Reuse of Textile Dyeing Effluents Treated with Coupled Nanofiltration and Electrochemical Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
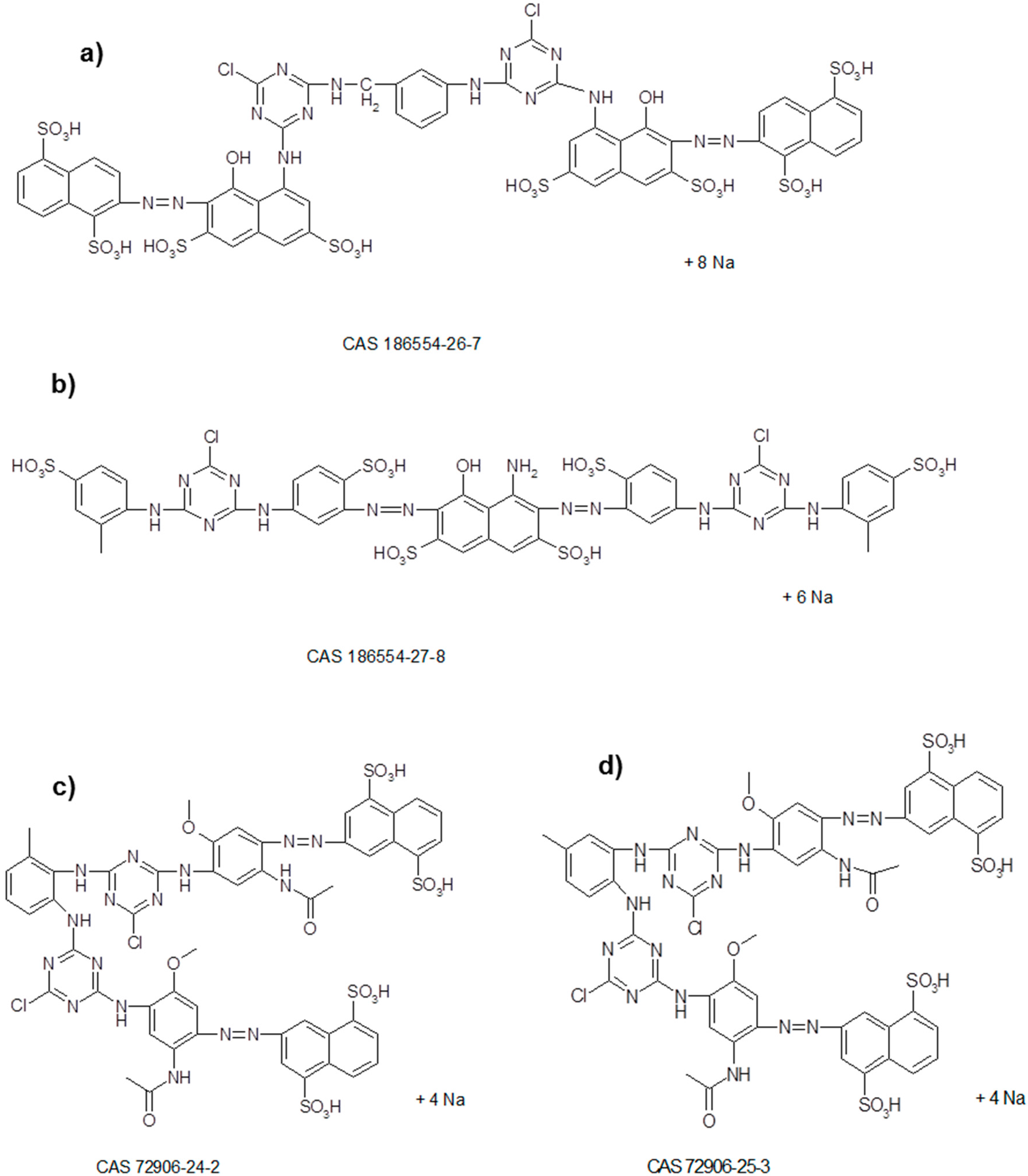
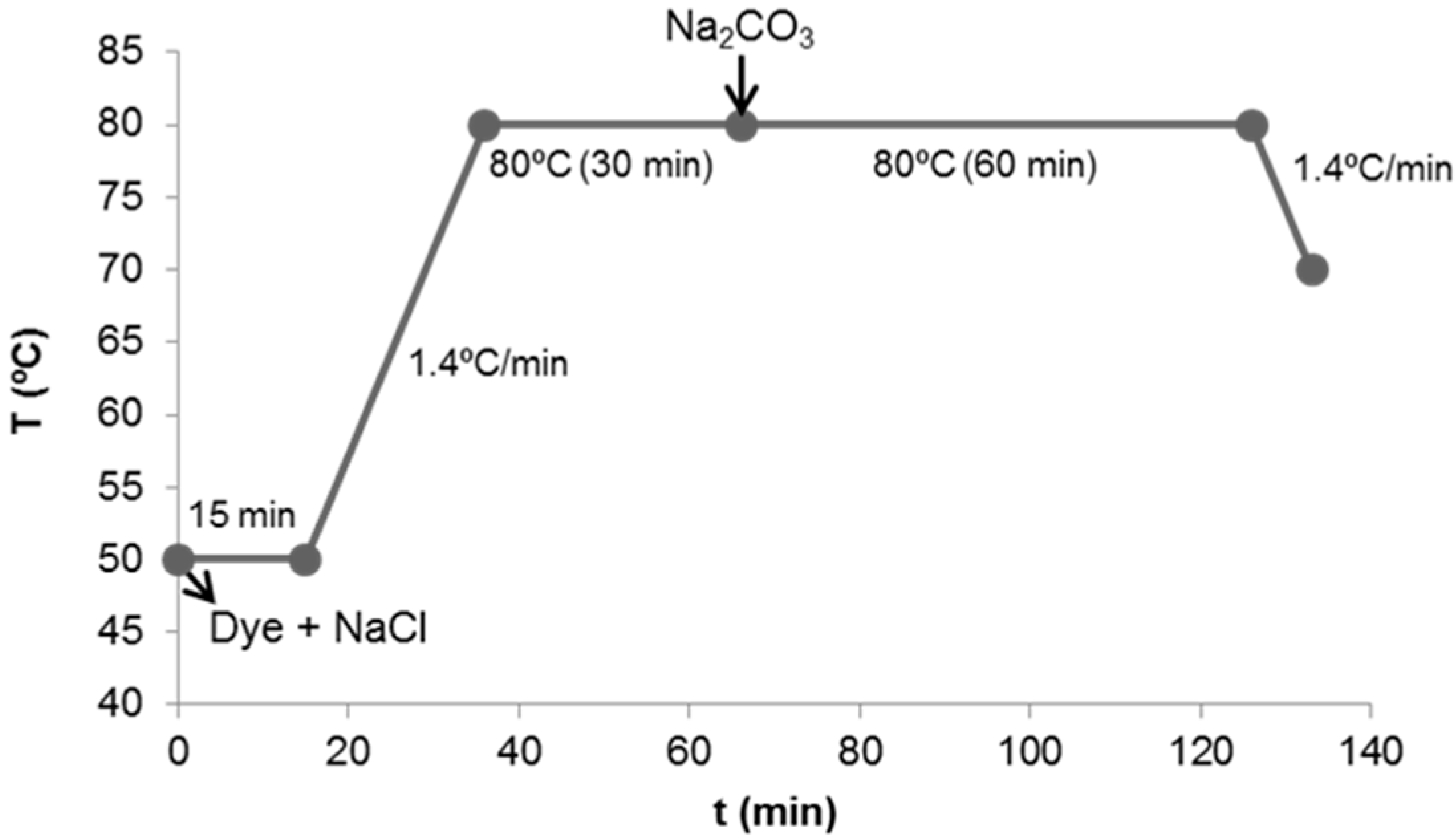
2.2. Reactive Synthetic Effluent Preparation
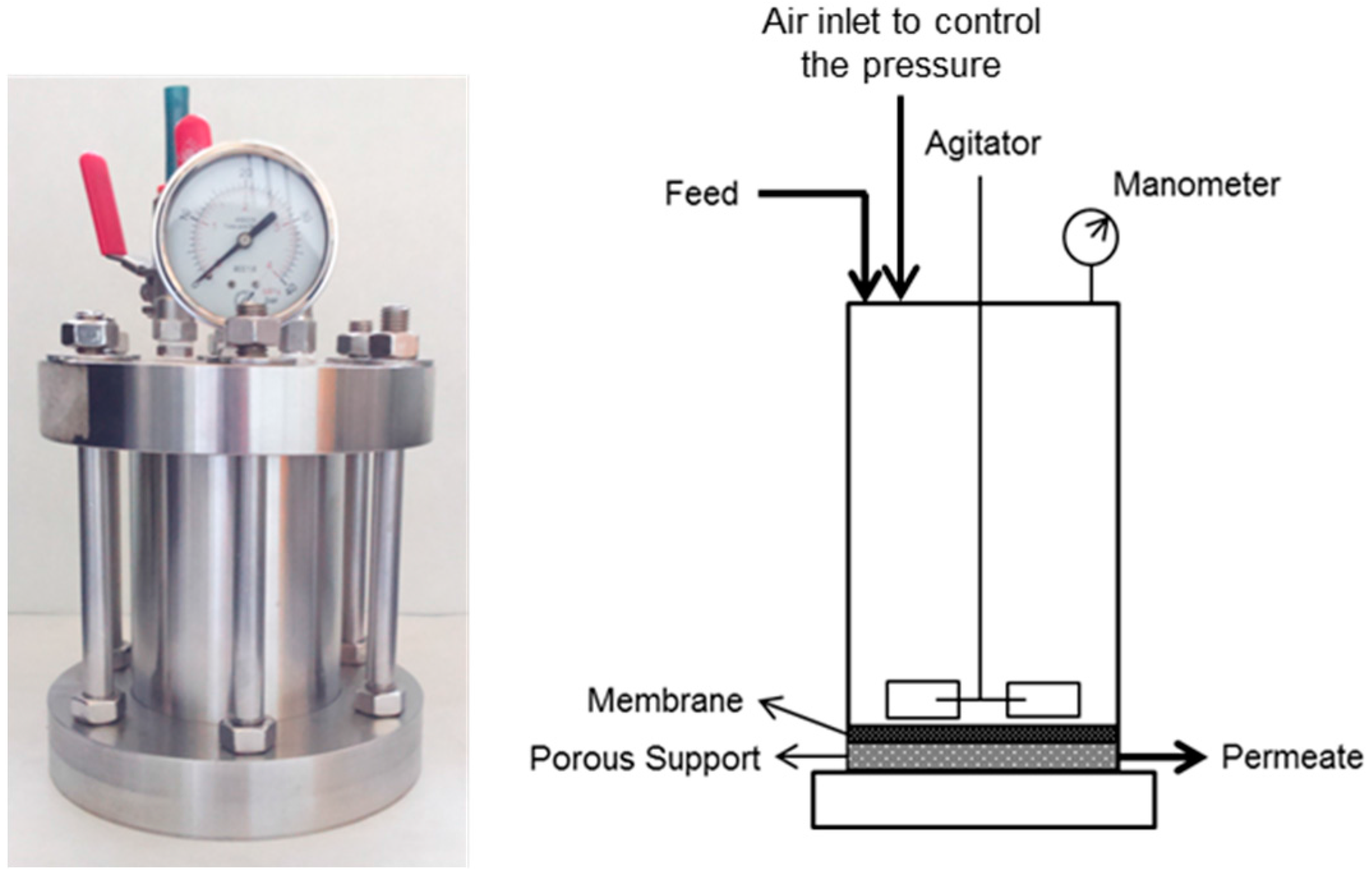
2.3. Membrane Treatment
- Soaking in deionized water for 10 min. This step was repeated three times;
- Membrane was immersed in a solution containing 0.005 g·L−1 active chlorine overnight;
- Rinsing with deionized water; and
- Soaking in deionized water for 10 min. This step was repeated three times.
2.4. Electrochemical Treatment
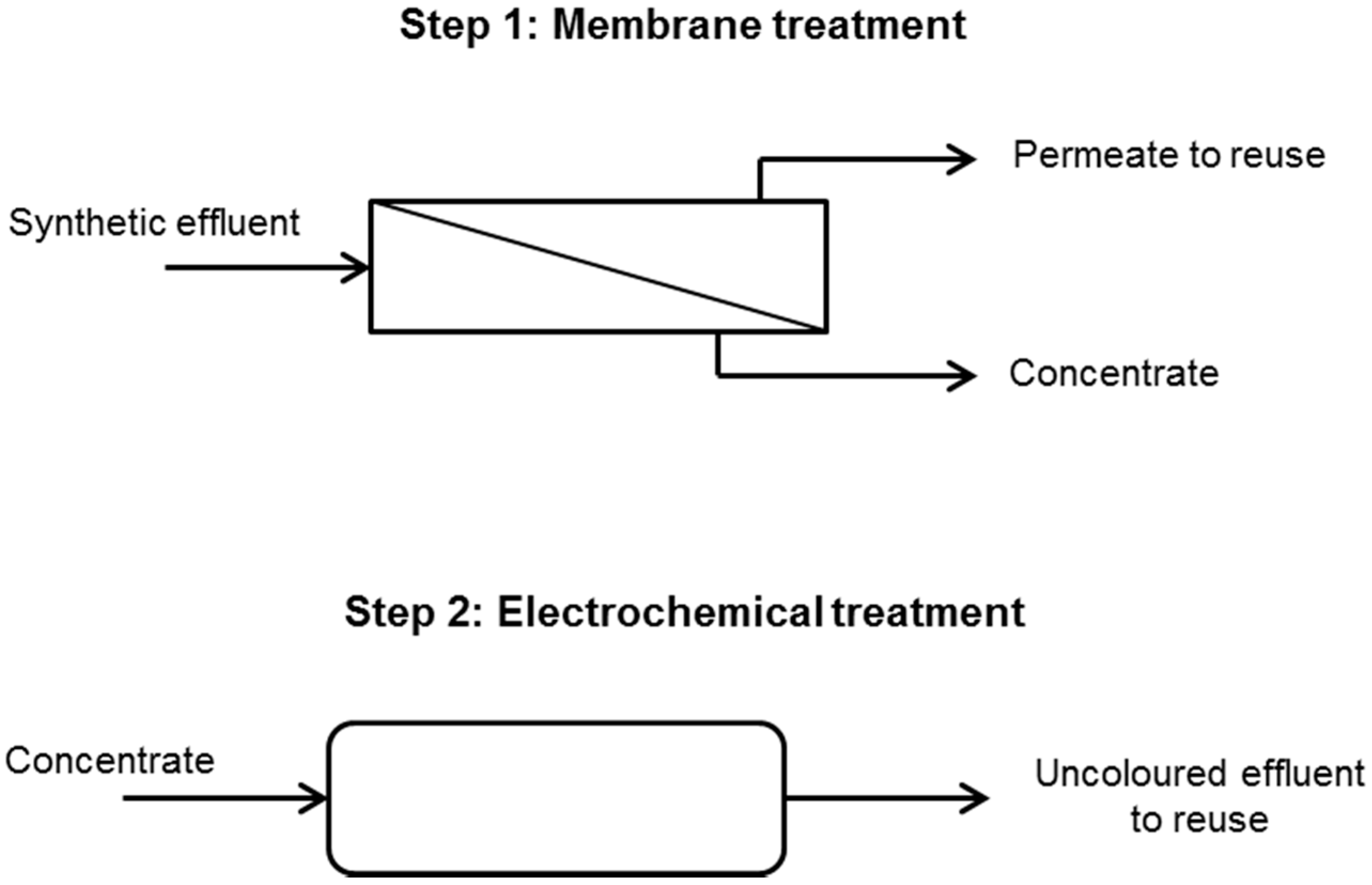
2.5. Effluent Reuse
- 1st–3rd: Cleaning with softened tap water at 50 °C for 10 min;
- 4th: Soap cleaning with 2 g·L−1 COTEMOLL TLTR at 95 °C for 15 min;
- 5th: Cleaning with softened tap water at 50 °C for 10 min;
- 6th: Soap cleaning with 2 g·L−1 COTEMOLL TLTR at 95 °C for 15 min; and
- 7th–9th: Cleaning with softened tap water at 50 °C for 10 min.
2.6. Analytical Methods and Measurements
3. Results and Discussion
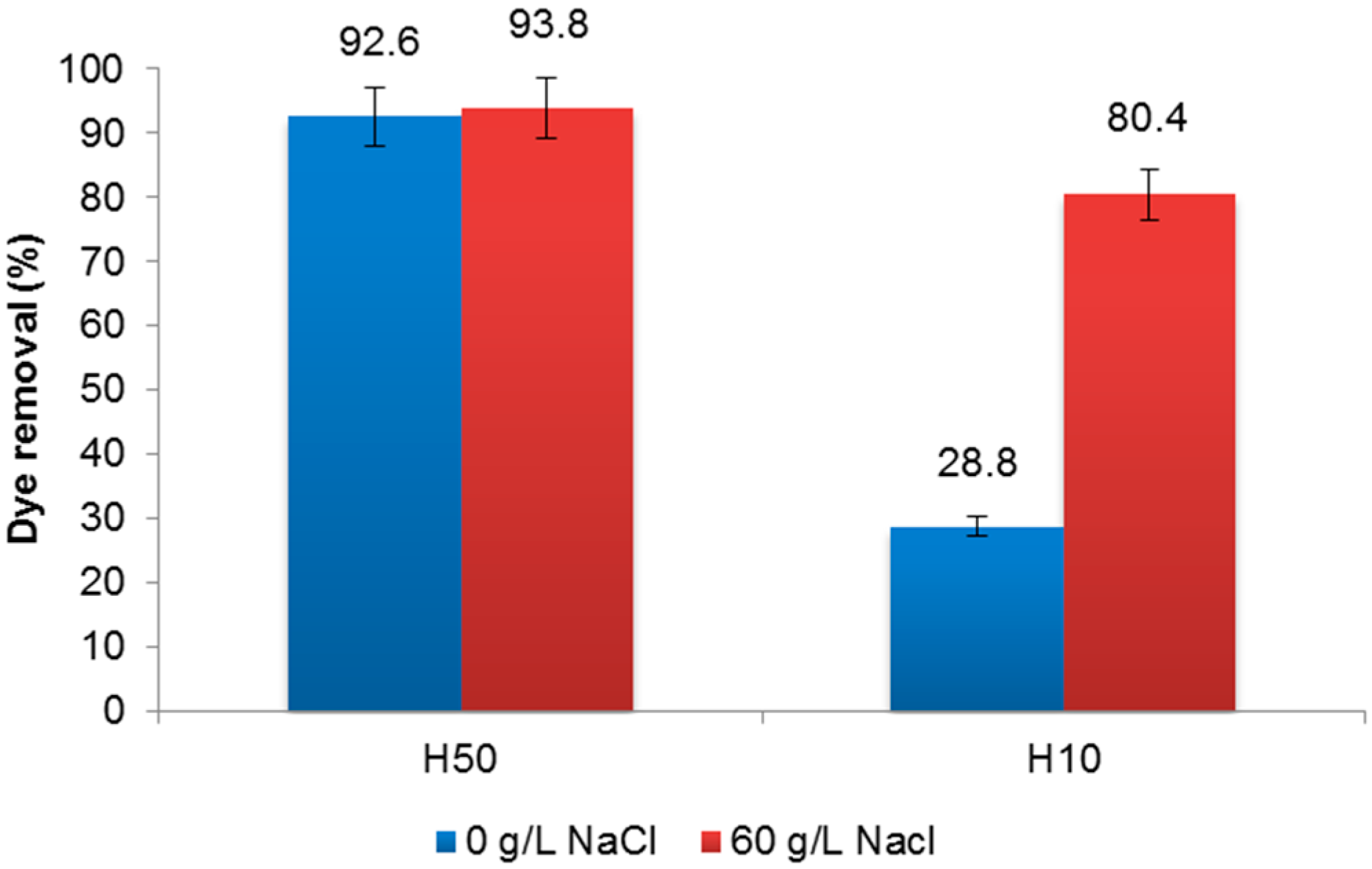
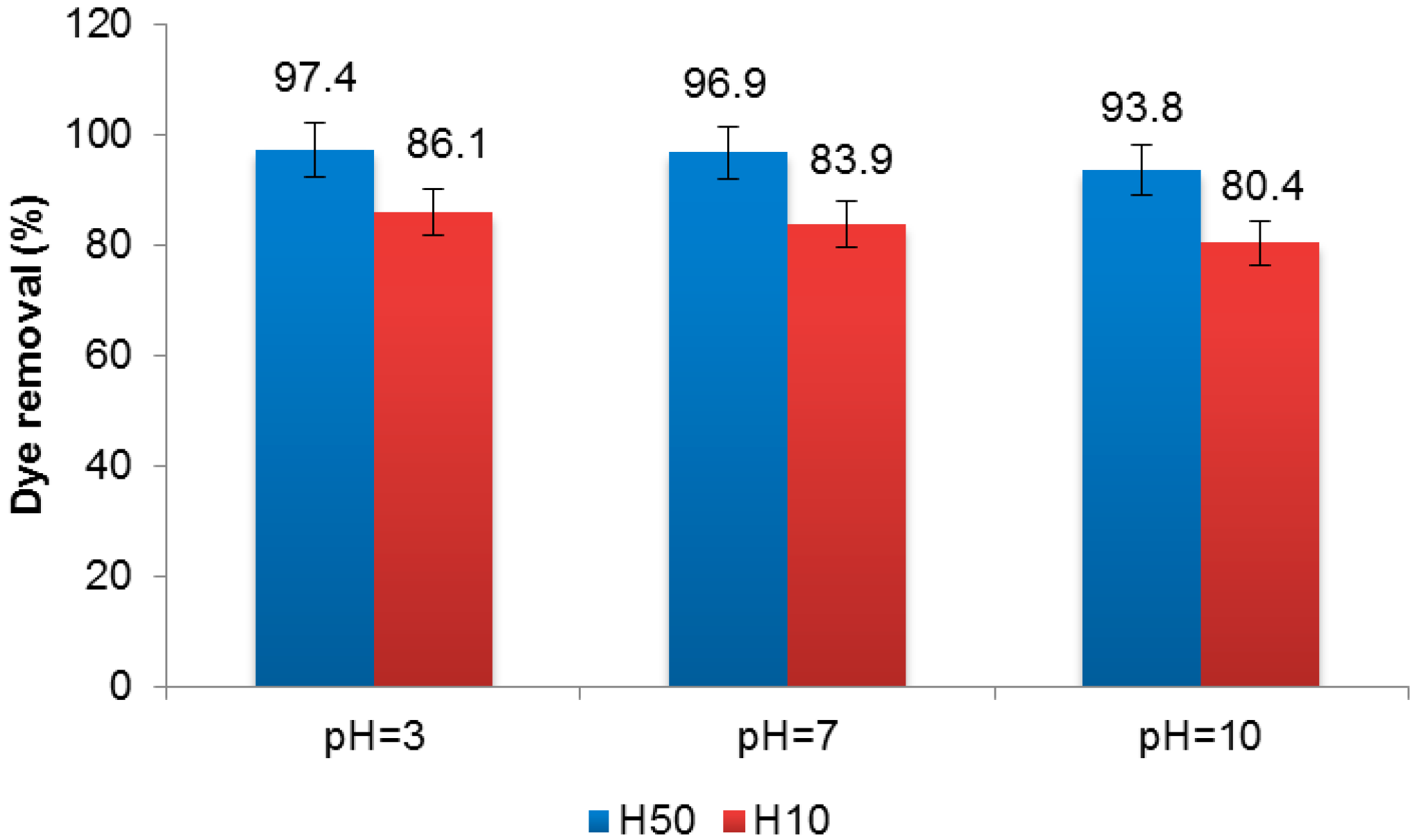
3.1. Membrane Treatment
3.2. Electrochemical Treatment
3.3. Effluent Reuse
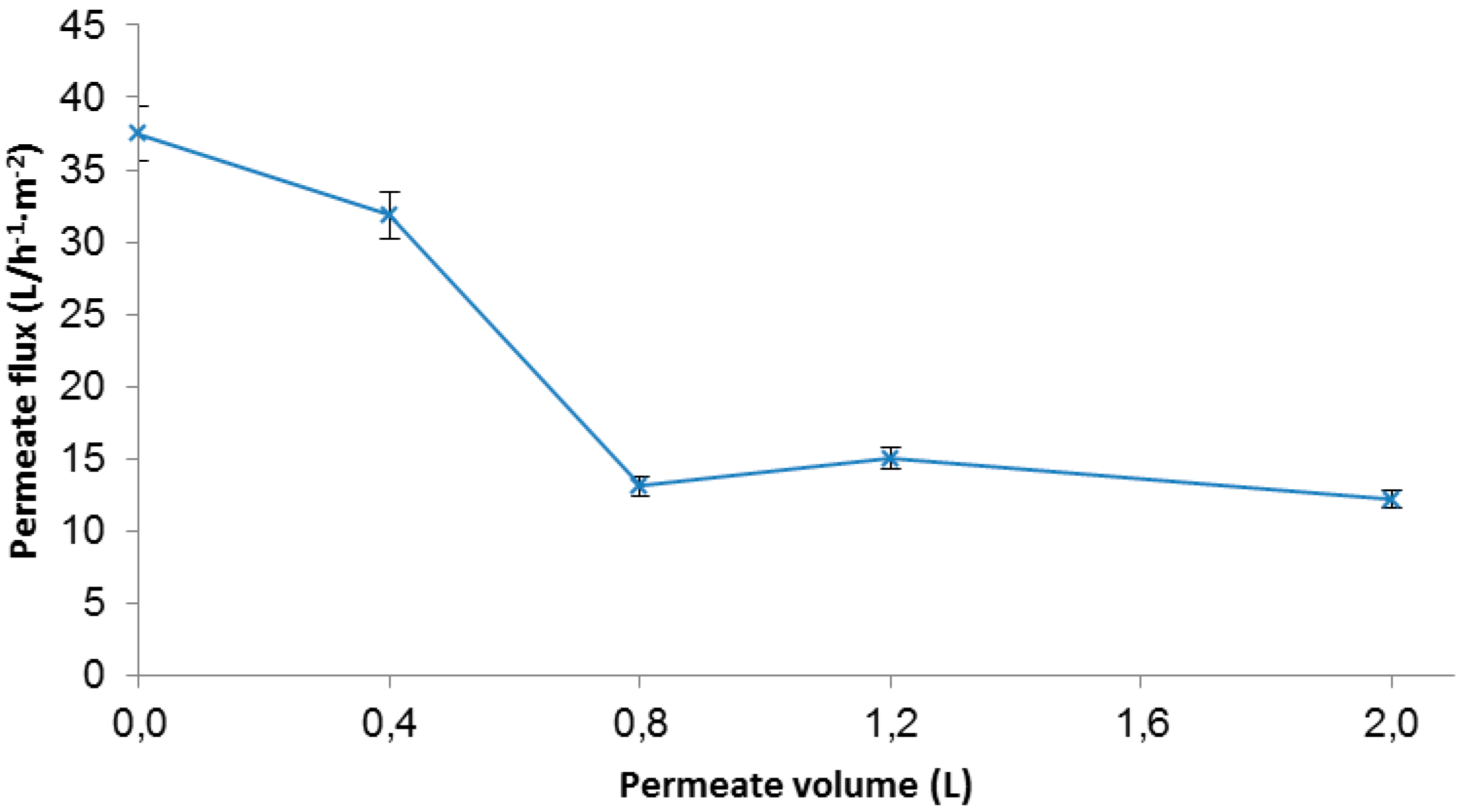
3.3.1. Permeate Reuse
3.3.2. Reuse of the Decoloured Concentrate Effluent
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cassardo, C.; Jones, A. Managing water in a changing world. Water 2011, 3, 618–628. [Google Scholar] [CrossRef]
- Water Withdrawal and Pressure on Water Resources (FAO). Available online: http://www.fao.org/nr/aquastat (accessed on 4 May 2016).
- Vajnhandl, S.; Valh, J.V. The status of water reuse in European textile sector. J. Environ. Manag. 2014, 141, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, S.; Shuai, S.; Zhou, Q.; Cheng, Q.; Liu, M.; Gao, C. Color removal and COD reduction of biologically treated textile effluent through submerged filtration using hollow fiber nanofiltration membrane. Desalination 2013, 314, 89–95. [Google Scholar] [CrossRef]
- Liu, M.; Lü, Z.; Chen, Z.; Yu, S.; Gao, C. Comparison of reverse osmosis and nanofiltration membranes in the treatment of biologically treated textile effluent for water reuse. Desalination 2011, 281, 372–378. [Google Scholar] [CrossRef]
- Bonakdarpour, B.; Vyrides, I.; Stuckey, D.C. Comparison of the performance of one stage and two stage sequential anaerobic–aerobic biological processes for the treatment of reactive-azo-dye-containing synthetic wastewaters. Int. Biodeterior. Biodegrad. 2011, 65, 591–599. [Google Scholar] [CrossRef]
- Vyrides, I.; Bonakdarpour, B.; Stuckey, D.C. Salinity effects on biodegradation of Reactive Black 5 for one stage and two stages sequential anaerobic aerobic biological processes employing different anaerobic sludge. Int. Biodeterior. Biodegrad. 2014, 95, 294–300. [Google Scholar] [CrossRef]
- Lotito, A.M.; De Sanctis, M.; Di Iaconi, C.; Bergna, G. Textile wastewater treatment: Aerobic granular sludge vs activated sludge systems. Water Res. 2014, 54, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Riera-Torres, M.; Gutiérrez-Bouzán, C.; Crespi, M. Combination of coagulation-flocculation and nanofiltration techniques for dye removal and water reuse in textile effluents. Desalination 2010, 252, 53–59. [Google Scholar] [CrossRef]
- Liang, C.Z.; Sun, S.-P.; Li, F.Y.; Ong, Y.K.; Chung, T.S. Treatment of highly concentrated wastewater containing multiple synthetic dyes by a combined process of coagulation/flocculation and nanofiltration. J. Membr. Sci. 2014, 469, 306–315. [Google Scholar] [CrossRef]
- Lau, Y.Y.; Wong, Y.S.; Teng, T.T.; Morad, N.; Rafatullah, M.; Ong, S.-A. Coagulation-flocculation of azo dye Acid Orange 7 with green refined laterite soil. Chem. Eng. J. 2014, 246, 383–390. [Google Scholar] [CrossRef]
- Babu, B.; Parande, A.; Raghu, S.; Prem, T. Cotton Textile Processing: Waste Generation and Effluent Treatment. J. Cotton Sci. 2007, 11, 141–153. [Google Scholar]
- Wong, P.; Teng, T.; Rahman, N. Efficiency of the coagulation-flocculation method for the treatment of dye mixtures contaning disperse and reactive dyes. Water Qual. Res. J. 2007, 42, 54–62. [Google Scholar]
- Barredo-Damas, S.; Alcaina-Miranda, M.I.; Bes-Piá, A.; Iborra-Clar, M.I.; Iborra-Clar, A.; Mendoza-Roca, J.A. Ceramic membrane behavior in textile wastewater ultrafiltration. Desalination 2010, 250, 623–628. [Google Scholar] [CrossRef]
- Barredo-Damas, S.; Alcaina-Miranda, M.I.; Iborra-Clar, M.I.; Mendoza-Roca, J.A.; Gemma, M. Effect of pH and MWCO on textile effluents ultrafiltration by tubular ceramic membranes. Desalin. Water Treat. 2011, 27, 81–89. [Google Scholar] [CrossRef]
- Blanco, J.; Torrades, F.; Morón, M.; Brouta-Agnésa, M.; García-Montaño, J. Photo-Fenton and sequencing batch reactor coupled to photo-Fenton processes for textile wastewater reclamation: Feasibility of reuse in dyeing processes. Chem. Eng. J. 2014, 240, 469–475. [Google Scholar] [CrossRef]
- Visa, T.; Sánchez, M.; López-Grimau, V.; Navarro, R.; Reche, S.; Gutiérrez-Bouzán, C. Photocatalysis with titanium dioxide to remove colour of exhausted reactive dyebaths without pH modification. Desalin. Water Treat. 2012, 45, 91–99. [Google Scholar] [CrossRef]
- Buscio, V.; Brosillon, S.; Mendret, J.; Crespi, M.; Gutiérrez-Bouzán, C. Photocatalytic Membrane Reactor for the Removal of C.I. Disperse Red 73. Materials 2015, 8, 3633–3647. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Buscio, V.; Marín, M.J.; Crespi, M.; Gutiérrez-Bouzán, C. Reuse of textile wastewater after homogenization-decantation treatment coupled to PVDF ultrafiltration membranes. Chem. Eng. J. 2015, 265, 122–128. [Google Scholar] [CrossRef]
- Buscio, V.; Crespi, M.; Gutiérrez-Bouzán, C. Sustainable dyeing of denim using indigo dye recovered with PVDF ultrafiltration membranes. J. Clean. Prod. 2015, 91, 201–207. [Google Scholar] [CrossRef]
- Debik, E.; Kaykioglu, G.; Coban, A.; Koyuncu, I. Reuse of anaerobically and aerobically pre-treated textile wastewater by UF and NF membranes. Desalination 2010, 256, 174–180. [Google Scholar] [CrossRef]
- Barredo-Damas, S.; Alcaina-Miranda, M.I.; Iborra-Clar, M.I.; Mendoza-Roca, J.A. Application of tubular ceramic ultrafiltration membranes for the treatment of integrated textile wastewaters. Chem. Eng. J. 2012, 192, 211–218. [Google Scholar] [CrossRef]
- Alventosa-deLara, E.; Barredo-Damas, S.; Zuriaga-Agustí, E.; Alcaina-Miranda, M.I.; Iborra-Clar, M.I. Ultrafiltration ceramic membrane performance during the treatment of model solutions containing dye and salt. Sep. Purif. Technol. 2014, 129, 96–105. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Ismail, F. Treatment of dyeing solution by NF membrane for decolorization and salt reduction. Desalin. Water Treat. 2012, 50, 245–253. [Google Scholar] [CrossRef]
- Aouni, A.; Fersi, C.; Cuartas-Uribe, B.; Bes-Pía, A.; Alcaina-Miranda, M.I.; Dhahbi, M. Reactive dyes rejection and textile effluent treatment study using ultrafiltration and nanofiltration processes. Desalination 2012, 297, 87–96. [Google Scholar] [CrossRef]
- Gozálvez-Zafrilla, J.M.; Sanz-Escribano, D.; Lora-García, J.; León Hidalgo, M.C. Nanofiltration of secondary effluent for wastewater reuse in the textile industry. Desalination 2008, 222, 272–279. [Google Scholar] [CrossRef]
- De Jager, D.; Sheldon, M.S.; Edwards, W. Colour removal from textile wastewater using a pilot-scale dual-stage MBR and subsequent RO system. Sep. Purif. Technol. 2014, 135, 135–144. [Google Scholar] [CrossRef]
- Kurt, E.; Koseoglu-Imer, D.Y.; Dizge, N.; Chellam, S.; Koyuncu, I. Pilot-scale evaluation of nanofiltration and reverse osmosis for process reuse of segregated textile dyewash wastewater. Desalination 2012, 302, 24–32. [Google Scholar] [CrossRef]
- Dasgupta, J.; Sikder, J.; Chakraborty, S.; Surcio, S.; Drioli, E. Remediation of textile effluents by membrane based techniques: A state of the art review. J. Environ. Manag. 2015, 147, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.H.B.; Gomes, M.M.S.; dos Santos, E.V.; de Moura, E.C.M.; da Silva, D.R.; Quiroz, M.A.; Martínez-Huitle, C.A. Electrochemical degradation of Novacron Yellow C-RG using boron-doped diamond and platinum anodes: Direct and Indirect oxidation. Electrochim. Acta 2014, 140, 419–426. [Google Scholar] [CrossRef]
- Sala, M.; López-Grimau, V.; Gutiérrez-Bouzán, C. Photo-Electrochemical Treatment of Reactive Dyes in Wastewater and Reuse of the Effluent: Method Optimization. Materials 2014, 7, 7349–7365. [Google Scholar] [CrossRef]
- AENOR. UNE-EN ISO105-J03; 2009; Test for Colour Fastness. Part J03: Calculation of Colour Differences; Spanish Association for the Standardization and Certification: Madrid, Spain, 2009. (In Spanish)
- Chidambaram, T.; Oren, Y.; Noel, M. Fouling of nanofiltration membranes by dyes during brine recovery from textile dye bath wastewater. Chem. Eng. J. 2015, 262, 156–168. [Google Scholar] [CrossRef]
- He, Y.; Li, G.; Wang, H.; Zhao, J.; Su, H.; Huang, Q. Effect of operating conditions on separation performance of reactive dye solution with membrane process. J. Membr. Sci. 2008, 321, 183–189. [Google Scholar] [CrossRef]
- Sala, M.; Gutiérrez-Bouzán, M.C. Electrochemical treatment of industrial wastewater and effluent reuse at laboratory and semi-industrial scale. J. Clean. Prod. 2014, 65, 458–464. [Google Scholar] [CrossRef]
- Kariyajjanavar, P.; Narayana, J.; Nayaka, Y.A. Degradation of textile dye C.I. Vat Black 27 by electrochemical method by using carbon electrodes. J. Environ. Chem. Eng. 2013, 1, 975–980. [Google Scholar] [CrossRef]
| Membrane Characteristics | H50 | H10 |
|---|---|---|
| Membrane Polymer | Sulfonated Polyethersulfone | Sulfonated Polyethersulfone |
| Molecular Weight Cut-off | 1000 daltons | 3000 daltons |
| Maximum Applied Pressure | 41 bar | 41 bar |
| Maximum Continuous Chlorine Concentration | 0.01 g·L−1 | 0.01 g·L−1 |
| Maximum Chlorine Concentration for Cleaning | 0.1 g·L−1 | 0.1 g·L−1 |
| Maximum Operating Temperature | 60 °C | 60 °C |
| Operating pH Range | 2–11 | 2–11 |
| Cleaning pH Range | 1–12 | 1–12 |
| Current Density (mA/cm2) | Intensity (A) | Voltage (V) | t (h) | Power Consumption (W·h·L−1) |
|---|---|---|---|---|
| 33 | 2 | 4.5 | 10 | 45 ± 0.03 |
| 83 | 5 | 5.2 | 4 | 52 ± 0.02 |
| 166 | 10 | 6.3 | 2 | 63 ± 0.08 |
| H50 Membrane | ||||
|---|---|---|---|---|
| Dye | DH | DL | DC | DECMC(2:1) |
| CY | 0.24 ± 0.04 | −1.03 ± 0.05 | 0.46 ± 0.04 | 0.42 ± 0.02 |
| PY | −0.74 ± 0.02 | −0.74 ± 0.05 | −0.07 ± 0.02 | 0.31 ± 0.02 |
| PC | 0.21 ± 0.03 | −0.28 ± 0.03 | 0.16 ± 0.07 | 0.18 ± 0.03 |
| PN | 0.30 ± 0.02 | −0.29 ± 0.02 | 0.02 ± 0.04 | 0.37 ± 0.04 |
| H10 Membrane | ||||
|---|---|---|---|---|
| Dye | DH | DL | DC | DECMC(2:1) |
| CY | −0.07 ± 0.02 | −0.51 ± 0.01 | 2.16 ± 0.04 | 0.28 ± 0.03 |
| PY | −0.40 ± 0.02 | −0.80 ± 0.05 | −0.44 ± 0.04 | 0.34 ± 0.02 |
| PC | 0.73 ± 0.06 | −0.28 ± 0.04 | 0.11 ± 0.03 | 0.28 ± 0.02 |
| PN | −0.08 ± 0.05 | 0.21 ± 0.05 | −0.34 ± 0.02 | 0.25 ± 0.01 |
| Dye | DH | DL | DC | DECMC(2:1) |
|---|---|---|---|---|
| CY | −0.10 ± 0.04 | 0.46 ± 0.10 | 1.29 ± 0.07 | 1.39 ± 0.04 |
| PY | 0.30 ± 0.05 | 0.16 ± 0.04 | −0.06 ± 0.04 | 0.87 ± 0.06 |
| PC | 0.24 ± 0.02 | 0.32 ± 0.05 | −0.31 ± 0.02 | 0.32 ± 0.06 |
| PN | 0.17 ± 0.06 | −0.18 ± 0.11 | −0.16 ± 0.02 | 0.35 ± 0.09 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buscio, V.; García-Jiménez, M.; Vilaseca, M.; López-Grimau, V.; Crespi, M.; Gutiérrez-Bouzán, C. Reuse of Textile Dyeing Effluents Treated with Coupled Nanofiltration and Electrochemical Processes. Materials 2016, 9, 490. https://doi.org/10.3390/ma9060490
Buscio V, García-Jiménez M, Vilaseca M, López-Grimau V, Crespi M, Gutiérrez-Bouzán C. Reuse of Textile Dyeing Effluents Treated with Coupled Nanofiltration and Electrochemical Processes. Materials. 2016; 9(6):490. https://doi.org/10.3390/ma9060490
Chicago/Turabian StyleBuscio, Valentina, María García-Jiménez, Mercè Vilaseca, Victor López-Grimau, Martí Crespi, and Carmen Gutiérrez-Bouzán. 2016. "Reuse of Textile Dyeing Effluents Treated with Coupled Nanofiltration and Electrochemical Processes" Materials 9, no. 6: 490. https://doi.org/10.3390/ma9060490
APA StyleBuscio, V., García-Jiménez, M., Vilaseca, M., López-Grimau, V., Crespi, M., & Gutiérrez-Bouzán, C. (2016). Reuse of Textile Dyeing Effluents Treated with Coupled Nanofiltration and Electrochemical Processes. Materials, 9(6), 490. https://doi.org/10.3390/ma9060490






