activity, by enhancing parasympathetic tone and activating the cholinergic anti-inflammatory pathway, is a therapeutic strategy to restrain chronic inflammation and prevent many chronic diseases. Med. Hypotheses 2013, 80, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Lambert, E.; Schlaich, M. Point: Chronic Activation of the Sympathetic Nervous System Is the Dominant Contributor to Systemic Hypertension. J. Appl. Physiol. 2010, 109, 1996–1998. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S. Adrenal Responses to Stress. Cell. Mol. Neurobiol. 2010, 30, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, B.K.S. Yoga The Path To Holistic Health, 2014th ed.; DK: London, UK, 2014. [Google Scholar]
- Khattab, K.; Khattab, A.A.; Ortak, J.; Richardt, G.; Bonnemeier, H. Iyengar Yoga Increases Cardiac Parasympathetic Nervous Modulation among Healthy Yoga Practitioners. Evid.-Based Complement. Altern. Med. 2007, 4, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Bandi, H.K.; Pal, P.; Pal, G.K.; Balachander, J.; Jayasettiaseelon, E.; Sreekanth, Y.; Sridhar, M.G.; Gaur, G.S. Effect of Yoga Therapy on Heart Rate, Blood Pressure and Cardiac Autonomic Function in Heart Failure. J. Clin. Diagn. Res. 2014, 8, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.J.; S-Parrilla, J.; Mirus, J.; Loadman, M.A.; Lien, D.C.; Feeny, D. An Assessment of the Effects of Iyengar Yoga Practice on the Health-Related Quality of Life of Patients with Chronic Respiratory Diseases: A Pilot Study. Can. Respir. J. 2013, 20, e17–e23. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.M.; Gerbarg, P.L.; Silveri, M.M.; Nielsen, G.H.; Owen, L.; Nyer, M.; Brown, R.P.; Streeter, C.C. Psychological Function, Iyengar Yoga, and Coherent Breathing. J. Psychiatr. Pract. 2019, 25, 437–450. [Google Scholar] [CrossRef]
- Michalsen, A.; Jeitler, M.; Brunnhuber, S.; Lüdtke, R.; Büssing, A.; Musial, F.; Dobos, G.; Kessler, C. Iyengar Yoga for Distressed Women: A 3-Armed Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2012, 2012, 408727. [Google Scholar] [CrossRef]
- Bhattacharyya, K.B. The stretch reflex and the contributions of C David Marsden. Ann. Indian Acad. Neurol. 2017, 20, 1–4. [Google Scholar] [CrossRef]
- Blank, S. Physiological Responses to Iyengar Yoga Performed by Trained Practitioners. J. Exerc. Physiol. (JEPonline) 2006, 9, 7–23. [Google Scholar]
- Wieser, M.; Buetler, L.; Vallery, H.; Schaller, J.; Mayr, A.; Kofler, M.; Saltuari, L.; Zutter, D.; Riener, R. Quantification of Clinical Scores through Physiological Recordings in low-responsive patients: A Feasibility Study. J. Neuroeng. Rehabil. 2012, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Statistical Notes for Clinical researchers:post-hocmultiple Comparisons. Restor. Dent. Endod. 2015, 40, 176. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Lundberg, S.; Allen, P.; Lee, S.I. A Unified Approach to Interpreting Model Predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).